Abstract
Background
Patients with spontaneous subarachnoid hemorrhage (SAH) frequently encounter cognitive dysfunction and mental health issues with negative effects on health-related quality of life (HR-QoL). Here, we aimed to describe the prevalence of cognitive deficits, mental health problems, and HR-QoL impairments 1 year after SAH.
Methods
In this prospective observational study, 177 patients with SAH admitted to our neurointensive care unit over a time span of ten years followed the invitation for an in-person 1-year follow-up, including a standardized neuropsychological test battery. Mental health issues (anxiety and depression) and HR-QoL were evaluated using questionnaires (Hospital Anxiety and Depression Scale; 36-item Short Form questionnaire). Functional outcome was assessed with the modified Rankin Scale (mRS) score.
Results
Patients were 54 years of age (interquartile range 47–62 years) and presented with a median Hunt and Hess score of 2 (interquartile range 1–3) at admission. Most patients (93%) achieved good functional 1-year outcomes (mRS score 0–2). Seventy-one percent of patients had deficits in at least one cognitive domain, with memory deficits being the most prevalent (51%), followed by deficits in executive functions (36%), visuoconstruction (34%), and attention (21%). Even patients with perimesencephalic SAH (18%) or with full functional recovery (mRS score = 0, 46%) had a comparable prevalence of cognitive deficits (61% and 60%, respectively). Symptoms of depression and anxiety were reported by 16% and 33% of patients, respectively. HR-QoL was impaired in 37% (55 of 147). Patients with cognitive deficits (p = 0.001) or mental health issues (p < 0.001) more frequently reported impaired HR-QoL.
Conclusions
Most patients with SAH have cognitive deficits and mental health issues 1 year after SAH. These deficits impair patients’ quality of life.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12028-023-01895-y.
Keywords: Subarachnoid hemorrhage, Neuropsychological evaluation, Cognitive deficits, Mental health outcomes, Quality of life
Introduction
Although mortality has declined substantially after spontaneous subarachnoid hemorrhage (SAH) in the past decades, case fatality rates remain high, ranging between 18 and 43% after the year 2000 [1]. With an average age at disease onset between 50 and 60 years, many patients are at the peak of their professional career and play an indispensable role for their family and socioeconomic environment. Using conventional outcome parameters, the majority of survivors (up to 60%) achieve favorable outcomes after SAH [2]. However, cognitive impairments are evident even in patients with good functional appearance and may remain unrecognized when only assessing functional outcome scores such as the modified Rankin Scale (mRS) score or the Glasgow Outcome Score. Numbers of patients with SAH who subsequently exhibit cognitive impairments are reported to be as high as 40–70% [3, 4]. Persisting cognitive deficits can have effects on day-to-day and social life and negatively influence patients’ activities of daily living, return-to-work, and health-related quality of life (HR-QoL) [5, 6]. To provide targeted cognitive rehabilitation strategies and facilitate workplace reintegration, identification of domain-specific cognitive deficits is crucial. Similarly, mental health and psychosocial problems, including depression and anxiety, affect almost every second patient 1 year after aneurysmal SAH based on pooled frequencies [4]. These symptoms substantially affect patients’ quality of life (QoL) [5].
In this prospective observational study, we aimed to describe multidimensional outcomes, including domain-specific cognitive functions, mental health, and HR-QoL, 1 year after spontaneous SAH. Furthermore, we aimed to identify factors that are associated with cognitive deficits. We hypothesized that cognitive deficits are prevalent and influence functional outcome and HR-QoL 1 year after SAH.
Methods
Study Design, Setting, and Participants
For this observational cohort study, patients with spontaneous SAH admitted to the neurological intensive care unit (ICU) of a tertiary hospital (Medical University of Innsbruck) were screened between April 2010 and December 2020. Of 425 patients, 177 patients fulfilled the following inclusion criteria: (1) diagnosis of a spontaneous aneurysmal and non-aneurysmal SAH (grades 1 to 5 on the Hunt and Hess [H&H] scale) confirmed by computed tomography (CT) scan or lumbar puncture, (2) age greater or equal to 18 years, (3) ICU stay for more than 24 h, (4) fluent in the German language, and (5) neuropsychological and clinical examination at the 1-year follow-up. Supplemental Fig. 1 shows the patient selection by a flowchart. The local institutional review board (Medical University of Innsbruck, AM4091-292/4.6) granted approval for this study. All patients provided informed consent according to local regulations in accordance with the Declaration of Helsinki. Results are reported based on the Strengthening the Reporting of Observational Studies in Epidemiology statement.
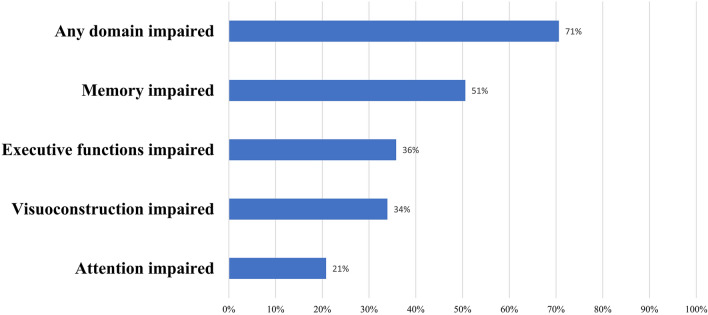
Fig. 1.
