Abstract
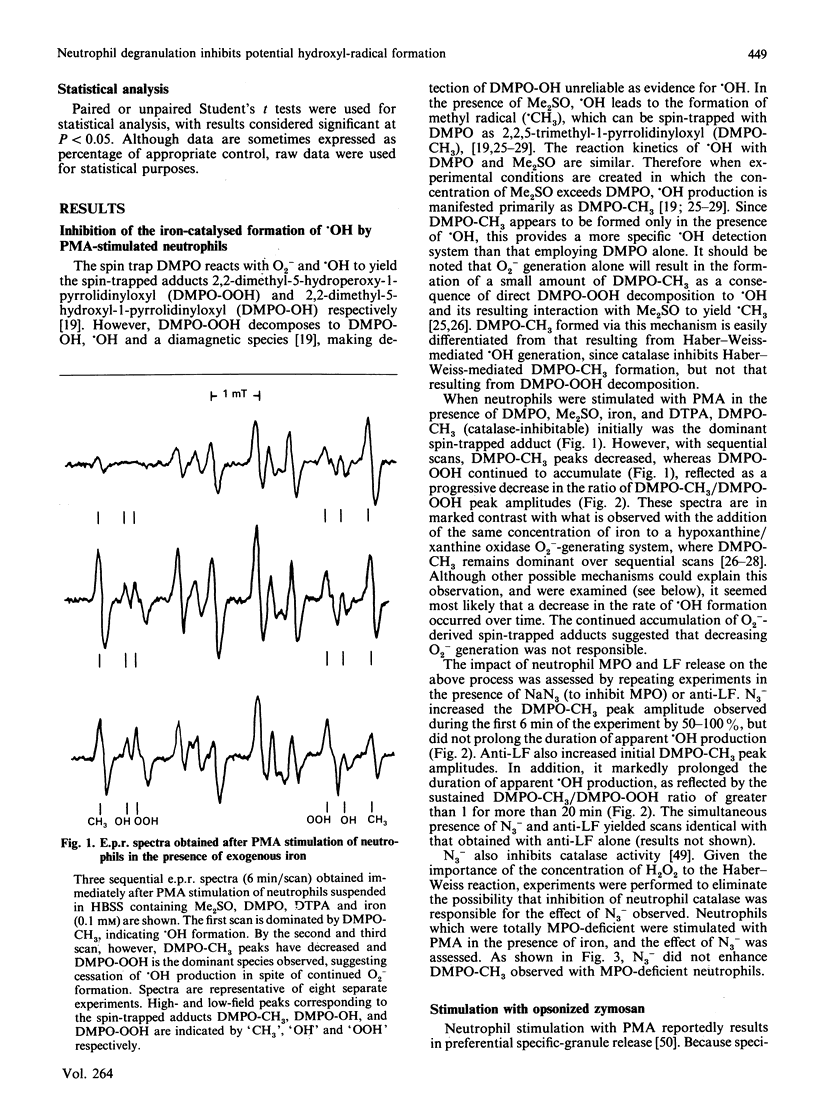
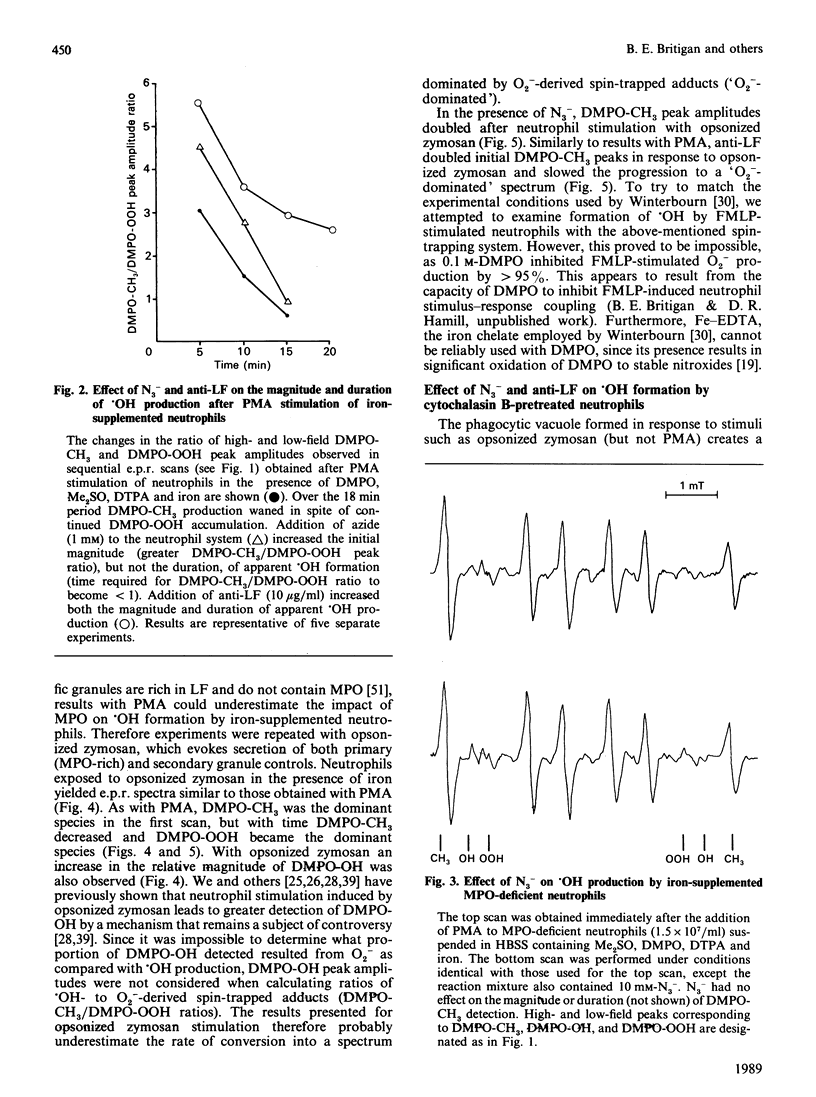
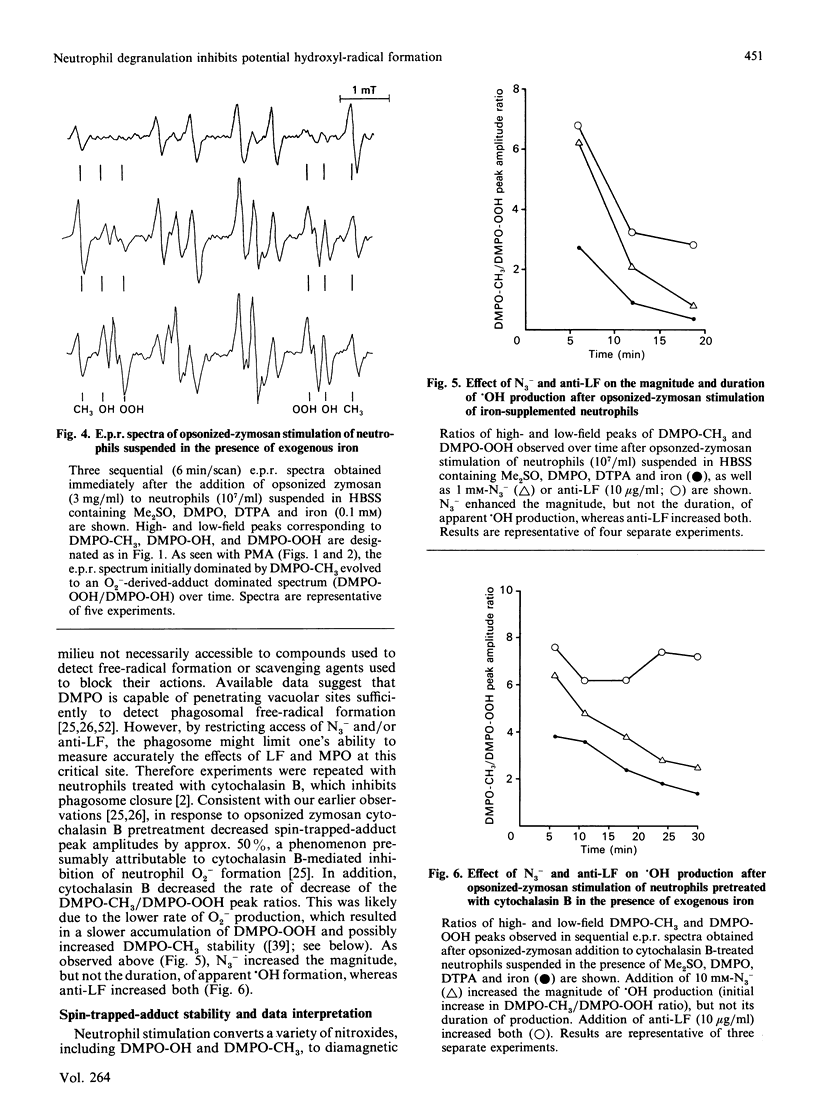
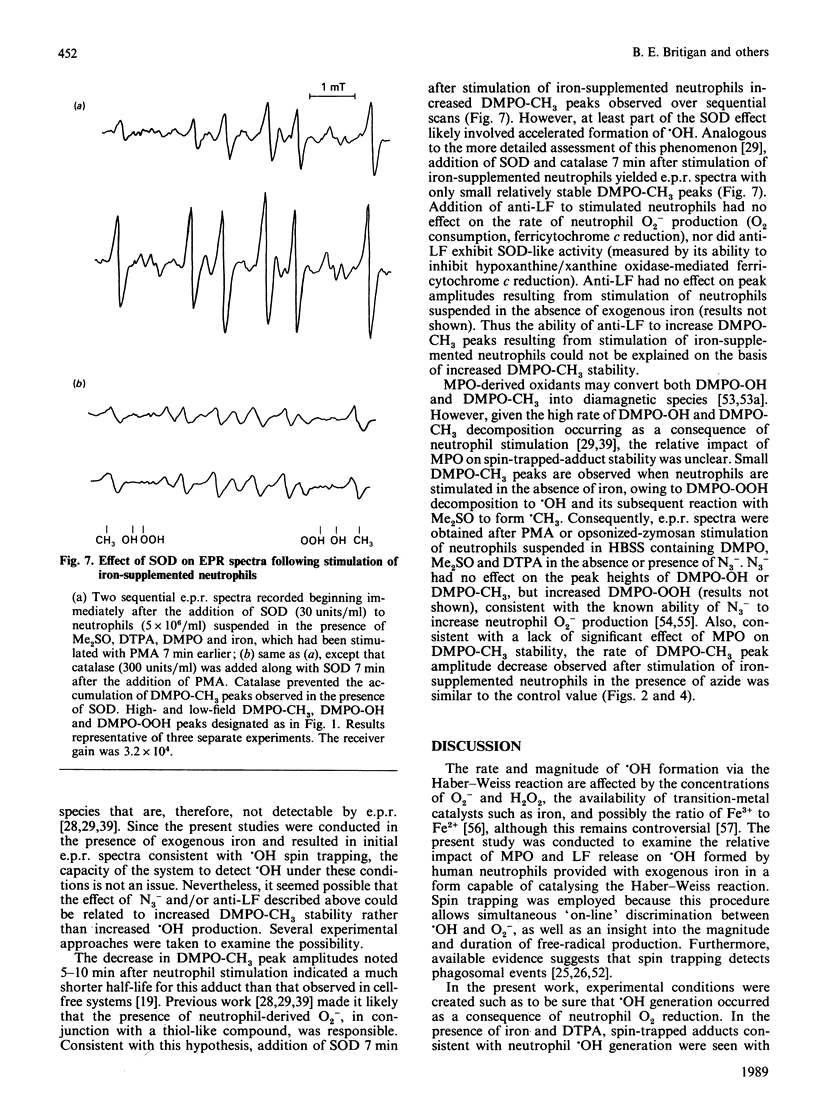
Hydroxyl radical (.OH) formation by neutrophils in vitro requires exogenous iron. Two recent studies [Britigan, Rosen, Thompson, Chai & Cohen (1986) J. Biol. Chem. 261, 17026-17032; Winterbourn (1987) J. Clin. Invest. 78, 545-550] both reported that neutrophil degranulation could potentially inhibit the formation of .OH, but differed in their conclusions as to the responsible factor, myeloperoxidase (MPO) or lactoferrin (LF). By using a previously developed spin-trapping system which allows specific on-line detection of superoxide anion (O2-) and .OH production, the impact of MPO and LF release on neutrophil .OH production was compared. When iron-diethylenetriaminepenta-acetic acid-supplemented neutrophils were stimulated with phorbol myristate acetate or opsonized zymosan, .OH formation occurred, but terminated prematurely in spite of continued O2- generation. Inhibition of MPO by azide increased the magnitude, but not the duration, of .OH formation. No azide effect was noted when MPO-deficient neutrophils were used. Anti-LF antibody increased both the magnitude and duration of .OH generation. Pretreatment of neutrophils with cytochalasin B to prevent phagosome formation did not alter the relative impact of azide or anti-LF on neutrophil .OH production. An effect of azide or anti-LF on spin-trapped-adduct stability was eliminated as a confounding factor. These data indicate that neutrophils possess two mechanisms for limiting .OH production. Implications for neutrophil-derived oxidant damage are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ainscough E. W., Brodie A. M., Plowman J. E., Bloor S. J., Loehr J. S., Loehr T. M. Studies on human lactoferrin by electron paramagnetic resonance, fluorescence, and resonance Raman spectroscopy. Biochemistry. 1980 Aug 19;19(17):4072–4079. doi: 10.1021/bi00558a026. [DOI] [PubMed] [Google Scholar]
- Alexander M. S., Husney R. M., Sagone A. L., Jr Metabolism of benzoic acid by stimulated polymorphonuclear cells. Biochem Pharmacol. 1986 Oct 15;35(20):3649–3651. doi: 10.1016/0006-2952(86)90643-x. [DOI] [PubMed] [Google Scholar]
