Abstract
Suicide is the second leading cause of death in youth, and depression is a strong proximal predictor of adolescent suicide. It is important to identify psychological factors that may protect against suicide ideation in depressed adolescents. Self-compassion may be such a factor. Converging evidence indicates the inverse association between self-compassion and suicide ideation, but the neural mechanisms underlying their link remain unknown. Because self-referential caudate activity is associated with both self-compassion and suicide ideation, its functional connectivity might explain their relationship. In this study, we examined the relationship between self-compassion and caudate functional connectivity during self-appraisals, a typical self-referential paradigm, and their associations with suicide ideation in both depressed and healthy youth. In the scanner, 79 depressed youth and 36 healthy controls evaluated, from various perspectives, whether phrases they heard were self-descriptive. Self-compassion and suicide ideation were rated with self-report and interview-based measures. We found that self-compassion was associated with stronger left caudate functional connectivity with bilateral posterior superior temporal sulcus/temporoparietal junction, the left middle temporal gyrus (MTG), and the left middle occipital gyrus during positive versus negative self-appraisals. Stronger left caudate connectivity with the left MTG explained the association between higher self-compassion and lower suicide ideation, even controlling for non-suicide ideation depression severity, anxiety severity, and non-suicidal self-injurious behavior. The findings suggest that the left caudate to MTG connectivity during positive versus negative self-referential processing could be a biomarker to be targeted by neural stimulation interventions for reducing suicide ideation in depressed youth, combined with self-compassion interventions.
Subject terms: Depression, Human behaviour
Introduction
Suicide is the second leading cause of death among adolescents [1, 2], and depression is a strong proximal predictor of suicidality in youth [3, 4]. It is important to identify psychological factors that may protect against the risks of suicide in depressed adolescents, an essential step in developing suicide prevention strategies [5, 6]. Self-compassion may be such a factor. Self-compassion refers to the tendency to be aware of and open to one’s own suffering, without avoiding or disconnecting from it, and without over-identifying with it, while simultaneously generating the desire to alleviate one’s suffering and to heal oneself with kindness [7]. Correlational and longitudinal studies have supported the inverse association between self-compassion and suicide ideation [8, 9], i.e., thoughts, ideas, and plans regarding attempting against one’s own life, including fleeting death wishes, and careful consideration of suicide behavior [10]. Nevertheless, the neural mechanisms underlying the association between self-compassion and suicide ideation remain unknown. Prior research has shown that distorted negative self-referential processing is a risk for suicide ideation [11], and caudate activity during self-processing is associated with both self-compassion and suicide ideation [12–14]. Thus, we examined the role of caudate circuitry during self-processing as a potential mediator in the association between self-compassion and suicide ideation in depressed youth.
It has been well established that depressed patients display distorted self-referential processing, which is in turn linked to suicide risks [11, 15]. Depressed suicide-attempting adolescents exhibited enhanced attention and arousal to negative versus positive self-referential stimuli [16]. At the neural level, general self-referential processing (regardless of emotional valence) engages the cortical midline structures (CMS), such as the medial prefrontal cortex, the anterior cingulate cortex, and the precuneus [17–19]. Positive self-referential stimuli usually activate the emotion-related regions such as the striatum more strongly than the negative ones in healthy individuals [20]. By contrast, those who are depressed with suicide ideation show a stronger response to negative than positive self-referential stimuli in the caudate, a striatal sub-region [12, 13]. These findings suggest the role of atypical caudate activity during emotionally valenced self-referential processing in suicide ideation. In terms of functional connectivity, prior findings showed that those who are depressed with suicide ideation showed aberrant reduced resting-state caudate functional connectivity with the anterior cingulate cortex, a sub-region in the CMS [21], and task-based connectivity between salience-related and task-relevant regions [22]. Given such past results, stronger caudate connectivity during negative vs. positive self-processing could be a biomarker of suicide risk.
Self-compassion is a psychological resource that helps manage stress and adversity [23]. At the behavioral level, prior research has consistently shown that self-compassion is related to lower levels of suicide ideation [8, 9]. At the neural level, self-compassion is associated with less CMS hyperactivity during self-processing tasks, especially during negative ones, among depressed adolescents [24–26]. This suggests that self-compassion may protect depressed adolescents from ruminative thinking linked to hyperactive CMS during negative self-processing. Self-compassion also engages the caudate [14, 27] and is associated with stronger caudate activity during positive self-referential processing, which is more blunted in depressed youth compared to healthy controls [12]. This suggests that self-compassion might strengthen or be associated with pleasure elicited by positive self-related stimuli, which further attenuates suicide ideation among depressed youth. So far, studies on the neural basis of self-compassion have focused on neural activity, and the functional connectivity correlates of self-compassion remain unknown. Because caudate activity engagement or its disengagement is associated with self-compassion and suicide ideation, respectively [12–14, 27], we explored the links between self-compassion and caudate circuitry during self-referential processing, as well as their associations with suicide ideation among depressed and healthy youth in the current study.
Based on prior findings, self-compassion might be linked to stronger caudate connectivity to CMS areas during positive vs. negative self-processing, which in turn reduces suicide ideation. Because self-referential processing engages the CMS, such as the medial prefrontal cortex, anterior cingulate cortex, precuneus, and mentalizing regions such as the posterior superior temporal sulcus (pSTS) or temporoparietal junction [17, 19, 28], we hypothesized that caudate connectivity with these regions during positive vs. negative self-processing would be associated with self-compassion and might mediate the inverse association between self-compassion and suicide ideation in depressed and healthy youth.
This study aimed to investigate: 1) the relationship between self-compassion and caudate circuitry during positive vs. negative self-appraisals, a well-established self-referential paradigm, and 2) whether caudate circuitry correlates of self-compassion would mediate the relationship between higher self-compassion and lower suicide ideation in depressed and healthy youth. Based on prior findings [12, 13, 19, 27], we explored whether self-compassion would be linked to greater caudate functional connectivity with regions engaged in self-referential processing, such as the medial prefrontal cortex, anterior cingulate cortex, precuneus, and the pSTS or temporoparietal junction, during positive vs. negative self-appraisals. We further tested whether caudate functional connectivity linked to self-compassion during positive vs. negative self-appraisals would mediate the relationship between higher self-compassion and lower suicide ideation in depressed and healthy youth, even controlling for confounding suicide risk factors (i.e., non-suicide ideation depression severity, anxiety severity, and non-suicidal self-injurious behavior).
Materials and methods
Participants
Adolescents (age: M ± SD = 14.82 ± 1.63 years, ranging from 11.30 - 17.80 years) and their caregiver(s) were recruited from psychiatric clinics at the Universities of Minnesota and Pittsburgh. Exclusion criteria included: IQ < 70, primary diagnosis other than depression, and left-handedness. Depression and other psychiatric disorders were diagnosed using the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL) interview [29]. All clinical interviews were videotaped and scored for diagnostic agreement. Three doctoral-level experts in child development reviewed and scored the videotapes with 98% agreement in symptom severity and diagnosis. Disagreements between the coders were mediated by the senior author, a licensed clinical psychologist (KQ). A total of 82 depressed adolescents (DEPs) and 37 healthy controls (HCs) consented to participate in the study. Four participants, due to image loss (n = 1), bad image quality (n = 1), or excessive movement in the scanner (n = 2), were excluded from analyses. The final sample consisted of 79 DEPs (62 scanned at the Minneapolis site and 17 at the Pittsburgh site) and 36 HCs (20 at the Minneapolis site and 16 at the Pittsburgh site). Sample size was not pre-determined but post-hoc analysis with G* Power 3.1 [30] showed that this sample size was sufficient to detect a medium effect size (r) of 0.30 with a power of 1 - β = 0.90 and α = 0.05. Sample characteristics are presented in Table 1.
