Abstract
Background
The probiotic potential of Lacticacid bacteria has been studied in various medical complications, from gastrointestinal diseases to antibiotic resistance infections recently. Moreover, diabetic ulcer (DU) is known as one of the most significant global healthcare concerns, which comprehensively impacts the quality of life for these patients. Given that the conventional treatments of DUs have failed to prevent later complications completely, developing alternative therapies seems to be crucial.
Methods
We designed the stable oleogel-based formulation of viable probiotic cells, including Lactobacillus rhamnosus (L. rhamnosus), Lactobacillus casei (L. casei), Lactobacillus fermentum (L. fermentum), and Lactobacillus acidophilus (L. acidophilus) individually to investigate their effect on wound healing process as an in vivo study. The wound repair process was closely monitored regarding morphology, biochemical, and histopathological changes over two weeks and compared it with the effects of topical tetracycline as an antibiotic approach. Furthermore, the antibiofilm activity of probiotic bacteria was assessed against some common pathogens.
Results
The findings indicated that all tested lactobacillus groups (excluded L. casei) included in the oleogel-based formulation revealed a high potential for repairing damaged skin due to the considerably more levels of hydroxyproline content of tissue samples along with the higher numerical density of mature fibroblasts cell and volume density of hair follicles, collagen fibrils, and neovascularization in comparison with antibiotic and control groups. L. acidophilus and L. rhamnosus showed the best potential of wound healing among all lactobacillus species, groups treated by tetracycline and control groups. Besides, L. rhamnosus showed a significant biofilm inhibition activity against tested pathogens.
Conclusions
This experiment demonstrated that the designed formulations containing probiotics, particularly L. acidophilus and L. rhamnosus, play a central role in manipulating diabetic wound healing. It could be suggested as an encouraging nominee for diabetic wound-healing alternative approaches, though further studies in detailed clinical trials are needed.
Subject terms: Preclinical research, Drug discovery, Diabetes complications
Introduction
The wound healing cascade is a complex multicellular process that initiates at the moment of injury, involving four sequential phases of hemostasis: inflammatory, proliferative, and re-epithelialization [1]. Each phase includes events regulated by numerous physical and biochemical factors and fibroblasts, which play a crucial role in all phases, particularly in the proliferation step [2]. Vascular endothelial growth factor (VEGF) is one of the significant factors in the wound-healing process that contributes to angiogenesis, epithelialization, and collagen deposition [3]. As collagen is a central component of the extracellular matrix, it effectively takes part in the regulation of the wound repair process via inducing the platelets activation, resulting in fibrin clots at the injury site, promoting fibroblast proliferation in the inflammatory stage, synthesis of the growth factors associated with angiogenesis, matrix remodeling, and facilitation of re-epithelialization stage [4, 5].
Diabetic ulcer (DU) pose a significant challenge to healthcare systems worldwide due to their increased rates of mortality and morbidity, as well as adversely impacting the health-related quality of life for patients with diabetes mellitus resulting from the complications of lower limb amputations [6]. The conventional treatment of DU emphasizes wound debridement, off-loading of weight, and antibiotic therapy to control infection. In contrast, the ever-increasing failures of treatment response have been reported in these patients [7]. Antibiotic-resistant diseases are the common reasons for this failure in some cases of severe conditions despite receiving antibiotic therapy, which could raise the necessity of amputation surgeries [8]. Therefore, some alternative approaches are crucial to help these patients to overcome DU.
Lactic acid bacteria (LAB) are generally considered live and safe microorganisms that could be used as probiotics in various medical applications [9–12]. The beneficial effects of probiotics have been proven in recent studies, which might be associated with different mechanisms of action, including improving the immune system and intestinal barrier functions, gut microbial modulation, and antipathogenic effects [13, 14]. The lactobacilli strains can inhibit and control infections caused by Gram-negative and Gram-positive pathogens such as Staphylococcus aureus and Pseudomonas aeruginosa. This property is probably linked to the organic acids like lactic acid they produce and release in biological environments. These antipathogenic properties could be potentially attributed to the morphological and physiological alterations that occur within the bacterial membrane due to exposure to lactic acid [15, 16]. Moreover, these probiotic bacteria can stimulate the expression and production of VEGF and promote fibroblast migration, affecting collagen synthesis and accelerating wound healing [17–20].
Given that the conventional treatments of DU have failed to prevent the complications of this disease completely, innovation and alternative therapies are required for better management of this challenge. Therefore, herein, we designed stable formulations containing four effective probiotic species including Lactobacillus rhamnosus (L. rhamnosus), Lactobacillus casei (L. casei), Lactobacillus fermentum (L. fermentum), and Lactobacillus acidophilus (L. acidophilus) to investigate their effect on different stages of wound healing in diabetic rat animal model. The changes in the morphology of the wounds and the biochemical and histopathological analysis were monitored to evaluate the therapeutic effects of each lactobacillus bacteria over two weeks individually and in comparison, with other tested species. According to the function of probiotics in improving the wound process, these microorganisms are expected to play a part in accelerating health-giving mechanisms in patients with DU.
Materials and methods
Materials
L. rhamnosus (Strain Number: IBRC-M 11409), L. acidophilus (Strain Number: IBRC-M 10815), L. casei (Strain Number: IBRC-M 10711), and L. fermentum (Strain Number: IBRC-M 10816) were purchased from the Iranian Biological Resource Center. All raw materials listed as follows were purchased from Sigma Chemical Co. (St. Louis, MO): streptozotocin (STZ), De Man, Rogosa, and Sharpe (MRS) medium, agar, glycerol (C3H8O3), paraffin, polyethylene glycol (PEG) 400 and 4000, formalin, and Mueller- Hinton broth. Tetracycline 3% ointment (TC) was purchased from Iran Darou Pharmaceutical Co, Iran. Xylazine hydrochloride and ketamine hydrochloride were purchased from Molteni Pharmaceutical Co., Italy. Thiopental was purchased from Loghman Pharmaceutical Co, Iran. Moreover, male Sprague-Dawley rats were purchased from the Laboratory Animals Research Center (Shiraz University of Medical Sciences, Iran).
Formulation development
The oleogel-based formulation was developed according to our prior study [21], containing a particular percentage of glycerol, PEG, and a particular precipitate concentration of each probiotic strain individually as an active pharmaceutical ingredient (API). Four Lactobacillus probiotic species of L. rhamnosus, L. casei, L. fermentum, and L. acidophilus included probiotic contents with a 1 × 109 colony forming unit (CFU) per ml of formulation.
Biofilm formation assay
Antibiofilm activity of L. rhamnosus was carried out against Escherichia coli ATCC 35150 (E. coli), Listeria monocytogenes ATCC 7644 (L. monocytogenes) and Salmonella typhimurium ATCC 14028 (S. typhimurium) biofilms, employing 12-well microtiter plates each containing 2 ml of MRS broth inoculated with 1% v/v of probiotic, were incubated at 30 °C for 48 h. The broth was discarded, and the biofilms formed on the plate wells were washed with 2 ml PBS (pH 7.1) to remove loosely adherent and planktonic cells. Pathogenic bacterial suspensions in TSB, with concentrations of 108 CFU/ml, were added to biofilms and incubated at 30 °C for 24, 48,72, and 96 h. Half of the broth in the plate wells (1 ml) was substituted with fresh broth every 24 h. After the incubation period, the removal of the planktonic cultures from the wells was executed carefully and the biofilms were suspended by shaking and scratching the well. The collected suspensions were employed to determine the adhering pathogen viable count in the biofilm. Saline solution 0.85% (w/v) was used to prepare the appropriate dilution of each suspension and plated on MacConkey sorbitol agar (SM) for E. coli, Modified Oxford agar (MOX) for L. monocytogenes and xylose lysine deoxycholate agar (XLD) for S. typhimurium. The bacterial count was determined after 48 h of incubation at 37 °C. Furthermore, the wells containing 108 CFU/ml of pathogenic bacterial suspension were considered as the control group of each pathogen. All assays were made in triplicate. of L. rhamnosus against three pathogens was calculated using the following formula:
where AC and A represent the log CFUs of the control group individually and log CFUs of each pathogen group treated by L. rhamnosus, respectively.
