Abstract
The oldest-old population, those aged ≥ 80 years, is the fastest-growing group in the United States (US), grappling with an increasingly heavy burden of dementia. We aimed to dissect the trends in dementia prevalence, mortality, and risk factors, and predict future levels among this demographic. Leveraging data from the Global Burden of Disease Study 2019, we examined the trends in dementia prevalence, mortality, and risk factors (with a particular focus on body mass index, BMI) for US oldest-old adults. Through decomposition analysis, we identified key population-level contributors to these trends. Predictive modeling was employed to estimate future prevalence and mortality levels over the next decade. Between 1990 and 2019, the number of dementia cases and deaths among the oldest-old in the US increased by approximately 1.37 million and 60,000 respectively. The population growth and aging were highlighted as the primary drivers of this increase. High BMI emerged as a growing risk factor. Females showed a disproportionately higher dementia burden, characterized by a unique risk factor profile, including BMI. Predictions for 2030 anticipate nearly 4 million dementia cases and 160,000 related deaths, with a marked increase in prevalence and mortality anticipated among those aged 80–89. The past 30 years have witnessed a notable rise in both the prevalence and mortality of dementia among the oldest-old in the US, accompanied by a significant shift in risk factors, with obesity taking a forefront position. Targeted age and sex-specific public health strategies that address obesity control are needed to mitigate the dementia burden effectively.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-024-01180-6.
Keywords: Dementia, Prevalence, Mortality, Body mass index, Public health
Introduction
Dementia is an important global public health issue characterized by symptoms that affect memory, thinking, and social abilities [1]. Alzheimer’s disease (AD) and AD-related dementias, among which vascular cognitive impairment and dementia (VCID) stands as the foremost cause, are the principal contributors to dementia. Forecasts suggest a steep rise in the global number of people with dementia, expected to reach 65.7 million by 2030 and swell to 115.4 million by 2050 [2]. The economic toll of dementia is equally staggering, amounting to $948 billion globally in 2016, with a yearly increment of 15.94% [3]. In the United States (US), the situation mirrors the global crisis, with 4.7 million individuals aged 65 or older affected in 2010 [4], making dementia the fifth leading cause of death among the elderly [1]. Particularly alarming is the scenario among the “oldest-old” adults—defined by the American Geriatric Society and the World Health Organization as those aged 80 or older—represent the most rapidly expanding segment of the population, bearing a disproportionately high burden of dementia [5]. Addressing the unique challenges and risk profiles of the oldest-old is crucial for developing targeted interventions aimed at mitigating the impact of dementia [6, 7].
However, large-scale epidemiological studies focusing on dementia among the US oldest-old adults have been sparse, leaving a significant knowledge gap. This void becomes especially critical in the context of the escalating obesity epidemic, which intertwines with dementia's prevalence and risk factors, underscoring the necessity for in-depth understanding and tailored strategies. This study aims to fill this gap by assessing the long-term trends in dementia prevalence, mortality, and risk factor patterns from 1990 to 2019 among US oldest-old adults, with a special focus on the obesity epidemic’s role as a pivotal risk factor. Additionally, we aim to project future prevalence and mortality rates over the coming decade, thereby providing insights essential for reducing dementia’s disease burden.
Leveraging comprehensive epidemiological data from the Global Burden of Disease (GBD) study, which covers 369 diseases and injuries across 204 countries and territories [8], this research offers a unique lens through which to view the epidemiological characteristics and trends of dementia within the US oldest-old demographic. Through a methodological approach that incorporates decomposition analysis and predictive modeling, this study will dissect the net contributions of key population-level factors—aging, growth, and epidemiological shifts—providing a nuanced understanding of how these elements influence dementia trends among the oldest-old.
The implications of our findings are far-reaching, with the potential to significantly impact public health policy and practice. By elucidating dementia trends and identifying critical risk factors, notably obesity, this study aims to arm policymakers and healthcare professionals with the information necessary to formulate targeted intervention strategies. By focusing on the health needs of the oldest-old and addressing modifiable risk factors like obesity, we envision paving the way towards more resilient healthcare systems, better equipped to support an aging population.
Methods
Data source
The US dementia data from 1990 to 2019 used in the present study were extracted from the public data of GBD study 2019 [9, 10] and GBD study was approved by University of Washington Institutional Review Board Committee (STUDY00009060) [8]. Briefly, the GBD 2019 provided comprehensive epidemiological data on 369 diseases and injuries for 204 countries and territories from 1990 to 2019 [9–11]. For the data source, the US data sources of dementia prevalence were the health and retirement study (HRS) and aging, demographics, and memory study (ADAMS, a sub-cohort from HRS) [12]. The same data sources were used to extract data on US dementia mortality.
Based on the data sources, the GBD 2019 study used the Disease Modelling Meta-Regression (DisMod-MR) 2.1 to generate estimation of dementia measures by sex, age group, year, etc. [10]. The DisMod-MR 2.1 is a Bayesian meta-regression tool and has been widely used in disease burden modeling (such as incidence and prevalence). Notably, DisMod-MR 2.1 could ensure consistency between the different epidemiological parameters by enforcing associations in a set of differential equations. This model has been validated and confirmed to be robust [13]. Focusing on oldest-old adults aged ≥ 80 years in the US, we extracted four age groups data on US dementia, including 80–84 years, 85–89 years, 90–94 years, and 95 + years. Additionally, for prevalence and mortality data, the absolute number (i.e., case), and the rates of the two measures were analyzed.
Decomposition analysis
To understand the net contribution of underlying population-level factors (i.e., aging of population, population growth, and epidemiological change) to the alterations of prevalence and mortality and their net contribution proportions from 1990 to 2019, we conducted decomposition analysis on changes in prevalence and mortality using the Das Gupta’s decomposition method, which has been widely used in epidemiological studies and demographic studies [14]. By the decomposition analysis, we could obtain the net contribution of the three population-level factors to the changes in prevalence and mortality. Here, we reported (1) the absolute changes due to the three population-level factors and (2) the contribution proportion of these factors. The proportion was positive when the change driven by the factor was positive; otherwise, the proportion was negative. Please see the supplementary method for more details of the calculation.
Risk factor analysis
Data of risk factors for dementia were also obtained from GBD 2019. Three modifiable risk factors, including smoking, high fasting plasma glucose, and high BMI, were analyzed in our study. First, the relative risk data of these risk factors were calculated by DisMod-MR 2.1 based on previously published papers through a systematic review by GBD group. Then, the population-attributable fractions (PAF) by agesexlocationyear were calculated [10, 15, 16]. We presented the PAF and the attributable number of US dementia: the PAF refers to the proportion of dementia that would be reduced if the risk factor’s historical exposure level had been reduced to the counterfactual level of the theoretical minimum risk exposure level [15, 17]; the attributable number refers to the absolute number of dementia cases attributable to the risk factor [18].
Predictions based on BAPC model
We used the Bayesian age-period-cohort (BAPC) model [19] to predict the values of dementia prevalence and mortality from 2020 to 2030. For the prediction method selection, we chose the BAPC model due to its better predictive performance [20]. The BAPC model is based on the assumption that the past effects of age, period, and cohort would continue into the future 10 years and applies a second-order random walk to smooth the priors for age, period, and cohort effects and predict posterior rates. In detail, the age effect represents the net impact of aging after adjusting for period and cohort effects; period effect represents the impact of external factors that equally affect all individuals at a particular calendar time, such as public health events and socioeconomic factors; cohort effect represents the impact of unique external factors among individuals born in different time periods or birth cohorts, such as historical, social, and environmental factors. The BAPC model utilizes an integrated nested Laplacian approximation to estimate marginal posterior distributions, which helps to circumvent the mixing and convergence issues commonly associated with the Markov Chain Monte Carlo sampling method typically used in Bayesian methodologies [19]. The BAPC package and INLA package in the R program were used for the prediction.
Statistical analysis
To quantify the temporal trends of number of prevalence and mortality, we calculated the percentage of relative changes between 1990 and 2019 according to the following formula [21]:
Relative changes = (value in 2019–value in 1990)/value in 1990 × 100%.
To quantify the temporal trend of rates of prevalence and mortality, we conducted joinpoint regression analysis [22] to calculate the average annual percentage change (AAPC) and the corresponding 95% confidence intervals (CIs) and the Monte Carlo methods were used for the significance tests. When the AAPC and the lower boundary of the 95% CI are > 0, the rate is in an upward trend; if the AAPC and the upper boundary of the 95% CI are < 0, the rate is in a downward trend; any other scenarios indicate that the rate remains stable over time. The Joinpoint software (National Cancer Institute, Rockville, MD, USA) was used to conduct joinpoint regression analysis. All analyses other than joinpoint regression analysis were performed with R (version 4.3.0) and a 2-tailed P value less than 0.05 was considered statistically significant.
