Abstract
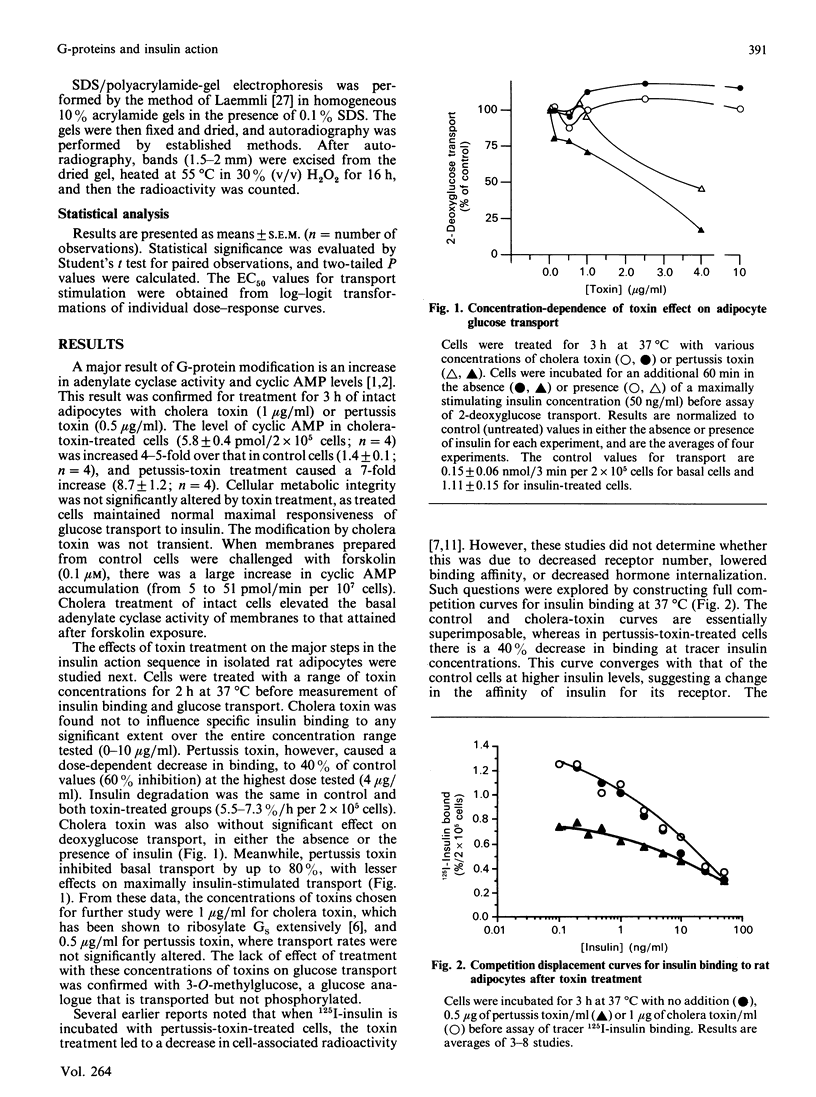
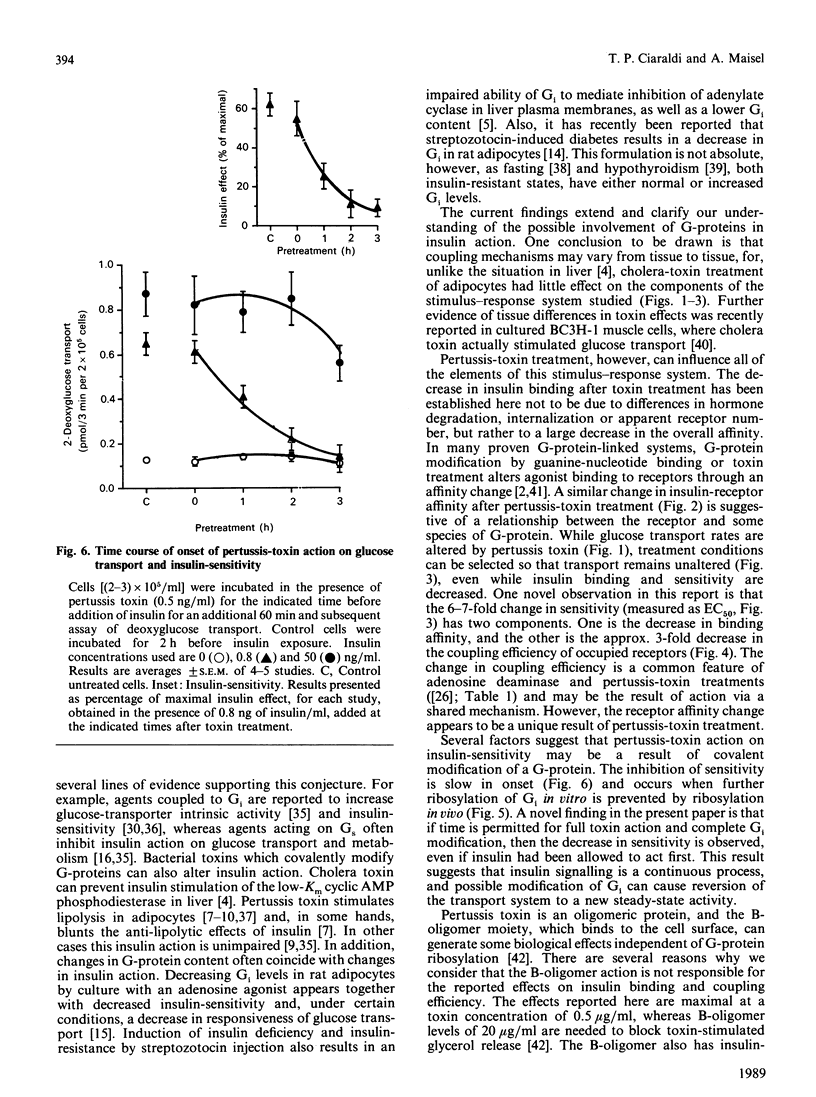
The potential role of guanine nucleotide regulatory proteins (G-proteins) in acute insulin regulation of glucose transport was investigated by using bacterial toxins which are known to modify these proteins. Cholera-toxin treatment of isolated rat adipocytes had no effect on either 2-deoxyglucose transport or insulin binding. Pertussis-toxin treatment resulted in an inhibition of both insulin binding and glucose transport. Insulin binding was decreased in pertussis-toxin-treated cells by up to 40%, owing to a lowering of the affinity of the receptor for hormone, with no change in hormone internalization. The dose-response curve for insulin stimulation of glucose transport was strongly shifted to the right by pertussis-toxin treatment [EC50 (half-maximally effective insulin concn.) = 0.31 +/- 0.04 ng/ml in control cells; 2.29 +/- 1.0 in treated cells), whereas cholera toxin had only a small effect (EC50 = 0.47 +/- 0.02 ng/ml). Correcting for the change in hormone binding, pertussis toxin was found to decrease the coupling efficiency of occupied receptors (50% of maximal insulin effect with 928 molecules bound/cell in control and 3418 in treated cells). Pertussis-toxin inhibition of insulin sensitivity was slow in onset, requiring 2-3 h for completion. Under conditions where pertussis-toxin inhibition of insulin sensitivity was maximal, a 41,000 Da protein similar to the alpha subunit of Gi (the inhibitory G-protein) was found to be fully ribosylated. These results are consistent with the concept that pertussis-toxin-sensitive G-protein(s) can modify the insulin-receptor/glucose-transport coupling system.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen I. S., Gaa S. T., Rogers T. B. Changes in expression of a functional Gi protein in cultured rat heart cells. Am J Physiol. 1988 Jul;255(1 Pt 1):C51–C59. doi: 10.1152/ajpcell.1988.255.1.C51. [DOI] [PubMed] [Google Scholar]
- Arsenis G., Livingston J. N. Isoproterenol reduces insulin stimulation of hexose uptake by rat adipocytes via a postinsulin binding alteration. Endocrinology. 1986 Jul;119(1):50–57. doi: 10.1210/endo-119-1-50. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chou C. K., Dull T. J., Russell D. S., Gherzi R., Lebwohl D., Ullrich A., Rosen O. M. Human insulin receptors mutated at the ATP-binding site lack protein tyrosine kinase activity and fail to mediate postreceptor effects of insulin. J Biol Chem. 1987 Feb 5;262(4):1842–1847. [PubMed] [Google Scholar]
- Ciaraldi T. P. The role of adenosine in insulin action coupling in rat adipocytes. Mol Cell Endocrinol. 1988 Nov;60(1):31–41. doi: 10.1016/0303-7207(88)90117-7. [DOI] [PubMed] [Google Scholar]