Experimental animals
Forty-eight male Sprague-Dawley rats (weighing 200–300 g, 8–10 weeks) were purchased from the laboratory animals research center of Shiraz University of Medical Sciences, Iran. The animals were monitored in the animal laboratory in stainless steel cages for two weeks to adapt to the controlled environment at 22–25 °C, humidity 55%, and lighting 12 h light/dark cycles. Their diet consisted of rat chow (Pars Dam Co., Tehran, Iran). The protocol of animal experiments was approved locally by the ethics committee of Shiraz University of Medical Sciences (Code: ir.sums.aec.1400.027), and the guidelines for the care, handling, and use of laboratory animals were precisely followed.
Ethics committee approval
The protocol of this study was approved by the Ethics Committee of Shiraz University of Medical Sciences and performed according to the Ethical Standards laid down in the Declaration of Helsinki of 1964 and its later amendments. It follows the ARRIVE (Animal Research: Reporting of in vivo Experiments) guidelines about using and coring experimental animals.
Induction of DM
The overnight fasted rats received a single dose of intraperitoneal injection of 60 mg/kg body weight STZ that was freshly dissolved in a 0.1 mol/L citrate buffer (pH 4.5) to induce diabetes in rats. After one-week, fasting blood sugar was monitored using the glucometer (Accu-Chek Active, Roche, Germany). The rats with blood glucose levels of more than 300 mg/dl were considered diabetic models and entered the study. Rats weight and water intake were screened throughout the study [22].
Skin wound model
The animals were generally anesthetized by intraperitoneal injection of 5 mg/kg xylazine hydrochloride and 80 mg/kg ketamine hydrochloride. Following the removal of hair and preparation of the dorsal skin with povidone-iodine solution, a biopsy punch is used to outline a circle of 2 cm in diameter under aseptic conditions.
Experimental design
In this experimental study, rats were randomly divided into eight groups of 6 rats each as follows: “control” group, nondiabetic rats without any treatment; the “DM” group, diabetic rats without any treatment; the “DM + B.g” group, diabetic rats received gel formulation without any bacteria once daily for 14 days and the “DM + TC” group, diabetic rats topically received TC, once daily for 14 days, as well as “DM + LBF”, “DM + LBR”, “DM + LBC” and “DM + LBA” groups which defined as diabetic animals received gel formulation containing L. fermentum, L. rhamnosus, L. casei, and L. acidophilus respectively once daily for 14 days. After the intervention, a morphological assay of wound sites and then the biological and histopathological analysis of tissue samples, including hydroxyproline contents, re-epithelization, hair follicle formation, fibroblast population, collagen deposition, and neovascularization been carried out to assess and compare the efficacy of probiotic formulations in the wound healing process. Finally, the euthanasia of all experimental rats was performed with a single dose of thiopental, 100 mg/kg.
Morphological analysis
To quantitatively and qualitatively evaluate the wound healing process in diabetic rats, the wound area was photographed on 0th, 3th, 7th, and 14th post-wounding days (n = 3). The illustrations were analyzed using Image Pro Plus software® V.6 (Media Cybernetics, Inc., Silver Spring, USA). Then, the wound closure percentage was calculated using the initial wound sizes using the following formula:
The higher wound closure percentage assigned to the effective wound healing performance.
Biological analysis
Tissue sampling
Tissue sampling is required before biological assays from biochemical to histopathological analysis. For doing so, the tissue samples were excised from the wound areas on 7th and 14th post-wounding days using a biopsy punch (n = 3). Vertical Uniform Random (VUR) method was used to create random and uniform cuts from tissue samples. Then, tissue samples were applied for biochemical tests. Furthermore, to perform the histopathological and stereological assay, the separated skins were fixed in formalin buffer solution (10%) and embedded in paraffin wax, followed by tissue passage and serial sections of sample blocks [23].
Biochemical analysis
The hydroxyproline content of skin tissues was analyzed as an index of collagen deposition in the wound site for biochemical studies. For this purpose, 500 μl homogenized tissue samples (in 2 ml deionized water, pH = 7.4) were digested in 1 ml of hydrochloric acid (6 N) for 8 h at 120 °C. Then, the suspension was mixed with an equal proportion of citrate-acetate buffer (pH = 6, 25 μl), and 500 μl of chloramines-t-solution, 56 mM, was added and left for 20 min at 25 °C. Finally, 500 μl of Ehrlich’s reagent containing 15 g of p-Dimethyl amino benzaldehyde in n-propanol/perchloric acid (2:1 v/v) was added to each sample, and the resultant mixture was incubated at 65 °C for 15 min. After cooling, the intensity of the developed color of samples was measured at 550 nm using an Ultrospec 2000® UV spectrophotometer (Pharmacia Biotech, Uppsala, Sweden).
Histopathological analysis
Histopathological analysis was performed on 7th and 14th post-wounding days. After the intervention, samples of rats (N = 3) of each group were collected from the wound area by biopsy punch and stored in 10% formaldehyde solution for further histopathological studies with standard procedures. For this purpose, the separated skins were blocked in paraffin after tissue passage and serial section. The histopathological slides were prepared by sectioning, staining, and mounting the paraffin-embedded tissues [23]. The volume and numerical density of skin tissue structures were determined to measure the re-epithelization, hair follicle formation, fibroblast population, collagen deposition, and neovascularization as the histopathological factors studied in the wound healing process. Finally, all animals were euthanized with a single dose of thiopental (100 mg/kg) after each tissue sampling.
The volume density of various components of skin tissue, including epidermis, collagen fibrils, hair follicles, and neovascularization, were determined by using the Delesse formula as follows, which involves counting cross points:
∑P (structure) is equal to the number of points that collided with different parts of the skin tissue, and ∑P (Reference) is similar to the total number of points that collided with the entire skin tissue [23].
Furthermore, the numerical density of the fibroblast cell was determined by the optical dissector method using a micrometer using the following formula:
∑Q is the number of fibroblast cells counted at the height of the dissector, ∑p is the total number of fields in which the counting was done, a/f is equal to the area of the counting frame in all microscopic fields, h is the height of the dissector, t is the average slice thickness in different sections, and BA is the total thickness of the cut [23].
Statistical analysis
Statistical analysis was performed using IBM SPSS software. One-way ANOVA and Tukey’s post hoc test were used to evaluate group differences. All experiments were carried out in triplicate, and a P value ≤ 0.05 was considered statistically significant.
Results
Biofilm formation assay
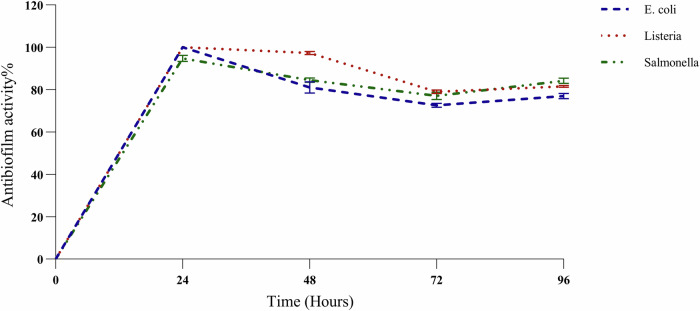
The biofilm formation of all tested pathogens including E. coli, L. monocytogenes, and S. typhimurium was inhibited by L. rhamnosus within 24, 48, 72, and 96 h compared to the control group of each pathogen significantly (P value ≤ 0.05). The highest level of pathogen inhibition was achieved after 24-hour incubation in all groups with antibiofilm activity of 100% in E. coli and L. monocytogenes, and 94.8% in S. typhimurium groups (Fig. 1). Although the Biofilm inhibition of L. rhamnosus was to some extent decreased during the time periods of incubation, 48, 72, and 96 h, this was significant in comparison with control groups of each pathogen with more than about 70% of antibiofilm activity.
