Abstract
Purpose
Debate persists regarding the feasibility of adopting an organ-preserving strategy as the treatment modality for clinical T2N0 rectal cancer. This study aimed to compare the outcomes of attempting organ-preserving strategies versus radical surgery in patients with clinical T2N0 mid to low rectal cancer.
Methods
Patients diagnosed with clinical T2N0 rectal cancer, with lesions located within 8 cm from the anal verge as determined by pre-treatment magnetic resonance imaging between January 2010 and December 2020 were included.
Results
Of 119 patients, 91 and 28 were categorized into the organ-preserving attempt group and the radical surgery group, respectively. The median follow-up duration was 48.8 months (range, 0–134 months). The organ-preserving attempt group exhibited a reduced incidence of stoma formation (44.0% vs. 75.0%; p = 0.004) and a lower occurrence of grade 3 or higher surgical complications (5.8% vs. 21.4%; p = 0.025). Univariate analyses revealed no significant association between treatment strategy and 3-year local recurrence-free survival (organ-preserving attempt 87.9% vs. radical surgery 96.2%; p = 0.129), or 3-year disease-free survival (79.6% vs. 84.9%; p = 0.429). Multivariate analysis did not identify any independent prognostic factors associated with oncologic outcomes.
Conclusion
Compared with radical surgery, attempted organ preservation resulted in lower incidences of stoma formation and severe surgical complications, whereas oncological outcomes were comparable. Attempting organ preservation may be a safe alternative to radical surgery for clinical T2N0 mid to low rectal cancer.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00384-024-04708-6.
Keywords: Rectal cancer, Radical surgery, Organ preservation
Introduction
Local excision (LE) may be considered for T1N0 rectal tumors with favorable histology, size, and location [1, 2]. However, for clinical T2N0 rectal cancer, total mesorectal excision (TME) as radical surgery is the standard recommendation, considering the risks of local recurrence and nodal involvement [1, 2]. Despite the oncological rationale for radical surgery, TME has been associated with numerous surgical complications, including urinary, sexual, and defecatory dysfunction [3–7]. Moreover, the formation of permanent stomas may be necessary for certain patients.
Many studies have investigated organ preservation strategies for rectal cancer, with some involving multidisciplinary approaches. Attempts have been made to perform LE or endoscopic resection as alternatives to TME for early rectal cancer [8, 9]. Additionally, a “watch-and-wait” (WW) approach has been implemented for patients who have achieved clinical complete responses (cCRs) after chemoradiotherapy (CRT), with the aim of observing them without surgical intervention [10–12]. Patients undergoing LE after good responses to CRT have exhibited similar oncological outcomes to those undergoing TME [13, 14]. Despite these findings, the debate over the risk of local recurrence continues, and current guidelines recommend limiting organ preservation to selected patients [1].
In recent years, our institution has explored organ-preserving strategies as the initial treatment modality for patients with clinical T2N0 mid to low rectal cancer. This study aimed to compare the outcomes between patients who underwent attempted organ preservation versus those who underwent radical surgery as primary treatment.
Methods
This study received approval from the relevant institutional review board (IRB No. CNUHH-2024–076). This cohort study was conducted in adherence to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) reporting guidelines [15]. Informed consent was waived owing to the study’s retrospective design. We conducted a retrospective review of data from patients diagnosed with clinical T2N0 mid to low rectal cancer (located less than 8 cm from the anal verge), as determined by pre-treatment magnetic resonance imaging (MRI) between January 2010 and December 2020. Exclusion criteria included patients with stage IV disease, those with synchronous or recurrent cancers, histological types other than adenocarcinoma, and patients who underwent emergency surgery.
Patients were categorized into two groups: those who received organ-preserving strategies and those who underwent TME as radical surgery for their initial treatment. The decision to pursue organ preservation or radical surgery was at the surgeon's discretion. Within the organ-preserving attempt group, patients initially underwent either LE or CRT. These patients were then either placed under surveillance or underwent adjuvant CRT, LE, or additional TME. Subsequent treatment decisions were based on the patient’s overall condition and the clinical stage confirmed by repeat MRI or the pathological stage confirmed by LE or TME. Complementary TME was defined as TME performed within 1 month after LE when prior treatment was deemed insufficient based on the pathological findings from LE.
