Abstract
Prostate cancer (PCa) is the second most prevalent malignancy among men worldwide. The aberrant activation of androgen receptor (AR) signaling has been recognized as a crucial oncogenic driver for PCa and AR antagonists are widely used in PCa therapy. To develop novel AR antagonist, a machine-learning MIEC-SVM model was established for the virtual screening and 51 candidates were selected and submitted for bioactivity evaluation. To our surprise, a new-scaffold AR antagonist C2 with comparable bioactivity with Enz was identified at the initial round of screening. C2 showed pronounced inhibition on the transcriptional function (IC50 = 0.63 μM) and nuclear translocation of AR and significant antiproliferative and antimetastatic activity on PCa cell line of LNCaP. In addition, C2 exhibited a stronger ability to block the cell cycle of LNCaP than Enz at lower dose and superior AR specificity. Our study highlights the success of MIEC-SVM in discovering AR antagonists, and compound C2 presents a promising new scaffold for the development of AR-targeted therapeutics.
Keywords: prostate cancer, androgen receptor antagonist, virtual screening, MIEC-SVM model, machine learning
Introduction
Prostate cancer (PCa) is a highly prevalent cancer that poses a significant threat to men’s health worldwide [1]. In 2022, PCa accounted for 27% of cancer diagnoses and 11% of cancer-related deaths in men [2]. The androgen receptor (AR), highly expressed in both normal and cancerous epithelial cells, plays a crucial role in prostate organogenesis and the progression of PCa by regulating epithelial differentiation and cell proliferation [3]. Due to its involvement in driving hormone dependency, it is widely recognized as an indispensable therapeutic target for PCa [4, 5]. Moreover, AR overexpression is commonly observed in PCa and is considered as one of the primary drivers of progression to castration-resistant prostate cancer (CRPC) [6, 7].
Similar to other members of the nuclear receptor (NR) family, AR comprised four functional domains: the C-terminus ligand-binding domain (LBD), the relatively unstructured N-terminal domain (NTD), the DNA-binding domain (DBD) and a flexible hinge region [4, 8]. Among these, the LBD contains several previously reported small molecule binding sites: the ligand-binding pocket (LBP) [9], the activation function 2 (AF2) site [8], the binding function 3 (BF3) site [10], and the dimer interface pocket (DIP) [11]. Activation of AR occurs through the binding of endogenous androgens, such as 5α-dihydrotestosterone (DHT) and testosterone, to the LBP. Numerous small molecules targeting AR have been discovered, however all approved nonsteroidal antagonists to date bind to the AR LBP to regulate the AR signaling pathway [12]. Both first-generation AR antagonists, such as flutamide and bicalutamide, and second-generation AR antagonists, like enzalutamide (Enz) and apalutamide (Apa, derivative of Enz), target this pocket [13–15]. Undoubtedly, the LBP is a critical site for the targeting and functioning of AR antagonists. However, the emergence of drug resistance and the limited structure diversity pose challenges to the clinical application of the AR antagonists. Therefore, there is an urgent need for the design and discovery of novel AR antagonists.
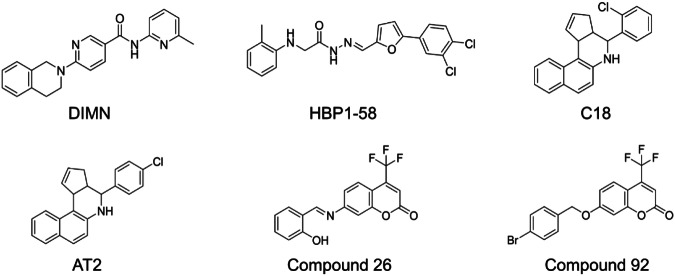
Virtual screening (VS) has been widely used as an efficient approach to discover novel and potent AR antagonists, especially those targeting the AR LBP. However, the bioactive compounds selected in the initial round of screening in previous studies have shown limited effectiveness. For example, DIMN (6-(3,4-dihydro-1H-isoquinolin-2-yl)-N-(6-methylpyridin-2-yl)nicotinamide) was the first application of structure-based virtual screening (SBVS) in this field, demonstrating stronger inhibition of prostate-specific antigen (PSA) (a biomarker of PCa) compared to bicalutamide. Nevertheless, DIMN only inhibited AR transactivation with the IC50 value at 3 μM [16]. Similarly, our group utilized SBVS to identify a series of novel antagonists targeting AR LBP with a hit rate of 8.62% in 2018. Among them, HBP1-58 showed most significant inhibitory effect in this round of screening, but the IC50 value only reached 3.25 µM [9]. In another study, the AR antagonist C18 (4-(2-chlorophenyl)-3a,4,5,11c-tetrahydro-3H-benzo[f]cyclopenta[c]quinoline) identified through SBVS demonstrated a modest IC50 value of 2.4 μM against AR transcriptional activity in the initial screening. After chemical modification, an analog, AT2 (4-(4-chlorophenyl)-3a,4,5,11c-tetrahydro-3H-benzo[f]cyclopenta[c]quinoline), exhibited improved potency with an IC50 value at a submicromolar level against AR transcriptional activity [17]. Subsequently, in 2021, a novel AR antagonist compound 26 (7-((2-hydroxybenzylidene)amino)-4-(trifluoromethyl)-2H-chromen-2-one) emerged as the leading compound through VS, but its IC50 value of 5.57 μM against AR transcriptional assay highlighted the limited effectiveness of the initial round of screening. A modified version of compound 26, compound 92 (7-((4-bromobenzyl)oxy)-4-(trifluoromethyl)-2H-chromen-2-one), exhibited an improved IC50 value reaching a submicromolar level [18]. The structures of these compounds are shown in Fig. 1. Overall, although VS provides various novel scaffolds for developing new AR antagonists, a micromolar level of antagonistic activity seems the limit that previous VS could achieve at the discovery of AR antagonist. Further intensive chemical modification or analog searching is required to obtain promising AR antagonist. It is meaningful for future studies to address the limitation and improve the efficiency of the initial screening process.
Fig. 1.
The structures of AR antagonists previously mentioned using the VS.
Recently, the effectiveness of the structure-based machine-learning method MIEC-SVM has been demonstrated in accurately classifying molecular categories in various systems. This method combines molecular interaction energy components (MIEC) derived from free energy decomposition with the support vector machine (SVM) [19]. Our group has successfully applied this approach to discriminate bioactive peptides in previous studies [20–22]. Additionally, we have discovered novel small-molecule inhibitors of anaplastic lymphoma kinase (ALK) using an established MIEC-SVM model [23]. Previous studies have shown that the MIEC-SVM model is an efficient method for distinguishing agonists and antagonists targeting the LBP of several NRs [24]. However, the study here represents the successful application of the MIEC-SVM model in the context of AR LBP.
The current study identified a new-scaffold AR antagonist C2 targeting the LBP by combining MIEC-SVM based SBVS and a series of bioassays. The crystal structure 1Z95 was utilized to construct the MIEC-SVM model. VS was performed on the ChemDiv database, followed by Glide HTVS docking and Glide SP docking to identify the top 20,000 molecules. Subsequently, MM-GBSA decomposition was conducted, and the MIEC model was constructed using the SVM algorithm for further screening. Finally, a total of 51 compounds were selected and evaluated. Therein, C2 exhibited significant inhibition on AR transcriptional activity (IC50 = 0.63 μM) and the proliferation of PCa cell line LNCaP (IC50 = 16.86 μM), while the IC50 values for Enz were 0.20 μM and 15.75 μM, respectively. Like Enz, C2 suppressed PCa cell colony formation and migration. In contrast to Enz, C2 displayed better specificity to GR and PR and stronger ability to induce the arrest of cell cycle at low concertation of 0.5 μM. Our study extends the application of MIEC-SVM model and provides a valuable strategy for developing novel AR antagonists with more effectiveness.
Materials and methods
Dataset and preparation
In this study, the MIEC-SVM model was used for the AR drug design. We used the crystal structure of PDB code 1Z95 [25] as the receptor for the MIEC model construction, where the mutation site of L741 was manually mutated back to W741. 310 active compounds (Ki ≤ 10 μM) targeting the LBP of AR were collected from the BindingDB database [26], and 31,000 molecules were randomly sampled from the ChemDiv database for using as decoy dataset, making the ratio of actives and inactives of 1:100. All the ligands were prepared with the LigPrep module in Schrodinger/2018 by using the default parameters [27].