Results
Trends in prevalence and mortality
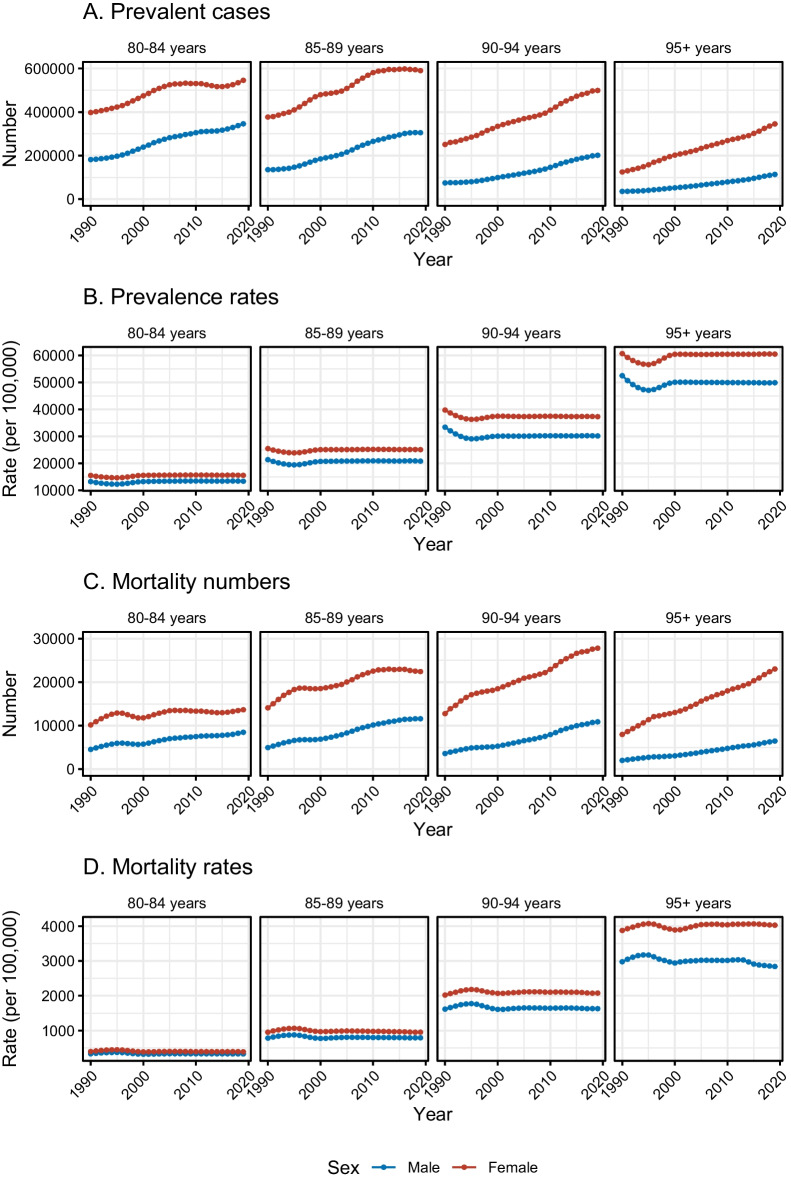
Overall, the absolute number of prevalent cases increased by about 1.37 million (86.74%) between 1990 and 2019 among US adults aged ≥ 80 years, from 1.58 (1.24 ~ 1.98) million to 2.95 (2.51 ~ 3.41) million (Table 1). Higher overall increase was seen in males (126.27%) than that in females (72.07%); the male-to-female ratios in prevalent cases from 1990 to 2019 were < 1.0, ranging from 0.2 to 0.7, but increased gradually (the sex difference narrowed) (Table 1 and Supplementary Fig. 1). For the four age groups, the older age group showed higher levels of increase, and the highest one was in the 95 + years group (186.27%), with similar patterns in males and females, although the cases in this age group were less than other groups (Table 1 and Fig. 1). Regarding prevalence rate, the age-standardized rate for US adults aged ≥ 80 years showed a slight decrease, with a mean decrease of -0.10% per year, from 22251.36 (17435.38 ~ 27849.03) per 100,000 to 21530.04 (18321.27 ~ 24964.11) per 100,000; the significant decline was seen in males but not in females (Table 1). The male-to-female ratios in prevalence rates from 1990 to 2019 ranged from 0.8 to 0.9, remaining relatively stable in the recent 20 years (Supplementary Fig. 1). For the four age groups, the highest decline for males and females was both in 90–94 years group (− 0.33% and − 0.21%) (Table 1 and Fig. 1).
Table 1.
Trends of prevalence and mortality among US oldest-old adults with dementia from 1990 to 2019
| Number of cases | Age-specific/age-standardized rates | |||||
|---|---|---|---|---|---|---|
| 1990 (thousand) (95% UI) | 2019 (thousand) (95% UI) | Changes (thousand) (%) | 1990 (per 100,000) (95% UI) | 2019 (per 100,000) (95% UI) | AAPCs (%) | |
| Prevalence | ||||||
| Both sexes | ||||||
| 80–84 years | 579.89 (450.12 ~ 740.23) | 892.03 (756.58 ~ 1041.56) | 312.14 (53.83) | 14,738.59 (11,440.29 ~ 18,813.84) | 14,652.07 (12,427.13 ~ 17,108.11) | − 0.01 (− 0.07 to 0.04) |
| 85–89 years | 512.71 (396.4 ~ 642.51) | 895.84 (762.5 ~ 1042.59) | 383.13 (74.73) | 24,254.05 (18,751.94 ~ 30,394.53) | 23,463.73 (19,971.37 ~ 27,307.35) | − 0.11 (− 0.15 to − 0.08) |
| 90–94 years | 325.71 (259.25 ~ 403.91) | 700.98 (600.69 ~ 806.38) | 375.27 (115.22) | 38,103.29 (30,328.94 ~ 47,252.03) | 34,931.31 (29,933.69 ~ 40,183.5) | − 0.3 (− 0.35 to − 0.24) |
| 95 + years | 160.4 (130.72 ~ 190.37) | 459.19 (389.8 ~ 521.73) | 298.79 (186.27) | 58,658.15 (47,804.22 ~ 69,615.76) | 57,476.12 (48,790.79 ~ 65,304.52) | − 0.06 (− 0.1 to − 0.03) |
| All ages/age-standardized | 1578.71 (1236.49 ~ 1977.02) | 2948.04 (2509.57 ~ 3412.26) | 1369.33 (86.74) | 22,251.36 (17,435.38 ~ 27,849.03) | 21,530.04 (18,321.27 ~ 24,964.11) | − 0.10 (− 0.17 to − 0.04) |
| Male | ||||||
| 80–84 years | 181.77 (140.41 ~ 232.47) | 346.06 (288.68 ~ 405.06) | 164.3 (90.39) | 13,258.37 (10,241.91 ~ 16,956.59) | 13,397.94 (11,176.52 ~ 15,682.19) | 0.05 (− 0.02 to 0.11) |
| 85–89 years | 135.41 (103.85 ~ 169.81) | 305.38 (256.07 ~ 356.75) | 169.97 (125.53) | 21,382.28 (16,398.2 ~ 26,813.93) | 20,834.67 (17,470 ~ 24,339.09) | − 0.08 (− 0.14 to − 0.02) |
| 90–94 years | 74.54 (58.34 ~ 93.32) | 201.61 (169.63 ~ 233.92) | 127.07 (170.47) | 33,403.01 (26,141.68 ~ 41,819.7) | 30,172.63 (25,385.59 ~ 35,008.01) | − 0.33 (− 0.36 to − 0.29) |
| 95 + years | 35.51 (28.5 ~ 42.81) | 113.64 (95.47 ~ 130.7) | 78.13 (220.03) | 52,482.97 (42,114.65 ~ 63,276.09) | 49,888.8 (41,910.92 ~ 57,375.72) | − 0.18 (− 0.2 to − 0.16) |
| All ages/age-standardized | 427.23 (331.09 ~ 538.41) | 966.7 (809.85 ~ 1126.44) | 539.48 (126.27) | 19,767.42 (15,340.51 ~ 24,854.1) | 19,175.06 (16,061.14 ~ 22,350.3) | − 0.1 (− 0.15 to − 0.05) |
| Female | ||||||
| 80–84 years | 398.12 (309.82 ~ 508.39) | 545.97 (467.49 ~ 635.02) | 147.85 (37.14) | 15,530.2 (12,085.55 ~ 19,831.62) | 15,576.24 (13,337.21 ~ 18,116.96) | 0.02 (− 0.03 to 0.06) |
| 85–89 years | 377.3 (291.69 ~ 471.87) | 590.46 (507.61 ~ 684.26) | 213.16 (56.5) | 25,482.33 (19,700.36 ~ 31,869.49) | 25,101.98 (21,579.89 ~ 29,089.7) | − 0.05 (− 0.08 to − 0.02) |
| 90–94 years | 251.17 (200.95 ~ 310.33) | 499.37 (429.76 ~ 574.43) | 248.2 (98.82) | 39,763.87 (31,813.46 ~ 49,129.41) | 37,306.82 (32,106.81 ~ 42,914.4) | − 0.21 (− 0.28 to − 0.15) |
| 95 + years | 124.89 (102.35 ~ 147.56) | 345.55 (294.1 ~ 391.68) | 220.65 (176.68) | 60,688.48 (49,732.49 ~ 71,704.85) | 60,502.35 (51,495.26 ~ 68,580.23) | 0 (− 0.04 to 0.03) |
| All ages/age-standardized | 1151.48 (904.8 ~ 1438.15) | 1981.34 (1698.96 ~ 2285.39) | 829.86 (72.07) | 23,330.91 (18,326.92 ~ 29,152.86) | 22,931.93 (19,668.61 ~ 26,508.49) | − 0.05 (− 0.11 to 0.01) |
| Mortality | ||||||
| Both sexes | ||||||
| 80–84 years | 14.71 (3.55 ~ 40.89) | 22.13 (5.39 ~ 60.4) | 7.42 (50.4) | 373.93 (90.12 ~ 1039.4) | 363.46 (88.61 ~ 992.16) | − 0.10 (− 0.2 to 0) |
| 85–89 years | 19.05 (4.68 ~ 50.39) | 34.02 (8.71 ~ 85.64) | 14.96 (78.54) | 901.34 (221.46 ~ 2383.76) | 891 (228.25 ~ 2242.99) | − 0.04 (− 0.12 to 0.04) |
| 90–94 years | 16.37 (4.19 ~ 41.65) | 38.68 (10.29 ~ 96.99) | 22.31 (136.31) | 1914.96 (490.11 ~ 4871.99) | 1927.6 (512.87 ~ 4833.13) | 0.03 (− 0.06 to 0.11) |
| 95 + years | 9.99 (2.49 ~ 25.51) | 29.48 (7.17 ~ 74.73) | 19.49 (195.05) | 3654.1 (910.12 ~ 9327.25) | 3690.23 (897.69 ~ 9354.25) | 0.03 (− 0.03 to 0.09) |