For radical surgery, TME was performed according to oncological surgical principles [16]. LE involved full-thickness tumor excision via a direct transanal approach. Those treated with CRT received 50.4 Gy of pelvic radiation concurrently with fluoropyrimidine-based chemotherapy [1]. Patients deemed to require further treatment after CRT, surgery, or both were given adjuvant chemotherapy, consisting of either oral capecitabine (1250 mg/m2, twice daily) or a combination of 5-fluorouracil (400 mg/m2/day, for 4 days) with leucovorin (20 mg/m2/day, for 4 days). Typically, a six-cycle regimen was prescribed; however, if concurrent chemotherapy with preoperative radiation was completed, the regimen was reduced to four cycles.
Postoperative complications were graded using the Clavien-Dindo classification [17]. After treatment completion, patients were followed up every 6 months, and laboratory tests, including serum carcinoembryonic antigen, chest, and abdominopelvic computed tomography, were conducted at each visit. For patients under WW management, the first follow-up was scheduled 3 months after CRT completion, with subsequent surveillance every 6 months. Surveillance colonoscopy was performed at 1, 3, and 5 years after initial treatment. Recurrence was defined as the reappearance of the disease confirmed through clinical, radiologic, or pathologic examination. Local recurrence-free survival (LRFS) was calculated as the duration from initial treatment to the date of local recurrence or death. Disease-free survival (DFS) was measured as the interval from initial treatment to the date of local recurrence, distant metastasis, or death.
Statistical analyses included the Student's t-test or Mann–Whitney U test for continuous variables and the chi-squared test or Fisher's exact test for categorical variables. Survival outcomes and risk factors for LRFS and DFS were identified using the Kaplan–Meier method and logistic regression analysis. Variables with p-value < 0.05 in univariate analyses and treatment strategy were included in multivariate analyses. Cox proportional hazards regression analysis was conducted to determine independent risk factors for survival outcomes, calculating hazard ratios (HRs) and 95% confidence intervals (CIs). A p-value < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS Statistics for Windows, version 27.0 (IBM Corp., Armonk, NY, USA).
Results
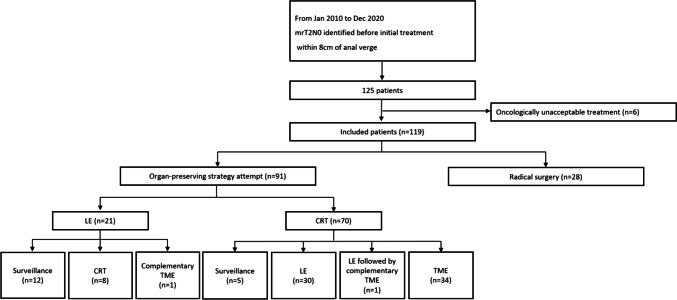
A total of 125 patients met our inclusion criteria (Fig. 1). Of these, six patients were determined to be receiving oncologically unacceptable treatment and were excluded from the study. Among the remaining 119 patients, 91 were categorized in the organ-preserving attempt group, and 28 who initially underwent TME were classified in the radical surgery group. Within the organ-preserving attempt group, 21 patients initially received LE, and 70 patients underwent CRT as initial treatment. Among those initially treated with LE, 12 were placed under surveillance without additional treatment, eight received adjuvant CRT, and one patient underwent complementary TME. Of the patients who initially received CRT, five were managed with a WW approach, 30 received additional LE, one underwent LE followed by complementary TME, and 34 proceeded to receive TME.
Fig. 1.
