Abstract
As individuals age, there is a gradual decline in cardiopulmonary function, often accompanied by cardiac pump dysfunction leading to increased pulmonary vascular resistance (PVR). Our study aims to investigate the changes in cardiac and pulmonary vascular function associated with aging. Additionally, we aim to explore the impact of phosphodiesterase 9A (PDE9A) inhibition, which has shown promise in treating cardiometabolic diseases, on addressing left ventricle (LV) dysfunction and elevated PVR in aging individuals. Young (3 months old) and aged (32 months old) male C57BL/6 mice were used. Aged mice were treated with the selective PDE9A inhibitor PF04447943 (1 mg/kg/day) through intraperitoneal injections for 10 days. LV function was evaluated using cardiac ultrasound, and PVR was assessed in isolated, ventilated lungs perfused under a constant flow condition. Additionally, changes in PVR were measured in response to perfusion of the endothelium-dependent agonist bradykinin or to nitric oxide (NO) donor sodium nitroprusside (SNP). PDE9A protein expression was measured by Western blots. Our results demonstrate the development of LV diastolic dysfunction and increased PVR in aged mice. The aged mice exhibited diminished decreases in PVR in response to both bradykinin and SNP compared to the young mice. Moreover, the lungs of aged mice showed an increase in PDE9A protein expression. Treatment of aged mice with PF04447943 had no significant effect on LV systolic or diastolic function. However, PF04447943 treatment normalized PVR and SNP-induced responses, though it did not affect the bradykinin response. These data demonstrate a development of LV diastolic dysfunction and increase in PVR in aged mice. We propose that inhibitors of PDE9A could represent a novel therapeutic approach to specifically prevent aging-related pulmonary dysfunction.
Keywords: Aging, Heart, Pump function, Pulmonary, Vascular resistance, Vascular endothelium dysfunction, Phosphodiesterase 9A, PF-04447943
Introduction
Aging is associated with a progressive deterioration of cardiopulmonary function leading to increased morbidity and mortality in the elderly [1–3]. Age-related changes in pulmonary vascular resistance, coupled with increased heterogeneity of alveolar ventilation and pulmonary perfusion, may lead to pulmonary pre-hypertension or progress toward pulmonary arterial hypertension. The consequences of age-associated increases in pulmonary vascular resistance and its progression toward pulmonary arterial pre- or fully developed pulmonary arterial hypertension include the development of right ventricular failure. The consequent impairment of the right ventricular – pulmonary artery coupling dramatically increases the mortality risk in the long term [4–11]. While pulmonary vascular dysfunction in the elderly is increasingly recognized, the underlying mechanisms are incompletely understood and therefore the preventive treatment options are very limited.
A significant aspect of aging-related cardiopulmonary dysfunction is the impaired left ventricular function [12, 13]. The hemodynamic interrelations in postcapillary pulmonary hypertension are complex [14–16]. Pulmonary vascular resistance is frequently elevated in patients with left ventricular failure as a result of dysregulation of left atrial mechanical function and structural remodeling of the pulmonary circulation [14, 17, 18]. Chronic elevation of the left-side filling pressure is associated with neurohormonal activation, which may cause excessive vasoconstriction with or without vascular remodeling leading to an increase in pulmonary vascular resistance. Mechanistically, studies have shown that under these conditions a reduced concentration of pulmonary vascular intracellular cGMP develops, an imbalance/dysregulation in the NO-cGMP-PKG pathway takes place, impairing vascular smooth muscle cell relaxation [19–24]. The subsequently decreased activation of the cGMP-dependent protein kinase G-I then promotes an increased pulmonary vascular resistance [25].
It is established that phosphodiesterase 5 (PDE5) inhibitors are valuable therapeutic options in the management of pulmonary hypertension [26–29]. PDE5 is abundantly expressed in the pulmonary vasculature, and by inhibiting the degradation of cGMP, PDE5 inhibitors efficiently promote relaxation of the pulmonary vascular smooth muscle, leading to vasodilation and an increase in blood flow in pulmonary hypertension. Some evidence suggests a role for PDE5 inhibitors in age-related changes in the peripheral microcirculation [30, 31]. While generally well-tolerated, there are certain considerations and potential risks associated with the use of PDE5 inhibitors in the elderly population. These include adverse cardiovascular effects, especially in elderly individuals, with pre-existing heart conditions, such as ischemic heart disease or heart failure [32, 33].
Interestingly, PDE9 inhibitors have recently emerged as promising agents in the treatment of cardiometabolic and cardiovascular diseases [34–36]. Studies have demonstrated that PDE9 inhibitors can enhance vasodilation, reduce inflammation, and improve insulin sensitivity [37]. Moreover, these inhibitors have shown promise in ameliorating cardiac remodeling and dysfunction [36]. By augmenting cGMP signaling, PDE9 inhibitors improve cardiac contractility and reduce hypertrophy [38]. Evidence supports that in this process PDE9 degrades specific spatial and physiological cGMP pools within cardiac myocytes; for this reason, inhibition of PDE9A remains efficacious even in states of disrupted upstream NO generation [34]. Given that, PDE9 inhibitors present a novel therapeutic avenue for addressing both heart and pulmonary dysfunction associated with aging. However, the impact of PDE9 inhibitors on cardiopulmonary performance remains unknown in aging.
Therefore, in this study we set out to examine the effect of aging on cardiopulmonary function and determine the impact of PDE9A inhibition. For this purpose, we utilized both young and aged mice to measure LV function with cardiac ultrasound and assessed pulmonary vascular function in isolated, ventilated, and perfused lung preparations. Key results from this study demonstrate that lungs of aged mice exhibit elevated pulmonary vascular resistance and increase in PDE9A protein expression. Moreover, pharmacological inhibition of PDE9A exerts significant beneficial effects on pulmonary vascular resistance in aged mice.
