Abstract
Background
Flaxseed has been widely used in animal diets to increase the omega-3 polyunsaturated fatty acid content in animal products and promote overall animal health, but little known about its effects on the productive performance and the mictobita of gut of laying duck.
Methods and results
Jinding duck, a Chinese indigenous breed, was used in the study. The corn-soybean basal diet supplemented with 0, 2%, 3% 4% and 5% flaxseed were provided to Control, 2% Fla, 3% Fla, 4% Fla and 5% Fla groups for 53 days, respectively. Compared with Control group, groups fed with flaxseed diets showed higher egg production, egg mass, ovary weight and more preovulatory follicles. The Docosahexaenoic Acid content of egg was extremely significantly elevated by flaxseed diets (P < 0.01), and the albumen height and haugh unit were elevated, especially in 4% Fla and/or 5% Fla group (P < 0.05). Groups 4% Fla and 5% Fla had highest ileal villus height, jejunal and ileal crypt depth. Moreover, Flaxseed diets significantly increased the levels of IgG and IgM in all Fla groups (P < 0.01), while increased IgA levels except for in 3% Fla group (P < 0.05). The results of 16s rDNA sequencing showed that flaxseed diet altered the microbial composition of gut and reduced the diversity and evenness of gut microbial communities except for 5% Fla. The correlation analysis identified Blautia, Butyricicoccus and Subdoligranulum positively associated with egg production. Genera Fourinierella, Fusobacterium and Intestinimonas positively associated with ovary weight, haught unit and album height. And Mucispirillum positively associated with haugh unit and album height.
Conclusion
This study has suggested that flaxseed play a positive role in productive performance, the overall or intestinal health of laying ducks.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11033-024-09858-y.
Keywords: Laying duck, Flaxseed, Omega-3 Polyunsaturated Fatty Acids, Gut microbiome
Introduction
Flaxseed (Linum usitatissimum) is a globally cultivated oil-seed crop known for its high oil content, comprising 35–45% of the seed. This oil is rich in α-linolenic acid (ALA, 18:3n-3), which accounts for 45–54% of the total fatty acids. Except ALA, flaxseed oil also include linoleic acid, oleic acid, and saturated fatty acids. Additionally, flaxseed is a valuable source of proteins, lignans, soluble fiber, and phenolic compounds. ALA, a natural omega-3 polyunsaturated fatty acid (ω-3 PUFA), serves as the precursor to eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) [1]. EPA and DHA have demonstrated preventive and therapeutic properties in various human diseases, including hypertriglyceridemia [2], cardiovascular disease [3], and Alzheimer’s disease [4]. Flaxseed and its extract are widely utilized as ingredients or food additives due to their health benefits, such as anti-inflammatory, antioxidant, and lipid-modulating properties [5].
The gastrointestinal tract of animals harbors a diverse microbial community, which plays essential roles in digestion, absorption, defense, and other physiological activities [6–8]. Diet composition affect the structure of the postnatal gut microbiota in animals and humans and entails complex interactions between the diet, microbiota, and the host [9]. Due to the prohibition of antibiotics in livestock and poultry farming, researchers have became more interested in the potential of herbal additives to improve the health and productivity of domesticated animals. Some beneficial effects of herbal additives may be attributed to their impact on the gut microbiota of these animals [10].
In recent years, flaxseed has been incorporated into the diets of domesticated animals to enhance the PUFA content in animal-derived products and promote animal health [11–13]. In ducks, addition of flaxseed in their diet has been shown to increase ALA, DHA, EPA, and total ω-3 PUFAs in meat or eggs [14, 15]. However, little is known about the effects of flaxseed supplementation on the gut microbiome of ducks. Therefore, this study aims to make a comprehensive knowledge of the effects of flaxseed on the growth, production and gut microbial communities of duck.
Materials and methods
Animal manipulation and sample collection
In the study, a total of 2250 healthy Jinding ducks, aged 140 days, were obtained from Hubei Chuda Duck Industry Co., Ltd (Hubei, China). Flaxseed was purchased from a biotechnology company (An You Biotechnology Inc.) and its composition was detected (Table S1). The ducks were randomly allotted into 15 replicates, each treatment had 3 replicates with 150 ducks in each replicate. These ducks were randomly reared in semi open rooms equipped with clean pool, feeders, defecation board and sufficient laying nests. The ducks were allowed to free access to feed and water during the experiment. A corn-soybean meal diet was formulated as the basal diet. Ducks were allotted to 5 dietary treatments, including the Control group (basal diet), 2% Fla group (basal diet supplemented with 2% flaxseed), 3% Fla group (basal diet supplemented with 3% flaxseed), 4% Fla group (basal diet supplemented with 4% flaxseed) and 5% Fla group (basal diet supplemented with 5% flaxseed). The four groups treated with flaxseed can be referred as Fla groups in the following description. The gross composition of the basal diet for the Control and Fla groups were formulated to meet the nutrient specifications recommended by the NRC (1994) (Tables S2 and S3).
The experiment lasted 53 days when the average daily laying rate of the five groups reached over 85%. During the experiment, daily feed intake (FI), egg weight (EW) and mass (EW), egg production (EP) and feed conversion ratio (FCR) of each group were assessed. The eeg production and FCR were calculated as rate of production per replicate per day and feed intake/egg mass. On the 53rd day, 30 ducks were randomly selected from five groups (two ducks in each replicate). The weight of body (BW), liver (LW), spleen (SW), pancreas (PW) and ovary (OW) of duck were recorded and the number of preovulatory follicles (NPF) were counted. The colon digest of 30 ducks (6 replicates of each group) was collected, transferred to sterile tubes, snap-frozen in liquid nitrogen, and stored at -80℃ for the 16s rDNA sequencing. The middle section of the duodenum, ileum and jejunum of three ducks of each group were randomly collected for morphological analysis. Meanwhile, three eggs of each group were collected and sent to the Institute of Oil Crops (Chinese Academy of Agricultural Sciences) to measure the concentrations of ALA, EPA, and DHA. Then ten eggs of each group were conducted quality determination.
Morphological analysis of gut
The segments derived from duodenum, jejunum and ileum of three ducks in each group were fixed using standard paraffin embedding method. Three cross sections of each intestinal segments were stained with hematoxylin and eosin (H&E). At least three well-oriented villi and the associated crypts of each segment were measured, and the villus/crypt ratio was calculated.
