Abstract
Human cytomegalovirus (hCMV) is a ubiquitous latent persistent herpesvirus infecting 60–90% of the population worldwide. hCMV carriage in immunocompetent people is asymptomatic; thus, hCMV can be considered a component of normative aging. However, hCMV powerfully modulates many features of the immune, and likely other, systems and organs. Questions remain as to how hCMV carriage affects the human host. We used anti-CMV antibody titers as a stratifying criterion to examine the impact of “intensity” of hCMV infection as a potential biomarker of aging, inflammation, and immune homeostasis in a cohort of 247 participants stratified into younger (21–40 years) and older (> 65 years of age) groups. We showed that anti-CMV antibody titers increased with age and directly correlated to increased levels of soluble tumor necrosis factor (sTNFR) I in younger but not older participants. CD8 + cell numbers were reduced in the older group due to the loss in CD8 + T naïve (Tn) cells. In CMV carriers and, in particular, in anti-CMV Ab-high participants, this loss was mitigated or reversed by an increase in the numbers of CD8 + T effector memory (Tem) and T effector memory reexpressing CD45RA (Temra) cells. Analysis of CD38, HLA-DR, and CD57 expression revealed subset (CD4 or CD8)-specific changes that correlated with anti-CMV Ab levels. In addition, anti-CMV Ab levels predicted anti-CMV CD8 T cell responsiveness to different CMV open reading frames (ORFs) selectively in older participants, which correlated to the transcriptional order of expression of specific CMV ORFs. Implications of these results for the potential predictive value of anti-CMV Ab titers during aging are discussed.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-024-01124-0.
Keywords: CMV, Immune aging, Immune profiles
Introduction
Human cytomegalovirus (hCMV) is one of the most ubiquitous persistent and latent microbial pathogens in the human population [1]. hCMV infects 40–100%, of the human population, with the prevalence increasing with age, worse socioeconomic status, and worse hygienic conditions. Even in the industrial countries, the older population, defined as those > 65 years of age, are > 70% infected with this virus [2]. The major characteristics of hCMV lifecycle are persistence with cycles of reactivation from latency over the course of a lifespan of its host. While hCMV has the potential to cause severe disease in immunocompromised hosts, including the developing fetus and those with innate or acquired immunodeficiencies [3–5], immunocompetent hosts are known to live long lives with hCMV and no apparent virus-related pathology making hCMV acquisition a part of the normative aging process in most humans [6]. hCMV is one of the master evaders of the immune system [7], and is known to modulate many parameters of systemic immunity more than any other known microbial pathogen [8]. Whether lifelong carriage of hCMV is detrimental to the host or not is a matter of considerable debate. In some studies, hCMV was connected to manifestations ranging from overall reduction in longevity [9], to type 2 diabetes [10], cardiovascular disease (CVD) [11, 12], immune aging [13], and other deleterious effects. Other studies, however, failed to substantiate this relationship [14, 15], including the large longitudinal studies that found little evidence for the association between hCMV and human chronic diseases typically associated with aging [2]. Moreover, studies in humans [16] and mice [17, 18] documented that CMV exhibits features of commensalism with its mammalian host to the effect of increasing host resistance to other infections via both innate [17] and adaptive [18] immune mechanisms.
Regardless of the net effect of hCMV on human health, there is no doubt that all CMVs, including hCMV, are powerful modulators of the immune system. Early studies examining immunodominance and epitope-specific responses to hCMV [19, 20] documented an exceptional breadth and intensity of anti-CMV CD8 T cell responses to the point that in certain humans, CMV responses occupied 50% or more of the entire T cell memory compartment [20]. Subsequently, in a landmark study of human monozygotic twins, Brodin and colleagues found that CMV singlehandedly modulated 58% out of > 130 cellular, signaling, and soluble parameters of immunity [8]. Of interest, there is considerable heterogeneity in human immune responses against hCMV, which raises the possibility that different individuals may be dealing with different loads of the virus at primary infection or with different frequencies of reactivations or reinfections, all of which could determine both their response to hCMV and the impact of hCMV on their health. If so, there may simply be different levels of “total lifetime hCMV load/exposure” in different individuals that individually, or with other stressors, may predispose a subset of people carrying hCMV to adverse outcomes. Questions remain on (i) whether and to what extent relations exist between “total hCMV load/exposure” and adverse outcomes; (ii) whether hCMV-mediated immune modulation correlates with chronological aging; and, perhaps more of practical value, (iii) whether the immune response against hCMV could be used as a biomarker of general, inflammatory, and/or immune aging.
With regard to the last question, levels of anti-CMV antibodies may provide a simple and straightforward surrogate to potentially quantify and describe the host’s reactivity to CMV and perhaps a proxy biomarker for immune, inflammatory, or general aging. Different studies performed with anti-CMV antibody titers so far have obtained different results in different populations of older adults. For example, Adriaensen et al. [21] found that low levels of anti-CMV antibodies and of differentiated T cells in the very old have correlated to poor health. By contrast, Vescovini et al. [22] described a positive association between high levels of anti-CMV Ab and anti-CMV CD4 responses and impaired health and cognitive status. Li et al. [23], in a small cohort of 15 females, found that neither Ab levels nor anti-CMV T cell levels changed over 12 years of follow-up and did not correlate with other immune or virological parameters. Lustig et al. [24] studied cytokines, telomere shortening, and anti-CMV antibodies and found no correlation with anti-CMV antibodies. Therefore, there are no definitive, accepted findings on the predictive values and cosegregation of immune phenotypic traits with anti-CMV Ab levels. Here, we sought to test in a larger study whether levels of antibodies can be used as a biomarker/proxy for overall immune profiles, hCMV immune, and overall inflammatory responses over the lifespan. We used quantitative measurements of total anti-CMV antibody (Ab) levels as a stratifying criterion and asked whether stratification into anti-CMV Ab-negative, -low, or -high groups may be informative in predicting immune profiles, including levels of inflammation, and non-specific or hCMV-specific immunity in an age-sensitive manner. Our results demonstrate a nuanced but clearly stratified inflammatory and immune profiles in adults and, even more so, in older adults as a function of their anti-hCMV antibody status.
Materials and methods
Study subjects and blood samples
This study was approved by the Institutional Review Boards at the University of Arizona (Tucson, AZ; # 1,510,182,734) and the Oregon Health and Science University (Portland, OR; # IRB00003007). Human samples were obtained from healthy donors, 21–101 years of age, recruited at the OHSU or the University of Arizona. The younger group (also called younger adults or adults) was 21–40 years of age; the older group (older or older adults) was ≥ 65 years of age. Exclusion criteria included known immunosuppressive treatments, HIV infection, stroke, cancer, or use of steroids within the last 5 years. Blood was drawn into heparinized vacutainer tubes (BD Bioscience, San Jose, CA, USA) and processed fresh to isolate peripheral blood mononuclear cells (PBMCs) and plasma. PBMCs were isolated using Ficoll-Paque (GE Healthcare, Chicago, IL, USA) density gradient media. PBMCs were viably cryopreserved using 90% fetal bovine serum (FBS)/10% DMSO. Some of the samples collected in Arizona were also processed and stored at the University of Arizona Health Sciences Biorepository. A parallel sample of whole blood was collected in K2-EDTA tubes to determine complete blood cell counts. The complete blood count (CBC) was determined by submitting samples to the Sonora Quest Laboratories (Phoenix, AZ).
Cytokine and chemokine measurements
Plasma cytokine and chemokine concentrations were measured as previously described [25] using cytokine bead array kits from Becton Dickinson. The sensitivity for this assay is 10 pg/ml for most cytokines and chemokines measured. We measured IL-1, IL-6, IL-10, IL-17a, IFN-γ, TNF-α, soluble TNFR 1 and 2, RANTES, CXCL9 (MIG), CXCL10 (IP-10), MCP-1 (CCL2), and IL-8.
Flow cytometry and T cell stimulation
Frozen PBMCs were thawed in RPMI medium supplemented with 10% FBS, penicillin, and streptomycin in the presence of DNase (Sigma, Saint Louis, MO), rested overnight in X-vivo medium (Lonza/Basel, Switzerland) supplemented with 5% human male AB serum. PBMCs were stained with anti-CD3-BV570, anti-CD4-BV750, anti-CD8b-BV785, anti-CD28-PEDazzle594, anti-CD95-BV421, anti-CD57-APC, anti-CD38-PerCPCy5.5, anti-HLA-DR-AF700, anti-CD56-BV650 (Biolegend), anti-CD45RA-APCE780 (eBioscience), anti-CCR7-PECy7 (BD Bioscience), anti-CD16-FITC (Invitrogen), anti-NKG2C-PE (R&D systems), and Zombie aqua (Biolegend). FCM acquisition was performed on the BD Fortessa instrument, using DiVa acquisition (BD Immunocytometry Systems, Mountain View, CA) and the FlowJo analysis software (Tree Star, Ashland, OR). Absolute CD4 and CD8 T cell numbers were calculated by multiplying the percentage by the total lymphocyte number determined from the CBC described above. The subsets of T cells were defined as follows: naïve (Tn), CD28intCD95lo; central memory (Tcm), CD28hiCD95hi; total effector memory (Tem), CD28lo or CD28loCD95hi; Tem, CD45RA− Tem; and Tem reexpressing CD45RA (Temra), CD45RA+ Tem [26]. Gate for CD57 expression was set by using a fluorescence minus one (FMO) control. NK cells were defined as CD3−CD16+CD56+ and gate for NKG2C expression was set by using FMO control.