Table 1.
Sample characteristics.
| Variable | Depressed Youth | Healthy Controls | Statistic |
|---|---|---|---|
| (n = 79) | (n = 36) | ||
| Age: M ± SD[Range] |
14.96 ± 1.66 [11.30 ~ 17.80] |
14.52 ± 1.54 [12.00 ~ 16.90] |
t(113) = 1.35 |
| Gender | χ2(1) = 4.68* | ||
| Male | 27 (34.2%) | 20 (55.6%) | |
| Female | 52 (65.8%) | 16 (44.4%) | |
| Race/Ethnicity: | χ2(6) = 8.73 | ||
| White | 45 (57.0%) | 27 (75.0%) | |
| African American | 8 (10.1%) | 1 (2.8%) | |
| Hispanic or Latino | 8 (10.1%) | 1 (2.8%) | |
| Native American | 1 (1.3%) | 0 | |
| Asian | 2 (2.5%) | 3 (8.3%) | |
| Multi-racial | 12 (15.2%) | 4 (11.1%) | |
| Others | 3 (3.8%) | 0 | |
| Puberty: Late State/Completed | 69 (87.3%) | 28 (77.8%) | χ2(1) = 1.71 |
| Annual Household Income: | χ2(4) = 11.73* | ||
| <$24,999 | 19 (24.1%) | 2 (5.6%) | |
| $25,000 ~ 49,999 | 21 (26.6%) | 5 (13.9%) | |
| $50,000 ~ 99,999 | 19 (24.1%) | 14 (38.9%) | |
| $100,000 ~ 149,999 | 12 (15.2%) | 11 (30.6%) | |
| >$150,000 | 5 (6.3%) | 4 (11.1%) | |
| Parent Marital Status | χ2(4) = 6.09 | ||
| Married | 44 (55.7%) | 29 (80.6%) | |
| Living with Partner | 8 (10.1%) | 2 (5.6%) | |
| Separated/Divorced | 13 (16.5%) | 3 (8.3%) | |
| Singer/Never Married | 11 (13.9%) | 2 (5.6%) | |
| Widowed | 1 (1.3%) | 0 (0%) | |
| Depression Diagnosis | |||
| Major Depressive Disorder | 55 (69.6%) | N/A | |
| Dysthymia | 3 (3.8%) | N/A | |
| Depressive Disorder-NOS | 21 (26.6%) | N/A | |
| Comorbid Anxiety | 57 (72.2%) | N/A | |
| Medication Use: (non-exclusive) | |||
| Antidepressant | 34 (43.0%) | N/A | |
| Antipsychotic | 6 (7.6%) | N/A | |
| Mood Stabilizing | 1 (1.3%) | N/A | |
| Stimulant | 10 (12.7%) | N/A | |
| Anxiolytic | 6 (7.6%) | N/A | |
| Any Medication Use | 41 (51.9%) | 2 (5.6%) | χ2(1) = 22.69*** |
| Suicide ideation (averaged standardized scores): M ± SD[Range] |
0.39 ± 0.85 [−0.95 ~ 2] |
−0.86 ± 0.10 [−0.95 ~ −0.63] |
t(113) = 12.97*** |
| Depression severity: M ± SD[Range] |
63.47 ± 14.63 [24 ~ 93] |
20.33 ± 5.83 [17 ~ 44] |
t(113) = 22.57*** |
| Self-compassion: M ± SD[Range] |
6.82 ± 4.44 [0 ~ 16] |
13.25 ± 3.42 [3 ~ 16] |
t(113) = -8.49*** |
M mean, SD standard deviation.
*p < .05, ***p < 0.001.
Significant results are shown in bold.
Measures
Self-compassion was measured using the Self-Compassionate Reactions Inventory (SCRI) [31], which lists 8 common negative events. For each event, participants were asked to endorse two responses from four given options, two of which are self-compassionate while the other two are not self-compassionate. The number of self-compassionate responses endorsed was summed up (ranging from 0–16) to represent self-compassion. The score of SCRI is highly correlated with that of Neff’s [23] Self-Compassion Scale (SCS; rs = 0.70 ~ 0.79) and the two scales have similar convergent and discriminant validity [31]. In the current study, the internal reliability of the scale is 0.92.
Suicide ideation was calculated by averaging standardized scores of suicide ideation items from three measures: K-SADS-PL, Children’s Depression Rating Scale-Revised (CDRS-R) [32] and Suicide ideation Questionnaire (SIQ) [33], whereas non-suicide ideation depression severity was indexed as total CDRS-R score minus suicide ideation items. The CDRS-R consists of 17 items, with item ratings from 1 to 7 or 1 to 5 (adding up to a total score from 17 to 113), whereas the SIQ consists of 15 items, with item ratings from 1 to 7 (adding up to a total score from 15 to 105). The decision to operationalize suicide ideation by averaging and standardizing scores from three instruments was to aim for greater reliability and validity conferred by using more items across both interview-based (K-SADS-PL & CDRS-R) and self-report (SIQ) measures.
Anxiety severity was measured with the Behavior Assessment System for Children, 2nd Edition (BASC-2) anxiety subscale [34]. Non-suicidal self-injurious behavior was determined using the K-SADS interview item regarding self-injury and was defined as any intentional act of self-injury without suicidal intent that caused tissue damage in the form of bleeding and/or scarring; this included cutting, burning, hitting, and scratching. Participants were classified as having engaged in non-suicidal self-injurious behavior if they reported at least four instances of self-injury within the last year.
Self-processing task
In the scanner, participants completed the Direct and Reflected Self-Appraisals Task (see Fig. S1), a well-studied experimental paradigm of self-referential processing [26, 18, 35]. The task required participants to evaluate whether phrases heard were self-descriptive from perspectives of themselves, their mother, classmates, and best friend. Half of the phrases belonged to the social domain and the other half to the academic domain, each of which contained equal numbers of intermixed positive and negative phrases. Thus, the task consisted of 8 blocks (4 perspectives by 2 domains across 2 valences). The blocks were presented in one of 8 orders counterbalanced across participants. Before each block, participants heard and read the following instructions: “What do I think about myself? I think…” or “What does my mother think about me? My mother thinks…” Participants pressed one of two buttons to indicate whether they endorsed or denied each phrase as self-descriptive. The presentation of phrases within blocks was algorithmically optimized [36] for detection of the difference between positive and negative phrases. The inter-stimulus interval varied (M = 6600 ms, SD = 1248.2 ms), allowing for aggregation of positive or negative phrases across perspectives and domains. The total task duration was 16 min and 50 s.
Data acquisition
Neuroimaging data were collected using two 3 T Siemens Trio MRI scanners located at the University of Minnesota and Pittsburgh, respectively. Structural 3D axial MPRAGE images were acquired in the same session (TE = 3.31 ms; TR = 2100 ms; TI = 1050; flip angle = 8°; FOV = 256 × 200 mm; matrix = 256 × 200; 176 slices; 1 mm thick). Mean BOLD images were acquired with a gradient echo EPI sequence covering 60 oblique axial slices (2 mm thick; TR/TE = 3340/30 ms; FOV = 200×200 mm; matrix = 80 × 80; flip angle = 90°).