Molecular docking
To derive the MIEC spectrum, protein–ligand interactions should be predicted at first. Here, the standard precision of Glide docking (Glide SP) [28–30] was used to derive the protein-ligand complex. And the docking box was set to 20 Å × 20 Å × 20 Å with the box center located at the center of mass of the co-crystalized ligand. The top1 docking pose of each system was employed as the initial structure for the MIEC spectrum calculation.
Molecular mechanics (MM) minimization
Before MIEC spectrum calculation, each system was optimized at first to relax the unfavorable contacts between the ligand and the receptor arising from the semi-flexible docking scheme in Glide SP. The protein and the ligands were parameterized with ff03 force field [31] and general amber force field (gaff version 1.7), [32] respectively. The AM1-BCC atomic charge [33] was employed for the ligands because of its balanced computational efficiency and accuracy [34]. Octahedral-shaped TIP3P [35] water box was added for each protein-ligand complex with 5 Å extended out of the solute. Counterions of Na+ or Cl− were added to neutralize the redundant charges of the solute. A cutoff of 8 Å was set to handle the short-range electrostatic and van der Waals interactions, while the Particle mesh Ewald (PME) [36] algorithm was employed to deal with the long-range electrostatic interactions.
A four-step MM minimization protocol was utilized to optimize the systems [37, 38], including (1) optimizing the hydrogen atoms with all the heavy atoms constrained at 5 kcal/mol·Å2; (2) releasing the heavy atoms in solvent (oxygen atoms in water and ions) additionally with heavy atoms in solute constrained at 5 kcal/mol·Å2; (3) minimizing the sidechain of the protein residues and ligand in addition with the heavy atoms in the protein backbone constrained at 5 kcal/mol·Å2; and (4) optimizing the whole system without any restraints. The first three steps were optimized for 1000 steps (500 cycles of steepest descent and 500 cycles of conjugate gradient minimization), while the last step was minimized for 3000 steps (1000 cycles of steepest descent and 2000 cycles of conjugate gradient minimization). All the MM minimizations were performed with the pmemd module in AMBER/14.
MIEC spectrum calculation
MM/GBSA decomposition was employed to calculate the pairwise residue-ligand interactions (∆Gresidue-ligand, Eq. 1), namely the MIEC spectrum, where the modified GB model developed by Onufriev et al. [39] and the ICOSA [40] algorithm were employed for the polar (∆GGB, εin = 1) and non-polar (∆GSA) solvation energy calculations. All the MM/GBSA decompositions were calculated with the MMPBSA.py [41] module in AMBER/14.
| 1 |
Model construction
In the MIEC model construction, the whole dataset was randomly split into 1:1 for using as the training and test sets (namely, 155 active compounds + 15,500 decoy compounds for both the training set and the test set). To prevent overfitting of the machine learning model, here we only employed the total energy of each residue-ligand pair within 5 Å of the co-crystalized ligand for the MIEC spectrum construction, and 28 residues were incorporated that represent the key residues for protein-ligand interaction in the LBP of AR. The SVM algorithm [42, 43] implemented in libsvm package [44] was employed for the model construction. The Radial Basis Function (RBF) was used as the kernel function in SVM with the hyperparameters cost (c) and gamma (γ) optimized with the grid-searching algorithm. Specifically, the hyperparameter searching on c and γ was designed exponentially growing against 2, namely 2n, where n denotes c or γ that goes from -2 to 10 or -10 to 2, respectively. The grid space was set to 0.5 for both c and γ. Thereby, a total of 525 models were constructed (the Model Optimization panel in Fig. 2). All the models were built based on 5-fold cross-validation using the Matthews Correlation Coefficient (MCC) value as the optimization objective. Besides, the ROC-AUC value and enrichment factor were also calculated on the test set to give a comparison.
Fig. 2.
Workflow of the MIEC-SVM machine learning model construction and virtual screening.
Virtual screening
The best-performed MIEC-SVM model was employed for the virtual screening of ChemDiv database (~3,500,000 compounds). All the compounds were screened at first with Glide HTVS docking, and then the top 500,000 compounds scored by Glide HTVS algorithm were submitted to Glide SP docking with the default parameters. Furthermore, the top 20,000 molecules ranked by the Glide SP score were submitted for MM minimization followed by MM/GBSA decomposition to derive the MIEC spectra, and afterwards all the retained molecules were screened by the MIEC-SVM model. Finally, the top 500 molecules ranked by the active compound-probability predicted by the MIEC model were clustered into 60 groups, and the top1 molecule in each group was purchased for experimental test (a total of 51 compounds available for purchase were tested).
Plasmids, cell lines, and cell culture
The plasmid of pGL4.36-MMTV was constructed by inserting MMTV fragment into pGL4.36[luc2P Hyg] (#E6731, Promega). pCMV-GR11 (#89105, Addgene) and pcDNA3-PRB (#89130, Addgene) were gifts from Elizabeth Wilson. The plasmid of pCMV-hMR was constructed by inserting MR fragment into pCMV-GR11 by the technology of seamless cloning (#C112-01, Vazyme). The cell lines LNCaP, 22Rv1, DU145, PC3, C4-2B, Ges-1 and Chang were cultured in phenol red RPMI-1640 medium (#R8758, Sigma-Aldrich) with 10% fetal bovine serum (FBS, #10099141C, Gibco). HEK293T cells were grown in DMEM (#BC-M-005, Bio-Channel) with 10% FBS. All the cultural media contained 1% Penicillin-Streptomycin solution (#C0222, Beyotime). All cell lines were cultured at 37 °C in 5% CO2 atmosphere. Dextran-coated charcoal (DCC) method was used to remove majority of hormones, growth factors and cytokines in FBS. Phenol red-free RPMI-1640 media and DMEM media containing 5% DCC-stripped serum-starvation (CSS) were used for starvation treatment.
AR transcriptional activity assay
The cells of LNCaP-ARR2PB-eGFP were used [9]. At first, the cells were subjected to a starvation period of 5 days in phenol red-free RPMI-1640 medium supplemented with 5% CSS. After starvation, the cells were plated in a 96-well plate with 3 × 104 cells/well, and cultured at 37 °C in 5% CO2 atmosphere. After 24 h, adhesive cells on plates were treated with test compounds at 10 μM or gradient concentrations (0‒50 μM) together with 5 nM DHT for 3 days. In agoniztic model assays, only a gradient concentration of compounds was required. Finally, the fluorescence intensities were measured by Synergy H1(BioTek. Excitation, 485 nm; Emission, 535 nm). Control wells with DMSO or DHT were included on each plate to define the 100% and 0% inhibition, respectively. Raw data values were transformed to % inhibition by the following calculation formula:
| 2 |
AR LBP competitive binding assay
The PolarScreen Androgen Receptor Competitor Assay Kit, Green (#A15880, Thermo Scientific), was employed to determine the AR LBP competitive binding affinity. Gradient concentrations of test compounds were prepared in opaque 384-well plates. The plates were protected from light and incubated at room temperature for 4 h. After that, the fluorescence polarization value (mP) was measured by Synergy H1 (BioTek, Excitation: 485 nm; Emission: 535 nm). Each plate included control wells with DHT or DMSO to establish the 100% and 0% effect, respectively. The calculation formula for compound % affinity in Table 2 is expressed as:
| 3 |
Table 2.