T cell stimulation was performed as previously described [27]. Briefly, PBMCs were stimulated with either PMA and ionomycin (cell stimulation cocktail, eBioscience), or with overlapping CMV IE1, or CMV pp65 peptide pools (15-mers overlapping by 9 amino acids, both from Miltenyi Biotec, San Diego, CA) for 3 h in the presence of Brefeldin A (eBioscience). Peptides were used according to the manufacturer’s instruction at approximately 1 µg/ml of each peptide. After the simulations, the surface proteins were stained with anti-CD3-BV570, anti-CD4-BV650, and anti-CD8b-ECD. For intracellular staining, cells were fixed and permeabilized with BD Cytofix/Cytoperm solution kit (BD Bioscience) and incubated with anti-IFN-γ-AF700 and anti-TNF-α-BV421 for 30 min at 4 °C. FCM acquisition and analysis were performed as described as above.
Antibody titers against CMV
Ninety-six well microtiter plates, coated with inactivated lysates of MRC-5 (diploid fibroblast) cells infected with CMV strain AD169, were obtained from EuroImmun (EI 2570–9601-G; Morris Plains, NJ). Serum samples with high immunofluorescence assay-scored antibody titers (i.e., 2560), obtained from prior studies, were used as the top standards for CMV as previously described [28]. Two-fold serial dilutions of the standards (2560, 1280, 640, 320, 160, 80, 40, and 20) were made with PBS in separate tubes. One hundred microliters of positive and negative controls, standards, and diluted patient samples (all dilutions were at 1:101 with PBS) were pipetted in duplicate into individual microplate wells followed by a 30-min incubation (all steps were carried out at room temperature). The plates were then washed 3 times with 350 µl wash buffer using an Embla microplate washer (Molecular Devices, Menlo Park, CA). Next, 100 µl of enzyme conjugate (peroxidase-labeled anti-human IgG) was pipetted into the wells followed by another 30-min incubation period. The plates were then washed 3 times, and 100 µl of chromogen substrate (TMB/H2O2) was pipetted into the wells. The plates were then covered to protect from direct light and incubated for 15 min. One hundred microliters of 0.5 M sulfuric acid was added to each well to stop the reaction. Absorbance was then read at 450 nm (reference wavelength 620 nm) using a SpectraMax Plus 384 (Molecular Devices). The values of the unknown samples were assigned in relation to the standard curve.
Neutralization antibody titer to CMV
Human foreskin fibroblast (HFF)-1 was purchased from ATCC (#SCRC-1041). HFF-1 cells were grown in complete DMEM (4.5 g glucose/L, 4 mM L-glutamine, 1 mM Sodium Pyruvate, 1X penicillin/streptomycin, and 15% FBS). GFP-expressing hCMV virus (strain TB40E) was kindly provided by Dr. Felicia Goodrum (University of Arizona) [29]. Human plasma from each subject was diluted in complete medium at 1:10, followed by two-fold dilutions 5 times. The diluted plasma was then mixed with an equal volume of medium containing 800 infectious unit (IU) of virus and incubated for 2 h at room temperature. HFF-1 monolayer in a 96-well plate was infected with half the amount of the plasma/virus mixture. No plasma control wells were infected with medium containing 400 IU of the virus. The plate was incubated in 37 °C CO2 incubator for 48 h. The number of GFP-positive cells per field was counted by Cytation 5 (Biotek). The cut-off value was calculated as 20% of no plasma control, which represents 80% reduction of the infection. The highest dilution rate, which exceeded 20% positive, was read as an 80% neutralizing titer (NT80).
Statistics
The following statistical analyses were performed using R v4.2.1 and GraphPad Prism 8.0.2. Statistical significance was declared at the significance level of 5%. Descriptive statistics were calculated for the demographic characteristics by age groups in the full sample and the stimulation subset. Demographics were summarized using median and quartiles for continuous variables and frequency and percent for categorical variables. Comparisons of anti-CMV antibody titers between age groups were assessed using two-sided Student’s t-tests. Based on anti-CMV antibody titers, each age group was divided into 3 subgroups by CMV serostatus (negative; low, Ab titer ≤ 200; and high, Ab titer > 200). Trichotomizing Ab titers allows us to accommodate non-linear relationships between outcomes and Ab titers. The two-way analysis of variance (ANOVA) was conducted to assess the interaction effects between age groups and CMV serostatus on each inflammatory and immune phenotype. For phenotypes that interaction effects were found to be significant, corresponding contrasts were tested for comparisons between age groups within each anti-CMV Ab group and comparisons between anti-CMV Ab groups within each age group. The main effects of age groups and anti-CMV Ab groups were reported for phenotypes without significant interaction effects. Holm’s method was used to adjust the p-values for comparing estimated marginal means within each phenotype [30]. A value of p < 0.05 was deemed significant after Holm’s adjustment.
T cell stimulation analysis was done for a subset of 88 younger participants and 87 older participants due to the limit of sample availability. To obtain the subset for stimulation analysis, 28–31 subjects were randomly selected by age group in each CMV titer. To examine if demographic characteristics shifted after subsampling, we compared demographic variables between the selected subset and non-selected subset using Fisher’s exact test for categorical variables and Wilcoxon rank sum test for continuous variables. Nonnegligible differences in distributions of sex (p-value = 0.0014) and ethnicity (p-value = 0.0014) were detected in the older participants. Although differences in age and race were statistically significant (p-values = 0.0452 and 0.0180), we found the size of the difference in age was negligible (median 72 vs 73 in selected and non-selected respectively), and we believed the difference in race was caused by random varying in race categories that had very small counts. Therefore, we only adjusted for sex and ethnicity in subset analyses. The age group by CMV serostatus interactions was evaluated by regression models adjusting for sex and ethnicity. Group comparisons were conducted following the previously described method. A p-value < 0.05 was deemed significant after Holm’s adjustment within each phenotype. The correlations between CMV Ab titer and percentages of cytokine-expressing cells were evaluated by Pearson correlation coefficients.
Results
Cohort description and stratification by anti-CMV antibody titers
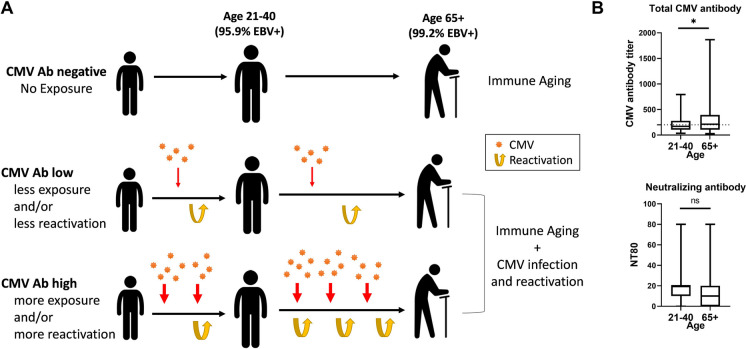
To assess the interaction between CMV antibody titer and immune aging, we randomly selected community-dwelling healthy study participants from Oregon and Arizona. Exclusion criteria comprised immunosuppressive conditions or therapy (including corticosteroid treatment within the last 5 years), stroke, cancer, or chemotherapy within the past 5 years. Age groups comprise 21–40 years (younger) and 65 and above (older participants). Based on anti-CMV antibody titers in plasma, each group was divided into 3 subgroups by CMV serostatus. The first group consisted of CMV seronegative participants, with CMV antibodies (Ab) below the level of detection that made up 37.2% of younger participants and 25.6% of older participants (Table 1; Fig. 1A, B). The other two groups were CMV Ab-low (detectable anti-CMV Ab titer ≤ 200) and CMV Ab-high (anti-CMV Ab titer > 200) (Fig. 1B). The threshold of 200 was arbitrarily chosen between median titers of CMV + younger participants (titer, 170) and CMV + older participants (titer, 213). In the CMV seropositive populations, the mean anti-CMV Ab titer in older participants was significantly higher than in younger participants (p = 0.0133, Fig. 1B top panel). By contrast, neutralizing antibody levels against CMV in the same populations were not sensitive to age (p = 0.4025, Fig. 1B bottom panel).
Table 1.
