Abstract
This single-arm phase 2 trial (ChiCTR2100046715) examined previously untreated patients with advanced esophageal squamous cell carcinoma (ESCC) who received four cycles of paclitaxel with carboplatin every 3 weeks. Toripalimab was infused intravenously every 3 weeks for 12 months, or until disease progression or intolerable toxicity. Radiotherapy that encompassed the primary lesions and metastases commenced in the third cycle. The median progression-free survival time was 9.8 months (95% confidence interval [CI]: 6.8–not estimable) in the intent-to-treat population, failing to meet the pre-specified primary endpoints. Secondary endpoints included an objective response rate of 45.5%, a disease control rate of 57.6%, and a median duration of response of 11.5 months (interquartile range, 6.4–15.0). The 1-year progression-free survival and overall survival rates were 41.9% (95% CI: 27.7–63.5) and 69.7% (95% CI: 55.7–87.3), respectively. Lymphopenia was the most frequent grade ≥3 adverse event (82%), and an esophageal fistula developed in three patients (9.1%). No treatment-related deaths occurred. In prespecified exploratory biomarker analysis, higher densities of CD8 + T cells, CD11c+ dendritic cells, and CD68+ macrophages correlated with improved tumor response and prognosis. Radiotherapy supplementation to first-line chemo-immunotherapy for treatment-naive advanced ESCC demonstrated some antitumor activity and manageable safety profiles, warranting further randomized controlled trials.
Subject terms: Radiotherapy, Tumour biomarkers, Cancer immunotherapy
Chemo-immunotherapy regimens are now recommended as first-line treatment for patients with advanced esophageal squamous cell carcinoma (ESCC), however survival outcomes remain unsatisfactory. Here the authors report the results of a phase 2 trial of toripalimab (anti-PD1) plus chemotherapy and radiotherapy in patients with treatment-naïve advanced ESCC.
Introduction
Esophageal cancer is associated with significant mortality, particularly in Asia, where esophageal squamous cell carcinoma (ESCC) is the most common histological subtype, accounting for approximately 90% of all cases. At the point of diagnosis, over two-thirds of patients already exhibit metastatic or locally advanced disease1. The National Comprehensive Cancer Network guidelines specify the use of chemo-immunotherapy as the first-line treatment for advanced esophageal cancer2. This recommendation was based on several large-scale phase III trials that reported substantial prolongation of progression-free survival (PFS) (5.7–7.3 months) and overall survival (OS) (12.6–17.2 months) compared to those using chemotherapy alone3–9. However, despite progress in therapeutic outcomes, the average 6-month PFS remains unsatisfactory, suggesting the development of drug resistance and disease progression during maintenance immunotherapy.
For cases with advanced esophageal cancer who have not received previous treatment, radiotherapy targeting both the primary and metastatic lesions is a vital therapeutic option. This intervention significantly improves dysphagia and nutritional status when directed at the primary lesion. It also alleviates pain and other symptoms, while inducing an abscopal effect in metastatic cases. Two retrospective studies indicated that compared to the use of chemotherapy alone, the combination of chemotherapy with radiotherapy modestly enhances the objective response rate and prognosis10,11. However, in the current landscape where chemo-immunotherapy is the standard for advanced esophageal cancer, radiotherapy is predominantly considered a salvage second-line treatment. Notably, reports on the use of radiotherapy with chemo-immunotherapy in cases of local recurrence and/or distant metastasis after first-line treatment failure have demonstrated clinical benefits and acceptable safety12,13. Furthermore, some studies have primarily focused on patients with metachronous oligometastatic esophageal cancer, considering radical radiotherapy exclusively for metastatic lesions14,15. In a recent prospective trial involving patients with a controlled esophageal primary lesion, the group receiving local treatment—mainly consisting of stereotactic body radiotherapy (SBRT) for metastatic lesions together with systemic therapy (chemotherapy or chemo-immunotherapy)—exhibited a median PFS of 15.3 months, compared to 6.4 months for systemic therapy alone15. Nonetheless, the safety and efficacy of this approach as a primary treatment strategy in treatment-naive advanced ESCC have yet to be substantiated by extensive research data, although multiple ongoing prospective clinical studies are anticipated to publish their findings soon16–18. Consequently, the optimal treatment approach for treatment-naive advanced ESCC and the role of radiotherapy within this regimen remains a subject of debate. This encompasses the decision on when to integrate radiotherapy with chemo-immunotherapy and the consideration of the simultaneous irradiation of primary and metastatic lesions.
Herein, we conduct a single-arm trial to assess the safety and efficacy of combining radiotherapy with chemo-immunotherapy as a first-line treatment for advanced ESCC. The addition of radiotherapy to first-line chemo-immunotherapy results in a median PFS of 9.8 months and demonstrates a manageable safety profile. Despite the primary endpoint (median PFS) was not met, this research highlights the feasibility of administering this combined treatment regimen to patients with treatment-naive advanced ESCC.
Results
Participants and treatment
From June 30, 2021 to September 30, 2022, we assessed 56 patients for eligibility; among them, 33 (median age: 59 years; range: 43–74; 29 men) were enrolled (Fig. 1). The most common site for oligometastasis was distant lymph nodes (63.6%), followed by the lungs (15.2%), and bones (9.1%) (Table 1). We observed that 27 (81.8%) patients had a total of 32 oligometastatic lesions, of which 12 were in distant organs and 15 in non-regional lymph nodes, whereas six patients (18.2%) had only regional lymph node metastases (cTanyN3M0). Treatment was permanently stopped prior to the commencement of radiotherapy in five patients for the following reasons: informed consent withdrawal (n = 1), supraventricular arrhythmia (n = 1), esophageal fistulae (n = 2), and disease progression (liver metastasis; n = 1). Among the 28 patients who started radiotherapy, one withdrew consent after completing three sessions of radiotherapy, and another refused radiotherapy for liver metastasis after completing radiotherapy for the primary lesion. A total of 26 patients (78.8% of the enrolled individuals) completed radiotherapy of all lesions (both esophageal and metastatic) and four chemotherapy cycles. Among these patients, 14 (42.4%) completed the planned 1-year treatment with toripalimab, with a median of 10 treatment cycles (interquartile range [IQR]: 4.5–14). The primary reasons for the early discontinuation of toripalimab included disease progression (n = 10), adverse events (AEs) (n = 4), and informed consent withdrawal (n = 5). Detailed information is available in Supplementary Table 1.
Fig. 1. Trial profile.
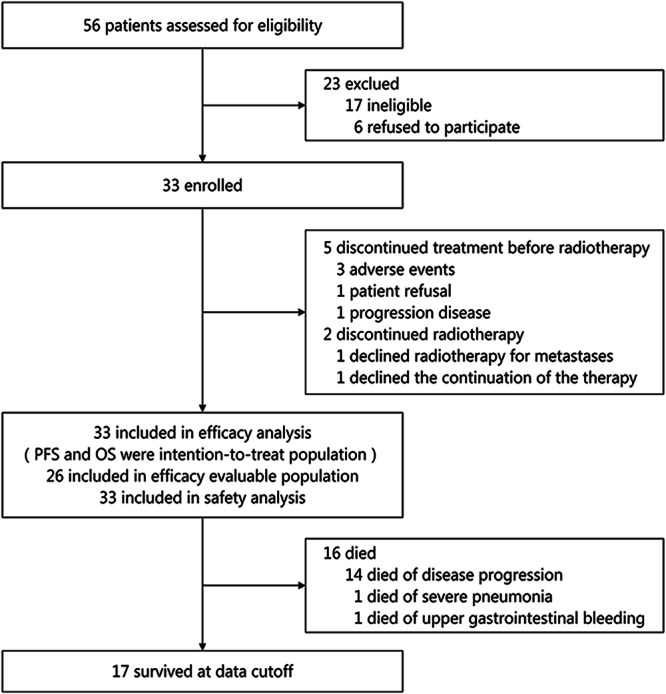
A total of 56 patients were screened, among them 23 were excluded and 33 patients were included. Five patients, after completing at least one cycle of chemo-immunotherapy, did not receive radiotherapy due to adverse effects, tumor progression, or withdrawal of informed consent. Two patients discontinued the study treatment as they did not complete the entire course of radiotherapy as per the study protocol.
Table 1.