Methods
Animals
The work involving experimental animals was conducted under the protocol approved by the Institutional Animal Care and Use Committee at Medical College of Georgia, Augusta University. All experimental animal procedures performed in this study were in compliance with the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes. Experiments were carried out in 32 months old aged male C57BL/6 J mice (obtained from the National Institute on Aging, Aged Rodent Colonies at the age of 22–24 months old) and 12-week-old male C57BL/6 J mice (Jackson Laboratory). The aged mouse (32 months old) is equivalent to older humans, around 80 years, whereas the 3 months old mouse is equivalent to 20-year-old humans. The mice were housed in the animal care facility and accessed rodent chow and tap water ad libitum with a 12-h light:dark cycle. Aged mice were randomly assigned to be treated either with vehicle (n = 12) or with the selective PDE9A inhibitor, PF-04447943 (1 mg/kg BW/d, Calbiochem, 538337, n = 9), by daily intraperitoneal injection for 10 days. PF-04447943 stock was prepared in DMSO (50 mg/ml) and diluted freshly in sterile PBS (100 µl of the injection volume per animal). Two mice died in the vehicle treated control group, one mouse has died and one was sacrificed due to health concerns in the PF-04447943 treated group.
Echocardiographic assessment
Using digital ultrasound micro-imaging system VEVO3100 and MX250 linear array transducer probe (VisualSonics, Toronto, ON, Canada), echocardiographic assessment was performed on anesthetized (1% − 2% inhaled isoflurane) young, aged 32-month-old mice with either vehicle or PF-04447943 treatment to measure left ventricular (LV) structural and functional parameters. LV volumes were measured during diastole and systole from the parasternal short-axis view in M-mode. Several ventricular wall dimensions were measured to assess for hypertrophy including left ventricular anterior wall thickness (LVAW; s/d), left ventricular posterior wall thickness (LVPW; s/d), and the left ventricular internal diameter at end diastole and end systole (LVID;d, LVID;s). The diastolic function of the left ventricle was evaluated from the apical 4-chamber view, in the pulsed-wave Doppler mode the gate was positioned above the mitral leaflets to measure mitral flow velocity tracings (peak velocity of early (E), late (A) mitral inflow, and deceleration time (DT) of early (E) filling of mitral inflow) and cardiac time intervals (isovolumic contraction time (IVCT), isovolumic relaxation time (IVRT), and aortic ejection time (AET)). Mitral valve motion parameters were evaluated from the apical 4-chamber view; the tissue doppler was positioned over the annulus of the mitral leaflets to measure mitral annular velocities (early diastolic myocardial relaxation velocity (e’) and myocardial velocity associated with atrial contraction (a’)). The EA ratio (the ratio between peak velocity of early (E) and late (A) mitral inflow), Ee’ ratio (the ratio between peak velocity of early (E) mitral inflow and early diastolic mitral annulus velocity (e’), as an indicator of LV filling pressures), ejection fraction (EF), and fractional shortening (FS) were compared between groups of experimental animals to assess systolic and diastolic function. All calculated parameters (ejection fraction, fractional shortening, estimated LV mass, myocardial performance index, etc.) were obtained from the VevoLab 4.5.1 analysis software. Animals heart rate, breathing, and ECG was monitored continuously throughout echocardiographic assessment [39, 40].
Isolated lung preparation
Mice were anesthetized with 3% isoflurane. The trachea was cannulated and immediately ventilated (respiratory rate 140 breaths/min with a tidal volume of 250 μl and positive end-expiratory pressure (PEEP) 1 cmH2O) using a small animal ventilator (RWD, VentStar R315) with 3% isoflurane mixed with room air. Sodium heparin (100 units per 100 g body weight) was injected into the vena cava and allowed to circulate for 1 min. After that, the animal was sacrificed through the exsanguination by transection of the vena cava (isoflurane supplementation shut down). The thoracic cavity was opened and the cannula was inserted and fixated with a ligature in the pulmonary artery. The left ventricle was opened and perfusion with Ca2+-free Krebs–Henseleit solution containing 2% dextran (MW 75000, Thermo Scientific, Cat#: J60989.22) was initiated at a constant flow of 0.3 ml/min. The perfusion solution was preliminarily filtered and pH was adjusted to 7.4 ± 0.05; throughout the experiment, the temperature of the perfusate was maintained at 37 °C and oxygenation was provided with 5% CO2, 95% O2. The outflow cannula was inserted and fixated in the left atrium after which, the preparation was dissected from the rib cage and attached to an isolated lung system (Fig. 3A). At this point, the perfusion buffer was switched from Ca2+-free to the Ca2+-containing (2.5 mM) solution. The outflow cannula connected to the outflow reservoir placed at the level of the ventricle, so the outflow pressure was controlled and held at a level of 0 ± 0.2 mmHg.
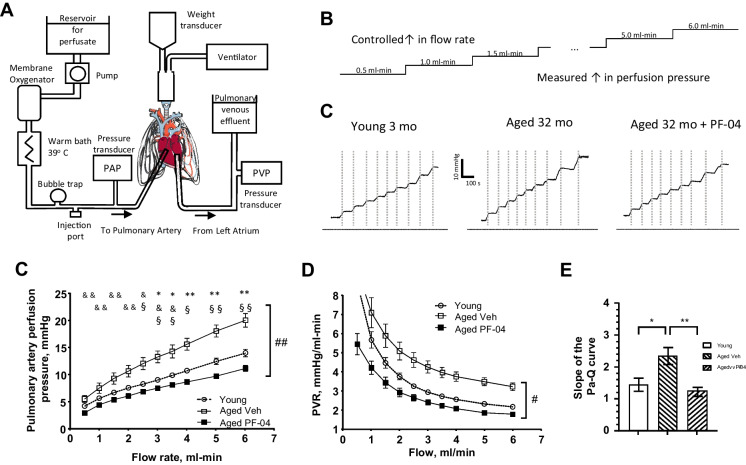
Fig. 3.