Image pre-processing
Images were preprocessed using SPM12 (https://www.fil.ion.ucl.ac.uk/spm/). Data for each participant was realigned to the first volume in the time series to correct for head motion. Realigned images were co-registered with the participant’s anatomical image, segmented, normalized to the MNI template, and spatially smoothed with a Gaussian kernel of 7 mm FWHM. Volumes with movement >2 mm, rotations >0.587, or global signal intensities >9 were removed from first-level analysis using the Artifact Detection Tools (ART) software (http://web.mit.edu/swg/software.htm).
Analyses
Imaging data was modeled with SPM12. First-level general linear models (GLMs) with predictors including 16 conditions: 2 Valences (positive, negative) by 4 Perspectives (self, mother, classmate, best friend) by 2 Domains (academic, social). Nuisance regressors including 6 movement parameters were estimated for each participant at each voxel, resulting in t-statistic images for each of the 16 conditions.
Self-compassion and caudate circuits during positive vs. negative self-appraisals
To investigate the relationship between self-compassion and caudate functional circuits during positive vs. negative self-appraisals, psychophysiological interaction (PPI) analysis was conducted [37]. First, left and right caudate seed regions were defined for each participant by creating two 7 mm spheres centered on coordinates of the highest peak activation within the masks of the left and the right caudate as defined by the PickAtlas toolbox [38]. Slightly different peak coordinates for the highest peak of caudate activity were thus yielded for each participant, but all activity time series were contained within the caudate masks. Next, signal time courses were extracted for each participant from the caudate seed regions for all conditions and convolved with the contrast of interest (positive versus negative self-appraisals), yielding 1st-level PPI maps for each participant representing functional connectivity estimates for the seed area convolved with the contrast of interest for the task (positive vs. negative self-appraisals). Finally, to examine the relationship between self-compassion and caudate circuits, a second-level regression model was estimated with self-compassion, diagnostic group, and their interaction as covariates of interest. Because scanning site, age, and puberty were potential confounding factors [39], and the DEP and HC group showed significant differences in gender, annual household income, and medication in the study (see Table 1), we included all of these variables (i.e., scanning site, age, puberty, gender, annual household income, and medication) as confounding covariates in the regression model (note that data of household income were missing for three out of the 115 participants, and the values for their household income in the model were replaced with the mean household income of all participants).
Furthermore, because self-compassion was moderately correlated with non-suicide ideation depression severity (r = −0.68, p < 0.001), anxiety severity (r = −0.62, p < 0.001), and non-suicidal self-injurious behavior (r = −0.45, p < 0.001) in this study, we estimated another model further controlling for these variables to rule out the possibility that the identified clusters were simply driven by the effects of those variables. Whole-brain cluster-extent thresholds of pFWE < 0.05 were calculated using Monte Carlo and 3dClustSim in AFNI 18 (https://afni.nimh.nih.gov/) with a voxel-height threshold of puncorr < 0.001, yielding a minimum cluster size of 98 voxels. Results surviving cluster-extent thresholds of pFWE < 0.1 were reported as trending results. BOLD-contrast strength of connectivity at the peak voxel in each cluster was extracted for visualization and further mediation analysis.
Mediation analysis
To examine whether caudate functional connectivity correlates of self-compassion (identified in the above-mentioned regression model) would mediate the relationship between self-compassion (independent variable) and suicide ideation (dependent variable), we conducted the following analyses. First, we conducted correlation analyses between the functional connectivity correlates of self-compassion and suicide ideation using SPSS version 26.0, controlling for non-suicide ideation depression severity, anxiety severity, and non-suicidal self-injurious behavior. Second, when there was evidence for significant correlations between suicide ideation and identified functional connectivity, we tested the hypothesized mediation model using Preacher and Hayes’s PROCESS package [40], controlling for non-suicide ideation depression severity, anxiety severity, and non-suicidal self-injurious behavior.
Results
Psychological dimensions
Correlation analyses showed self-compassion negatively correlated with suicide ideation (r = −0.65, p < 0.001), non-suicide ideation depression severity (r = −0.68, p < 0.001), anxiety severity (r = −0.62, p < 0.001), and non-suicidal self-injurious behavior (r = −0.45, p < 0.001); suicide ideation positively correlated with non-suicide ideation depression severity (r = 0.71, p < 0.001), anxiety severity (r = 0.58, p < 0.001), and non-suicidal self-injurious behavior (r = 0.56, p < 0.001). More importantly, the significant correlation between self-compassion and suicide ideation held even after non-suicide ideation depression severity, anxiety severity, and non-suicidal self-injurious behavior were controlled for (rp = −0.27, p = 0.003), suggesting that the association between higher self-compassion and lower suicide ideation could not be completely accounted by these variables. Independent-sample t-tests showed that, as expected, DEPs reported lower level of self-compassion than HCs, t(113) = −8.49, p < 0.001 (DEP: 6.82 ± 4.44, HC: 13.25 ± 3.42), and higher level of suicide ideation than HCs, t(113) = 12.97, p < 0.001 (DEP: 0.39 ± 0.85, HC: −0.86 ± 0.10). Regression analysis revealed that the effects of self-compassion (β = −0.34, p < 0.001), diagnostic group (β = 0.47, p < 0.001), and their interaction effect (β = −0.18, p = 0.034) on suicide ideation were all significant. The simple slope analysis revealed that the effect of diagnostic group on suicide ideation was larger for individuals with lower level of self-compassion: for low scores (mean minus one standard deviation), β = 0.64, p < 0.001; for average scores, β = 0.47, p < 0.001; and for high scores (mean plus one standard deviation), β = 0.29, p = 0.004. On the other hand, the correlation between self-compassion and suicide ideation among DEP (r = −0.48, p < 0.001) and HC (r = −0.53, p < 0.001) did not significantly differ from each other (z = 0.35, p = 0.726). Results regarding response time and endorsement in the self-appraisal task [26] are in the supplements.
Self-compassion and caudate circuits during positive vs. negative self-appraisals
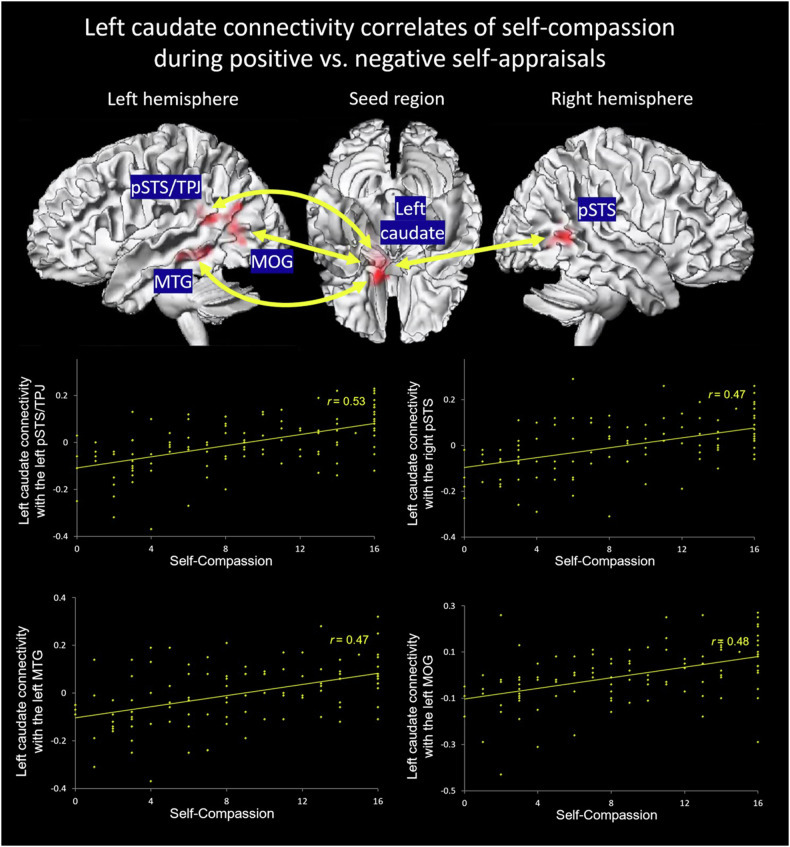
The regression analysis showed that a higher level of self-compassion was positively associated with greater left caudate functional connectivity with bilateral posterior superior temporal sulcus (pSTS)/temporoparietal junction (BA39; left pSTS/temporoparietal junction: r = 0.53; right pSTS: r = 0.47), the left middle temporal gyrus (MTG, BA 21; r = 0.47), and the left middle occipital gyrus (r = 0.48) at a cluster-level threshold of pFWE < 0.05, as well as the dorsomedial prefrontal cortex (BA8; r = 0.46) at a cluster-level threshold of pFWE < 0.1, during positive vs. negative self-appraisals (Table 2; Fig. 1 & S2). Moreover, the results remained unchanged (with the clusters of the left pSTS/temporoparietal junction and the left middle occipital gyrus merging into a bigger one, and the dorsomedial prefrontal cortex became significant at the cluster-level threshold of pFWE < 0.05) when depression severity (minus suicide items), anxiety severity, and non-suicidal self-injurious behavior were added to the regression model (Table S2). We found no right caudate circuits associated with self-compassion, and there were no left or right caudate circuits associated with the diagnostic group or its interaction with self-compassion either.