Domain-specific cognitive deficits (percentage) 1 year after subarachnoid hemorrhage in 177 patients
Clinical Management
All patients received treatment according to evidence-based international guidelines (except for nimodipine being administered intravenously in poor-grade patients) [7–9]. Prophylactic nimodipine treatment was administered in all patients. Treatment decisions regarding endovascular coiling or neurosurgical clipping for early aneurysm occlusion were based on interdisciplinary case-by-case discussions. Repetitive transcranial color-coded duplex sonography (LOGIQ S8; GE Healthcare, Chicago, IL) was performed to detect large-vessel sonographic vasospasm, which was defined as an elevation of mean velocities > 120 cm/s in the middle or anterior cerebral artery or a daily change in mean velocities > 50 cm/s. In the setting of severe vasospasm, intra-arterial nimodipine was administered after confirmation by catheter cerebral angiogram. Delayed cerebral ischemia was defined as neurological deterioration (new focal neurological deficits and/or a ≥ 2-point decline on the Glasgow Coma Scale) and/or new ischemic lesions on follow-up imaging (CT or magnetic resonance imaging) not attributable to any other cause [10].
Neurorehabilitation after acute ICU treatment followed a multimodal concept. The stages of treatment extend from phase A, the acute phase, to phase E, in which social and professional reintegration takes place. The goal setting was adapted to the individual needs of the patients. According to local regulations, patients received a maximum of 3 h of therapy on working days, and the overall rehabilitation time conformed to the patient’s needs [11].
Outcome Instruments
Functional outcome was assessed at discharge and 3 and 12 months after bleeding using the mRS. Favorable outcome was defined as mRS scores 0–2. At the 1-year follow-up, each patient underwent a medical examination by a doctor of the study team.
All included patients underwent detailed neuropsychological testing performed by experienced neuropsychologists who were blinded to the clinical course. Using a standardized test battery, several cognitive domains were screened as follows:
Executive function was evaluated using six different tests: the Frontal Assessment Battery, which consists of six subtests exploring conceptualization, phonematic verbal fluency, motor programming, sensitivity to interference, inhibitory control, and environmental autonomy [12, 13]; the semantic verbal fluency test (animals per minute), or the Regensburger Verbal Fluency Test (Regensburger Wortflüssigkeitstest; RWT), to assess executive language functions [14]; the Trail Making Test (TMT) A and B to examine processing speed and mental flexibility [15]; the digit span backward subtest of the Wechsler Memory Scale-Revised to assess verbal working memory [16]; and a clock drawing task (CLOX) to assess figure planning [17]. The TMT-B was adjusted for education years with normative values. Impairment of executive function was assumed if the patient scored below the tenth percentile (according to age and/or education stratified norms) in two of six tests.
To assess memory function, two important measures were used: (1) the delayed recall subtest of the Verbal Learning and Memory Test (Verbaler Lern- und Merkfähigkeitstest; VLMT) [18] and (2) the delayed recall subtest of the Rey–Osterrieth complex figure test (ROCF) [19]. Impairment in memory function was assumed if the patient scored below the tenth percentile (according to age stratified norms) in one of two tests.
Attention deficits were assumed if the patient scored below the tenth percentile (according to age norms) in the digit span forward subtest of the Wechsler Memory Scale-Revised [13].
Deficits in visuoconstruction were assessed with the copy subtest of the ROCF [19] and scored in the setting of scores below the tenth percentile.
Overall cognitive functions were screened using the Mini-Mental State Examination, and impairment was classified in patients who scored ≤ 24 out of 30 points [20].
The Hospital Anxiety and Depression Scale (HADS) was applied to detect symptoms of anxiety and depression in survivors of SAH [21]. It consists of an anxiety (HADS‐A) and depression (HADS‐D) subscale, each testing seven items scored from 0 to 3. Scores range from 0 to 21 in each subscale. Lower scores are linked to less severe anxiety‐ and depression‐related symptoms. Scores > 7 suggest anxiety disorder or depression.
HR-QoL was assessed with the 36-item Short Form (SF-36). The SF-36 is a self-report questionnaire and rates the subjective health condition [22]. It provides scores of eight health domains, which can be classified into the physical component summary and mental component summary, each ranging from 0 to 100 points. Higher levels indicate a better health condition. Scores below 40 are considered impaired according to norm-based scoring.
Statistical Analysis
All statistical analyses were generated using IBM SPSS Statistics for Windows (IBM Corp., released 2020, Version 27.0; IBM Corp, Armonk, NY). Numerical data were assessed for normality and are given as medians and interquartile ranges (IQRs); categorical data are reported as counts and proportions. Univariate analysis was done with use of the t-test or Mann–Whitney U-test for continuous variables and Fisher’s exact test for categorical variables, as appropriate. To identify clinically relevant risk factors assessed during the acute phase of disease for cognitive impairments, significantly associated factors (p < 0.1) in univariate analysis were included stepwise in multivariable logistic regression models using generalized linear models and were retained if significant (p < 0.05). Independent associations between cognitive/mental health status and functional outcome at the 1-year follow-up were calculated with generalized linear models adjusted for the H&H score on admission and age as established outcome parameters. The mRS score at 12 months served as an ordinal dependent variable. To check for changes in the mRS score between discharge and the 1-year follow-up, the McNemar test was used.
Results
Patient Population
Of 425 patients, 177 patients were able to complete the 1-year neuropsychological evaluation in-person. At ictus, patients had a median age of 54 (IQR 47–62) years, and 105 (59%) were women (Table 1). The median H&H grade at admission of 2 (IQR 1–3) was significantly lower compared with that for the excluded patient group, who died or did not undergo neuropsychological testing (H&H grade 3 [IQR 1–5], p < 0.001), indicating a less severely injured subpopulation. Still, patients of all disease severity groups were included in the final analysis (Table 1). Included patients were younger (p < 0.001; Supplemental Table 1). The diagnosis of dementia was not recorded in any patient. The median follow-up interval from ICU admission was 374 (IQR 365–387) days.