Serum protein profiling
Total protein (TP), albumin (ALB), globulin (GLOB) in serum were assessed using fully-automatic blood biochemical analyzer, and the concentrations of IgA, IgG and IgM (3 replicates of each group) were determined following the protocol of ELISA Kits (DRE-D5713b, DRE-D5712b, DRE-C5715b; Kamai Shu Biotechnology, China) under the sensitivities of 1.0 μg/mL,10 μg/mL and 10 μg/mL, respectively. The coefficients of variation inter-assay and intra-assay were all less than 15%.
DNA extraction and PCR amplification
Microbial DNA was extracted from the contents of the colon using the Magnetic Soil and Stool DNA Kit (TianGen, China, Catalog #: DP712). The 16S rRNA genes of the V3-V4 region were amplified using the specific primers 341F (5′-CCTAYGGGRBGCASCAG-3′) and 806R (5′-GGACTACNNGGGTATCTAAT-3′). The PCR reaction mixture consisted of 15 μL of Phusion® High-Fidelity PCR Master Mix (New England Biolabs), 0.2 μL of forward and reverse primers, and approximately 10 ng of template DNA. The amplification protocol involved an initial denaturation step at 98 ℃ for 1 min, followed by 30 cycles of denaturation at 98 ℃ for 10 s, annealing at 50 ℃ for 30 s, and elongation at 72 ℃ for 30 s, and a final extension at 72 ℃ for 5 min. The quality of the amplified DNA was assessed using 2% agarose gel electrophoresis. The purified PCR products were then subjected to purification using the Universal DNA Purification Kit (Tianjin, China).
16s rDNA sequencing process and data analysis
Sequencing libraries were prepared using the NEB Next® UltraTM II FS DNA PCR-free Library Prep Kit (New England Biolabs, USA), following the manufacturer’s recommendations. Indexes were incorporated during library preparation. The quality of the library was assessed using Qubit and real-time PCR. A bioanalyzer were applied to analyze the size distribution analysis. After quantification, the libraries were pooled and sequenced on the Illumina NovaSeq platform, generating 250 bp paired-end reads. Raw tags of each sample were obtained after removing barcodes and primers using FLASH software (V1.2.11, http://ccb.jhu.edu/software/FLASH/) [16]. Effective tags were finally obtained through filtering out tags with low quality and removing chimera sequences using fast software (Version 0.23.1) [17] and the UCHIME Algorithm (http://www.drive5.com/usearch/manual/uchime_algo.html) [18]. The final Amplicon Sequence Variants (ASVs) were obtained after denoising and then assigned to species annotation and phylogenetic analysis. Subsequent analysis were conducted based on the normalized data of each sample.
The alpha diversity indices, Goods coverage, Chao 1, Simpson and Pielou_e were generated and analyzed using wilcox test. The beta diversity were calculated based on unweighted unifrac distances and analyzed using wilcox test. Multiple Response Permutation Procedure (MRPP) based on Bray–Curtis distances and Principal Coordinates Analysis (PCoA) were performed in R. Differential microbiota based on the relative abundance at the phylum and genus levels were identified using MetaStat analysis in R.
Correlation analysis
Spearman’s correlation analysis was conducted in R (R-4.3.2) to study the relationship between micorobial species and productive performance of duck. The results were presented by the heatmap plot and the P-value were also concerned.
Statistical analysis
The data related to the growth and production of duck, morphological analysis of gut and serum parameters were all checked for normality of variance and homogeneity of variance using the Shapiro–Wilk test and bartlett test in R, respectively. One-way analysis of variance (ANOVA) and Duncan’s multiple range tests in R were carried out for the analysis of data that obeyed the conditions of ANOVA. The data that disobeyed the conditions were analyzed using the Kruskal–Wallis test in R. The P-value below 0.05 were considered statistically significant.
Result
Effects of flaxseed on the growth and productive performance of duck
As shown in Table 1, there were no statistically significant differences in the BW, LW, SW and PW among groups. Compared with control group, flaxseed did promote the OW and the NPF, which showed the highest levels in 4% Fla group.
Table 1.
Effects of flaxseed on the growth of duck
| Group | BW (g) | LW (g) | SW (g) | PW(g) | OW (g) | NPF |
|---|---|---|---|---|---|---|
| Control | 1220 ± 110 | 41.68 ± 7.58 | 0.60 ± 0.30 | 4.83 ± 1.10 | 38.30 ± 3.28c | 5.17 ± 0.41b |
| 2%Fla | 1290 ± 90 | 42.90 ± 4.74 | 0.68 ± 0.19 | 4.33 ± 0.43 | 45.94 ± 3.50b | 6.00 ± 0.63ab |
| 3%Fla | 1260 ± 100 | 45.63 ± 8.24 | 0.70 ± 0.13 | 5.05 ± 0.50 | 49.71 ± 5.58b | 5.67 ± 0.52ab |
| 4%Fla | 1400 ± 140 | 41.39 ± 7.92 | 0.71 ± 0.21 | 5.45 ± 1.17 | 56.98 ± 9.89a | 6.50 ± 1.05a |
| 5%Fla | 1330 ± 110 | 41.10 ± 5.17 | 0.72 ± 0.12 | 5.04 ± 0.61 | 46.76 ± 4.91b | 5.67 ± 0.52ab |
Different superscript lowercase letter in one column indicated statistically difference at P < 0.05
Both production and quality of egg are the most important characteristics of laying duck. As shown in Table 2, the FI increased as the proportion of flaxssed in diet elevated even though there were no significant differences in FI among groups. And no significant difference in EW among groups were observed. However, the EP of laying ducks was extremely significantly elevated in 2% Fla, 3% Fla and 4% Fla groups (P < 0.01) compared to control group and 5% Fla group. Ducks fed with 3% flaxseed diet showed significantly higher average EM than other groups (P < 0.05). And the EM in 2% Fla and 4% Fla groups was significantly higher than that in 5% Fla and control groups (P < 0.05). The FCR value of 5% Fla group was the highest, followed by Control and 4% Fla groups, and the FCR value of 3% Fla group was the lowest.
Table 2.
