ABSTRACT
Duplications in chromosomal locus 2q24.3 region that solely involve SCN2A remain less explored. Favorable outcomes have been reported in patients with SCN2A gene duplications in cases of mild epilepsy with onset during the neonatal to infantile period, or in infantile epileptic spasm syndrome. Herein, we report a case of microduplications, including SCN2A gene duplications, wherein developmental/epileptic encephalopathy with spike-wave activation during sleep (D/EE-SWAS) developed. A 3-day-old girl without birth complications exhibited tonic seizures in her right limb with eye deviation to the right. She developed drug-resistant seizures, including atypical absence seizures, at 1 year and 6 months old. Despite achieving seizure freedom at 9 years old, she experienced academic difficulties. D/EE-SWAS was diagnosed based on the long-term electroencephalogram findings. Following a corpus callosotomy at 11 years old, her academic performance and emotional expression improved. Comprehensive genetic analysis at 10 years old revealed a microduplication spanning approximately 300 kb within the 2q24.3 region, which included a segment of the SCN2A gene and an adjacent CSRNP3 gene. In conclusion, we reported a rare case of duplications solely encompassing SCN2A. Corpus callosotomy resolved the D/EE-SWAS.
Keywords: D/EE-SWAS, duplication, epilepsy, SCN2A
Voltage-gated sodium (Na2+) channels play important roles in neural transmission and are strongly implicated in the onset of epilepsy.1 The genes encoding voltage-gated Na2+ channels, namely SCN1A, SCN2A, SCN3A, and SCN8A, are located in the chromosome 2q24.3 region.1 Pathogenic variants of the SCN2A gene have been associated with a spectrum of conditions, ranging from relatively mild disorders, such as self-limited familial neonatal infantile epilepsy, to severe forms of epilepsy, including Dravet syndrome, early infantile developmental and epileptic encephalopathy, paroxysmal ataxia, and neurodevelopmental disorders.2,3,4,5,6 Specifically, duplications involving SCN2A, usually encompassing SCN1A and SCN3A, have been reported to lead to neonatal-onset drug-resistant epilepsy and intellectual disabilities.7 However, reports concerning duplications involving only SCN2A (excluding other Na2+-channel genes) are limited. There have been few cases of neonatal or infantile epilepsy caused by gene duplication of SCN2A with favorable seizure outcomes.1, 8, 9
Here, we report the case of a female child who carried a duplication involving SCN2A who was afflicted by drug-resistant epilepsy with onset in the neonatal period. She was additionally diagnosed with developmental/epileptic encephalopathy with spike-wave activation during sleep (D/EE-SWAS) during childhood.
PATIENT REPORT
Clinical course
A 3-day-old girl visited our hospital with a chief complaint of tonic seizures in the right upper and lower limbs, accompanied by eye deviation to the right. She was born without asphyxia at a gestational age of 38 weeks and weighed 3,000 g. She was the third child of healthy, non-consanguineous parents, and her paternal uncle developed epileptic seizures at 17 years of age. Serum laboratory tests, head computed tomography, and electroencephalography (EEG) revealed no abnormalities. She experienced recurrence at five months of age, and EEG showed multifocal spike waves. Oral administration of phenobarbital (PB) controlled the seizures.
From 1 year and 6 months of age, daily atypical absence seizures occurred, as evidenced with ictal EEG with continuous spike and wave discharges in the right middle temporal and left centroparietal regions. From 3 years of three years, she developed various types of seizures, including extension of the right lower limb and eye blinking with dizziness. The developmental quotient using the Kinder Infant Development Scale at 3 years and 6 months of age was 85. Although the clinical seizures were intractable to multiple antiseizure medications, after 9 years of age, she achieved seizure freedom with treatment with ethosuximide, valproic acid, clobazam, and topiramate.