| All ages/age-standardized | 60.13 (14.91 ~ 158.44) | 124.31 (31.57 ~ 317.76) | 64.18 (106.74) | 855.26 (212.11 ~ 2251.54) | 849.58 (215.62 ~ 2180.49) | − 0.02 (− 0.08 to 0.05) |
| Male | ||||||
| 80–84 years | 4.54 (1.05 ~ 13.06) | 8.47 (2 ~ 23.86) | 3.93 (86.46) | 331.49 (76.24 ~ 952.76) | 328.07 (77.46 ~ 923.81) | − 0.04 (− 0.11 to 0.02) |
| 85–89 years | 4.96 (1.18 ~ 13.67) | 11.59 (2.87 ~ 30.79) | 6.63 (133.66) | 783.05 (186.14 ~ 2159.11) | 790.52 (195.95 ~ 2100.92) | 0.03 (− 0.05 to 0.11) |
| 90–94 years | 3.61 (0.87 ~ 9.45) | 10.88 (2.74 ~ 28.22) | 7.28 (201.75) | 1616.12 (388.86 ~ 4233.46) | 1628.66 (410.5 ~ 4223.62) | 0.02 (− 0.05 to 0.09) |
| 95 + years | 2.01 (0.48 ~ 5.4) | 6.47 (1.52 ~ 17.77) | 4.46 (221.15) | 2977.57 (702.98 ~ 7986.18) | 2840.37 (665.98 ~ 7799.26) | − 0.18 (− 0.29 to − 0.07) |
| All ages/age-standardized | 15.12 (3.57 ~ 41.59) | 37.41 (9.13 ~ 100.64) | 22.29 (147.37) | 732.67 (173.17 ~ 2004.43) | 728.99 (177.9 ~ 1962.65) | − 0.03 (− 0.1 to 0.04) |
| Female | ||||||
| 80–84 years | 10.17 (2.48 ~ 27.83) | 13.65 (3.36 ~ 36.53) | 3.49 (34.29) | 396.63 (96.59 ~ 1085.73) | 389.54 (95.99 ~ 1042.29) | − 0.05 (− 0.21 to 0.1) |
| 85–89 years | 14.09 (3.49 ~ 36.78) | 22.43 (5.83 ~ 55.27) | 8.34 (59.15) | 951.93 (235.53 ~ 2484.25) | 953.61 (247.74 ~ 2349.76) | 0.00 (− 0.09 to 0.09) |
| 90–94 years | 12.76 (3.32 ~ 32.09) | 27.8 (7.58 ~ 68.75) | 15.04 (117.82) | 2020.54 (525.8 ~ 5080.97) | 2076.84 (565.96 ~ 5136.14) | 0.09 (0.01 to 0.16) |
| 95 + years | 7.98 (2.01 ~ 20.11) | 23.01 (5.68 ~ 57.78) | 15.03 (188.46) | 3876.53 (978.23 ~ 9770) | 4029.21 (995.34 ~ 10,116.77) | 0.12 (0.08 to 0.17) |
| All ages/age-standardized | 45 (11.3 ~ 116.82) | 86.9 (22.45 ~ 218.33) | 41.89 (93.09) | 904.66 (226.97 ~ 2350.29) | 914.53 (235.94 ~ 2307.82) | 0.04 (− 0.02 to 0.11) |
AAPC average annual percentage change; UI uncertainty intervals. For number of cases, the percentage of relative changes (%) between 1990 and 2019 was calculated to quantify the temporal trends; the absolute changes of number of cases (thousand) were also presented. For the age-specific rates and age-standardized rates, the average annual percentage changes (AAPCs, %) were calculated to quantify the temporal trends
Fig. 1.
Trends of prevalence and mortality among US oldest-old adults with dementia in the four age groups from 1990 to 2019. A Trend of absolute number of prevalent cases. B Trend of prevalence rates (per 100,000). C Trend of mortality numbers. D Trend of mortality rates (per 100,000)
The number of mortality cases increased by 64.18 thousand (106.74%) between 1990 and 2019 among US adults aged ≥ 80 years, from 60.13 (14.91 ~ 158.44) thousand to 124.31 (31.57 ~ 317.76) thousand. Males experienced a higher increase (147.37%) than females (93.09%) (Table 1). The male-to-female ratios in mortality cases in the past 30 years were < 1.0, ranging from 0.20 to 0.65, but increased gradually (the sex difference narrowed) (Table 1 and Supplementary Fig. 2). In terms of specific age groups, the increase was greater in the older age groups, and the 95 + years group showed the highest increasing level (195.05%), with a similar phenomenon in both sexes (Table 1 and Fig. 1). For the mortality rate, the age-standardized rate stayed stable during the past 30 years in both sexes. The ratios of male-to-female mortality rates from 1990 to 2019 ranged from 0.70 to 0.85, which remained relatively stable for the past 20 years in the 80–94 age group, but showed a declining trend in the 95 + age group (the sex difference widened) (Supplementary Fig. 2). With regard to specific age groups, for males, a significant decline in mortality rate was seen in 95 + years (− 0.18, − 0.29 to − 0.07); for females, however, the statistically significant increasing trends were seen in 90–94 years (0.09, 0.01 to 0.16) and 95 + years (0.12, 0.08 to 0.17) (Table 1 and Fig. 1).
Decomposition analysis on changes in prevalence and mortality
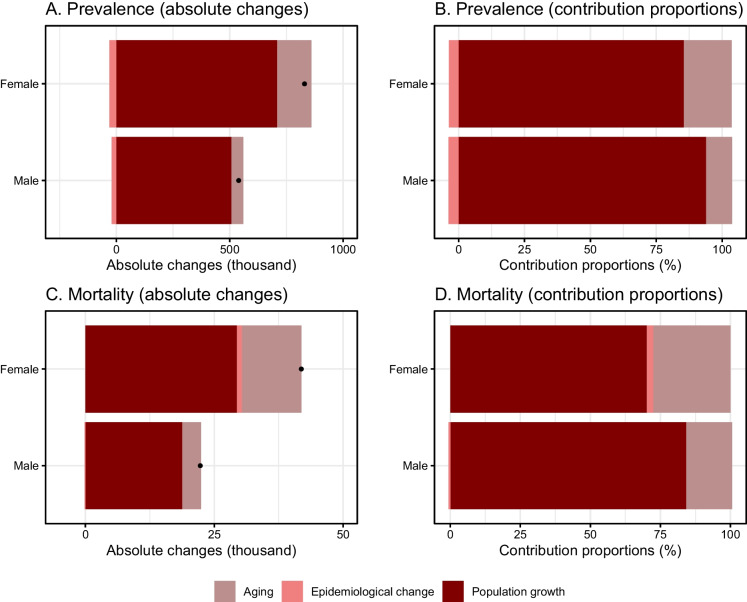
There are three population-level factors, including aging of population, population growth, and epidemiological change, commonly used in epidemiological studies and demographic studies [23]. The population aging (i.e., changes in age structure) and population growth are two aspect of population and they should be separated to provide accurate estimation of the net effect of them [23]. Accordingly, conducting decomposition analysis is necessary to determine net contribution of the population-level factors. The decomposition analysis on changes in dementia prevalence identified that population growth was a major driver for the rise in prevalence cases during the past 30 years, and a higher contribution in males was seen (93.88% vs. 85.42%). Namely, 93.88% (about 0.51 million) of men and 85.42% (about 0.71 million) of women of the total changes in prevalent cases were due to population growth. The aging of population was the second positive factor and females (18.22%) showed a higher contribution proportion than males (9.9%). Additionally, the contribution of prevalence rate change (i.e., epidemiological change) was negative with contribution of − 3.78% (about − 0.02 million) in men and − 3.65% (about − 0.03 million) in women (Fig. 2 and Supplementary Table 1).
