Abstract
TGF-β1 activation of hepatic stellate cells (HSCs), transcriptional activator 3 (Stat3) activation and short chain fatty acids (SCFAs), metabolite of intestinal bacteria, is closely associated with hepatic fibrosis. Previous studies have shown that Lactucin has significant anti-inflammatory and hepatoprotective effects; however, the mechanism of Lactucin's role in liver fibrosis associated with SCFAs remains unknown. This study was intended to investigate whether effect of Lactucin on liver fibrosis was mediated by TGF-β1/Stat3 and SCFAs. We found that Lactucin induced apoptosis in HSC-T6 cells, and inhibition of nuclear translocation of Stat3 and p-Stat3. And Smad3 and TGF-β1 protein expression was significantly inhibited, while TLR4 and Smad7 protein expression was significantly enhanced. For in vivo experiments, we demonstrated that Lactucin alleviated liver fibrosis in mice, as evidenced by a reduction in inflammatory factors, collagen deposition, liver injury and fibrosis-related factors expression, especially the expression of Smad3 and TGF-β1 proteins was significantly suppressed and Smad7 protein expression was significantly increased in the liver. In addition, the levels of acetic acid, butyric acid and valeric acid in the intestine of Lactucin-treated mice were significantly higher than those in the intestine of liver fibrosis mice. In conclusion, based on the results of in vivo and in vitro experiments, preventive mechanism of Lactucin against liver fibrosis in mice may be to improve the enterohepatic circulation by regulating the metabolites of intestinal microorganisms, acetic acid and butyric acid, and to further regulate the Stat3 and TGF-β1 signaling pathway through the "gut-liver axis" to combat liver fibrosis.
Keywords: Liver fibrosis, Short-chain fatty acid, Lactucin, TGF-β1, STAT3
Subject terms: Cell biology, Drug discovery, Plant sciences, Gastroenterology, Molecular medicine
Introduction
Hepatic fibrosis (HF) is a self-injury repair response of the body secondary to acute and chronic liver injury caused by multiple causes, which can lead to cirrhosis and liver cancer in severe cases and seriously threaten human health1. There are many factors that cause HF, such as alcohol, toxins, drugs, inflammation, viral infections, etc.2. More and more scholars believe that intestinal flora dysbiosis is closely related to the development of liver fibrosis3, and it is now believed that intestinal flora affects the development of liver disease mainly through metabolites such as short-chain fatty acids (SCFAs), amino acids and bile acids4,5. Studies have shown that improving the composition of intestinal microorganisms and then acting on the liver through the "gut-liver axis" cycle can reduce liver inflammation and improve liver fibrosis6,7. However, it is worthwhile to investigate whether the role of gut microbes in reducing inflammation through the "gut-liver axis" to further improve liver fibrosis is related to the SCFAs they metabolize.
SCFAs are metabolites of intestinal flora that break down carbohydrates or amino acids, including acetic acid, propionic acid, butyric acid, valeric acid, hexanoic acid, isobutyric acid, isovaleric acid, etc. Among them, acetic acid, propionic acid and butyric acid are more abundant8. It has been shown that butyric and propionic acids can ameliorate S. aureus-induced inflammatory responses by inhibiting NF-κB, IFN-β/STAT1, and HDAC, which attenuates NO production in macrophages9. A study showed that butyric acid, a major energy source for colonocytes, can restore ZO-1 and occludin proteins, decrease plasma LPS, and improve intestinal histological appearances10. Maintaining the intestinal mucosal barrier, improving the "enterohepatic axis" circulation, and reducing hepatic inflammation have very positive effects on the treatment of liver fibrosis.
In the liver, HF is the final result of a chronic inflammatory response, and the main cause of inflammation-induced liver fibrosis is that it promotes the activation of Hepatic Stellate Cells (HSCs), which are the main collagen-producing cells11, whose activation has been demonstrated in the pathogenesis of HF. Transforming growth factor-β1 (TGF-β1) is one of the most potent pro-fibrotic cytokines12, promoting the activation of HSCs in both autocrine and paracrine forms, activating downstream signaling pathways to promote the phosphorylation of Smad2 and Smad313, converting hepatocytes to myofibroblasts and producing large amounts of extracellular matrix14,15, thereby promoting the generation of liver fibrosis. Therefore, inhibiting the activation of HSCs, promoting the apoptosis of HSCs and regulating related cytokines are effective methods to treat liver fibrosis. On the other hand, TGF-β1 induces inflammatory cells and fibroblasts to secrete other inflammatory factors16,17, including interleukin (IL) -6, IL-1β, Tumor necrosis factor (TNF) -α, etc. IL-6 is one of the most important cytokines that activate signal transduction and transcriptional activator 3 (Stat3) in the liver, and the activation of Stat 3 is also closely related to the development of liver fibrosis18. It was found that in the CCl4-induced liver fibrosis model, the JAK/STAT3 signaling pathway could be inhibited by reducing the serum levels of inflammatory cytokines TNF-α and IL-6, and the expression of α-smooth muscle agonist protein (α-SMA), a marker of hepatic stellate cell activation, was reduced, thus exerting an anti-fibrotic effect19. In conclusion, it is possible to reduce the release of inflammatory factors by regulating the SCFAs metabolized by gut microbes, and further regulate the Stat3 signaling pathway and the TGF-β1 signaling pathway through the "gut-liver axis" to synergize against the formation of liver fibrosis.