The competitive binding affinity to AR LBP and antagonistic activity of 51 compounds.
| Comp. ID | Competitive binding affinity (% control) ± SEM | Antagonistic activity (% control) ± SEM | Comp. ID | Competitive binding affinity (% control) ± SEM | Antagonistic activity (% control) ± SEM |
|---|---|---|---|---|---|
| C1 | 148.67 ± 17.79 | 27.30 ± 5.06 | C27 | −0.76 ± 4.36 | 8.98 ± 1.82 |
| C2 | 50.19 ± 4.13 | 87.48 ± 9.11 | C28 | 0.00 ± 4.08 | −35.76 ± 25.34 |
| C3 | 65.02 ± 1.78 | −614.94 ± 112.60 | C29 | −0.38 ± 5.43 | −70.69 ± 5.50 |
| C4 | NA | −107.52 ± 4.36 | C30 | 3.04 ± 7.78 | −26.50 ± 1.42 |
| C5 | 3.042 ± 15.89 | −15.03 ± 22.18 | C31 | 2.66 ± 8.09 | −12.11 ± 4.03 |
| C6 | 58.56 ± 4.47 | 36.78 ± 11.04 | C32 | 1.52 ± 11.42 | −60.15 ± 42.6 |
| C7 | −7.22 ± 3.87 | −129.79 ± 21.17 | C33 | 45.25 ± 12.69 | −51.60 ± 3.38 |
| C8 | 12.92 ± 3.38 | 62.55 ± 11.96 | C34 | 9.89 ± 8.94 | 36.34 ± 2.402 |
| C9 | 7.22 ± 1.94 | −28.62 ± 19.57 | C35 | 24.72 ± 2.31 | −1.942.49 |
| C10 | 1.90 ± 2.16 | −103.65 ± 8.83 | C36 | 14.45 ± 5.38 | −2.95 ± 2.67 |
| C11 | −3.04 ± 1.94 | -46.72 ± 24.79 | C37 | 9.12 ± 1.16 | −65.68 ± 3.40 |
| C12 | −3.04 ± 5.43 | −151.44 ± 23.66 | C38 | 17.87 ± 7.24 | −62.49 ± 2.83 |
| C13 | −6.84 ± 2.01 | −48.68 ± 22.76 | C39 | −12.93 ± 2.05 | −131.39 ± 26.2 |
| C14 | 38.78 ± 2.42 | 63.53 ± 10.39 | C40 | −15.59 ± 1.02 | −55.58 ± 2.14 |
| C15 | −10.65 ± 8.66 | 39.60 ± 19.46 | C41 | −7.60 ± 2.16 | 1.25 ± 3.58 |
| C16 | −18.25 ± 4.65 | −73.53 ± 11.95 | C42 | 3.80 ± 11.83 | −12.15 ± 2.13 |
| C17 | 4.18 ± 10.28 | 52.73 ± 3.71 | C43 | −13.69 ± 0.01 | 9.76 ± 6.50 |
| C18 | −1.90 ± 10.41 | −87.43 ± 11.02 | C44 | 0.76 ± 1.69 | −85.19 ± 2.77 |
| C19 | −5.32 ± 15.63 | −108.80 ± 6.85 | C45 | 75.29 ± 4.40 | −87.32 ± 2.14 |
| C20 | −8.36 ± 6.52 | −90.05 ± 22.91 | C46 | 0.38 ± 1.40 | 18.69 ± 4.72 |
| C21 | 22.81 ± 11.09 | −109.80 ± 1.67 | C47 | 12.93 ± 2.16 | −0.66 ± 3.32 |
| C22 | 40.68 ± 5.38 | −137.20 ± 3.71 | C48 | 5.70 ± 0.67 | −14.59 ± 7.02 |
| C23 | 23.57 ± 8.45 | −1.17 ± 3.23 | C49 | 6.46 ± 0.39 | −30.31 ± 1.00 |
| C24 | 66.92 ± 1.55 | −41.01 ± 5.90 | C50 | 3.42 ± 2.93 | 0.89 ± 2.10 |
| C25 | 1.90 ± 0.39 | −122.30 ± 20.29 | C51 | 3.42 ± 0.67 | −70.55 ± 1.64 |
| C26 | 23.57 ± 4.71 | 0.28 ± 14.76 | Enz | 87.07 ± 0.93 | 100.00 ± 0.14 |
Biolayer interferometry (BLI) assay
The expression and purification of human AR LBD protein were performed as described previously [8]. The purified AR LBD protein was biotinylated using a Genemore Biotinylation Kit (#G-MM-IGT, GENEMORE). To assess the interaction between the small molecules and AR LBD, a BLI assay was performed using the Octet K2 apparatus (FortéBio). The experimental procedure involved several steps. Initially, a baseline acquisition was conducted followed by immobilization of the protein onto Octet® SSA biosensors (#18-5057, Sartorius) at a concentration exceeding 0.2 μg/μL to achieve an immobilization level of up to 5 nm. Sensors without protein served as controls during subsequent analysis. Then for the association of C2, interaction assays were carried out at 30 °C with various concentrations of C2, ranging from 6.25 to 100 μM, in PBS supplemented with 0.02% Tween 20 (PBST). The final step was the dissociation of C2 in PBST. Data analysis was performed using FortéBio Data Analysis 7.0 software (Pall FortéBio) with double reference subtraction. Further processing and visualization of the results were carried out using GraphPad 9.0 software.
Cell proliferation and cytotoxicity assay
In general, the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) colorimetric assay was performed. The PCa cell lines including LNCaP, C4-2B, PC3, 22Rv1, and DU145, as well as non-cancerous Chang and Ges-1 cells were utilized. The cell lines were seeded in 96-well plates with RPMI-1640 medium containing 10% FBS. The seeding densities for each cell line were as follows: LNCaP (5×103 cells/well), C4-2B (3.5×103 cells/well), PC3 (5×103 cells/well), 22Rv1 (6×103 cells/well), DU145 (3.5×103 cells/well), Chang (5×103 cells/well), and Ges-1 (5×103 cells/well). After allowing the cells to attach at 37 °C for 24 h, various concentrations of Enz and C2 were administered. Following a 3-day treatment period, 10 μL of MTT solution (5 mg/mL in PBS) was added to each well and incubated for 3-4 h. Subsequently, a 100 μL of triplex mixture containing 10% sodium dodecyl sulfate (SDS), 5% isobutyl alcohol, and 0.012 mol/L hydrochloric acid (HCl) was added to dissolve the formazan crystals generated by viable cells for over 12 h to allow complete dissolution. Finally, the absorbance at 570 nm was measured with the reference wavelength at 650 nm using a spectrophotometer (Eon, Bioteck).
q-PCR
LNCaP cells were cultured in 5% CSS RPMI-1640 without phenol-red for 3 days. The starved cells were seeded in 6-well plates (3×105 cells/well) and incubated for 24 h. For treatment, two compounds, Enz or C2, were administered at concentrations of either 0.5 or 5 μM, along with 5 nM DHT, while a control group received DMSO alone. After 48 h of treatment, total mRNA was extracted using EZ-10 DNA away RNA Mini-Preps Kit (#B618133, Sangon Biotech) according to the manufacturer’s protocol. Then cDNA was prepared using Hifair III 1st Strand cDNA Synthesis SuperMix for qPCR (#11141ES10, YEASEN). Subsequently, the cDNA was mixed with qPCR SYBR Green Master Mix (#11202ES03, YEASEN) and specific oligonucleotides and subjected for q-PCR. The entire experiment and data processing were conducted according to the operation manuals and the relative mRNA levels were calculated using the 2–ΔΔCt method based on the control of GAPDH (QuantStudio 3, Applied Biosystems, Graphpad Prism 9).
Western blot assay
LNCaP cells were starved in phenol-red-free RPMI-1640 (5% CSS) for 3 days and then cultured in 6-well plates (3×105 cells/well). After incubating at 37 °C for 24 h, Enz or C2 (at concentrations of 0, 0.5 or 5 μM) was added to plates along with 5 nM DHT. The control group was treated with DMSO alone. After 48 h of treatment, the cells were lysed in cell lysis buffer (#P0013, Beyotime), collected in tubes, and boiled at 100 °C for 5 to 10 min. Subsequently, these lysates were subjected to 10% SDS-polyacrylamide gel electrophoresis for Western blot analysis. Proteins in gel were transferred onto the PVDF membranes (#IPFL00005, Millipore). The membranes were then probed with rabbit anti-AR (#5153, Cell Signaling Technology (CST), 1:10000), rabbit anti-PSA/KLK3 (#5356, CST, 1:2000), along with β-actin rabbit mAb (#4970, CST, 1:1500). After that, membranes were incubated with anti-rabbit HRP-conjugated secondary anti-bodies (# D110058-0100, Sangon Biotech,1:5000) and the protein bands were visualized using Super ECL Detection Reagent (#36208ES60, YEASEN).