Table 2.
The left caudate functional connectivity correlates of self-compassion during positive vs. negative self-appraisals (puncorr < 0.001 at voxel level, cluster-level pFWE < 0.05).
| Left caudate functional connectivity with regions associated with self-compassion | Direction | Cluster Size (K) | Hemisphere | MNI coordinate | T | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| pSTS/temporoparietal junction (posterior middle temporal gyrus & angular gyrus), BA39/13/22 | Positive | 220 | Left | −38 | −48 | 18 | 4.68 |
| Middle occipital gyrus | Positive | 152 | Left | −34 | −66 | 12 | 4.10 |
| pSTS (posterior middle temporal gyrus), BA39/22 | Positive | 157 | Right | 38 | −52 | 8 | 4.02 |
| MTG, BA21 | Positive | 159 | Left | −44 | −32 | −8 | 4.02 |
| Dorsomedial prefrontal cortex | Positive | 91† | Left/Right | 8 | 36 | 44 | 3.87 |
pSTS posterior superior temporal sulcus, MTG middle temporal gyrus.
†Trending results (puncorr < 0.001 at voxel level, cluster-level pFWE < 0.1).
There were no supra-threshold clusters linked to self-compassion for the right caudate connectivity. No left or right caudate connectivity is associated with the diagnostic group or its interaction with self-compassion.
Fig. 1.
Self-compassion was associated with greater left caudate functional connectivity with bilateral posterior superior temporal sulcus (pSTS) and temporoparietal junction (TPJ), the left middle temporal gyrus (MTG), and the middle occipital gyrus (MOG) during positive vs. negative self-appraisals in depressed and healthy adolescents.
Mediation analysis
Suicide ideation and functional connectivity reported above were all significantly correlated (left pSTS/temporoparietal junction: r = −0.38, p < 0.001; right pSTS: r = −0.25, p = 0.007; left MTG: r = −0.44, p < 0.001; left middle occipital gyrus: r = −0.29, p = 0.001; dorsomedial prefrontal cortex: r = −0.27, p = 0.004). After controlling for non-suicide ideation depression severity, anxiety severity, and non-suicidal self-injurious behavior, however, only the left caudate functional connectivity with the left pSTS/temporoparietal junction (r = −0.22, p = 0.021) and the left MTG (r = −0.33, p < 0.001) held significant. This suggests that only the links between self-compassion and these two couplings cannot be fully explained by those three variables (see detailed results for the correlations between self-compassion, suicide ideation and the caudate circuitry in the full and different sub-samples in Table S2; note that correlations for the different sub-samples are for additional information and results interpretation, and our main findings were not based on such analyses).
Based on these results, mediation analysis with those two circuits as the mediating variables between self-compassion (independent variable) and suicide ideation (dependent variable), controlling for non-suicide ideation depression severity, anxiety severity, and non-suicidal self-injurious behavior, was conducted. Figure 2 showed that the direct effect of higher self-compassion upon reduced suicide ideation became insignificant once the two functional circuits were added to the model. The indirect effect via the left caudate-MTG connectivity was significant (95% CI: [−0.035, −0.004]), but the left caudate-pSTS/temporoparietal junction connectivity was not significant (95% CI: [−0.026, 0.010]). The results remained essentially unchanged when the two circuits were estimated as mediating variables separately (95% CI = [−0.037, −0.004] for the caudate-MTG connectivity; 95% CI = [−0.032, 0.008] for the caudate to pSTS and temporoparietal junction connectivity) or when only depressed participants (N = 79) were included in the model (Figure S3). We also conducted mediation models in only the non-medicated (Fig. S4) or the medicated depressed participants (Fig. S5). Perhaps due to a small sample (N = 38 for the non-medicated depressed sub-sample and N = 41 for the medicated depressed sub-sample), the indirect effect via the left caudate-MTG connectivity was not significant in either model. Given that the path coefficient from the left caudate-MTG connectivity to suicide ideation was similar in both models, it appears improbable that the lack of significance was due to an absence of effect in either sub-sample. These results indicate that greater left caudate-MTG functional connectivity during positive vs. negative self-appraisals fully explained the relationship between a higher level of self-compassion and a lower level of suicide ideation. On the other hand, the indirect effect via the left caudate-pSTS/temporoparietal junction connectivity was significant in the non-medicated but not in the medicated sub-sample. Larger sample sizes are needed to explore these effects.
Fig. 2.
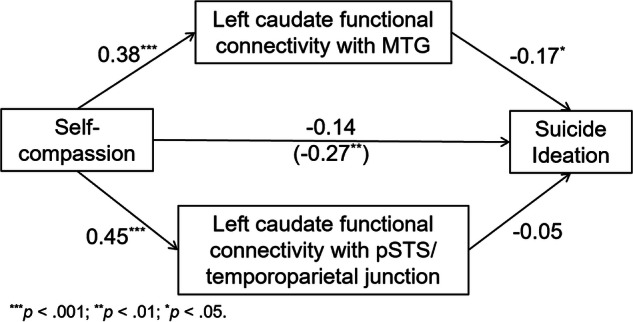
Greater left caudate functional connectivity with the left middle temporal gyrus (MTG) during positive vs. negative self-appraisals mediated the relationship between higher self-compassion and lower suicide ideation (controlling for depression severity minus suicide ideation, anxiety severity, and non-suicidal self-injurious behavior) in depressed and healthy adolescents. The number in the parenthesis is the partial correlation coefficient between self-compassion and suicide ideation (controlling for depression severity minus suicide ideation, anxiety severity, and non-suicidal self-injurious behavior).