- Ambruso D. R., Johnston R. B., Jr Lactoferrin enhances hydroxyl radical production by human neutrophils, neutrophil particulate fractions, and an enzymatic generating system. J Clin Invest. 1981 Feb;67(2):352–360. doi: 10.1172/JCI110042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruoma O. I., Halliwell B., Laughton M. J., Quinlan G. J., Gutteridge J. M. The mechanism of initiation of lipid peroxidation. Evidence against a requirement for an iron(II)-iron(III) complex. Biochem J. 1989 Mar 1;258(2):617–620. doi: 10.1042/bj2580617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruoma O. I., Halliwell B. Superoxide-dependent and ascorbate-dependent formation of hydroxyl radicals from hydrogen peroxide in the presence of iron. Are lactoferrin and transferrin promoters of hydroxyl-radical generation? Biochem J. 1987 Jan 1;241(1):273–278. doi: 10.1042/bj2410273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin D. A., Jenny E. R., Aisen P. The effect of human serum transferrin and milk lactoferrin on hydroxyl radical formation from superoxide and hydrogen peroxide. J Biol Chem. 1984 Nov 10;259(21):13391–13394. [PubMed] [Google Scholar]
- Bannister J. V., Bannister W. H., Hill H. A., Thornalley P. J. Enhanced production of hydroxyl radicals by the xanthine-xanthine oxidase reaction in the presence of lactoferrin. Biochim Biophys Acta. 1982 Mar 15;715(1):116–120. doi: 10.1016/0304-4165(82)90056-3. [DOI] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. A mechanism for the production of ethylene from methional. The generation of the hydroxyl radical by xanthine oxidase. J Biol Chem. 1970 Sep 25;245(18):4641–4646. [PubMed] [Google Scholar]
- Biemond P., van Eijk H. G., Swaak A. J., Koster J. F. Iron mobilization from ferritin by superoxide derived from stimulated polymorphonuclear leukocytes. Possible mechanism in inflammation diseases. J Clin Invest. 1984 Jun;73(6):1576–1579. doi: 10.1172/JCI111364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N., Heiple J. M., Simons E. R., Clark R. A. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983 Jul;97(1):52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britigan B. E., Coffman T. J., Adelberg D. R., Cohen M. S. Mononuclear phagocytes have the potential for sustained hydroxyl radical production. Use of spin-trapping techniques to investigate mononuclear phagocyte free radical production. J Exp Med. 1988 Dec 1;168(6):2367–2372. doi: 10.1084/jem.168.6.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britigan B. E., Cohen M. S., Rosen G. M. Detection of the production of oxygen-centered free radicals by human neutrophils using spin trapping techniques: a critical perspective. J Leukoc Biol. 1987 Apr;41(4):349–362. doi: 10.1002/jlb.41.4.349. [DOI] [PubMed] [Google Scholar]
- Britigan B. E., Rosen G. M., Chai Y., Cohen M. S. Do human neutrophils make hydroxyl radical? Determination of free radicals generated by human neutrophils activated with a soluble or particulate stimulus using electron paramagnetic resonance spectrometry. J Biol Chem. 1986 Apr 5;261(10):4426–4431. [PubMed] [Google Scholar]
- Britigan B. E., Rosen G. M., Thompson B. Y., Chai Y., Cohen M. S. Stimulated human neutrophils limit iron-catalyzed hydroxyl radical formation as detected by spin-trapping techniques. J Biol Chem. 1986 Dec 25;261(36):17026–17032. [PubMed] [Google Scholar]
- Cohen M. S., Britigan B. E., Hassett D. J., Rosen G. M. Do humans neutrophils form hydroxyl radical? Evaluation of an unresolved controversy. Free Radic Biol Med. 1988;5(2):81–88. doi: 10.1016/0891-5849(88)90033-0. [DOI] [PubMed] [Google Scholar]
- Cohen M. S., Cooney M. H. A bacterial respiratory burst: stimulation of the metabolism of Neisseria gonorrhoeae by human serum. J Infect Dis. 1984 Jul;150(1):49–56. doi: 10.1093/infdis/150.1.49. [DOI] [PubMed] [Google Scholar]
- Cohen M. S., Metcalf J. A., Root R. K. Regulation of oxygen metabolism in human granulocytes: relationship between stimulus binding and oxidative response using plant lectins as probes. Blood. 1980 Jun;55(6):1003–1010. [PubMed] [Google Scholar]
- Finkelstein E., Rosen G. M., Rauckman E. J. Spin trapping of superoxide and hydroxyl radical: practical aspects. Arch Biochem Biophys. 1980 Mar;200(1):1–16. doi: 10.1016/0003-9861(80)90323-9. [DOI] [PubMed] [Google Scholar]