- Elks M. L., Watkins P. A., Manganiello V. C., Moss J., Hewlett E., Vaughan M. Selective regulation by pertussis toxin of insulin-induced activation of particulate cAMP phosphodiesterase activity in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 1983 Oct 31;116(2):593–598. doi: 10.1016/0006-291x(83)90565-x. [DOI] [PubMed] [Google Scholar]
- Gawler D., Milligan G., Spiegel A. M., Unson C. G., Houslay M. D. Abolition of the expression of inhibitory guanine nucleotide regulatory protein Gi activity in diabetes. Nature. 1987 May 21;327(6119):229–232. doi: 10.1038/327229a0. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Goren H. J., Northup J. K., Hollenberg M. D. Action of insulin modulated by pertussis toxin in rat adipocytes. Can J Physiol Pharmacol. 1985 Aug;63(8):1017–1022. doi: 10.1139/y85-167. [DOI] [PubMed] [Google Scholar]
- Green A. Adenosine receptor down-regulation and insulin resistance following prolonged incubation of adipocytes with an A1 adenosine receptor agonist. J Biol Chem. 1987 Nov 15;262(32):15702–15707. [PubMed] [Google Scholar]
- Green A. Catecholamines inhibit insulin-stimulated glucose transport in adipocytes, in the presence of adenosine deaminase. FEBS Lett. 1983 Feb 21;152(2):261–264. doi: 10.1016/0014-5793(83)80392-5. [DOI] [PubMed] [Google Scholar]
- Haigler H. T., Maxfield F. R., Willingham M. C., Pastan I. Dansylcadaverine inhibits internalization of 125I-epidermal growth factor in BALB 3T3 cells. J Biol Chem. 1980 Feb 25;255(4):1239–1241. [PubMed] [Google Scholar]
- Hewlett E. L., Cronin M. J., Moss J., Anderson H., Myers G. A., Pearson R. D. Pertussis toxin: lessons from biological and biochemical effects in different cells. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:173–182. [PubMed] [Google Scholar]
- Heyworth C. M., Houslay M. D. Insulin exerts actions through a distinct species of guanine nucleotide regulatory protein: inhibition of adenylate cyclase. Biochem J. 1983 Aug 15;214(2):547–552. doi: 10.1042/bj2140547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch J., Gallian E. Methods for the determination of adipose cell size in man and animals. J Lipid Res. 1968 Jan;9(1):110–119. [PubMed] [Google Scholar]
- Hsia J. A., Moss J., Hewlett E. L., Vaughan M. ADP-ribosylation of adenylate cyclase by pertussis toxin. Effects on inhibitory agonist binding. J Biol Chem. 1984 Jan 25;259(2):1086–1090. [PubMed] [Google Scholar]
- Izawa T., Komabayashi T., Shinoda S., Suda K., Tsuboi M., Koshimizu E. Possible mechanism of regulating adenylate cyclase activity in adipocyte membranes from exercise-trained male rats. Biochem Biophys Res Commun. 1988 Mar 30;151(3):1262–1268. doi: 10.1016/s0006-291x(88)80502-3. [DOI] [PubMed] [Google Scholar]
- Joost H. G., Göke R., Steinfelder H. J. Dual effect of isoprenaline on glucose transport and response to insulin in isolated adipocytes. Biochem Pharmacol. 1985 Mar 1;34(5):649–653. doi: 10.1016/0006-2952(85)90259-x. [DOI] [PubMed] [Google Scholar]