Baseline patient characteristics
| Characteristics | Total (n = 33) |
|---|---|
| Age, years; median (IQR) | 59 (55–69) |
| Sex | |
| Male | 29 (87.9%) |
| Female | 4 (12.1%) |
| Smoking history | |
| Yes | 25 (75.8%) |
| No | 8 (24.2%) |
| Alcohol history | |
| Yes | 24 (72.7%) |
| No | 9 (27.3%) |
| Bodyweight loss | |
| <10% | 30 (90.9%) |
| ≥10% | 3 (9.1%) |
| ECOG performance status | |
| 0 | 15 (45.5%) |
| 1 | 18 (54.5%) |
| Tumor location | |
| Upper | 6 (18.2%) |
| Middle | 12 (36.4%) |
| Lower | 15 (45.5%) |
| Primary tumor length (cm) | |
| ≤5 | 7 (21.2%) |
| >5 | 26 (78.8%) |
| Clinical T stage | |
| T3 | 26 (78.8%) |
| T4 | 7 (21.2%) |
| Clinical N stage | |
| N2 | 14 (42.4%) |
| N3 | 19 (57.6%) |
| Clinical M stage | |
| M0 | 6 (18.2%) |
| M1 | 27 (81.8%) |
| Clinical TNM stage | |
| IV | 33 (100%) |
| Site of metastases | |
| Distant lymph nodes | 22 (66.7%) |
| Lung | 6 (18.2%) |
| Liver | 1 (3.0%) |
| Bone | 3 (9.1%) |
| Spleen | 1 (3.0%) |
| Adrenal gland | 1 (3.0%) |
Data are presented as n (%). ECOG Eastern Cooperative Oncology Group, IQR interquartile range.
Efficacy outcomes in patients who completed radiotherapy of all lesions
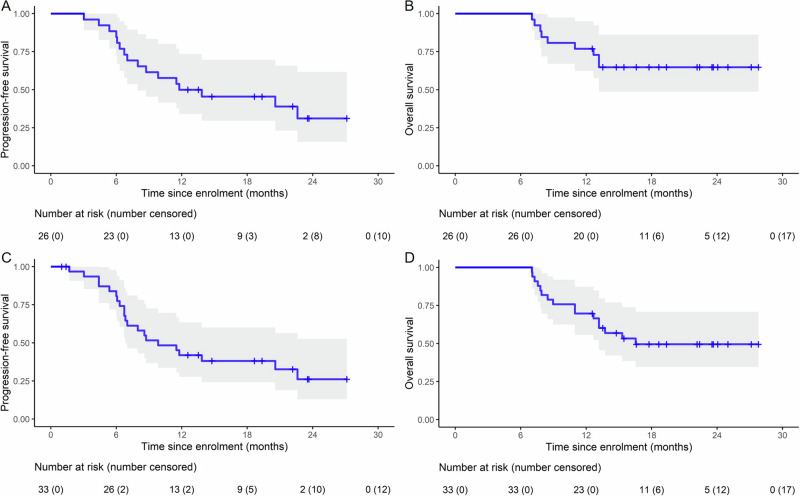
We evaluated the efficacy of the treatment regimen in the 26 patients who completed radiotherapy of all lesions (esophageal and metastatic) and four chemotherapy cycles. Assessment at 3 months after completing the radiotherapy revealed partial response (PR) in 15 patients (57.7%), stable disease (SD) in four patients (15.4%), and disease progression in seven patients (26.9%). Notably, the objective response rate (ORR) was 57.7% (15/26; 95% confidence interval [CI]: 36.9–76.7%), whereas the disease control rate (DCR) was 73.1% (19/26, 95% CI: 52.2–88.4%) (Supplementary Table 2). The 1-year PFS and OS rates were 50.0% (95% CI: 34–73.4%) and 76.9% (95% CI: 62.3–94.9%), respectively. The median PFS was 12.8 months (95% CI: 8.0 months–not estimable) (Fig. 2A) and the median OS was not attained (Fig. 2B).
Fig. 2. Kaplan−Meier estimates of survival.
A, B PFS and OS in the efficacy-evaluable population (n = 26). C, D PFS and OS in the ITT population (n = 33). The gray shaded area represents the 95% CI. Source data are provided as a Source Data file. CI confidence interval, PFS progression-free survival, OS overall survival, ITT intention-to-treat.
Efficacy outcomes in intent-to-treat (ITT) patients
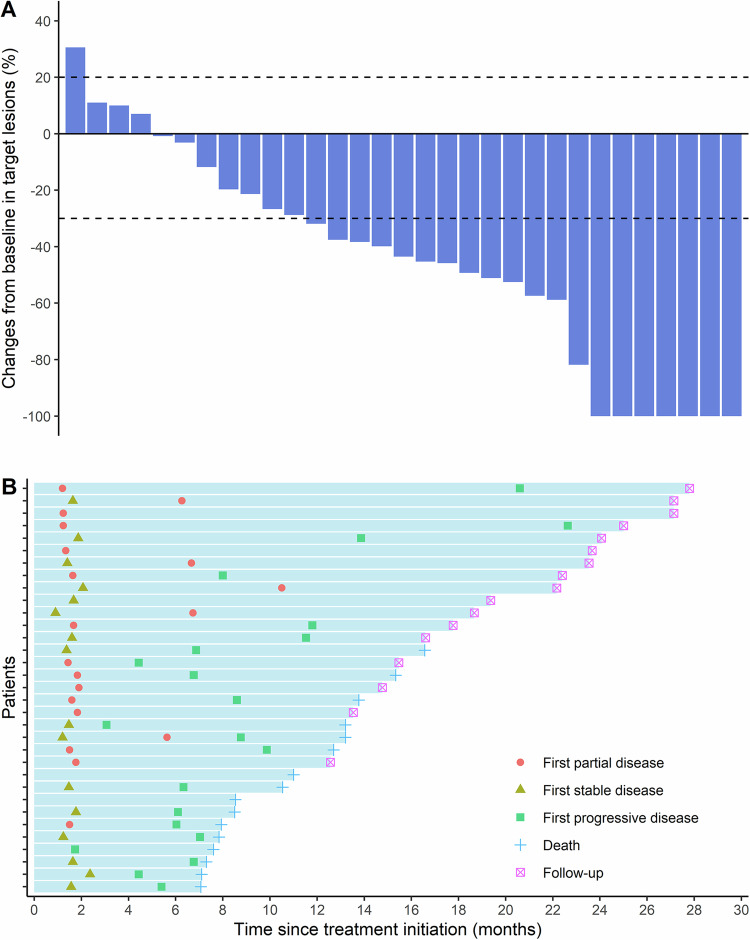
Analysis of the best overall response showed reductions in the sizes of target lesions after treatment compared to baseline in 27 of the 33 enrolled patients (Fig. 3A). Responses included complete response (CR) in seven cases, PR in 13, and SD in seven cases. Four patients exhibited increases in the target lesion size, including one patient with disease progression, while two patients could not be evaluated. Three months after the completion of radiotherapy, an efficacy evaluation was conducted on the ITT population. The results showed an ORR of 45.5% (15/33; 95% CI: 28.1–63.7%) and a DCR of 57.6% (19/33; 95% CI: 39.2–74.5%) (Supplementary Table 2). A summary of the tumor responses observed following two cycles of chemo-immunotherapy is provided in Supplementary Table 3.
Fig. 3. Tumor responses.
A Best percentage changes in target lesion sizes from baseline (n = 31). Dashed lines at +20% and −30% represent thresholds for disease progression and partial response, respectively, according to the RECIST 1.1 criteria. Among the total 33 patients, One patient withdrew informed consent after one cycle of chemo-immunotherapy. One patient experienced severe cardiac adverse events after two cycles of chemo-immunotherapy. Both patients refused further assessment. Therefore, only 31 patients had a baseline and at least one post-baseline radiologic assessment. B Onset of response, duration of response, and outcome (n = 33). Source data are provided as a Source Data file. RECIST Response Evaluation Criteria in Solid Tumors.
Overall, the median follow-up was 22.2 (range: 16.3–28.1) months and the median duration of response (DoR) was 11.5 (IQR, 6.4–15.0) months. Among the 33 patients, 21 experienced recurrences (64%), and 16 died because of the disease (48%). A summary of the information on recurrence (pattern, site, and reasons for death) is provided in Supplementary Table 4. Responses and outcomes are summarized in Fig. 3B. The 1-year PFS and OS rates were 41.9% (95% CI: 27.7–63.5%) and 69.7% (95% CI: 55.7–87.3%), respectively. The median PFS was 9.8 months (95% CI: 6.83 months–not estimable; primary endpoint was not met) (Fig. 2C), while the median OS was 16.5 months (95% CI: 13.2 months–not estimable) (Fig. 2D). Subsequent treatments after recurrence are provided in Supplementary Table 5.
