Abstract
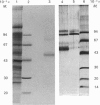
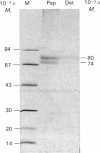
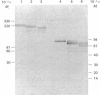
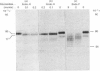
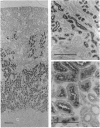
The phosphoramidon-insensitive endopeptidase-2 in rat renal brush borders was investigated by immunochemical approaches with a rabbit polyclonal antibody raised to the purified enzyme released from the membrane by papain. An immunoaffinity column successfully purified the detergent-solubilized form of endopeptidase-2. This preparation had an apparent subunit Mr of 80,000, and did not show the two subunits, of Mr 80,000 and 74,000, consistently found in the papain-solubilized forms, indicating that the latter resulted from proteolysis by papain. SDS/polyacrylamide-gel electrophoresis of non-reduced samples of the enzyme revealed a band of Mr 220,000, confirming the presence of disulphide-bridged subunits. Treatment with endoglycosidases H and F generated smaller molecular forms, indicating that endopeptidase-2 contained about 30% asparagine-linked carbohydrate and that a few of these oligosaccharide chains were of the high-mannose type. Treatment with phosphatidylinositol-specific phospholipase indicated that the enzyme did not possess a glycolipid membrane anchor. A survey of rat tissues examined immunohistochemically and by immunoblotting revealed that only the kidney and intestinal tract expressed the antigen in significant amounts. Although some weak staining was seen in salivary glands and thyroid, other organs and tissues including brain and spinal cord were negative by both immunochemical techniques. In the kidney the antigen was confined to the lumen of the proximal tubule and was seen mainly in the population of juxtamedullary nephrons. In the gut, luminal staining was observed throughout its whole length, from duodenum to rectum. Excellent cross-reactivity of the antibody with Balb/c mouse tissues was observed. Immunohistochemistry of mouse kidney and gut revealed a distribution identical with that observed in the rat. Immunopurification of the detergent-solubilized mouse kidney antigen showed it to be a protein containing disulphide-linked subunits of Mr 90,000. It possessed endopeptidase-2-like activity, but was more efficient in hydrolysing azo-casein and less efficient in hydrolysing a model substrate than the rat enzyme. The close similarity between rat endopeptidase-2 and mouse meprin is further supported by these results.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes K., Matsas R., Hooper N. M., Turner A. J., Kenny A. J. Endopeptidase-24.11 is striosomally ordered in pig brain and, in contrast to aminopeptidase N and peptidyl dipeptidase A ('angiotensin converting enzyme'), is a marker for a set of striatal efferent fibres. Neuroscience. 1988 Dec;27(3):799–817. doi: 10.1016/0306-4522(88)90184-4. [DOI] [PubMed] [Google Scholar]
- Beynon R. J., Shannon J. D., Bond J. S. Purification and characterization of a metallo-endoproteinase from mouse kidney. Biochem J. 1981 Dec 1;199(3):591–598. doi: 10.1042/bj1990591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond J. S., Beynon R. J. Meprin: a membrane-bound metallo-endopeptidase. Curr Top Cell Regul. 1986;28:263–290. doi: 10.1016/b978-0-12-152828-7.50009-3. [DOI] [PubMed] [Google Scholar]
- Bond J. S., Butler P. E., Beynon R. J. Metalloendopeptidases of the mouse kidney brush border: meprin and endopeptidase-24.11. Biomed Biochim Acta. 1986;45(11-12):1515–1521. [PubMed] [Google Scholar]
- Bond J. S., Shannon J. D., Beynon R. J. Certain mouse strains are deficient in a kidney brush-border metallo-endopeptidase activity. Biochem J. 1983 Jan 1;209(1):251–255. doi: 10.1042/bj2090251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth A. G., Hubbard L. M., Kenny A. J. Proteins of the kidney microvillar membrane. Immunoelectrophoretic analysis of the membrane hydrolases: identification and resolution of the detergent- and proteinase-solubilized forms. Biochem J. 1979 May 1;179(2):397–405. doi: 10.1042/bj1790397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth A. G., Kenny A. J. A rapid method for the preparation of microvilli from rabbit kidney. Biochem J. 1974 Sep;142(3):575–581. doi: 10.1042/bj1420575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler P. E., Bond J. S. A latent proteinase in mouse kidney membranes. Characterization and relationship to meprin. J Biol Chem. 1988 Sep 15;263(26):13419–13426. [PubMed] [Google Scholar]