- Gallin J. I. Neutrophil specific granule deficiency. Annu Rev Med. 1985;36:263–274. doi: 10.1146/annurev.me.36.020185.001403. [DOI] [PubMed] [Google Scholar]
- Gannon D. E., Varani J., Phan S. H., Ward J. H., Kaplan J., Till G. O., Simon R. H., Ryan U. S., Ward P. A. Source of iron in neutrophil-mediated killing of endothelial cells. Lab Invest. 1987 Jul;57(1):37–44. [PubMed] [Google Scholar]
- Green M. R., Hill H. A., Okolow-Zubkowska M. J., Segal A. W. The production of hydroxyl and superoxide radicals by stimulated human neutrophils- measurements by EPR spectroscopy. FEBS Lett. 1979 Apr 1;100(1):23–26. doi: 10.1016/0014-5793(79)81123-0. [DOI] [PubMed] [Google Scholar]
- Green T. R., Fellman J. H., Eicher A. L. Myeloperoxidase oxidation of sulfur-centered and benzoic acid hydroxyl radical scavengers. FEBS Lett. 1985 Nov 11;192(1):33–36. doi: 10.1016/0014-5793(85)80037-5. [DOI] [PubMed] [Google Scholar]
- Greenwald R. A., Rush S. W., Moak S. A., Weitz Z. Conversion of superoxide generated by polymorphonuclear leukocytes to hydroxyl radical: a direct spectrophotometric detection system based on degradation of deoxyribose. Free Radic Biol Med. 1989;6(4):385–392. doi: 10.1016/0891-5849(89)90084-1. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Paterson S. K., Segal A. W., Halliwell B. Inhibition of lipid peroxidation by the iron-binding protein lactoferrin. Biochem J. 1981 Oct 1;199(1):259–261. doi: 10.1042/bj1990259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D. C., Aisen P. Facilitation of Fe(II) autoxidation by Fe(3) complexing agents. Biochim Biophys Acta. 1973 Nov 2;329(1):156–158. doi: 10.1016/0304-4165(73)90019-6. [DOI] [PubMed] [Google Scholar]
- Hawley D. A., Kleinhans F. W., Biesecker J. L. Determination of alternate pathway complement kinetics by electron spin resonance spectroscopy. Am J Clin Pathol. 1983 Jun;79(6):673–677. doi: 10.1093/ajcp/79.6.673. [DOI] [PubMed] [Google Scholar]
- Janzen E. G., Jandrisits L. T., Barber D. L. Studies on the origin of the hydroxyl spin adduct of DMPO produced from the stimulation of neutrophils by phorbol-12-myristate-13-acetate. Free Radic Res Commun. 1987;4(2):115–123. doi: 10.3109/10715768709088096. [DOI] [PubMed] [Google Scholar]