Fig. 1. Antibiofilm assay.
Quantification of antibiofilm activity of L. rhamnosus against E. coli, L. monocytogenes, and S. typhimurium, after 0,24,48,72, and 96 h.
Morphological analysis
The appearance and wound closure percentage of wound sites on 0th, 3th, 7th, and 14th post-wounding days of all experimental groups were represented in Fig. 2. The results show that all experimental groups have a continuous reduction of the wound area with a variety of healing times. The highest wound closure percentage was found in Lactobacilli groups, with about 89–96% of DM + LBA and DM + LBR on 14th post-wounding day. At the same time, the least was related to the base gel of formulation (DM + B.g, 62%), which implies that the efficacy of the developed formulation on the healing process is highly linked with its API, probiotic contents, and the base gel has almost showed no remarkable effect. Among tested lactobacilli strain groups, L. acidophilus and L. rhamnosus significantly contributed to wound closure compared to DM and TC as the topical treatment in DU. L. fermentum (DM + LBF) and L. casei (DM + LBC) wound closure efficacy was more potent than the DM group. However, there was no significant difference with TC in the L. fermentum group (DM + LBF), with 85.31% compared to 89.66% and even less potent in the L. casei group (DM + LBC) with 79.96% closure percentage on the 14th day.
Fig. 2. Wound area measurement.
The appearance of wound area (A) and wound closure percentage graph (B) on 0th, 3th, 7th, and 14th post-wounding days of the control, DM, DM + TC, and DM + B.g, DM + LBR, DM + LBF, DM + LBC and, DM + LBA groups. Values are expressed as mean ± S.D. (n = 6 animals). The symbols *† and **†† represent two levels of significant statistical differences (*†P < 0.05, **††P < 0.01).
Biological analysis
Biochemical analysis
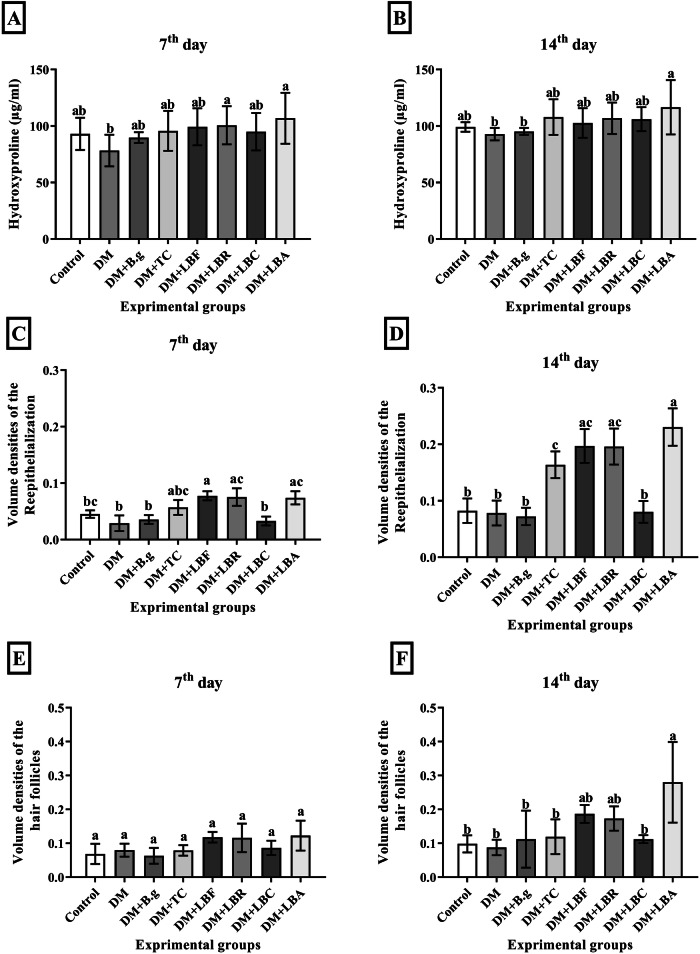
Hydroxyproline content in tissue samples of the experimental groups is reported in Fig. 3A, B. The results showed that the highest and lowest levels of hydroxyproline content were found in the DM + LBA and DM groups for 14 days, 116.67 and 92.84 mcg/ml, respectively. Comparison of the biochemical analysis of samples related to lactobacilli groups with DM and TC showed that DM + LBA and DM + LBR approximately represented a greater hydroxyproline content than DM and DM + TC groups over the experiment period. Moreover, significant differences have not been revealed in hydroxyproline content between lactobacilli groups and comparison with the DM + TC group as well; however, this content in all lactobacilli groups was found more than in the DM group, which was statistically meaningful on the day 7 in DM + LBA and DM + LBR groups, and after 14 days in DM + LBA group not been revealed in hydroxyproline content between lactobacilli groups and comparison with the DM + TC group as well; however, this content in all lactobacilli groups was found more than in the DM group, which was statistically meaningful on the day 7 in DM + LBA and DM + LBR groups, and after 14 days in DM + LBA group.
Fig. 3. Biochemical and Histopathological assay of Tissue samples.
The hydroxyproline content (A and B), volume densities of the re-epithelialization process (C and D) and volume density of hair follicles formation (E and F) of the control, DM, DM + TC, and DM + B.g, DM + LBR, DM + LBF, DM + LBC and, DM + LBA groups on 0th, 3th, 7th, and 14th post-wounding days respectively. Values are expressed as mean ± S.D. (n = 6 animals). Different symbols * † $ in two groups represent significant statistical differences between experimental groups (P < 0.05).
Histopathological analysis
Re-epithelization and hair follicle formation
Figure 3C, D revealed the re-epithelialization rate and volume density of hair follicles of tissue samples in wound sites in all groups in 2 weeks. The results showed the re-epithelialization rate of wound tissues increased during the intervention. Re-epithelization was improved with time in all groups. The highest and lowest volume densities of epithelium were associated with the DM + LBA and DM + B.g groups with 0.23 and 0.07, respectively, after 14 days. Moreover, a comparison of the re-epithelialization rate of samples related to lactobacilli groups with DM and TC showed that all lactobacilli groups except DM + LBC had a higher level of re-epithelialization rate than DM + TC and DM groups on both days, which was significantly compared to DM groups. In contrast, the re-epithelization results related to the DM + LBC group were almost no different from the DM group in both 7 and 14 days, which implies that L. casei is not sufficiently effective in the re-epithelialization process. Furthermore, Fig. 3E, F represents the analysis of the volume density of hair follicle formation of tissue samples in wound tissues during the experiment. The most and least volume density of hair follicles were related to DM + LBA and DM groups with 0.28 and 0.09, respectively, on day 14.