Discussion
Self-compassion was associated with greater left caudate functional connectivity with bilateral posterior superior temporal sulcus (pSTS)/temporoparietal junction, the left medial temporal gyrus (MTG), and the left middle occipital gyrus during positive vs. negative self-appraisals across depressed and healthy youth. Among these circuits, only the left caudate-MTG connectivity mediated the association between higher self-compassion and lower suicide ideation, even after controlling for non-suicide ideation depression severity, anxiety severity, and non-suicidal self-injurious behavior. While prior research has focused on the neural activity correlates of self-compassion, our study provided the first evidence for the functional connectivity correlates of self-compassion and the close associations between self-compassion, self-referential caudate connectivity, and suicide ideation in depressed and healthy adolescents. These findings not only provide insight into the neural mechanisms underlying the association between self-compassion and suicide ideation but also have implications for reducing suicide ideation in adolescents.
Self-compassion and caudate functional connectivity
Self-compassion was associated with greater left caudate functional connectivity with bilateral pSTS/temporoparietal junction, the left MTG, and the left middle occipital gyrus, as well as the dorsomedial prefrontal cortex to a lesser degree, during positive vs. negative self-appraisals across depressed and healthy adolescents. The caudate is associated with learning via evaluation of outcomes and behavioral anticipation [41–43], and it supports the self-serving bias, i.e., attributing the causation of positive events internally and negative events externally [44] in healthy individuals. Prior research has also shown that self-compassion engages the caudate among other reward-related regions [14, 27, 45]. The present findings suggest that self-compassionate adolescents may show enhanced behavioral anticipation for rewarding vs. punishing self-relevant cues. Given the caudate function [41–43], this means that self-compassionate youth expect, or are more prepared to receive, positive self-relevant evaluations than negative ones. These processes are in coordination with cognitive processes enabled by the pSTS, temporoparietal junction, MTG, and middle occipital gyrus, as well as, to a lesser degree, the dorsomedial prefrontal cortex, during positive versus negative self-appraisals. This is in line with prior findings on the negative link between self-compassion and negative self-processing as well as the positive link between self-compassion and positive self-processing in depressed youth [12, 21, 24, 26].
As expected, self-compassion was associated with caudate to pSTS and temporoparietal junction functional connectivity during positive vs. negative self-appraisals. The regions of pSTS and temporoparietal junction enable self-processing [18, 19, 28, 46]. In a meta-analysis [19], bilateral pSTS/temporoparietal junction were found among other regions to be activated during self- vs. other-referential processing. The regions of pSTS and temporoparietal junction have been widely reported to be associated with self-other distinction [47–49], mentalizing [50–53], or social attention [54, 55]. In the present study, the circuit comprised by the pSTS/temporoparietal junction and the caudate may be engaged by judging whether phrases heard are self-descriptive from the participant’s own as well as others’ perspectives, and with pleasure elicited by positive versus negative self-descriptors. We also found a mild association between self-compassion and caudate to dorsomedial prefrontal cortex connectivity. The dorsomedial prefrontal cortex is part of the CMS, a robust substrate of self-referential processing [17–19]. The medial prefrontal cortex enables social information integration, such as representation of traits across time, enabling conceptual self-representation [19, 52]. Together with the caudate enabling reward learning [41–43], our results suggest that positive self-referential stimuli elicit more pleasure or rewarding feelings among self-compassionate youth across social perspectives.
Intriguingly, self-compassion was also associated with greater caudate to MTG and middle occipital gyrus functional connectivity during positive vs. negative self-appraisals. The engagement of MTG and the middle occipital gyrus suggests that basic cognitive processes such as language, auditory, and visual processing involved in the self-referential task were coordinated with caudate responses. The left MTG or BA21 is part of the language network [56, 57], which interposes auditory and visual processing streams [58], and is associated with multimodal semantic processing [59]. The middle occipital gyrus is part of the visual cortex, which is engaged in emotion category encoding in addition to visual processing [60, 61]. Collectively, the connections observed between self-compassion and the left caudate’s functional connectivity with MTG and the middle occipital gyrus indicate that the heightened behavioral anticipation for positive self-referential phrases among self-compassionate adolescents may stem from fundamental cognitive processes. These processes involve auditory and visual emotion categorization, as well as semantic processing, working in coordination with reward systems and behavioral anticipation specifically for positive self-referential stimuli.
More importantly, these associations became stronger when non-suicide ideation depression severity, anxiety severity, and non-suicidal self-injurious behavior were controlled for (with the clusters of the left pSTS/temporoparietal junction and the left middle occipital gyrus merging into a bigger cluster, as well as the cluster of the dorsomedial prefrontal cortex becoming significant and two more clusters being yielded (i.e., the right MTG and the left precentral/middle frontal gyrus). No associations were found between any caudate circuitry and non-suicide ideation depression severity, anxiety severity, or non-suicidal self-injurious behavior. These results lent further support to the associations between self-compassion and caudate-MTG connectivity, as well as caudate circuits in general. They suggest that these links cannot be explained by their associations with non-suicide ideation depression severity, anxiety severity, or non-suicidal self-injurious behavior, even though those symptoms were moderately correlated with self-compassion.
Because the caudate is mainly engaged in reward and punishment processing [62] and emotionally valenced self-processing (e.g., positive vs. negative) [12, 44], the caudate and its connectivity are likely not engaged in neutral self-referential processing. To our knowledge, however, there is no available research regarding caudate engagement during non-valenced self-referential processing. Given the dopaminergic nature of this neural circuit, it is doubtful that its engagement would be prompted by mental activities or behavior devoid of emotional judgments. In other words, self-compassion may be associated primarily with caudate circuits during positive vs. negative rather than neutral self-appraisals. Because we did not measure neural responses during neutral self-appraisals (e.g., “I have two legs”), however, this assumption needs to be tested in future studies.
It should be noted that self-compassion was correlated with only the left but not the right caudate circuits. The left caudate has been reported to be engaged in language control [63, 64]. The specific engagement of the left caudate circuits in self-compassion during self-referential processing may be associated with the left caudate serving as a hub for receiving signals related to language processing of specific self-referential information. In other words, the fact that participants were evaluating language-based self-referential information might have primed preponderant engagement of the left hemisphere. However, further research is needed to test this assumption.
The mediating effect of caudate-MTG connectivity between self-compassion and suicide ideation
The left caudate connectivity with the left pSTS/ temporoparietal junction and the left MTG during positive vs. negative self-appraisals was negatively correlated with suicide ideation, even after non-suicide ideation depression severity, anxiety severity, and non-suicidal self-injurious behavior were controlled for. These findings suggest that stronger anticipation for, or higher pleasure during, positive vs. negative self-descriptors in coordination with mentalizing and semantic processing may reduce suicide ideation. This might mean that pleasure derived from hearing positive self-descriptors is associated with lower suicide ideation. By contrast, individuals with lower self-compassion may be incapable of experiencing self-referential reward upon hearing positive self-referential descriptors or phrases. Moreover, these links were not driven by the links between non-suicide ideation depression severity, anxiety severity, or non-suicidal self-injurious behavior and the left caudate to pSTS and temporoparietal junction or MTG connectivity. This is consistent with previous findings that suicide ideation or attempts showed neural patterns distinct from related symptoms, such as non-suicide ideation depression severity [65, 66]. Specifically for task-based functional connectivity, our findings provide further support to the extant literature that suicide ideation or attempts are associated with reduced connectivity between salience-related and task-relevant regions [22, 65, 67].