Patient inclusion and classification flow diagram. TME, total mesorectal excision; LE, local excision; CRT, chemoradiotherapy
Clinicopathologic characteristics based on treatment strategy are presented in Table 1. In the organ-preserving attempt group, the proportion of low rectal cancer was significantly higher than in the radical surgery group (53.8% vs. 25.0%, p = 0.007). Additionally, the organ-preserving attempt group had a lower incidence of stoma formation (44.0% vs. 75.0%, p = 0.004), fewer surgical complications of grade 3 or higher (5.8% vs. 21.4%, p = 0.025), and a higher rate of adjuvant chemotherapy administration (37.4% vs. 14.3%, p = 0.022). The pathological stages based on treatment strategy are presented in Table S1.
Table 1.
Clinicopathologic characteristics based on treatment strategy
| Organ-preserving attempt (n = 91) | Radical surgery (n = 28) | p | ||
|---|---|---|---|---|
| Sex | Male | 58 (63.7) | 17 (60.7) | 0.772 |
| Female | 33 (36.3) | 11 (39.3) | ||
| Age (years) | 66.8 ± 10.7 | 63.6 ± 10.3 | 0.162 | |
| BMI (kg/m2) | 24.1 ± 2.8 | 23.0 ± 5.0 | 0.137 | |
| ASA score | 1/2 | 82 (90.1) | 27 (96.4) | 0.449 |
| 3/4 | 9 (9.9) | 1 (3.6) | ||
| Tumor level | Middle (> AV 4 cm) | 42 (46.2) | 21 (75.0) | 0.007 |
| Low (≤ AV 4 cm) | 49 (53.8) | 7 (25.0) | ||
| Tumor size (cm) | < 2 | 17 (18.7) | 5 (17.9) | 0.922 |
| ≥ 2 | 74 (81.3) | 23 (82.1) | ||
| Tumor circumferential | Quarter | 41 (45.1) | 17 (60.7) | 0.169 |
| involvement | Half | 45 (49.5) | 10 (35.7) | |
| Full | 5 (5.5) | 1 (3.6) | ||
| CEA level before | < 5 | 74 (83.1) | 21 (75.0) | 0.336 |
| treatment (ng/mL) | ≥ 5 | 15 (16.9) | 7 (25.0) | |
| Differentiation | w/d, m/d | 86 (97.7) | 28 (100.0) | 1.000 |
| p/d, mucinous | 2 (2.3) | 0 (0.0) | ||
| Stoma formation | Performed | 40 (44.0) | 21 (75.0) | 0.004 |
| Not performed | 51 (56.0) | 7 (25.0) | ||
| Permanent stoma | Formed | 11 (12.1) | 2 (7.1) | 0.730 |
| Not formed | 80 (87.9) | 26 (92.9) | ||
| Surgical complication | 0/1/2 | 81 (94.2) | 22 (78.6) | 0.025 |
| 3 | 5 (5.8) | 6 (21.4) | ||
| Adjuvant | Performed | 34 (37.4) | 4 (14.3) | 0.022 |
| chemotherapy | Not performed | 57 (62.6) | 24 (85.7) | |
Data are presented as mean ± standard deviation or number (percentage)
BMI body mass index, ASA American Society of Anesthesiologists, CEA carcinoembryonic antigen, w/d well differentiated, m/d moderately differentiated, p/d poorly differentiated
The median follow-up duration for the included patients was 48.8 months (range, 0–134 months). The follow-up period for patients in the organ-preserving attempt group was 48.5 months (range, 0–134 months), compared to 50.0 months (range, 9–103 months) for the radical surgery group (p = 0.801).
Four patients underwent salvage TME due to local regrowth identified during the follow-up period. Of these, three patients had received CRT with additional LE as their treatment strategy, while the remaining patient had received LE followed by adjuvant CRT.
Univariate analyses were performed to identify factors associated with survival outcomes (Table 2). Male gender and poor differentiation were identified as significantly associated with LRFS (p = 0.036 and p = 0.024, respectively). No factors were significantly associated with DFS. The 3-year LRFS rates of the organ-preserving attempt and radical surgery groups were 87.9% and 96.2%, respectively (p = 0.129), and the 3-year DFS rates were 79.6% and 84.9%, respectively (p = 0.429) (Fig. 2). Multivariate analysis, which included sex, differentiation, and treatment strategy as covariates, revealed no independent prognostic factors for LRFS (Table 3). Furthermore, treatment strategy was not associated with either LRFS (HR = 4.246; 95% CI, 0.551–32.705; p = 0.165) or DFS (HR = 1.541; 95% CI, 0.521–4.560, p = 0.434).