- Kaur H., Fagerheim I., Grootveld M., Puppo A., Halliwell B. Aromatic hydroxylation of phenylalanine as an assay for hydroxyl radicals: application to activated human neutrophils and to the heme protein leghemoglobin. Anal Biochem. 1988 Aug 1;172(2):360–367. doi: 10.1016/0003-2697(88)90456-3. [DOI] [PubMed] [Google Scholar]
- Kettle A. J., Sangster D. F., Gebicki J. M., Winterbourn C. C. A pulse radiolysis investigation of the reactions of myeloperoxidase with superoxide and hydrogen peroxide. Biochim Biophys Acta. 1988 Aug 31;956(1):58–62. doi: 10.1016/0167-4838(88)90297-x. [DOI] [PubMed] [Google Scholar]
- Kettle A. J., Winterbourn C. C. Superoxide modulates the activity of myeloperoxidase and optimizes the production of hypochlorous acid. Biochem J. 1988 Jun 1;252(2):529–536. doi: 10.1042/bj2520529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J., Hamon C. B. Role of myeloperoxidase-mediated antimicrobial systems in intact leukocytes. J Reticuloendothel Soc. 1972 Aug;12(2):170–196. [PubMed] [Google Scholar]
- Klebanoff S. J. Iodination catalyzed by the xanthine oxidase system: role of hydroxyl radicals. Biochemistry. 1982 Aug 17;21(17):4110–4116. doi: 10.1021/bi00260a030. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Rosen H. Ethylene formation by polymorphonuclear leukocytes. Role of myeloperoxidase. J Exp Med. 1978 Aug 1;148(2):490–506. doi: 10.1084/jem.148.2.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Minotti G., Aust S. D. The requirement for iron (III) in the initiation of lipid peroxidation by iron (II) and hydrogen peroxide. J Biol Chem. 1987 Jan 25;262(3):1098–1104. [PubMed] [Google Scholar]
- Nauseef W. M., Metcalf J. A., Root R. K. Role of myeloperoxidase in the respiratory burst of human neutrophils. Blood. 1983 Mar;61(3):483–492. [PubMed] [Google Scholar]
- Nauseef W. M. Myeloperoxidase biosynthesis by a human promyelocytic leukemia cell line: insight into myeloperoxidase deficiency. Blood. 1986 Apr;67(4):865–872. [PubMed] [Google Scholar]