Safety
All 33 cases experienced treatment-related AEs (TRAEs) of varying grades (Table 2). No unexpected AEs or treatment-related deaths occurred. Commonly observed TRAEs included myelosuppression, weight loss, and anorexia, and commonly observed grade 3 or higher AEs were lymphopenia (27/33, 82%), neutropenia (9/33, 27%), and leukopenia (8/33, 24%). More specifically, radiotherapy-related AEs mainly included radiation esophagitis (24/28, 86%), radiation dermatitis (21/28, 75%), esophageal/epigastric pain (18/28, 64%), and radiation pneumonitis (6/28, 22%) (Table 3). The most frequent immune-related AEs were hypertriglyceridemia (21/33, 64%), hypothyroidism (18/33, 54%), and rash (4/33, 12%). Three patients (9.1%) developed grade 3 esophageal fistula; two immediately after two cycles of chemo-immunotherapy, and one at 4 months after the completion of radiotherapy. All three patients who developed esophageal fistula had T4 tumors (tumor length >5 cm).
Table 2.
Treatment-related adverse events in all patients
| Total (n = 33) | ||||
|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Treatment-related adverse events, n (%) | ||||
| Lymphopenia | 0 | 2 (6%) | 18 (55%) | 9 (27%) |
| Leukopenia | 1 (3%) | 14 (42%) | 7 (21%) | 1 (3%) |
| Neutropenia | 6 (18%) | 7 (21%) | 8 (24%) | 1 (3%) |
| Thrombocytopenia | 14 (42%) | 6 (18%) | 1 (3%) | 0 |
| Anemia | 22 (67%) | 5 (15%) | 3 (9%) | 1 (3%) |
| Elevated triglyceride | 17 (52%) | 2 (6%) | 2 (6%) | 0 |
| Bilirubin elevation | 6 (18%) | 2 (6%) | 0 | 0 |
| Hypoproteinemia | 31 (94%) | 2 (6%) | 0 | 0 |
| Creatinine increased | 3 (9%) | 0 | 0 | 0 |
| AST elevation | 17 (52%) | 1 (3%) | 0 | 0 |
| ALP elevation | 10 (30%) | 1 (3%) | 0 | 0 |
| Gamma-glutamyl transferase elevation | 9 (27%) | 1 (3%) | 2 (6%) | 0 |
| ALP elevation | 7 (21%) | 0 | 1 (3%) | 0 |
| Hypothyroidism | 14 (42%) | 4 (12%) | 0 | 0 |
| Anorexia | 3 (9%) | 8 (24%) | 0 | 0 |
| Fatigue | 2 (6%) | 1 (3%) | 0 | 0 |
| Nausea or vomiting | 3 (9%) | 5 (15%) | 2 (6%) | 0 |
| Constipation | 4 (12%) | 3 (9%) | 0 | 0 |
| Rash | 2 (6%) | 1 (3%) | 1 (3%) | 0 |
| Arrhythmia | 1 (3%) | 1 (3%) | 1 (3%) | 0 |
| Weight loss | 7 (21%) | 5 (15%) | 0 | 0 |
| Fever | 1 (3%) | 0 | 0 | 0 |
| Diarrhea | 1 (3%) | 1 (3%) | 0 | 0 |
| Esophageal fistula | 0 | 0 | 3 (9.1%) | 0 |
ALP alkaline phosphatase, ALT alanine aminotransferase, AST aspartate aminotransferase, GGT gamma-glutamyl transferase.
Table 3.
Radiotherapy-related adverse events
| Total (n = 28) | ||||
|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Radiotherapy-related adverse events, n (%) | ||||
| Esophagitis | 9 (32%) | 12 (43%) | 3 (11%) | 0 |
| Radiation dermatitis | 21 (75%) | 0 | 0 | 0 |
| Cough | 1 (4%) | 8 (29%) | 0 | 0 |
| Pneumonitis | 0 | 3 (11%) | 3 (11%) | 0 |
| Esophageal/epigastric pain | 12 (43%) | 6 (21%) | 0 | 0 |
Biomarkers for treatment response and outcomes
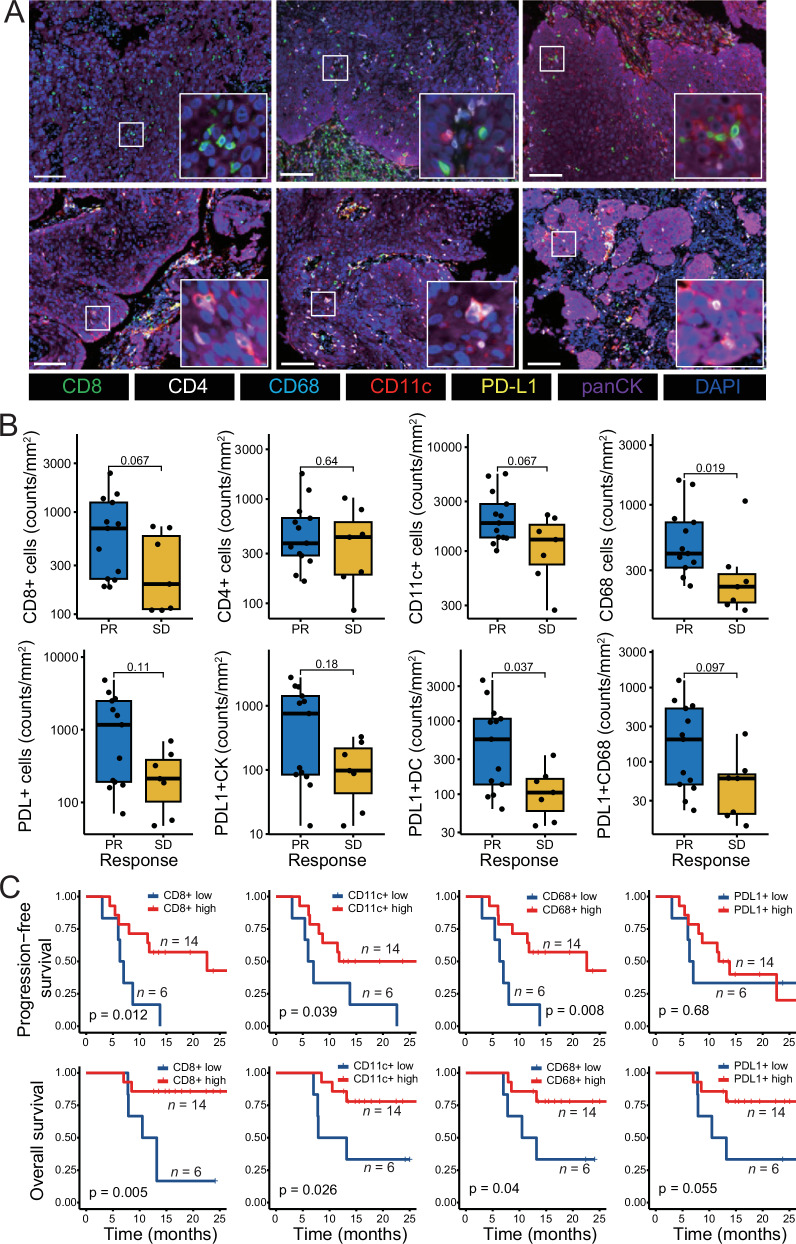
We conducted a prespecified exploratory analysis of biomarkers to assess treatment response and outcomes. Baseline tumor biopsies from 76.9% (20/26) of patients were available for multiple immunofluorescence (mIF) analysis. Representative mIF images showed higher infiltration of immune cells in three patients who achieved PR (Fig. 4A, upper panel) compared to the corresponding in three patients who achieved SD (Fig. 4A, lower panel). Patients with clinical PR exhibited significantly greater densities of CD68+ macrophages (p = 0.019) compared to those with SD (Fig. 4B). Additionally, there was a trend toward increased densities of CD8 + T cells and CD11c+ dendritic cells (DCs) in the PR group. Furthermore, higher densities of overall PDL1+ cells, including PDL1+ tumor cells, PDL1+ DCs, and PDL1+ macrophages, were observed in the PR group compared to those in the SD group, with a statistically significant difference found in PDL1+ DCs (p = 0.037) (Fig. 4B). Importantly, infiltration of PDL1+ DCs and PDL1+ macrophages in the stromal compartment positively correlated with better treatment response (Supplementary Fig. 1A).
Fig. 4. Biomarkers in the tumor microenvironment.