Table 2.
Univariate analysis of factors associated with local recurrence-free survival and disease-free survival
| local recurrence-free survival | disease-free survival | ||||
|---|---|---|---|---|---|
| 3-years LRFS(%) | p | 3-years DFS(%) | p | ||
| Age (years) | < 70 | 90.3 | 0.674 | 81.3 | 0.853 |
| ≥ 70 | 89.4 | 80.6 | |||
| Sex | male | 86.3 | 0.036 | 79.5 | 0.446 |
| female | 96.7 | 83.8 | |||
| Tumor level | Middle (〉AV 4 cm) | 94.6 | 0.237 | 86.2 | 0.347 |
| Low (≤ AV 4 cm) | 84.7 | 74.9 | |||
| Tumor size (cm) | < 2 | 83.3 | 0.241 | 74.3 | 0.279 |
| ≥ 2 | 91.5 | 82.6 | |||
| Tumor circumferential | Quarter | 85.0 | 0.318 | 72.3 | 0.069 |
| involvement | Half | 93.4 | 89.4 | ||
| Full | 100.0 | 83.3 | |||
| CEA | < 5 | 91.6 | 0.278 | 82.9 | 0.271 |
| ≥ 5 | 81.1 | 70.7 | |||
| Treatment strategy | Organ-preserving attempt | 87.9 | 0.129 | 79.6 | 0.429 |
| Radical surgery | 96.2 | 84.9 | |||
| Differentiation | w/d, m/d | 90.6 | 0.024 | 81.4 | 0.145 |
| p/d, mucinous | 50.0 | 50.0 | |||
LRFS local recurrence-free survival, DFS disease-free survival, CEA carcinoembryonic antigen, w/d well differentiated, m/d moderately differentiated, p/d poorly differentiated
Fig. 2.
Survival outcomes based on treatment strategy. A, local recurrence-free survival. B, disease-free survival
Table 3.
Multivariate analysis of survival outcomes
| local recurrence-free survival | disease-free survival | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Differentiation | 4.155 | 0.526–32.795 | 0.177 | |||
| w/d, m/d vs. p/d, mucinous | ||||||
| Sex | 6.346 | 0.823–48.926 | 0.076 | |||
| Female vs. Male | ||||||
| Treatment strategy | 4.246 | 0.551–32.705 | 0.165 | 1.541 | 0.521–4.560 | 0.434 |
| Radical surgery vs. Organ-preserving attempt | ||||||
HR hazard ratio, CI confidence interval, w/d well differentiated, m/d moderately differentiated, p/d poorly differentiated
We conducted a subgroup analysis among patients in the organ-preserving attempt group between those who initially underwent LE and those who initially received CRT. We compared the characteristics of these patients, as shown in Table S2, and found that up-front LE was more frequently performed in middle rectal cancer, while up-front CRT was more used in low rectal cancer (low rectal cancer: LE 28.6% vs. CRT 61.4%, p = 0.008). When comparing the oncologic outcomes between the up-front CRT and up-front LE groups, we found no significant differences between the subgroups in 3-year LRFS (LE 88.8% vs. CRT 87.6%, p = 0.982) or 3-year DFS (LE 88.8% vs. CRT 76.8%, p = 0.409) (Figure S1). Additionally, we compared survival outcomes based on initial treatment and subsequent strategies within the organ-preserving attempt group (Table S3).
Apart from the initial classification, the included patients were divided into an organ preservation group and a radical surgery group based on their final treatment for further analysis. The characteristics of the patients according to final treatment are shown in Table S4. Univariate analyses revealed no significant intergroup differences in 3-year LRFS (organ preservation 90.8% vs. radical surgery 89.4%, p = 0.889) or 3-year DFS (organ preservation 88.8% vs. radical surgery 74.7%, p = 0.165) (Figures S2). Moreover, final treatment was not identified as independently associated with survival outcomes in the multivariate analysis (LRFS, p = 0.956; DFS, p = 0.173).