- Parry M. F., Root R. K., Metcalf J. A., Delaney K. K., Kaplow L. S., Richar W. J. Myeloperoxidase deficiency: prevalence and clinical significance. Ann Intern Med. 1981 Sep;95(3):293–301. doi: 10.7326/0003-4819-95-3-293. [DOI] [PubMed] [Google Scholar]
- Pou S., Cohen M. S., Britigan B. E., Rosen G. M. Spin-trapping and human neutrophils. Limits of detection of hydroxyl radical. J Biol Chem. 1989 Jul 25;264(21):12299–12302. [PubMed] [Google Scholar]
- Pou S., Rosen G. M., Britigan B. E., Cohen M. S. Intracellular spin-trapping of oxygen-centered radicals generated by human neutrophils. Biochim Biophys Acta. 1989 Jun 27;991(3):459–464. doi: 10.1016/0304-4165(89)90073-1. [DOI] [PubMed] [Google Scholar]
- Pryor W. A., Tang R. H. Ethylene formation from methional. Biochem Biophys Res Commun. 1978 Mar 30;81(2):498–503. doi: 10.1016/0006-291x(78)91562-0. [DOI] [PubMed] [Google Scholar]
- Pryzwansky K. B., Martin L. E., Spitznagel J. K. Immunocytochemical localization of myeloperoxidase, lactoferrin, lysozyme and neutral proteases in human monocytes and neutrophilic granulocytes. J Reticuloendothel Soc. 1978 Sep;24(3):295–310. [PubMed] [Google Scholar]
- Repine J. E., Eaton J. W., Anders M. W., Hoidal J. R., Fox R. B. Generation of hydroxyl radical by enzymes, chemicals, and human phagocytes in vitro. Detection with the anti-inflammatory agent, dimethyl sulfoxide. J Clin Invest. 1979 Dec;64(6):1642–1651. doi: 10.1172/JCI109626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repine J. E., Fox R. B., Berger E. M. Hydrogen peroxide kills Staphylococcus aureus by reacting with staphylococcal iron to form hydroxyl radical. J Biol Chem. 1981 Jul 25;256(14):7094–7096. [PubMed] [Google Scholar]
- Repine J. E., Johansen K. S., Berger E. M. Hydroxyl radical scavengers produce similar decreases in the chemiluminescence responses and bactericidal activities of neutrophils. Infect Immun. 1984 Jan;43(1):435–437. doi: 10.1128/iai.43.1.435-437.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Cohen M. S. The microbicidal mechanisms of human neutrophils and eosinophils. Rev Infect Dis. 1981 May-Jun;3(3):565–598. doi: 10.1093/clinids/3.3.565. [DOI] [PubMed] [Google Scholar]