Experiments on ventilated, isolated and perfused whole lungs from young (n = 8), aged vehicle (n = 5) and PF-04447943 (PF-04)-treated (n = 6) animals used to generate pulmonary artery perfusion pressure vs flow curves. A A schema of the experimental setup for the isolated, ventilated and perfused whole lung preparation. B A schema of the experimental protocol where flow controlled by a peristaltic pump and increased stepwise, while pulmonary artery perfusion pressure is monitored. C Representative traces recorded from the lung experiments in all assigned experimental groups. D Summary graphs of pulmonary artery perfusion pressure vs flow relations in experimental groups. E Summery graphs of the calculated pulmonary vascular resistance for each flow rate. F Bar graphs of the calculated slopes for the pressure-flow relations (at flows 1 through 6 ml-min). Data presented as means ± SEM and analyzed using two-way ANOVA with mixed effects and Tukey’s multiple comparison test or a one-way ANOVA with a Tukey’s multiple comparison test was used when appropriate. *—p < 0.05, **—p < 0.01, ***—p < 0.001; in D and E *—young vs aged veh, &—young vs aged PDE9I, §—aged veh vs aged PDE9I
In the experimental protocol, flow (Q) was established with a peristaltic pump (Masterflex® Ismatec® Reglo ICC Digital Pump, model#: 78,001–80). The pulmonary arterial perfusion pressure (Pa) and the pulmonary venous perfusion pressure Pv were monitored at different levels of the flow rate (Fig. 3B). Pulmonary vascular resistance was calculated: PVR = (Pa-Pv)/Q. The slope of the Pa-Q curves was calculated for the flow interval of 2.0 – 6.0 ml/min with GraphPad Prism 10.1, using simple linear regression analysis. In agonist-response experiments, a preconstrictor, U46619 (1 × 10−6 M), was added to a perfusion solution. When perfusion pressure stabilized, 50 µl Ca2+-containing Krebs solution was injected as a negative control for the perfusion pressure change. Following, 50 µl bolus injections of increasing concentrations of bradykinin (Bk, 10–9.5, 10–8.5, 10–7.5, 10–5.5 M) or sodium nitroprusside (SNP, 10–6.5, 10–5.5, 10–4.5 M) were injected through the injection port, and the decrease in perfusion pressure was monitored and interpreted as a reflection of pulmonary vasculature relaxation. The response was calculated as the percent of perfusion pressure after drug exposure to the U46619-induced maximum perfusion pressure.
Western immunoblotting
The lungs were homogenized in radio-immunoprecipitation assay (RIPA, R0278, Sigma) buffer mixed with 1% protease inhibitor cocktail (Sigma). Protein concentration was measured by BCA assay (Cat#: 23,227, Thermo Scientific). Equal amounts of protein were loaded for gel electrophoresis. After blotting, membranes (Hybond-P, GE Healthcare) were probed with anti-PDE5 primary antibody (1:1000, Cat# PA5-79,796, Invitrogen) or PDE9A antibody (1:1000, Cat# NBP1-00641, Novus Biologicals) followed by incubation with HRP linked secondary antibody (1:2000, Cat# 7074S, Cell Signaling Technologies). Enhanced chemiluminescence was visualized autoradiographically by Fluochem E system, and analyzed in ImageJ 1.54f. Protein expression was normalized for the total loaded protein.
Statistical analysis
All statistical analyses were performed using GraphPad Prism Software. Data comparisons between groups repeatedly over time were analyzed by one-way ANOVA (cardiac ultrasound measurements) or by two-way repeated-measures ANOVA (isolated lung experiments) followed by Tukey’s post-hoc test for multiple comparisons or with two-tailed, unpaired Student t-test (Western blots), as appropriate. Data are expressed as mean ± SEM with individual data points. P < 0.05 was considered statistically significant.
Results
Development of left ventricular diastolic dysfunction in aged mice unaffected by PDE9 inhibitor treatment
Previous studies have indicated that aging leads to alterations in cardiovascular structure and cardiac diastolic function, while systolic function (ejection fraction) remains relatively preserved [41, 42]. In this study, we aimed to determine age-related changes in cardiac pump function and to investigate whether PDE9A inhibition exerts beneficial effects on cardiopulmonary function. To that end, first we performed echocardiographic analysis to assess left ventricular systolic and diastolic performance in 32-months-old aged mice treated with either vehicle or PDE9 inhibitor, PF-04447943, with young 3-month-old mice serving as controls. A summary of all parameters from the echocardiographic analysis is provided in Table 1.
Table 1.