- Joost H. G., Weber T. M., Cushman S. W., Simpson I. A. Insulin-stimulated glucose transport in rat adipose cells. Modulation of transporter intrinsic activity by isoproterenol and adenosine. J Biol Chem. 1986 Aug 5;261(22):10033–10036. [PubMed] [Google Scholar]
- Kahn C. R. The molecular mechanism of insulin action. Annu Rev Med. 1985;36:429–451. doi: 10.1146/annurev.me.36.020185.002241. [DOI] [PubMed] [Google Scholar]
- Katada T., Ui M. ADP ribosylation of the specific membrane protein of C6 cells by islet-activating protein associated with modification of adenylate cyclase activity. J Biol Chem. 1982 Jun 25;257(12):7210–7216. [PubMed] [Google Scholar]
- Kather H., Aktories K., Schulz G., Jakobs K. H. Islet-activating protein discriminates the antilipolytic mechanism of insulin from that of other antilipolytic compounds. FEBS Lett. 1983 Sep 5;161(1):149–152. doi: 10.1016/0014-5793(83)80749-2. [DOI] [PubMed] [Google Scholar]
- Kather H., Bieger W., Michel G., Aktories K., Jakobs K. H. Human fat cell lipolysis is primarily regulated by inhibitory modulators acting through distinct mechanisms. J Clin Invest. 1985 Oct;76(4):1559–1565. doi: 10.1172/JCI112137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi A., Kozawa O., Kaibuchi K., Katada T., Ui M., Takai Y. Direct evidence for involvement of a guanine nucleotide-binding protein in chemotactic peptide-stimulated formation of inositol bisphosphate and trisphosphate in differentiated human leukemic (HL-60) cells. Reconstitution with Gi or Go of the plasma membranes ADP-ribosylated by pertussis toxin. J Biol Chem. 1986 Sep 5;261(25):11558–11562. [PubMed] [Google Scholar]
- Klein H. H., Ciaraldi T. P., Freidenberg G. R., Olefsky J. M. Adenosine modulates insulin activation of insulin receptor kinase in intact rat adipocytes. Endocrinology. 1987 Jun;120(6):2339–2345. doi: 10.1210/endo-120-6-2339. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Olefsky J. M. Effects of streptozotocin-induced diabetes on insulin binding, glucose transport, and intracellular glucose metabolism in isolated rat adipocytes. Diabetes. 1979 Feb;28(2):87–95. doi: 10.2337/diab.28.2.87. [DOI] [PubMed] [Google Scholar]
- Krupinski J., Rajaram R., Lakonishok M., Benovic J. L., Cerione R. A. Insulin-dependent phosphorylation of GTP-binding proteins in phospholipid vesicles. J Biol Chem. 1988 Sep 5;263(25):12333–12341. [PubMed] [Google Scholar]
- Kuroda M., Honnor R. C., Cushman S. W., Londos C., Simpson I. A. Regulation of insulin-stimulated glucose transport in the isolated rat adipocyte. cAMP-independent effects of lipolytic and antilipolytic agents. J Biol Chem. 1987 Jan 5;262(1):245–253. [PubMed] [Google Scholar]