Effects of flaxseed on productive performance of laying duck
| Group | FI (g/d/bird) | EP (%) | EW (g) | EM (g/d/bird) | FCR |
|---|---|---|---|---|---|
| Control | 134.28 ± 7.22 | 51.55 ± 1.01D | 57.87 ± 2.86 | 29.84 ± 1.66c | 4.50 ± 0.08b |
| 2% Fla | 134.38 ± 6.05 | 60.84 ± 1.39B | 57.70 ± 1.96 | 35.11 ± 1.72b | 3.83 ± 0.14c |
| 3% Fla | 137.01 ± 5.00 | 65.11 ± 0.32A | 58.59 ± 2.49 | 38.15 ± 1.62a | 3.60 ± 0.24c |
| 4% Fla | 138.54 ± 7.02 | 57.83 ± 1.22C | 58.60 ± 2.76 | 33.87 ± 1.25b | 4.10 ± 0.30bc |
| 5% Fla | 158.07 ± 11.09 | 53.27 ± 1.31D | 57.34 ± 3.26 | 30.52 ± 1.00c | 5.18 ± 0.40a |
Different superscript lowercase letter and capital letter in one column indicated statistically difference at P < 0.05 and P < 0.01, respectively
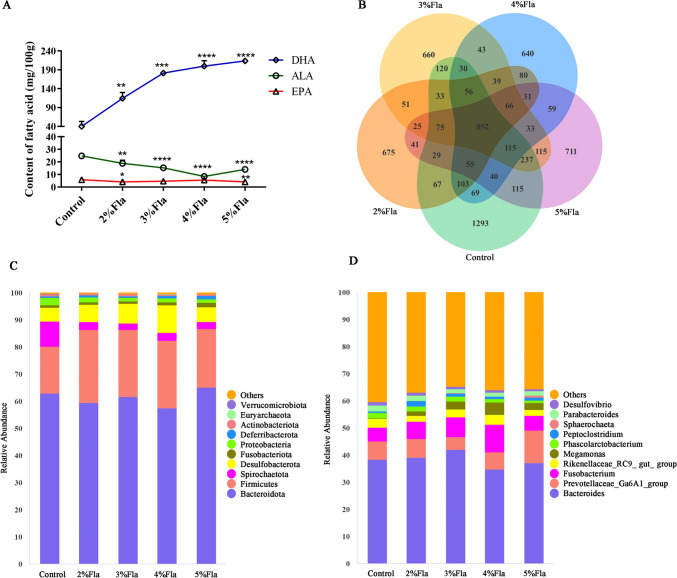
On the other hand, the unsaturated fatty acid content of the eggs and egg quality were also measured. Flaxseed diet significantly increased the content of DHA, but decreased the content of ALA (Fig. 1A; P < 0.01). The content of EPA remained a relatively low level although it significantly decreased in 2% and 5% Fla groups compared with Control group (P < 0.05). Moreover, The album height (AH) increased as the proportion of flaxseed elevated and it was significantly higher in 5% Fla group compared with Control group. Likewise, groups with 3–5% flaxseed had higher haugh unit (HU) of egg (P < 0.05). But flaxseed had no significant effects on the shell thickness (STE) (Table 3).
Fig. 1.
Effects of flaxseed on the content of ω-3 PUFAs in egg and microbial compositon of gut. A The content of DHA, EPA and ALA in eggs; B Venn diagram of ASVs. Each pear-like plot represents a group; The top 10 abundant ASVs at phylum C and genus D level. Others represents the proportion of ASVs unannotated or with low abundance at the phylum and genus level, respectively. * represents the P-value is below 0.05; ** represents the P-value is below 0.01; *** represents the P-value is below 0.001; **** represents the P-value is below 0.0001
Table 3.
Effects of flaxseed on the quality of egg
| Group | AH | HU | STE |
|---|---|---|---|
| Control | 4.28 ± 1.07b | 61.37 ± 10.70b | 0.44 ± 0.05 |
| 2% Fla | 4.45 ± 0.94ab | 63.65 ± 7.97b | 0.49 ± 0.05 |
| 3% Fla | 5.48 ± 1.80ab | 72.08 ± 13.51ab | 0.40 ± 0.04 |
| 4% Fla | 6.57 ± 1.06ab | 81.13 ± 7.61a | 0.42 ± 0.06 |
| 5% Fla | 7.68 ± 1.75a | 85.71 ± 10.97a | 0.45 ± 0.06 |
Different superscript lowercase letter in one column indicated statistically difference at P < 0.05
Effects of flaxseed on the gut morphology and serum parameters of duck
The crypt depth, villi height and the ratio of villi height to crypt depth are often used to value the absorption capacity and mucosal damage of intestine, respectively. In the study, morphological analysis for duodenum, jejunum and ileum were conducted (Table 4). Groups fed with diets containing 4% and 5% flaxeed had higher ileal villus height (VH) than other groups (P < 0.05). 4% Fla group had the highest jejunal and duodenal VH even though there was no significant difference between 4% Fla and Control groups. No significant differences in crypt depths (CD) between Control and Fla groups were observed, but they were upregulated in 4% and 5% Fla groups compared to Control group. As for the ratio of VH to CD (VCR), The dominant group varied in different parts of the gut and there were no significant differences between Control and Fla groups (P > 0.05).
Table 4.