Participants’ characteristics
| Overall | Age 20–40 | Age 65 + | |||
|---|---|---|---|---|---|
| (n = 247) | All (n = 122) | Stim (n = 88)* | All (n = 125) | Stim (n = 87)* | |
| Age, median (Q1, Q3) | 65.0 (29.4, 72.2) | 29.4 (25.0, 33.6) | 29.0 (25.0, 33.0) | 72.0 (69.0, 80.2) | 71 (68.0, 76.0) |
| Sex, n (%) | |||||
| Female | 139 (56.3%) | 61 (50.0%) | 41 (46.6%) | 78 (62.4%) | 46 (52.9%) |
| Male | 108 (43.7%) | 61 (50.0%) | 47 (53.4%) | 47 (37.6%) | 41 (47.1%) |
| Ethnicity, n (%) | |||||
| Hispanic or Latino | 74 (30.1%) | 46 (37.7%) | 32 (36.4%) | 28 (22.6%) | 14 (16.1%) |
| Not Hispanic or Latino | 172 (69.9%) | 76 (62.3%) | 56 (63.6%) | 96 (77.4%) | 73 (83.9%) |
| Race, n (%) | |||||
| White | 224 (91.1%) | 106 (86.9%) | 76 (86.4%) | 118 (95.2%) | 85 (97.7%) |
| Black or African American | 7 (2.8%) | 5 (4.1%) | 4 (4.5%) | 2 (1.6%) | 1 (1.1%) |
| Asian | 8 (3.3%) | 7 (5.7%) | 6 (6.8%) | 1 (0.8%) | 1 (1.1%) |
| American Indian/Alaska Native | 1 (0.4%) | 1 (0.8%) | 1 (1.1%) | 0 (0%) | 0 (0%) |
| Pacific Islander | 1 (0.4%) | 1 (0.8%) | 1 (1.1%) | 0 (0%) | 0 (0%) |
| Other | 5 (2.0%) | 2 (1.6%) | 0 (0%) | 3 (2.4%) | 0 (0%) |
| CMV antibody titer, n (%) | |||||
| Negative (< 20) | 77 (31.3%) | 45 (37.2%) | 29 (33.0%) | 32 (25.6%) | 28 (32.2%) |
| Low (20–199) | 88 (35.8%) | 45 (37.2%) | 29 (33.0%) | 43 (34.4%) | 31 (35.6%) |
| High (200–1865) | 81 (32.9%) | 31 (25.6%) | 30 (34.1%) | 50 (40.0%) | 28(32.2%) |
*PMA stim and CMV stim experiment (Fig. 6) was performed on Stim Cohort only due to the limited sample availability
Fig. 1.
Cohort stratification by age, CMV and EBV serostatus and anti-CMV antibody response magnitude in relation to expected relationship to CMV exposure and reactivation
To obtain better insight into the relationships between anti-CMV Ab titers and immunity or aging, we profiled numerous immune parameters with the three anti-CMV Ab-stratified categories of participants, further stratified by age.
Relation between anti-CMV antibody titers and inflammatory status across aging
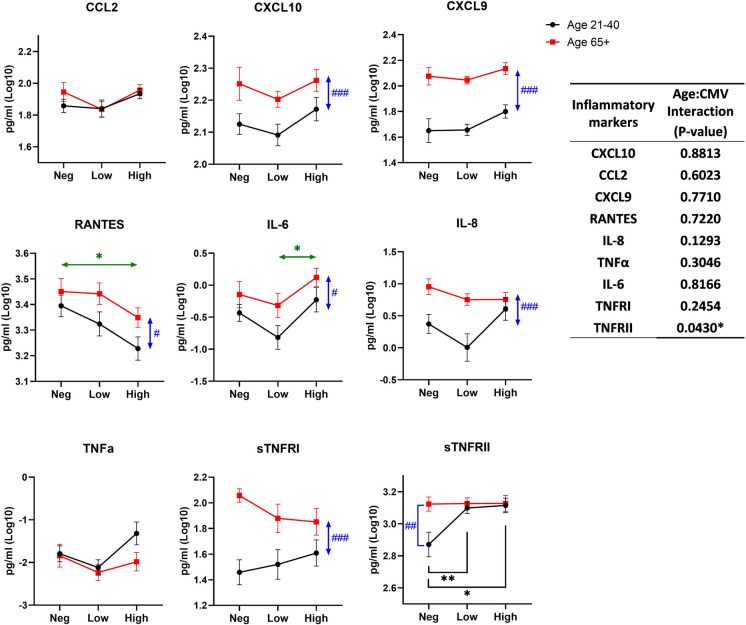
Aging alone can be accompanied by a subclinical increase in pro-inflammatory mediators, including IL-6, CRP, and soluble TNF receptors, depending on the cohort [31]. CMV reactivation is known to activate interferon responses that if intense enough, would further increase some pro-inflammatory mediators. To assess the relationships between aging and CMV seropositivity and Ab levels, we measured inflammatory cytokines and chemokines in plasma and compared the level of inflammatory markers between groups by CMV and age. Several cytokines were below detection levels (IL-2, IL-4, IL-10, IL-17A, IFN-γ, and TNF-α) in the majority of the samples and were not fitted for analysis (data not shown), Detectable inflammatory cytokines are shown in Fig. 2 CCL2 and TNF-α did not show any significant differences between CMV serostatus groups regardless of age or between age groups in all CMV groups. CXCL9, CXCL10, IL-8, and sTNFRI levels in older participants were higher relative to younger counterparts (p-values: < 0.0001, 0.0008, 0.0002, and < 0.0001, respectively) and were not affected by CMV status. RANTES was also higher in older participants (p = 0.0201), but also showed a significant decline in the anti-CMV Ab-high titer group relative to the CMV-negative group (p = 0.0129). Conversely, IL-6 also exhibited differences by age, but these were pronounced in both younger and older participants with the increase in anti-CMV Ab titer. Finally, a significant interaction between age and CMV serostatus was found only in sTNFRII (p = 0.043). The sTNFRII levels were increased with seropositivity in younger participants (p-value for negative vs low, 0.0094, and p-value for negative vs high, 0.012). In older participants, sTNFRII was already high in the anti-CMV Ab-negative group and did not further increase as a function of increasing anti-CMV titers.
Fig. 2.
Effect of age and CMV serostatus on inflammatory markers in plasma. The interaction of age and CMV serostatus for each inflammatory marker level in plasma is analyzed by ANOVA (inset table). Data are presented as mean ± SE. Black circle and line, age 21–40; red square and line, age 65 + . Neg, anti-CMV antibody negative; low, anti-CMV antibody titer low; high, anti-CMV antibody titer high. Vertical double-head arrows (blue) represent the comparison between age groups regardless of CMV status and horizontal double-head arrows (Green) represent the comparison between CMV groups regardless of age. p-values for group comparisons were adjusted by Holm’s method. Statistical significance between age groups is shown as #p < 0.05, ##p < 0.01, and ###p < 0.001. Statistical significance between CMV groups is shown as *p < 0.05, **p < 0.01, and ***p < 0.001
Overall, this analysis showed that CMV seropositivity/titer did not correlate to inflammatory markers in older participants. There was an effect of CMV serostatus on RANTES (decline), IL-6, and sTNFRII (increase with increased titer), but overall, the effect of age in the inflammatory response was dominant over the effect of CMV serostatus and titers, particularly on CXCL9 and 10.
T cell memory inflation correlates with CMV antibody regardless of age
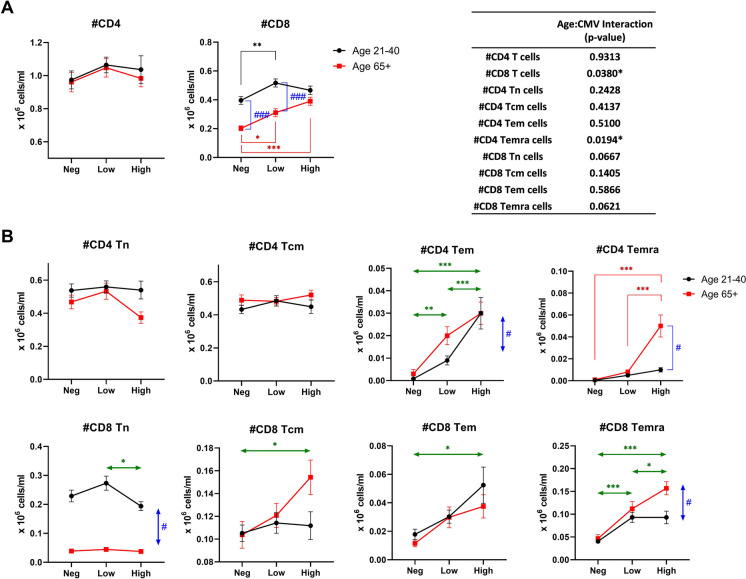
We used multicolor flow cytometry to simultaneously follow 14 lineage, activation, and differentiation markers on T cell subsets (see gating schema, Suppl. Figure 1). It has been established in the murine MCMV model that T cell memory inflation, an absolute numerical increase over time in T memory cell populations specific for certain CMV antigens, occurs in the course of a CMV infection [32–34]. We confirmed that that is the case in our current cohort (Fig. 3). While the absolute number of CD4 T cells in blood did not show any significant numerical changes with aging, CMV serostatus, or anti-CMV Ab titer (Fig. 3A, left panel), the absolute number of CD8 T cells showed a significant interaction between age and CMV serostatus (Fig. 3A, left panel and inset table). The number of CD8 T cells was reduced in CMV-negative older participants compared to in CMV-negative younger participants but increased with CMV-positivity and with increasing anti-CMV antibody titer. As a result, the absolute number of CD8 T cells in older participants with high CMV antibody titer was similar to those in younger counterparts.
Fig. 3.