- Root R. K., Metcalf J. A. H2O2 release from human granulocytes during phagocytosis. Relationship to superoxide anion formation and cellular catabolism of H2O2: studies with normal and cytochalasin B-treated cells. J Clin Invest. 1977 Dec;60(6):1266–1279. doi: 10.1172/JCI108886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen G. M., Britigan B. E., Cohen M. S., Ellington S. P., Barber M. J. Detection of phagocyte-derived free radicals with spin trapping techniques: effect of temperature and cellular metabolism. Biochim Biophys Acta. 1988 May 13;969(3):236–241. doi: 10.1016/0167-4889(88)90057-2. [DOI] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Hydroxyl radical generation by polymorphonuclear leukocytes measured by electron spin resonance spectroscopy. J Clin Invest. 1979 Dec;64(6):1725–1729. doi: 10.1172/JCI109637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadrzadeh S. M., Graf E., Panter S. S., Hallaway P. E., Eaton J. W. Hemoglobin. A biologic fenton reagent. J Biol Chem. 1984 Dec 10;259(23):14354–14356. [PubMed] [Google Scholar]
- Sagone A. L., Jr, Decker M. A., Wells R. M., Democko C. A new method for the detection of hydroxyl radical production by phagocytic cells. Biochim Biophys Acta. 1980 Feb 21;628(1):90–97. doi: 10.1016/0304-4165(80)90354-2. [DOI] [PubMed] [Google Scholar]
- Sagone A. L., Jr, Husney R. M. Oxidation of salicylates by stimulated granulocytes: evidence that these drugs act as free radical scavengers in biological systems. J Immunol. 1987 Apr 1;138(7):2177–2183. [PubMed] [Google Scholar]
- Sagone A. L., Jr, Wells R. M., DeMocko C. Evidence that OH. production by human PMNs is related to prostaglandin metabolism. Inflammation. 1980 Mar;4(1):65–71. doi: 10.1007/BF00914104. [DOI] [PubMed] [Google Scholar]
- Samuni A., Black C. D., Krishna C. M., Malech H. L., Bernstein E. F., Russo A. Hydroxyl radical production by stimulated neutrophils reappraised. J Biol Chem. 1988 Sep 25;263(27):13797–13801. [PubMed] [Google Scholar]
- Tauber A. I., Babior B. M. Evidence for hydroxyl radical production by human neutrophils. J Clin Invest. 1977 Aug;60(2):374–379. doi: 10.1172/JCI108786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber A. I., Gabig T. G., Babior B. M. Evidence for production of oxidizing radicals by the particulate O-2-forming system from human neutrophils. Blood. 1979 Apr;53(4):666–676. [PubMed] [Google Scholar]