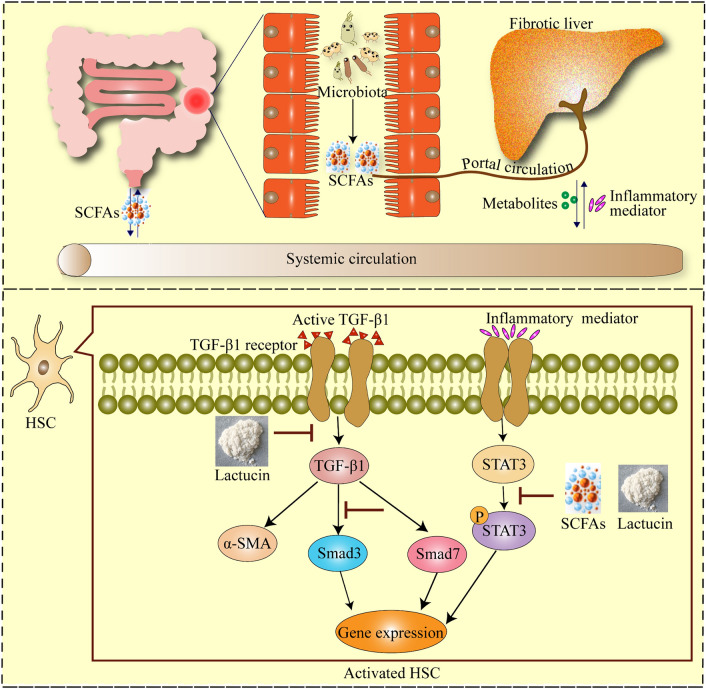
Cichorium glandulosum Boiss. et Huet. (CG) is commonly used by Uyghur doctors as a medicinal herb, and is often used to treat liver disorders. Lactucin, the most abundant natural terpenoid in CG, a sesquiterpene lactone, has significant anti-inflammatory, antibacterial, and lipogenic inhibitory effects6,20,21. A study showed that Lactucin could inhibit adipogenesis by downregulating the JAK/STAT3 signaling pathway22, and our previous study showed that Lactucin could regulate the MAPK signaling pathway and inhibit p38 protein phosphorylation thus acting as an anti-inflammatory agent, and Lactucin was present in up to 6% of Cichorium pumilum Jacq ethyl acetate extract (CGEA)23, which has been shown to regulate intestinal flora in rats, and also to inhibit the TGF-β/SMAD signaling pathway to significantly improve liver fibrosis in rats24,25. Based on the above description, we predicted that Lactucin could improve HF by modulating intestinal microbial metabolites and further modulating Stat3 and TGF-β1 signaling pathways. In conclusion, this study demonstrated that Lactucin had a significant anti-liver fibrosis effect and explored whether this anti-liver fibrosis effect was mediated by SCFAs and TGF-β1/STAT3 signaling pathway, the mechanism of action is shown in Fig. 1.
Figure 1.
Lactucin reverses liver fibrosis by inhibiting TGF-β1/STAT3 signaling pathway and regulating short-chain fatty acid metabolism.
Materials and methods
Preparation of Lactucin
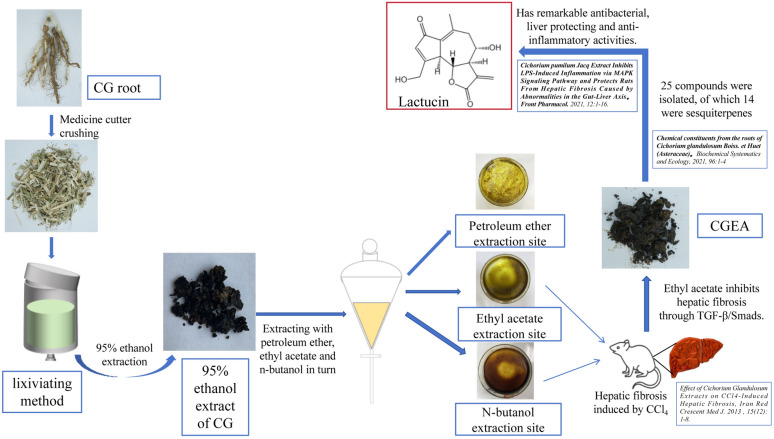
Lactucin was prepared by our research team as follows: 1 kg of dried roots of CG (Xinjiang Medicines Co., Ltd., Xinjiang, China. identified by Prof. Mehmet Nur Ayhoi at Xinjiang Uygur Medical College) was soaked in 95% ethanol for 5 times at room temperature (2 L each time for 3 days), the extract was suspended in water, extracted 1:1 with ethyl acetate and water, and the solvent was volatilized at room temperature to obtain Cichorium pumilum Jacq ethyl acetate extract (CGEA, 7.644 g). The CGEA fraction was fractionated by a silica gel column chromatograph (CC) eluting with a gradient of CH2Cl2-MeOH (from 30:1 to 3:1) to give subfractions B. Fraction B was subjected to an MCI CC to remove pigment eluted excessively with 70, 80, 90 and 100% methyl alcohol to give subfractions B1. Fraction B1 was separated by an RP C18 silica gel CC to give compound 10 (38 mg), and the compound 10 was identified by 1H NMR and 13C NMR spectroscopy as Lactucin6,23, the spectroscopic characterization of Lactucin is shown in the Supplementary Figure (Fig 1). The discovery process of Lactucin is shown in Fig. 2.
Figure 2.
Discovery and preparation of Lactucin.
High performance liquid chromatography analysis6
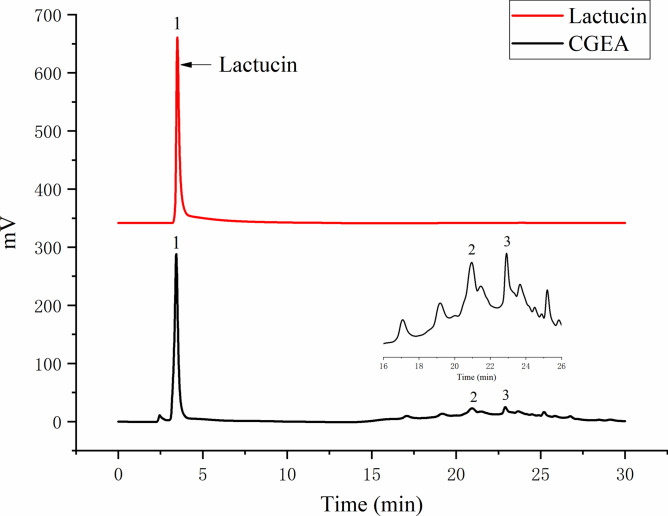
The composition of CGEA was analyzed using Agilent 1290 Infinity II HPLC (Agilent, Germany), and the retention time of the detected peaks was compared to that of the Lactucin standard (The standard was prepared by the research group with a purity of ≥ 95%) for comparison and determination of the Lactucin composition and content in CGEA. HPLC was equipped with a (4.6 × 150 mm, 5 μm) Agilent TC-C18 liquid chromatography column (Agilent, Germany). Mobile phase: Methanol (A)-0.1% formic acid (B), Elution gradient: 0–10 min, 25% A-30% A; 10–20 min, 30% A-70% A; 20–30 min, 70% A-70% A, flow rate 1.0 ml/min, column temperature 30 °C, detection wavelength 275 nm, injection volume 5 μL. And the observed peak at 275 nm was identified as Lactucin (Fig. 3) at a concentration of 6%.