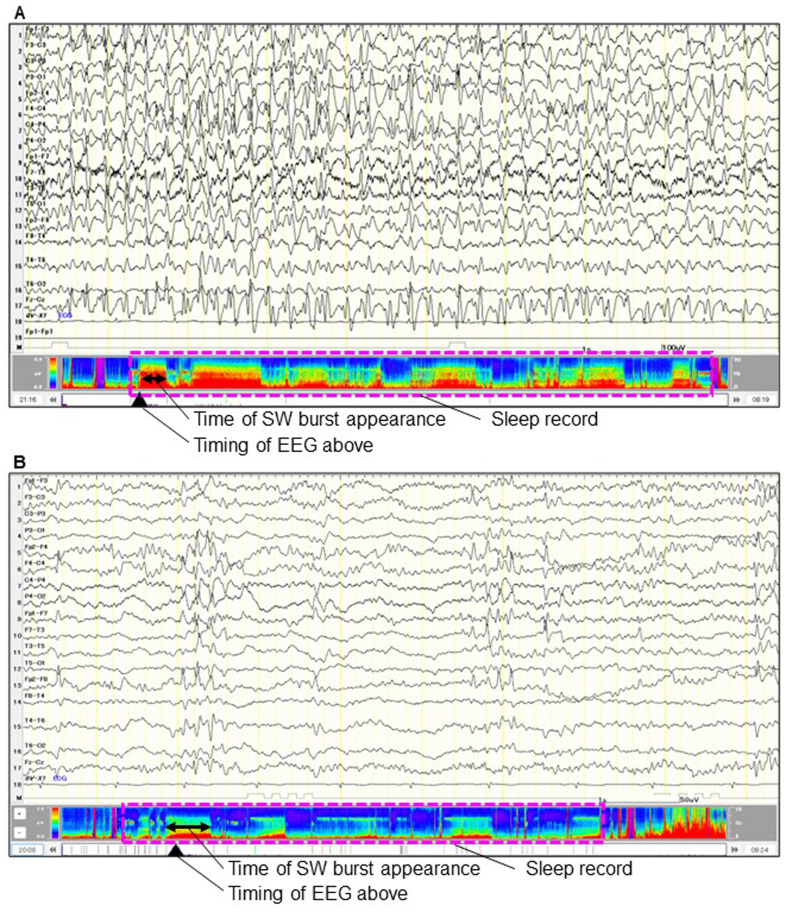
Owing to the poor academic performance, we conducted long-term EEG monitoring at 9 years and 7 months of age, which revealed diffuse and continuous spikes and waves during sleep (Fig. 1A). The spike-wave index reached a maximum value of 93%. The intelligence quotient (IQ) on the Tanaka Binet Intelligence Scale was 33 at 9 years and 9 months of age. Subsequently, the patient was diagnosed with D/EE-SWAS. High-dose diazepam and methylprednisolone pulse therapy did not reduce the discharge rate.
Fig. 1.
(A) Long-term EEG, before the corpus callosotomy (9 years and 7 months of age), revealed a continuous diffuse spike-slow wave burst during non-REM sleep (low frequency filter, 1.59 Hz; high frequency filter, 30 Hz; sensitivity, 20 μV; time constant, 0.3). (B) After the corpus callosotomy (13 years and 7 months of age), the distribution and appearance time of spile-slow wave burst reduced (low frequency filter, 1.59 Hz; high frequency filter, 30 Hz; sensitivity, 10 μV; Tc, 0.3). The density spectral array shows the reduction of the time ratios and powers of the spike-slow wave burst during sleep [pink-dash boxes in (A) and (B)].
Preoperative cranial MRI and FDG-PET examinations did not reveal any evident epileptic foci. After the corpus callosotomy, EEG discharges were markedly reduced and occurred independently in both hemispheres (Fig. 1B). After the corpus callosotomy, the parents felt that she became able to express her emotion and intent in words, and behave appropriately by observing the surrounding situation. She also became able to concentrate during classes, resulting in decreased distraction and forgetfulness. The Attention Deficit Hyperactivity Disorder Rating Scale and the Parent-Interview Autism Spectrum Disorder Rating Scale improved from 15 to 8 points, and from 14 to 7 points, respectively. The Tanaka Binet Intelligence Scale showed a stable IQ of 31 at 13 years of age. The EEG discharges were further reduced, with a shift in dominance from the left to the right hemisphere.