A Representative mIF images of CD8, CD68, CD11c, and CD4 cells in the tumor immune microenvironment from patients achieving clinical PR (upper panel, n = 3 samples) and SD (lower panel, n = 3 samples). Scale bars: 100 μm. B Immune cell infiltration levels in the tumor tissues between patients achieving PR (n = 13 samples) and those achieving SD (n = 7 samples) assessed by mIF. For box plots, the central line represents the median value, the bottom and top of the box represent the values of the 25th and 75th percentile, and the lower and upper whiskers represent the minimum and maximum value of the data, respectively. The p-values are derived from a two-tailed Mann–Whitney test. C Progression-free and overall survival analyses for the tumor infiltration levels of diverse immune cells. Patients were grouped into low- and high-expression of each variable as described in the Methods (n = 20 samples). The p-values are derived from a two-tailed Log-rank test. Source data are provided as a Source Data file. Abbreviations: mIF multiplex immunofluorescence, PR partial response, SD stable disease.
Higher infiltration of CD8 + T cells (PFS, p = 0.012; OS, p = 0.005), CD11c+ DCs (PFS, p = 0.039; OS, p = 0.026), and CD68+ macrophages (PFS, p = 0.008; OS, p = 0.04) correlated with PFS and OS (Fig. 4C). Elevated numbers of PDL1+ cells showed a tendency toward better OS (p = 0.055) but not PFS (p = 0.68) (Fig. 4C). Moreover, higher densities of PDL1+ macrophages in the stroma were associated with improved PFS and OS (Supplementary Fig. 1B).
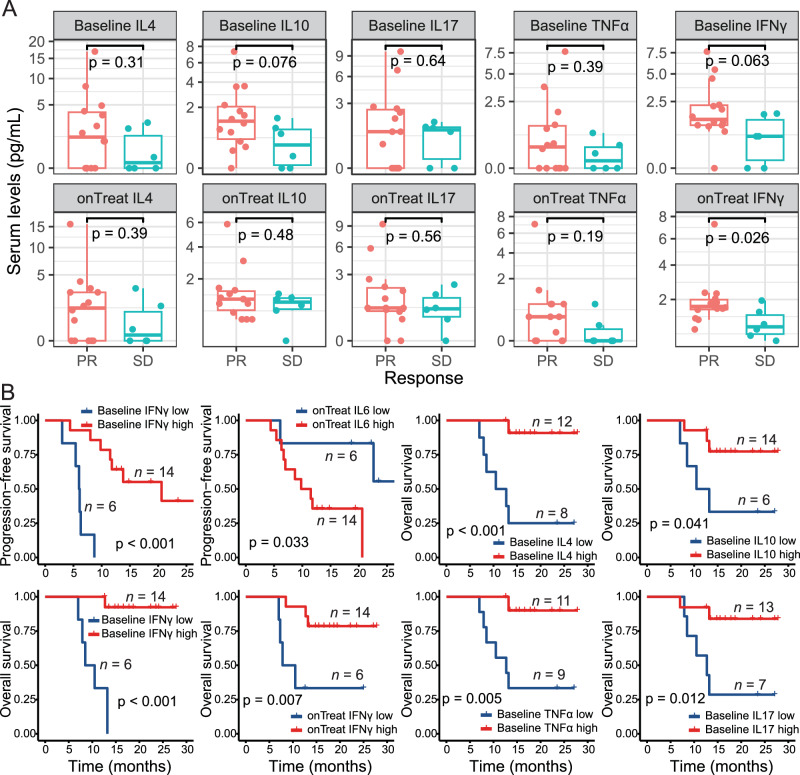
To further investigate whether peripheral cytokines could predict treatment response and patient outcomes, sera from 25 patients were analyzed at both baseline and during therapy (20 baseline samples and 20 treatment samples, with 15 paired samples; Supplementary Fig. 2A). The results indicated that levels of the eight tested cytokines were similar between the baseline and on-treatment groups (Supplementary Fig. 2B). Specifically, on-treatment levels of interferon-gamma (IFN-γ) were significantly higher in patients who achieved PR compared to those who achieved SD (Fig. 5A, p = 0.026). While there was a tendency for higher baseline levels of IFN-γ and IL-10 in the PR group, no significant differences were observed for interleukin (IL)−2, IL-4, IL-6, IL-17, IL-37, or tumor necrosis factor-alpha (TNF-α) (Fig. 5A, Supplementary Fig. 3).
Fig. 5. Peripheral cytokines predict treatment response and patient survival.
A Serum levels of different cytokines at baseline or on-treatment between patients achieving a PR (n = 14 samples) and those achieving SD (n = 6 samples). For box plots, the central line represents the median value, the bottom and top of the box represent the values of the 25th and 75th percentile, and the lower and upper whiskers represent the minimum and maximum value of the data, respectively. The p-values are derived from a two-tailed Mann–Whitney test. B Progression-free and overall survival analyses for different periphery cytokines. Patients were grouped into low- and high-expression of each variable as described in the Methods (n = 20 samples). The p-values are derived from a two-tailed Log-rank test. Exact p-values: baseline IFN-γ for PFS, p = 0.000044; baseline IFN-γ for OS, p = 0.0000075; baseline IL4 for OS, p = 0.00099. Source data are provided as a Source Data file. Abbreviations: IFN-γ interferon-gamma, IL interleukin, PR, partial response, TNF tumor necrosis factor.
Moreover, higher baseline levels of IFN-γ (p < 0.001) and lower on-treatment levels of IL-6 (p = 0.033) were associated with improved PFS (Fig. 5B). Furthermore, higher baseline levels of IL-4 (p < 0.001), baseline IL-10 (p = 0.041), baseline IFN-γ (p < 0.001), on-treatment IFN-γ (p = 0.007), baseline TNF-α (p = 0.005), and baseline IL-17 (p = 0.012) were significantly associated with better OS (Fig. 5B). No associations with PFS or OS were found for other serum cytokine levels (Supplementary Fig. 4).
Discussion
In the regimen of combining chemotherapy with immunotherapy for advanced esophageal cancer, the role of radiotherapy remains unknown. This phase 2 trial reported the safety, efficacy, and identification of candidate biomarkers for radiotherapy outcomes combined with chemo-immunotherapy in treatment-naive advanced ESCC cases. Our results revealed that radiotherapy targeting the primary esophageal and metastatic lesions is promisingly effective and has manageable toxicity when combined with chemo-immunotherapy.
In clinical practice, chemo-immunotherapy remains the preferred approach for advanced esophageal cancer3,19. However, adding radiotherapy to primary and metastatic lesions has been shown, in certain retrospective studies, to enhance local tumor control, potentially delaying disease progression and prolonging survival5,6,20–22. Despite this, chemo-immunotherapy remains the standard treatment, and evidence supporting the safety and therapeutic benefits of adding radiotherapy is currently lacking in prospective clinical research, especially regarding the potential additive toxicity of combined radio- and immunotherapy. In the present study, we observed a median PFS of 9.8 months and a 1-year PFS rate of 41.9% in the ITT population. Although the results did not meet the pre-specified primary endpoints, they compared favorably to the median PFS reported in previous studies, such as the KEYNOTE-590 (6.3 months), JUPITER-06 (5.7 months), and Checkmate-648 (5.8 months) trials3,4,7. Moreover, our Kaplan–Meier estimates of PFS indicated a plateau after 1 year, whereas the curves for OS appeared to plateau after approximately 16 months, suggesting long-term survival benefits in cases of advanced ESCC following treatment with radiotherapy plus chemo-immunotherapy. Notably, most of our studied patients had synchronous oligometastatic disease, with some patients exhibiting locally advanced N3 disease. Increasing evidence suggests that higher numbers of individuals with oligometastatic disease can attain long-term survival using local treatment together with systemic therapy than those with multiple metastases23–25. However, longer-term follow-up studies together with prospective randomized controlled trials are required to verify these findings. Additionally, there is no definitive evidence regarding the optimal duration of immune checkpoint inhibitor (ICI) therapy in patients with advanced esophageal cancer. A 2-year immunotherapy maintenance period may pose greater toxic side effects for patients who do not respond to the treatment. Therefore, we explored whether short-course ICI therapy (1-year immunotherapy maintenance) combined with systemic therapy and radiotherapy could enhance efficacy while reducing drug toxicity and financial burden for patients.