Figure 3.
High performance liquid chromatography (HPLC) of Lactucin and CGEA.
Cell culture and processing
Rat hepatic stellate cells (HSC-T6) were donated by Xinjiang Uygur Autonomous Region Medical Research Institute. Cells were routinely cultured in DMEM high-glucose medium (HyClone, Logan, Utah, United States) containing 10% v/v fetal bovine serum (FBS, Gibco, United States) and 1% penicillin–streptomycin (HyClone, Logan, Utah, United States) at 37 ℃ and 5% CO2, and subcultured every 2–3 days to maintain logarithmic growth. Different concentrations of Lactucin solution were prepared using Dimethyl sulfoxide (DMSO) (Beijing Solarbio Technology Co., Beijing, China) and added to the cell culture medium. The percentage of DMSO (Solarbio) in the cell culture medium was 0.1% (v/v).
Effect of Lactucin on the viability of HCS-T6 cells
The effect of Lactucin on the viability of HSC-T6 cells was determined by MTT (Solarbio). HSC-T6 cells were inoculated in 96-well plates at a density of 1 × 105 cells and incubated overnight, and then the cells were treated with medium containing different concentrations (Fig. 4A) of Lactucin for 24 h. Then the culture medium was carefully aspirated and fresh medium containing 10% MTT solution was added, and the culture was continued for 4 h. Then the culture medium was aspirated and 110 μL of Formazan solution was added to each well, and the wells were shaken at low speed for 10 min, and then the absorbance (OD) of each well was measured at 490 nm by an enzyme marker (Thermo Fisher, United States). And the cell viability was calculated by the following equation: Cell Survival Rate (%) = (OD1/OD0) × 100%. The OD1 indicates the absorbance value of cells in each treatment group, and the OD0 indicates the absorbance value of normal cultured cells.
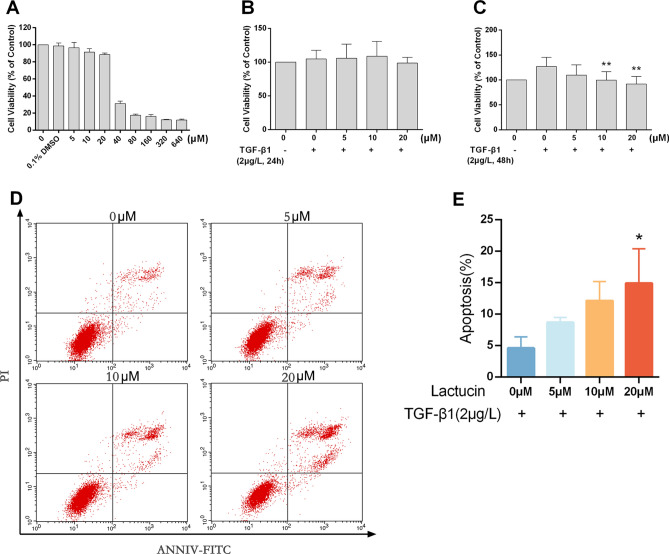
Figure 4.
Lactucin exerts its anti-liver fibrosis effect by inducing HSC-T6 apoptosis. (A–C) The effect of Lactucin on HSC-T6 cell viability was detected by MTT method (n = 5, SD; *P < 0.05; **P < 0.01); (D,E) The effect of Lactucin on HSC-T6 cell apoptosis was detected by flow cytometry (n = 3, SD; *P < 0.05; **P < 0.01).
Effect of TGF-β1 cell growth factor on the viability of HSC-T6 cells
HSC-T6 cells were inoculated in 96-well plates at a density of 1 × 105 cells and incubated overnight, then medium solutions containing different concentrations (Fig. 4B,C) of Lactucin were added, while TGF-β1 cell growth factor at a final concentration of 2 μg/L was added to each well to co-stimulate the cells for 24 h and 48 h, and then the cell viability of HSC-T6 was measured by MTT assay.
Detection of the effect of Lactucin on apoptosis of HSC-T6 cells by flow cytometry
After co-stimulation of Lactucin and TGF-β1 for 48 h, cells of each experimental group were digested with EDTA-free trypsin (Gibco, United States) and 5 × 105 cells were collected. The cells were washed twice using PBS and 500 μL of EDTA-free trypsin was added to each well to digest the cells. The cells were then collected and 195 μL of cell binding solution, 5 μL of AnnexinV-FITC, and 10 μL of propidium iodide (PI) were added to each tube. Finally, the cells were incubated for 30 min under protection from light and detected by flow cytometry (BD FACSCalibur, United States). The experiment was repeated three times to take the average value to calculate the apoptosis rate.
Apoptosis rate (%) = number of apoptotic cells/(number of apoptotic cells + number of normal cells) × 100%.
Cell immunofluorescence assay
Double immunofluorescence staining method was used. Pretreated HSC-T6 cells from each group were digested with trypsin and transferred to cell crawl sheets in equal amounts. Lactucin and TGF-β1 were used to co-stimulate the cells for 48 h, and then the cells were fixed with 4% paraformaldehyde. After washing, 0.3% Triton X-100 was added and incubated for 10 min. The cells were then blocked with 5% bovine serum albumin (BSA) for 1 h. Primary antibodies including Stat3 (1:1000, Cell Signaling Technology, United States), p-Stat3 (1:1000, Cell Signaling Technology, United States) were incubated overnight at 4 ℃. Then cells were incubated with Alexa Fluor 594 or Alexa Fluor 488-coupled secondary antibody (1:1000, Proteintech) for 1 h at room temperature. Finally, the cells were incubated with 4, 6-diamidino-2-phenylindole (DAPI, leagene) for 15 min and the staining was observed using laser confocal microscopy (Nikon, Japan). This part of the experiment was entrusted to Wuhan Xavier Biotechnology Co.