Echocardiographic parameters
| Parameter | Young | Aged | p-value | |
|---|---|---|---|---|
| 3 month | 32 month + Vehicle | 32 month + PDE9I | ||
| Heart Rate, bpm | 427.4 ± 11.3 | 431.5 ± 11.35 | 417.6 ± 19.57 | 0.370 |
| Diameter;s, mm | 2.26 ± 0.085 | 2.9 ± 0.2 | 2.73 ± 0.32 | 0.150 |
| Diameter;d, mm | 3.65 ± 0.08 | 4.16 ± 0.19 | 4.1 ± 0.28 | 0.170 |
| Volume;s, µl | 17.84 ± 1.7 | 35.3 ± 5.7 | 36.36 ± 6.01 | 0.060 |
| Volume;d, µl | 56.9 ± 3.21 | 79.2 ± 8.1 | 77.62 ± 12.16 | 0.160 |
| Stroke Volume, µl | 39 ± 1.97 | 43.9 ± 3.4 | 46 ± 5.62 | 0.240 |
| Ejection Fraction, % | 69 ± 1.6 | 61 ± 3.5 | 57.83 ± 2.3 | 0.082 |
| Fractional Shortening, % | 38.13 ± 1.25 | 32.58 ± 1.86 | 30.7 ± 1.46 | 0.058 |
| Cardiac Output, ml/min | 16.69 ± 0.93 | 18.8 ± 1.35 | 19.15 ± 2.3 | 0.100 |
| LV Mass, mg | 117.8 ± 5.1 | 189.6 ± 13.47 *** | 209.9 ± 11.07 **** |
< 0.0001 **** |
| LV Mass, mg | 94.2 ± 4.1 | 151.9 ± 10.78 *** | 168 ± 8.85 **** |
< 0.0001 **** |
| LVAW;s, mm | 1.39 ± 0.05 | 1.45 ± 0.12 | 1.49 ± 0.08 | 0.820 |
| LVAW;d, mm | 0.87 ± 0.04 | 0.99 ± 0.09 | 0.99 ± 0.07 | 0.480 |
| LVPW;s, mm | 1.31 ± 0.031 | 1.54 ± 0.1 | 1.77 ± 0.194 * | 0.0413 * |
| LVPW;d, mm | 0.91 ± 0.052 | 1.16 ± 0.076 | 1.37 ± 0.18 * | 0.0084 ** |
| MV Decel Time, ms | 30.44 ± 2.7 | 20.5 ± 1.75 * | 18 ± 1.87 ** | 0.003 ** |
| MV E/A | 1.36 ± 0.05 | 1.32 ± 0.07 | 1.42 ± 0.09 | 0.790 |
| MV E/E’ (abs) | 25.7 ± 1.04 | 34.1 ± 1.9 * | 28.3 ± 1.75 | 0.032 * |
| IVRT, ms | 19.3 ± 0.9 | 18.4 ± 1.0 | 18.47 ± 1.33 | 0.250 |
| IVCT, ms | 13.4 ± 1.18 | 16.9 ± 1.3 | 16.01 ± 1.9 | 0.290 |
| AET, ms | 53.1 ± 1.63 | 58.45 ± 3.74 | 58.46 ± 2.79 | 0.150 |
| LV MPI IV | 0.61 ± 0.02 | 0.623 ± 0.04 | 0.6 ± 0.04 | 0.088 |
| PA VTI, mm | 31.2 ± 0.91 | 22.96 ± 1.23 ** | 22.9 ± 1.42 ** | 0.0013 ** |
| PAT/PET | 0.27 ± 0.007 | 0.28 ± 0.0075 | 0.3 ± 0.02 | 0.190 |
*—Compared to young group
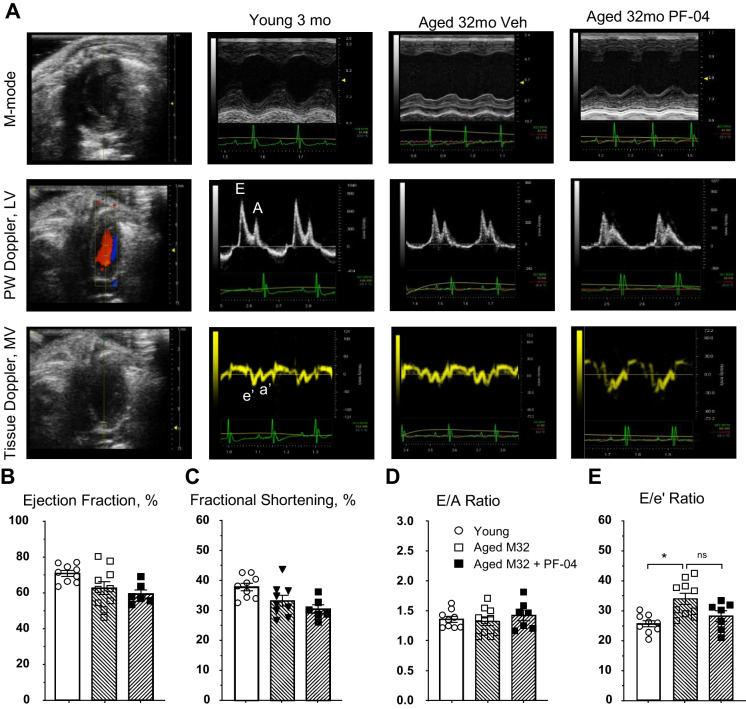
Aged mice treated with the vehicle showed a significant increase in the E/e’ ratio, while aged mice treated with PF-04447943 exhibited a non-significant decrease in the E/e’ ratio, reaching levels similar to those observed in young mice (Fig. 1, Table 1). Additionally, we did not observe significant changes in the E/A ratio between groups (Fig. 1D). Although fractional shortening decreased in aged mice treated with PF-04447943, the ejection fraction remained above 55% without a significant change (Fig. 1B, C). Morphologically, we observed a gradual increase in left ventricular posterior wall thickness in aged animals, which reached significance in PF-04447943-treated aged mice. This increase in left ventricular wall thickness was also reflected in the significantly higher estimated left ventricular mass (Table 1).
Fig. 1.