Table 1.
Demographics of 177 patients with subarachnoid hemorrhage
| N = 177 | |
|---|---|
| Baseline characteristics | |
| Age, years | 54 (47–62) |
| Female sex | 105 (59.3%) |
| Hypertension history | 69 (38.9%) |
| Diabetes mellitus II | 8 (4.5%) |
| Smoking history | 72 (40.7%) |
| Years of education | 10 (9–12) |
| Admission variables | |
| Loss of consciousness at ictus | 51 (28.8%) |
| Parenchymal bleeding at admission | 28 (16.1%) |
| Hunt and Hess Score at admission | 2 (1–3) |
| 1 | 59 (33.3%) |
| 2 | 52 (29.4%) |
| 3 | 32 (18.1%) |
| 4 | 12 (6.8%) |
| 5 | 22 (12.4%) |
| Modified Fisher Scale at admission | 3 (2–4) |
| SEBES score at admission | 1 (0–2) |
| Hjidra score | 11 (6–18) |
| Hjidra ventricle score | 1 (0–4) |
| Aneurysm location | |
| Anterior circulation | 83 (46.9%) |
| Posterior circulation | 42 (23.7%) |
| No aneurysm | 51 (28.8%) |
| Unknown/other | 1 (0.6%) |
| Aneurysm treatment | |
| Coiling | 88 (49.7%) |
| Clipping | 39 (22.0%) |
| No intervention | 51 (28.8%) |
| Hospital complications | |
| Hydrocephalus requiring EVD | 71 (40.1%) |
| Large-vessel vasospasm | 81 (45.8%) |
| Delayed cerebral ischemia | 22 (12.4%) |
| Ventriculitis | 21 (11.9%) |
| Pneumonia | 61 (34.5%) |
| Urinary tract infection | 41 (23.2%) |
| Sepsis/bacteremia | 18 (10.2%) |
| Outcomes | |
| Length of ICU stay, days | 17 (10–27) |
| Hospital mortality | 0 (0%) |
| mRS at discharge | 2 (1–4) |
| mRS at 3 months | 1 (0–2) |
| mRS at 12 months | 1 (0–2) |
| 0 | 82 (46.3%) |
| 1 | 47 (26.6%) |
| 2 | 35 (19.8%) |
| 3 | 9 (5.1%) |
| 4 | 3 (1.7%) |
| 5 | 1 (0.6%) |
Data are n (%) or median (interquartile range)
EVD, external ventricular drain, ICU, intensive care unit, mRS, modified Rankin Scale score, SEBES, subarachnoid hemorrhage early brain edema score
Cognitive Outcomes 1 Year After SAH
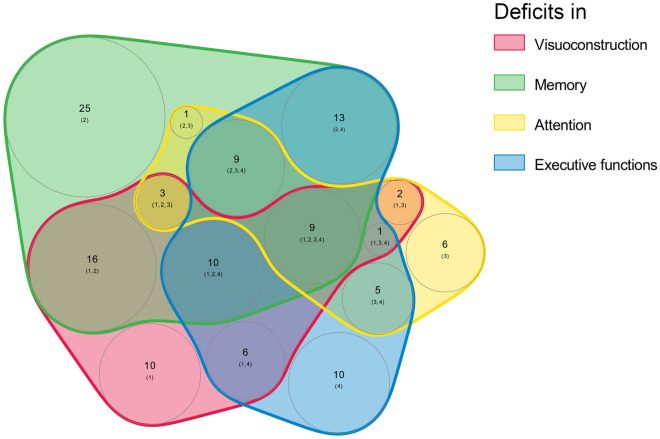
Test results of single examinations 1 year after SAH are given in Table 2. Overall, 71% (125 of 177) of patients had deficits in at least one cognitive domain, with memory deficits being the most prevalent (51%), followed by deficits in executive functions (36%), visuoconstruction (34%), and attention (21%; Fig. 1). More precisely, verbal memory was impaired in 37% (VLMT delayed recall), and deficits in visual memory were evident in 30% of patients (ROCF delayed recall). Executive dysfunctions comprised deficits in cognitive flexibility (27%, TMT-B), deficits in working memory (23%, WMS backward), decreased processing speed (21%, TMT-A), and decreased semantic verbal fluency (13%, RWT, animals/minute). Although co-occurrence of multiple cognitive deficits was frequent (7% of patients with deficits in all tested domains), other patients had deficits in a single domain only (Fig. 2).
Table 2.