Effects of flaxseed on the gut morphology
| Item | Groups | ||||
|---|---|---|---|---|---|
| Control | 2%Fla | 3%Fla | 4%Fla | 5%Fla | |
| Duodenum | |||||
| VH(μm) | 788.32.92 ± 21.73 | 778.25 ± 37.00 | 768.87 ± 40.19 | 971.28 ± 94.88 | 762.09 ± 50.82 |
| CD(μm) | 357.38 ± 17.67ab | 320.74 ± 23.41ab | 283.65 ± 10.15b | 428.31 ± 20.20a | 326.69 ± 68.87ab |
| VCR | 2.21 ± 0.17ab | 2.44 ± 0.20ab | 2.72 ± 0.19a | 2.02 ± 0.18b | 2.39 ± 0.36ab |
| Jejunum | |||||
| VH(μm) | 935.24 ± 12.20ab | 890.22 ± 71.08ab | 836.13 ± 57.73b | 1204.77 ± 29.00a | 1074.26 ± 237.61ab |
| CD(μm) | 199.90 ± 9.47 | 195.89 ± 6.8 | 205.90 ± 11.39 | 210.51 ± 23.17 | 217.85 ± 15.03 |
| VCR | 4.69 ± 0.22ab | 4.56 ± 0.51ab | 4.08 ± 0.43b | 5.77 ± 0.60a | 4.91 ± 0.80ab |
| ileum | |||||
| VH(μm) | 713.61 ± 15.41b | 755.20 ± 30.71ab | 709.42 ± 45.86b | 836.51 ± 41.01a | 789.47 ± 23.54a |
| CD(μm) | 169.70 ± 38.60 | 144.25 ± 10.78 | 177.95 ± 16.06 | 185.46 ± 13.65 | 183.34 ± 25.33 |
| VCR | 4.4 ± 0.96ab | 5.26 ± 0.43a | 4.01 ± 0.44b | 4.54 ± 0.51ab | 4.36 ± 0.50ab |
Different superscript lowercase letter in one column indicated statistically difference at P < 0.05
Moreover, the content of ALB, TP, GLOB, IgA, IgG and IgM were measured to assess the effects of flaxseed on the health of duck. As shown in Table 5, addition of flaxseed extremely significantly elevated the concentrations of IgG and IgM in most Fla groups compared to Control group (P < 0.01). and except for 3% Fla group, the concentrations of IgA were significantly elevated in other Fla groups compared to Control group (P < 0.05). There were no significant differences in ALB, TP and GLOB.
Table 5.
Effects of flaxseed on serum parameters of duck
| Item | Control | 2%Fla | 3%Fla | 4%Fla | 5%Fla |
|---|---|---|---|---|---|
| ALB (g/L) | 24.67 ± 1.15 | 24.33 ± 0.58 | 25.67 ± 2.08 | 26.67 ± 1.53 | 27.33 ± 0.58 |
| TP (g/L) | 61.67 ± 1.15 | 56.00 ± 4.36 | 57.67 ± 7.02 | 59.67 ± 4.51 | 65.67 ± 5.13 |
| GLOB (g/L) | 37.00 ± 2.00 | 31.67 ± 4.93 | 32.00 ± 5.00 | 33.00 ± 3.00 | 38.33 ± 4.73 |
| IgA (μg/mL) | 124.41 ± 3.98d | 257.56 ± 17.97a | 133.61 ± 19.34d | 172.98 ± 3.89b | 157.20 ± 8.78c |
| IgG (μg/mL) | 1191.34 ± 61.10C | 1740.88 ± 66.32B | 2068.18 ± 16.94A | 2135.82 ± 69.46A | 1691.36 ± 56.81B |
| IgM (μg/mL) | 1200.79 ± 95.31B | 1492.50 ± 80.36A | 1161.83 ± 36.18B | 1540.95 ± 63.44A | 1480.15 ± 45.57A |
Different superscript lowercase letter and capital letter in one column indicated statistically difference at P < 0.05 and P < 0.01, respectively
Data summary of 16s rDNA sequencing
A total of 4,082,163 raw reads were obtained, and an average of 108,310 clean reads were generated (Table S4). After removing duplicate sequences, a total of 6558 unique ASVs were obtained. The Venn chart showed that there were 852 common ASVs among the five groups, accounting for 25.90% of Control group, 37.40% of 2% Fla group, 33.41% of 3% Fla group, 36.87% of 4% Fla group, and 32.78% of 5% Fla group. Additionally, each group also has unique ASVs, with 1293 ASVs in the Control group, 675 ASVs in the 2% Fla group, 660 ASVs in the 3% Fla group, 640 ASVs in the 4% Fla group, and 711 ASVs in the 5% Fla group (Fig. 1B).
Flaxseed altered the microbial composition and diversity of duck’s colon
At the phylum level (Fig. 1C), the most abundant ASVs were Bacteroidota, Firmicutes, Proteobacteria, Fusobacteriota, Spirochaetota, Desulfobacterota, Deferribacterota, Actinobacteriota, Euryarchaeota, and Verrucomicrobiota. Bacteroidota accounted for more than 55% of the ASVs in each group, making it the most dominant group. The average percentage of Firmicutes of Fla groups was 1.4 times higher than that of Control group. There was a slight increase in the percentage of Fusobacteriota and Deferribacterota in each Fla group compared to Control group. However, there was a substantial decline of about 70% in the percentage of Proteobacteria in each Fla group. Moreover, the percentage of Verrucomicrobiota in Control group was one order of magnitude higher than that in each Fla group. At the genus level (Fig. 1D), the top 10 abundant ASVs were Bacteroides, Prevotellaceae_Ga6A1_group, Fusobacterium, Rikenellaceae_RC9_gut_group, Megamonas, Phascolarctobacterium, Peptoclostridium, Sphaerochaeta, Parabacteroides, and Desulfovibrio. Among them, the percentage of Megamonas increased from 0.24% in Control group to an average of 2.94% in Fla groups. The abundance of Peptoclostridium also increased by almost an order of magnitude in Fla groups. Prevotellaceae_Ga6A1_group and Fusobacterium showed significant increases in 5% Fla and 4% Fla group, respectively. Conversely, the percentages of Phascolarctobacterium and Desulfovibrio declined gradually with increased flaxseed intake.