Fibroblast population
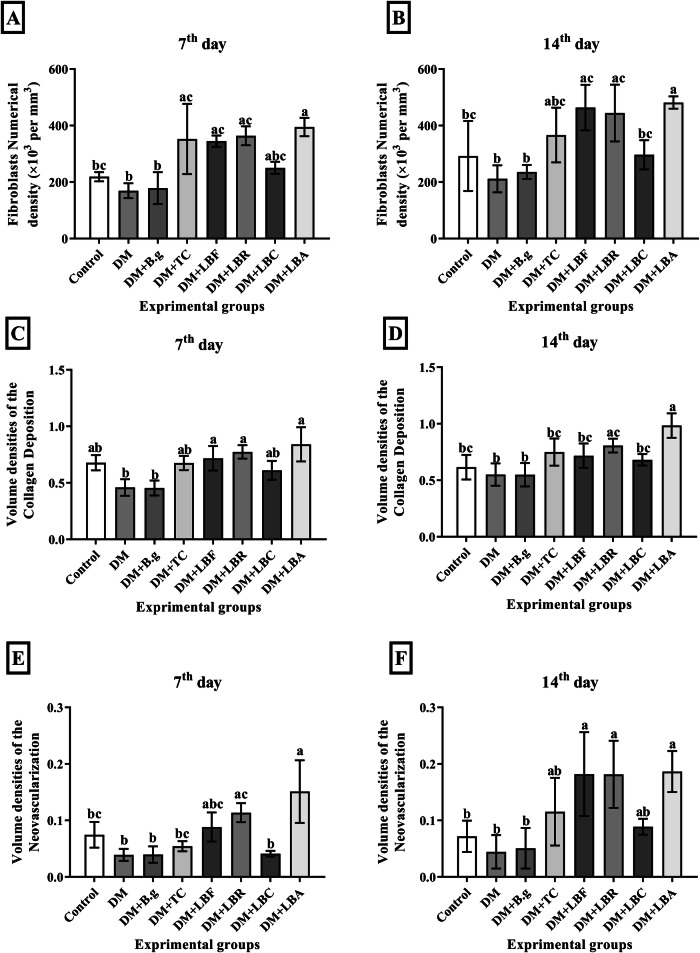
Figure 4A, B demonstrates the numerical density of fibroblast cells in experimental groups on 7th and 14th post-wounding days. The analysis indicated that the DM + LBA group had the highest skin fibroblast population (481.31 ×103/mm3), while the DM group had the lowest (211.51 ×103/mm3) after two weeks. A comparison of the results related to lactobacilli groups with DM and TC indicated that all groups, excluding the DM + LBC group, showed a higher skin fibroblast population than DM + TC groups and statistically more in comparison with DM on both days. The DM + LBC group showed a higher population than DM (296.59 ×103/mm3), without statistical significance.
Fig. 4. Histopathological assay of Tissue samples.
The fibroblast numerical density (A and B), volume density of collagen deposition (C and D) and volume density of neovascularization (E and F) of the control, DM, DM + TC, and DM + B.g, DM + LBR, DM + LBF, DM + LBC and, DM + LBA groups on 7th and 14th post-wounding days respectively. Values are expressed as mean ± S.D. (n = 6 animals). Different symbols * † $ in two groups represent significant statistical differences between experimental groups (P < 0.05).
Collagen deposition and neovascularization
The volume density of collagen deposition and neovascularization of tissue samples related to that of resected specimens are represented in Fig. 4C, D. As shown in the results, the collagen deposition density increased during the wound-healing process within the injured site in all groups. Along that line, the highest and lowest volume density of collagen deposition corresponded to the DM + LBA with 0.23 and DM and DM + B.g groups with 0.08 and 0.07, respectively, on day 14 of the experiment. The comparison between collagen volume density related to lactobacilli groups and DM and TC showed that collagen volume density levels in DM + LBA and DM + LBR were significantly greater than in DM and DM + TC groups after 14 days of intervention. Moreover, there was more in DM + LBF and DM + LBC compared to DM but less than DM + TC, without statistical significance. Besides, the highest and lowest neovascularization profiles were found in DM + LBA and DM groups with a volume density of 0.19 and 0.04 after two weeks of intervention (Fig. 4E, F). The results showed a higher neovascularization profile was observed in all lactobacilli groups compared to DM on day 14, excluding DM + LBC with DM + TC.
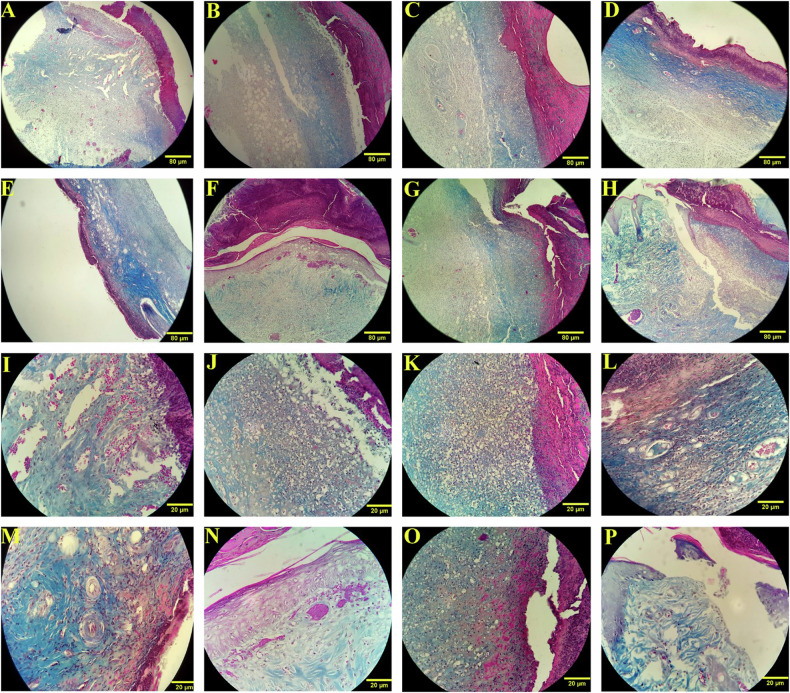
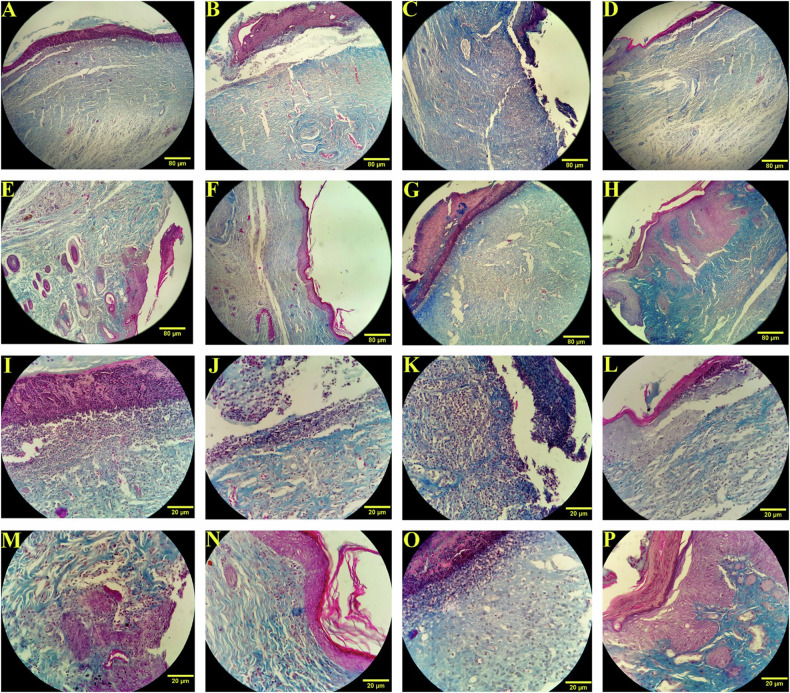
Photomicrographs analysis
The photomicrograph analysis of all experimental groups is illustrated in Figs. 5 and 6. As displayed in images, overall, lactobacilli bacteria, excluding L. casei (Figs. 4O and 5O), represented a higher level of epidermis thickness, hair follicle formation, collagen fibrils maturation, neovascularization, and fibroblast population in comparison with the other groups including control, DM, DM + B.g, and DM + TC on both days 7 and 14. Furthermore, our findings indicated that the strain of L. acidophilus and L. rhamnosus poses the most wound repair potential among all tested lactobacilli groups. The photomicrographs of the group treated by L. acidophilus and L. rhamnosus showed that the epidermis and collagen bundles were noticeably formed in a suitable thickness on both days (Figs. 5P, N and 6P, N respectively). Moreover, a desirable number of fibroblast cells and hair follicles with proper morphology and effective vascular regeneration are observable in microscopic and microscopic images during the experiment period (Figs. 2, 5, and 6). At the same time, the results of the group treated with a formulation containing L. casei showed negligible evidence of spinous cell migration associated with epidermis formation, rarity of neovascularization, and collagen bundle organization (Figs. 5O and 6O). The collagen cells were observed in microscopic images without significant elongated fibers. Moreover, the results revealed an insignificant fibroblast proliferation activity and distinct hair structure.