Neuropsychological test performance 1 year after subarachnoid hemorrhage in 177 patients
| Cognitive domain | Test | n (%) | Median (IQR) | n (%) average | n (%) slightly impaired | n (%) impaired |
|---|---|---|---|---|---|---|
| Global mental status | MMSE | 176 (99.4) | 29 (28–30) | 147 (83.5) | n.a | 29 (16.5) |
| Executive function | CLOX | 174 (98.3) | 13 (11–14) | 151 (86.8) | n.a | 23 (13.2) |
| Executive function | RWT | 172 (97.2) | 21 (16–26) | 150 (87.2) | 11 (6.4) | 11 (6.4) |
| Executive function | TMT-A | 168 (94.9) | 37.5 (26–54) | 133 (79.2) | 22 (13.1) | 13 (7.7) |
| Executive function | TMT-B | 148 (83.6) | 89 (60.5–120) | 108 (73.0) | 16 (10.8) | 24 (16.2) |
| Executive function | FAB total | 173 (97.7) | 16 (14–18) | 98 (56.6) | 30 (17.3) | 45 (26.0) |
| Executive function | WMS-R, digit span backward | 172 (97.2) | 6 (4–7) | 132 (76.7) | 34 (19.8) | 6 (3.5) |
| Attention | WMS-R, digit span forward | 173 (97.7) | 7 (6–8) | 137 (79.2) | 28 (16.2) | 8 (4.6) |
| VLMT learning, total score | 170 (96.0) | 41 (34–51) | 129 (75.9) | 39 (22.9) | 2 (1.2) | |
| VLMT early recall | 171 (96.6) | 9 (5–11) | 117 (68.4) | 47 (27.5) | 7 (4.1) | |
| Memory function | VLMT delayed recall | 170 (96.0) | 8 (6–11) | 107 (62.9) | 57 (33.5) | 6 (3.5) |
| Memory function | ROCF delayed recall | 162 (91.5) | 16 (10.5–20.5) | 114 (70.4) | 16 (9.9) | 32 (19.8) |
| VLMT recognition | 169 (95.5) | 12 (8–14) | 131 (77.5) | 23 (13.6) | 15 (8.9) | |
| Visuoconstruction | ROCF copy | 168 (94.9) | 33 (30–35) | 111 (66.1) | 17 (10.1) | 40 (23.8) |
| ROCF immediate recall | 163 (92.1) | 17 (11.5–21) | 121 (74.2) | 21 (12.9) | 21 (12.9) | |
| ROCF recognition | 159 (89.8) | 20 (19–22) | 136 (85.5) | 10 (6.3) | 13 (8.2) |
CLOX, clock drawing task, FAB, Frontal Assessment Battery, IQR, interquartile range, MMSE, Mini-Mental State Examination, n.a., not applicable, ROCF, Rey–Osterrieth complex figure test, RWT, Regensburger Wortflüssigkeitstest, TMT A&B, Trail Making Test A&B, VLMT, Verbaler Lern- und Merkfähigkeitstest, WMS-R, Wechsler Memory Scale-Revised
Fig. 2.
Number of patients with co-occurrence of multiple cognitive deficits versus single occurrence of one domain-specific cognitive deficit
Factors Associated with Cognitive Outcomes 1 Year After SAH
Patient and disease related factors associated with single cognitive deficits in univariate analysis are given in Supplemental Table 2. In multivariable analysis, delayed cerebral ischemia (adjusted odds ratio [aOR] 3.80, 95% confidence interval [CI] 1.13–12.77, p = 0.021), a higher mRS score at discharge (aOR 1.43, 95% CI 1.15–1.77, p = 0.001), and fewer years of education (aOR 0.86, 95% CI 0.75–0.98, p = 0.019) were associated with more frequent memory deficits 1 year after SAH. Similarly, a higher mRS score at discharge (aOR 1.65, 95% CI 1.33–2.04, p < 0.001) and fewer years of education (aOR 0.81, 95% CI 0.70–0.94, p = 0.006) predicted the occurrence of executive dysfunctions. Patients with an additional intraparenchymal hemorrhage on the admission CT scan (aOR 3.50, 95% CI 1.35–9.04, p = 0.010), loss of consciousness at ictus (aOR 2.57, 95% CI 1.15–5.73, p = 0.022), and fewer years of education (aOR 0.75, 95% CI 0.61–0.92, p = 0.005) were at higher risk of attention deficits at the 1-year follow-up.
Cognitive Outcomes in Patients with Perimesencephalic SAH
Of 177 patients, 31 (18%) presented with perimesencephalic SAH. Although not significant, these patients had lower prevalence of cognitive deficits (61%) in comparison with patients with non-perimesencephalic SAH (71%, p = 0.277): 42% (vs. 51%, p = 0.324) exhibited memory deficits, 23% (vs. 36%, p = 0.102) exhibited executive dysfunctions, 23% (vs. 34%, p = 0.207) exhibited deficits in visuoconstruction, and 13% (vs. 21%, p = 0.329) exhibited deficits in attention.
Mental Health Outcomes 1 Year After SAH
Depressive and anxiety symptoms (HADS score > 7) were reported by 16% (27 of 165) and 33% (54 of 165) of patients, respectively. In comparison, at SAH onset, 21 (12%) patients were treated for depression and 8 (5%) patients were treated for anxiety. There was no association between premedical history of depression or anxiety and self-reported depressive (p = 0.103) or anxiety symptoms (p = 1.000) at the 1-year follow-up. Patients with depressive symptoms at the 1-year follow-up more commonly had deficits in memory than nondepressive patients (74% vs. 44%; p = 0.006), which was especially true for visual memory (p = 0.046) but not for verbal memory (p = 0.077). No difference was found for other cognitive domains (all p > 0.05). Patients with or without anxiety symptoms had similar frequencies of cognitive deficits (all p > 0.05). Premedical history of depression or anxiety had no effect on cognitive deficits (all p > 0.05).