- Butler P. E., McKay M. J., Bond J. S. Characterization of meprin, a membrane-bound metalloendopeptidase from mouse kidney. Biochem J. 1987 Jan 1;241(1):229–235. doi: 10.1042/bj2410229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig S. S., Reckelhoff J. F., Bond J. S. Distribution of meprin in kidneys from mice with high- and low-meprin activity. Am J Physiol. 1987 Oct;253(4 Pt 1):C535–C540. doi: 10.1152/ajpcell.1987.253.4.C535. [DOI] [PubMed] [Google Scholar]
- Danielsen E. M., Norén O., Sjöström H., Ingram J., Kenny A. J. Proteins of the kidney microvillar membrane. Aspartate aminopeptidase: purification by immunoadsorbent chromatography and properties of the detergent- and proteinase-solubilized forms. Biochem J. 1980 Sep 1;189(3):591–603. doi: 10.1042/bj1890591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubray G., Bezard G. A highly sensitive periodic acid-silver stain for 1,2-diol groups of glycoproteins and polysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 15;119(2):325–329. doi: 10.1016/0003-2697(82)90593-0. [DOI] [PubMed] [Google Scholar]
- Fulcher I. S., Kenny A. J. Proteins of the kidney microvillar membrane. The amphipathic forms of endopeptidase purified from pig kidneys. Biochem J. 1983 Jun 1;211(3):743–753. doi: 10.1042/bj2110743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan D., Yoshioka M., Erickson R. H., Heizer W., Kim Y. S. Protein digestion in human and rat small intestine: role of new neutral endopeptidases. Am J Physiol. 1988 Aug;255(2 Pt 1):G212–G220. doi: 10.1152/ajpgi.1988.255.2.G212. [DOI] [PubMed] [Google Scholar]
- Hausen P., Dreyer C. The use of polyacrylamide as an embedding medium for immunohistochemical studies of embryonic tissues. Stain Technol. 1981 Sep;56(5):287–293. doi: 10.3109/10520298109067329. [DOI] [PubMed] [Google Scholar]
- Hooper N. M., Low M. G., Turner A. J. Renal dipeptidase is one of the membrane proteins released by phosphatidylinositol-specific phospholipase C. Biochem J. 1987 Jun 1;244(2):465–469. doi: 10.1042/bj2440465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper N. M., Turner A. J. Ectoenzymes of the kidney microvillar membrane. Aminopeptidase P is anchored by a glycosyl-phosphatidylinositol moiety. FEBS Lett. 1988 Mar 14;229(2):340–344. doi: 10.1016/0014-5793(88)81152-9. [DOI] [PubMed] [Google Scholar]
- Kenny A. J., Fulcher I. S., Ridgwell K., Ingram J. Microvillar membrane neutral endopeptidases. Acta Biol Med Ger. 1981;40(10-11):1465–1471. [PubMed] [Google Scholar]
- Kenny A. J., Ingram J. Proteins of the kidney microvillar membrane. Purification and properties of the phosphoramidon-insensitive endopeptidase ('endopeptidase-2') from rat kidney. Biochem J. 1987 Jul 15;245(2):515–524. doi: 10.1042/bj2450515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Low M. G. Biochemistry of the glycosyl-phosphatidylinositol membrane protein anchors. Biochem J. 1987 May 15;244(1):1–13. doi: 10.1042/bj2440001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsas R., Kenny A. J., Turner A. J. An immunohistochemical study of endopeptidase-24.11 ("enkephalinase") in the pig nervous system. Neuroscience. 1986 Aug;18(4):991–1012. doi: 10.1016/0306-4522(86)90113-2. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Stephenson S. L., Kenny A. J. The metabolism of neuropeptides. Hydrolysis of peptides by the phosphoramidon-insensitive rat kidney enzyme 'endopeptidase-2' and by rat microvillar membranes. Biochem J. 1988 Oct 1;255(1):45–51. doi: 10.1042/bj2550045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterchi E. E., Green J. R., Lentze M. J. Non-pancreatic hydrolysis of N-benzoyl-l-tyrosyl-p-aminobenzoic acid (PABA-peptide) in the human small intestine. Clin Sci (Lond) 1982 May;62(5):557–560. doi: 10.1042/cs0620557. [DOI] [PubMed] [Google Scholar]
- Sterchi E. E., Green J. R., Lentze M. J. Nonpancreatic hydrolysis of N-benzoyl-L-tyrosyl-p-aminobenzoic acid (PABA peptide) in the rat small intestine. J Pediatr Gastroenterol Nutr. 1983;2(3):539–547. doi: 10.1097/00005176-198302030-00024. [DOI] [PubMed] [Google Scholar]
- Sterchi E. E., Naim H. Y., Lentze M. J. Biosynthesis of N-benzoyl-L-tyrosyl-p-aminobenzoic acid hydrolase: disulfide-linked dimers are formed at the site of synthesis in the rough endoplasmic reticulum. Arch Biochem Biophys. 1988 Aug 15;265(1):119–127. doi: 10.1016/0003-9861(88)90377-3. [DOI] [PubMed] [Google Scholar]
- Sterchi E. E., Naim H. Y., Lentze M. J., Hauri H. P., Fransen J. A. N-benzoyl-L-tyrosyl-p-aminobenzoic acid hydrolase: a metalloendopeptidase of the human intestinal microvillus membrane which degrades biologically active peptides. Arch Biochem Biophys. 1988 Aug 15;265(1):105–118. doi: 10.1016/0003-9861(88)90376-1. [DOI] [PubMed] [Google Scholar]