Patients with advanced esophageal cancer include those with synchronous metastasis, metachronous metastasis, and locoregional recurrence. Thus, in studies on advanced esophageal cancer combining radiotherapy with chemo-immunotherapy, the enrolled patients exhibited substantial heterogeneity. In the ESO-SHANGHAI 13 study15, approximately 90% of the patients developed metachronous oligometastatic disease after curative treatment, with radiotherapy limited to metastatic lesions. Thus, the efficacy and safety of simultaneously combining radiotherapy with systemic treatment for the primary lesion remains unclear. The ongoing EC-CRT-003 trial is enrolling patients with treatment-naive stage IVb ESCC17, and is considering adding thoracic radiotherapy (45–50 Gy/25–28 f) after 4–6 cycles of standard chemo-immunotherapy, without irradiating metastatic lesions. Similar clinical studies are in progress26–31. Collectively, these study designs reveal considerable debate over the optimal timing and target area of radiotherapy intervention in chemo-immunotherapy for advanced esophageal cancer. This debate centers on the following issues: first, whether radiotherapy is added during first-line systemic treatment in treatment-naive patients, after completing primary lesion treatment, or as a second-line treatment for tumor recurrence and metastasis; and second, whether radiotherapy targets only the primary lesion, metastatic lesions, or both.
Our study enrolled treatment-naive patients with advanced esophageal cancer, with 81% of the patients being oligometastatic, indicating a higher tumor burden at initial treatment compared to the ESO-SHANGHAI 13 study15. Therefore, in our study, radiotherapy was conducted concurrently after two cycles of systemic treatment to reduce the tumor burden and shrink the radiotherapy target volume, thus mitigating radiotherapy toxicity. During the third cycle of chemo-immunotherapy, concurrent radiotherapy was performed because of the apparent synergistic mechanism of radiotherapy and chemo-immunotherapy. Radiotherapy of the primary lesion can increase the production of tumor antigens, thus, enhancing antigen presentation via DCs32. Moreover, SBRT for metastatic lesions may achieve systemic antitumor immunity through local activation33. Notably, our radiotherapy covered both primary and metastatic lesions. An opinion piece reported that targeting all lesions could enhance the likelihood of successfully initiating an antitumor immune response, overcome the problem of tumor heterogeneity, and enhance the destruction of drug-resistant subclones34. However, in our study, the primary endpoint was not met in the ITT population, which may be related to the sandwich treatment strategy we used. Unless radiotherapy and chemo-immunotherapy are fully synchronized during the initial treatment, various circumstances, including disease progression and treatment side effects, may prevent the administration of radiotherapy following chemo-immunotherapy in advanced esophageal cancer, as observed in this study. The results from the efficacy-evaluable group indicate that patients who can tolerate and respond to chemo-immunotherapy are more suitable candidates for the addition of local radiotherapy.
An increasing body of evidence now indicates that the interaction between the tumor microenvironment and cancer cells is a key factor in tumor progression35. Our analyses using multiple immunohistochemistry/immunofluorescence (mIHC/mIF) techniques indicate that higher densities of CD8 + T cells, CD11c+ DCs, and CD68+ macrophages correlate with improved tumor response and prognosis. This effect is attributed to the enhanced tumor immune response from the adequate infiltration of these immune cells in the immune microenvironment, a relationship well-established in prior studies36–38. However, there have been conflicting reports on the predictive value of PD-L1 status in esophageal cancer3,4,7,36. This variation in findings may arise because the studies investigating this have typically used immunohistochemistry to detect PD-L1 expression, with the combined positive score (CPS) being the most commonly used. However, CPS only includes tumor-associated immune cells, such as macrophages and lymphocytes, which are in close proximity to the tumor cells. Conversely, our study examined PD-L1 expression in a broader range of cells, including tumor cells, macrophages, and dendritic cells. Our results show that a high expression of PD-L1 on tumor and immune cells is associated with better tumor outcomes. This finding suggests that comprehensive PD-L1 detection across all relevant cells may more accurately predict a patient’s treatment response. Moreover, our findings are in concordance with existing studies4–6 that highlight the beneficial predictive value of PD-L1 in cancer therapy.
Further, our findings demonstrate a significant correlation between elevated baseline serum IFN-γ levels and reduced on-treatment IL-6 levels and an extended PFS in patients with advanced ESCC. A previous investigation established a correlation between reduced IL-6 levels and improved prognosis in advanced melanoma39, and attributed this to the role of IL-6 in accelerating tumor progression through the inhibition of cancer cell apoptosis and the promotion of angiogenesis. In contrast, IFN-γ activates antitumor immune cells and suppresses immunoregulatory cells in immune antigens40. Therefore, IFN-γ and IL-6 could serve as reliable predictors of response to combined immunotherapy and chemoradiotherapy in advanced esophageal cancer cases. IL-4, IL-10, and TNF-α play multifaceted roles in cancer immunity, activating antitumor immune cells and facilitating tumor immune suppression and escape. IL-10, IL-17, and TNF-α, known for their immunosuppressive properties, promote tumor growth and are associated with poor prognosis, as corroborated by several clinical studies, whereas IL-4 is considered an enhancer of immune cell antitumor activity41–44. Our results showed that higher baseline levels of IL-4, IL-10, and IFN-γ, as well as elevated treatment levels of IFN-γ, TNF-α, and IL-17, were associated with improved OS. This result contrasts with previous findings and highlights the complexity and variability of the local tumor immune microenvironment. As different cancers harbor distinct immunosuppressive cells and cytokines within their tumor microenvironment, and various ICIs employ unique mechanisms of action, the relationship between cytokines and immunotherapy efficacy warrants further exploration.
A key issue in combining first-line radiotherapy with chemo-immunotherapy is safety. The present findings corroborate those of earlier clinical trials combining ICIs with radiotherapy for esophageal cancer36,45. In this study, we did not observe any unexpected safety signals, and most TRAEs were of grades 1–2. Grade 3 or higher TRAEs predominantly included myelosuppression, with no treatment-related deaths. Of note, 17 (51.5%) patients in this trial were still alive at the time of cut-off. Importantly, among the three cases of esophageal fistula, two occurred after two cycles of chemoradiotherapy and prior to radiotherapy. Retrospective analysis indicated that the incidence of esophageal perforation or fistula in patients with the T4 stage was as high as 30.1%. Hence, there may be a link between the occurrence of esophageal fistula and the clinical stages of the primary tumors, as all three patients presented with cT4 stage with tumor length >5 cm. In summary, the precise influence of the addition of PD-1 inhibitors with chemoradiotherapy on fistula risk requires further investigation.
This study had several limitations. First, it was a single-arm study conducted at a single center with a small sample size, which may have led to selection bias, thus, limiting the generalizability of the findings. Second, although all patients received the necessary imaging and multidisciplinary evaluation before enrollment, not all metastatic lesions were pathologically confirmed. Third, this study investigated biomarkers; however, the limited sample size precludes definitive conclusions. These results may inform the design of future large-scale trials. To enhance the reliability of our findings, we are currently participating in a multi-center, randomized, controlled, phase III clinical trial to examine the effect of first-line radiotherapy combined with chemo-immunotherapy in 100 patients (ClinicalTrials.gov identifier: ChiCTR2300070300).
In conclusion, in patients with treatment-naive, advanced ESCC, first-line radiotherapy to both primary and metastatic lesions in combination with chemo-immunotherapy demonstrated some antitumor activity with a manageable safety profile. Furthermore, our findings provide insights into potential biomarkers for assessing clinical effectiveness.
Methods
Study design and patients
This single-arm, open-label, phase 2 trial was conducted at Sichuan Cancer Hospital (Chengdu, Sichuan Province, China). The study was performed in compliance with the Declaration of Helsinki and Good Clinical Practice guidelines, and was approved by the Institutional Review Board of Sichuan Cancer Hospital, Sichuan, China (Ethics number: SCCHEC-02-2021-021). An interim analysis, authorized by the Institutional Review Board of Sichuan Cancer Hospital at a later stage, included data on some secondary endpoints (ORR, DCR, toxicity) to provide an early assessment of efficacy and to help identify and address safety issues early. All patients provided written informed consent before any procedure. No participation compensation was provided. The trial was registered with chictr.org.cn (ChiCTR2100046715) on May 27, 2021. Analyses were conducted as planned in the preregistration. There were some minor deviations from the preregistration regarding the radiotherapy scheme, follow-up, statistical analyses, and other aspects. These deviations are explicitly indicated at the end of the Supplementary Note (see section “Summary of Amendments to Protocol”). The first patient was enrolled on June 30, 2021, and the last patient on September 30, 2022.