Effect of age and CMV serostatus on numbers of T cell subsets in blood. The interaction of age and CMV serostatus for each T cell subset is analyzed by ANOVA (inset table). A Comparison of total CD4 and CD8 T cells between the age/CMV groups. B Comparison of T cell subsets between the age/CMV groups. Tn, naïve T cells; Tcm, central memory T cells; Tem, effector memory T cells; Temra, RA-positive effector memory T cells. Black circle and line, age 21–40; red square and line, age 65 + . Neg, anti-CMV antibody negative; low, anti-CMV antibody titer low; high, anti-CMV antibody titer high. Vertical double-head arrows (blue) represent the comparison between age groups regardless of CMV status and horizontal double-head arrows (Green) represent the comparison between CMV groups regardless of age. Data are presented as mean ± SE. p-values for group comparisons were adjusted by Holm’s method. Statistical significance between age groups is shown as #p < 0.05, ##p < 0.01, and ###p < 0.001. Statistical significance between CMV groups is shown as *p < 0.05, **p < 0.01, and ***p < 0.001
Analysis of T cell subsets stratified by differentiation status (Suppl. Figure 1) into T naïve (Tn, CD28int/hiCD95loCD45RAhi), T central memory (Tcm, CD28int/hiCD95hiCD45RAhi), T effector memory (Tem, CD28loCD95intCD45RAlo), and T effector memory reexpressing CD45 RA (Temra, CD28loCD95hiCD45RAhi) revealed further breakdown by CMV serostatus and antibody levels. Circulating CD4 Tn and Tcm cells showed no numerical changes with age or CMV seropositivity/Ab levels, whereas CD8 Tn cells showed an age-related absolute reduction irrespective of CMV serostatus/Ab levels (Fig. 3B), consistent with our prior work [35, 36]. CD8 Tcm cells and Tem cells accumulated in anti-CMV Ab-high participants, suggesting effects of CMV exposure length, infection dose, reactivation and/or reinfection on memory inflation (Fig. 3B).
Tem and Temra CD4 and CD8 cell subset numbers were the most sensitive correlates of CMV serostatus and anti-CMV Ab titers, as one may expect from the subsets directly engaged in responding to the virus [19, 37–43]. The numbers of all four subsets were the lowest in the CMV-negative group and progressively increased from anti-CMV Ab-low to anti-CMV Ab-high participants, demonstrating effector memory cell inflation in both age groups. Tem CD8 cell numbers were not age-sensitive relative to anti-CMV Ab levels, as they exhibited comparable inflation in both age groups, increasing comparably from anti-CMV Ab-negative to Ab-low to Ab-high (Fig. 3B, bottom third panel from left). There was a minor effect of age on Tem CD4 cell number that essentially followed the same, but less pronounced, inflation pattern observed in Tem CD8 cells (Fig. 3B, top third panels from left). By contrast, Temra cell numbers exhibited significantly larger accumulation selectively in older anti-CMV Ab-high participants, for both CD4 and CD8 populations (Fig. 3B, rightmost panels, top and bottom). This suggests progressive differentiation of T cells into the Temra fate with aging and the higher anti-CMV Ab levels.
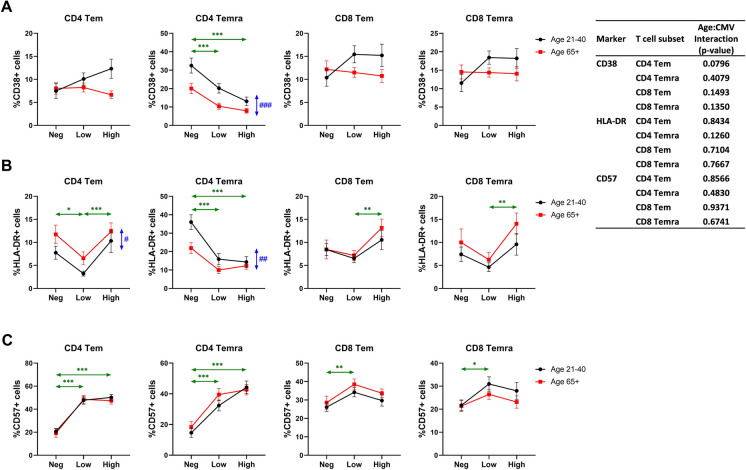
CMV serostatus correlates to expression of activation and differentiation markers on Tem cells in an age-dependent manner
To further elucidate the relationship between CMV serostatus/Ab levels and Tem and Temra cell differentiation across ages, we examined markers associated with Tem function and/or stipulated to be involved in their senescence. CD38 and HLA-DR are considered markers associated with T cell activation [44–46]. No significant differences were found in the expression of CD38 in Tem CD4 or CD8 cells or in CD8 Temra cells (Fig. 4A; HLA-DR expression in CD8 Tem and Temra was significantly higher in CMV Ab-high compared to CMV Ab-low (Fig. 4B). Some statistically significant changes were found in the expression of HLA-DR in CD4 Tem and Temra cells (Fig. 4B) suggesting that HLA-DR may be a more sensitive marker than CD38 in examining long-term activation in response to a persisting virus. Both CD38 and HLA-DR exhibited a clear pattern of reduced expression on CD4 Temra cells with CMV seropositivity and were significantly higher in the younger participants (Fig. 4A, B).
Fig. 4.
Effect of age and CMV serostatus on expression of functional markers on effector memory T cells. The interaction of age and CMV serostatus for each expression level of CD38 (A), HLA-DR (B), or CD57 (C) on effector memory CD4 and CD8 T cells is analyzed by ANOVA (inset table). Tem, effector memory T cells; Temra, RA-positive effector memory T cells. Black circle and line, age 21–40; red square and line, age 65 + . Neg, anti-CMV antibody negative; low, anti-CMV antibody titer low; high, anti-CMV antibody titer high. Vertical double-head arrows (blue) represent the comparison between age groups regardless of CMV status and horizontal double-head arrows (Green) represent the comparison between CMV groups regardless of age. Data are presented as mean ± SE. p-values for group comparisons were adjusted by Holm’s method. Statistical significance between age groups is shown as #p < 0.05, ##p < 0.01, and ###p < 0.001. Statistical significance between CMV groups is shown as *p < 0.05, **p < 0.01, and ***p < 0.001
We further analyzed the frequency of CD57 expression in Tem and Temra cell populations. CD57 is expressed on terminally differentiated cells and it has been considered by some authors as a senescence marker [47, 48], although definitive biological support for this classification is lacking. Perhaps surprisingly, we found no effect of age on the frequency of CD57 + CD4 and CD8Tem or Temra cells (Fig. 4C). Instead, CMV seropositivity, but not anti-CMV Ab levels, greatly contributed to the increase of CD57 + cell representation among both Tem and Temra CD4 T cells (Fig. 4C). The effect of CMV positivity on CD8 Tem and Temra was also observed in lesser degree.
We did not find any significant interaction of age and CMV serostatus for T cell functional markers, suggesting all changes were regulated by age or CMV status independently.
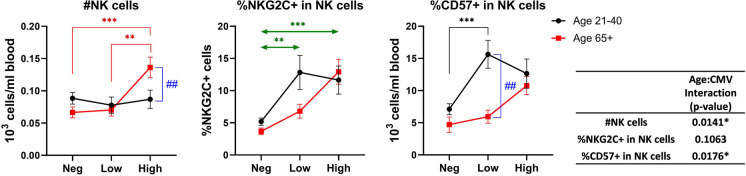
Increase of NK cells and NKG2C + NK cells in CMV + individual is both anti-CMV Ab titer and age-dependent
NK cells play a critical role in controlling acute and reactivating CMV infection and the homeostatic characteristics of NK cells are known to change after the CMV infection. We determined NK cell numbers and frequencies of NKG2C + (a receptor for CMV UL40 [49]) and CD57 + NK cells, both of which are known to expand with CMV infection [50]. NK numbers and frequency of CD57 + NK cells showed a significant interaction between age and CMV serostatus (Fig. 5). Specifically, the numbers of NK cells in blood were increased selectively in older participants with the anti-CMV Ab-high titers (Fig. 5, left panel). Expression of NKG2C and of CD57 was increased with CMV-seropositivity, regardless of antibody titer, but was more graded and most pronounced in older participants that were anti-CMV Ab-high (Fig. 5, middle and right panels).
Fig. 5.
Effect of age and CMV serostatus on NK cells. The interaction of age and CMV serostatus for the number of NK cells, frequency of NKG2C + cells in total NK cells, and frequency of CD57 + cells in total NK cells in blood is analyzed by ANOVA (inset table). Black circle and line, age 21–40; red square and line, age 65 + . Neg, anti-CMV antibody negative; low, anti-CMV antibody titer low; high, anti-CMV antibody titer high. Vertical double-head arrows (blue) represent the comparison between age groups regardless of CMV status and horizontal double-head arrows (Green) represent the comparison between CMV groups regardless of age. Data are presented as mean ± SE. p-values for group comparisons were adjusted by Holm’s method. Statistical significance between age groups is shown as #p < 0.05, ##p < 0.01, and ###p < 0.001. Statistical significance between CMV groups is shown as *p < 0.05, **p < 0.01, and ***p < 0.001
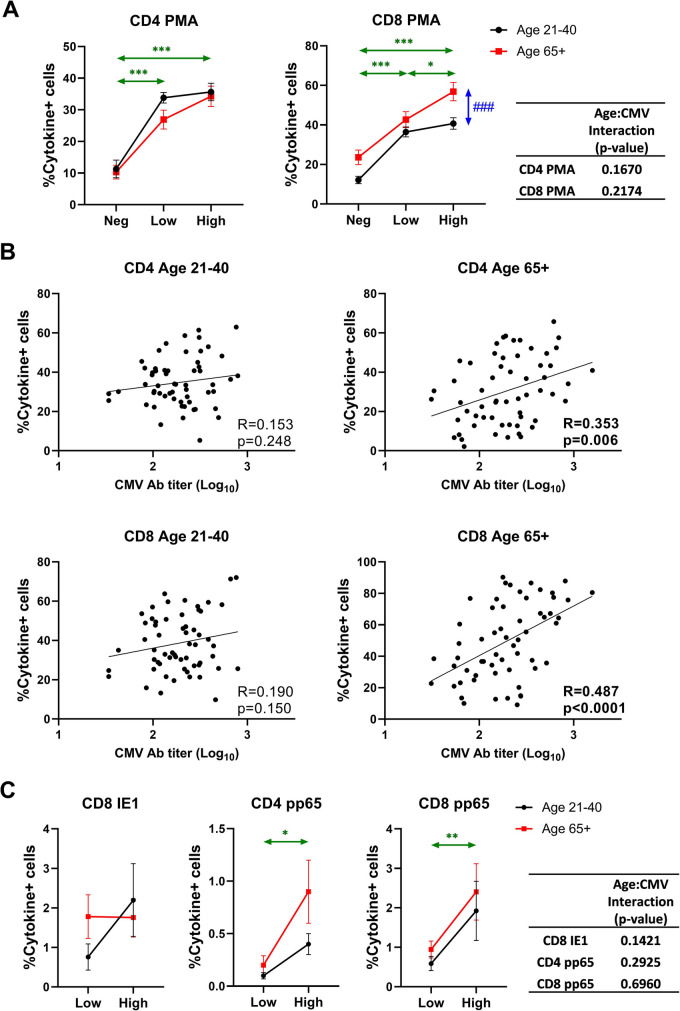
Prediction of T cell function by anti-CMV Ab titers
We next correlated CMV serostatus and antibody levels to polyclonal and antigen-specific responsiveness of CD4 and CD8 T cells. This experiment was done in a smaller cohort (Stim Cohort in Table 1) due to fewer samples available. In response to phorbol 12-myristate 13-acetate (PMA) and ionomycin, we found that both CD4 and CD8 T cells exhibited much more robust (~ 2–threefold, p < 0.001) cytokine response in CMV-seropositive participants as measured by intracellular production of IFNγ and TNFα (Fig. 6A). We found more cytokine expressing cells in CD8 T cells of older, compared to those of younger participants. Furthermore, this response correlated with anti-CMV Ab titers in CD8T cells of older, but not of younger participants (Fig. 6A, B), again pointing out to more extensive virus-related CD8 T cell activation with aging.