Fig. 5. Microscope-based assay of wound healing process.
Photomicrographs of the wound site on 7th post-wounding day with 10× (A–H) and 40× (I–P) magnification. control (A and I), DM (B and J), DM + B.g (C and K), DM + TC (D and L), DM + LBF (E and M), DM + LBR (F and N), DM + LBC (G and O) and DM + LBA (H and P) group. Masson’s trichrome staining.
Fig. 6. Microscope-based assay of wound healing process.
Photomicrographs of the wound site on 14th post-wounding day with 10×(A–H) and 40× (I–P) magnification. control (A and I), DM (B and J), DM + B.g (C and K), DM + TC (D and L), DM + LBF (E and M), DM + LBR (F and N), DM + LBC (G and O) and DM + LBA (H and P) group. Masson’s trichrome staining.
Discussion
DU, a severe complication in diabetic patients, has posed a serious challenge for healing due to the heightened risks of treatment resistant infections, which dramatically impact quality of life. On the other hand, the rise of resistant microorganism species to various antimicrobial agents as a worldwide health concern has garnered significant interest in developing alternative approaches to treating infected DU in recent decades. In the other hand probiotics, as the natural bioactive compounds, demonstrate the significant beneficial effects and considerably contribution into wound healing process, though the exact molecular mechanism of action remains unknown yet [24]. Probiotics compared to the small molecules widely contribute to multiple aspects of the wound healing process [25]. It seems that the molecular mechanism of probiotic in wound healing process is to influence essential mediators such as cytokines, chemokines, and growth factors [26]. Besides, these microorganisms facilitate wound healing by inhibiting pathogen adhesion, biofilm colonization, and regulating anti-inflammatory responses. Mohammedsaeed et al. demonstrated that the lysate of L. rhamnosus GG could enhance the re-epithelialization of keratinocytes through upregulation of the chemokine CXCL2 and CXCR2 [27]. Moreover, L. plantarum could reduce the biofilms activity of Staphylococcus aureus in wound areas and enhances the healing process by boosting the host’s innate immunity [28]. Accordingly, given that different probiotic strains represented high therapeutic potentials to cope with a wide range of resistant pathogens, enhance human immunity, and increase the influential factors of wound healing, we studied the contribution of four lactobacilli strains (L. rhamnosus, L. casei, L. fermentum, and L. acidophilus) included as API in a developed oleogel-based formulation to the wound healing process in diabetic animal models. The experiments were conducted individually in each strain for two weeks, and the following evaluations, including morphological, biochemical, and histopathological [29] analysis of tissue samples, were performed.
Biofilm formation assay
The findings of antibiofilm activity exhibited that L. Rhamnosus could effectively inhibit the growth of E. coli, L. monocytogenes, and S. Typhimurium biofilms over the 96 hours. This was in consistent with similar results of Gómez et al. study [1], which was assessed the antibiofilm effects of various probiotics strains on same pathogens. There are a few studies that have explored the therapeutic potential of various probiotics in infected wounds associated with pathogens biofilm, with promising results by disrupting biofilms and reducing bacterial load for faster healing and decreased infection risk [2, 3]. Zhou et al. reported in an in vivo study the significant inhibition activity of bacteriocins isolated from L. rhamnosus against Staphylococcus aureus infection following orthopedic surgery [4]. Moreover, L. acidophilus exhibited antibacterial potentials in Staphylococcal infections by decreasing biofilm formation and bacterial adhesion [5]. The promising antibiofilm findings of probiotics strains make them as a great candidate for topical formulations particularly in wound healing process. Besides, as one of the common complications with DU is the microbiological resistance to conventional treatments, our previous findings showed the antibacterial activity of probiotics as the API of topical formulation in wound healing process [6]. In this study, we assessed the antibacterial activities of the topical formulation containing L. Rhamnosus against two well-known hospital resistant pathogens, vancomycin-resistant Enterococcus (VRE) and methicillin-resistant Staphylococcus aureus (MRSA). The findings indicated that this probiotic formulation could be effective against antibiotic-resistant species particularly MRSA and VRE with MIC value 32 and 16 μg/ml respectively. While assessing the microbiological load post-treatment is crucial for wound healing which should be taken into account in future investigations in this regard.
Morphological analysis
The evaluation of the wound morphology is a primary aspect of wound healing studies, offering valuable information about visual tissue changes indicating cellular and tissue-level events occurring during the wound healing process, which could draw on the efficacy of various therapeutic interventions and monitoring the overall recovery at the wound area [18]. Our findings indicated that the Lactobacillus strains, in particular L. acidophilus and L. rhamnosus, seem to be useful as topical administration for promoting wound closure rate, which is in line with previous studies reporting the application of Lactobacillus strains in the wound healing process [30–32]. Mohseni et al. evaluated the effect of an oral probiotic supplement capsule containing L. acidophilus, L. casei, L. fermentum, and Bifidobacterium bifidum on wound healing in subjects with diabetic foot ulcer [33]. This study, which was conducted for 12 weeks, showed the promising effects of probiotic supplementation on the size of diabetic wounds. Moreover, the efficacy of Lactobacillus bulgaricus (L. bulgaricus) and Lactobacillus plantarum (L. plantarum) have been shown as supplements in promoting the treatment of diabetic rats with cutaneous wounds [19]. Their findings indicated that orally administrating probiotics facilitated the healing process of diabetic wounds by regulating the inflammatory cells. It has been suggested that these bacteria may have been found to modulate the immune response, enhancing the body’s defense mechanisms against infections and improving wound closure rates [30].
Furthermore, our findings indicated that the administration of certain Lactobacillus strains, such as L. acidophilus, could be a helpful intervention in accelerating wound closure even more effective than TC over a 2-week study period, which proposed as an effective topical antibiotic in this regard [34, 35].
Biochemical analysis
Hydroxyproline is a crucial component of collagen, an essential protein for wound healing. Its concentration in wound tissues estimates the collagen amount produced during the healing process [36, 37], a higher amount of hydroxyproline, a more advanced stage, and a higher rate of wound healing [38, 39]. Our experiments confirm previous studies in support of the positive efficacy of probiotics in different forms in improving wound healing rates by increasing hydroxyproline content and collagen deposition. Golkar et al. studied a cold cream formulation containing postbiotics obtained from L. fermentum, Lactobacillus. reuteri and Bacillus subtilis natto to increase wound healing process in animal models [40]. They found that rats treated with postbiotics formulations showed higher amounts of hydroxyproline tissue contents than the control group, making this topical formulation a promising nominee for wound healing approaches. Furthermore, previous studies evaluated the healing potentials of probiotics suspension (medium with probiotics) included in the topical formulation on skin burn wounds [41]. The findings revealed that topical gel formulation containing L. plantarum could significantly increase the amount of hydroxyproline in wound site tissue samples of animal models compared to the control and the base gel groups.