Discussion
We analyzed the surgical and oncological outcomes of attempting organ-preserving strategies versus radical surgery in clinical T2N0 mid to low rectal cancer. Our findings suggest that organ-preserving strategies significantly reduce the incidence of stoma formation and severe surgical complications without compromising oncological safety, as evidenced by comparable 3-year LRFS and DFS rates to those of radical surgery.
Organ preservation, in the context of rectal cancer, typically involves administering CRT as the initial treatment [18]. Following CRT, patients may be monitored through a WW approach if a cCR is observed, potentially avoiding surgery. Some studies have indicated cCR rates ranging between 0 and 25% when a WW approach is applied to patients with rectal cancer, with local recurrence rates varying between 5 and 50% [19–22]. However, these studies primarily involved patients with cT2 to cT4, N0 to N2, M0 disease. In contrast, a study focusing specifically on cT2 rectal cancer demonstrated early tumor growth to be less common than that observed in association with cT3/4 disease (3% vs. 30%; p = 0.007); the 1-year LRFS was also significantly better in association with cT2 rectal cancer (96% vs. 69%; p = 0.009) [23]. Another study found that in cases of local regrowth during WW, patients with baseline cT2 were more likely than those with baseline cT3/4 to undergo organ-preserving surgery for salvage (56.2% vs. 26.5%; p = 0.03) [24].
Several studies have investigated the feasibility of CRT followed by LE for rectal cancer treatment. Garcia-Aguilar et al. [25] found that in patients with distal T2N0 rectal cancer who underwent LE following CRT, the local recurrence rate was 5.1%, and the systemic recurrence rate was 6.3%. Park et al. [26] observed a rectal sparing rate of 52.2% with acceptable oncological outcomes after CRT followed by LE. Additionally, preoperative CRT with LE has been shown to result in similar oncological outcomes to those associated with TME but with fewer complications requiring reoperation [27]. These findings support current guidelines that recommend CRT followed by LE for selected patients with rectal cancer [1].
The primary advantage of initially performing LE as an organ-preserving strategy is its potential to address the limitations of preoperative examinations. MRI is considered the most accurate method for staging rectal cancer; thus, pre-treatment MRI was used to determine clinical stages in this cohort. Nevertheless, discrepancies between MRI findings and pathological stages in early rectal cancer remain a challenge, as confirmed by previous research [28, 29]. In the study by Part et al., pT1 was identified in 18.5% of clinical T2N0 rectal cancer patients who underwent upfront surgery [26]. Similarly, our study found that 44.9% of patients who initially underwent surgery were pathologically confirmed as pT1. Therefore, performing LE initially to confirm accurate pathological staging may be a viable strategy for reducing overtreatment associated with TME. In our cohort, 12 patients diagnosed with carcinoma in situ or pT1 cancer through LE were observed without additional treatments, and no recurrences occurred during follow-up. Moreover, even if the pathological stage after LE was pT2 or higher, adjuvant CRT could be administered. This approach aligns with findings from a study indicating similar outcomes for CRT following LE and LE following CRT [30]. Furthermore, our subgroup analysis found no significant difference in oncologic outcomes between patients who initially underwent LE and those who initially received CRT, both aimed at organ preservation.
Various organ-preserving strategies have been explored for rectal cancer; however, a consensus on the optimal method and selection criteria has not yet been established. Consequently, the initial treatment option for rectal cancer often depends on the surgeon’s preference [31, 32]. Moreover, the selection of subsequent treatments based on the outcomes of initial interventions varies widely. Further studies are needed to determine acceptable organ-preserving strategies for rectal cancer.