To synchronize the cell cycle, LNCaP cells were incubated in 1640 medium supplemented with 0.5% FBS overnight. Following this, the medium was replaced with complete medium (2 mL per well) containing various concentrations of C2 (0, 20, 40, 60, 80, and 100 μM). After 48 h, the cells were collected for a Western blot assay using the same procedure described above. Proteins were probed with rabbit anti-p27 (#25614-1-AP, Proteintech, 1:5000), rabbit anti-CDK2 (#10122-1-AP, Proteintech, 1:1000), rabbit anti-Cyclin D1 (#26939-1-AP, Proteintech, 1:5000) and rabbit anti-Lamin B1 (#AF1408, Beyotime, 1:1000).
Secreted PSA assays
Secreted PSA levels were evaluated after the LNCaP-ARR2PB-eGFP cellular AR transcription assay. The supernatant from each sample was collected and sent to Cancer Hospital of University of Chinese Academy of Sciences, Zhejiang Cancer Hospital (Hangzhou, Zhejiang, China). The IMMULITE 2000 XPi Immunoassay System (Siemens Ltd. Erlangen, Germany) was used for analyzing PSA.
Nuclear and cytoplasmic protein extraction and analysis
LNCaP cells were seeded at a density of 1 × 106 cells. They were treated with either 10 μM Enz or C2 along with 5 nM DHT for 16 h. The extraction of nuclear and cytoplasmic proteins from LNCaP cells was performed using NE-PER Nuclear and Cytoplasmic Extraction Reagents (#78835, Thermo Fisher) according to its User’s Guide. The nuclear and cytoplasmic proteins were then analyzed by Western blot assay with rabbit anti-AR (#5153, CST, 1:10000), rabbit anti-β-Tubulin (#2128, CST, 1:1000) and rabbit anti-Lamin B1 (#AF1408, Beyotime, 1:1000).
Cell colony assay
A density of 1.5 × 103 cells/well of LNCaP cells was cultured in a 6-well plate. Following incubation for a period of two weeks with DMSO, Enz (0.5 and 5 μM), or C2 (0.5 and 5 μM), the growth medium was carefully removed, and the cell colonies on the plate were washed with PBS. The cell colonies were fixed with methanol (#10014108, Sinopharm) at room temperature for 30 min, and subsequently stained with crystal violet (#C0121, Beyotime) for 30 min. The colony images were captured by the Fusion FX SPECTRA (Vilber).
Wound healing assay
1 × 106 LNCaP cells were cultured in six-well plates with complete medium. Once the cells reached 90% confluence, a wound was intentionally created at the center using a 200 μL micropipette tip. The cells were then washed trice with PBS to eliminate any debris from the wound. After culturing in 1% FBS 1640 medium for 2 h at 37 °C, the cells were incubated with 0, 0.5 or 5 μM Enz or C2. Then, the migration into the scratched area was evaluated and imaged using an inverted microscope at time points 0, 12, and 24 h (#CKX41, Olympus).
Transwell assay
An alternative method employed to evaluate cell migration involves the utilization of a Transwell chamber with an 8 μm pore size (#14141, LABSELECT). In brief, cells in the logarithmic growth phase were enzymatically detached, washed with PBS, resuspended in 0.5% FBS-1640 medium, and the cell density was adjusted to 1 × 105. Then, 400 μL of cell suspension was added to the upper compartment of a six-well Transwell culture chamber. The lower compartment was filled with 1.5 mL of 10% FBS-1640 medium. Following 1 h incubation at 37 °C to allow for cell adherence, DMSO, 0.5 μM Enz, 5 μM Enz, 0.5 μM C2 or 5 μM C2 were added into both the upper and lower mediums, and co-cultured for 48 h. To remove non-migratory cells, a cotton swab was used to wipe away the cells on the upper side of the filter, and those on the reverse side of the filter were fixed in methanol (#10014108, Sinopharm) for 30 min. Then, these cells were stained with crystal violet (#C0121, Beyotime) for 30 min and counted using an inverted microscope (#CKX41, Olympus).
Luciferase reporter assays
HEK293T cells were cultured overnight in opaque 96-well plates using 5% DCC DMEM at a density of 1.2 × 104 cells/well. For progesterone receptor (PR), mineralocorticoid receptor (MR) and glucocorticoid receptor (GR) induction assays, the HG-TransGene transfection reagent (#TG-10012, Genomeditech) was used to transfect HEK293T cells with 70 ng of pCMV-GR11, pCMV-MR, or pcDNA3-PRB per well. Additionally, 24 ng of pGL4.36-MMTV and 5 ng of Renilla were co-transfected into the cells. The transfection process was carried out for 6 h. For antagonistic models, the cells were stimulated with 50 nM dexamethasone for GR assays, 5 nM aldosterone for MR assays, and 5 nM progesterone for PR assays, respectively. The compounds were triple-diluted from 50 μM and added to the cells. In agoniztic model assays, only a gradient concentration of compounds was required. The luciferase activity was measured by the Dual-Glo Luciferase system (#E1910, Promega).
Flow cytometry for cell cycle analysis
The effect of C2 on the cell cycle was assessed through flow cytometry analysis. When reaching 50%‒60% confluence, LNCaP cells were incubated in 0.5% FBS 1640 medium overnight to synchronize the cell cycle. Then, the medium was removed, and Enz or C2 (at concentrations of 0, 0.5, and 5 μM) mixed with 2 mL of complete medium was added to each well. After 48 h, cells were collected, washed with 4 °C PBS and fixed with 70% ethanol for 2 h at 4 °C. Following fixation, the cells were treated with PI and RNase A in the dark at 25 °C for 30 min using the Cell Cycle and Apoptosis Analysis Kit (#40301ES50, YEASEN) according to the provided protocol. Samples were analyzed by CytoFLEX LX (BECKMAN COULTER), collecting 10,000 events for each sample.
Statistical analysis
All data were analyzed by GraphPad Prism 9 software (GraphPad Software, La Jolla, CA, USA). Results were presented as mean ± SD, and P < 0.05 was considered as significant.
Results and discussion
Establishment of the MIEC-SVM model
In our study, the workflow of the SBVS protocol is emerged in Fig. 2.
Here we employed the MIEC algorithm for model construction. As shown in Table 1, high performance is shown of the MIEC-SVM model in the AR system with the best MCC values of 0.786 and 0.785 on the training and test sets, respectively, implying that the constructed model is robust enough for downstream tasks. Moreover, the ROC-AUC value and the enrichment factor were also calculated on the test set (Table 1), where the MIEC-SVM model shows remarkably better results than the traditional docking results (Glide SP) with the ROC-AUC of 0.941 versus 0.857 (Fig. 3a), and the average enrichment factor ~70% versus ~25% (averaged from the top 20 to 140 molecules, Fig. 3b) for the MIEC-SVM model and Glide SP docking, respectively. These findings suggest that the specially designed MIEC-SVM model is a superior solution for drug design compared with the traditional method (at least in the AR system). Therefore, we employed the best-performed MIEC model for the VS of AR antagonists in our study. In total, 51 compounds were selected and subjected to bioassays.
Table 1.
Performance of the best-performed MIEC-SVM model and the comparison between Glide-SP docking on the test set.
| MCCtrain | SEtest | SPtest | MCCtest | ROC-AUCtest | |
|---|---|---|---|---|---|
| MIEC-SVM | 0.786 | 0.892 | 0.996 | 0.785 | 0.941 |
| Glide-SP | - | 0.857 | |||
Fig. 3. Performance of the best-performed MIEC model.
a, b The ROC curve (a) and enrichment factor (b) of the test set were compared with the results of MIEC model and Glide SP docking shown in green and orange lines, respectively, in the two panels.