- Lacasa D., Agli B., Giudicelli Y. Increased sensitivity of fat cell adenylate cyclase to stimulatory agonists during fasting is not related to impaired inhibitory coupling system. FEBS Lett. 1986 Jul 7;202(2):260–266. doi: 10.1016/0014-5793(86)80698-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Litosch I., Fain J. N. Regulation of phosphoinositide breakdown by guanine nucleotides. Life Sci. 1986 Jul 21;39(3):187–194. doi: 10.1016/0024-3205(86)90529-1. [DOI] [PubMed] [Google Scholar]
- Lu Z. D., Piñeyro M. A., Kirkland J. L., Li Z. H., Gregerman R. I. Prostaglandin-sensitive adenylyl cyclase of cultured preadipocytes and mature adipocytes of the rat: probable role of Gi in determination of stimulatory or inhibitory action. J Cell Physiol. 1988 Jul;136(1):1–12. doi: 10.1002/jcp.1041360102. [DOI] [PubMed] [Google Scholar]
- Lönnroth P., Davies J. I., Lönnroth I., Smith U. The interaction between the adenylate cyclase system and insulin-stimulated glucose transport. Evidence for the importance of both cyclic-AMP-dependent and -independent mechanisms. Biochem J. 1987 May 1;243(3):789–795. doi: 10.1042/bj2430789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisel A. S., Wright C. M., Carter S. M., Ziegler M., Motulsky H. J. Tachyphylaxis with amrinone therapy: association with sequestration and down-regulation of lymphocyte beta-adrenergic receptors. Ann Intern Med. 1989 Feb 1;110(3):195–201. doi: 10.7326/0003-4819-110-3-195. [DOI] [PubMed] [Google Scholar]
- Malbon C. C., Rapiejko P. J., Garciá-Sáinz J. A. Pertussis toxin catalyzes the ADP-ribosylation of two distinct peptides, 40 and 41 kDa, in rat fat cell membranes. FEBS Lett. 1984 Oct 29;176(2):301–306. doi: 10.1016/0014-5793(84)81184-9. [DOI] [PubMed] [Google Scholar]
- Milligan G., Spiegel A. M., Unson C. G., Saggerson E. D. Chemically induced hypothyroidism produces elevated amounts of the alpha subunit of the inhibitory guanine nucleotide binding protein (Gi) and the beta subunit common to all G-proteins. Biochem J. 1987 Oct 1;247(1):223–227. doi: 10.1042/bj2470223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G. Techniques used in the identification and analysis of function of pertussis toxin-sensitive guanine nucleotide binding proteins. Biochem J. 1988 Oct 1;255(1):1–13. doi: 10.1042/bj2550001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno F. J., Mills I., García-Sáinz J. A., Fain J. N. Effects of pertussis toxin treatment on the metabolism of rat adipocytes. J Biol Chem. 1983 Sep 25;258(18):10938–10943. [PubMed] [Google Scholar]
- Morgan D. O., Roth R. A. Acute insulin action requires insulin receptor kinase activity: introduction of an inhibitory monoclonal antibody into mammalian cells blocks the rapid effects of insulin. Proc Natl Acad Sci U S A. 1987 Jan;84(1):41–45. doi: 10.1073/pnas.84.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. M., Houslay M. D., Milligan G., Siddle K. The insulin receptor tyrosyl kinase phosphorylates holomeric forms of the guanine nucleotide regulatory proteins Gi and Go. FEBS Lett. 1987 Feb 23;212(2):281–288. doi: 10.1016/0014-5793(87)81361-3. [DOI] [PubMed] [Google Scholar]