Contrary to our expectations, when caudate-pSTS/temporoparietal junction and caudate-MTG connectivity were included in the mediation model, only the caudate functional connectivity with MTG rather than pSTS/temporoparietal junction explained the association between higher self-compassion and lower suicide ideation (controlling for non-suicide ideation depression severity, anxiety severity, and non-suicidal self-injurious behavior). The results remained unchanged when only depressed participants were included in the model. This suggests that it is the behavioral anticipation/pleasure for positive vs. negative self-referential phrases coordinated with semantic rather than mentalizing processing that may mediate the relationship between higher self-compassion and lower suicide ideation during the self-processing task. Based on the potential meaning of caudate-MTG connectivity discussed above, self-compassion might prepare adolescents more readily for positive rather than negative self-referential phrases or other self-related stimuli as early as the stages of semantic processing. Nevertheless, this assumption needs to be tested using higher temporal resolution methods, such as magnetoencephalography (MEG).
Of note, when only depressed youth (N = 79) were tested, the left caudate-MTG connectivity still significantly mediated the relationship between self-compassion and suicide ideation. Furthermore, when only non-medicated depressed youth were tested, the role of the left caudate-pSTS/temporoparietal junction connectivity as a mediator between self-compassion and suicide ideation became significant. While depressed youth reported lower self-compassion and higher suicide ideation versus heathy controls, our results held even when healthy controls were excluded. This suggests that diagnostic differences were not driving our results. Instead, self-compassion’s wide range of scores within depressed youth (see Fig. S2) likely allowed us to detect associations between the three variables despite diagnostic mean differences. This encouraging result suggests that individual differences in self-compassion within depressed youth may protect some from suicide risks and could be relied upon to foster recovery. Finally, because even non-medicated depressed youth showed caudate circuitry mediating the relationship between self-compassion and suicide ideation, we can venture that the caudate circuitry’s mediating role is not facilitated by medication, which is again a promising result. It suggests that therapies based on self-compassion, and/or those up-regulating caudate circuitry during positive vs. negative self-processing, may bear additional benefits and suicide prevention above and beyond those conferred by medication. However, this finding relies on a small sample (N = 38) and thus requires validation.
Self-compassion, suicide risk factors, caudate connectivity, and suicide ideation
Consistent with previous findings [8, 9, 68], the behavioral results revealed that self-compassion not only moderated the effect of depression diagnosis on suicide ideation but also predicted lower suicide ideation beyond depression diagnosis. These findings suggest that self-compassion not only mitigates the negative effect of depression (diagnosis) on suicide ideation, but also has a direct and independent impact on suicide ideation. The imaging findings that the caudate connectivity uniquely correlated with self-compassion and mediated the relationship between self-compassion and suicide ideation even after controlling for other suicide risk factors (i.e., non-suicide ideation depression severity, anxiety severity, and non-suicidal self-injury) lent further support to this important role of self-compassion. It suggests that in addition to a buffering effect, self-compassion has an additional protective effect for suicide ideation beyond these risk factors, which is facilitated by the self-referential caudate connectivity. This direct and independent effect of self-compassion highlights its unique role and importance among various factors that affect suicide ideation. As a protective factor for suicide, self-compassion does not simply indicate the absence of suicide risk factors, such as depression, anxiety, self-injury, as well as self-hate [8, 69, 70]. Though self-compassion can alleviate these risk factors by nurturing a more positive attitude toward self, it entails proactive components such as self-kindness and mindfulness that directly contribute to reduced suicide ideation. Future studies should further investigate the relationship between self-compassion and other self-related risk factors, such as self-hate, in their association with suicide ideation as well as the underlying neural mechanisms.
Limitations
This is a correlational study. It cannot determine the causal relationship between self-compassion, caudate circuitry, and suicide ideation. Intervention studies are required to confirm this. Additionally, we used a unidimensional scale to measure self-compassion, so we cannot determine which components of self-compassion (e.g., self-kindness, common humanity, or mindfulness) drove the findings. Future studies ought to replicate the findings using a multidimensional scale of self-compassion such as Neff’s scale [23]. Finally, we only examined the caudate circuits, but other striatal regions such as putamen or ventral striatum deserve further investigations.
Conclusions
Self-compassion is associated with left caudate to left MTG connectivity during positive vs. negative self-appraisals, and this circuitry mediates the association between higher self-compassion and lower suicide ideation among depressed and healthy youth. These results advance our understanding of the neural mechanisms underlying the protective role of self-compassion for suicide risks. They suggest that left caudate to MTG circuitry during positive vs. negative self-referential processing could be targeted with neural stimulation to reduce suicide ideation in depressed youth, either alone or combined with self-compassion interventions.
Supplementary information
Acknowledgements
The authors would like to thank Hannah Scott, Garry Smyda, Hoang-Giang Nguyen, and Carmen Santana-Gonzalez for their assistance in analyses and writing.
Author contributions
GL: Conceptualization, Methodology, Formal Analysis, Writing – Original draft preparation & Editing. GH: Formal Analysis, Review & Editing. JR, ND, CS and LY: Review & Editing. KQ: Conceptualization, Methodology, Supervision, Resources, Writing – Review & Editing.
Funding
Funding was provided by K01MH092601, R61MH122634-01, and two NARSAD grants to the last author KQ. Grant support from 2023XS-0074 to GL is acknowledged.
Data availability
Data and research materials for this study are available upon request.
Competing interests
The authors declare no competing interests.
Ethics approval
This study was conducted in accordance with the Helsinki Declaration and approved by the Institutional Review Board at University of Minnesota (IRB#: 1209M21381) and at University of Pittsburgh (IRB#: REN11090197/PRO10100210). All participants and their parents gave informed consent.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41398-024-03037-0.