Western blot analysis
HSC-T6 cells were inoculated at a density of 1 × 106 cells in a 6-well flat-bottom plate for 16 h. Cells were pre-protected with the corresponding concentration of Lactucin for 30 min, and then co-stimulated with TGF-β1 for 48 h. After washing cells twice with cold PBS, cells were collected by scraping, and 100 μL of radio immunoprecipitation assay (RIPA) lysis buffer (CoWin Biosciences Co., Beijing, China) was added, shaken thoroughly and placed in a refrigerator at 4 ℃, lysed for 30 min, and then centrifuged at 4 ℃ and 12,000 g for 10 min, extracted the total cellular protein, and determined the protein concentration using bicinchoninic acid (BCA) protein concentration determination kit (Solarbio Life Sciences, Beijing, China). The proteins (50 μg) in each sample were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF, Millipore, Billerica, MA, United States) membranes. The PVDF membranes were then incubated overnight at 4 ℃ with primary antibodies including TLR4 antibody (1:1000), Smad7 antibody (1:1000), Smad3 antibody (1:1000), all from (Cell Signaling Technology, United States). α-SMA antibody (1:2000) and TGF-β1 antibody (1:2000) are from (Boster Biological technology, WuHan, China), GAPDH antibody (1:2000, Beyotime Biotechnology, Shanghai, China). Then the membranes were then incubated for 1 h with horseradish peroxidase (HRP)-conjugated goat anti-rabbit/mouse IgG antibody (1:5000, Zhongshan Jinqiao Biotechnology Co., Beijing, China), and the immunoreactive proteins were visualized using the enhanced chemiluminescence reagent (ECL, Solarbio Technology Co., Beijing, China). The images were obtained using ChemiDoc™ Omnimaging System (Bio-Rad, Hercules, CA, United States) and developed using Image J (NIH, United States) Software for quantification.
Protocol of animal experiments in vivo
Ethical statement
The present study was conducted in compliance with the ARRIVE guidelines. All animal experiments were conducted in strict compliance with the Chinese Guidelines for Ethical Review of Laboratory Animal Welfare (GB/T 35892-2018) and approved by the Medical Ethics Committee of the First Affiliated Hospital of Shihezi University School of Medicine (Approval number: A2020-035-01). Every effort was made to minimize animal suffering and to limit the number of experimental mice. (The data presented here were collected from 60 mice).
Animals
Sixty specific pathogen free (SPF)-grade male C57BL/6 mice weighting 22 ± 2 g purchased from Henan Skeleton Biotechnology Co., Ltd. with experimental animal production license number: SCXK (Yu) 2020-0005. They were housed in the same room in a 14 h-light 10 h-dark cycle in a controlled environment (temperature 22–25 ℃, humidity 55% ± 5). Mice were fed a standard rodent diet and had ad libitum access to water.
The mice were randomly divided into 6 groups of 10 mice each, namely normal control group, model group, Lactucin high dose intervention group (8 mg/kg), medium dose intervention group (4 mg/kg), low dose intervention group (2 mg/kg) and silymarin positive drug control group (4 mg/kg). After 7 days of adaptive feeding, a mouse liver fibrosis model was established by intraperitoneal injection of 10% CCl4 oil solution twice a week for 6 weeks24 in all experimental groups except the normal control group. At the beginning of the seventh week, the intraperitoneal injection of CCl4 was stopped, and each dose group was gavaged with the corresponding test drug, and the blank control group and the model group were gavaged with an equal amount of saline once a day for 28 days. At the end of the experiment, and the blood samples were collected from the retro-orbital venous plexus of mice under gaseous anaesthesia (The mice were put into the anesthesia box containing isoflurane, and the concentration of induced anesthesia was 2–2.5%. When the mice showed a slight squinting of the eyes and a slight trembling, it meant that they had been completely anesthetized, and at this time, the mice were removed and placed on the surgical platform with an anesthesia mask, and the noses of the mice were placed in the anesthesia mask, and the mice were given isoflurane at a concentration of 1.5% continuously to maintain anesthesia and the anesthesia state of the mice was checked constantly. The anesthesia was maintained by continuous inhalation of 1.5% isoflurane). Serums were obtained after centrifugation of the blood at 1300 g for 10 min and frozen at – 80 ℃ until use. When blood collection was completed the mice were rapidly cervical dislocated and executed. Liver and colonic feces were collected from the mice immediately, and a small portion of fresh liver tissue samples were collected and fixed with 4% paraformaldehyde. The remaining tissues and freshly collected feces were rapidly frozen in liquid nitrogen and stored at – 80 ℃ for tissue homogenization kit analysis and metabolomics analysis.
Determination of serum biomarkers
To determine liver function and biochemical parameters, according to the manufacturer's commercial kit instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), the activities of aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (AKP), lactate dehydrogenase (LDH), γ-glutamate transferase (γ-GT) and hydroxyproline (HyP) in serum were determined by enzyme colorimetric method.
Assay of IL-6, IL-1β and TNF-α in the liver
Approximately 1 g of liver tissue was weighed and 9 times ice normal saline was added, and the tissue was cut as much as possible with small ophthalmic scissors and ground into a 10% tissue homogenate using a tissue homogenizer. The prepared homogenate was prepared with centrifuge at 4 ℃, 800 g, 15 min centrifugation for supernatant. The levels of IL-6, IL-1β and TNF-α in liver tissue were determined according to the enzyme-linked immunosorbent assay (ELISA) kit instructions (Shanghai Yaji Biological Co., Ltd., Shanghai, China).
Pathological evaluation of liver tissues
The liver tissues were fixed in 4% paraformaldehyde. These tissues were subsequently dehydrated in a graded ethanol series (75–100%) and embedded in paraffin wax. Tissue thickness was 4 μm in sections, and liver tissue was stained with hematoxylin and eosin (H&E) and Masson, digitally photographed by light microscopic at total magnifications of × 100.