Relationship Between Cognitive/Mental Health Status and Functional Outcome 1 Year After SAH
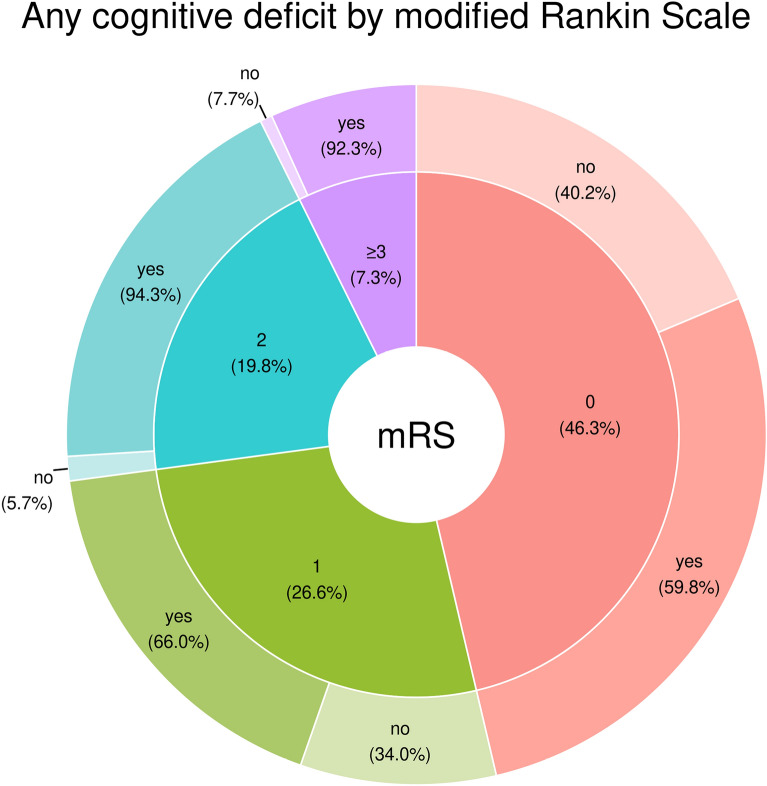
Functional outcomes as assessed with the mRS improved from 2 (IQR 1–4) at discharge to 1 (IQR 0–2) 1 year after SAH (p < 0.001; Supplemental Fig. 2). Accordingly, 93% achieved good functional 1-year outcome (mRS score 0–2). However, 69% of patients with good functional outcome had a cognitive deficit in at least one cognitive domain (Fig. 3, Supplemental Fig. 3). More specifically, in patients presenting without any functional deficit (mRS score = 0, n = 82, 46%), detailed neuropsychological testing revealed cognitive deficits in 60% (n = 49), with the following distribution: impairments in memory (37%), visuoconstruction (30%), executive functions (21%), or attention (16%).
Fig. 3.
Percentages of cognitive deficits (any domain) across scores on the modified Rankin Scale (mRS) in 177 patients with subarachnoid hemorrhage
Functional outcome 1 year after SAH was affected by deficits in memory (p = 0.001) and executive functions (p < 0.001) after correcting for the H&H score and age. Impairments in visuoconstruction (p = 0.177) and attention (p = 0.065) did not influence functional outcome (Table 3). Self-reported symptoms of depression (p < 0.001) and anxiety (p = 0.028) co-occurred with worse functional outcome irrespective of the H&H score and age (Table 3).
Table 3.
Associations between cognitive/mental health status and functional outcome 1 year after SAH
| Variable | Adjusted OR | 95% CI | p value | n |
|---|---|---|---|---|
| Any cognitive deficit | 2.86 | 1.47–5.57 | 0.002 | 176 |
| Executive deficits | 3.23 | 1.72–6.07 | < 0.001 | 175 |
| Visuoconstructive deficits | 1.52 | 0.83–2.82 | 0.177 | 168 |
| Memory deficits | 2.71 | 1.49–4.95 | 0.001 | 170 |
| Attention deficits | 1.98 | 0.96–4.09 | 0.065 | 172 |
| HADS-A > 7 | 2.01 | 1.08–3.74 | 0.028 | 165 |
| HADS-D > 7 | 4.64 | 2.14–10.10 | < 0.001 | 165 |
Multivariable regression models were each calculated with generalized linear models with the modified Rankin Scale score at 12 months serving as an ordinal dependent variable. All models were adjusted for the Hunt and Hess score on admission and age as established outcome parameters. One patient with a modified Rankin Scale score of 5 was excluded from analysis.
CI, confidence interval, HADS-A, Hospital Anxiety and Depression Scale anxiety subscale, HADS-D, Hospital Anxiety and Depression Scale depression subscale, OR, odds ratio, SAH, subarachnoid hemorrhage
HR-QoL 1 Year After SAH
HR-QoL was impaired in 37% (55 of 147) of patients, with 21% reporting restrictions in the physical component summary, 27% reporting restrictions in the mental component summary, and 11% reporting restrictions in both components. Patients with cognitive deficits at the 1-year follow-up (p = 0.001) or mental health issues (p < 0.001) more frequently reported impaired HR-QoL. Moreover, functional outcome was worse in patients with impaired HR-QoL (p < 0.001; Supplemental Table 3).
Return-to-Work
Based on available data (n = 119 of 177), 45% were retired. Of the remaining, 34% returned to their previous occupation, 4% returned but worked fewer hours, 14% failed to return, and 3% were not employed before the bleeding.
Discussion
We describe multidimensional 1-year outcomes, including detailed domain-specific cognitive outcomes, mental health outcomes, functional outcomes, and QoL measures, from a large cohort of patients with spontaneous SAH who were treated over a time span of ten years. We found that seven of ten patients had evident cognitive deficits, which was highest for memory deficits, followed by deficits in executive functions, visuoconstruction, and attention. Even in patients with excellent functional recovery, cognitive deficits were evident in 61% of cases. The most consistent prognostic factors for impairments across all cognitive domains were worse functional status at ICU discharge and fewer years of education. Every third or sixth patient reported anxiety or depressive symptoms, respectively. Cognitive deficits and restrictions in mental health co-occurred with worse functional outcome and HR-QoL.