Fig. 2.
Decomposition analyses on changes in dementia prevalence and mortality and contributions of population-level determinants among US oldest-old adults. A Absolute changes of prevalence attributed to the three factors. B Contribution proportions in changes of prevalence attributed to the three factors. C Absolute changes of mortality attributed to the three factors. D Contribution proportions in changes of mortality attributed to the three factors. The black dot in A and C represented the combined effect of all three factor, including aging, population growth, and epidemiological change
For mortality, according to the decomposition analysis, the population growth was also the major positive driver for the increase in mortality numbers and a higher contribution in males than females (84.17% vs. 70.19%). Namely, 84.17% (about 0.02 million) of men and 70.19% (about 0.03 million) of women of the total changes in mortality numbers were due to population growth. Another important positive driver is aging and a higher contribution proportion of aging was observed in females (27.6% vs. 16.41%). Regarding mortality rate change (i.e., epidemiological change), the positive contribution was seen in females (2.21%, about 0.9 thousand), while a negative contribution was seen in males (− 0.58%, about − 0.1 thousand) (Fig. 2 and Supplementary Table 2).
Trends and pattern transitions of risk factor
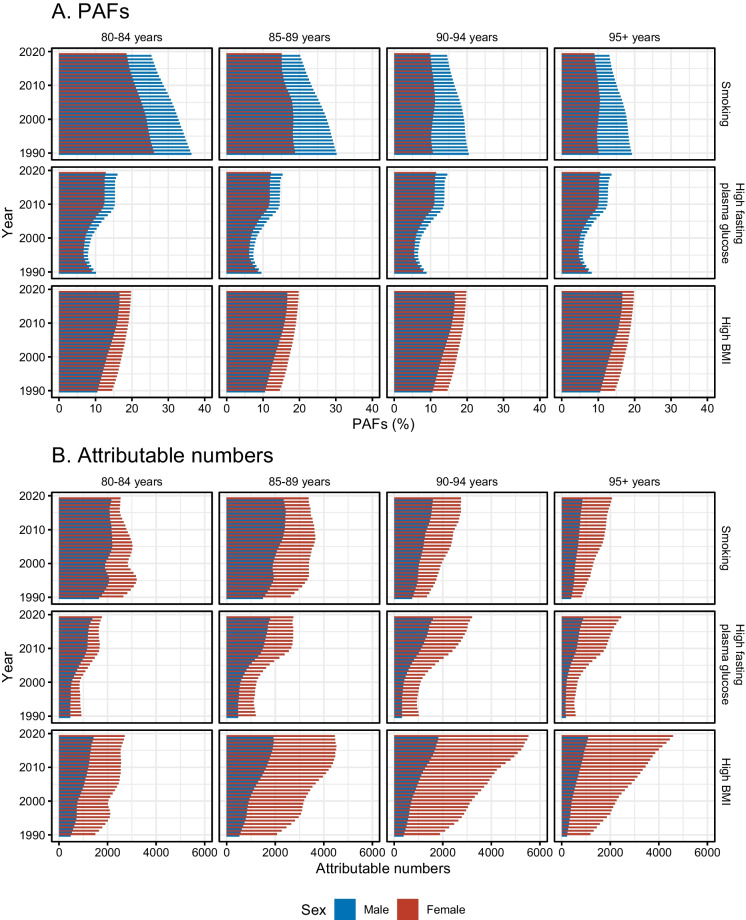
The PAFs of dementia mortality due to risk factors were shown in Fig. 3A and Supplementary Fig. 3. For age group of 80–84 years, smoking was a major risk factor with the highest PAF and an overall decreasing trend; while the PAFs of high BMI increased. On the contrary, in the 95 + years group, high BMI was an important risk factor with the highest PAF and an overall increasing trend, indicating a pattern transition of risk factors among age groups. Notably, for smoking and high fasting plasma glucose, higher PAFs were seen in males; however, females showed higher PAFs of high BMI.
Fig. 3.
PAFs and attributable numbers of dementia mortality due to risk factors among US oldest-old males and females in the four age groups from 1990 to 2019. A PAFs of dementia mortality due to risk factors. B Attributable numbers of dementia mortality due to risk factors. PAF = population-attributable fractions
The attributable numbers of dementia mortality due to risk factors were displayed in Fig. 3B and Supplementary Fig. 4. Higher attributable numbers due to smoking were seen in age groups of 80–84 years and 85–89 years, while higher attributable numbers due to high BMI were seen in the groups of 90–94 years and 95 + years, indicating a pattern transition of risk factors among age groups. Other than the 80–84 years and 85–89 years groups for smoking, increasing trends in attributable numbers of dementia mortality were seen, with the highest increase in attributable numbers due to high BMI among women. More importantly, compared with males, females showed higher levels of attributable numbers of dementia mortality due to these risk factors in all age groups, especially for attributable numbers due to high BMI. The male-to-female ratios in attributable number of dementia mortality were < 1.0 and presented in Supplementary Fig. 5, the lowest male-to-female ratios (the largest sex difference) were seen in 95 + year group of high BMI, ranging from 0.17 to 0.24.
Predictions of prevalence and mortality
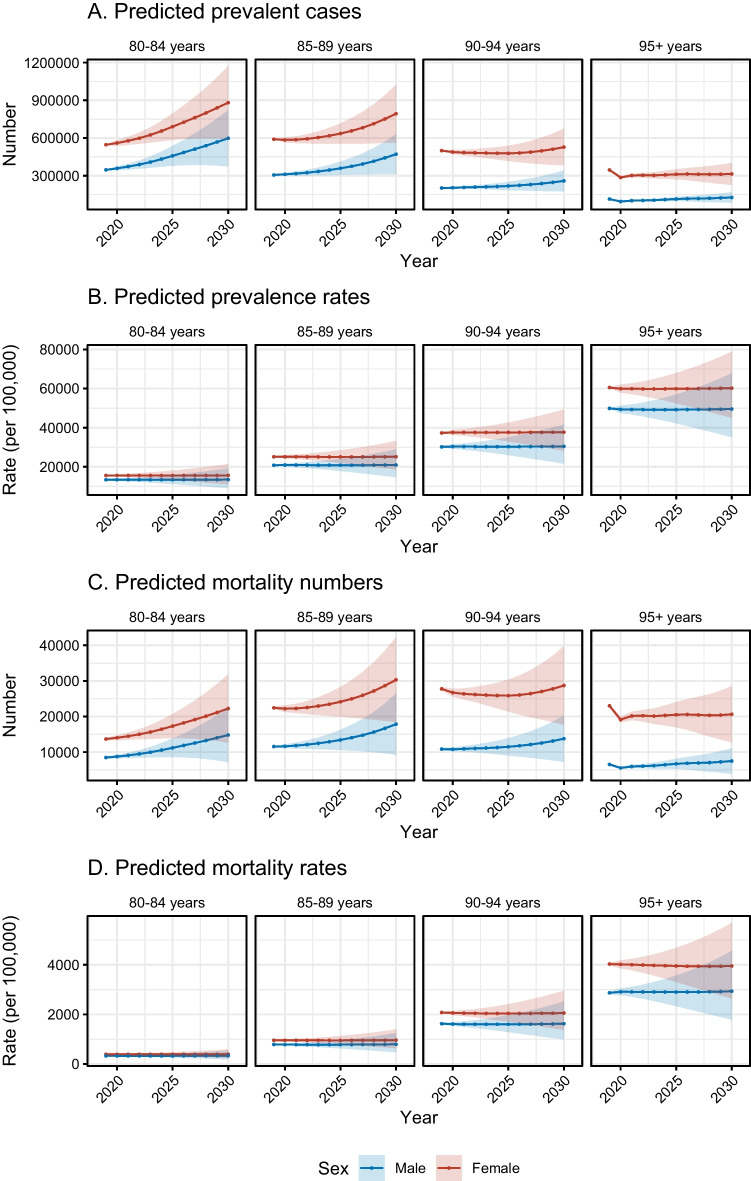
By 2030, there will be around 4 million dementia cases in total among those aged 80 and above based on the BAPC model, as indicated in Fig. 4A and Supplementary Table 3. In groups of 80–84 years and 85–89 years, the absolute number of prevalent cases will show an increasing trend for both sexes and were predicted to be about 1.5 million and 1.25 million by 2030. The prevalent cases number was predicted to stay relatively stable in the upcoming 10 years in groups of 90–94 years and 95 + years. In terms of prevalence rate, it was predicted to show relatively stable trends in both sexes (Fig. 4B and Supplementary Table 4).