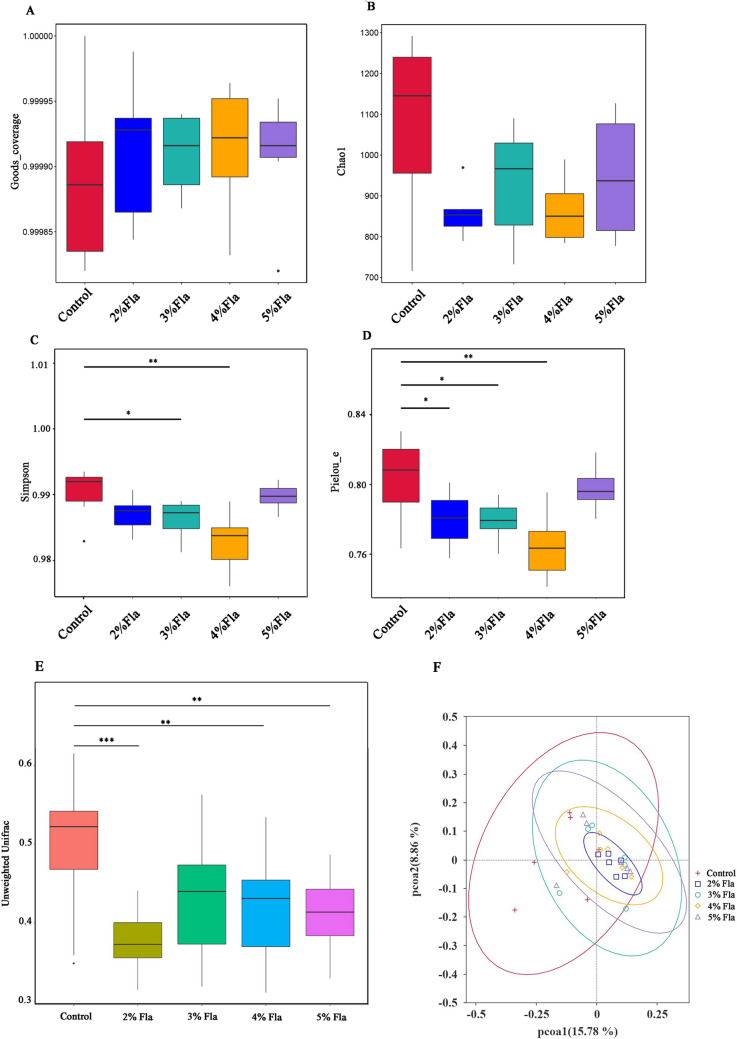
Alpha diversities were assessed using several indices, including Good’s coverage, Chao1, Simpson, and Pielou_e (Fig. 2A–D). The Good’s coverage values for all five groups were above 0.9998, indicating that the sequencing results were representative and reliable. No significant difference in richness of bacterial community among these groups were found according to the Chao1 index. Whereas it seemed that the diversity and evenness of gut microbiota were affected by flaxseed diet according to the Simpson and Pielou_e indices, respectively. The diversities of bacterial community of gut gradually decreased and it was significantly down-regulated in 3% and 4% Fla groups (P < 0.05). Similarly, the evenness of gut microbiota gradually decreased as the proportion of flaxseed increased to 4%. However, the diversity and evenness seemed to be unaffected in 5% Fla group compared to Control group.
Fig. 2.
Effects of flaxseed on the compositon and diversities of intestinal microbiota. Analysis of alpha indices including Goods Coverage A, Chao1 B, Simpson C and Pielou_e D. Beta diversity analysis basing on unweighted UniFrac distances matrices E. PCoA analysis F.* represents the P-value is below 0.05; ** represents the P-value is below 0.01; *** represents the P-value is below 0.001
Beta diversity analysis based on unweighted UniFrac distance matrices demonstrated a significant changes in microbial communities in most Fla groups compared to Control group (Fig. 2E, P < 0.01). Moreover, the analysis of MRPP and PCoA showed that the composition and structure of bacterial community of Control group was distinct from that of Fla groups (Fig. 2F; Table S5).
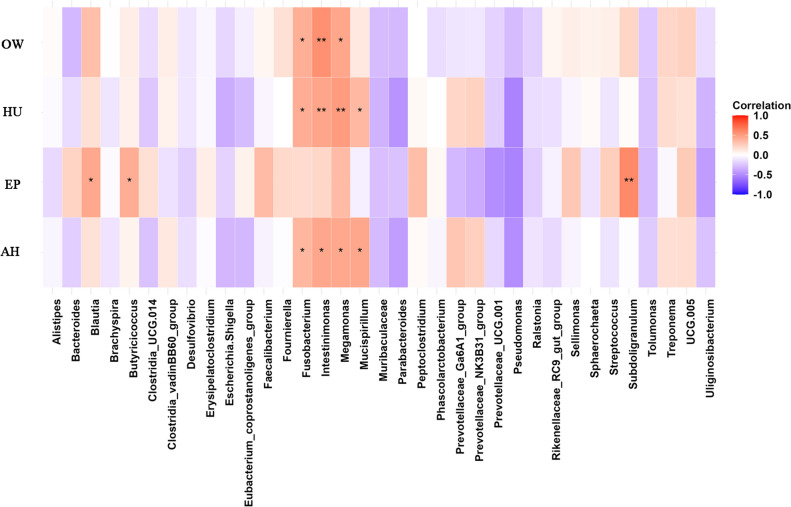
Correlation analysis of microbial species with productive characteristics of duck
Correlations between the top 35 most abundant genera and EP, OW, HU and AH were analyzed (Fig. 3). Genera Blautia, Butyricicoccus and Subdoligranulum showed positive relationship with EP. Genera Fourinierella, Fusobacterium and Intestinimonas positively associated with OW, HU and AH. And Mucispirillum positively associated with HU and AH.
Fig. 3.
Heatmap based on the correlation analysis between the top 35 abundant genera and OW, HU, EP and AH
Discussion
Jinding ducks are a kind of indigenous laying duck in China known for their annual egg production of 260~300 eggs and a 2-year egg production period. Previous researches have showed that flaxseed consumption may be inversely associated with body weight of human [19, 20]. However, no significant difference in body weight between Fla and Control groups were found in the present study even though it slightly increased in the Fla groups. It has been widely proved that intestinal histomophometric characteristics and microbiota tightly connect with weight gain of human and animals [21]. In the present study, 4% and 5% Fla groups had higher VH, CD and VCR values in jejunum and ileum than Control group. Similarly, addition of flaxseed increased the ileal VH of pigs [22], and ω-3 PUFAs enriched diets upregulated the duodenal VH and VCR of hens [23]. Moreover, the dominant intestinal phyla Firmicutes and Bacteroidota have been proved to play a role in energy metabolism. And an increment of the relative abundance of Firmicutes with respect to Bacteroidota (F:B ratio) has been associated with obesity [24]. In this study, the F:B ratio were slightly upregulated in each Fla group, which seemed to be coincident with the elevation of body weight in Fla groups.