The overall cognitive performance was worse than that in the normal population, with a high prevalence of cognitive impairments (71%) in our patients. In comparison to the existing literature, in which prevalence rates range from 40 to 70% after aneurysmal SAH, our results are at the upper end of this range [3]. Interestingly, all tested domains of cognitive function were affected with high rates of co-occurrence, which may reflect a more global injury after SAH. As confirmed by others, the highest rate of domain-specific deficits was related to memory dysfunction in our population (51%), which ranges from 14 to 61% in the literature [5, 6]. The estimated prevalence of executive and attention dysfunctions after SAH varies significantly in previous reports, ranging from 3 to 76%, thus making a comparison challenging [3, 5]. There is a limited body of literature reporting cognitive outcomes after perimesencephalic SAH [23, 24], and only low patient numbers were included in respective studies [25, 26]. One study including 18 patients with perimesencephalic SAH found impairments in at least one cognitive domain in 72% (13 of 18 patients); visual memory was impaired in 39%, immediate memory was impaired in 33%, abstraction was impaired in 33%, and verbal fluency was impaired in 28% [25] between 3 months and 6 years after SAH. Another study reported deficits in attention in 25% (3 of 12 patients), poor or impaired memory in 83% (10 of 12 patients), and executive dysfunctions in 33% 12 months after SAH [26]. Thus, these studies suggest slightly higher prevalence rates of cognitive impairments in comparison with our cohort (any cognitive deficit: 61%; memory deficits: 42%; executive dysfunctions: 23%, deficits in visuoconstruction: 23%; deficits in attention: 13%). However, low patient numbers, as well as differing applied tests and definitions of abnormalities, make comparisons challenging. The high variability of cognitive outcome measures among studies has several reasons, as nonstandardized neuropsychological testing batteries with a heterogeneity of scale-specific metrics and inconsistent cutoff scores, variable timing of the testing, and heterogenous study populations were reported [3, 5]. On the other hand, detailed neuropsychological testing is not feasible in patients with SAH with poor recovery [6]. Our data reflect this selection bias of patients with good functional recovery 1 year after the bleeding. This means that we may have likely underestimated the true prevalence of cognitive impairments after SAH. Still, especially in physically independent patients, it is important to screen for and be alert of cognitive deficits that may interact with day-to-day life and the capacity to return to work because these patients may best benefit from tailored rehabilitative measures.
We found that a worse functional outcome at ICU discharge and fewer years of education were consistent risk factors for all tested domains of cognition. However, as only test scores of the TMT-B were adjusted for education years using published normative values, we cannot exclude the preexistence of cognitive deficits in patients with fewer education years given the fact that lower education years have previously been linked to lower memory performance irrespective of brain injury. Still, our finding is in agreement with previous reports [27, 28] and underlines the advantage of individual patients with an increased cognitive reserve before SAH. A higher mRS score at discharge reflects the sum of early and secondary brain injury together with non-disease-specific adverse events during the ICU course, all of which would in principle be modifiable to some part. Mechanisms of early and secondary brain injury, such as increased intracranial pressure, ischemia, blood–brain barrier breakdown, excitotoxicity, cortical spreading depolarizations, toxic effect of blood degradation products [29, 30], neuroinflammation [31], autoinflammation [32], and mitochondrial dysfunction [33, 34], can trigger diffuse neurodegeneration and axonal injury [30, 35], resulting in focal and diffuse global damage to the brain tissue [5, 36]. It is well accepted that disease severity parameters are associated with cognitive impairments in the long term after SAH [3, 5]. Interestingly, we could not replicate previous results by linking a higher clinical initial disease severity grade with poor cognitive performance. Although significant in univariate analysis, the H&H score did not remain significant in multivariable analysis. This may be explainable be the relatively high proportion of good-grade patients included in our cohort (H&H score 1–3, 81%).
Our findings confirm the debilitating nature of SAH-related cognitive dysfunctions, with negative effects on QoL even in patients with good functional outcome. Cognitive deficits impact patients’ behaviors, such as activities of daily living, social and leisure activities, and the ability to return to work. This qualifies cognitive impairment after SAH as a potential candidate to target in future clinical trials. Promising neuroprotective treatment strategies targeting white matter injury are not yet ready for clinical practice [37]. Still, individualized rehabilitative measures, such as neurocognitive training and reintegration programs, are of importance, which is only feasible if comprehensive neuropsychological examinations are incorporated and patients are made aware of the cognitive consequences after SAH [4]. Patients benefit from tailored neurocognitive rehabilitation programs to improve cognitive function and to learn coping strategies or use compensatory tools [11, 38]. In this regard, it is important to particularly pay attention to mental health symptoms, which are associated with the level of patients’ participation in rehabilitation programs. In accordance with previous literature, we found higher prevalence rates of mental health issues, including depression and anxiety, after SAH than in the general population [5, 39]. Both depression and anxiety were linked to poor QoL and functional outcome in our cohort [5]. Consistent with others, only depression was linked to memory deficits [40].
Some limitations of this study deserve mention. First, we did not include a control group to assess for real-life differences with the general population. However, to overcome this issue, we used age-based normative data for all cognitive test domains. Second, we do not have baseline data before the disease, so we cannot assume that all cognitive impairments in our patients are solely disease related. Still, normative data and the relatively young patient age help to minimize this bias. Third, we do not have longitudinal data to conclude on temporal evolutions of neuropsychological outcomes in our patients. Repeated detailed and time-consuming neuropsychological testing, which would imply the bias of training effects, would not be feasible. We think that the timing of 1 year post injury is useful because most patients have finished intensive rehabilitation and are after their active phase of recovery. A future healthy control group–matched multicenter study should be undertaken to confirm our results. Last, we included patients with aneurysmal and non-aneurysmal SAH, limiting the comparability to other studies in which only patients with aneurysmal SAH were included. We therefore provide a subanalysis for patients with perimesencephalic SAH, who are known to have fewer complications and better long-term outcomes. From a pathophysiological point of view, patients with an aneurysmal pattern of blood distribution on imaging modalities, even without the detection of an aneurysm, have similar complications and therefore similar outcomes as patients with aneurysmal SAH.