Fig. 4.
Predicted prevalence and mortality of US oldest-old adults with dementia in the four age groups from 2020 to 2030. A Predicted absolute number of prevalent cases. B Predicted prevalence rate (per 100,000). C Predicted mortality numbers. D Predicted mortality rate (per 100,000)
We estimated that there will be around 160 thousand deaths due to dementia among US adults aged 80 or older according to the BAPC prediction analyses. Similarly, we found an increasing trend of mortality numbers for both sexes in the groups of 80–84 years and 85–89 years; the number of mortality numbers was predicted to be about 37 thousand and 48 thousand by 2030 in the two groups. Regarding the groups of 90–94 years and 95 + years, the mortality numbers were predicted to remain relatively stable in the upcoming 10 years (Fig. 4C and Supplementary Table 5). For mortality rate, it was also predicted to show relatively stable trends in both sexes (Fig. 4D and Supplementary Table 6).
Discussion
Utilizing data from the GBD study 2019, our comprehensive analysis from 1990 to 2019 revealed that while the absolute numbers of dementia prevalence and mortality have increased, the rates of both have remained relatively stable. This trend underscores the significant influence of population growth and aging on the rising dementia cases, highlighting the critical need for targeted public health interventions. Notably, our study observed a shift in risk factors across age groups, with smoking decreasing as a major risk factor in individuals aged 80–84 years, and high BMI emerging as a significant risk factor, especially in those over 90 years, showcasing an overall increasing trend. These findings indicate the evolving nature of dementia risk factors and underscore the obesity epidemic’s role in shaping future public health strategies. Our projections for the next decade suggest an increase in both prevalence and mortality numbers among individuals aged 80–89 years, with stable rates expected to continue. This projection, alongside the observed higher burden of dementia in females compared to males, calls for sex-targeted strategies to mitigate the disease’s impact. Females exhibited higher levels of prevalence and mortality, more pronounced epidemiological changes, and higher attributable numbers to all risk factors, particularly high BMI.
By focusing on the oldest-old population, defined as those aged 80 or older [5], our study sheds light on the significant long-term increase in dementia's absolute numbers, a slight decrease in age-standardized prevalence rates, and a relatively stable trend in age-standardized mortality rates. These findings align with other studies [4, 24, 25] but also highlight the need for continued attention to age and sex differences in dementia burden and the effectiveness of intervention strategies. In terms of mortality rate, a report based on data from the US National Center for Health Statistics found an increasing trend from 2000 to 2017 among the general population [26]. Notably, the estimated prevalence of dementia could be influenced by the choice of diagnostic criteria, and the recent editions of diagnostic criteria tend to yield a higher prevalence compared to older editions [27]. Given the marked increase in dementia prevalence and mortality predicted for 2030, potential limitations in the current healthcare infrastructure to meet the demands of this population should be noted. With a rise in the number of patients, the number of dementia specialists to evaluate and diagnose patients may become limited. In addition, the healthcare system may not have sufficient capacity to handle the increased number of dementia cases, which could result in prolonged waiting times for patients. Furthermore, the dementia care workforce may not be large enough or equipped with the necessary skills to meet the growing demand [28, 29].
The decomposition analysis underscores that population growth and the aging demographic are pivotal factors driving the increase in dementia prevalence and mortality in the US. Over the next few decades, the segment of the US population aged 90 years and older is anticipated to expand significantly, with projections suggesting a rise to over 8.7 million by 2050, according to the US Census Bureau [30]. Particularly noteworthy is the aging of the baby boom generation, defined as individuals born between 1946 and 1964, whose numbers exceed 65 million [31]. As the vanguard of this generation approaches the age of 80, they represent a demographic shift that will pose substantial challenges for public health systems, primarily due to their substantial size and the elevated risk of dementia associated with advanced age [30]. Our projections, informed by the BAPC model, indicate a gradual increase in dementia case numbers and mortality rates within the age groups of 80–84 and 85–89 years by 2030. This trend aligns with findings from national studies [32], reinforcing the imperative for ongoing and enhanced public health interventions over the forthcoming decades to address the anticipated rise in dementia cases. These measures are crucial for mitigating the impact of dementia on this rapidly growing segment of the population, ensuring that public health infrastructure can accommodate the rising demand for dementia care and support services. This includes enhancing geriatric care training for healthcare professionals, expanding long-term care services, and increasing dementia care support for families and caregivers. Research into innovative care models and technologies that support aging in place can also play a pivotal role in addressing the needs of this growing demographic.
Our analysis meticulously examines the modifiable risk factors that drive dementia morbidity among the oldest-old adults in the US, identifying high BMI as a predominant factor across various age groups. The obesity epidemic represents a substantial public health crisis within the US, bearing profound implications, especially for the elderly demographic. In this population, the widespread prevalence of obesity introduces distinct challenges and amplifies existing vulnerabilities. In 2019, 31.4% of the US population was obese [33], while the prevalence of obesity over the age of 65 was estimated somewhat higher at 35% [34]. The occurrence of obesity is most likely to increase in this age group, as statistical data show a continuously increasing trend of obesity between 2013 and 2021 in those ages 65 and above [35]. Interestingly, the prevalence of obesity starts decreasing above 70 years of age [36]. This observed tendency, however, may be due to healthy survival bias, meaning that the decrease in prevalence in more advanced ages is more strongly connected to the higher mortality rates and shorter survival of patients with obesity. This also points in the direction that this demographic is increasingly bearing the brunt of obesity's adverse health effects, which include a heightened risk of chronic diseases and death. For older adults, obesity not only diminishes quality of life but also complicates the management of existing health conditions, increases dependency, and places a significant strain on healthcare resources. The causes of obesity in this age group are multifaceted, involving a combination of sedentary lifestyles, metabolic changes associated with aging, and socio-economic factors that limit access to healthy food choices and physical activity.
As obesity rates continue to climb, the repercussions for older adults are especially concerning, given the strong link between obesity, cardiovascular and cerebrovascular diseases, diabetes, and dementia. A meta-analysis highlighted the elevated relative risks for dementia in individuals with obese BMI in midlife compared to those with a normal BMI, pegging it at 1.64 (95% CI: 1.34–2.00) [37]. As for the effect of late life obesity, the results are often mixed with certain studies finding that obesity in late life may not necessarily be a risk factor for dementia [38]. This is also seen in a Whitehall II study that found that the BMI of patients with dementia peaks in midlife but begins to decrease in late life, shortly before the diagnosis of dementia [39]. This indicates that future trends of dementia may be more strongly influenced by the proportion of adults who have obesity in midlife than in late life. This is still aggravating, as approximately 40–45% of individuals older than 80 years of age are either currently or formerly obese [36]. Since obesity is linked to several comorbidities, such as diabetes [40] or hypertension [41] that increase the risk of dementia independently from bodyweight, it is still of utmost importance to tackle the obesity epidemic not only in midlife but also in later stages of life.
Obesity increases the risk of developing dementia through several potential mechanisms. For the old population specifically, visceral fat plays a key role in the development of dementia. Visceral fat produces multiple cytokines that promote inflammation, thereby increasing the risk of cognitive impairment and dementia by promoting oxidative stress and endothelial dysfunction, disrupting the function and integrity of the blood brain barrier and by negatively impacting cellular energy metabolism [38]. Another possible mechanism explaining how obesity leads to dementia may be connected to its altering effect related to the production of certain adipokines, such as leptin and resistin, which may negatively impact microcirculation and promote insulin resistance [38]. Insulin resistance, especially in the hippocampus has been shown to negatively impact spatial learning and neuroplasticity [38]. Moreover, insulin resistance also elevates the risk of developing diabetes, which is associated with increased risk of dementia [42–45]. Other factors explaining the relationship of obesity and dementia may reside in cellular senescence responsible for the accelerated vascular ageing observed in obesity and in the increased permeability of the gut-brain axis leading to neuronal damage [38].
The obesity epidemic places a substantial burden on healthcare systems, increasing the demand for long-term care services and specialized dementia care. Addressing obesity, therefore, is not merely a matter of improving individual health outcomes but is crucial for mitigating the broader impact on public health infrastructure, particularly in light of the aging population. Effective strategies to combat obesity could lead to a substantial decrease in the incidence of dementia and other obesity-related conditions, ultimately enhancing quality of life and reducing healthcare costs of elderly. The obesity epidemic’s effect underscores the urgent need for comprehensive public health initiatives aimed at promoting healthier lifestyles and interventions targeted specifically at weight management within this vulnerable demographic. Public health strategies should prioritize nutritional education, physical activity promotion, and access to weight management programs. Notably, weight loss is linked to marked improvements in cognitive functions, such as attention and memory, further emphasizing the importance of addressing obesity as part of a broader strategy to combat dementia [46]. Moreover, healthcare systems should integrate routine obesity and dementia risk screenings for the elderly. Research aimed at exploring the effectiveness of these interventions and their impact on dementia incidence among older adults with high BMI could further inform policy and practice.