Of the 119 patients included in this study, 91 (76.5%) underwent attempted organ-preserving treatment; however, only 55 patients (46.2%) ultimately maintained organ preservation as their final treatment approach. Approximately 60% of those who were initiated on organ-preserving treatment were able to achieve organ preservation and avoid radical surgery. When comparing patients who received radical surgery as their final treatment with those who did not, no significant differences were observed in 3-year LRFS or 3-year DFS. The absence of a significant difference in survival between those who achieved organ preservation and those who ultimately received radical surgery might suggest that both approaches are viable for managing rectal cancer, given appropriate patient selection.
This study had several limitations. First, the study’s retrospective design means that selection bias cannot be ruled out. Second, the study included a small cohort of patients because it was limited to those who underwent pre-treatment MRI for clinical staging. Third, the inclusion of patients with insufficient follow-up periods may have affected survival outcomes. Fourth, the application of organ-preserving strategies was widely heterogeneous, depending on the surgeon’s discretion without clearly defined criteria for implementation. This diversity in treatment modalities, timing, and surgical approaches prevented a comprehensive analysis of pathological characteristics such as pathological T and N staging, lymphovascular invasion, and perineural invasion as prognostic factors. Fifth, owing to missing data, there was a lack of evaluation regarding the adverse effects and quality of life associated with CRT or chemotherapy. However, we standardized diagnostic imaging with MRI to enhance accuracy in patients with clinical T2N0 disease and attempted to evaluate all organ-preserving strategies used in actual clinical practice, such as the initial LE or WW approaches.
Conclusion
Compared with radical surgery, attempted organ preservation resulted in lower incidences of stoma formation and severe surgical complications, while oncological outcomes remained similar between the two management approaches. Consequently, organ-preserving strategies that consider characteristics such as tumor level may represent a viable and safe alternative to radical surgery for patients with clinical T2N0 mid to low rectal cancer.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
None
Author Contribution
Hyeong Rok Kim conceived the study. Hyeung-min Park and Soo Young Lee contributed to the study design. Hyeung-min Park also wrote the main manuscript, examined the statistical aspects of this study, and prepared figures. Jaram Lee and Chang Hyun Lim provided advice on the study design. All authors reviewed the manuscript.
Funding
None reported.
Data Availability
No datasets were generated or analysed during the current study.
Declarations
Competing Interests
The authors declare no competing interests.
Financial Disclosure
None reported.
Disclosure
The authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Benson AB, Venook AP, Al-Hawary MM, Azad N, Chen YJ, Ciombor KK et al (2022) Rectal Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 20(10):1139–1167. 10.6004/jnccn.2022.0051 [DOI] [PubMed] [Google Scholar]
- 2.Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A et al (2017) Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 28(suppl_4):iv22–iv40. 10.1093/annonc/mdx224 [DOI] [PubMed] [Google Scholar]
- 3.Bregendahl S, Emmertsen KJ, Lindegaard JC, Laurberg S (2015) Urinary and sexual dysfunction in women after resection with and without preoperative radiotherapy for rectal cancer: a population-based cross-sectional study. Colorectal Dis 17(1):26–37. 10.1111/codi.12758 [DOI] [PubMed] [Google Scholar]