Analysis of liver fibrosis-related protein expression in liver
Fresh mouse liver tissue was weighed 100 mg, 100 μL of cold RIPA lysis buffer was added, quickly ground using a tissue homogenizer, and then placed in a refrigerator at 4 ℃ for 30 min for lysis, centrifuged at 4 ℃ and 12,000 g for 10 min to extract total tissue protein, and protein concentration was determined using a BCA protein concentration inhibition kit (Solarbio Life Sciences, Beijing, China). The proteins (50 μg) in each sample were separated by SDS-PAGE and transferred to PVDF membranes. The PVDF membranes were then incubated overnight at 4 ℃, including primary antibodies TLR4 (1:1000), Smad7 (1:1000), Smad3 (1:1000), α-SMA (1:2000), TGF-β1 (1:2000), Stat3 (1:1000), p-Stat3 (1:1000), GAPDH (1:2000). Then the membranes were then incubated for 1 h with HRP-conjugated goat anti-rabbit/mouse IgG antibody (1:5000), and the immunoreactive proteins were visualized using the ECL. The images were obtained using ChemiDoc™ Omnimaging system and developed using Image J Software for quantification.
Determination of SCFAs in feces
Fresh mouse feces was collected in a 2 mL centrifuge tube, 50 μL of 15% phosphoric acid was added, then 100 μL of 125 μg/mL of internal standard (isocaproic acid) solution and 400 μL of ether were homogenized for 1 min, and the supernatant was centrifuged at 12,000 g at 4 ℃ for 10 min. Then, the supernatant was taken for the determination of SCFAs using a Thermo TRACE 1310-ISQ LT gas chromatograph- mass spectrometer (GC–MS, Thermo, United States).
GC–MS detection: Chromatographic conditions: Agilent HP-INNOWAX capillary column (30 m × 0.25 mm ID × 0.25 μm); injection volumes was 1 μL with split injection, and split ratio was 10:1. Inlet temperature, ion source temperature, transmission line temperature and quadrupole temperature were 250 ℃, 230 ℃, 250 ℃ and 150 ℃, respectively. The program temperature was increased from 90 ℃ to 120 ℃ at 10 ℃/min, then to 150 ℃ at 5 ℃/min, and finally to 250 ℃ at 25 ℃/min for 2 min. The carrier gas was helium at a flow rate of 1.0 mL/min.
MS conditions: electron bombardment ionization (EI) source, SIM scanning mode, electron energy 70 eV.
Based on the assay results, targeted quantification of the detected samples was performed, and based on the quantification results, relevant data analysis was performed.
Growth effect of Lactucin on microorganisms
We measured the minimal inhibit concentration (MIC) of Lactucin on both S. aureus and E. faecalis at 250 μmol/L after preliminary experiments. 2 MIC concentrations of Lactucin were then used to co-culture the bacteria for 8 h. Bacteria were collected, washed three times with sterile PBS, fixed in 2.5% glutaraldehyde fixative, embedded and sectioned, and observed using SEM and TEM to observe the morphology of bacteria and take pictures.
Statistical analysis
Quantitative data are expressed as the means ± standard deviation (SD). One-way analysis of variance (ANOVA) was used to compare differences among multiple groups, and unpaired Student’s t-test was used to analyze the significance between two groups. Statistical analyses were performed with IBM SPSS statistics version 22.0 (IBM, United States, URL: https://sps.server:9080/analyticserver). GraphPad Prism 6.0 (GraphPad Software, United States, URL: https://www.graphpad.com) was used for image production. *P < 0.05, **P < 0.01 were used to denote statistical significance.
Ethics approval
The study was approved by the Ethics Committee for the Protection of Welfare of Experimental Animals of First Affiliated Hospital, Shihezi University, School of Medicine (number A2020-035-01).
Results
Lactucin inhibits the activity of HSC-T6 cells activated by TGF-β1
High concentration of Lactucin significantly inhibited the growth of HSC-T6 cells, and the cell survival rate was 88.59% when the concentration of Lactucin was 20 μM (Fig. 4A), and it can be considered that Lactucin was not cytotoxic to HSC-T6 cells. There was no significant difference in the cell survival rate when using TGF-β1 and Lactucin to co-interact with cells for 24 h (Fig. 4B), but when co-interacting for 48 h, TGF-β1 could significantly activate the activation of HSC-T6 and promote the growth of HSC-T6 differentiation (P < 0.01), while both 20 μM and 10 μM Lactucin could significantly inhibit the activation of HSC-T6 cells activated by TGF-β1 (P < 0.01) (Fig. 4C).
Analysis of apoptosis
Apoptosis is the programmed death of cells, which is a normal death process. When normal cells undergo abnormal proliferation or differentiation, resulting in their failure to apoptosis normally, it induces the development of many diseases. Here we verified the way in which Lactucin induces death to occur in HSC-T6 cells by Annexin V-PI double staining combined with flow cytometry. The apoptosis flow chart shows that when HSC-T6 was co-cultured with TGF-β1, the apoptosis rate of HSC-T6 cells without Lactucin pre-protection was 4.59%, which increased to 9.30%, 12.12% and 14.88% after Lactucin treatment (P < 0.05) with a significant dose-dependent effect, indicating that Lactucin could significantly promote apoptosis of HSC-T6 cells, and the results were mainly in the middle and late stages of apoptosis (Fig. 4D,E).
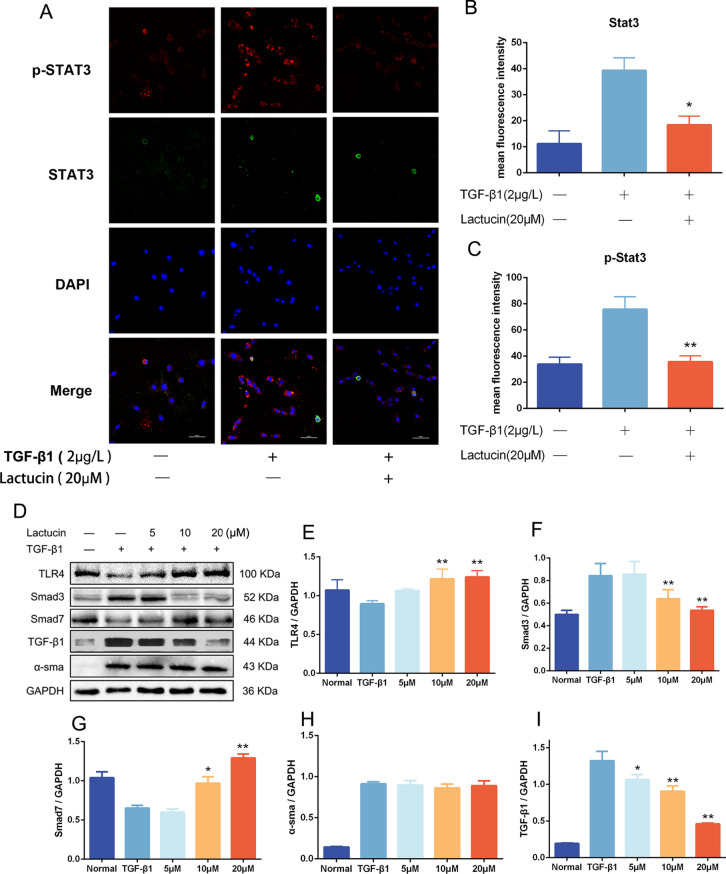
Cellular immunofluorescence staining
We evaluated the potential effect of Stat3 and p-Stat3 translocation from the cytoplasm to the nucleus using immunofluorescence assays. Intracellular Stat3 and p-Stat3 translocation from the cytoplasm to the nucleus was observed after TGF-β1 treatment compared to untreated cells. This nuclear translocation was significantly inhibited by Lactucin pretreatment (P < 0.01), and the results are shown in Fig. 5A–C.