Genetic analysis
Comprehensive exome analyses were conducted on the patient and both parents at 10 years of age. As a result, a microduplication of approximately 300 kb in size (chr2:166,152,233-166,451,823) was detected in the 2q24.3 region of the patient’s chromosome. This microduplication, encompassing a portion of the SCN2A gene and an adjacent CSRNP3 gene, was exclusively observed in the patient. Furthermore, a heterozygous single-base variant (NM_212482.1: c.4223C > T: p. (Ser1408Phe)) was identified in the gene encoding Fibronectin 1 (FN1) located in the 2q35 region.
DISCUSSION
We report a patient with neonatal-onset drug-resistant epilepsy who developed D/EE-SWAS associated with a duplication solely in the SCN2A gene among Na2+ channel genes. There have been no reports of a patient with D/EE-SWAS with duplication of the Na2+ channel solely affecting SCN2A. In this case, corpus callosotomy was effective in improving both EEG patterns and cognitive function.
Pathogenic variants in the SCN2A gene, including point mutations and deletions, have been associated with various epilepsy severities, including developmental and epileptic encephalopathy, benign familial neonatal infantile seizures, paroxysmal ataxia, and neurodevelopmental disorders, such as autism spectrum disorder.2,3,4,5,6 Most cases of SCN2A duplications in previous reports also involved SCN1A and SCN3A, often leading to neonatal-onset epilepsy with a course of drug resistance and severe cognitive outcomes.7 There are only a few reports on SCNA2 duplications, and most cases exhibit mild epilepsy with onset during the neonatal period.1 Thuresson et al. reported the case of a boy who experienced tonic-clonic seizures beginning at the age of 5 years, which were controlled by valproate monotherapy. The patient also exhibited a moderate intellectual disability and attention-deficit hyperactivity disorder.8 Boutry-Kryza et al. reported a case of infantile epileptic spasm syndrome9; however, specific details remain uncertain. In our patient, we identified a duplication involving SCN2A without the involvement of other Na2+ channel genes, which resulted in drug-resistant epilepsy and the development of D/EE-SWAS.
In this patient, a partial duplication of the CSRNP3 gene and mutations in the FN1 gene were identified. Although CSRNP3 is known for its role in promoting apoptosis and transcription by RNA polymerase II,13 there have been no reports of this gene linking to the onset of epilepsy or intellectual problems. It is difficult to discuss whether the mutation affected the patient’s condition. FN1 gene is involved in cellular adhesion and migration processes. While epilepsy has not been directly associated with FN1 gene mutations in previous literature, its expression in neuronal cells suggests a potential role in the present condition’s pathogenesis.
Moreover, the patient underwent corpus callosotomy. Although corpus callosotomy is primarily performed as a palliative surgery for patients with drop attacks, including epileptic spasms, tonic seizures, and atonic seizures, this procedure is also adapted for patients with diffuse spike-wave discharges that affect cognitive function.10,11,12 In our patient, corpus callosotomy reduced the rate of epileptic discharge and resulted in improvements in emotional and developmental disorder symptoms. Similarly, Peltola et al. reported improvements in cognitive function and behavior following corpus callosotomy, particularly in patients with atypical absence seizures.11 Yokosako et al. also reported improvements in the spike-wave index, cognitive function, and seizure frequency, and reduced antiepileptic medication following corpus callosotomy for D/EE-SWAS.12 Accordingly, these reports and our case highlight the benefits of callosotomy in the treatment of D/EE-SWAS.
In conclusion, we report the case of a patient with duplications at 2q24.3 solely encompassing SCN2A among all sodium channel genes. The patient developed various types of seizures and progressive drug resistance. Corpus callosotomy resolved D/EE-SWAS.
Acknowledgments
Acknowledgements: We sincerely thank the doctors with whom we collaborated to treat the patient.
Footnotes
Informed consent: The authors obtained written informed consent from the patient’s parents for publication.
The authors declare no conflict of interest.