Histopathological analysis
Re-epithelialization is an essential phase in wound healing, which depends on various molecules and cellular processes that elaborate and facilitate it [42]. Our results suggested the beneficial effects of probiotics on the re-epithelialization rate in wound healing, which is consistent with previous studies. Moussavi Amin et al. proposed that the supernatant of Bifidobacterium bifidum in hydrogel could improve re-epithelization in topical administration for dressing wounds [43]. Furthermore, the soluble fraction from the lysate of L. acidophilus, L. plantarum, and Streptococcus thermophilus was suggested to significantly promote the re-epithelialization of immortalized human keratinocytes, physically affect the wound healing process [22]. A similar study by Mei et al. also proved that using hydrogels loaded with L. rhamnosus improves the re-epithelialization rate in infected wound repair in animal models [44]. The hair follicle formation rate could be an excellent index for assessing injury site healing progress. The higher rate of hair follicle formation indicates a more accelerating healing process [45]. According to our results the hair follicle formation rate was found to be higher in almost all lactobacilli groups compared to DM and DM + TC groups, which was significant in the DM + LBA group on day 14, which is in line with previous findings, including Young Min Woo et al. study [46]. They suggested the hydrolyzed protein of L. plantarum as a practical component driving the potential to regenerate hair follicles of wound sites, which is significantly assigned to wound repair rate.
Fibroblast cells contribute to wound repair by producing growth factors such as VEGF and collagen fibers, which are effective in wound re-epithelialization acceleration [47]. Our findings indicate that all lactobacilli strains, except L. casei, considerably increased the skin fibroblast population. This aligns with previous studies that provided evidence to determine the regeneration potential of Kefir as a natural probiotic compound by promoting fibroblast cell migration in animal models [48].
Collagens play an essential role in the wound environment by facilitating various processes, including platelet aggregation, modulation of inflammation, re-epithelialization, and angiogenesis [4, 49]. Our experiments demonstrate that probiotic formulation, mainly containing L. acidophilus, could considerably increase the volume density levels of collagen deposition and neovascularization of tissue samples, which appear well substantiated by previous studies[20, 50].
Furthermore, VEGF known, a primary neovascularization marker, is released by many different cell types, including macrophages, tumor cells, keratinocytes, and platelets, to cope with hypoxia conditions in the wound site and increase the angiogenesis and neovascularization of the endothelial cells [51]. It stimulates several mechanisms of the antigenic cascade, leading to the initiation of capillary tube formation. This, in turn, facilitates the subsequent formation of a new basement membrane, thereby enhancing tissue repair and regeneration [3, 52]. Our findings revealed that treatment with L. acidophilus and L. Rhamnosus substantially enhanced collagen deposition and neovascularization compared to the control group, DM group, and DM + B.g group on 7th and 14th post-wounding days. These findings are consistent with the Hina Khan et al. investigation, which administered the topical form of L. acidophilus in rat wounds and monitored several factors, including neovascularization and fibroblast proliferation. They observed that angiogenesis was increased, and the wound repair at the injury site was accelerated in the group that received topical probiotics compared to other groups [53]. Similarly, Campos LF et al. designed a study to evaluate the effect of orally administered probiotic supplements on the skin healing process of diabetic rats. Their results showed that the probiotic supplemented group had increased deposition of type I collagen and more neovascularization compared to the control group, indicating improved skin healing in the supplemented group [54].
In general, there are several studies in support of the positive efficacy of probiotics on various biomedical applications, particularly wound repair process using different forms of formulation, including probiotics suspensions (media with a certain concentration of probiotics), probiotics precipitate (microorganism cells harvested by centrifugation), cell-free supernatant or bioactive compounds and metabolic compositions secreted by microorganisms known as postbiotic included formulations in improving wound healing rate by increasing hydroxyproline content and collagen deposition [10, 55, 56]. In this experimental study, we suggested a novel formulation containing viable lactobacilli species as API in the form of precipitates in the healing process of diabetic wounds. The evidence intimates that the most therapeutic potential was associated with L. acidophilus and L. Rhamnosus among all groups. At the same time, all lactobacilli groups, excluding L. casei, also considerably impacted diabetic wound repair. It infers that the type of probiotic species also plays a decisive role in the therapeutic efficacy of this formulation. This lower therapeutic efficacy of L. casei could be related to some bioactive exopolysaccharides and peptides released by these bacteria that proposed to inhibit the action of thrombin to regulate the immuno-coagulative response [50, 51]. However, more detailed investigations are required in this regard. It is noteworthy that the difference in therapeutic potentials of LBA with DM and TC was statistically significant on both 7th and 14th post-wounding days healing (Table 1), which implies that this probiotic formulation was influential not only more than the control group and diabetic groups without any treatment, but also than topical antibiotic of TC. Besides, the efficacy of the developed formulation on the healing process is highly linked with its API, probiotic contents, and the base gel of formulation has almost shown no remarkable effect due to demonstrate a negligible impact on wound healing markers.
Table 1.
Maximum (Max) and minimum (Min) parameters associated to the biochemical and histopathological analysis among all groups.
| Max or Min/ post-wounding days | Hydroxyproline content | Re-epithelialization level | Hair follicles population | Fibroblasts population | Collagen deposition level | Neovascularization level |
|---|---|---|---|---|---|---|
| Max/7th | DM + LBAa | DM + LBFa | DM + LBA | DM + LBAa | DM + LBAa | DM + LBAa,b |
| Max/14th | DM + LBAa | DM + LBAa,b | DM + LBAa,b | DM + LBAa | DM + LBAa,b | DM + LBAa |
| Min/7th | DM | DM | DM + B.g | DMb | DM + B.g | DM |
| Min/14th | DM | DM + B.gb | DM | DM | DM + B.g | DM |
aStatistically significant difference in comparison with the DM group.
bStatistically significant difference in comparison with DM + TC group.
Taken together, biochemical and histopathological analysis, including the re-epithelialization, hair follicle formation, fibroblast proliferation, collagen synthesis, and neovascularization, is observed in lactobacilli groups excluded L. casei, particularly in the DM + LBA and DM + LBR group, which implies the remarkable wound healing potential of this probiotic formulation. Furthermore, the findings of antibiofilm studies showed that the antibiofilm and antibacterial potentials of probiotic species could not be negligible in the therapeutic efficacy of this topical formulation. While further investigation and clinical trials are required to introduce this as an alternative approach to managing diabetic wound treatment.
Conclusion
Probiotic therapy is a potent approach to address various complications, from antibiotic resistance as a significant global health issue to chronic wounds. The findings of current study offered the considerable evidence supporting the therapeutic potentials of designed gel formulations containing live Lactobacillus in expediting wound healing in diabetic rats and improving biochemical and histopathological factors, which was observable in both microscopic and microscopic images during the experiment period. This novel formulation, which mainly contains L. acidophilus and L. Rhamnosus, is a potential candidate as a probiotic agent for an alternative approach to managing diabetic wound treatment. In addition, the results demonstrated that the antibacterial and antibiofilm capabilities of the probiotic strains effectively contribute to the therapeutic potential of this topical product. However, further investigation and clinical trials are required in this regard.
Author contributions
Ahmad Gholami, Nima Montazeri-Najafabady, and Amir Azadi were responsible for designing the protocol, writing the protocol and report. Farkhonde Karimi was responsible for conducting the search, screening potentially eligible studies, extracting and analyzing data, interpreting results, updating reference lists and creating ’Summary of findings’ tables. Also, Farkhonde Karimi and Fatemeh Mohammadi were responsible for writing the main manuscript text and prepared figures. Farhad Koohpeyma designing the protocol and screening potentially eligible studies. He contributed to writing the report, extracting and analyzing data, interpreting results and creating ’Summary of findings’ tables. He also contributed to data extraction. Ahmad Gholami, Nima Montazeri-Najafabady,and Amir Azadi provided feedback on the report.