Figure 5.
Lactucin exerts antifibrotic effects by impacting the expression of fibrosis-related factors. (A–C) The immunofluorescence assay was used to assess the potential effect on translocation of Stat3, Image Scale 50 μm (n = 3, SD; *P < 0.05; **P < 0.01); (D–I) The levels of TLR4, Smad3, Smad7, TGF-β1 and α-SMA were determined by Western blot (n = 3, SD; *P < 0.05; **P < 0.01).
Western blot analysis
The TGF-β/Smad signaling pathway is an important pathway for TGF-β1 pro-fibrosis. We examined the protein expression of Toll Like Receptor 4 (TLR4), Smad 3, Smad 7, TGF-β1, and α-SMA in HSC-T6 cells by Western blot, and the results showed that after TGF-β1 growth factor stimulation in HSC-T6 cells, the cell TLR4 and Smad7 protein expression was significantly decreased, while Smad3, α-SMA and TGF-β1 protein expression was significantly increased in HSC-T6 cells after TGF-β1 growth factor stimulation(Fig. 5D). After pre-protection with different concentrations of Lactucin, it could significantly promote the protein expression of TLR4 and Smad7 (P < 0.01) (Fig. 5E,G) and significantly inhibit the protein expression of Smad3 and TGF-β1 (P < 0.01) (Fig. 5F,I) with a certain dose dependence, and the effect of Lactucin on the protein expression of α-SMA was not significant (Fig. 5H).
Lactucin reduces liver fibrosis in mice
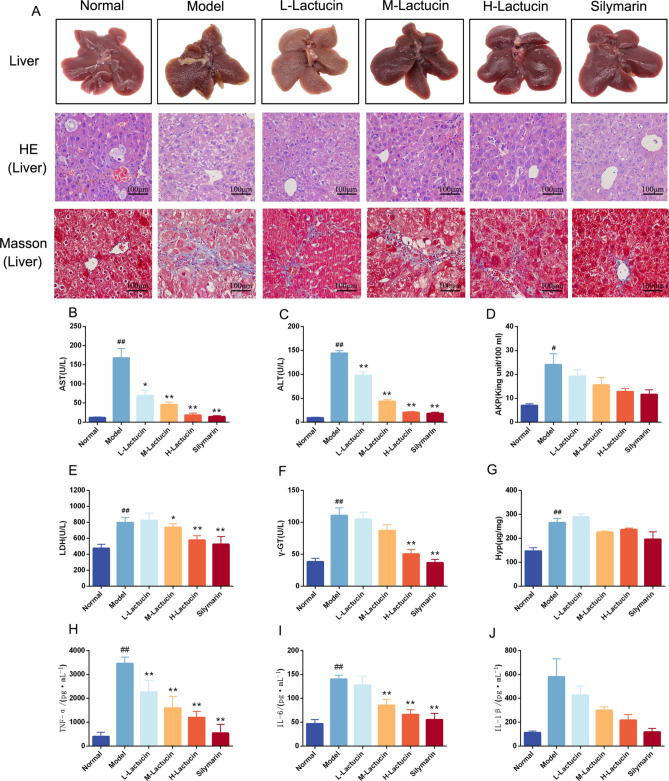
The model of liver fibrosis caused by long-term intraperitoneal injection of CCl4 is an accepted classical model of liver fibrosis. The results of this study showed that long-term intraperitoneal administration of CCl4 to mice resulted in significantly higher concentrations of aspartate transaminase (AST), alanine transaminase (ALT) and lactate dehydrogenase (LDH) in serum (P < 0.01) and significantly higher activity of alkaline phosphatase (AKP) (P < 0.05), as well as significantly higher concentrations of γ-glutamyl transpeptidase (γ-GT) and hydroxyproline (Hyp) in liver tissue homogenates (P < 0.01) compared with normal controls, and administration of Lactucin intervention treatment significantly reduced the concentrations of AST, ALT, LDH, and γ-GT (P < 0.01) with a certain dose-dependent effect, and a tendency to reduce AKP and Hyp, although not significantly, and the results are shown in Fig. 6B–G.
Figure 6.
Lactucin ameliorates liver damage and hepatic fibrosis in mice with CCl4-induced liver fibrosis. (A) HE staining and Masson’s trichrome staining (400 × magnification); (B–G) The levels of AST, ALT, AKP, LDH, γ-GT and Hyp were measured by Biochemical kits (n = 10); (H–J) The levels of TNF-α, IL-6 and IL-1β in liver tissue were measured by ELISA (n = 6). The data are presented as the means ± SD in each group. *P < 0.05, **P < 0.01 with the Model Group; #P < 0.05, ##P < 0.01 with the Normal Group; Normal healthy mice, Model mice with liver fibrosis, L-Lactucin Lactucin low-dose treatment group, M-Lactucin Lactucin middle-dose treatment group, H-Lactucin Lactucin high-dose treatment group, AST aspartate transaminase, ALT alanine transaminase, AKP alkaline phosphatase, LDH lactate dehydrogenase, γ-GT γ-glutamyl transpeptidase, Hyp hydroxyproline.
