Abstract
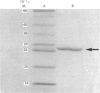
The Escherichia coli hemD gene, encoding the enzyme uroporphyrinogen III synthase (co-synthase), was cloned into multi-copy plasmids in E. coli cells that were used to generate strains producing up to 1000 times the concentration of the synthase in the wild-type. The enzyme was purified to homogeneity from these strains in milligram amounts. The enzyme is a monomer of Mr 28,000 with an isoelectric point of 5.2 and a pH optimum of 7.8. The specific activity of the purified synthase is 1500 units/mg and the Km for the substrate, pre-uroporphyrinogen, is 5 microM. The N-terminal sequence of the enzyme is Ser-Ile-Leu-Val-Thr-Arg-Pro-Ser-Pro-Ala-Gly-, in agreement with the gene-derived protein sequence. The enzyme contains four 5,5'-dithiobis-(2-nitrobenzoic acid)-titratable groups, one reacting rapidly with the reagent and three further groups having lower reactivity. The enzyme is heat-sensitive, and during heat inactivation all four thiol groups become equally available for reaction.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOGORAD L. The enzymatic synthesis of porphyrins from porphobilinogen. I. Uroporphyrin I. J Biol Chem. 1958 Aug;233(2):501–509. [PubMed] [Google Scholar]
- BOGORAD L. The enzymatic synthesis of porphyrins from porphobilinogen. II. Uroporphyrin III. J Biol Chem. 1958 Aug;233(2):510–515. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Echelard Y., Dymetryszyn J., Drolet M., Sasarman A. Nucleotide sequence of the hemB gene of Escherichia coli K12. Mol Gen Genet. 1988 Nov;214(3):503–508. doi: 10.1007/BF00330487. [DOI] [PubMed] [Google Scholar]
- Hart G. J., Battersby A. R. Purification and properties of uroporphyrinogen III synthase (co-synthetase) from Euglena gracilis. Biochem J. 1985 Nov 15;232(1):151–160. doi: 10.1042/bj2320151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P. M., Berry A. Preuroporphyrinogen, a universal intermediate in the biosynthesis of uroporphyrinogen III. FEBS Lett. 1980 Mar 24;112(1):86–88. doi: 10.1016/0014-5793(80)80134-7. [DOI] [PubMed] [Google Scholar]
- Jordan P. M., Mgbeje B. I., Alwan A. F., Thomas S. D. Nucleotide sequence of hemD, the second gene in the hem operon of Escherichia coli K-12. Nucleic Acids Res. 1987 Dec 23;15(24):10583–10583. doi: 10.1093/nar/15.24.10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P. M., Mgbeje B. I., Thomas S. D., Alwan A. F. Nucleotide sequence for the hemD gene of Escherichia coli encoding uroporphyrinogen III synthase and initial evidence for a hem operon. Biochem J. 1988 Jan 15;249(2):613–616. doi: 10.1042/bj2490613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P. M., Seehra J. S. Purification of porphobilinogen synthase from bovine liver. Methods Enzymol. 1986;123:427–434. doi: 10.1016/s0076-6879(86)23053-0. [DOI] [PubMed] [Google Scholar]
- Jordan P. M. Uroporphyrinogen III cosynthetase: a direct assay method. Enzyme. 1982;28(2-3):158–169. doi: 10.1159/000459099. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Raich N., Romeo P. H., Dubart A., Beaupain D., Cohen-Solal M., Goossens M. Molecular cloning and complete primary sequence of human erythrocyte porphobilinogen deaminase. Nucleic Acids Res. 1986 Aug 11;14(15):5955–5968. doi: 10.1093/nar/14.15.5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasarman A., Nepveu A., Echelard Y., Dymetryszyn J., Drolet M., Goyer C. Molecular cloning and sequencing of the hemD gene of Escherichia coli K-12 and preliminary data on the Uro operon. J Bacteriol. 1987 Sep;169(9):4257–4262. doi: 10.1128/jb.169.9.4257-4262.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythe E., Williams D. C. Rat liver uroporphyrinogen III synthase has similar properties to the enzyme from Euglena gracilis, including absence of a requirement for a reversibly bound cofactor for activity. Biochem J. 1988 Jul 1;253(1):275–279. doi: 10.1042/bj2530275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S. D., Jordan P. M. Nucleotide sequence of the hemC locus encoding porphobilinogen deaminase of Escherichia coli K12. Nucleic Acids Res. 1986 Aug 11;14(15):6215–6226. doi: 10.1093/nar/14.15.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. F., Bishop D. F., Desnick R. J. Human uroporphyrinogen III synthase: molecular cloning, nucleotide sequence, and expression of a full-length cDNA. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7049–7053. doi: 10.1073/pnas.85.19.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. F., Bishop D. F., Desnick R. J. Purification and properties of uroporphyrinogen III synthase from human erythrocytes. J Biol Chem. 1987 Jan 25;262(3):1268–1273. [PubMed] [Google Scholar]
- Wetmur J. G., Bishop D. F., Cantelmo C., Desnick R. J. Human delta-aminolevulinate dehydratase: nucleotide sequence of a full-length cDNA clone. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7703–7707. doi: 10.1073/pnas.83.20.7703. [DOI] [PMC free article] [PubMed] [Google Scholar]