Echocardiographic assessment of the cardiac function in young (n = 9), aged 24mo (n = 9) and aged 32-month-old vehicle (n = 10) and PF-04447943 (PF-04) treated (n = 7) animals. A Representative figures for the ultrasound analysis in all groups in parasternal short axis for M-mode systolic function measurements (Ejection Fraction, B, and Fractional Shortening, C), and four-chamber apical view for diastolic function assessments in Pulse-Wave Doppler mode (E/A ratio, D) and Tissue Doppler mode (e’ and consecutively E/e’ ratio, E). Data presented as means ± SEM and analyzed using ordinary one-way ANOVA with a Tukey’s multiple comparison test; *—p < 0.05
PDE9A protein expression is increased in the lung of aged mice
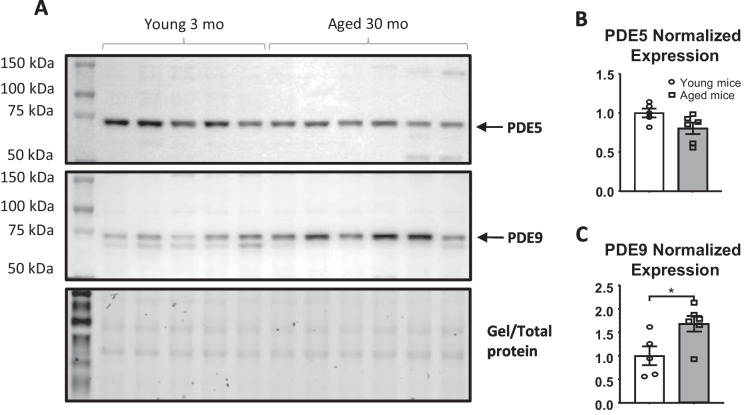
PDE9A expression has been observed to increase in human heart biopsies obtained from heart failure patients [34]. In our study, we measured PDE9A protein expression in the lungs of both young and aged mice. Using Western blot analysis, we detected an increase in PDE9A protein expression in whole lung lysates from aged mice (Fig. 2A,B), while the protein expression of PDE5 remained unchanged in aged mice compared to young controls (Fig. 2A,B).
Fig. 2.
PDE9 but not PDE5 expression is significantly upregulated in aged animals (n = 6), when compared to young controls (n = 5). A Representative images of Western immunoblot for detecting protein expression of PDE5, PDE9 and the picture of total unstained gel for the total protein loading control. B, C Quantified protein expression for PDE9 and PDE5 respectively, normalized for a total protein. Data presented as means ± SEM and analyzed using unpaired t-test; * p < 0.05
PDE9A inhibition improves increased pulmonary vascular resistance in aged mice
To assess pulmonary vascular function independently of in vivo cardiac influences, we utilized the isolated, ventilated, and perfused lung preparation ex vivo. Data from the distensible vessel model, fitting in accordance with the ohmic-Starling resistor model and calculated for slope, were collected from young mice, aged mice treated with the vehicle, and aged mice treated with PF-04447943 (Fig. 3A,B). These data points were then plotted to generate a multipoint Pa-Q relationship, as previously described [43–45].
At every flow rate, Pa was elevated in aged mice treated with the vehicle compared to young mice, with significant increases noted for higher flow (Q) levels. Treatment with PF-04447943 led to a significant decrease in Pa, trending lower but not significantly different from that of young controls. Upon further data analysis, we calculated pulmonary vascular resistance (PVR) at each point of the Pa-Q curve and determined the slope of the curve within the interval of 1.5 to 6 ml-min flow rates. The trend observed in the data mirrored the Pa-Q curve, indicating elevated vascular resistance in aged animals compared to young mice, which was reversed following PF-04447943 treatment (Fig. 3C-D).
PDE9A inhibition in aged mice improves pulmonary vasodilator dysfunction
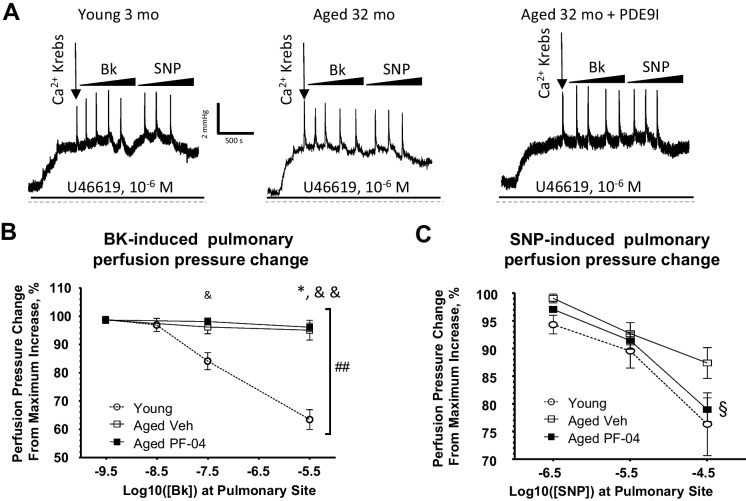
To assess the roles of vascular endothelium and smooth muscle in the increased pulmonary vascular resistance seen in aged animals and to understand the potential impact of PDE9A inhibition, we initially tested pulmonary vasodilation function in isolated lung preparations using pharmacological agonists such as bradykinin or sodium nitroprusside (SNP).
A bolus injection of the endothelium-dependent agonist bradykinin, with increasing concentrations, led to a significant reduction in perfusion pressure in the lungs of young animals. However, in aged animals treated with the vehicle, this response was almost completely absent, and treatment with PF-04447943 did not improve it (Fig. 4A, B). To evaluate NO-dependent relaxation independently of the endothelium, we administered bolus injections of SNP at increasing concentrations. The SNP-induced reduction in perfusion pressure was reduced in aged mice; however, in PF-04447943-treated mice, responses were comparable to those observed in young mice (Fig. 4A,C).
Fig. 4.