References
- 1.Heron MP. Deaths: leading causes for 2018. Natl Vital- Stat Rep. 2021;70:1–115. [PubMed] [Google Scholar]
- 2.Thompson MP, Swartout K. Epidemiology of suicide attempts among youth transitioning to adulthood. J Youth Adolescence. 2018;47:807–17. 10.1007/s10964-017-0674-8 [DOI] [PubMed] [Google Scholar]
- 3.Foley DL, Goldston DB, Costello EJ, Angold A. Proximal psychiatric risk factors for suicidality in youth: the Great Smoky Mountains Study. Arch Gen Psychiatr. 2006;63:1017–24. 10.1001/archpsyc.63.9.1017 [DOI] [PubMed] [Google Scholar]
- 4.Nock MK, Green JG, Hwang I, McLaughlin KA, Sampson NA, Zaslavsky AM, et al. Prevalence, correlates, and treatment of lifetime suicidal behavior among adolescents: results from the National Comorbidity Survey Replication Adolescent Supplement. JAMA Psychiatr. 2013;70:300–10. 10.1001/2013.jamapsychiatry.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mann JJ, Michel CA, Auerbach RP. Improving suicide prevention through evidence-based strategies: a systematic review. Am J Psychiatr. 2021;178:611–24. 10.1176/appi.ajp.2020.20060864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brent DA, Mann JJ. Familial pathways to suicidal behavior-understanding and preventing suicide among adolescents. N. Engl J Med. 2006;355:2719. 10.1056/NEJMp068195 [DOI] [PubMed] [Google Scholar]
- 7.Neff KD. Self-compassion: An alternative conceptualization of a healthy attitude toward oneself. Self identity. 2003;2:85–101. 10.1080/15298860309032 [DOI] [Google Scholar]
- 8.Suh H, Jeong J. Association of self-compassion with suicidal thoughts and behaviors and non-suicidal self injury: A meta-analysis. Front Psychol. 2021;12:633482. 10.3389/fpsyg.2021.633482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cleare S, Gumley A, O’Connor RC. Self‐compassion, self‐forgiveness, suicidal ideation, and self‐harm: A systematic review. Clin Psychol Psychother. 2019;26:511–30. 10.1002/cpp.2372 [DOI] [PubMed] [Google Scholar]
- 10.Reynolds WM. Psychometric characteristics of the Adult Suicidal Ideation Questionnaire in college students. J Personal Assess. 1991;56:289–307. 10.1207/s15327752jpa5602_9 [DOI] [PubMed] [Google Scholar]
- 11.Burke TA, Connolly SL, Hamilton JL, Stange JP, Abramson LY, Alloy LB. Cognitive risk and protective factors for suicidal ideation: a two year longitudinal study in adolescence. J Abnorm Child Psychol. 2016;44:1145–60. 10.1007/s10802-015-0104-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quevedo K, Teoh JY, Liu G, Santana-Gonzalez C, Forbes EE, Engstrom M. Neural substrates of rewarding and punishing self representations in depressed suicide-attempting adolescents. J Psychiatr Res. 2022;148:204–13. 10.1016/j.jpsychires.2022.01.037 [DOI] [PubMed] [Google Scholar]
- 13.Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatr. 2009;166:702–10. 10.1176/appi.ajp.2008.08081201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Longe O, Maratos FA, Gilbert P, Evans G, Volker F, Rockliff H, et al. Having a word with yourself: Neural correlates of self-criticism and self-reassurance. NeuroImage. 2010;49:1849–56. 10.1016/j.neuroimage.2009.09.019 [DOI] [PubMed] [Google Scholar]
- 15.LeMoult J, Kircanski K, Prasad G, Gotlib IH. Negative self-referential processing predicts the recurrence of major depressive episodes. Clin Psychol Sci. 2017;5:174–81. 10.1177/2167702616654898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allison GO, Benau EM, Asbaghi S, Pagliacco D, Stewart JG, Auerbach RP. Neurophysiological markers related to negative self-referential processing differentiate adolescent suicide ideators and attempters. Biol Psych Glob Open Sci. 2021;1:16–27. 10.1016/j.bpsgos.2021.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Northoff G, Bermpohl F. Cortical midline structures and the self. Trends Cogn Sci. 2004;8:102–7. 10.1016/j.tics.2004.01.004 [DOI] [PubMed] [Google Scholar]
- 18.Northoff G, Heinzel A, De Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain—a meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–57. 10.1016/j.neuroimage.2005.12.002 [DOI] [PubMed] [Google Scholar]
- 19.Hu C, Di X, Eickhoff SB, Zhang M, Peng K, Guo H, et al. Distinct and common aspects of physical and psychological self-representation in the brain: A meta-analysis of self-bias in facial and self-referential judgements. Neurosci Biobehav Rev. 2016;61:197–207. 10.1016/j.neubiorev.2015.12.003 [DOI] [PubMed] [Google Scholar]
- 20.Brühl AB, Rufer M, Kaffenberger T, Baur V, Herwig U. Neural circuits associated with positive and negative self-appraisal. Neuroscience. 2014;265:48–59. 10.1016/j.neuroscience.2014.01.053 [DOI] [PubMed] [Google Scholar]
- 21.Qiu H, Cao B, Cao J, Li X, Chen J, Wang W, et al. Resting-state functional connectivity of the anterior cingulate cortex in young adults depressed patients with and without suicidal behavior. Behavioural Brain Res. 2020;384:112544. 10.1016/j.bbr.2020.112544 [DOI] [PubMed] [Google Scholar]
- 22.Auerbach RP, Pagliaccio D, Allison GO, Alqueza KL, Alonso MF. Neural correlates associated with suicide and nonsuicidal self-injury in youth. Biol Psych. 2021;89:119–33. 10.1016/j.biopsych.2020.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neff KD. The development and validation of a scale to measure self-compassion. Self Identity. 2003;2:223–50. 10.1080/15298860309027 [DOI] [Google Scholar]
- 24.Liu G, Zhang N, Teoh JY, Egan C, Zeffiro TA, Davidson RJ, et al. Self-compassion and dorsolateral prefrontal cortex activity during sad self-face recognition in depressed adolescents. Psychol Med. 2022;52:864–73. 10.1017/S0033291720002482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradley KA, Colcombe S, Henderson SE, Alonso CM, Milham MP, Gabbay V. Neural correlates of self-perceptions in adolescents with major depressive disorder. Dev Cogn Neurosci. 2016;19:87–97. 10.1016/j.dcn.2016.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu G, Santana-Gonzalez C, Zeffiro TA, Zhang N, Engstrom M, Quevedo K. Self-compassion and neural activity during self-appraisals in depressed and healthy adolescents. J Affect Disord. 2023;339:717–24. 10.1016/j.jad.2023.07.012 [DOI] [PubMed] [Google Scholar]
- 27.Lutz J, Berry MP, Napadow V, Germer C, Pollak S, Gardiner P, et al. Neural activations during self-related processing in patients with chronic pain and effects of a brief self-compassion training–a pilot study. Psych Res: Neuroimaging. 2020;304:111155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sui J, Gu X. Self as Object: Emerging Trends in Self Research. Trends Neurosci. 2017;40:643–53. 10.1016/j.tins.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 29.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–8. 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- 30.Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G* Power 3.1: Tests for correlation and regression analyses. Behav Res methods. 2009;41:1149–60. 10.3758/BRM.41.4.1149 [DOI] [PubMed] [Google Scholar]
- 31.Terry ML, Leary MR, Mehta S, Henderson K. Self-compassionate reactions to health threats. Personal Soc Psychol Bull. 2013;39:911–26. 10.1177/0146167213488213 [DOI] [PubMed] [Google Scholar]
- 32.Poznanski EO & Mokros HB. Children’s depression rating scale, revised (CDRS-R). (Western Psychological Services Los Angeles, 1996).
- 33.Reynolds WM. Suicidal ideation questionnaire (SIQ). Odessa, FL:Psychological Assessment Resources (1987).
- 34.Reynolds CR & Kamphaus RW. Behavior assessment system for children, (BASC-2). Circle Pines, MN: American Guidance Service (2004).