Flaxseed had also increased the ovary weight and the number of preovulatory follicles of laying ducks in this experiment. Consistently, laying ducks in 2% Fla, 3% Fla and 4% Fla groups had significantly higher EP, EM but lower FCR. Previous researches have proved the regulatory role. of flaxseed on the female reproduction including ovarian growth, follicle development, puberty, hormone release and the resulting reproductive cycle [25, 26]. And This indicates that moderate supplementation of flaxseed can improve the reproductive performance of laying ducks. Short-chain ω-3 PUFAs, such as ALA, are commonly found in plant oils like flaxseed and soybean oil. While long-chain ω-3 PUFAs, such as DHA and EPA are primarily obtained from marine products like fish oil. Previous researches have implied that DHA and EPA are modulators of inflammatory and immune response [27, 28]. Although endogenous DHA and EPA can be converted from ALA, the conversion efficiency is quite low in human [29, 30], especially in infants and elderly individuals [31, 32]. In the present study, addition of flaxseed significantly increased the content of easily absorbed DHA in duck eggs, but decreased the content of ALA. Previous research showed that ALA and DHA were both upregulated in the egg yolk of laying hens or ducks fed with full-fat flaxseed meal or flaxseed oil [14, 15]. Whether if the duck breed or procession method of flaxseed contributed to this discrepancy needs further research. On the other hand, flaxseed might enhanced the overall health of laying ducks owing to the elevated levels of IgA, IgG and IgM in Fla groups. This might reflect the positive role of ω-3 PUFAs in immunity [33, 34].
Dietary flaxseed have been proved to improve the health of gut through enhancing the intestinal barrier or regulating the composition of gut microbiota [35, 36]. Analysis of ASVs revealed that the Control and Fla groups shared a quite large number of ASVs, although the relative abundance of some ASVs exhibited significant differences. At the phylum level, 7/10 microbiota were common to both ducks and hens. And the proportion of Firmicutes was significantly increased by the flaxseed diet in the hen’s ceca, which was consistent with the findings of this study [37]. Unlike Firmicutes, the relative abundance of Proteobacteria and Desulfobacterota decreased in all Fla groups. Proteobacteria consists of many pathogens, such as Vibrio cholerae, Shigella and Salmonella. Abnormal expansion of Proteobacteria has been demonstrated to be positively correlated chronic colitis [38]. Desulfobacterota is a group of sulfate-reducing anaerobic bacterium that can bind to human colonic mucin, which has been proved to have pro-inflammatory and pathological effects in the gut [39, 40]. Nowadays, a growing number of researches have shown the relationship between intestinal microbiota with productive performance in poultry [41, 42]. In this study, seven genera were identifed positively correlated with the production and/or quality of egg in this experiment. Among them, Intestinimonas and Butyricicoccus are two butyrate-producing micrbiota which have been proved to benefits for intestinal barrier functions, resistance to pathogenic bacteria and anti-inflammation [43–46]. Another Blautia has been reported to be able to alleviate inflammatory diseases and metabolic diseases and prevent pathogen colonization by producing bacteriocins [47, 48]. Whereas, Fusobacterium has been identified as a kind of opportunist bacteria, some of them can induce the host to produce pro-inflammatory cytokines [49]. These findings suggested that flaxseed could indirectly improving productive performance through promoting the health of gut.
In summary, addition of flaxseed improved the productive performance including egg production, egg mass, feed conversion rate, egg quality and the content of DHA in eggs of laying ducks. Flaxseed diets may improve the body immunity through the elevating the plasma levels of IgA, IgG and IgM. Moreover, dietary flaxseed altered the composition of intestinal microbiota and increased the relative abundance of several anti-inflammatory bacteria, some of which showed positive correlation with several indices of production.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
LY, YS, TZ and ZY conceived and designed the study. LY, YS, WZ, LZ, DZ and KZ performed the experiments. YS and WZ analyzed the data. LY and YS wrote the manuscript. ZY and TZ supervised the experiment. All authors reviewed and approved the final version of the manuscript.
Funding
This study was financially supported by the Natural Science Foundation Program (Joint Fund Project) in Hubei Province (Grant No.2023AFD051), the Key Science and Technology Plan Project in Xiangyang (Grant No. 2022-31663) and the Science Research Program Guidance Project of Education Department in Huber Province (Grant No. B2022537).
Data availability
The datasets generated for this study has been deposited in the Sequence Read Archive (https://www.ncbi.nlm.nih.gov/sra, Accession Number PRJNA1085023) at NCBI.
Declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
All experimental procedure with animals were conducted with care and obey the “Guidelines for Experimental Animals”of the Ministry of Science and Technology (Beijing, China). This study was supervised and approved by the Experimental Ethics Committee of Xiangyang Polytechnic (permit No.2022000600).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Liyun Yuan and Wenhao Zhang have contributed equally to the current work.
Contributor Information
Yu Shi, Email: 18227551690@163.com.
Zhihang Yuan, Email: zhyuan2016@hunau.edu.cn.
Tao Zhao, Email: 534686238@qq.com.