- Thomas C. E., Morehouse L. A., Aust S. D. Ferritin and superoxide-dependent lipid peroxidation. J Biol Chem. 1985 Mar 25;260(6):3275–3280. [PubMed] [Google Scholar]
- Thomas M. J., Shirley P. S., Hedrick C. C., DeChatelet L. R. Role of free radical processes in stimulated human polymorphonuclear leukocytes. Biochemistry. 1986 Dec 2;25(24):8042–8048. doi: 10.1021/bi00372a037. [DOI] [PubMed] [Google Scholar]
- Wang-Iverson P., Pryzwansky K. B., Spitznagel J. K., Cooney M. H. Bactericidal capacity of phorbol myristate acetate-treated human polymorphonuclear leukocytes. Infect Immun. 1978 Dec;22(3):945–955. doi: 10.1128/iai.22.3.945-955.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J. Oxygen, ischemia and inflammation. Acta Physiol Scand Suppl. 1986;548:9–37. [PubMed] [Google Scholar]
- Weiss S. J., Rustagi P. K., LoBuglio A. F. Human granulocyte generation of hydroxyl radical. J Exp Med. 1978 Feb 1;147(2):316–323. doi: 10.1084/jem.147.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston G. W., Eibschutz O. M., Strekas T., Cederbaum A. I. Complex-formation and reduction of ferric iron by 2-oxo-4-thiomethylbutyric acid, and the production of hydroxyl radicals. Biochem J. 1986 Apr 15;235(2):521–529. doi: 10.1042/bj2350521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C. Lactoferrin-catalysed hydroxyl radical production. Additional requirement for a chelating agent. Biochem J. 1983 Jan 15;210(1):15–19. doi: 10.1042/bj2100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C. Myeloperoxidase as an effective inhibitor of hydroxyl radical production. Implications for the oxidative reactions of neutrophils. J Clin Invest. 1986 Aug;78(2):545–550. doi: 10.1172/JCI112607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C. The ability of scavengers to distinguish OH. production in the iron-catalyzed Haber-Weiss reaction: comparison of four assays for OH. Free Radic Biol Med. 1987;3(1):33–39. doi: 10.1016/0891-5849(87)90037-2. [DOI] [PubMed] [Google Scholar]