Funding
This study was funded by Shiraz University of Medical Sciences (Grant number: 23627).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved locally by the Ethics Committee of Shiraz University of Medical Sciences (Code: ir.sums.aec.1400.027).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.desJardins-Park HE, Foster DS, Longaker MT. Fibroblasts and wound healing: an update. Future Med. 2018;13:491–5. [DOI] [PubMed] [Google Scholar]
- 2.Cialdai F, Risaliti C, Monici M. Role of fibroblasts in wound healing and tissue remodeling on Earth and in space. Front Bioeng Biotechnol. 2022;10:958381. 10.3389/fbioe.2022.958381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bao P, Kodra A, Tomic-Canic M, Golinko MS, Ehrlich HP, Brem H. The role of vascular endothelial growth factor in wound healing. J Surgical Res. 2009;153:347–58. 10.1016/j.jss.2008.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathew-Steiner SS, Roy S, Sen CK. Collagen in wound healing. Bioengineering. 2021;8:63. 10.3390/bioengineering8050063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhee S. Fibroblasts in three dimensional matrices: cell migration and matrix remodeling. Exp Mol Med. 2009;41:858–65. 10.3858/emm.2009.41.12.096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Everett E, Mathioudakis N. Update on management of diabetic foot ulcers. Ann N Y Acad Sci. 2018;1411:153–65. 10.1111/nyas.13569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eleftheriadou I, Tentolouris A, Tentolouris N, Papanas N. Advancing pharmacotherapy for diabetic foot ulcers. Expert Opin Pharmacother. 2019;20:1153–60. 10.1080/14656566.2019.1598378 [DOI] [PubMed] [Google Scholar]
- 8.Chang M, Nguyen TT. Strategy for Treatment of Infected Diabetic Foot Ulcers. Acc Chem Res. 2021;54:1080–93. 10.1021/acs.accounts.0c00864 [DOI] [PubMed] [Google Scholar]
- 9.Eid R, Jakee J, Rashidy A, Asfour H, Omara S, Kandil M, et al. Potential antimicrobial activities of probiotic Lactobacillus strains isolated from raw milk. J Probiotics Health 2016;4:138. [Google Scholar]
- 10.Azarang A, Farshad O, Ommati MM, Jamshidzadeh A, Heidari R, Abootalebi SN, et al. Protective role of probiotic supplements in hepatic steatosis: a rat model study. BioMed Res Int. 2020;2020:5487659. 10.1155/2020/5487659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gholami A, Dabbaghmanesh MH, Ghasemi Y, Koohpeyma F, Talezadeh P, Montazeri-Najafabady N. The ameliorative role of specific probiotic combinations on bone loss in the ovariectomized rat model. BMC Complement Med Ther. 2022;22:1–11. 10.1186/s12906-022-03713-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montazeri-Najafabady N, Kazemi K, Gholami A. Recent advances in antiviral effects of probiotics: Potential mechanism study in prevention and treatment of SARS-CoV-2. Biologia. 2022;77:3211–28. 10.1007/s11756-022-01147-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng S, Hart A, Kamm M, Stagg A, Knight SC. Mechanisms of action of probiotics: recent advances. Inflamm Bowel Dis. 2009;15:300–10. 10.1002/ibd.20602 [DOI] [PubMed] [Google Scholar]
- 14.Montazeri-Najafabady N, Ghasemi Y, Dabbaghmanesh MH, Ashoori Y, Talezadeh P, Koohpeyma F, et al. Exploring the bone sparing effects of postbiotics in the post-menopausal rat model. BMC Complement Med Ther. 2021;21:155. 10.1186/s12906-021-03327-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma C, Singh BP, Thakur N, Gulati S, Gupta S, Mishra SK, et al. Antibacterial effects of Lactobacillus isolates of curd and human milk origin against food-borne and human pathogens. 3 Biotech. 2017;7:1–9. 10.1007/s13205-016-0591-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gemechu T. Review on lactic acid bacteria function in milk fermentation and preservation. Afr J Food Sci. 2015;9:170–5. 10.5897/AJFS2015.1276 [DOI] [Google Scholar]
- 17.Lam EK, Yu L, Wong HP, Wu WK, Shin VY, Tai EK, et al. Probiotic Lactobacillus rhamnosus GG enhances gastric ulcer healing in rats. Eur J Pharmacol. 2007;565:171–9. 10.1016/j.ejphar.2007.02.050 [DOI] [PubMed] [Google Scholar]
- 18.Dharmani P, De Simone C, Chadee K. The probiotic mixture VSL# 3 accelerates gastric ulcer healing by stimulating vascular endothelial growth factor. PLoS One. 2013;8:e58671. 10.1371/journal.pone.0058671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohtashami M, Mohamadi M, Azimi‐Nezhad M, Saeidi J, Nia FF, Ghasemi A. Lactobacillus bulgaricus and Lactobacillus plantarum improve diabetic wound healing through modulating inflammatory factors. Biotechnol Appl Biochem. 2021;68:1421–31. [DOI] [PubMed] [Google Scholar]
- 20.Gudadappanavar AM, Hombal PR, Timashetti SS, Javali S. Influence of Lactobacillus acidophilus and Lactobacillus plantarum on wound healing in male Wistar rats-an experimental study. Int J Appl Basic Med Res. 2017;7:233. 10.4103/ijabmr.IJABMR_329_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karimi F, Azadi A, Omidifar N, Najafabady NM, Mohammadi F, Kazemi R, et al. Pharmacotechnical aspects of a stable probiotic formulation toward multidrug-resistance antibacterial activity: design and quality control. BMC Complement Med Ther. 2023;23:391. 10.1186/s12906-023-04224-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lombardi F, Palumbo P, Mattei A, Augello FR, Cifone MG, Giuliani M, et al. Soluble fraction from lysates of selected probiotic strains differently influences re-epithelialization of HaCaT scratched monolayer through a mechanism involving nitric oxide synthase 2. Biomolecules. 2019;9:756. 10.3390/biom9120756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howard V, Reed M. Unbiased stereology: three-dimensional measurement in microscopy. London: Garland Science; 2004.