- 4.Lim SL, Wan Zain WZ, Zahari Z, Zakaria AD, Hashim MNM, Wong MP et al (2023) Risk factors associated with low anterior resection syndrome: a cross-sectional study. Ann Coloproctol 39(5):427–434. 10.3393/ac.2022.00227.0032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pieniowski EHA, Palmer GJ, Juul T, Lagergren P, Johar A, Emmertsen KJ et al (2019) Low Anterior Resection Syndrome and Quality of Life After Sphincter-Sparing Rectal Cancer Surgery: A Long-term Longitudinal Follow-up. Dis Colon Rectum 62(1):14–20. 10.1097/dcr.0000000000001228 [DOI] [PubMed] [Google Scholar]
- 6.Son GM, Lee IY, Yun MS, Youn JH, An HM, Kim KH et al (2022) Analgesic effect of structured anal skin care for perianal dermatitis after low anterior resection in the rectal cancer patients: prospective, single-center, open-label, therapeutic confirmatory, randomized clinical trial. Ann Surg Treat Res 103(6):360–371. 10.4174/astr.2022.103.6.360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whelan S, Burneikis D, Kalady MF (2022) Rectal cancer: Maximizing local control and minimizing toxicity. J Surg Oncol 125(1):46–54. 10.1002/jso.26743 [DOI] [PubMed] [Google Scholar]
- 8.Hyun JH, Alhanafy MK, Park HC, Park SM, Park SC, Sohn DK et al (2022) Initial local excision for clinical T1 rectal cancer showed comparable overall survival despite high local recurrence rate: a propensity-matched analysis. Ann Coloproctol 38(2):166–175. 10.3393/ac.2021.00479.0068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.São Julião GP, Celentano JP, Alexandre FA, Vailati BB (2017) Local Excision and Endoscopic Resections for Early Rectal Cancer. Clin Colon Rectal Surg 30(5):313–323. 10.1055/s-0037-1606108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Habr-Gama A, Gama-Rodrigues J, São Julião GP, Proscurshim I, Sabbagh C, Lynn PB et al (2014) Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys 88(4):822–828. 10.1016/j.ijrobp.2013.12.012 [DOI] [PubMed] [Google Scholar]
- 11.Habr-Gama A, Sabbaga J, Gama-Rodrigues J, São Julião GP, Proscurshim I, Bailão Aguilar P et al (2013) Watch and wait approach following extended neoadjuvant chemoradiation for distal rectal cancer: are we getting closer to anal cancer management? Dis Colon Rectum 56(10):1109–1117. 10.1097/DCR.0b013e3182a25c4e [DOI] [PubMed] [Google Scholar]
- 12.Lee C, Park IJ, Lim SB, Yu CS, Kim JC (2022) The watch-and-wait strategy versus radical resection for rectal cancer patients with a good response (≤ycT2) after neoadjuvant chemoradiotherapy. Ann Surg Treat Res 103(6):350–359. 10.4174/astr.2022.103.6.350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin YS, Yu CS, Park JH, Kim JC, Lim SB, Park IJ et al (2017) Total Mesorectal Excision Versus Local Excision After Favorable Response to Preoperative Chemoradiotherapy in “Early” Clinical T3 Rectal Cancer: A Propensity Score Analysis. Int J Radiat Oncol Biol Phys 99(1):136–144. 10.1016/j.ijrobp.2017.05.009 [DOI] [PubMed] [Google Scholar]
- 14.Yu CS, Yun HR, Shin EJ, Lee KY, Kim NK, Lim SB et al (2013) Local excision after neoadjuvant chemoradiation therapy in advanced rectal cancer: a national multicenter analysis. Am J Surg 206(4):482–487. 10.1016/j.amjsurg.2013.01.042 [DOI] [PubMed] [Google Scholar]
- 15.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP (2007) Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 335(7624):806–808. 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacFarlane JK, Ryall RD, Heald RJ (1993) Mesorectal excision for rectal cancer. Lancet 341(8843):457–460. 10.1016/0140-6736(93)90207-w [DOI] [PubMed] [Google Scholar]
- 17.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD et al (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250(2):187–196. 10.1097/SLA.0b013e3181b13ca2 [DOI] [PubMed] [Google Scholar]
- 18.Varela C, Kim NK (2021) Surgical Treatment of Low-Lying Rectal Cancer: Updates. Ann Coloproctol 37(6):395–424. 10.3393/ac.2021.00927.0132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ayloor Seshadri R, Kondaveeti SS, Jayanand SB, John A, Rajendranath R, Arumugam V et al (2013) Complete clinical response to neoadjuvant chemoradiation in rectal cancers: can surgery be avoided? Hepatogastroenterology 60(123):410–414. 10.5754/hge12354 [DOI] [PubMed] [Google Scholar]