References
- 1.Christon R, Haloui RB, Durand G (1995) Dietary polyunsaturated fatty acids and aging modulate glutathione-related antioxidants in rat liver. J Nutr 125:3062–3070. 10.1093/jn/125.12.3062 [DOI] [PubMed] [Google Scholar]
- 2.Doggrell SA (2019) Clinical trials of eicosapentaenoic acid (EPA) prescription products for the treatment of hypertriglyceridemia. Expert Opin Pharmacother 20:1221–1225. 10.1080/14656566.2019.1609942 [DOI] [PubMed] [Google Scholar]
- 3.Elagizi A, Lavie CJ, O’Keefe E, Marshall K, O’Keefe JH, Milani RV (2021) An update on Omega-3 polyunsaturated fatty acids and cardiovascular health. Nutrients. 10.3390/nu13010204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patrick RP (2019) Role of phosphatidylcholine-DHA in preventing APOE4-associated alzheimer’s disease. FASEB J 33:1554–1564. 10.1096/fj.201801412R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parikh M, Maddaford TG, Austria JA, Aliani M, Netticadan T, Pierce GN (2019) Dietary flaxseed as a strategy for improving human health. Nutrients. 10.3390/nu11051171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salvucci E (2016) Microbiome, holobiont and the net of life. Crit Rev Microbiol 42:485–494. 10.3109/1040841x.2014.962478 [DOI] [PubMed] [Google Scholar]
- 7.Postler TS, Ghosh S (2017) Understanding the holobiont: how microbial metabolites affect human health and shape the immune system. Cell Metab 26:110–130. 10.1016/j.cmet.2017.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simon JC, Marchesi JR, Mougel C, Selosse MA (2019) Host-microbiota interactions: from holobiont theory to analysis. Microbiome 7:5. 10.1186/s40168-019-0619-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu J, Zhao Y, Wang X, Kong L, Johnston LJ, Lu L, Ma X (2022) Dietary nutrients shape gut microbes and intestinal mucosa via epigenetic modifications. Crit Rev Food Sci Nutr 62:783–797. 10.1080/10408398.2020.1828813 [DOI] [PubMed] [Google Scholar]
- 10.Yang M, Yin Y, Wang F, Bao X, Long L, Tan B, Yin Y, Chen J (2021) Effects of dietary rosemary extract supplementation on growth performance, nutrient digestibility, antioxidant capacity, intestinal morphology, and microbiota of weaning pigs. J Anim Sci. 10.1093/jas/skab237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anjum FM, Haider MF, Khan MI, Sohaib M, Arshad MS (2013) Impact of extruded flaxseed meal supplemented diet on growth performance, oxidative stability and quality of broiler meat and meat products. Lipids Health Dis 12:13. 10.1186/1476-511x-12-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen W, Jiang YY, Wang JP, Yan BX, Huang YQ, Wang ZX (2015) Effect of flaxseed on the fatty acid profile of egg yolk and antioxidant status of their neonatal offspring in Huoyan geese. Animal 9:1749–1755. 10.1017/s1751731115001287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sobeková A, Piešová E, Maková Z, Szabóová R, Sopková D, Andrejčáková Z, Vlčková R, Faixová D, Faixová Z (2023) Duration of the flaxseed supplementation affects antioxidant defence mechanisms and the oxidative stress of fattening pigs. Vet Sci. 10.3390/vetsci10090586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shahid MS, Wu Y, Xiao Z, Raza T, Dong X, Yuan J (2019) Duration of the flaxseed diet promotes deposition of n-3 fatty acids in the meat and skin of Peking ducks. Food Nutr Res. 10.29219/fnr.v63.3590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du X, Liu Y, Lu L, Wang W, Zeng T, Tian Y, Xu X, Shen J, Niu D, Lu Y (2017) Effects of dietary fats on egg quality and lipid parameters in serum and yolks of Shan Partridge Duck. Poult Sci 96:1184–1190. 10.3382/ps/pew348 [DOI] [PubMed] [Google Scholar]
- 16.Magoč T, Salzberg SL (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963. 10.1093/bioinformatics/btr507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bokulich NA, Subramanian S, Faith JJ, Gevers D, Gordon JI, Knight R, Mills DA, Caporaso JG (2013) Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat Methods 10:57–59. 10.1038/nmeth.2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200. 10.1093/bioinformatics/btr381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohammadi-Sartang M, Mazloom Z, Raeisi-Dehkordi H, Barati-Boldaji R, Bellissimo N, Totosy de Zepetnek JO (2017) The effect of flaxseed supplementation on body weight and body composition: a systematic review and meta-analysis of 45 randomized placebo-controlled trials. Obes Rev 18:1096–1107. 10.1111/obr.12550 [DOI] [PubMed] [Google Scholar]
- 20.Bongartz U, Hochmann U, Grube B, Uebelhack R, Alt F, Erlenbeck C, Peng LV, Chong PW, De Costa P (2022) Flaxseed mucilage (IQP-LU-104) reduces body weight in overweight and moderately obese individuals in a 12-week, three-arm, double-blind, randomized, and placebo-controlled clinical study. Obes Facts 15:395–404. 10.1159/000522082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Hul M, Cani PD (2023) The gut microbiota in obesity and weight management: microbes as friends or foe? Nat Rev Endocrinol 19:258–271. 10.1038/s41574-022-00794-0 [DOI] [PubMed] [Google Scholar]
- 22.Ndou SP, Tun HM, Kiarie E, Walsh MC, Khafipour E, Nyachoti CM (2018) Dietary supplementation with flaxseed meal and oat hulls modulates intestinal histomorphometric characteristics, digesta- and mucosa-associated microbiota in pigs. Sci Rep 8:5880. 10.1038/s41598-018-24043-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nain S, Renema RA, Zuidhof MJ, Korver DR (2012) Effect of metabolic efficiency and intestinal morphology on variability in n-3 polyunsaturated fatty acid enrichment of eggs. Poult Sci 91:888–898. 10.3382/ps.2011-01661 [DOI] [PubMed] [Google Scholar]
- 24.Ley RE, Turnbaugh PJ, Klein S, Gordon JI (2006) Microbial ecology: human gut microbes associated with obesity. Nature 444:1022–1023. 10.1038/4441022a [DOI] [PubMed] [Google Scholar]
- 25.Jelodar G, Masoomi S, Rahmanifar F (2018) Hydroalcoholic extract of flaxseed improves polycystic ovary syndrome in a rat model. Iran J Basic Med Sci 21:645–650. 10.22038/ijbms.2018.25778.6349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vlčková R, Andrejčáková Z, Sopková D, Kozioł K, Hertelyová Z, Koziorowska A, Gancarčíková S (2022) Effects of supplemental flaxseed on the ovarian and uterine functions of adult cycling mice. Gen Physiol Biophys 41:205–219. 10.4149/gpb_2022003 [DOI] [PubMed] [Google Scholar]