Patients (aged 18–75 years) with histologically diagnosed unresectable, treatment-naive, stage IV ESCC based on the eighth Edition of the American Joint Committee on Cancer (AJCC) tumor, node, metastasis (TNM) staging system, with multiple lymph node metastases (N3) or distant oligometastases (M1) were eligible for inclusion. Other inclusion criteria included having a minimum of one measurable lesion, in terms of the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0–1, a life expectancy ≥6 months, and sufficient bone marrow and organ function. The exclusion criteria included a history of any other malignancy, metastases to the central nervous system, a previous history of immunotherapy, a history of autoimmune or interstitial lung disease, or serious comorbidities, such as congestive heart failure or uncontrolled diabetes. The study protocol is presented as Supplementary Note in the Supplementary information file.
Definition of oligometastasis
In our study, oligometastasis was defined as ≤5 metastatic lesions in ≤3 metastatic organs; notably, the involvement of a single non-regional lymph node station was also considered an oligometastasis46. For example, the presence of ≥1 lymph node metastases in the left axilla was considered a single oligometastatic lesion.
Definition of regional lymph nodes and distant (non-regional) lymph nodes
In this study, the eighth edition of the AJCC TNM staging system was used47. The regional lymph nodes, irrespective of the primary tumor site, are those within the esophageal drainage area, including the coeliac axis nodes and paraesophageal nodes in the neck, but not supraclavicular nodes. This includes nodes in regions 1 R/1 L/2 R/2 L/4 R/4 L/7/8U/8 M/8 L/9 R/9 L/15/16/17/18/19/20. Specifically, lymph nodes located above the upper boundary of Region 1 (above the apex of the lung) or below the lower boundary of Region 20 (below the coeliac artery) are considered distant (non-regional) lymph nodes. Additionally, lymph nodes located in the anterior mediastinum, supraclavicular region, axilla, and groin, beyond the previously defined regions, are considered distant metastatic lymph nodes (M1).
Treatments
Each chemo-immunotherapy cycle lasted for 3 weeks and consisted of 240 mg toripalimab and 135–175 mg/m2 paclitaxel plus carboplatin (area under the curve, 4–6) on day 1. Concurrent radiotherapy was initiated on the third chemo-immunotherapy cycle. Primary lesions were treated with intensity-modulated radiotherapy at 50–50.4 Gy in 25–28 fractions 5 days/week. The gross tumor volume (GTV) included the primary tumor (GTV-P), metastatic lymph nodes (GTV-N), and metastatic lesions (GTV-M). The planning target volume was defined as GTV with an additional 1–2 cm at the proximal and distal margins and a radical margin of 0.5–1.0 cm. For distant lymph nodes, such as supraclavicular and retroperitoneal lymph nodes, conventionally fractionated radiotherapy (50–50.4 Gy in 25–28 fractions) was administered at the physician’s discretion, in consideration of adjacent organs at risk, such as the trachea, stomach, and intestines. SBRT was recommended in cases with suitable oligometastatic lesions in the liver, lungs, or bones, with consideration for the same factors. SBRT was administered to all metastatic lesions at doses of 30–40 Gy in 3–5 fractions. The delineation of the target area is shown in Supplementary Figs. 5, 6.
Upon completion of four chemo-immunotherapy cycles, chemotherapy was discontinued; toripalimab was continued at 240 mg every 3 weeks for a maximum of 1 year or until the patient exhibited disease progression or evidence of intolerable toxicity. Dose reduction was permitted for paclitaxel and carboplatin but not for toripalimab. Chemotherapy was suspended or deferred if grade ≥3 AEs occurred.
Follow-up and outcomes
Baseline computed tomography examination was performed within 14 days before treatment initiation. Tumor evaluations were conducted at 6-weekly intervals (± 7 days) during chemotherapy. Meanwhile, during chemoradiotherapy, tumor evaluations were conducted once every 12 weeks (± 7 days) to the end of year 2, at 6-monthly intervals during the 3rd and 4th years, and annually thereafter. Efficacy assessments were performed in accordance with the RECIST 1.1 criteria. Laboratory analyses, including complete blood count, blood chemical tests, electrocardiography, routine urine analysis and stool examination, coagulation testing, and thyroid function testing, were conducted once every 3 weeks. AEs were identified and monitored using the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0.
The primary endpoint was PFS, defined as the time between beginning treatment and tumor progression, patient death, or the last follow-up. Secondary endpoints included the ORR, DCR, DoR, 1- and 2-year OS rates, patient-reported health-related quality of life, and AEs. Objective responses included CR and PR. Disease control represented CR, PR, and SD, while DoR was determined as the interval between the first objective response to the first documentation of progression or all-cause death. OS was assessed from the initiation of therapy to all-cause death. Exploratory outcomes included the relationship between clinical outcomes with immune cell types in the tumor microenvironment, and biomarkers in peripheral blood (e.g., soluble PD-L1 and cytokines). Two-year OS rates, quality of life, and soluble PD-L1 in peripheral blood are not reported in this article because of data immaturity; these results will be reported in future.
mIHC/mIF
Tumor biopsy sections were analyzed via mIHC/mIF analysis using the Opal 7-color kit (NEL811001KT; Akoya Biosciences, Marlborough, MA, USA), in accordance with the manufacturer’s instructions. Following antigen retrieval in EDTA buffer (pH 9.0; 3 min, 125 °C) and cooling to room temperature (RT), the sections were washed with ddH2O followed by TBST/0.5% Tween (repeated three times for 2 min each time). Then, the slides were blocked with a blocking buffer at RT for 10 min and treated with primary anti-PDL1 (ZA-0629, 1:50; ZSGB Biotech, Beijing, China) at 37 °C for 60 min followed by rinsing in TBS. The slides were incubated with an HRP-conjugated secondary antibody (10 min, 37 °C) followed by TSA dye 620 (1:100) for 5 min after further TBST washes. The same procedure was repeated for the other primary antibodies; namely, anti-CD68 (ZM-0060, 1:100, dye480; ZSGB Biotech), CD8 (ZA-0508, 1:100, dye570; ZSGB Biotech), CD11c (45581, 1:400, dye520; Abcam, Cambridge, UK), CD4 (ZA-0509, 1:100, dye690; ZSGB Biotech), and pan-cytokeratin (ZM-0067, 1:100, dye780; ZSGB Biotech). Additional rounds of antigen retrieval were undertaken in EDTA (pH 6.0) buffer using a pressure cooker for 2 min. DAPI was used for nuclear staining (100 μL DAPI, 5 min, RT).
Whole slide images were scanned using the TissueFAXS SL system (7.1.120; Tissue Gnostics, Vienna, Austria). Digital images were analyzed using the HALO™ software (v 3.5). Immune cell densities were assessed as positively stained cell counts per mm2. The cell density was calculated in total, tumor, and stromal areas, respectively.
Periphery cytokines
Sera were obtained at baseline and during treatment (after two cycles of chemo-immunotherapy and before radiotherapy). Inflammation-related cytokines, namely IL-2, IL-4, IL-6, IL-10, IL-17, TNF-α, and IFN-γ, were assessed using a magnetic beads kit (281004; Wellgrow, Beijing, China). Briefly, 50 μL of the serum sample or reference standards was added to 50 μL of capture beads suspension, mixed thoroughly, and incubated at RT in the dark for 1 h. The supernatant was removed after magnetic precipitation, and the beads were incubated with 100 μL of a fluorescent-labeled antibody at RT for 1 h. After washing, the beads were assessed using flow cytometry (BD FACSCanto™; BD Biosciences, San Jose, CA, USA). List of antibody clones utilized for flow cytometry are included in Supplementary Data 1. The flow cytometry gating strategy is presented in Supplementary Fig. 7. The data were analyzed using the FCAP ArrayTM Software (Version 3.0; BD Biosciences, Franklin Lakes, NJ, USA). IL-37 levels were assessed using the human IL-37 ELISA Kit (ab213798, Abcam), following the manufacturer’s instructions.
Statistical analysis
The necessary sample size was assessed based on an increase in median PFS from 6.3 to 12 months with chemo-immunotherapy, as previously described3. This calculation assumed a significance level of 0.05 (one-sided), a statistical power of 80%, and a 20% dropout rate. The follow-up duration was calculated from the time of enrollment to the date of the last follow-up. To observe 16 PFS events, we calculated that 32 patients were needed. This was based on an assumption of a uniform accrual accomplished over a period of approximately 12 months, with an additional 12 months of follow-up subsequent to the enrollment of the last patient. The data collection cut-off date was October 12, 2023.