Discovery of C2 from 51 selected candidates
The initial evaluation involved testing the 51 compounds for their ability to displace a potent fluorescent ligand (fluorophore) at the AR LBP. We used a fluorescence polarization (FP) assay and set the competition ability of the endogenous androgen DHT as a 100% control. The antiandrogen Enz displayed a competition of 87.02% in this assay. Among the 51 compounds, C1, C2, C3, C6, C24 and C45 exhibited considerable binding affinity to AR LBP, with competition percentages of 148.67%, 50.19%, 65.02%, 58.56%, 66.92%, and 75.29%, respectively, at a concentration of 10 μM. This indicates that these compounds adopt a similar LBP-mediated mechanism as Enz (Table 2). Subsequently, the 51 compounds were assessed using an AR transcriptional activity assay [9]. The antagonistic activity of 100% was defined as the one without DHT induction. Compounds C2, C8, C14, and C17 demonstrated anti-androgen effects of over 50% at 10 μM, and Enz reached 100% under the same condition (Table 2). For the other identified binders, as shown in Figure S1, C3 demonstrated robust agonistic activity, and C45 displayed weaker agonistic activity at higher concentrations above 10 μM. And C1, C6 and C24 were ineffective in suppressing AR transcriptional activity (Fig. S1). Taken together, C2 displayed considerable competitive binding affinity (50.19% of DHT) and antagonistic activity (87.48% of Enz) at 10 μM, making it a promising candidate for further comprehensive investigation.
Predicted binding mechanism of C2 to AR
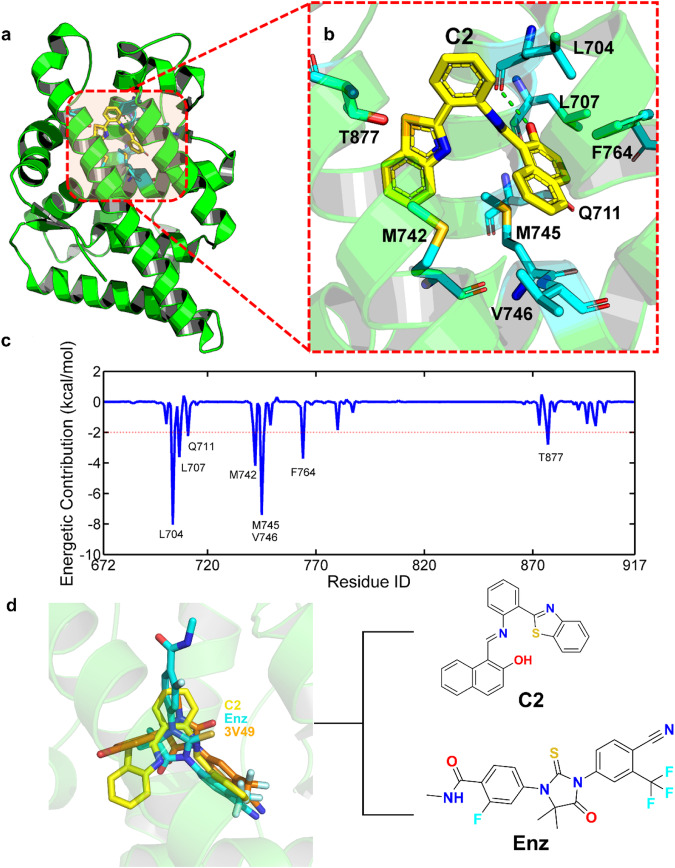
Molecular dynamics simulations were further performed to predict the dynamic binding behavior of C2 to the AR LBP. The binding mode (panels A and B) and energetic contribution of the key residues (pane c) to compound C2 were illustrated in Fig. 4, where residues with the energetic contribution larger than 2 kcal/mol to C2 were identified as key residues (red dotted line in Fig. 4c), and 8 residues were identified (Fig. 4b, c). Further analysis reveals the crucial role of hydrophobic interactions in the binding of compound C2, with 6 out of 8 of the key residues being hydrophobic amino acids (L704, L707, M742, M745, V746 and F764 in Fig. 4c). Moreover, a hydrogen bond was observed between the backbone oxygen atom of L704 and the hydroxyl group of C2 (green dotted line in Fig. 4b), further stabilizing the ligand to bind with the LBP of AR. Compound C2 and Enz were aligned with the crystal structure of 3V49, which had been co-crystallized with a similar compound to Enz. The Fig. 4d shows the binding poses of compound C2 (highlighted in yellow), Enz (highlighted in cyan), and the co-crystallized ligand (highlighted in orange) in crystal structure (PDB ID: 3V49) to the LBP of AR. The figure demonstrates a significant overlap between compound C2 and Enz with the co-crystallized ligand, particularly in the hetero-aromatic fragment, located in the exact position within the LBP. Moreover, despite the dissimilar scaffold of C2 compared to the other two ligands, it binds to AR with the center of mass overlapped with the other two ligands as well, indicating the credibility of the binding mode of C2. These findings reveal the basic binding mechanism between C2 and AR, encouraging us to further explore the compound.
Fig. 4. Predicted binding mechanism.
a, b Predicted binding mechanism of C2 to AR. c The corresponding energetic contribution of the key residues. d Binding mode of compound C2 (yellow), Enz (cyan) and the co-crystalized ligand in 3V49 (orange).
C2 significantly inhibited AR signaling
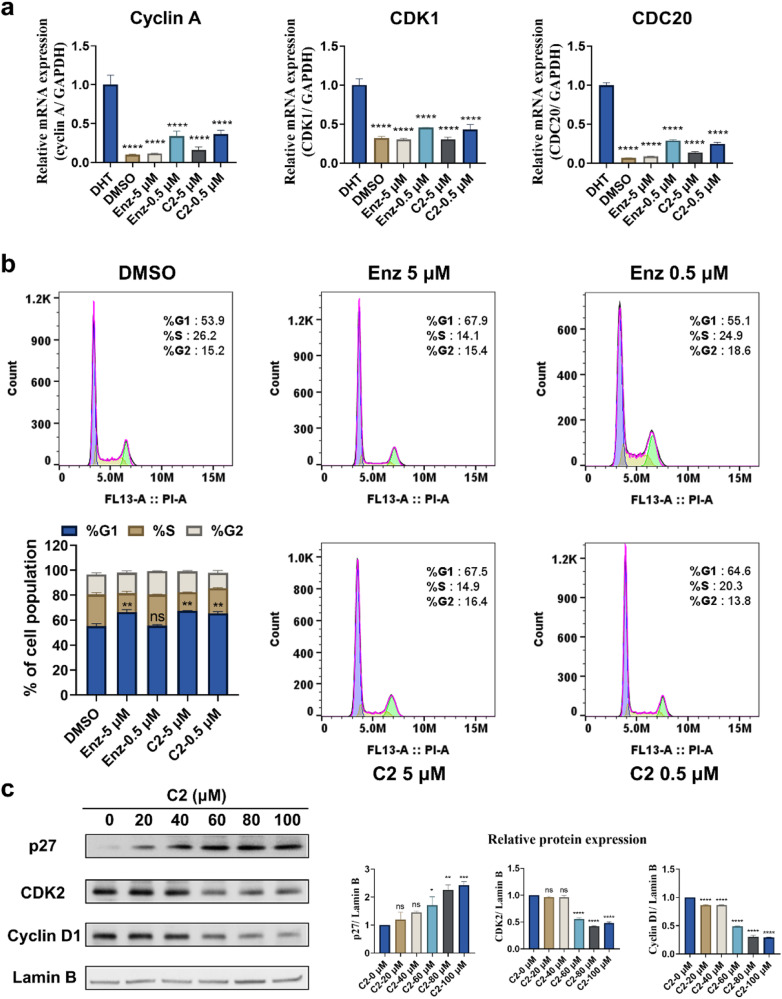
To confirm the inhibition of AR transcriptional activity, compound C2 was further tested at gradient concentrations. It exhibited a dose-dependent manner with an IC50 value of 0.63 μM, while Enz had an IC50 value of 0.20 μM (Fig. 5a). Then the secreted PSA in the transcription assay was examined. It showed that the secretion of PSA decreased also dose-dependently with IC50 values of 0.18 μM, while that of Enz was 0.13 μM (Fig. 5b). Subsequently, AR LBP competitive binding assay was carried out for C2. As shown in Fig. 5c, C2 is competitively bound to AR LBP (IC50 = 10.58 μM), while the IC50 value for Enz was 4.17 μM. To confirm the direct binding of C2 to AR LBD protein, a BLI assay was performed as well. A strong binding affinity of C2 was observed to AR LBD with a KD value of 4.28×10-5 M, while the reported KD for Enz was 4.02 × 10−5 M [8] (Fig. 5d). Further, the antagonistic effects of C2 on the expression of typical AR-regulated genes including KLK3 (PSA), TMPRSS2, FKBP5 and UBE2C were assessed using q-PCR (Fig. 5e). Compared to the cells treated with DHT alone, C2 reduced the DHT-induced mRNA expression of PSA to 20.35% at 5 μM. Furthermore, C2 could drive down the mRNA expression of other AR-regulated genes TMPRSS2, FKBP5 and UBE2C to 36.63%, 40.50% and 28.99%, respectively, at a concentration of 0.5 μM. The inhibitory effect of C2 on PSA protein level was also evident by Western blotting in Fig. 5f. In parallel, C2 had minimal impact on AR mRNA level while Enz had no effect on it. It is suggested that C2 may have additional mechanism of action that could influence AR expression at the mRNA level.