This approach is in harmony with the consensus on tackling modifiable dementia risk factors, which, beyond obesity, include smoking, diabetes, and hypertension [47–49]. Cohort studies highlight the substantial risks posed by these factors: for example, persistent or heavy smoking in midlife significantly escalates the risk of developing dementia, with hazard ratios (HRs) between 2.14 and 2.28 [50, 51]. Additionally, elevated glucose levels [52] and diabetes [40] have been associated with an increased risk of dementia, emphasizing the need for targeted interventions to mitigate these risks. For instance, former smokers show a significantly lower risk of dementia compared to those who continue smoking (HR: 0.68; 95% CI: 0.48–0.96) [53]. Moreover, the application of metformin, typically used for diabetes management, has shown potential in reducing dementia risk, although results vary [54, 55]. These insights support the potential of multidomain lifestyle interventions in mitigating dementia risk, underscoring the importance of age-specific strategies tailored to address distinct risk factors effectively, thereby reducing the overall burden of dementia among the oldest-old population in the US [56, 57].
Our analysis revealed a significantly higher dementia burden among US oldest-old women compared to men, demonstrated by elevated prevalence, mortality rates, and higher attributable numbers across all risk factors. This sex disparity aligns with findings from a national epidemiological study [58] and a recent meta-analysis [59], which also reported higher dementia prevalence among elderly females. Interestingly, our trend analysis indicates a narrowing sex gap in both prevalence and mortality over time, echoing observations from other national studies [24, 60]. Building on the identified sex disparities in dementia burden among the US oldest-old population, several mechanisms may underlie the differential effects of sex on AD and VCID. These mechanisms are complex and multifactorial, involving biological, genetic, and possibly lifestyle factors. Firstly, biological differences, such as hormonal variations, particularly estrogen levels, are hypothesized to play a significant role [61–64]. Estrogen has been shown to have neuroprotective effects, and the decline in estrogen levels post-menopause may increase vulnerability to neurodegeneration among women [61–64]. Research suggests that estrogen promotes brain health by improving neural plasticity, increasing antioxidant activity, and reducing the accumulation of amyloid-beta plaques, a hallmark of AD pathology [61–64]. Secondly, genetic factors may contribute to sex disparities in dementia. The presence of the ApoE4 allele, the most significant genetic risk factor for sporadic AD, has been associated with a higher risk of AD in women than in men [63]. This difference may be related to interactions between ApoE4 and sex hormones or other sex-specific genetic and environmental factors. Thirdly, cerebral vascular health, which is crucial in VCID, shows sex differences in its impact on cognitive decline [65]. Women are generally protected against vascular diseases earlier in life, potentially due to estrogen’s vasoprotective effects [65, 66]. Importantly, estrogen confers both anti-atherogenic effects and promotes microvascular health [67]. However, as women age, the risk of vascular diseases and consequently VCID increases, possibly surpassing that of men in the oldest-old age group. This shift could be attributed to the loss of estrogen’s protective effects and a lifetime exposure to other risk factors such as hypertension, which has a different impact on men and women [65–67]. Furthermore, lifestyle factors and social determinants of health, which often differ between sexes, might influence the progression and manifestation of dementia. Women may experience higher lifetime stress levels, less access to education in early life (particularly in older cohorts), and different patterns of employment and caregiving roles, which can affect cognitive reserve and vulnerability to dementia. Lastly, sex differences in brain structure and function, such as variations in brain volume, connectivity, and response to neurodegenerative processes, may influence the susceptibility and progression of AD and VCID. Women might have a higher cognitive reserve due to denser connectivity in certain brain regions, which could delay the onset of dementia symptoms. However, once the disease process begins, the decline could be more rapid compared to men. Importantly, our study observed that females exhibited higher PAFs due to high BMI, consistent across all age groups from 1990 to 2019. Supporting our findings, in terms of biological factors, Malpetti et al. unraveled that females with high BMI can withstand a lower degree of brain dysfunction and show greater vulnerability of brain networks, reflecting a sex effect of high BMI in AD [68]. In addition, socioeconomic and lifestyle factors associated with high BMI, such as lower socioeconomic status, unhealthy diet, and physical inactivity, may disproportionately affect females and contribute to their higher risk of dementia [69]. This finding suggests a sex-specific vulnerability to high BMI, corroborated by research indicating that women with high BMI may experience greater cognitive decline, pointing towards the necessity of sex-specific approaches in public health interventions among this oldest-old population. First, education programs for females may raise awareness about the importance of lifestyle factors in dementia prevention, such as regular physical activity, healthy diet, weight management. Second, with higher attributable numbers due to risk factors among females, ensuring access to female-cantered healthcare services that address the sex-specific risk factors (i.e., high BMI) may improve engagement and effectiveness of preventive strategies. Additionally, in terms of care, in order to provide proper care for older females with high BMI, it is necessary to implement training programs that focus on daily care.
Our study showcases several notable strengths that contribute significantly to the dementia research field, particularly regarding the oldest-old adults in the US. By concentrating on four distinct age groups aged 80 years and above, we have provided a detailed analysis of the long-term temporal trends of dementia, examining both the absolute numbers of cases and rates of prevalence and mortality by age and sex over the past three decades. Moreover, our predictive analysis extends these insights into the next decade, offering a forward-looking perspective on the dementia burden. This comprehensive approach has yielded valuable data for the formulation of targeted strategies aimed at mitigating the impact of dementia across the US. Importantly, our findings underscore the necessity for age and sex-specific interventions that address high-risk populations and modifiable risk factors, thereby presenting a pathway to alleviate the overall burden of dementia. Despite its contributions, our study is not without limitations. A significant constraint is the reliance on the GBD 2019 dataset, which lacks detailed information on the subtypes of dementia, limiting our ability to conduct more nuanced analyses. Furthermore, the dataset does not encompass data on certain risk factors known to influence dementia, such as air pollution and physical inactivity, precluding a comprehensive risk factor analysis that includes environmental and lifestyle considerations. Additionally, the estimations and predictions based on the GBD data, while derived from robust mathematical modeling, require further validation to confirm their accuracy [13]. In terms of the prediction based on the BAPC model, the potential impact of recent global health events, such as the COVID-19 pandemic, on future dementia prevalence and mortality introduces an element of uncertainty into our predictions [70]. Emerging evidence suggests that COVID-19 may elevate the risk of developing dementia by accelerating the conversion from mild cognitive impairment to dementia [71–73]. Accordingly, the period effect in the BAPC model may change after the year of 2019, and the current predictions may be under-estimated. The intersection of COVID-19 with dementia emphasizes the need for ongoing surveillance and research into the pandemic’s long-term effects on cognitive health.
This current study has reported risk factor analyses for dementia among the US oldest-old demographic including smoking, high fasting plasma glucose, and high BMI. However, additional modifiable risk factors for dementia, such as physical inactivity, diet, and social isolation, warrant further exploration to provide a broader perspective on intervention strategies. Moreover, our study has specifically focused on the US, which is one of the high-income countries having increasing burden of dementia among oldest-old adults. Future comparative analysis with other high-income countries is needed to offer insights into how the US’s healthcare policies and social determinants of health impact dementia trends among the oldest-old.
In conclusion, our study leverages the GBD 2019 data to highlight the dynamic and increasing burden of dementia among the oldest-old in the US, driven by population aging and the obesity epidemic. Our findings reveal significant shifts in risk factors, notably the rise of high BMI as key contributor, alongside notable sex disparities in dementia burden. These insights emphasize the critical importance of implementing risk factor-targeted strategies to mitigate the impact of dementia. Moreover, the projection of growing dementia cases due to demographic changes calls for adaptable and responsive public health policies and research priorities aimed at reducing dementia burden and enhancing care for the aging population.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We appreciate the works by the Global Burden of Disease Study 2019.