Hyp is a non-essential amino acid, an important product of collagen metabolism, which can objectively reflect the degree of liver fibrosis. Although the effect of Lactucin on reducing the concentration of Hyp in serum was not significant, it could be seen from the pathological sections that compared with the normal group, the model group had blurred contours of liver lobules, significantly damaged structures, and a large number of inflammatory cells infiltrating liver lobules (Fig. 6A). Masson staining results showed that the model group mice had increased collagen fibrous tissue proliferation and collagen deposition around the central vein and in the fusion zone of the liver, forming obvious fibrous septa with typical histological features of liver fibrosis. Compared with the normal group, the collagen fibers in the Lactucin group were mainly distributed in the hepatic interstitium and were significantly reduced (Fig. 6A). In conclusion, the mice in this experiment had typical characteristics of liver fibrosis, and Lactucin significantly reduced the content of collagen fibers in the liver and had significant anti-fibrotic activity.
Lactucin reduces the levels of liver inflammatory factors in mice
To verify the effect of Lactucin on liver fibrosis in experimental mice by reducing liver inflammation, we measured the levels of IL-6, TNF-α and IL-1β in mouse liver tissues by ELISA. TNF-α, IL-6 and IL-1β are important inflammatory response factors in the organism, and they promote the formation of fibrosis by inducing an inflammatory response in the liver. The results of this study showed that the levels of inflammatory response factors IL-6, TNF-α and IL-1β were significantly increased in the liver of mice in the model group compared with mice in the normal group (P < 0.01), while Lactucin dose-dependently decreased the levels of TNF-α and IL-6 (P < 0.01), and the effect on the level of IL-1β, although there was a tendency to decrease but not significantly (P > 0.05), and the results are shown in Fig. 6H–J.
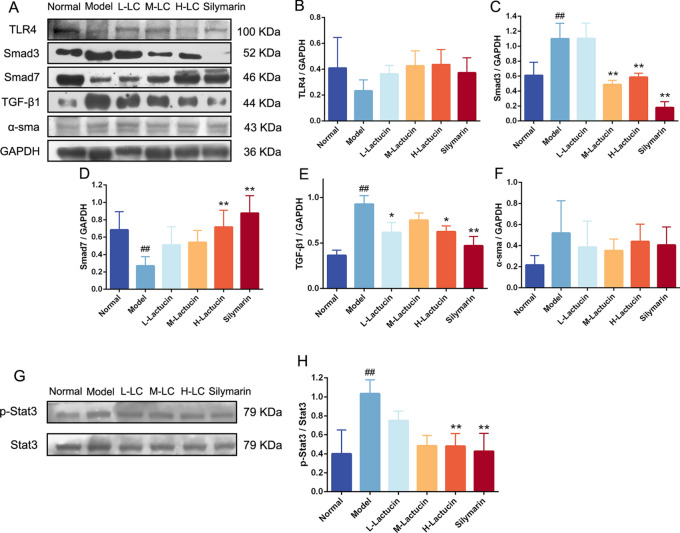
Effect of Lactucin on TGF-β/Smad signaling pathway in mouse liver
To further investigate the effect of Latucin on the TGF-β/Smad signaling pathway, we performed protein immunoblot analysis on the livers of mice, and the results showed that the expression of Smad3 and TGF-β1 proteins was significantly increased (P < 0.01) and Smad7 protein expression level was significantly decreased (P < 0.01) in the livers of mice in the model group, while after Lactucin intervention, high doses of Lactucin significantly inhibited Smad3 and TGF-β1 protein expression (P < 0.01) and promoted Smad7 protein expression (P < 0.01) (Fig. 7), and interestingly Lactucin still had no significant effect on α-SMA protein expression in vivo. In addition, we also examined Stat3 protein phosphorylation in the liver, and the results showed that Stat3 protein was significantly phosphorylated in the liver of mice in the model group (P < 0.01), and after Lactucin intervention treatment, Stat3 protein phosphorylation could be significantly reduced (P < 0.01) (Fig. 7).
Figure 7.
Lactucin exert antifibrotic effects in mice by affecting the expression of fibrosis-related factors. The levels of TLR4, Smad3, Smad7, TGF-β1, α-SMA and Stat3 were determined by Western blot (n = 3). The data are presented as the means ± SD in each group. *P < 0.05, **P < 0.01 with the Model Group; #P < 0.05, ##P < 0.01 with the Normal Group. Normal healthy mice, Model mice with liver fibrosis, L-Lactucin Lactucin low-dose treatment group, M-Lactucin Lactucin middle-dose treatment group, H-Lactucin Lactucin high-dose treatment group.
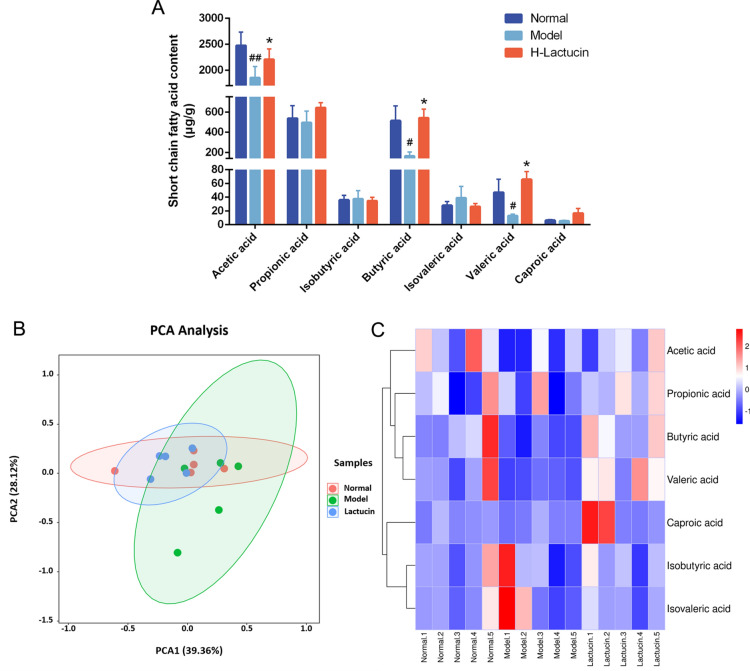
Lactucin alters the content of SCFAs in the feces of mice
Intestinal bacteria are important indicators of intestinal and systemic status, and our previous study showed that ethyl acetate extract of hairy chicory promoted the growth of beneficial bacteria and improved microbial diversity in the intestine of rats with liver fibrosis, thereby reducing liver inflammation and alleviating liver fibrosis. It was shown that intestinal flora affecting the course of liver disease mainly through its metabolites, such as SCFAs, bile acid metabolism, and increased endogenous ethanol. Therefore, to further investigate the effect of Lactucin on the metabolites of mouse intestinal flora, we used an animal model of liver fibrosis as a basis to explore the role of intestinal bacterial metabolites in the process of liver fibrosis and the effect on the TGF-β/Smad signaling pathway. The intestinal contents of mice were collected, and SCFAs were measured by gas-mass spectrometry. The results showed that acetic acid (P < 0.01), butyric acid (P < 0.05) and valeric acid (P < 0.05) were significantly reduced in the intestine of mice in the model group compared with normal controls, and after Lactucin intervention treatment, it could significantly increase acetic acid (P < 0.05), butyric acid (P < 0.05) and valeric acid (P < 0.05) levels. In addition, we also found some decrease in the content of propionic and caproic acids and a relative increase in the content of isobutyric and isovaleric acids in the intestine of liver fibrosis mice, and Lactucin could improve this trend, but none of these changes were significant (P > 0.05), the results are shown in Fig. 8A.