- 27.Troesch B, Eggersdorfer M, Laviano A, Rolland Y, Smith AD, Warnke I, Weimann A, Calder PC (2020) Expert opinion on benefits of long-chain Omega-3 fatty acids (DHA and EPA) in aging and clinical nutrition. Nutrients. 10.3390/nu12092555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miles EA, Childs CE, Calder PC (2021) Long-chain polyunsaturated fatty acids (LCPUFAs) and the developing immune system: a narrative review. Nutrients. 10.3390/nu13010247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emken EA, Adlof RO, Gulley RM (1994) Dietary linoleic acid influences desaturation and acylation of deuterium-labeled linoleic and linolenic acids in young adult males. Biochim Biophys Acta 1213:277–288. 10.1016/0005-2760(94)00054-9 [DOI] [PubMed] [Google Scholar]
- 30.Pawlosky RJ, Hibbeln JR, Novotny JA, Salem N Jr (2001) Physiological compartmental analysis of alpha-linolenic acid metabolism in adult humans. J Lipid Res 42:1257–1265 [PubMed] [Google Scholar]
- 31.Gerster H (1998) Can adults adequately convert alpha-linolenic acid (18:3n–3) to eicosapentaenoic acid (20:5n–3) and docosahexaenoic acid (22:6n–3)? Int J Vitam Nutr Res 68:159–173 [PubMed] [Google Scholar]
- 32.Brenna JT, Salem N Jr, Sinclair AJ, Cunnane SC (2009) alpha-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot Essent Fatty Acids 80:85–91. 10.1016/j.plefa.2009.01.004 [DOI] [PubMed] [Google Scholar]
- 33.Che L, Zhou Q, Liu Y, Hu L, Peng X, Wu C, Zhang R, Tang J, Wu F, Fang ZJF, Lin Y, Xu S, Feng B, Li J, Jiang P, Wu D, Chen D (2019) Flaxseed oil supplementation improves intestinal function and immunity, associated with altered intestinal microbiome and fatty acid profile in pigs with intrauterine growth retardation. Food Funct 10:8149–8160 [DOI] [PubMed] [Google Scholar]
- 34.Swanson D, Block R, Mousa SA (2012) Omega-3 fatty acids EPA and DHA: health benefits throughout life. Adv Nutr 3:1–7. 10.3945/an.111.000893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu Z, Chen W, Deng Q, Huang Q, Wang X, Yang C, Huang F (2020) Flaxseed oligosaccharides alleviate DSS-induced colitis through modulation of gut microbiota and repair of the intestinal barrier in mice. Food Funct 11:8077–8088. 10.1039/d0fo01105c [DOI] [PubMed] [Google Scholar]
- 36.Mueed A, Shibli S, Korma SA, Madjirebaye P, Esatbeyoglu T, Deng Z (2022) Flaxseed bioactive compounds: chemical composition, functional properties, food applications and health benefits-related gut microbes. Foods. 10.3390/foods11203307 [DOI] [PMC free article] [PubMed]
- 37.Lee JY, Kang SK, Heo YJ, Shin DW, Park TE, Han GG, Jin GD, Lee HB, Jung E, Kim HS, Na Y, Kim EB, Choi YJ (2016) Influence of flaxseed oil on fecal microbiota, egg quality and fatty acid composition of egg yolks in Laying Hens. Curr Microbiol 72:259–266. 10.1007/s00284-015-0946-z [DOI] [PubMed] [Google Scholar]
- 38.Rooks MG, Veiga P, Wardwell-Scott LH, Tickle T, Segata N, Michaud M, Gallini CA, Beal C, van Hylckama-Vlieg JE, Ballal SA, Morgan XC, Glickman JN, Gevers D, Huttenhower C, Garrett WS (2014) Gut microbiome composition and function in experimental colitis during active disease and treatment-induced remission. ISME J 8:1403–1417. 10.1038/ismej.2014.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB (2012) Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature 487:104–108. 10.1038/nature11225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maldonado-Arriaga B, Sandoval-Jiménez S, Rodríguez-Silverio J, Lizeth Alcaráz-Estrada S, Cortés-Espinosa T, Pérez-Cabeza de Vaca R, Licona-Cassani C, Gámez-Valdez JS, Shaw J, Mondragón-Terán P, Hernández-Cortez C, Suárez-Cuenca JA, Castro-Escarpulli G (2021) Gut dysbiosis and clinical phases of pancolitis in patients with ulcerative colitis. Microbiologyopen 10:e1181. 10.1002/mbo3.1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clavijo V, Flórez MJV (2018) The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: a review. Poult Sci 97:1006–1021. 10.3382/ps/pex359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo M, Hao G, Wang B, Li N, Li R, Wei L, Chai T (2016) Dietary administration of bacillus subtilis enhances growth performance, immune response and disease resistance in cherry valley ducks. Front Microbiol 7:1975. 10.3389/fmicb.2016.01975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Louis P, Flint HJ (2017) Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol 19:29–41. 10.1111/1462-2920.13589 [DOI] [PubMed] [Google Scholar]
- 44.Eeckhaut V, Machiels K, Perrier C, Romero C, Maes S, Flahou B, Steppe M, Haesebrouck F, Sas B, Ducatelle R, Vermeire S, Van Immerseel F (2013) Butyricicoccus pullicaecorum in inflammatory bowel disease. Gut 62:1745–1752. 10.1136/gutjnl-2012-303611 [DOI] [PubMed] [Google Scholar]
- 45.Scott SA, Fu J, Chang PV (2020) Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A 117:19376–19387. 10.1073/pnas.2000047117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qu W, Yuan X, Zhao J, Zhang Y, Hu J, Wang J, Li J (2017) Dietary advanced glycation end products modify gut microbial composition and partially increase colon permeability in rats. Mol Nutr Food Res. 10.1002/mnfr.201700118 [DOI] [PubMed] [Google Scholar]
- 47.Khattab MSA, Tawab AMAE, Fouad MT (2017) Isolation and characterization of anaerobic bacteria from frozen rumen liquid and its potential characterizations. Int J Dairy Sci 12:47–51 [Google Scholar]
- 48.Kalyana Chakravarthy S, Jayasudha R, Sai Prashanthi G, Ali MH, Sharma S, Tyagi M, Shivaji S (2018) Dysbiosis in the gut bacterial microbiome of patients with uveitis, an inflammatory disease of the eye. Indian J Microbiol 58:457–469. 10.1007/s12088-018-0746-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Engevik MA, Danhof HA, Ruan W, Engevik AC, Chang-Graham AL, Engevik KA, Shi Z, Zhao Y, Brand CK, Krystofiak ES, Venable S, Liu X, Hirschi KD, Hyser JM, Spinler JK, Britton RA, Versalovic J (2021) Fusobacterium nucleatum secretes outer membrane vesicles and promotes intestinal inflammation. MBio. 10.1128/mBio.02706-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Mueed A, Shibli S, Korma SA, Madjirebaye P, Esatbeyoglu T, Deng Z (2022) Flaxseed bioactive compounds: chemical composition, functional properties, food applications and health benefits-related gut microbes. Foods. 10.3390/foods11203307 [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The datasets generated for this study has been deposited in the Sequence Read Archive (https://www.ncbi.nlm.nih.gov/sra, Accession Number PRJNA1085023) at NCBI.