Conclusions
Our study suggests that domain-specific cognitive deficits, specifically memory deficits, together with mental health issues, including anxiety and depression, are common after SAH despite good functional recovery. Both cognitive and mental health deficits had negative effects on HR-QoL measures. This calls for a thorough screening of neurocognitive as well as mental health outcomes in patients with SAH to offer a more tailored rehabilitative program with the aim to improve patients’ QoL and the return-to-work rate. Furthermore, cognitive deficits should serve as an outcome parameter for the ultimate goal to develop neuroprotective treatment.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank the nursing staff and the entire team of the neurological ICU.
Author contributions
VR and RH designed and oversaw the study. VR, KA, LZ, AL, MK, MG, B-AI, LP, PK, MD, AJS, RB, BP, and RH collected the data. VR and KA performed statistical analysis and wrote the manuscript. All the authors read and approved the final manuscript.
Funding
Open access funding provided by University of Innsbruck and Medical University of Innsbruck.
Source of Support
None.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of interest
None.
Ethical Approval and Informed Consent
This article adheres to ethical guidelines. The conduct of the study was approved by the local ethics committee (Medical University of Innsbruck, AM4091-292/4.6). Written informed consent was obtained according to local regulations.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Verena Rass and Klaus Altmann have contributed equally.
References
- 1.Lovelock CE, Rinkel GJ, Rothwell PM. Time trends in outcome of subarachnoid hemorrhage: population-based study and systematic review. Neurology. 2010;74(19):1494–501. 10.1212/WNL.0b013e3181dd42b3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamdan A, Barnes J, Mitchell P. Subarachnoid hemorrhage and the female sex: analysis of risk factors, aneurysm characteristics, and outcomes. J Neurosurg. 2014;121(6):1367–73. 10.3171/2014.7.JNS132318 [DOI] [PubMed] [Google Scholar]
- 3.Nussbaum ES, Mikoff N, Paranjape GS. Cognitive deficits among patients surviving aneurysmal subarachnoid hemorrhage. A contemporary systematic review. Br J Neurosurg. 2021;35(4):384–401. 10.1080/02688697.2020.1859462 [DOI] [PubMed] [Google Scholar]
- 4.Nwafor DC, Kirby BD, Ralston JD, et al. Neurocognitive sequelae and rehabilitation after subarachnoid hemorrhage: optimizing outcomes. J Vasc Dis. 2023;2(2):197–211. [Google Scholar]
- 5.Al-Khindi T, Macdonald RL, Schweizer TA. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke. 2010;41(8):e519–36. 10.1161/STROKEAHA.110.581975 [DOI] [PubMed] [Google Scholar]
- 6.Mayer SA, Kreiter KT, Copeland D, et al. Global and domain-specific cognitive impairment and outcome after subarachnoid hemorrhage. Neurology. 2002;59(11):1750–8. 10.1212/01.WNL.0000035748.91128.C2 [DOI] [PubMed] [Google Scholar]
- 7.Connolly ES Jr, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012;43(6):1711–37. 10.1161/STR.0b013e3182587839 [DOI] [PubMed] [Google Scholar]
- 8.Steiner T, Juvela S, Unterberg A, et al. European Stroke Organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis. 2013;35(2):93–112. 10.1159/000346087 [DOI] [PubMed] [Google Scholar]
- 9.Hoh, B.L., Ko, N.U., Amin-Hanjani, S., et al. 2023 Guideline for the management of patients with aneurysmal subarachnoid hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke 2023. [DOI] [PubMed]
- 10.Vergouwen MD, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010;41(10):2391–5. 10.1161/STROKEAHA.110.589275 [DOI] [PubMed] [Google Scholar]
- 11.Lindner, A., Brunelli, L., Rass, V., et al. Long-term clinical trajectory of patients with subarachnoid hemorrhage: linking acute care and neurorehabilitation. Neurocrit Care 2022. [DOI] [PMC free article] [PubMed]
- 12.Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a frontal assessment battery at bedside. Neurology. 2000;55(11):1621–6. 10.1212/WNL.55.11.1621 [DOI] [PubMed] [Google Scholar]
- 13.Benke T, Karner E, Delazer M. FAB-D: German version of the frontal assessment battery. J Neurol. 2013;260(8):2066–72. 10.1007/s00415-013-6929-8 [DOI] [PubMed] [Google Scholar]
- 14.Aschenbrenner, S., Tucha, O.Lange, K.W., Regensburger Wortflüssigkeits‐Test (RWT)2001, Göttingen: Hogrefe.