Fig. 5. The effects of C2 on AR signaling.
a The antagonistic activity to AR transcriptional function. b The inhibition on PSA secretion. c The competitive binding affinity to AR LBP. d The BLI assay. e The effects on DHT-induced mRNA expression of PSA, TMPRSS2, FKBP5 and UBE2C at 5 or 0.5 μM in LNCaP cells. The DHT group was normalized to 1. *P < 0.05, ****P< 0.0001 was considered significance compared with DHT control; ns not significant. f The effects on the protein expression of AR and PSA in LNCaP cells. g The AR levels in nuclear and cytoplasmic fractions of LNCaP cells treated with 10 μM Enz or C2. β-tubulin served as the cytoplasmic fraction control, while Lamin B served as the nuclear fraction control. The DHT group was normalized to 1. *P < 0.05, **P < 0.01 versus DHT group; ns not significant.
After being activated by androgens, the AR in cells would translocate into the nucleus to work as a transcription factor. The second generation of AR antagonist Enz inhibits the nuclear translocation of AR [45]. Therefore, to evaluate the effect of C2 on DHT-induced AR translocation, the cytoplasmic and nuclear fractions of LNCaP cells treated by C2 were subjected to Western blot assay. As shown in Fig. 5g, C2, similar to Enz, effectively prevented the translocation of AR to the nucleus compared to the DHT-induced control group. In summary, these results indicated that compound C2 targets AR and suppresses AR signaling.
C2 retarded the cell cycle progression of LNCaP
The effect of C2 on cell cycle progression was then explored via q-PCR assay with Enz as control. The genes associated with cell cycle driven by DHT were analyzed. Similar to Enz, treatment with 5 μM C2 resulted in substantial reduction of the mRNA levels of Cyclin A, CDK1 and CDC20 to 16.19%, 30.83% and 14.00%, respectively (Fig. 6a). In order to figure out how C2 affects cell cycle progression in LNCaP cells, we further conducted flow cytometry experiments. Adherent cells were treated with the indicated concentrations of C2 or Enz. The percentage of cells at the G1 phase was significantly higher in the C2 treatment group than in the DMSO group, indicating that C2 could restrain the cell cycle progression at the G1 phase (Fig. 6b). And the percentage of cells at the S phase was lower in the C2 treatment group compared with the Enz group at the same concentration (0.5 μM), indicating that C2 has a stronger ability to block the cell cycle than Enz at lower dose. Then, to investigate the molecular mechanism underlying the G1 phase arrest induced by C2, the expression level of specific proteins involved in the G1/S checkpoint was analyzed. The proteins of p27, CDK2, and Cyclin D1 were focused. As shown in Fig. 6c, the expression of p27 increased and the levels of Cyclin D1 and CDK2 decreased in a dose-dependent manner. To sum up, these findings suggested that C2 inhibited cell cycle progression of LNCaP cells by inducing G1 phase arrest and the effect was mediated through the regulation of p27, CDK2, and Cyclin D1.
Fig. 6. C2 attenuated the cell cycle progression of LNCaP cells.
a The effect of C2 on DHT-induced mRNA expression of Cyclin A, CDK1, and CDC20. ****P < 0.0001 versus DHT group. b Cycle-synchronized cells treated with DMSO, Enz, and C2, respectively. **P < 0.01 versus DMSO group; ns not significant. c The influence of C2 on the protein expression of p27, CDK2, and Cyclin D1 analyzed by Western blot assay. The C2-0 μM group was normalized to 1.
C2 Suppressed the proliferation of PCa cells like Enz
In vitro tumor inhibition of C2 on PCa cells was evaluated by MTT assay. Five different PCa cell lines were included in the study: LNCaP, an AR-positive and androgen-dependent PCa cell line; C4-2B, an AR-positive but hormone-insensitive PCa cell line; PC3 and DU145, AR-negative cell lines that do not rely on androgens for survival; and 22Rv1, an androgen-independent cell line expressing high levels of AR variants. As shown in Fig. 7a, C2 inhibited LNCaP cells in a dose-dependent manner with an IC50 value of 16.86 μM comparable with that of Enz (IC50 = 15.75 μM). Moreover, C2 inhibited C4-2B cells with an IC50 value of 49.84 μM while that for Enz is 41.66 μM (Fig. 7b). However, treatment with Enz or C2 had minimal effects on the proliferation of PC3, DU145, and 22Rv1 (Fig. 7c–e). These findings suggested that C2 selectively suppressed AR signaling pathway. Then the cytotoxicity of C2 on non-cancerous cells, including human liver cells (Chang) and human gastric epithelial cells (Ges-1), was evaluated. Similar as Enz, C2 exhibited negligible suppression of the cell growth of Chang and Ges-1 under the concentrations lower than 50 μM (Fig. 7f, g), suggesting its tolerability along with its specialized anti-PCa tumor capability. Additionally, a colony formation assay was conducted to assess the long-term growth inhibition effect of C2 on LNCaP cells. After 14 days of treatment, the number and size of the colonies were significantly reduced compared to the DMSO groups, indicating that C2 significantly weakened the clonogenicity of LNCaP cells (Fig. 7h).
Fig. 7. The cytotoxicity of C2 on PCa cell lines.
a‒e Inhibition on five different PCa cell lines. f, g Assessment of the cytotoxicity of C2 and Enz against Chang and Ges-1 cells. h Clonogenic assay of LNCaP treated with DMSO, Enz, and C2, respectively. *P < 0.05, **P < 0.01 versus DMSO group.
C2 inhibited the migration of LNCaP Cells in vitro
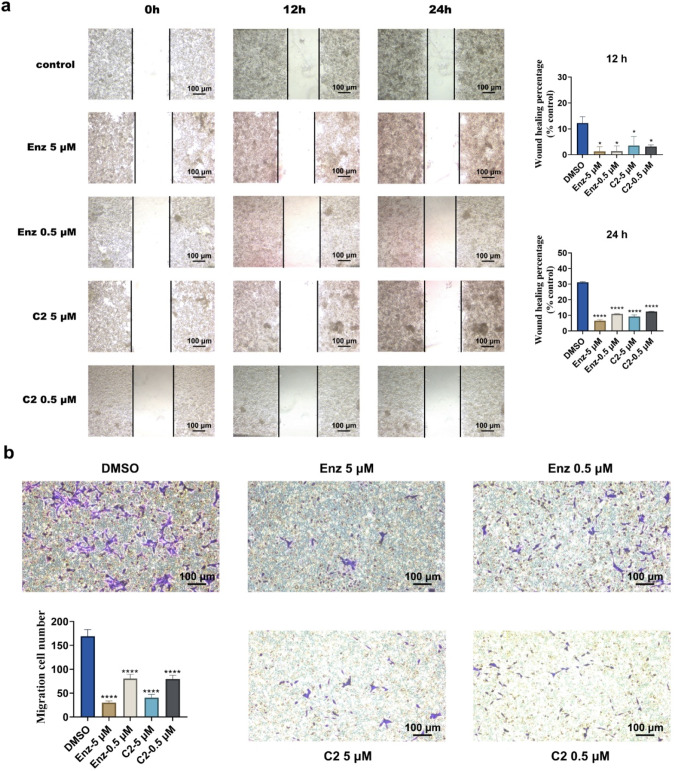
Androgens play a regulatory role in the migration process of PCa cells, which is crucial for initiating metastasis development [46]. Therefore, two distinct approaches were employed to investigate the effect of C2 on the metastasis of LNCaP. Firstly, a wound-healing assay was conducted. As shown in Fig. 8a, after healing for 24 h, the wound in the control group was significantly narrower, while treatment with Enz or C2 exhibited the opposite effects. The wound healing percentages of LNCaP cells treated with 5 and 0.5 μM C2 were 9.20% and 12.30% after 24 h, while that for the DMSO group was 31.45%. Meanwhile, a Transwell migration assay was performed in parallel. Representative images showing the number of cells migrating through the Transwell chamber are presented in Fig. 8b. As expected, C2 significantly impaired the cell motility of LNCaP cells in a dose-dependent manner, with inhibition rates of 11.91% (0.5 μM) and 23.52% (5 μM). These findings suggested that C2 possesses the essential ability to suppress the migration of LNCaP cells and restrain the malignancy of cancer cells.