Fig. 6.
Effect of age and CMV serostatus on non-specific and CMV-specific T cell response. PBMCs were stimulated by PMA/ionomycin or CMV peptides and IFN-γ and TNF-α positive cells were quantified by intracellular staining followed by flow cytometry. Cytokine + cells are defined by IFN-γ + and/or TNF-α + cells. A Effect of age and CMV serostatus on non-specific T cell response. B Correlation analysis between CMV antibody titer and frequency of cytokine + cells in CD4 or CD8 of different age groups. Pearson’s R and correlation p-values are shown at the bottom right corner of each scatter plot. C Effect of age and CMV serostatus on CMV-specific T cell response. A and C Black circle and line, age 21–40; red square and line, age 65 + . Neg, anti-CMV antibody negative; low, anti-CMV antibody titer low; high, anti-CMV antibody titer high. Vertical double-head arrows (blue) represent the comparison between age groups regardless of CMV status and horizontal double-head arrows (Green) represent the comparison between CMV groups regardless of age. Data are presented as mean ± SE. The interaction of age and CMV serostatus for frequency of cytokine + cells in CD4 or CD8 T cells is analyzed by ANOVA. p-values for group comparisons were adjusted by Holm’s method. Statistical significance between age groups is shown as #p < 0.05, ##p < 0.01, and ###p < 0.001. Statistical significance between CMV groups is shown as *p < 0.05, **p < 0.01, and ***p < 0.001
We further stimulated CD4 and CD8 T cells from our CMV-seropositive participants with overlapping peptides spanning two highly immunodominant open reading frames (ORF) of hCMV, pp65, and IE1. CD4 T cell responses to the immediate early 1 ORF, IE1, were extremely low (0.02–0.05%) and did not differ with age or Ab titer levels (Fig. S2). While CD8 T cell responses to IE1 were much larger, they did not differ by age or anti-CMV antibody titers (Fig. 6C). However, anti-pp65 CD4 and CD8 responses showed antibody-level dependency (higher responses in anti-CMV Ab-hi groups, p = 0.0112 in CD4, p = 0.0048 in CD8), which was independent of age (Fig. 6C).
Discussion
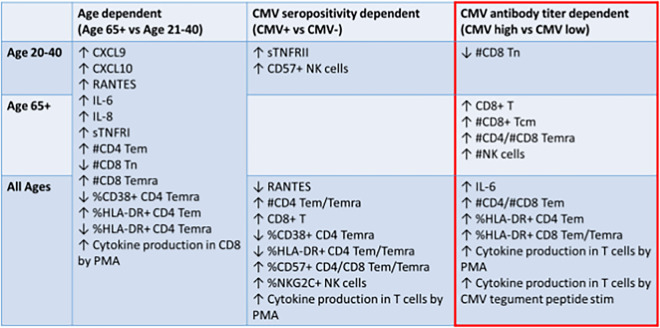
In this study, we have demonstrated that anti-CMV antibody levels can serve as a proxy of immune profiles that characterize certain aspects of inflammatory and immune aging. Specifically, CD8 immunity and, in particular, Temra cell numbers and activation, as well as NK cell activation, segregated with high anti-CMV antibody levels particularly in older participants (Table 2). This is consistent with the idea that CMV infection or reactivation causes the coordinate change in these parameters in blood. Whether and to what extent this may correlate to health outcomes will require further studies.
Table 2.
Aggregate results
The association of CMV with several different health conditions has been studied extensively by many researchers. CMV seropositivity has been associated with CVDs [11, 51, 52], rheumatoid arthritis [53], diabetes [10], systemic lupus erythematosus (SLE) [54], and/or all-cause mortalities [9, 11]. In addition to this, several reports showed correlation of high anti-CMV antibody titer with CVDs [55–59], atherosclerosis [60], SLE [61], and/or all-cause mortalities [9, 56]. This suggests that the CMV infection marked by high levels of anti-CMV antibodies can be a risk factor for those diseases and that anti-CMV Ab levels may be used to predict their outcomes. However, correlations were weak and the topic remains controversial [62]. More recently, a thorough and seminal study by Maier’s group [2] showed in large longitudinal cohorts that no associations held between CMV positivity and serostatus and any of the clinical outcomes including cardiovascular diseases and all-time mortality.
In part, the above results may reflect the fact that we do not precisely know what the level of CMV-specific IgG reflects. B cells are not considered a primary control mechanism of CMV infection; however, they are involved in a lifelong dance with the virus. Higher CMV-specific IgG likely represents the higher level of B cell exposure to CMV antigens [63], which could occur due to any combination of (i the higher initial exposure to the virus (viral dose at original infection; (ii more frequent re-exposures to the virus; (iii more frequent re-activation events and, perhaps linked to this, infection earlier in life; and (iv more robust virus replication in the event of infection, reinfection, and/or re-activation (which could include impaired/delayed viral clearance by other arms of antiviral immunity. It is further possible, if not likely, that certain major conditions and/or stressors (e.g., HIV infection, organ transplantation may jointly contribute to the adverse effects of hCMV in only a subset of hCMV-positive individuals. The task, then, is to attempt to stratify various human populations and try to unravel the associations between hCMV and health or disease. As we can rarely ascertain the time of human infection during the lifespan, and even less so the dose and other parameters of infection in humans, we rely on imperfect approximations in both cross-sectional and longitudinal studies, using anti-CMV Ab titers.
Within these confines, we sought to evaluate whether and to what extent anti-CMV Ab titers can serve as a potential stratifying biomarker for immune profiling across aging, cross-correlating them to soluble mediators, T and NK cellular phenotypes and T cell function in a cross-sectional cohort of 247 participants. We used the CMV-negative group as controls ascertaining the effects of age alone, without CMV. While other persistent human pathogens can also be highly ubiquitous, none so far has been described to have an impact similar to CMV on the immune system. For example, EBV is another ubiquitous herpes virus that persistently infects most of the population (> 95%). However, many studies have shown that EBV does not cause global changes in immune phenotypes in the same manner like CMV [16, 36, 38, 64]. In that regard, our cohort was 97.4% EBV positive in total (95.8% in age 21–40, 99.1% in age 65 +), meaning that none of the observed differences can be explained by the presence or absence of EBV.
For cellular phenotypes, we have relied more dominantly on absolute cell numbers and less on fractional representation (percentages) to avoid artifacts secondary to changes in other populations examined. Aggregate results are shown in Table 2. Some parameters were age-sensitive regardless of CMV serostatus, like CD8Tn cell numbers, declining with age as described in previous publications [35, 36], and many of inflammatory marker levels in plasma increasing with age alone [31, 65] (Table 2, left column). Others were sensitive to CMV seropositivity alone, including those sensitive at all ages, that as expected involved increases in CD4 Tem and Temra numbers, and increased cytokine production from T cells in response to PMA, as a consequence of larger numbers of Tem and Temra cells (Table 2, middle column). Elevated percentages of CD57 + T cells and reduced percentages of CD4 Tem and Temra cells expressing HLA-DR and CD38 were in this same category, but selectively did not apply to CD8 counterparts. As HLA-DR and CD38 are related to relatively acute activation, one could speculate that their reduction in CD4 Temra could be caused by extensive differentiation in the presence of CMV. Why is this decline in CD38 and HLA-DR + not seen in CD8 Tem and Temra cells (Table 2, middle column) is unclear at the moment. One could speculate that virus-specific CD8 Tem and Temra cells with this “acutely activated” phenotype may represent descendants of recently activated CD8 cells as a consequence of reactivation, and that CD4 T cells, as a second line of defense, would not be activated unless the reactivation is massive and pronounced, which is less likely in healthy participants. Finally, a few traits were segregated with CMV seropositivity in only younger participants (increased sTNFRII, CD57 + NK cells, and CD8 + Tcm cells). We explain these as reflecting perhaps the relatively more recent infection of the participants that preferentially mobilized NK cells as an early defense mechanism and CD8 Tcm as a consequence of the relatively recent conversion of T effector cells into Tcm, which is then replaced by dominant inflation of memory CD8 T cells and differentiation into Tem/emra in older participants as a consequence of many decades of life with the virus.