- 15.Reitan R, Wolfson D. The Halstead-Reitan Neuropsycholgical Test Battery: Therapy and clinical interpretation. Tucson: Neuropsychological Press; 1985. [Google Scholar]
- 16.Härting, C., Markowitsch, H.-J., Neufeld, H., et al., Wechsler memory scale—Revised Edition2000, Bern: Huber
- 17.Royall DR, Cordes JA, Polk M. CLOX: an executive clock drawing task. J Neurol Neurosurg Psychiatry. 1998;64(5):588–94. 10.1136/jnnp.64.5.588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helmstaedter C, Durwen HF. The verbal learning and retention test. A useful and differentiated tool in evaluating verbal memory performance. Schweiz Arch Neurol Psychiatr. 1990;141(1):21–30. [PubMed] [Google Scholar]
- 19.Zhang X, Lv L, Min G, et al. Overview of the complex figure test and its clinical application in neuropsychiatric disorders. Includ Copy Recall Front Neurol. 2021;12: 680474. 10.3389/fneur.2021.680474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 21.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 22.Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. 10.1097/00005650-199206000-00002 [DOI] [PubMed] [Google Scholar]
- 23.Burke T, Hughes S, Carr A, Javadpour M, Pender N. A systematic review of cognitive outcomes in angiographically negative subarachnoid haemorrhage. Neuropsychol Rev. 2018;28(4):453–69. 10.1007/s11065-018-9389-1 [DOI] [PubMed] [Google Scholar]
- 24.Kapadia A, Schweizer TA, Spears J, Cusimano M, Macdonald RL. Nonaneurysmal perimesencephalic subarachnoid hemorrhage: diagnosis, pathophysiology, clinical characteristics, and long-term outcome. World Neurosurg. 2014;82(6):1131–43. 10.1016/j.wneu.2014.07.006 [DOI] [PubMed] [Google Scholar]
- 25.Madureira S, Canhao P, Guerreiro M, Ferro JM. Cognitive and emotional consequences of perimesencephalic subarachnoid hemorrhage. J Neurol. 2000;247(11):862–7. 10.1007/s004150070074 [DOI] [PubMed] [Google Scholar]
- 26.Mukerji N, Holliman D, Baisch S, et al. Neuropsychologic impact of treatment modalities in subarachnoid hemorrhage: clipping is no different from coiling. World Neurosurg. 2010;74(1):129–38. 10.1016/j.wneu.2010.05.009 [DOI] [PubMed] [Google Scholar]
- 27.Kreiter KT, Copeland D, Bernardini GL, et al. Predictors of cognitive dysfunction after subarachnoid hemorrhage. Stroke. 2002;33(1):200–8. 10.1161/hs0102.101080 [DOI] [PubMed] [Google Scholar]
- 28.Haug T, Sorteberg A, Finset A, et al. Cognitive functioning and health-related quality of life 1 year after aneurysmal subarachnoid hemorrhage in preoperative comatose patients (Hunt and Hess Grade V patients). Neurosurgery. 2010;66(3):475–84 (discussion 84-5). 10.1227/01.NEU.0000365364.87303.AC [DOI] [PubMed] [Google Scholar]
- 29.Helbok, R., Rass, V., Kofler, M., et al. Intracerebral iron accumulation may be associated with secondary brain injury in patients with poor grade subarachnoid hemorrhage. Neurocrit Care 2021. [DOI] [PMC free article] [PubMed]
- 30.Scherfler C, Schiefecker AJ, Delazer M, et al. Longitudinal profile of iron accumulation in good-grade subarachnoid hemorrhage. Ann Clin Transl Neurol. 2016;3(10):781–90. 10.1002/acn3.341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schiefecker AJ, Dietmann A, Beer R, et al. Neuroinflammation is associated with brain extracellular TAU-protein release after spontaneous subarachnoid hemorrhage. Curr Drug Targets. 2017;18(12):1408–16. 10.2174/1389450117666160201111804 [DOI] [PubMed] [Google Scholar]
- 32.Needham, E.J., Stoevesandt, O., Thelin, E.P., et al. Complex autoantibody responses occur following moderate to severe traumatic brain injury. J Immunol 2021. [DOI] [PMC free article] [PubMed]
- 33.Rass V, Helbok R. Early brain injury after poor-grade subarachnoid hemorrhage. Curr Neurol Neurosci Rep. 2019;19(10):78. 10.1007/s11910-019-0990-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rass, V., Helbok, R. How to diagnose delayed cerebral ischaemia and symptomatic vasospasm and prevent cerebral infarction in patients with subarachnoid haemorrhage. Curr Opin Crit Care 2021; Publish Ahead of Print. [DOI] [PubMed]
- 35.Helbok R, Schiefecker A, Delazer M, et al. Cerebral tau is elevated after aneurysmal subarachnoid haemorrhage and associated with brain metabolic distress and poor functional and cognitive long-term outcome. J Neurol Neurosurg Psychiatry. 2015;86(1):79–86. 10.1136/jnnp-2013-307326 [DOI] [PubMed] [Google Scholar]
- 36.Bendel P, Koivisto T, Aikia M, et al. Atrophic enlargement of CSF volume after subarachnoid hemorrhage: correlation with neuropsychological outcome. AJNR Am J Neuroradiol. 2010;31(2):370–6. 10.3174/ajnr.A1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ru X, Gao L, Zhou J, et al. Secondary white matter injury and therapeutic targets after subarachnoid hemorrhage. Front Neurol. 2021;12: 659740. 10.3389/fneur.2021.659740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilewski M. Treating patients with frontal neurocognitive deficits after SAH and stroke. Acta Neurochir Suppl. 2020;127:175–8. 10.1007/978-3-030-04615-6_27 [DOI] [PubMed] [Google Scholar]
- 39.Powell J, Kitchen N, Heslin J, Greenwood R. Psychosocial outcomes at 18 months after good neurological recovery from aneurysmal subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2004;75(8):1119–24. 10.1136/jnnp.2002.000414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mavaddat N, Sahakian BJ, Hutchinson PJ, Kirkpatrick PJ. Cognition following subarachnoid hemorrhage from anterior communicating artery aneurysm: relation to timing of surgery. J Neurosurg. 1999;91(3):402–7. 10.3171/jns.1999.91.3.0402 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
None.
The data that support the findings of this study are available from the corresponding author upon reasonable request.