Experiments on ventilated, isolated and perfused whole lungs from young (n = 5), aged vehicle (n = 5) and PF-04447943 (PF-04)-treated (n = 6) animals used to test the vasodilator action on the perfusion pressure in preconstricted pulmonary vasculature. A Representative traces recorded from the lung experiments in response to increasing concentrations of the vasodilator drugs in all assigned experimental groups. B Summary graphs of the pulmonary artery perfusion pressure decrease in response to a increasing concentrations of bradykinin in a whole lung vasculature, preconstricted with U46619 (10–6 M). C Summary graphs of the pulmonary artery perfusion pressure decrease in response to a increasing concentrations of sodium nitroprusside (SNP, 10–6.5) in a whole lung vasculature, preconstricted with U46619 (10–6 M). Data presented as means ± SEM and analyzed using two-way ANOVA with mixed effects and Tukey’s multiple comparison test was used. *—p < 0.05, **—p < 0.01, ***—p < 0.001; in B and C *—young vs aged veh, &—young vs aged PDE9I, §—aged veh vs aged PDE9I
Discussion
In this study, we present evidence that age-related left ventricular diastolic dysfunction and increases in pulmonary vascular resistance develop independently. Our findings reveal increased PDE9A protein expression in the lungs of aged mice, correlating with elevated pulmonary vascular resistance and impaired pulmonary vasodilation. Pharmacological inhibition of PDE9A reversed age-related changes selectively in pulmonary vascular function, highlighting its organ specific beneficial effects.
Aging is commonly associated with progressive cardiopulmonary dysfunction, contributing to increased morbidity and mortality among the elderly [1–3]. One of the major concerns is the development of the increase in pulmonary vascular resistance, leading to pre-hypertension or pulmonary arterial hypertension, ultimately resulting in right ventricular failure and increased mortality risks [4–11]. While these age-related changes are recognized, the underlying mechanisms and treatment options remain limited. Consistent with previous studies [12, 13], we observed the development of impaired left ventricular diastolic function in aged mice. It is important to note the left ventricle diastolic dysfunction is often associated with increased pulmonary vascular resistance due to dysregulated left atrial mechanical function and remodeling of the pulmonary vasculature. Indeed, chronic elevation of the left-side filling pressures trigger complex pathological changes resulting in increased pulmonary arterial vasoconstriction and structural vascular remodeling. To assess age-related changes in pulmonary vascular function independently of in vivo cardiac influences, in this study we employed the isolated, ventilated, and perfused ex vivo lung preparation. The results from these experiments suggest that increased pulmonary vascular resistance in aged mice may develop independently or manifest independently from increased left atrial and ventricular filling pressures in these older mice.
PDE5 inhibitors have been established as therapeutic options in pulmonary hypertension by promoting pulmonary vascular smooth muscle relaxation through cGMP signaling [26–29]. However, concerns exist using PDE5 inhibitors, especially in elderly populations with cardiovascular comorbidities [32, 33]. Previous studies observed that in HFpEF patients with left ventricular diastolic dysfunction, PDE9A expression is upregulated in the myocardium [34]. Elevated PDE9A levels may diminish cGMP-PKG mediated responses, which is supported by positive results observed in preclinical studies using selective PDE9A inhibitors [35, 46]. In this study, we observed an increase in PDE9A levels in the lungs of aged mice, while the levels of PDE5 remained unchanged. Additionally, the PDE9A inhibitor, PF-04447943 treatment resulted in decreased pulmonary vascular resistance in the aged mice. These findings underscore the specific role of PDE9A in age-related changes within the pulmonary circulation. Additionally, we observed that the treatment with PF-04447943 was not effective in restoring bradykinin-induced, vascular endothelium-dependent, NO-mediated pulmonary arterial relaxation in aged mice. In contrast, the smooth muscle acting, direct NO donor SNP showed significantly improved relaxation. Our study thus introduces PDE9A inhibitors as promising alternatives, highlighting their potential to enhance pulmonary vasodilation in aging, even in conditions with endothelial dysfunction and disrupted endothelial NO generation. However, further studies are necessary to investigate the direct mechanistic connections and the underlying molecular signaling mechanisms between PDE9A inhibition and improved pulmonary vascular responses in aging.
Previous studies with PDE9A inhibitors have noted beneficial effects even under conditions of significant oxidative stress [47], which is known to interfere with NO-mediated responses. Interestingly, some studies have even suggested direct antioxidant effects of PDE9A inhibitors in the treatment of Alzheimer’s disease [48]. Additionally, PDE9A inhibitors have been shown to reduce inflammation, improve insulin sensitivity, and ameliorate cardiometabolic dysfunction in previous studies [35, 46, 49]. However, in our study, we did not evaluate changes in aging-related, oxidative stress, inflammation and metabolic function, nor did we assess the potential effects of PDE9A inhibitors in these processes. Further studies are needed to explore these aspects and effects by PDE9A inhibitors in aging models.
The potential of PDE9A inhibitors in treating age-related pulmonary complications, such as acutely or chronically elevated PVR, may exhibit sex-specific differences, which have not yet been thoroughly investigated. In our current study, we exclusively used male mice to assess the short-term beneficial effects of PDE9A inhibition (10-day treatment) on pulmonary vasodilator function. We did not examine indicators of pulmonary vascular wall remodeling or the effects of a more prolonged PDE9A inhibition in this context. Interestingly, a prior study by Mishra et al. found that while PDE9A inhibition for two months in young male and ovariectomized female mice suppressed diet-induced metabolic defects and improved LV dysfunction, it had negligible impact on these cardiometabolic parameters in non-ovariectomized young female mice [35]. Therefore, it is possible that PDE9A inhibition could offer both acute and chronic benefits for pulmonary vascular function as well as vascular wall remodeling, and may exhibit sexual dimorphism in this context. This intriguing hypothesis warrants investigation in future studies. In addition, clinical trials in human subjects, especially in elderly populations with cardiopulmonary diseases, will be crucial in translating these findings into clinical practice and improving outcomes.
In conclusion, our study reveals an increase in PDE9A protein expression in the lungs of aged mice, which correlates with elevated pulmonary vascular resistance. Pharmacological inhibition of PDE9A showed beneficial effects on reducing pulmonary vascular resistance in aged mice. These findings highlight therapeutic potential and novel avenues for managing age-related cardiopulmonary dysfunction using PDE9 inhibitors.