The ORR, DCR, and DoR were assessed in the following two populations: the ITT group, which included all participants, and the efficacy-evaluable group, comprising patients who actually received radiotherapy of all lesions and underwent at least one post-baseline disease assessment. Safety was assessed in all cases where a minimum of one dose of the study drug had been administered. OS, PFS, and the corresponding 95% CIs were determined using the Kaplan–Meier method. Survival outcomes were analyzed using Log-rank tests. Clinical and demographic features, together with TRAEs, were analyzed using descriptive statistical methods.
Differences in tumor-infiltrating immune cell densities and periphery levels of cytokines between the PR and SD groups were compared using the Mann–Whitney test. For PFS and OS analyses, patients were categorized into those having low- and high-levels of immune cell densities based on the 30th percentile values: CD8+ cells, 210 cells/mm2; CD11c+ cells, 1318 cells/mm2; CD68+ cells, 257 cells/mm2; PDL1+ cells, 190 cells/mm2. Similarly, cutoffs for peripheral cytokines were based on the 30th percentile values of each variable.
Associations between biomarkers and PFS and OS were assessed using the Log-rank test. Because of the exploratory nature of this clinical study, no adjustments were made for multiple comparisons. Statistical analyses were conducted using R software (v4.3.1; Vienna, Austria), SPSS 22.0 (IBM Corp., Armonk, NY, USA), and SAS 9.4 (SAS Institute; Cary, NC, USA). Differences were considered statistically significant at p < 0.05.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
The authors thank the participating patients and their families and the study centers and investigators for their contributions to the study. The authors also thank Dongmei Tang, Qin Ren, and Jianchao Lu for their assistance in data collection and sample checking. This trial was supported by grants from the Science and Technology Department of Sichuan Province (2023YFS0488 and 2023YFQ0055) and the Immunotherapy Research Fund of the Radiotherapy Oncology Branch of the Chinese Medical Association. All investigators received no remuneration. Shanghai Junshi Biosciences provided toripalimab but had no role in the study design or writing of the manuscript. The manuscript was edited and proofread by Editage.
Author contributions
W.Q.F., C.B.R., and L.Y. were the principal investigators and participated in trial design, study management, data and toxicity review, review of the report, supervision of the study, and final approval of the report. W.L., Z.J., and L.L. contributed to the writing of the protocol, recruitment and treatment of the patients, data and trial management, data analysis and interpretation, and writing of manuscript. L.B.S., W.G, W.Y., and Z.W. participated in the recruitment and treatment of the patients, data and trial management, and report preparation. L.X.F., H.W.W., H.S.Y., Z.Y.H., Y.L., and W.D.S. were responsible for statistical analysis and interpretation as well as data review. Z.W.C., P.Q.S., L.T., P.L., H.Y.T., and L.J.Y. contributed to patient accrual, toxicity review, and review of the completed report. All authors have reviewed and approved the final draft. All authors had full access to all data in the study. The corresponding author held final responsibility for the decision to submit the manuscript for publication.
Peer review
Peer review information
Nature Communications thanks Xiumei Ma and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
All data requests will undergo review by Sichuan Cancer Hospital and the study sponsor, Shanghai Junshi Biosciences Co., Ltd., to assess any potential intellectual property or confidentiality obligations. A proposal detailing the study objectives and statistical analysis plan will be required for evaluation. Additional materials may also be requested during the evaluation process. Data will be available upon request 12 months after the publication of this article. Detailed individual data are available under restricted access for both legal and ethical concerns. Requests for access to de-identified participant data from this study can be submitted via email to wangqifeng@scszlyy.org.cn, accompanied by a detailed proposal for approval. Please allow 1 month for a response to the request. Access to the shared data will require signing a data access agreement with the sponsor. The raw identifying individual participant data are protected and are not available due to data privacy laws. The study protocol is available as Supplementary Note in the Supplementary Information file. The remaining data are available within the Article, Supplementary Information, or Source Data file. Source data are provided with this paper and are also available in Figshare at: 10.6084/m9.figshare.26387656. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Lei Wu, Baisen Li, Gang Wan.
These authors jointly supervised this work: Yang Liu, Bangrong Cao, Qifeng Wang.
Contributor Information
Yang Liu, Email: liuyanglyon@uestc.edu.cn.
Bangrong Cao, Email: caobangrong@uestc.edu.cn.
Qifeng Wang, Email: wangqifeng@scszlyy.org.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-51105-2.
References
- 1.Zheng, R. S. et al. Cancer statistics in China, 2016. Zhonghua Zhong Liu Za Zhi45, 212–220 (2023). [DOI] [PubMed] [Google Scholar]
- 2.Ajani, J. A. et al. Esophageal and Esophagogastric Junction Cancers. version 2.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl Compr. Canc. Netw.21, 393–422 (2023). 10.6004/jnccn.2023.0019 [DOI] [PubMed] [Google Scholar]
- 3.Sun, J. M. et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): A randomised, placebo-controlled, phase 3 study. Lancet398, 759–771 (2021). 10.1016/S0140-6736(21)01234-4 [DOI] [PubMed] [Google Scholar]
- 4.Doki, Y. et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N. Engl. J. Med.386, 449–462 (2022). 10.1056/NEJMoa2111380 [DOI] [PubMed] [Google Scholar]
- 5.Luo, H. et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: The ESCORT-1st randomized clinical trial. JAMA326, 916–925 (2021). 10.1001/jama.2021.12836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu, Z. et al. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): Multicentre, randomised, double blind, phase 3 trial. BMJ377, e068714 (2022). 10.1136/bmj-2021-068714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang, Z. X. et al. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): A multi-center phase 3 trial. Cancer Cell40, 277–288.e3 (2022). 10.1016/j.ccell.2022.02.007 [DOI] [PubMed] [Google Scholar]
- 8.Xu, J. et al. Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first-line treatment for advanced or metastatic oesophageal squamous cell carcinoma (RATIONALE-306): A global, randomised, placebo-controlled, phase 3 study. Lancet Oncol.24, 483–495 (2023). 10.1016/S1470-2045(23)00108-0 [DOI] [PubMed] [Google Scholar]
- 9.Song, Y. et al. First-line serplulimab or placebo plus chemotherapy in PD-L1-positive esophageal squamous cell carcinoma: A randomized, double-blind phase 3 trial. Nat. Med.29, 473–482 (2023). 10.1038/s41591-022-02179-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li, L. Q. et al. Chemoradiotherapy versus chemotherapy alone for advanced esophageal squamous cell carcinoma: The role of definitive radiotherapy for primary tumor in the metastatic setting. Front. Oncol.12, 824206 (2022). 10.3389/fonc.2022.824206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyu, J. et al. Outcomes of concurrent chemoradiotherapy versus chemotherapy alone for stage IV esophageal squamous cell carcinoma: A retrospective controlled study. Radiat. Oncol.13, 233 (2018). 10.1186/s13014-018-1183-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu, X. et al. Immunotherapy with or without radiotherapy for metastatic or recurrent esophageal squamous cell carcinoma: A real-world study. Clin. Transl. Radiat. Oncol.38, 130–137 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao, W. et al. Radiotherapy plus camrelizumab and irinotecan for oligometastatic esophageal squamous cell carcinoma patients after first-line immunotherapy plus chemotherapy failure: An open-label, single-arm, phase II trial. Radiother. Oncol.184, 109679 (2023). 10.1016/j.radonc.2023.109679 [DOI] [PubMed] [Google Scholar]
- 14.Liu, Q. et al. Phase 2 study of stereotactic body radiation therapy for patients with oligometastatic esophageal squamous cell carcinoma. Int. J. Radiat. Oncol. Biol. Phys.108, 707–715 (2020). 10.1016/j.ijrobp.2020.05.003 [DOI] [PubMed] [Google Scholar]
- 15.Liu, Q. et al. Systemic therapy with or without local intervention for oligometastatic oesophageal squamous cell carcinoma (ESO-Shanghai 13): An open-label, randomised, phase 2 trial. Lancet Gastroenterol. Hepatol.9, 45–55 (2024). 10.1016/S2468-1253(23)00316-3 [DOI] [PubMed] [Google Scholar]
- 16.Efficacy and safety of tislelizumab and nabpaclitaxel combined with low-dose radiotherapy in patients with stage IVb esophageal squamous cell carcinoma. Identifier: NCT05547828. https://ClinicalTrials.gov/show/NCT05547828 (2022).
- 17.University, S. Y.-S. Concurrent chemoradiotherapy for stage IVB esophageal squamous cell carcinoma (EC-CRT-003). https://ClinicalTrials.gov/show/NCT05512520 (2022).