- 24.Heydari Nasrabadi M, Tajabadi Ebrahimi M, Dehghan, Banadaki S. Study of cutaneous wound healing in rats treated with Lactobacillus plantarum on days 1, 3, 7, 14 and 21. Afr J Pharm Pharmacol. 2011;5:2395–401. 10.5897/AJPP11.568 [DOI] [Google Scholar]
- 25.Pratap K, Taki AC, Johnston EB, Lopata AL, Kamath SD. A comprehensive review on natural bioactive compounds and probiotics as potential therapeutics in food allergy treatment. Front Immunol. 2020;11:996. 10.3389/fimmu.2020.00996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nam Y, Kim J, Baek J, Kim W. Improvement of cutaneous wound healing via topical application of heat-killed lactococcus chungangensis cau 1447 on diabetic mice. Nutrients. 2021;13:2666. 10.3390/nu13082666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohammedsaeed W, Cruickshank S, McBain AJ, O’Neill CA. Lactobacillus rhamnosus GG lysate increases re-epithelialization of keratinocyte scratch assays by promoting migration. Sci Rep. 2015;5:16147. 10.1038/srep16147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ong JS, Taylor TD, Yong CC, Khoo BY, Sasidharan S, Choi SB, et al. Lactobacillus plantarum USM8613 aids in wound healing and suppresses Staphylococcus aureus infection at wound sites. Probiotics Antimicrobial Proteins. 2020;12:125–37. 10.1007/s12602-018-9505-9 [DOI] [PubMed] [Google Scholar]
- 29.Knackstedt R, Knackstedt T, Gatherwright J. The role of topical probiotics on wound healing: A review of animal and human studies. Int Wound J. 2020;17:1687–94. 10.1111/iwj.13451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel BK, Patel KH, Huang RY, Lee CN, Moochhala SM. The Gut-Skin Microbiota Axis and Its Role in Diabetic Wound Healing—A Review Based on Current Literature. Int J Mol Sci. 2022;23:2375. 10.3390/ijms23042375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsiouris CG, Tsiouri MG. Human microflora, probiotics and wound healing. Wound Med. 2017;19:33–38. 10.1016/j.wndm.2017.09.006 [DOI] [Google Scholar]
- 32.Bekiaridou A, Karlafti E, Oikonomou IM, Ioannidis A, Papavramidis TS. Probiotics and their effect on surgical wound healing: a systematic review and new insights into the role of nanotechnology. Nutrients. 2021;13:4265. 10.3390/nu13124265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohseni S, Bayani M, Bahmani F, Tajabadi‐Ebrahimi M, Bayani MA, Jafari P, et al. The beneficial effects of probiotic administration on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double‐blind, placebo‐controlled trial. Diabetes Metab Res Rev. 2018;34:e2970. 10.1002/dmrr.2970 [DOI] [PubMed] [Google Scholar]
- 34.Nakao C, Angel M, Di Mateo S, Komesu MC. Effects of topical tetracycline in wound healing on experimental diabetes in rats. Open Diabetes J. 2009;2:53–59. 10.2174/1876524600902010053 [DOI] [Google Scholar]
- 35.Forson O, Ayanka E, Olu-Taiwo M, Pappoe-Ashong P, Ayeh-Kumi P. Bacterial infections in burn wound patients at a tertiary teaching hospital in Accra, Ghana. Ann Burns Fire Disasters. 2017;30:116. [PMC free article] [PubMed]
- 36.Li P, Wu G. Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids. 2018;50:29–38. 10.1007/s00726-017-2490-6 [DOI] [PubMed] [Google Scholar]
- 37.Phang JM, Liu W, Zabirnyk O. Proline metabolism and microenvironmental stress. Annu Rev Nutr. 2010;30:441–63. 10.1146/annurev.nutr.012809.104638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caetano GF, Fronza M, Leite MN, Gomes A, Frade MAC. Comparison of collagen content in skin wounds evaluated by biochemical assay and by computer-aided histomorphometric analysis. Pharm Biol. 2016;54:2555–9. 10.3109/13880209.2016.1170861 [DOI] [PubMed] [Google Scholar]
- 39.Nayak BS, Kanhai J, Milne DM, Pereira LP, Swanston WH. Experimental evaluation of ethanolic extract of Carapa guianensis L. leaf for its wound healing activity using three wound models. Evid Based Complement Alternative Med. 2011;2011:419612. 10.1093/ecam/nep160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Golkar N, Ashoori Y, Heidari R, Omidifar N, Abootalebi SN, Mohkam M, et al. A novel effective formulation of bioactive compounds for wound healing: preparation, in vivo characterization, and comparison of various postbiotics cold creams in a rat model. Evid Based Complement Alternative Med. 2021;2021:8577116. 10.1155/2021/8577116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salaran M, Oryan A, Nikahval B, Kamali A, Ghaemi M, Abbasi-Teshnizi F, et al. Topical application of Lactobacillus plantarum on burn wound healing in diabetic rats. Iran J Vet Surg. 2019;14:60–72. [Google Scholar]
- 42.Rousselle P, Braye F, Dayan G. Re-epithelialization of adult skin wounds: Cellular mechanisms and therapeutic strategies. Adv Drug Deliv Rev. 2019;146:344–65. 10.1016/j.addr.2018.06.019 [DOI] [PubMed] [Google Scholar]
- 43.Tabatabaee Bafroee AS, Khalili Hadad B. Evaluating the Effect of Supernatant Collected from the Culture of Bifidobacterium bifidum on the Increase of Angiogenesis in Acute Wound Healing. Iran J Med Microbiol. 2021;15:552–68. 10.30699/ijmm.15.5.552 [DOI] [Google Scholar]
- 44.Mei L, Zhang D, Shao H, Hao Y, Zhang T, Zheng W, et al. Injectable and self-healing probiotics-loaded hydrogel for promoting superbacteria-infected wound healing. ACS Appl Mater Interfaces. 2022;14:20538–50. 10.1021/acsami.1c23713 [DOI] [PubMed] [Google Scholar]
- 45.Jimenez F, Poblet E, Izeta A. Reflections on how wound healing‐promoting effects of the hair follicle can be translated into clinical practice. Exp Dermatol. 2015;24:91–94. 10.1111/exd.12521 [DOI] [PubMed] [Google Scholar]
- 46.Woo YM, Kim OJ, Jo ES, Jo MY, Ahn MY, Lee Y-H, et al. The effect of Lactobacillus plantarum hydrolysates promoting VEGF production on vascular growth and hair growth of C57BL/6 mice. J Anal Sci Technol. 2019;10:1–9. 10.1186/s40543-019-0178-0 [DOI] [Google Scholar]
- 47.Bainbridge P. Wound healing and the role of fibroblasts. J Wound Care. 2013;22:407–8. 10.12968/jowc.2013.22.8.407 [DOI] [PubMed] [Google Scholar]
- 48.Oryan A, Alemzadeh E, Eskandari MH. Kefir Accelerates Burn Wound Healing Through Inducing Fibroblast Cell Migration In Vitro and Modulating the Expression of IL-1ß, TGF-ß1, and bFGF Genes In Vivo. Probiotics Antimicrob Proteins. 2019;11:874–86. 10.1007/s12602-018-9435-6 [DOI] [PubMed] [Google Scholar]
- 49.Chattopadhyay S, Raines RT. Collagen‐based biomaterials for wound healing. Biopolymers. 2014;101:821–33. 10.1002/bip.22486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elçi MP, Fatsa T, Sinem K, Ersoy N, Alpay M, Özgürtaş T. Overview of the angiogenic effect of probiotics (Lactobacillus acidophilus and Lactobacillus rhamnosus) at human umbilical vein endothelial cells. J Health Sci Med. 2022;5:765–70. [Google Scholar]
- 51.Duffy AM, Bouchier-Hayes DJ, Harmey JH. Vascular endothelial growth factor (VEGF) and its role in non-endothelial cells: autocrine signalling by VEGF. In: Madame Curie Bioscience Database. Austin, Texas: Landes Bioscience; 2013.
- 52.Sinno H, Prakash S. Complements and the wound healing cascade: an updated review. Plast Surg Int. 2013;2013:146764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khan H, Memon S, Korai S, Memon AM, Hameed F, Kamran S. Probiotics Accelerate the Process of Neovascularization in Wound Healing: A Comparative Study in Rats. Natl Editor Advisory Board. 2020;31:124. [Google Scholar]
- 54.Campos LF, Tagliari E, Casagrande TAC, Noronha LD, Campos ACL, Matias JEF. Effects of probiotics supplementation on skin wound healing in diabetic rats. Arq Bras Cir Dig. 2020;33:e1498. 10.1590/0102-672020190001e1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ommati MM, Li H, Jamshidzadeh A, Khoshghadam F, Retana-Márquez S, Lu Y, et al. The crucial role of oxidative stress in non-alcoholic fatty liver disease-induced male reproductive toxicity: the ameliorative effects of Iranian indigenous probiotics. Naunyn Schmiedebergs Arch Pharm. 2022;395:247–65. 10.1007/s00210-021-02177-0 [DOI] [PubMed] [Google Scholar]
- 56.Ashoori Y, Mohkam M, Heidari R, Abootalebi SN, Mousavi SM, Hashemi SA, et al. Development and in vivo characterization of probiotic lysate-treated chitosan nanogel as a novel biocompatible formulation for wound healing. BioMed Res Int. 2020;2020:1–9. 10.1155/2020/8868618 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.