Author contributions
Z.B. conceptualized the project; Z.B., V.B., K.A.F., and L.L. performed the experiments. V.B and Z.B wrote the original draft of the manuscript. V.B., K.A.F, D.V.I, A.V., and Z.B. reviewed and edited the manuscript. Z.B. supervised the research.
Funding
This work was supported by an award from the American Heart Association [917057 to VB].
Data availability
We declare that the data supporting the findings of this study are available within the article and its Supplementary Information files and from the corresponding authors upon request.
Declarations
Conflict of interests
The authors declare no conflict of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lam CS, et al. Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation. 2009;119(20):2663–70. 10.1161/CIRCULATIONAHA.108.838698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodgers JL, et al. Cardiovascular risks associated with gender and aging. J Cardiovasc Dev Dis. 2019;6(2):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wannamethee SG, et al. Lung function and airway obstruction: associations with circulating markers of cardiac function and incident heart failure in older men-the British Regional Heart Study. Thorax. 2016;71(6):526–34. 10.1136/thoraxjnl-2014-206724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351(16):1655–65. 10.1056/NEJMra035488 [DOI] [PubMed] [Google Scholar]
- 5.Badesch DB, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54(1 Suppl):S55–66. 10.1016/j.jacc.2009.04.011 [DOI] [PubMed] [Google Scholar]
- 6.Ryan JJ, et al. Right ventricular adaptation and failure in pulmonary arterial hypertension. Can J Cardiol. 2015;31(4):391–406. 10.1016/j.cjca.2015.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huston JH, et al. Association of mild echocardiographic pulmonary hypertension with mortality and right ventricular function. JAMA Cardiol. 2019;4(11):1112–21. 10.1001/jamacardio.2019.3345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guazzi M, et al. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: an index of right ventricular contractile function and prognosis. Am J Physiol Heart Circ Physiol. 2013;305(9):H1373–81. 10.1152/ajpheart.00157.2013 [DOI] [PubMed] [Google Scholar]
- 9.Santas E, et al. Usefulness of right ventricular to pulmonary circulation coupling as an indicator of risk for recurrent admissions in heart failure with preserved ejection fraction. Am J Cardiol. 2019;124(4):567–72. 10.1016/j.amjcard.2019.05.024 [DOI] [PubMed] [Google Scholar]
- 10.Jentzer JC, et al. Right ventricular pulmonary artery coupling and mortality in cardiac intensive care unit patients. J Am Heart Assoc. 2021;10(7): e019015. 10.1161/JAHA.120.019015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parasca CA, et al. Right ventricle to pulmonary artery coupling after transcatheter aortic valve implantation-Determinant factors and prognostic impact. Front Cardiovasc Med. 2023;10:1150039. 10.3389/fcvm.2023.1150039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenkranz S, et al. Left ventricular heart failure and pulmonary hypertension. Eur Heart J. 2016;37(12):942–54. 10.1093/eurheartj/ehv512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toma M, et al. Left heart disease phenotype in elderly patients with pulmonary arterial hypertension: insights from the Italian PATRIARCA registry. J Clin Med. 2022;11(23):7136. 10.3390/jcm11237136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai YC, Wang L, Gladwin MT. Insights into the pulmonary vascular complications of heart failure with preserved ejection fraction. J Physiol. 2019;597(4):1143–56. 10.1113/JP275858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolsk E, et al. The influence of age on hemodynamic parameters during rest and exercise in healthy individuals. JACC Heart Fail. 2017;5(5):337–46. 10.1016/j.jchf.2016.10.012 [DOI] [PubMed] [Google Scholar]
- 16.Tedford RJ, et al. Pulmonary capillary wedge pressure augments right ventricular pulsatile loading. Circulation. 2012;125(2):289–97. 10.1161/CIRCULATIONAHA.111.051540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huston JH, Shah SJ. Understanding the pathobiology of pulmonary hypertension due to left heart disease. Circ Res. 2022;130(9):1382–403. 10.1161/CIRCRESAHA.122.319967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freed BH, et al. Prognostic utility and clinical significance of cardiac mechanics in heart failure with preserved ejection fraction: importance of left atrial strain. Circ Cardiovasc Imaging. 2016;9:e003754. [DOI] [PMC free article] [PubMed]
- 19.Ungvari Z, et al. Mechanisms of vascular aging. Circ Res. 2018;123(7):849–67. 10.1161/CIRCRESAHA.118.311378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baliga RS, et al. Synergy between natriuretic peptides and phosphodiesterase 5 inhibitors ameliorates pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;178(8):861–9. 10.1164/rccm.200801-121OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bubb KJ, et al. Inhibition of phosphodiesterase 2 augments cGMP and cAMP signaling to ameliorate pulmonary hypertension. Circulation. 2014;130(6):496–507. 10.1161/CIRCULATIONAHA.114.009751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evgenov OV, et al. Inhibition of phosphodiesterase 1 augments the pulmonary vasodilator response to inhaled nitric oxide in awake lambs with acute pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2006;290(4):L723–9. 10.1152/ajplung.00485.2004 [DOI] [PubMed] [Google Scholar]
- 23.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263–71. 10.1016/j.jacc.2013.02.092 [DOI] [PubMed] [Google Scholar]