- 20.Dalton RS, Velineni R, Osborne ME, Thomas R, Harries S, Gee AS et al (2012) A single-centre experience of chemoradiotherapy for rectal cancer: is there potential for nonoperative management? Colorectal Dis 14(5):567–571. 10.1111/j.1463-1318.2011.02752.x [DOI] [PubMed] [Google Scholar]
- 21.Glynne-Jones R, Hughes R (2012) Critical appraisal of the “wait and see” approach in rectal cancer for clinical complete responders after chemoradiation. Br J Surg 99(7):897–909. 10.1002/bjs.8732 [DOI] [PubMed] [Google Scholar]
- 22.Smith JJ, Strombom P, Chow OS, Roxburgh CS, Lynn P, Eaton A et al (2019) Assessment of a Watch-and-Wait Strategy for Rectal Cancer in Patients With a Complete Response After Neoadjuvant Therapy. JAMA Oncol 5(4):e185896. 10.1001/jamaoncol.2018.5896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Habr-Gama A, São Julião GP, Gama-Rodrigues J, Vailati BB, Ortega C, Fernandez LM et al (2017) Baseline T Classification Predicts Early Tumor Regrowth After Nonoperative Management in Distal Rectal Cancer After Extended Neoadjuvant Chemoradiation and Initial Complete Clinical Response. Dis Colon Rectum 60(6):586–594. 10.1097/dcr.0000000000000830 [DOI] [PubMed] [Google Scholar]
- 24.Fernandez LM, Figueiredo NL, Habr-Gama A, São Julião GP, Vieira P, Vailati BB et al (2020) Salvage Surgery With Organ Preservation for Patients With Local Regrowth After Watch and Wait: Is It Still Possible? Dis Colon Rectum 63(8):1053–1062. 10.1097/dcr.0000000000001707 [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Aguilar J, Renfro LA, Chow OS, Shi Q, Carrero XW, Lynn PB et al (2015) Organ preservation for clinical T2N0 distal rectal cancer using neoadjuvant chemoradiotherapy and local excision (ACOSOG Z6041): results of an open-label, single-arm, multi-institutional, phase 2 trial. Lancet Oncol 16(15):1537–1546. 10.1016/s1470-2045(15)00215-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park MY, Yu CS, Kim TW, Kim JH, Park JH, Lee JL et al (2023) Efficacy of preoperative chemoradiotherapy in patients with cT2N0 distal rectal cancer. Ann Coloproctol 39(3):250–259. 10.3393/ac.2022.00066.0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lynn PB, Van der Valk MJM, Claassen YHM, Shi Q, Widmar M, Bastiaannet E et al (2023) Chemoradiation and Local Excision Versus Total Mesorectal Excision for T2N0 Rectal Cancer: Comparison of Short- and Long-Term Outcomes From 2 Prospective Studies. Ann Surg 277(1):e96–e102. 10.1097/sla.0000000000005052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Detering R, van Oostendorp SE, Meyer VM, van Dieren S, Bos A, Dekker JWT et al (2020) MRI cT1-2 rectal cancer staging accuracy: a population-based study. Br J Surg 107(10):1372–1382. 10.1002/bjs.11590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosén R, Nilsson E, Rahman M, Rönnow CF (2022) Accuracy of MRI in early rectal cancer: national cohort study. Br J Surg 109(7):570–572. 10.1093/bjs/znac059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee L, Kelly J, Nassif GJ, Atallah SB, Albert MR, Shridhar R et al (2017) Chemoradiation and Local Excision for T2N0 Rectal Cancer Offers Equivalent Overall Survival Compared to Standard Resection: a National Cancer Database Analysis. J Gastrointest Surg 21(10):1666–1674. 10.1007/s11605-017-3536-5 [DOI] [PubMed] [Google Scholar]
- 31.Al-Sawat A, Bae JH, Kim HH, Lee CS, Han SR, Lee YS et al (2022) Short- and long-term outcomes of local excision with adjuvant radiotherapy in high-risk T1 rectal cancer patients. Ann Surg Treat Res 102(1):36–45. 10.4174/astr.2022.102.1.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dulskas A, Caushaj PF, Grigoravicius D, Zheng L, Fortunato R, Nunoo-Mensah JW et al (2023) International Society of University Colon and Rectal Surgeons survey of surgeons’ preference on rectal cancer treatment. Ann Coloproctol 39(4):307–314. 10.3393/ac.2022.00255.0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.