Fig. 8. C2 inhibited the migration of LNCaP cells.
a Representative images of the wound healing assay. A pipette tip was used to create a scratch wound when the cells reached 70%‒80% confluence. The migration percentage was then counted after 12 h and 24 h. Experiments were performed in duplicate. b Representative images of the Transwell chamber assays after incubation at 37 °C for 48 h. The number of the cells that had migrated through the membrane was counted under a microscope. Three images per well were used to quantify the migrated cells. *P < 0.05, ****P < 0.0001 versus DMSO group.
C2 showed better selectivity than Enz
The LBD sequences of PR, MR, GR and AR exhibit the highest evolutionary conservation among 48 human NRs [11]. To investigate the selectivity of C2 for different NRs and determine its specificity towards AR, we analyzed the LBDs of these four NR by multiple sequence alignment. It showed that the LBDs of PR, MR, and GR shared high sequence identities with that of AR, indicating their similarity at the molecular level (Fig. 9a). We then conducted a luciferase reporter assay to assess the selectivity of C2. As shown in Fig. 9b, spironolactone demonstrated MR antagonistic activity with an IC50 of 0.1 μM, while Enz and C2 displayed negligible antagonistic activity towards MR. In the GR and PR antagonistic model, mifepristone exhibited extremely strong antagonistic activity. However, C2 exhibited no effect on GR and moderate antagonistic activity to PR (IC50 = 5.17 μM), while Enz had weak but obvious inhibitory effect on GR and noticeable antagonistic activity to PR (IC50 = 0.73 μM). As for the agonistic model, neither Enz nor C2 showed any effect towards MR, GR, or PR. In conclusion, C2 demonstrated higher selectivity than Enz.
Fig. 9. Selectivity of C2 to nuclear receptors MR, GR and PR.
a The multiple sequence alignment of AR, PR, MR, and GR. b Assessment of the antagonistic and agonistic activities of Enz and C2 towards MR, GR, and PR.
Conclusion
The AR LBP site, which remains the exclusive binding site for approved nonsteroidal antagonists, continues to hold significant research potential for exploring innovative therapeutic agents against PCa. In our study, an MIEC-SVM model was established and utilized in VS to identify novel antagonists targeting AR LBP. Among 51 candidates selected by the VS, six compounds showed good binding affinity with AR. Notably, compound C2 emerged as a novel AR antagonist with remarkable inhibition of AR signaling and appreciable binding to AR. Then we further investigated the effect of C2 on PCa cell proliferation, colony formation, and migration, as well as on the signal transduction in the cell cycle. At a concentration of 5 μM, C2 suppressed the colony formation of LNCaP cells by 23.57%, and profoundly impaired their motility with inhibition rate reaching up to 11.91% in the Transwell migration assay. Furthermore, C2 inhibited cell cycle progression of LNCaP cells by inducing G1 phase arrest and this outcome was facilitated through the regulation of p27, CDK2, and Cyclin D1. And like Enz, the administration of C2 hindered AR nuclear translocation. In contrast to Enz, C2 exhibited a superior ability to induce the arrest of cell cycle at low concertation of 0.5 μM and demonstrated better selectivity. However, it should be recognized that C2, as a new scaffold AR antagonist, can still be subject to further structural optimization. In addition, the utility of the MIEC-SVM model is constrained by the absence of a crystal structure depicting the antagonistic conformation of AR, thus restricting its ability to substantially increase the hit rate.
In summary, this study represents a successful application of MIES-SVM model for developing novel AR antagonist targeting AR LBP. And C2 stands out as a potential leading compound and a novel scaffold providing important clues for the development of novel therapeutics for PCa.
Supplementary information
Acknowledgements
This research was supported by Zhejiang Provincial Natural Science Foundation of China (LD22H300001, 2023C03110) and the National Natural Science Foundation of China (U21A20301).
Author contributions
DL, TJH, and HYS initiated and supervised the research. XYW, XC, and HYS conducted virtual screening, compound validations and biological assays. LHS, XHX, and LX performed part of in vitro experiments and interpreted part of the data. XYW, XC, HYS, and DL wrote the manuscript, and other authors contributed specific parts of the manuscript. HYS and DL assume responsibility for the manuscript in its entirety. All authors have given approval to the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Contributor Information
Hui-yong Sun, Email: huiyongsun@cpu.edu.cn.
Dan Li, Email: lidancps@zju.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41401-024-01284-x.
References
- 1.Wang G, Zhao D, Spring DJ, DePinho RA. Genetics and biology of prostate cancer. Genes Dev. 2018;32:1105–40. 10.1101/gad.315739.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 3.Rebello RJ, Oing C, Knudsen KE, Loeb S, Johnson DC, Reiter RE, et al. Prostate cancer. Nat Rev Dis Prim. 2021;7:9. 10.1038/s41572-020-00243-0 [DOI] [PubMed] [Google Scholar]
- 4.Li D, Zhou WF, Pang JP, Tang Q, Zhong BL, Shen C, et al. A magic drug target: androgen receptor. Med Res Rev. 2019;39:1485–514. 10.1002/med.21558 [DOI] [PubMed] [Google Scholar]
- 5.Lv S, Song Q, Chen G, Cheng E, Chen W, Cole R, et al. Regulation and targeting of androgen receptor nuclear localization in castration-resistant prostate cancer. J Clin Invest. 2021;131:e141335. [DOI] [PMC free article] [PubMed]
- 6.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–9. 10.1038/nm972 [DOI] [PubMed] [Google Scholar]
- 7.Zhang B, Zhang M, Yang Y, Li Q, Yu J, Zhu S, et al. Targeting KDM4A-AS1 represses AR/AR-Vs deubiquitination and enhances enzalutamide response in CRPC. Oncogene. 2022;41:387–99. 10.1038/s41388-021-02103-x [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Chai X, Sun H, Zhou W, Chen C, Shan L, Yang Y, et al. Discovery of N-(4-(Benzyloxy)-phenyl)-sulfonamide derivatives as novel antagonists of the human androgen receptor targeting the activation function 2. J Med Chem. 2022;65:2507–21. 10.1021/acs.jmedchem.1c01938 [DOI] [PubMed] [Google Scholar]
- 9.Zhou W, Duan M, Fu W, Pang J, Tang Q, Sun H, et al. Discovery of novel androgen receptor ligands by structure-based virtual screening and bioassays. Genom Proteom Bioinform. 2018;16:416–27. 10.1016/j.gpb.2018.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munuganti RS, Hassona MD, Leblanc E, Frewin K, Singh K, Ma D, et al. Identification of a potent antiandrogen that targets the BF3 site of the androgen receptor and inhibits enzalutamide-resistant prostate cancer. Chem Biol. 2014;21:1476–85. 10.1016/j.chembiol.2014.09.012 [DOI] [PubMed] [Google Scholar]
- 11.Fu W, Yang H, Hu C, Liao J, Gong Z, Zhang M, et al. Small-molecule inhibition of androgen receptor dimerization as a strategy against prostate cancer. ACS Cent Sci. 2023;9:675–84. 10.1021/acscentsci.2c01548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh SM, Gauthier S, Labrie F. Androgen receptor antagonists (antiandrogens): structure-activity relationships. Curr Med Chem. 2000;7:211–47. 10.2174/0929867003375371 [DOI] [PubMed] [Google Scholar]
- 13.Kim W, Ryan CJ. Androgen receptor directed therapies in castration-resistant metastatic prostate cancer. Curr Treat Options Oncol. 2012;13:189–200. 10.1007/s11864-012-0188-2 [DOI] [PubMed] [Google Scholar]