REFERENCES
- 1.Brunklaus A,Du J,Steckler F,Ghanty II,Johannesen KM,Fenger CD,et al. . Biological concepts in human sodium channel epilepsies and their relevance in clinical practice. Epilepsia. 2020;61:387-99. 10.1111/epi.16438 [DOI] [PubMed] [Google Scholar]
- 2.Zeng Q,Yang Y,Duan J,Niu X,Chen Y,Wang D,et al. . SCN2A-Related Epilepsy: The Phenotypic Spectrum, Treatment and Prognosis. Front Mol Neurosci. 2022;15:809951. 10.3389/fnmol.2022.809951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reynolds C,King MD,Gorman KM. The phenotypic spectrum of SCN2A-related epilepsy. Eur J Paediatr Neurol. 2020;24:117-22. 10.1016/j.ejpn.2019.12.016 [DOI] [PubMed] [Google Scholar]
- 4.Spratt PWE,Ben-Shalom R,Keeshen CM,Burke KJ Jr,Clarkson RL,Sanders SJ,et al. . The Autism-Associated Gene Scn2a Contributes to Dendritic Excitability and Synaptic Function in the Prefrontal Cortex. Neuron. 2019;103:673-685.e5. 10.1016/j.neuron.2019.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maksemous N,Smith RA,Sutherland HG,Sampaio H,Griffiths LR. Whole-Exome Sequencing Implicates SCN2A in Episodic Ataxia, but Multiple Ion Channel Variants May Contribute to Phenotypic Complexity. Int J Mol Sci. 2018;19:3113. 10.3390/ijms19103113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heyne HO,Artomov M,Battke F,Bianchini C,Smith DR,Liebmann N,et al. . Targeted gene sequencing in 6994 individuals with neurodevelopmental disorder with epilepsy. Genet Med. 2019;21:2496-503. 10.1038/s41436-019-0531-0 [DOI] [PubMed] [Google Scholar]
- 7Goeggel Simonetti B,Rieubland C,Courage C,Strozzi S,Tschumi S,Gallati S,et al. . Duplication of the sodium channel gene cluster on 2q24 in children with early onset epilepsy. Epilepsia. 2012;53:2128-34. 10.1111/j.1528-1167.2012.03676.x [DOI] [PubMed] [Google Scholar]
- 8.Thuresson A-C,Van Buggenhout G,Sheth F,Kamate M,Andrieux J,Clayton Smith J,et al. . Whole gene duplication of SCN2A and SCN3A is associated with neonatal seizures and a normal intellectual development. Clin Genet. 2017;91:106-10. 10.1111/cge.12797 [DOI] [PubMed] [Google Scholar]
- 9.Boutry-Kryza N,Labalme A,Ville D,de Bellescize J,Touraine R,Prieur F,et al. . Molecular characterization of a cohort of 73 patients with infantile spasms syndrome. Eur J Med Genet. 2015;58:51-8. 10.1016/j.ejmg.2014.11.007 [DOI] [PubMed] [Google Scholar]
- 10.Fujimoto A,Okanishi T. Corpus Callosotomy: editorial. [Editorial]. Brain Sci. 2022;12:1006. 10.3390/brainsci12081006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peltola ME,Liukkonen E,Granström ML,Paetau R,Kantola-Sorsa E,Valanne L,et al. . The effect of surgery in encephalopathy with electrical status epilepticus during sleep. Epilepsia. 2011;52:602-9. 10.1111/j.1528-1167.2010.02783.x [DOI] [PubMed] [Google Scholar]
- 12.Yokosako S,Muraoka N,Watanabe S,Kosugi K,Takayama Y,Iijima K,et al. . Corpus callosotomy in pediatric patients with non-lesional epileptic encephalopathy with electrical status epilepticus during sleep. Epilepsy Behav Rep. 2021;16:100463. 10.1016/j.ebr.2021.100463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gingras S,Pelletier S,Boyd K,Ihle JN. Characterization of a family of novel cysteine- serine-rich nuclear proteins (CSRNP). PLoS One. 2007;2:e808. 10.1371/journal.pone.0000808 [DOI] [PMC free article] [PubMed] [Google Scholar]