The most informative set of data is summarized in Table 2, right column (red box), showing the potential power of Ab titer stratification. At older ages, anti-CMV Ab titers positively correlated with numbers of total NK cells, total CD8 + T cells, CD8 + Tcm cells, and number of CD4/CD8 Temra (Table 2, right column). There was a loss of CD8 + Tn cells with increased anti-CMV Ab titers in younger participants that were not different with titers in older participants because levels of the CD8 Tn are already low. Regardless of age, the number of CD4/CD8 Tem, %HLA-DR in CD4/CD8 Tem, and responsiveness of T cells to both non-specific stimulation and CMV tegument protein (pp65) were elevated in the anti-CMV Ab-hi groups.
The main limitations of this study include the cross-sectional design, with no knowledge of the time of infection, and no true study of time as a continuous variable, as well as lower diversity of the older cohort, particularly with regard to Hispanic participation in the subset of participants where T cell stimulation assays were done. Within these limitations, the above findings are consistent with time/age being an important factor in shaping the immune phenotypes in the presence of CMV, and therefore, levels of anti-CMV Ab correlate well with the anti-CMV-specific and overall T cell effector and Temra accumulation. Clearly, it would be very important to follow and validate the observed changes longitudinally over time and, even more so, to discern whether they correlate with, or even possibly predict, health outcomes over time, particularly with age-related frailty [66] and chronic diseases. In that regard, a positive correlation of anti-CMV Ab levels with IL-6, as well as with percentages and numbers of highly differentiated T cells, recently implicated in driving age-related disturbances outside of the immune system (Mogilenko et al., 2021, 2022) would be of potential clinical importance. Other topics of interest to expand upon would be to evaluate whether IgM antibodies, sometimes found with hCMV reactivation, and/or the affinity of either IgM or IgG antibodies, may further improve predictive values of the above studies. These questions are topics of our major efforts, and at the present, we are in the process of following this cohort, as well as utilizing confirmatory, validation cohorts, to address them.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Dr. David Harris and the staff at Arizona Health Sciences Center Biorepository for the biospecimen processing and biobanking.
Author contribution
M.W., L.D., P.S., M.J., J.L.U., M.J.S. R.C.W, and R.P.S. conducted experiments and acquired data. M.J., K.K., G.W., H.T., and J.N. contributed to the study design. M.W., P.F.C., and J.R. analyzed and interpreted the data. M.W., L.E.F., and B.D. performed recruitment, specimen collection, and database maintenance. M.W., J. R., H.T., L.E.F., and J.N. contributed to writing the manuscript.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Consent to participate
All patients provided written informed consent to the study.
Conflict of interest
The authors declare no competing interests.
Footnotes
Supported in part by the USPHS Awards AG060900 to H.T.III,, J.Ž. N., and G.W. and AG054317 and AG020719 and the Bowman Professorship in Medical Sciences to J. Ž. N.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010;20(4):202–13. 10.1002/rmv.655. 10.1002/rmv.655 [DOI] [PubMed] [Google Scholar]
- 2.Chen S, Pawelec G, Trompet S, Goldeck D, Mortensen LH, Slagboom PE, Christensen K, Gussekloo J, Kearney P, Buckley BM, Ford I, Jukema JW, Westendorp RGJ, Maier AB. Associations of cytomegalovirus infection with all-cause and cardiovascular mortality in multiple observational cohort studies of older adults. J Infect Dis. 2021;223(2):238–46. 10.1093/infdis/jiaa480. 10.1093/infdis/jiaa480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brizic I, Hirsl L, Britt WJ, Krmpotic A, Jonjic S. Immune responses to congenital cytomegalovirus infection. Microbes Infect. 2018;20(9–10):543–51. 10.1016/j.micinf.2017.12.010. 10.1016/j.micinf.2017.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mocarski ES Jr. Biology and replication of cytomegalovirus. Transfus Med Rev. 1988;2(4):229–34. 10.1016/s0887-7963(88)70050-4. 10.1016/s0887-7963(88)70050-4 [DOI] [PubMed] [Google Scholar]
- 5.Nelson CT, Demmler GJ. Cytomegalovirus infection in the pregnant mother, fetus, and newborn infant. Clin Perinatol. 1997;24(1):151–60 https://www.ncbi.nlm.nih.gov/pubmed/9099507. 10.1016/S0095-5108(18)30189-1 [DOI] [PubMed] [Google Scholar]
- 6.Nikolich-Zugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat Rev Immunol. 2008;8(7):512–22. 10.1038/nri2318. 10.1038/nri2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Powers C, DeFilippis V, Malouli D, Fruh K. Cytomegalovirus immune evasion. Curr Top Microbiol Immunol. 2008;325:333–59. 10.1007/978-3-540-77349-8_19. 10.1007/978-3-540-77349-8_19 [DOI] [PubMed] [Google Scholar]
- 8.Brodin P, Jojic V, Gao T, Bhattacharya S, Angel CJ, Furman D, Shen-Orr S, Dekker CL, Swan GE, Butte AJ, Maecker HT, Davis MM. Variation in the human immune system is largely driven by non-heritable influences. Cell. 2015;160(1–2):37–47. 10.1016/j.cell.2014.12.020. 10.1016/j.cell.2014.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang GC, Kao WH, Murakami P, Xue QL, Chiou RB, Detrick B, McDyer JF, Semba RD, Casolaro V, Walston JD, Fried LP. Cytomegalovirus infection and the risk of mortality and frailty in older women: a prospective observational cohort study. Am J Epidemiol. 2010;171(10):1144–52. 10.1093/aje/kwq062. 10.1093/aje/kwq062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen S, de Craen AJ, Raz Y, Derhovanessian E, Vossen AC, Westendorp RG, Pawelec G, Maier AB. Cytomegalovirus seropositivity is associated with glucose regulation in the oldest old. Results from the Leiden 85-plus Study. Immun Ageing. 2012;9(1):18. 10.1186/1742-4933-9-18. 10.1186/1742-4933-9-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simanek AM, Dowd JB, Pawelec G, Melzer D, Dutta A, Aiello AE. Seropositivity to cytomegalovirus, inflammation, all-cause and cardiovascular disease-related mortality in the United States. PLoS ONE. 2011;6(2):e16103. 10.1371/journal.pone.0016103. 10.1371/journal.pone.0016103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soderberg-Naucler C. Does cytomegalovirus play a causative role in the development of various inflammatory diseases and cancer? J Intern Med. 2006;259(3):219–46. 10.1111/j.1365-2796.2006.01618.x. 10.1111/j.1365-2796.2006.01618.x [DOI] [PubMed] [Google Scholar]
- 13.Pawelec G, Derhovanessian E, Larbi A, Strindhall J, Wikby A. Cytomegalovirus and human immunosenescence. Rev Med Virol. 2009;19(1):47–56. 10.1002/rmv.598. 10.1002/rmv.598 [DOI] [PubMed] [Google Scholar]
- 14.Mathei C, Vaes B, Wallemacq P, Degryse J. Associations between cytomegalovirus infection and functional impairment and frailty in the BELFRAIL Cohort. J Am Geriatr Soc. 2011;59(12):2201–8. 10.1111/j.1532-5415.2011.03719.x. 10.1111/j.1532-5415.2011.03719.x [DOI] [PubMed] [Google Scholar]
- 15.Vallejo AN, Hamel DL Jr, Mueller RG, Ives DG, Michel JJ, Boudreau RM, Newman AB. NK-like T cells and plasma cytokines, but not anti-viral serology, define immune fingerprints of resilience and mild disability in exceptional aging. PLoS ONE. 2011;6(10):e26558. 10.1371/journal.pone.0026558. 10.1371/journal.pone.0026558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furman D, Jojic V, Sharma S, Shen-Orr SS, Angel CJ, Onengut-Gumuscu S, Kidd BA, Maecker HT, Concannon P, Dekker CL, Thomas PG, Davis MM. Cytomegalovirus infection enhances the immune response to influenza. Sci Transl Med. 2015;7(281):281ra243. 10.1126/scitranslmed.aaa2293. 10.1126/scitranslmed.aaa2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barton ES, White DW, Cathelyn JS, Brett-McClellan KA, Engle M, Diamond MS, Miller VL, Virgin HW. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature. 2007;447(7142):326–9. 10.1038/nature05762. 10.1038/nature05762 [DOI] [PubMed] [Google Scholar]