- 14.Moilanen AM, Riikonen R, Oksala R, Ravanti L, Aho E, Wohlfahrt G, et al. Discovery of ODM-201, a new-generation androgen receptor inhibitor targeting resistance mechanisms to androgen signaling-directed prostate cancer therapies. Sci Rep. 2015;5:12007. 10.1038/srep12007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bambury RM, Scher HI. Enzalutamide: development from bench to bedside. Urol Oncol. 2015;33:280–8. 10.1016/j.urolonc.2014.12.017 [DOI] [PubMed] [Google Scholar]
- 16.Song CH, Yang SH, Park E, Cho SH, Gong EY, Khadka DB, et al. Structure-based virtual screening and identification of a novel androgen receptor antagonist. J Biol Chem. 2012;287:30769–80. 10.1074/jbc.M112.379107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang Q, Fu W, Zhang M, Wang E, Shan L, Chai X, et al. Novel androgen receptor antagonist identified by structure-based virtual screening, structural optimization, and biological evaluation. Eur J Med Chem. 2020;192:112156. 10.1016/j.ejmech.2020.112156 [DOI] [PubMed] [Google Scholar]
- 18.Fu W, Zhang M, Liao J, Tang Q, Lei Y, Gong Z, et al. Discovery of a novel androgen receptor antagonist manifesting evidence to disrupt the dimerization of the ligand-binding domain via attenuating the hydrogen-bonding network between the two monomers. J Med Chem. 2021;64:17221–38. 10.1021/acs.jmedchem.1c01287 [DOI] [PubMed] [Google Scholar]
- 19.Hard R, Li N, He W, Ross B, Mo GCH, Peng Q, et al. Deciphering and engineering chromodomain-methyllysine peptide recognition. Sci Adv. 2018;4:eaau1447. 10.1126/sciadv.aau1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hou T, Zhang W, Case DA, Wang W. Characterization of domain-peptide interaction interface: a case study on the amphiphysin-1 SH3 domain. J Mol Biol. 2008;376:1201–14. 10.1016/j.jmb.2007.12.054 [DOI] [PubMed] [Google Scholar]
- 21.Hou T, Xu Z, Zhang W, McLaughlin WA, Case DA, Xu Y, et al. Characterization of domain-peptide interaction interface: a generic structure-based model to decipher the binding specificity of SH3 domains. Mol Cell Proteom. 2009;8:639–49. 10.1074/mcp.M800450-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou TJ, Li N, Li YY, Wang W. Characterization of domain-peptide interaction interface: prediction of SH3 domain-mediated protein-protein interaction network in yeast by generic structure-based models. J Proteome Res. 2012;11:2982–95. 10.1021/pr3000688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun H, Pan P, Tian S, Xu L, Kong X, Li Y, et al. Constructing and validating high-performance MIEC-SVM models in virtual screening for kinases: a better way for actives discovery. Sci Rep. 2016;6:24817. 10.1038/srep24817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Q, Wang Z, Tian S, Wang L, Tang R, Yu Y, et al. Determination of molecule category of ligands targeting the ligand-binding pocket of nuclear receptors with structural elucidation and machine learning. J Chem Inf Model 2022;62:3993–4007. 10.1021/acs.jcim.2c00851 [DOI] [PubMed] [Google Scholar]
- 25.Bohl CE, Gao W, Miller DD, Bell CE, Dalton JT. Structural basis for antagonism and resistance of bicalutamide in prostate cancer. Proc Natl Acad Sci USA. 2005;102:6201–6. 10.1073/pnas.0500381102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu T, Lin Y, Wen X, Jorissen RN, Gilson MK. BindingDB: a web-accessible database of experimentally determined protein–ligand binding affinities. Nucleic Acids Res. 2007;35:D198–D201. 10.1093/nar/gkl999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schrödinger, version 9.0, Schrödinger, LLC, New York, NY, 2009. http://www.schrodinger.com.
- 28.Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA, et al. Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein−ligand complexes. J Med Chem. 2006;49:6177–96. 10.1021/jm051256o [DOI] [PubMed] [Google Scholar]
- 29.Halgren TA, Murphy RB, Friesner RA, Beard HS, Frye LL, Pollard WT, et al. Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J Med Chem. 2004;47:1750–9. 10.1021/jm030644s [DOI] [PubMed] [Google Scholar]
- 30.Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, et al. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem. 2004;47:1739–49. 10.1021/jm0306430 [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Cieplak P, Kollman PA. How well does a restrained electrostatic potential (RESP) model perform in calculating conformational energies of organic and biological molecules? J Comput Chem. 2000;21:1049–74. [DOI] [Google Scholar]
- 32.Wang J, Wolf RM, Caldwell JW, Kollman PA, Case DA. Development and testing of a general amber force field. J Comput Chem. 2004;25:1157–74. 10.1002/jcc.20035 [DOI] [PubMed] [Google Scholar]
- 33.Jakalian A, Jack DB, Bayly CI. Fast, efficient generation of high-quality atomic charges. AM1-BCC model: II. Parameterization and validation. J Comput Chem. 2002;23:1623–41. 10.1002/jcc.10128 [DOI] [PubMed] [Google Scholar]
- 34.Xu L, Sun H, Li Y, Wang J, Hou T. Assessing the performance of MM/PBSA and MM/GBSA methods. 3. The impact of force fields and ligand charge models. J Phys Chem B. 2013;117:8408–21. 10.1021/jp404160y [DOI] [PubMed] [Google Scholar]
- 35.Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. Comparison of simple potential functions for simulating liquid water. J Chem Phys. 1983;79:926–35. 10.1063/1.445869 [DOI] [Google Scholar]
- 36.Darden T, York D, Pedersen L. Particle mesh Ewald: an N log(N) method for Ewald sums in large systems. J Chem Phys. 1993;98:10089–92. 10.1063/1.464397 [DOI] [Google Scholar]
- 37.Tang R, Chen P, Wang Z, Wang L, Hao H, Hou T, et al. Characterizing the stabilization effects of stabilizers in protein–protein systems with end-point binding free energy calculations. Brief Bioinform. 2022;23:bbac127. 10.1093/bib/bbac127 [DOI] [PubMed] [Google Scholar]
- 38.Yu Y, Wang Z, Wang L, Tian S, Hou T, Sun H. Predicting the mutation effects of protein-ligand interactions via end-point binding free energy calculations: strategies and analyses. J Cheminform. 2022;14:56. 10.1186/s13321-022-00639-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Onufriev A, Bashford D, Case DA. Exploring protein native states and large-scale conformational changes with a modified generalized born model. Proteins Struct Funct Bioinform. 2004;55:383–94. 10.1002/prot.20033 [DOI] [PubMed] [Google Scholar]
- 40.Gohlke H, Kiel C, Case DA. Insights into protein-protein binding by binding free energy calculation and free energy decomposition for the Ras-Raf and Ras-RalGDS complexes. J Mol Biol. 2003;330:891–914. 10.1016/S0022-2836(03)00610-7 [DOI] [PubMed] [Google Scholar]
- 41.Miller III BR, McGee TD, Swails JM, Homeyer N, Gohlke H, Roitberg AE. J Chem Theory Comput. Vol. 8, 3314–21 (2012). [DOI] [PubMed]
- 42.Vapnik V. The nature of statistical learning theory (Springer Science & Business Media, 2013).
- 43.Cortes C, Vapnik V. Support-vector networks. Mach Learn. 1995;20:273–97. 10.1007/BF00994018 [DOI] [Google Scholar]
- 44.Chang C-C, Lin C-J. LIBSVM: a library for support vector machines. ACM Trans Intell Syst Technol. 2001;2:1–27. 10.1145/1961189.1961199 [DOI] [Google Scholar]
- 45.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–90. 10.1126/science.1168175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Culig Z, Santer FR. Androgen receptor signaling in prostate cancer. Cancer Metastasis Rev. 2014;33:413–27. 10.1007/s10555-013-9474-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.