Author contributions
Conceptualization: XC, YG, and VFP. Data acquisition: YG, MW, and YM. Data analysis: YG, MZ, YM. Data interpretation: XC, MW, and YG. Drafting manuscript: XC, VFP, YG. Revising manuscript: MW, MZ, YM, DM, AL, and YG. Approving final version of the manuscript: All authors.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 82103727), the fellowship of China Postdoctoral Science Foundation (grant number 2021M702221), Guangdong Basic and Applied Basic Research Foundation (grant number 2022A1515010957), Shenzhen Science and Technology Program (grant number RCBS20210706092408008), the National Research, Development and Innovation Fund of Hungary (grant number TKP2021-NKTA-47), the National Cardiovascular Laboratory Program (grant number RRF-2.3.1–21-2022–00003), the National Research, Development and Innovation Fund of Hungary K_20 funding scheme (grant number 135784), the National Research, Development and Innovation Fund of Hungary New National Excellence Program of the Ministry for Innovation and Technology (grant number ÚNKP-24–3-II-SE-14), and the European University for Well-Being (EUniWell) program (grant number 101004093/ EUniWell/EAC-A02-2019 / EAC-A02-2019–1). The funding sources had no role in the writing of the manuscript; and in the decision to submit the article for publication.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xueshan Cao, Minmin Wang, and Mengge Zhou contributed equally to this work.
References
- 1.Alzheimer's Association. Alzheimer’s disease facts and figures. Alzheimers Dement. 2019;15:321–387.
- 2.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement: J Alzheimers Assoc. 2013;9:63-75.e62. 10.1016/j.jalz.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 3.Xu J, Zhang Y, Qiu C, Cheng F. Global and regional economic costs of dementia: a systematic review. The Lancet. 2017;390:S47. 10.1016/S0140-6736(17)33185-9 [DOI] [Google Scholar]
- 4.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80:1778–83. 10.1212/WNL.0b013e31828726f5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Q, Gu D. Oldest-Old Adults. In: Gu D, Dupre ME, editors. Encyclopedia of gerontology and population aging. Cham: Springer International Publishing; 2021. p. 3637–53. [Google Scholar]
- 6.Pierce AL, Kawas CH. Dementia in the oldest old: Beyond Alzheimer disease. PLoS Med. 2017;14:e1002263. 10.1371/journal.pmed.1002263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broyles IH, Li Q, Palmer LM, DiBello M, Dey J, Oliveira I, et al. Dementia’s unique burden: function and health care in the last 4 years of life. J Gerontol A Biol Sci Med Sci. 2023;78:1053–9. 10.1093/gerona/glad003 [DOI] [PubMed] [Google Scholar]
- 8.Collaborators GDaI. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet (London, England). 2020;396:1204–22. 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.GHDx-Link. GBD Results tool. https://vizhub.healthdata.org/gbd-results/. last accessed: 27 June 2023.
- 10.GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. The Lancet Public Health. 2022;7:e105–e125. [DOI] [PMC free article] [PubMed]
- 11.GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet (London, England). 2020;396:1204–1222. [DOI] [PMC free article] [PubMed]
- 12.Qin Z, Liu Z, Li R, Luo Y, Wei Z, He L, et al. Association between BMI trajectories in late-middle age and subsequent dementia risk in older age: a 26-year population-based cohort study. BMC Geriatr. 2023;23:773. 10.1186/s12877-023-04483-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peterson HM, Flaxman AD. Meta-regression with DisMod-MR: how robust is the model? The Lancet. 2013;381:S110. 10.1016/S0140-6736(13)61364-1 [DOI] [Google Scholar]
- 14.Aryannejad A, Saeedi Moghaddam S, Mashinchi B, Tabary M, Rezaei N, Shahin S, et al. National and subnational burden of female and male breast cancer and risk factors in Iran from 1990 to 2019: results from the Global Burden of Disease study 2019. Breast Cancer Res. 2023;25:47. 10.1186/s13058-023-01633-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avan A, Hachinski V. Global, regional, and national trends of dementia incidence and risk factors, 1990–2019: a global burden of disease study. Alzheimers Dement : J Alzheimers Assoc. 2023;19:1281–91. 10.1002/alz.12764 [DOI] [PubMed] [Google Scholar]
- 16.GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet (London, England). 2020;396:1223–1249. [DOI] [PMC free article] [PubMed]
- 17.Mansournia MA, Altman DG. Population attributable fraction. BMJ (Clin Res ed). 2018;360:k757. 10.1136/bmj.k757 [DOI] [PubMed] [Google Scholar]
- 18.Graubard BI, Flegal KM, Williamson DF, Gail MH. Estimation of attributable number of deaths and standard errors from simple and complex sampled cohorts. Stat Med. 2007;26:2639–49. 10.1002/sim.2734 [DOI] [PubMed] [Google Scholar]
- 19.Riebler A, Held L. Projecting the future burden of cancer: Bayesian age-period-cohort analysis with integrated nested Laplace approximations. Biom J. 2017;59:531–49. 10.1002/bimj.201500263 [DOI] [PubMed] [Google Scholar]
- 20.Liu Z, Xu K, Jiang Y, Cai N, Fan J, Mao X, et al. Global trend of aetiology-based primary liver cancer incidence from 1990 to 2030: a modelling study. Int J Epidemiol. 2021;50:128–42. 10.1093/ije/dyaa196 [DOI] [PubMed] [Google Scholar]
- 21.Cao G, Liu J, Liu M. Global, regional, and national incidence and mortality of neonatal preterm birth, 1990–2019. JAMA Pediatr. 2022;176:787–96. 10.1001/jamapediatrics.2022.1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–51. [DOI] [PubMed] [Google Scholar]
- 23.Cheng X, Yang Y, Schwebel DC, Liu Z, Li L, Cheng P, et al. Population ageing and mortality during 1990–2017: a global decomposition analysis. PLoS Med. 2020;17:e1003138. 10.1371/journal.pmed.1003138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hudomiet P, Hurd MD, Rohwedder S. Trends in inequalities in the prevalence of dementia in the United States. Proc Natl Acad Sci USA. 2022;119:e2212205119. 10.1073/pnas.2212205119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langa KM, Larson EB, Crimmins EM, Faul JD, Levine DA, Kabeto MU, et al. A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Intern Med. 2017;177:51–8. 10.1001/jamainternmed.2016.6807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kramarow EA, Tejada-Vera B. Dementia mortality in the United States, 2000–2017. Natl Vital Stat Rep: Center Dis Control Prev, Natl Center Health Stat, Natl Vital Stat Syst. 2019;68:1–29. [PubMed] [Google Scholar]
- 27.Wetterberg H, Najar J, Rydberg Sterner T, Kern S, Skoog I. The Effect of Diagnostic criteria on dementia prevalence - a population-based study from gothenburg, sweden. The American journal of geriatric psychiatry: Off J Am Assoc Geriatr Psychiatr. 2024;32(2):230–243. [DOI] [PubMed]
- 28.Liu JL, Hlavka JP, Hillestad R, Mattke S. Assessing the preparedness of the U.S. health care system infrastructure for an alzheimer's treatment. Santa Monica, CA: RAND Corporation. https://www.rand.org/content/dam/rand/pubs/research_reports/RR2200/RR2272/RAND_RR2272.pdf. Accessed 10 April 2024.
- 29.Lyons B, Watts MOM. Addressing the shortage of direct care workers: insights from seven states (Commonwealth Fund, Mar. 2024). 10.26099/czzn-m038. last accessed: 8 April 2024.
- 30.Bullain SS, Corrada MM. Dementia in the oldest old. Continuum (Minneapolis, Minn). 2013;19:457–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogerson PA, Kim D. Population distribution and redistribution of the baby-boom cohort in the United States: recent trends and implications. Proc Natl Acad Sci USA. 2005;102:15319–24. 10.1073/pnas.0507318102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rajan KB, Weuve J, Barnes LL, McAninch EA, Wilson RS, Evans DA. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimers Dement: J Alzheimers Assoc. 2021;17:1966–75. 10.1002/alz.12362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boutari C, Mantzoros CS. A 2022 update on the epidemiology of obesity and a call to action: as its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism. 2022;133:155217. 10.1016/j.metabol.2022.155217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alexis M. McKee, MD and John E, Morley, MB, BCh. Obesity in the Elderly. https://www.ncbi.nlm.nih.gov/books/NBK532533/. Accessed 10 April 2024.