- 18.Day, F. et al. Chemoradiotherapy with concurrent durvalumab for the palliative treatment of oligometastatic oesophageal and gastrooesophageal carcinoma with dysphagia: A single arm phase II clinical trial (PALEO, sponsored by the Australasian Gastro-Intestinal Trials Group). BMC Cancer22, 1324 (2022). 10.1186/s12885-022-10407-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Obermannová, R. et al. Oesophageal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol.33, 992–1004 (2022). 10.1016/j.annonc.2022.07.003 [DOI] [PubMed] [Google Scholar]
- 20.Chen, Y. et al. Outcomes of concurrent chemoradiotherapy versus chemotherapy alone for esophageal squamous cell cancer patients presenting with oligometastases. J. Thorac. Dis.11, 1536–1545 (2019). 10.21037/jtd.2019.03.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi, Z. et al. Survival impact of concurrent chemoradiotherapy for elderly patients with synchronous oligometastatic esophageal squamous cell carcinoma: A propensity score matching and landmark analyses. Radiother. Oncol.164, 236–244 (2021). 10.1016/j.radonc.2021.09.033 [DOI] [PubMed] [Google Scholar]
- 22.Li, B. et al. Development and validation of a nomogram prognostic model for esophageal cancer patients with oligometastases. Sci. Rep.10, 11259 (2020). 10.1038/s41598-020-68160-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pitroda, S. P., Chmura, S. J. & Weichselbaum, R. R. Integration of radiotherapy and immunotherapy for treatment of oligometastases. Lancet Oncol.20, e434–e442 (2019). 10.1016/S1470-2045(19)30157-3 [DOI] [PubMed] [Google Scholar]
- 24.Iyengar, P. et al. Consolidative radiotherapy for limited metastatic non-small-cell lung cancer: A Phase 2 randomized clinical trial. JAMA Oncol.4, e173501 (2018). 10.1001/jamaoncol.2017.3501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomez, D. R. et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: Long-term results of a multi-institutional, Phase II, randomized study. J. Clin. Oncol.37, 1558–1565 (2019). 10.1200/JCO.19.00201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ji, Y., Du, X. & Chen, M. A multicenter, randomized controlled, phase II clinical study of first-line chemotherapy and camrelizumab with or without radiotherapy in the treatment of oligometastatic esophageal cancer. Identifier: NCT05183958. Available at: https://Clinicaltrials.gov/show/NCT05183958 (2021).
- 27.Gao, S. Clinical control study of immunotherapy and concurrent chemoradiotherapy in patients with esophageal cancer recurrence. Identifier: NCT04404491. https://ClinicalTrials.gov/show/NCT04404491 (2020).
- 28.A clinical study of camrelizumab with or without radiotherapy in the treatment of esophageal cancer. Identifier: NCT04512417. https://ClinicalTrials.gov/show/NCT04512417 (2020).
- 29.Tislelizumab combined with chemotherapy or radiotherapy in the treatment of advanced or recurrent metastatic elderly esophageal cancer. Identifier: NCT05628610. https://ClinicalTrials.gov/show/NCT05628610 (2022).
- 30.Study of PD-1 antibody combined with chemoradiotherapy in oligometastatic esophageal cancer. Identifier: NCT04821765. https://ClinicalTrials.gov/show/NCT04821765 (2020).
- 31.Camrelizumab combined with CRT for treatment of patients with local recurrence of esophageal cancer. Identifier: NCT04390945. https://ClinicalTrials.gov/show/NCT04390945 (2021).
- 32.Demaria, S., Golden, E. B. & Formenti, S. C. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol.1, 1325–1332 (2015). 10.1001/jamaoncol.2015.2756 [DOI] [PubMed] [Google Scholar]
- 33.Theelen, W. S. M. E. et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: A pooled analysis of two randomised trials. Lancet Respir. Med.9, 467–475 (2021). 10.1016/S2213-2600(20)30391-X [DOI] [PubMed] [Google Scholar]
- 34.Brooks, E. D. & Chang, J. Y. Time to abandon single-site irradiation for inducing abscopal effects. Nat. Rev. Clin. Oncol.16, 123–135 (2019). 10.1038/s41571-018-0119-7 [DOI] [PubMed] [Google Scholar]
- 35.de Visser, K. E. & Joyce, J. A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell41, 374–403 (2023). 10.1016/j.ccell.2023.02.016 [DOI] [PubMed] [Google Scholar]
- 36.Zhu, Y. et al. Toripalimab combined with definitive chemoradiotherapy in locally advanced oesophageal squamous cell carcinoma (EC-CRT-001): A single-arm, phase 2 trial. Lancet Oncol.24, 371–382 (2023). 10.1016/S1470-2045(23)00060-8 [DOI] [PubMed] [Google Scholar]
- 37.Tzeng, T. C. et al. CD11c(hi) dendritic cells regulate the re-establishment of vascular quiescence and stabilization after immune stimulation of lymph nodes. J. Immunol.184, 4247–4257 (2010). 10.4049/jimmunol.0902914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding, H. et al. Tumor-associated macrophages induce lymphangiogenesis in cervical cancer via interaction with tumor cells. APMIS122, 1059–1069 (2014). 10.1111/apm.12257 [DOI] [PubMed] [Google Scholar]
- 39.Laino, A. S. et al. Serum interleukin-6 and C-reactive protein are associated with survival in melanoma patients receiving immune checkpoint inhibition. J. Immunother. Cancer8, e000842 (2020). 10.1136/jitc-2020-000842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thapa, R. J. et al. NF-kappaB protects cells from gamma interferon-induced RIP1-dependent necroptosis. Mol. Cell. Biol.31, 2934–2946 (2011). 10.1128/MCB.05445-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao, S., Wu, D., Wu, P., Wang, Z. & Huang, J. Serum IL-10 predicts worse outcome in cancer patients: A meta-analysis. PLOS ONE10, e0139598 (2015). 10.1371/journal.pone.0139598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ho, M. Y. et al. TNF-α induces epithelial-mesenchymal transition of renal cell carcinoma cells via a GSK3β-dependent mechanism. Mol. Cancer Res.10, 1109–1119 (2012). 10.1158/1541-7786.MCR-12-0160 [DOI] [PubMed] [Google Scholar]
- 43.Han, X. et al. Pan-cancer analysis reveals interleukin-17 family members as biomarkers in the prediction for immune checkpoint inhibitor curative effect. Front. Immunol.13, 900273 (2022). 10.3389/fimmu.2022.900273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ji, S. et al. Peripheral cytokine levels as predictive biomarkers of benefit from immune checkpoint inhibitors in cancer therapy. Biomed. Pharmacother.129, 110457 (2020). 10.1016/j.biopha.2020.110457 [DOI] [PubMed] [Google Scholar]
- 45.Zhang, W. et al. Safety and feasibility of radiotherapy plus camrelizumab for locally advanced esophageal squamous cell carcinoma. Oncologist26, e1110–e1124 (2021). 10.1002/onco.13797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kroese, T. E. et al. Definition, diagnosis and treatment of oligometastatic oesophagogastric cancer: A Delphi consensus study in Europe. Eur. J. Cancer185, 28–39 (2023). 10.1016/j.ejca.2023.02.015 [DOI] [PubMed] [Google Scholar]
- 47.Rice, T. W. et al. Cancer of the esophagus and esophagogastric junction: An Eighth Edition Staging Primer. J. Thorac. Oncol.12, 36–42 (2017). 10.1016/j.jtho.2016.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All data requests will undergo review by Sichuan Cancer Hospital and the study sponsor, Shanghai Junshi Biosciences Co., Ltd., to assess any potential intellectual property or confidentiality obligations. A proposal detailing the study objectives and statistical analysis plan will be required for evaluation. Additional materials may also be requested during the evaluation process. Data will be available upon request 12 months after the publication of this article. Detailed individual data are available under restricted access for both legal and ethical concerns. Requests for access to de-identified participant data from this study can be submitted via email to wangqifeng@scszlyy.org.cn, accompanied by a detailed proposal for approval. Please allow 1 month for a response to the request. Access to the shared data will require signing a data access agreement with the sponsor. The raw identifying individual participant data are protected and are not available due to data privacy laws. The study protocol is available as Supplementary Note in the Supplementary Information file. The remaining data are available within the Article, Supplementary Information, or Source Data file. Source data are provided with this paper and are also available in Figshare at: 10.6084/m9.figshare.26387656. Source data are provided with this paper.