- 18.Smithey MJ, Venturi V, Davenport MP, Buntzman AS, Vincent BG, Frelinger JA, Nikolich-Zugich J. Lifelong CMV infection improves immune defense in old mice by broadening the mobilized TCR repertoire against third-party infection. Proc Natl Acad Sci U S A. 2018;115(29):E6817–25. 10.1073/pnas.1719451115. 10.1073/pnas.1719451115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang KS, Moris A, Gouttefangeas C, Walter S, Teichgraber V, Miller M, Wernet D, Hamprecht K, Rammensee HG, Stevanovic S. High frequency of human cytomegalovirus (HCMV)-specific CD8+ T cells detected in a healthy CMV-seropositive donor. Cell Mol Life Sci. 2002;59(6):1076–80. 10.1007/s00018-002-8488-5. 10.1007/s00018-002-8488-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, Nelson JA, Picker LJ. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202(5):673–85. 10.1084/jem.20050882. 10.1084/jem.20050882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adriaensen W, Derhovanessian E, Vaes B, Van Pottelbergh G, Degryse JM, Pawelec G, Hamprecht K, Theeten H, Mathei C. CD4:8 ratio >5 is associated with a dominant naive T-cell phenotype and impaired physical functioning in CMV-seropositive very elderly people: results from the BELFRAIL study. J Gerontol A Biol Sci Med Sci. 2015;70(2):143–54. 10.1093/gerona/glu018. 10.1093/gerona/glu018 [DOI] [PubMed] [Google Scholar]
- 22.Vescovini R, Biasini C, Telera AR, Basaglia M, Stella A, Magalini F, Bucci L, Monti D, Lazzarotto T, Dal Monte P, Pedrazzoni M, Medici MC, Chezzi C, Franceschi C, Fagnoni FF, Sansoni P. Intense antiextracellular adaptive immune response to human cytomegalovirus in very old subjects with impaired health and cognitive and functional status. J Immunol. 2010;184(6):3242–9. 10.4049/jimmunol.0902890. 10.4049/jimmunol.0902890 [DOI] [PubMed] [Google Scholar]
- 23.Li H, Weng P, Najarro K, Xue QL, Semba RD, Margolick JB, Leng SX. Chronic CMV infection in older women: longitudinal comparisons of CMV DNA in peripheral monocytes, anti-CMV IgG titers, serum IL-6 levels, and CMV pp65 (NLV)-specific CD8(+) T-cell frequencies with twelve year follow-up. Exp Gerontol. 2014;54:84–9. 10.1016/j.exger.2014.01.010. 10.1016/j.exger.2014.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lustig A, Liu HB, Metter EJ, An Y, Swaby MA, Elango P, Ferrucci L, Hodes RJ, Weng NP. Telomere shortening, inflammatory cytokines, and anti-cytomegalovirus antibody follow distinct age-associated trajectories in humans. Front Immunol. 2017;8:1027. 10.3389/fimmu.2017.01027. 10.3389/fimmu.2017.01027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou JJ, Zhai J, Zhou H, Chen Y, Guerra S, Robey I, Weinstock GM, Weinstock E, Dong Q, Knox KS, Twigg HL 3rd. Supraglottic lung microbiome taxa are associated with pulmonary abnormalities in an HIV longitudinal cohort. Am J Respir Crit Care Med. 2020;202(12):1727–31. 10.1164/rccm.202004-1086LE. 10.1164/rccm.202004-1086LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahnke YD, Brodie TM, Sallusto F, Roederer M, Lugli E. The who’s who of T-cell differentiation: human memory T-cell subsets. Eur J Immunol. 2013;43(11):2797–809. 10.1002/eji.201343751. 10.1002/eji.201343751 [DOI] [PubMed] [Google Scholar]
- 27.Pulko V, Davies JS, Martinez C, Lanteri MC, Busch MP, Diamond MS, Knox K, Bush EC, Sims PA, Sinari S, Billheimer D, Haddad EK, Murray KO, Wertheimer AM, Nikolich-Zugich J. Human memory T cells with a naive phenotype accumulate with aging and respond to persistent viruses. Nat Immunol. 2016;17(8):966–75. 10.1038/ni.3483. 10.1038/ni.3483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stowe RP, Ruiz RJ, Fagundes CP, Stowe RH, Chen M, Glaser R. An ELISA method to compute endpoint titers to Epstein-Barr virus and cytomegalovirus: application to population-based studies. J Immunol Methods. 2014;408:64–9. 10.1016/j.jim.2014.05.006. 10.1016/j.jim.2014.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Umashankar M, Petrucelli A, Cicchini L, Caposio P, Kreklywich CN, Rak M, Bughio F, Goldman DC, Hamlin KL, Nelson JA, Fleming WH, Streblow DN, Goodrum F. A novel human cytomegalovirus locus modulates cell type-specific outcomes of infection. PLoS Pathog. 2011;7(12):e1002444. 10.1371/journal.ppat.1002444. 10.1371/journal.ppat.1002444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6(2):65–70 http://www.jstor.org/stable/4615733. [Google Scholar]
- 31.Tanaka, T., Biancotto, A., Moaddel, R., Moore, A. Z., Gonzalez-Freire, M., Aon, M. A., Candia, J., Zhang, P., Cheung, F., Fantoni, G., consortium, C. H. I., Semba, R. D., & Ferrucci, L. (2018). Plasma proteomic signature of age in healthy humans. Aging Cell, 17(5), e12799. 10.1111/acel.12799 [DOI] [PMC free article] [PubMed]
- 32.Dekhtiarenko, I., Ratts, R. B., Blatnik, R., Lee, L. N., Fischer, S., Borkner, L., Oduro, J. D., Marandu, T. F., Hoppe, S., Ruzsics, Z., Sonnemann, J. K., Mansouri, M., Meyer, C., Lemmermann, N. A., Holtappels, R., Arens, R., Klenerman, P., Fruh, K., Reddehase, M. J., . . . Cicin-Sain, L. (2016). Peptide processing is critical for T-cell memory inflation and may be optimized to improve immune protection by CMV-based vaccine vectors. PLoS Pathog, 12(12), e1006072. 10.1371/journal.ppat.1006072 [DOI] [PMC free article] [PubMed]
- 33.Holtappels R, Podlech J, Pahl-Seibert MF, Julch M, Thomas D, Simon CO, Wagner M, Reddehase MJ. Cytomegalovirus misleads its host by priming of CD8 T cells specific for an epitope not presented in infected tissues. J Exp Med. 2004;199(1):131–6. 10.1084/jem.20031582. 10.1084/jem.20031582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sims S, Bolinger B, Klenerman P. Increasing inflationary T-cell responses following transient depletion of MCMV-specific memory T cells. Eur J Immunol. 2015;45(1):113–8. 10.1002/eji.201445016. 10.1002/eji.201445016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thyagarajan B, Faul J, Vivek S, Kim JK, Nikolich-Zugich J, Weir D, Crimmins EM. Age-related differences in T-cell subsets in a nationally representative sample of people older than age 55: findings from the health and retirement study. J Gerontol A Biol Sci Med Sci. 2022;77(5):927–33. 10.1093/gerona/glab300. 10.1093/gerona/glab300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wertheimer AM, Bennett MS, Park B, Uhrlaub JL, Martinez C, Pulko V, Currier NL, Nikolich-Zugich D, Kaye J, Nikolich-Zugich J. Aging and cytomegalovirus infection differentially and jointly affect distinct circulating T cell subsets in humans. J Immunol. 2014;192(5):2143–55. 10.4049/jimmunol.1301721. 10.4049/jimmunol.1301721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hadrup SR, Strindhall J, Kollgaard T, Seremet T, Johansson B, Pawelec G, thor Straten, P., & Wikby, A. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J Immunol. 2006;176(4):2645–53. 10.4049/jimmunol.176.4.2645. 10.4049/jimmunol.176.4.2645 [DOI] [PubMed] [Google Scholar]
- 38.Khan N, Hislop A, Gudgeon N, Cobbold M, Khanna R, Nayak L, Rickinson AB, Moss PA. Herpesvirus-specific CD8 T cell immunity in old age: cytomegalovirus impairs the response to a coresident EBV infection. J Immunol. 2004;173(12):7481–9. 10.4049/jimmunol.173.12.7481. 10.4049/jimmunol.173.12.7481 [DOI] [PubMed] [Google Scholar]
- 39.Khan N, Shariff N, Cobbold M, Bruton R, Ainsworth JA, Sinclair AJ, Nayak L, Moss PA. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol. 2002;169(4):1984–92. 10.4049/jimmunol.169.4.1984. 10.4049/jimmunol.169.4.1984 [DOI] [PubMed] [Google Scholar]
- 40.Kuijpers TW, Vossen MT, Gent MR, Davin JC, Roos MT, Wertheim-van Dillen PM, Weel JF, Baars PA, van Lier RA. Frequencies of circulating cytolytic, CD45RA+CD27-, CD8+ T lymphocytes depend on infection with CMV. J Immunol. 2003;170(8):4342–8. 10.4049/jimmunol.170.8.4342. 10.4049/jimmunol.170.8.4342 [DOI] [PubMed] [Google Scholar]
- 41.Pita-Lopez ML, Gayoso I, DelaRosa O, Casado JG, Alonso C, Munoz-Gomariz E, Tarazona R, Solana R. Effect of ageing on CMV-specific CD8 T cells from CMV seropositive healthy donors. Immun Ageing. 2009;6:11. 10.1186/1742-4933-6-11. 10.1186/1742-4933-6-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pourgheysari B, Khan N, Best D, Bruton R, Nayak L, Moss PA. The cytomegalovirus-specific CD4+ T-cell response expands with age and markedly alters the CD4+ T-cell repertoire. J Virol. 2007;81(14):7759–65. 10.1128/JVI.01262-06. 10.1128/JVI.01262-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vescovini R, Telera A, Fagnoni FF, Biasini C, Medici MC, Valcavi P, di Pede P, Lucchini G, Zanlari L, Passeri G, Zanni F, Chezzi C, Franceschi C, Sansoni P. Different contribution of EBV and CMV infections in very long-term carriers to age-related alterations of CD8+ T cells. Exp Gerontol. 2004;39(8):1233–43. 10.1016/j.exger.2004.04.004. 10.1016/j.exger.2004.04.004 [DOI] [PubMed] [Google Scholar]