- 35.Pfeifer JH, Masten CL, Borofsky LA, Dapretto M, Fuligni AJ, Lieberman MD. Neural correlates of direct and reflected self-appraisals in adolescents and adults: When social perspective-taking informs self-perception. Child Dev. 2009;80:1016–38. 10.1111/j.1467-8624.2009.01314.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wager TD, Nichols TE. Optimization of experimental design in fMRI: a general framework using a genetic algorithm. Neuroimage. 2003;18:293–309. 10.1016/S1053-8119(02)00046-0 [DOI] [PubMed] [Google Scholar]
- 37.Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–29. 10.1006/nimg.1997.0291 [DOI] [PubMed] [Google Scholar]
- 38.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. 10.1016/S1053-8119(03)00169-1 [DOI] [PubMed] [Google Scholar]
- 39.Pfeifer JH, Kahn LE, Merchant JS, Peake SJ, Veroude K, Masten CL, et al. Longitudinal change in the neural bases of adolescent social self-evaluations: effects of age and pubertal development. J Neurosci. 2013;33:7415–9. 10.1523/JNEUROSCI.4074-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res methods. 2008;40:879–91. 10.3758/BRM.40.3.879 [DOI] [PubMed] [Google Scholar]
- 41.Haruno M, Kawato M. Different neural correlates of reward expectation and reward expectation error in the putamen and caudate nucleus during stimulus-action-reward association learning. J Neurophysiol. 2006;95:948–59. 10.1152/jn.00382.2005 [DOI] [PubMed] [Google Scholar]
- 42.Grahn JA, Parkinson JA, Owen AM. The cognitive functions of the caudate nucleus. Prog Neurobiol. 2008;86:141–55. 10.1016/j.pneurobio.2008.09.004 [DOI] [PubMed] [Google Scholar]
- 43.Haruno M, Kuroda T, Doya K, Toyama K, Kimura M, Samejima K, et al. A neural correlate of reward-based behavioral learning in caudate nucleus: a functional magnetic resonance imaging study of a stochastic decision task. J Neurosci. 2004;24:1660–5. 10.1523/JNEUROSCI.3417-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blackwood N, Bentall R, Simmons A, Murray R, Howard R. Self-responsibility and the self-serving bias: an fMRI investigation of causal attributions. NeuroImage. 2003;20:1076–85. 10.1016/S1053-8119(03)00331-8 [DOI] [PubMed] [Google Scholar]
- 45.Novak L, Malinakova K, Mikoska P, van Dijk JP, Tavel P. Neural correlates of compassion–An integrative systematic review. Int J Psychophysiol. 2022;172:46–59. 10.1016/j.ijpsycho.2021.12.004 [DOI] [PubMed] [Google Scholar]
- 46.Qin P, Northoff G. How is our self related to midline regions and the default-mode network? Neuroimage. 2011;57:1221–33. 10.1016/j.neuroimage.2011.05.028 [DOI] [PubMed] [Google Scholar]
- 47.Quesque F, Brass M. The role of the temporoparietal junction in self-other distinction. Brain Topogr. 2019;32:943–55. 10.1007/s10548-019-00737-5 [DOI] [PubMed] [Google Scholar]
- 48.Steinbeis N. The role of self–other distinction in understanding others’ mental and emotional states: neurocognitive mechanisms in children and adults. Philos Trans R Soc B: Biol Sci. 2016;371:20150074. 10.1098/rstb.2015.0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Northoff G. Is the self a higher-order or fundamental function of the brain? The “basis model of self-specificity” and its encoding by the brain’s spontaneous activity. Cogn Neurosci. 2016;7:203–22. 10.1080/17588928.2015.1111868 [DOI] [PubMed] [Google Scholar]
- 50.Frith U, Frith CD. Development and neurophysiology of mentalizing. Philos Trans R Soc Lond B: Biol Sci. 2003;358:459–73. 10.1098/rstb.2002.1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gallagher HL, Frith CD. Functional imaging of ‘theory of mind’. Trends Cogn Sci. 2003;7:77–83. 10.1016/S1364-6613(02)00025-6 [DOI] [PubMed] [Google Scholar]
- 52.Van Overwalle F. Social cognition and the brain: a meta‐analysis. Hum Brain Mapp. 2009;30:829–58. 10.1002/hbm.20547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Overwalle F, Baetens K. Understanding others’ actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage. 2009;48:564–84. 10.1016/j.neuroimage.2009.06.009 [DOI] [PubMed] [Google Scholar]
- 54.Nummenmaa L, Calder AJ. Neural mechanisms of social attention. Trends Cogn Sci. 2009;13:135–43. 10.1016/j.tics.2008.12.006 [DOI] [PubMed] [Google Scholar]
- 55.Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends Cogn Sci. 2000;4:267–78. 10.1016/S1364-6613(00)01501-1 [DOI] [PubMed] [Google Scholar]
- 56.Acheson DJ, Hagoort P. Stimulating the brain’s language network: syntactic ambiguity resolution after TMS to the inferior frontal gyrus and middle temporal gyrus. J Cogn Neurosci. 2013;25:1664–77. 10.1162/jocn_a_00430 [DOI] [PubMed] [Google Scholar]
- 57.Ashtari M, Lencz T, Zuffante P, Bilder R, Clarke T, Diamond A, et al. Left middle temporal gyrus activation during a phonemic discrimination task. Neuroreport. 2004;15:389–93. 10.1097/00001756-200403010-00001 [DOI] [PubMed] [Google Scholar]
- 58.Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb cortex. 2009;19:2767–96. 10.1093/cercor/bhp055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Visser M, Jefferies E, Embleton KV, Lambon Ralph MA. Both the middle temporal gyrus and the ventral anterior temporal area are crucial for multimodal semantic processing: distortion-corrected fMRI evidence for a double gradient of information convergence in the temporal lobes. J Cogn Neurosci. 2012;24:1766–78. 10.1162/jocn_a_00244 [DOI] [PubMed] [Google Scholar]
- 60.Kragel PA, Reddan MC, LaBar KS, Wager TD. Emotion schemas are embedded in the human visual system. Sci Adv. 2019;5:eaaw4358. 10.1126/sciadv.aaw4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bo K, Yin S, Liu Y, Hu Z, Meyyappan S, Kim S, et al. Decoding Neural Representations of Affective Scenes in Retinotopic Visual Cortex. Cereb Cortex. 2021;31:3047–63. 10.1093/cercor/bhaa411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Delgado M, Locke H, Stenger VA, Fiez J. Dorsal striatum responses to reward and punishment: effects of valence and magnitude manipulations. Cogn, Affect, Behav Neurosci. 2003;3:27–38. 10.3758/CABN.3.1.27 [DOI] [PubMed] [Google Scholar]
- 63.Tan AP, Ngoh ZM, Yeo SSP, Koh DXP, Gluckman P, Chong YS, et al. Left lateralization of neonatal caudate microstructure affects emerging language development at 24 months. Eur J Neurosci. 2021;54:4621–37. 10.1111/ejn.15347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crinion J, Turner R, Grogan A, Hanakawa T, Noppeney U, Devlin JT, et al. Language control in the bilingual brain. Science. 2006;312:1537–40. 10.1126/science.1127761 [DOI] [PubMed] [Google Scholar]
- 65.Alarcón G, Sauder M, Teoh JY, Forbes EE, Quevedo K. Amygdala functional connectivity during self-face processing in depressed adolescents with recent suicide attempt. J Am Acad Child Adolesc Psychiatry. 2019;58:221–31. 10.1016/j.jaac.2018.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ai H, van Tol M-J, Marsman J-BC, Veltman DJ, Ruhé HG, van der Wee NJ, et al. Differential relations of suicidality in depression to brain activation during emotional and executive processing. J Psychiatr Res. 2018;105:78–85. 10.1016/j.jpsychires.2018.08.018 [DOI] [PubMed] [Google Scholar]
- 67.Pan L, Hassel S, Segreti A, Nau S, Brent DA, Phillips ML. Differential patterns of activity and functional connectivity in emotion processing neural circuitry to angry and happy faces in adolescents with and without suicide attempt. Psychological Med. 2013;43:2129–42. 10.1017/S0033291712002966 [DOI] [PubMed] [Google Scholar]
- 68.Johnson J, Wood AM, Gooding P, Taylor PJ, Tarrier N. Resilience to suicidality: The buffering hypothesis. Clin Psychol Rev. 2011;31:563–91. 10.1016/j.cpr.2010.12.007 [DOI] [PubMed] [Google Scholar]
- 69.Franklin JC, Ribeiro JD, Fox KR, Bentley KH, Kleiman EM, Huang X, et al. Risk factors for suicidal thoughts and behaviors: A meta-analysis of 50 years of research. Psychological Bull. 2017;143:187. 10.1037/bul0000084 [DOI] [PubMed] [Google Scholar]
- 70.Büge B, Bilge Y. Reliability and validity of self-hate scale in Turkish community sample. J Happiness Health. 2022;2:61–69. 10.47602/johah.v2i2.17 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and research materials for this study are available upon request.