- 35.Percentage of adults aged 65 years or older in the U.S. who were obese from 2013 to 2021. Statista. https://www.statista.com/statistics/720268/elderly-obesity-united-states/. Accessed 10 April 2024
- 36.Stokes A, Ni Y, Preston SH. Prevalence and trends in lifetime obesity in the U.S., 1988–2014. Am J Prev Med. 2017;53:567–75. 10.1016/j.amepre.2017.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes Rev: Off J Int Assoc Study Obes. 2011;12:e426-437. 10.1111/j.1467-789X.2010.00825.x [DOI] [PubMed] [Google Scholar]
- 38.Bray GA, Bouchard C. (Eds.). Handbook of Obesity - Volume 2: Clinical Applications 5th ed. CRC Press; 2023. 10.1201/9781003432807
- 39.Singh-Manoux A, Dugravot A, Shipley M, Brunner EJ, Elbaz A, Sabia S, et al. Obesity trajectories and risk of dementia: 28 years of follow-up in the Whitehall II Study. Alzheimers Dement. 2018;14:178–86. 10.1016/j.jalz.2017.06.2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chatterjee S, Peters SA, Woodward M, Mejia Arango S, Batty GD, Beckett N, et al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care. 2016;39:300–7. 10.2337/dc15-1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sierra C. Hypertension and the risk of dementia. Front Cardiovasc Med. 2020;7:5. 10.3389/fcvm.2020.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luchsinger JA, Cheng D, Tang MX, Schupf N, Mayeux R. Central obesity in the elderly is related to late-onset Alzheimer disease. Alzheimer Dis Assoc Disord. 2012;26:101–5. 10.1097/WAD.0b013e318222f0d4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ntlholang O, McCarroll K, Laird E, Molloy AM, Ward M, McNulty H, et al. The relationship between adiposity and cognitive function in a large community-dwelling population: data from the Trinity Ulster Department of Agriculture (TUDA) ageing cohort study. Br J Nutr. 2018;120:517–27. 10.1017/S0007114518001848 [DOI] [PubMed] [Google Scholar]
- 44.Item F, Konrad D. Visceral fat and metabolic inflammation: the portal theory revisited. Obes Rev: Off J Int Assoc Study Obes. 2012;13(Suppl 2):30–9. 10.1111/j.1467-789X.2012.01035.x [DOI] [PubMed] [Google Scholar]
- 45.Abbatecola AM, Lattanzio F, Spazzafumo L, Molinari AM, Cioffi M, Canonico R, et al. Adiposity predicts cognitive decline in older persons with diabetes: a 2-year follow-up. PLoS One. 2010;5:e10333. 10.1371/journal.pone.0010333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veronese N, Facchini S, Stubbs B, Luchini C, Solmi M, Manzato E, et al. Weight loss is associated with improvements in cognitive function among overweight and obese people: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2017;72:87–94. 10.1016/j.neubiorev.2016.11.017 [DOI] [PubMed] [Google Scholar]
- 47.Balasubramanian P, Kiss T, Tarantini S, Nyul-Toth A, Ahire C, Yabluchanskiy A, et al. Obesity-induced cognitive impairment in older adults: a microvascular perspective. Am J Physiol Heart Circ Physiol. 2021;320:H740–61. 10.1152/ajpheart.00736.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement: J Alzheimers Assoc. 2015;11:718–26. 10.1016/j.jalz.2015.05.016 [DOI] [PubMed] [Google Scholar]
- 49.Ungvari Z, Toth P, Tarantini S, Prodan CI, Sorond F, Merkely B, et al. Hypertension-induced cognitive impairment: from pathophysiology to public health. Nat Rev Nephrol. 2021;17:639–54. 10.1038/s41581-021-00430-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rusanen M, Kivipelto M, Quesenberry CP Jr, Zhou J, Whitmer RA. Heavy smoking in midlife and long-term risk of Alzheimer disease and vascular dementia. Arch Intern Med. 2011;171:333–9. 10.1001/archinternmed.2010.393 [DOI] [PubMed] [Google Scholar]
- 51.Ohara T, Ninomiya T, Hata J, Ozawa M, Yoshida D, Mukai N, et al. Midlife and late-life smoking and risk of dementia in the community: the Hisayama study. J Am Geriatr Soc. 2015;63:2332–9. 10.1111/jgs.13794 [DOI] [PubMed] [Google Scholar]
- 52.Crane PK, Walker R, Hubbard RA, Li G, Nathan DM, Zheng H, et al. Glucose levels and risk of dementia. N Engl J Med. 2013;369:540–8. 10.1056/NEJMoa1215740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi D, Choi S, Park SM. Effect of smoking cessation on the risk of dementia: a longitudinal study. Ann Clin Transl Neurol. 2018;5:1192–9. 10.1002/acn3.633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campbell JM, Stephenson MD, de Courten B, Chapman I, Bellman SM, Aromataris E. Metformin use associated with reduced risk of dementia in patients with diabetes: a systematic review and meta-analysis. J Alzheimers Dis: JAD. 2018;65:1225–36. 10.3233/JAD-180263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.AreosaSastre A, Vernooij RW, González-ColaçoHarmand M, Martínez G. Effect of the treatment of type 2 diabetes mellitus on the development of cognitive impairment and dementia. Cochrane Database Syst Rev. 2017;6:003804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosenberg A, Ngandu T, Rusanen M, Antikainen R, Bäckman L, Havulinna S, et al. Multidomain lifestyle intervention benefits a large elderly population at risk for cognitive decline and dementia regardless of baseline characteristics: the FINGER trial. Alzheimers Dement: J Alzheimers Assoc. 2018;14:263–70. 10.1016/j.jalz.2017.09.006 [DOI] [PubMed] [Google Scholar]
- 57.Sindi S, Solomon A, Kåreholt I, Hovatta I, Antikainen R, Hänninen T, et al. Telomere length change in a multidomain lifestyle intervention to prevent cognitive decline: a randomized clinical trial. J Gerontol A Biol Sci Med Sci. 2021;76:491–8. 10.1093/gerona/glaa279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–32. 10.1159/000109998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eikelboom WS, Pan M, Ossenkoppele R, Coesmans M, Gatchel JR, Ismail Z, et al. Sex differences in neuropsychiatric symptoms in Alzheimer’s disease dementia: a meta-analysis. Alzheimers Res Ther. 2022;14:48. 10.1186/s13195-022-00991-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eastman J, Bahorik A, Kornblith E, Xia F, Yaffe K. Sex differences in the risk of dementia in older veterans. J Gerontol A Biol Sci Med Sci. 2022;77:1250–3. 10.1093/gerona/glac029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bagit A, Hayward GC, MacPherson REK. Exercise and estrogen: common pathways in Alzheimer’s disease pathology. Am J Physiol Endocrinol Metab. 2021;321:E164–8. 10.1152/ajpendo.00008.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jett S, Malviya N, Schelbaum E, Jang G, Jahan E, Clancy K, et al. Endogenous and exogenous estrogen exposures: how women’s reproductive health can drive brain aging and inform Alzheimer’s prevention. Front Aging Neurosci. 2022;14:831807. 10.3389/fnagi.2022.831807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Valencia-Olvera AC, Maldonado Weng J, Christensen A, LaDu MJ, Pike CJ. Role of estrogen in women’s Alzheimer’s disease risk as modified by APOE. J Neuroendocrinol. 2023;35:e13209. 10.1111/jne.13209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao L, Woody SK, Chhibber A. Estrogen receptor beta in Alzheimer’s disease: from mechanisms to therapeutics. Ageing Res Rev. 2015;24:178–90. 10.1016/j.arr.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nguyen DH, Cunningham JT, Sumien N. Estrogen receptor involvement in vascular cognitive impairment and vascular dementia pathogenesis and treatment. Geroscience. 2021;43:159–66. 10.1007/s11357-020-00263-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Deer RR, Stallone JN. Effects of estrogen on cerebrovascular function: age-dependent shifts from beneficial to detrimental in small cerebral arteries of the rat. Am J Physiol Heart Circ Physiol. 2016;310:H1285-1294. 10.1152/ajpheart.00645.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Davezac M, Buscato M, Zahreddine R, Lacolley P, Henrion D, Lenfant F, et al. Estrogen receptor and vascular aging. Front Aging. 2021;2:727380. 10.3389/fragi.2021.727380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Malpetti M, Sala A, Vanoli EG, Gianolli L, Luzi L, Perani D. Unfavourable gender effect of high body mass index on brain metabolism and connectivity. Sci Rep. 2018;8:12584. 10.1038/s41598-018-30883-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li R, Li R, Xie J, Chen J, Liu S, Pan A, et al. Associations of socioeconomic status and healthy lifestyle with incident early-onset and late-onset dementia: a prospective cohort study. The Lancet Healthy Longev. 2023;4:e693–702. 10.1016/S2666-7568(23)00211-8 [DOI] [PubMed] [Google Scholar]
- 70.Li C, Hua R, Gao D, Zheng F, Xie W. Cognitive decline before and during COVID-19 pandemic among older people with multimorbidity: a longitudinal study. J Am Med Dir Assoc. 2023;24:419-425.e410. 10.1016/j.jamda.2023.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Owens CD, Bonin Pinto C, Mukli P, Szarvas Z, Peterfi A, Detwiler S, et al. Vascular mechanisms leading to progression of mild cognitive impairment to dementia after COVID-19: protocol and methodology of a prospective longitudinal observational study. PLoS One. 2023;18:e0289508. 10.1371/journal.pone.0289508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kirkpatrick A, Delpirou NC, Husain F, Xu C, Vincent A, Yabluchanskiy A, et al. MCI patients with COVID-19 have increased progression to dementia at 18 months (S39. 010). Neurology. 2023;100.
- 73.Matsuzono K, Mashiko T, Anan Y, Koide R, Yoshizumi H, Fujimoto S. Impact of COVID-19 pandemic on mortality and cognitive function of dementia patients: Tochigi Dementia Cohort Study. J Neurol Sci. 2024;456:122840. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.