- 44.Baecher-Allan C, Wolf E, Hafler DA. MHC class II expression identifies functionally distinct human regulatory T cells. J Immunol. 2006;176(8):4622–31. 10.4049/jimmunol.176.8.4622. 10.4049/jimmunol.176.8.4622 [DOI] [PubMed] [Google Scholar]
- 45.Quarona V, Zaccarello G, Chillemi A, Brunetti E, Singh VK, Ferrero E, Funaro A, Horenstein AL, Malavasi F. CD38 and CD157: a long journey from activation markers to multifunctional molecules. Cytometry B Clin Cytom. 2013;84(4):207–17. 10.1002/cyto.b.21092. 10.1002/cyto.b.21092 [DOI] [PubMed] [Google Scholar]
- 46.Viallard JF, Blanco P, Andre M, Etienne G, Liferman F, Neau D, Vidal E, Moreau JF, Pellegrin JL. CD8+HLA-DR+ T lymphocytes are increased in common variable immunodeficiency patients with impaired memory B-cell differentiation. Clin Immunol. 2006;119(1):51–8. 10.1016/j.clim.2005.11.011. 10.1016/j.clim.2005.11.011 [DOI] [PubMed] [Google Scholar]
- 47.Focosi D, Bestagno M, Burrone O, Petrini M. CD57+ T lymphocytes and functional immune deficiency. J Leukoc Biol. 2010;87(1):107–16. 10.1189/jlb.0809566. 10.1189/jlb.0809566 [DOI] [PubMed] [Google Scholar]
- 48.Strioga M, Pasukoniene V, Characiejus D. CD8+ CD28- and CD8+ CD57+ T cells and their role in health and disease. Immunology. 2011;134(1):17–32. 10.1111/j.1365-2567.2011.03470.x. 10.1111/j.1365-2567.2011.03470.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hammer, Q., Ruckert, T., Borst, E. M., Dunst, J., Haubner, A., Durek, P., Heinrich, F., Gasparoni, G., Babic, M., Tomic, A., Pietra, G., Nienen, M., Blau, I. W., Hofmann, J., Na, I. K., Prinz, I., Koenecke, C., Hemmati, P., Babel, N., . . . Romagnani, C. (2018). Peptide-specific recognition of human cytomegalovirus strains controls adaptive natural killer cells. Nat Immunol, 19(5):453–463. 10.1038/s41590-018-0082-6 [DOI] [PubMed]
- 50.Reed RG, Presnell SR, Al-Attar A, Lutz CT, Segerstrom SC. Perceived stress, cytomegalovirus titers, and late-differentiated T and NK cells: between-, within-person associations in a longitudinal study of older adults. Brain Behav Immun. 2019;80:266–74. 10.1016/j.bbi.2019.03.018. 10.1016/j.bbi.2019.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muhlestein JB, Horne BD, Carlquist JF, Madsen TE, Bair TL, Pearson RR, Anderson JL. Cytomegalovirus seropositivity and C-reactive protein have independent and combined predictive value for mortality in patients with angiographically demonstrated coronary artery disease. Circulation. 2000;102(16):1917–23. 10.1161/01.cir.102.16.1917. 10.1161/01.cir.102.16.1917 [DOI] [PubMed] [Google Scholar]
- 52.Spyridopoulos I, Martin-Ruiz C, Hilkens C, Yadegarfar ME, Isaacs J, Jagger C, Kirkwood T, von Zglinicki T. CMV seropositivity and T-cell senescence predict increased cardiovascular mortality in octogenarians: results from the Newcastle 85+ study. Aging Cell. 2016;15(2):389–92. 10.1111/acel.12430. 10.1111/acel.12430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pierer M, Rothe K, Quandt D, Schulz A, Rossol M, Scholz R, Baerwald C, Wagner U. Association of anticytomegalovirus seropositivity with more severe joint destruction and more frequent joint surgery in rheumatoid arthritis. Arthritis Rheum. 2012;64(6):1740–9. 10.1002/art.34346. 10.1002/art.34346 [DOI] [PubMed] [Google Scholar]
- 54.Esen BA, Yilmaz G, Uzun S, Ozdamar M, Aksozek A, Kamali S, Turkoglu S, Gul A, Ocal L, Aral O, Inanc M. Serologic response to Epstein-Barr virus antigens in patients with systemic lupus erythematosus: a controlled study. Rheumatol Int. 2012;32(1):79–83. 10.1007/s00296-010-1573-4. 10.1007/s00296-010-1573-4 [DOI] [PubMed] [Google Scholar]
- 55.Blum A, Giladi M, Weinberg M, Kaplan G, Pasternack H, Laniado S, Miller H. High anti-cytomegalovirus (CMV) IgG antibody titer is associated with coronary artery disease and may predict post-coronary balloon angioplasty restenosis. Am J Cardiol. 1998;81(7):866–8. 10.1016/s0002-9149(98)00019-8. 10.1016/s0002-9149(98)00019-8 [DOI] [PubMed] [Google Scholar]
- 56.Gkrania-Klotsas E, Langenberg C, Sharp SJ, Luben R, Khaw KT, Wareham NJ. Seropositivity and higher immunoglobulin g antibody levels against cytomegalovirus are associated with mortality in the population-based European prospective investigation of Cancer-Norfolk cohort. Clin Infect Dis. 2013;56(10):1421–7. 10.1093/cid/cit083. 10.1093/cid/cit083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haarala A, Kahonen M, Lehtimaki T, Aittoniemi J, Jylhava J, Hutri-Kahonen N, Taittonen L, Laitinen T, Juonala M, Viikari J, Raitakari OT, Hurme M. Relation of high cytomegalovirus antibody titres to blood pressure and brachial artery flow-mediated dilation in young men: the Cardiovascular Risk in Young Finns Study. Clin Exp Immunol. 2012;167(2):309–16. 10.1111/j.1365-2249.2011.04513.x. 10.1111/j.1365-2249.2011.04513.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roberts ET, Haan MN, Dowd JB, Aiello AE. Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow-up. Am J Epidemiol. 2010;172(4):363–71. 10.1093/aje/kwq177. 10.1093/aje/kwq177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang FJ, Shu KH, Chen HY, Chen IY, Lay FY, Chuang YF, Wu CS, Tsai WC, Peng YS, Hsu SP, Chiang CK, Wang G, Chiu YL. Anti-cytomegalovirus IgG antibody titer is positively associated with advanced T cell differentiation and coronary artery disease in end-stage renal disease. Immun Ageing. 2018;15:15. 10.1186/s12979-018-0120-0. 10.1186/s12979-018-0120-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blum A, Peleg A, Weinberg M. Anti-cytomegalovirus (CMV) IgG antibody titer in patients with risk factors to atherosclerosis. Clin Exp Med. 2003;3(3):157–60. 10.1007/s10238-003-0019-7. 10.1007/s10238-003-0019-7 [DOI] [PubMed] [Google Scholar]
- 61.Barzilai O, Sherer Y, Ram M, Izhaky D, Anaya JM, Shoenfeld Y. Epstein-Barr virus and cytomegalovirus in autoimmune diseases: are they truly notorious? A preliminary report. Ann N Y Acad Sci. 2007;1108:567–77. 10.1196/annals.1422.059. 10.1196/annals.1422.059 [DOI] [PubMed] [Google Scholar]
- 62.Aiello AE, Chiu YL, Frasca D. How does cytomegalovirus factor into diseases of aging and vaccine responses, and by what mechanisms? Geroscience. 2017;39(3):261–71. 10.1007/s11357-017-9983-9. 10.1007/s11357-017-9983-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iglesias-Escudero M, Moro-Garcia MA, Marcos-Fernandez R, Garcia-Torre A, Alvarez-Arguelles ME, Suarez-Fernandez ML, Martinez-Camblor P, Rodriguez M, Alonso-Arias R. Levels of anti-CMV antibodies are modulated by the frequency and intensity of virus reactivations in kidney transplant patients. PLoS ONE. 2018;13(4):e0194789. 10.1371/journal.pone.0194789. 10.1371/journal.pone.0194789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramendra R, Isnard S, Lin J, Fombuena B, Ouyang J, Mehraj V, Zhang Y, Finkelman M, Costiniuk C, Lebouche B, Chartrand-Lefebvre C, Durand M, Tremblay C, Ancuta P, Boivin G, Routy JP. Cytomegalovirus seropositivity is associated with increased microbial translocation in people living with human immunodeficiency virus and uninfected controls. Clin Infect Dis. 2020;71(6):1438–46. 10.1093/cid/ciz1001. 10.1093/cid/ciz1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Armas, L. R., Pallikkuth, S., George, V., Rinaldi, S., Pahwa, R., Arheart, K. L., & Pahwa, S. (2017). Reevaluation of immune activation in the era of cART and an aging HIV-infected population. JCI Insight, 2(20). 10.1172/jci.insight.95726 [DOI] [PMC free article] [PubMed]
- 66.Schmaltz HN, Fried LP, Xue QL, Walston J, Leng SX, Semba RD. Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J Am Geriatr Soc. 2005;53(5):747–54. 10.1111/j.1532-5415.2005.53250.x. 10.1111/j.1532-5415.2005.53250.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.