- 24.Hirata Y, et al. Plasma concentrations of alpha-human atrial natriuretic polypeptide and cyclic GMP in patients with heart disease. Am Heart J. 1987;113(6):1463–9. 10.1016/0002-8703(87)90663-6 [DOI] [PubMed] [Google Scholar]
- 25.Zhao YD, et al. Protein kinase G-I deficiency induces pulmonary hypertension through Rho A/Rho kinase activation. Am J Pathol. 2012;180(6):2268–75. 10.1016/j.ajpath.2012.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galie N, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353(20):2148–57. 10.1056/NEJMoa050010 [DOI] [PubMed] [Google Scholar]
- 27.Desai K, et al. Safety and efficacy of sildenafil for group 2 pulmonary hypertension in left heart failure. Children (Basel). 2023;10(2):270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prasad S, Wilkinson J, Gatzoulis MA. Sildenafil in primary pulmonary hypertension. N Engl J Med. 2000;343(18):1342. 10.1056/NEJM200011023431814 [DOI] [PubMed] [Google Scholar]
- 29.Wharton J, et al. Antiproliferative effects of phosphodiesterase type 5 inhibition in human pulmonary artery cells. Am J Respir Crit Care Med. 2005;172(1):105–13. 10.1164/rccm.200411-1587OC [DOI] [PubMed] [Google Scholar]
- 30.Sokanovic SJ, et al. Long-term inhibition of PDE5 ameliorates aging-induced changes in rat testis. Exp Gerontol. 2018;108:139–48. 10.1016/j.exger.2018.04.007 [DOI] [PubMed] [Google Scholar]
- 31.Aversa A, et al. Androgen deficiency and phosphodiesterase type 5 expression changes in aging male: therapeutic implications. Front Endocrinol (Lausanne). 2019;10:225. 10.3389/fendo.2019.00225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holt A, et al. Adverse events associated with coprescription of phosphodiesterase type 5 inhibitors and oral organic nitrates in male patients with ischemic heart disease : a case-crossover study. Ann Intern Med. 2022;175(6):774–82. 10.7326/M21-3445 [DOI] [PubMed] [Google Scholar]
- 33.Triposkiadis F, et al. Therapeutic augmentation of NO-sGC-cGMP signalling: lessons learned from pulmonary arterial hypertension and heart failure. Heart Fail Rev. 2022;27(6):1991–2003. 10.1007/s10741-022-10239-5 [DOI] [PubMed] [Google Scholar]
- 34.Lee DI, et al. Phosphodiesterase 9A controls nitric-oxide-independent cGMP and hypertrophic heart disease. Nature. 2015;519(7544):472–6. 10.1038/nature14332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mishra S, et al. Inhibition of phosphodiesterase type 9 reduces obesity and cardiometabolic syndrome in mice. J Clin Invest. 2021;131(21). [DOI] [PMC free article] [PubMed]
- 36.Richards DA, et al. CRD-733, a novel PDE9 (Phosphodiesterase 9) inhibitor, reverses pressure overload-induced heart failure. Circ Heart Fail. 2021;14(1): e007300. 10.1161/CIRCHEARTFAILURE.120.007300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Genders AJ, et al. cGMP phosphodiesterase inhibition improves the vascular and metabolic actions of insulin in skeletal muscle. Am J Physiol Endocrinol Metab. 2011;301(2):E342–50. 10.1152/ajpendo.00691.2010 [DOI] [PubMed] [Google Scholar]
- 38.Blanton RM. Phosphodiesterase 9 inhibition in models of heart failure with preserved left ventricular ejection fraction: should we focus on the positive or negative? Circ Heart Fail. 2020;13(5): e007107. 10.1161/CIRCHEARTFAILURE.120.007107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindsey ML, et al. Guidelines for measuring cardiac physiology in mice. Am J Physiol-Heart Circ Physiol. 2018;314(4):H733–52. 10.1152/ajpheart.00339.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Respress JL, Wehrens XHT. Transthoracic echocardiography in mice. J Visual Exp. 2010;(39). [DOI] [PMC free article] [PubMed]
- 41.Yang B, Larson DF, Watson R. Age-related left ventricular function in the mouse: analysis based on in vivo pressure-volume relationships. Am J Physiol. 1999;277(5):H1906–13. [DOI] [PubMed] [Google Scholar]
- 42.Medrano G, et al. Left atrial volume and pulmonary artery diameter are noninvasive measures of age-related diastolic dysfunction in mice. J Gerontol A Biol Sci Med Sci. 2016;71(9):1141–50. 10.1093/gerona/glv143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Linehan JH, et al. A simple distensible vessel model for interpreting pulmonary vascular pressure-flow curves. J Appl Physiol (1985). 1992;73(3):987–94. 10.1152/jappl.1992.73.3.987 [DOI] [PubMed] [Google Scholar]
- 44.Nelin LD, et al. A distensible vessel model applied to hypoxic pulmonary vasoconstriction in the neonatal pig. J Appl Physiol (1985). 1993;74(5):2049–56. 10.1152/jappl.1993.74.5.2049 [DOI] [PubMed] [Google Scholar]
- 45.Lewis GD, et al. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation. 2013;128(13):1470–9. 10.1161/CIRCULATIONAHA.112.000667 [DOI] [PubMed] [Google Scholar]
- 46.Methawasin M, et al. Phosphodiesterase 9a inhibition in mouse models of diastolic dysfunction. Circ Heart Fail. 2020;13(5): e006609. 10.1161/CIRCHEARTFAILURE.119.006609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dunkerly-Eyring B, Kass DA. Myocardial phosphodiesterases and their role in cGMP regulation. J Cardiovasc Pharmacol. 2020;75(6):483–93. 10.1097/FJC.0000000000000773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang C, et al. Discovery of novel PDE9A inhibitors with antioxidant activities for treatment of Alzheimer’s disease. J Enzyme Inhib Med Chem. 2018;33(1):260–70. 10.1080/14756366.2017.1412315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shao YX, et al. Discovery of a phosphodiesterase 9A inhibitor as a potential hypoglycemic agent. J Med Chem. 2014;57(24):10304–13. 10.1021/jm500836h [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We declare that the data supporting the findings of this study are available within the article and its Supplementary Information files and from the corresponding authors upon request.