- Obermaier-Kusser B., Mühlbacher C., Mushack J., Rattenhuber E., Fehlmann M., Haring H. U. Regulation of glucose carrier activity by AlCl3 and phospholipase C in fat-cells. Biochem J. 1988 Dec 1;256(2):515–520. doi: 10.1042/bj2560515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olansky L., Myers G. A., Pohl S. L., Hewlett E. L. Promotion of lipolysis in rat adipocytes by pertussis toxin: reversal of endogenous inhibition. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6547–6551. doi: 10.1073/pnas.80.21.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky J. M. Mechanisms of the ability of insulin to activate the glucose-transport system in rat adipocytes. Biochem J. 1978 Apr 15;172(1):137–145. doi: 10.1042/bj1720137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens J. R., Frame L. T., Ui M., Cooper D. M. Cholera toxin ADP-ribosylates the islet-activating protein substrate in adipocyte membranes and alters its function. J Biol Chem. 1985 Dec 15;260(29):15946–15952. [PubMed] [Google Scholar]
- Parsons W. J., Stiles G. L. Heterologous desensitization of the inhibitory A1 adenosine receptor-adenylate cyclase system in rat adipocytes. Regulation of both Ns and Ni. J Biol Chem. 1987 Jan 15;262(2):841–847. [PubMed] [Google Scholar]
- Pessin J. E., Gitomer W., Oka Y., Oppenheimer C. L., Czech M. P. beta-Adrenergic regulation of insulin and epidermal growth factor receptors in rat adipocytes. J Biol Chem. 1983 Jun 25;258(12):7386–7394. [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Rapiejko P. J., Northup J. K., Evans T., Brown J. E., Malbon C. C. G-proteins of fat-cells. Role in hormonal regulation of intracellular inositol 1,4,5-trisphosphate. Biochem J. 1986 Nov 15;240(1):35–40. doi: 10.1042/bj2400035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg P. L., Kahn C. R. Insulin inhibits pertussis toxin-catalyzed ADP-ribosylation of G-proteins. Evidence for a novel interaction between insulin receptors and G-proteins. J Biol Chem. 1988 Oct 25;263(30):15546–15552. [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Segura M., Saborío J. L. Separation of the mRNAs coding for different forms of chick embryo gizzard tropomyosin. J Biol Chem. 1982 Aug 10;257(15):8867–8871. [PubMed] [Google Scholar]
- Spiegel A. M. Signal transduction by guanine nucleotide binding proteins. Mol Cell Endocrinol. 1987 Jan;49(1):1–16. doi: 10.1016/0303-7207(87)90058-x. [DOI] [PubMed] [Google Scholar]
- Tamura M., Nogimori K., Yajima M., Ase K., Ui M. A role of the B-oligomer moiety of islet-activating protein, pertussis toxin, in development of the biological effects on intact cells. J Biol Chem. 1983 Jun 10;258(11):6756–6761. [PubMed] [Google Scholar]