Figure 8.
Effect of Lactucin on short-chain fatty acid metabolism of mice with CCl4-induced hepatic fibrosis. (A) The relative content of SCFAs in the feces of each group of mice; (B) PCA analysis of the relative content of SCFAs in the feces of each group of mice; (C) Heat map analysis of the SCFAs. n = 5 per group. The data are presented as the means ± SD in each group. *P < 0.05, **P < 0.01 with the Model Group; #P < 0.05, ##P < 0.01 with the Normal Group. Normal healthy mice, Model mice with liver fibrosis, H-Lactucin Lactucin high-dose treatment group.
To more visually observe the effect of Lactucin on the metabolism of SCFAs in mice, we performed principal component analysis (PCA) and heat map analysis on SCFAs in mice feces (Fig. 8B,C). The results showed that the difference in SCFAs content in the intestine of the normal control group and the model group was more obvious, while the difference was less obvious compared with the Lactucin intervention treatment group, further indicating that Lactucin can regulate the abnormal SCFAs metabolism phenomenon caused by liver fibrosis.
Effect of Lactucin on microbial growth
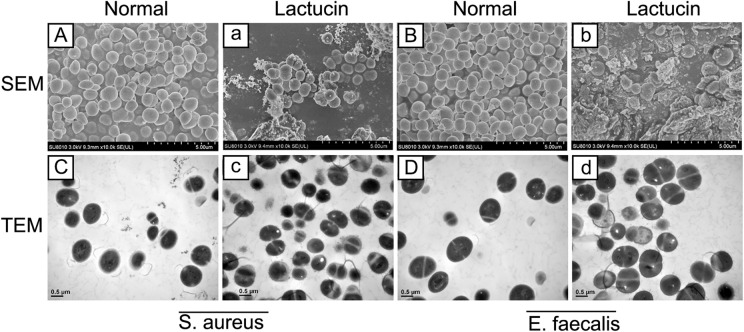
In order to further observe the effect of Lactucin on microbial growth, we used scanning electron microscopy (SEM) and transmission electron microscope (TEM) to observe the morphology of S. aureus and E. faecalis, and the results of SEM plots showed that the surface of the bacteria in the normal group was smooth, with clear borders between the bacteria and arranged in clusters (Fig. 9A,B). In contrast, the bacteria that were co-cultured with Lactucin for 8 h were loosely arranged, with a large reduction in the number of bacteria and blurred borders (Fig. 9a,b). The results from the TEM map showed that the bacterial cell structure of the normal group was intact and clear, with obvious cytoplasm and nucleoplasm, and obvious cell division gap (Fig. 9C,D), while the intracellular material of the bacteria was lost, the intracellular vacuole was obvious, the cell boundary was blurred, and the division was abnormal after the intervention with Lactucin (Fig. 9c,d), and the results indicated that Lactucin could destroy the cell wall of bacteria to play bactericidal effect.
Figure 9.
Effect of Lactucin on the growth of bacteria. (A,a) Morphology of S. aureus under SEM; (B,b) Morphology of E. faecalis under SEM; (C,c) Morphology of S. aureus under TEM; (D,d) Morphology of E. faecalis under TEM. Normal Bacteria without intervention, Lactucin 2 MIC Lactucin intervenes for 8 h in bacteria, SEM scanning electron microscope, TEM transmission electron microscope.
Discussion
In the present study, we demonstrated that Lactucin alleviates liver fibrosis caused by CCl4 and proposed that the beneficial effect of Lactucin on liver fibrosis is achieved by modulating SCFAs and thus the TGF-β1/STAT3 signaling pathway through the "gut-liver axis".
Studies had demonstrated that SCFAs, metabolites of intestinal flora, are linked to the liver to some degree via the gut-liver axis and are involved in inflammation, liver injury, and the development of chronic liver fibrosis26,27. SCFAs are an organic fatty acid with 1–6 carbon atoms, which is produced by fermentation of dietary fiber by microorganisms in the intestinal tract. The thick-walled phylum mainly produced butyrate, and the bacteriophage mainly produced acetate and propionate28,29. The results of this study showed that Lactucin significantly increased the levels of acetate, butyrate and valeric acid in the intestine of liver fibrosis mice, which may be related to its significant anti-harmful bacterial growth and promotion of thick-walled bacterial phylum growth. SCFAs are absorbed by the colon and partially enter the body circulation to act30. Depending on the fermentation substrate, bacterial species and other factors, the types and amounts of SCFAs produced vary, and they play different roles in the intestine31. Acetic acid can protect the intestinal mucosal barrier by activating the innate immune response through multiple pathways and inhibiting intestinal bacterial translocation32,33, which is consistent with our results from a previous study in which ethyl acetate extract of hairy chicory improved the intestinal mucosal barrier in rats by modulating intestinal flora6. The present study further demonstrated that Lactucin significantly increased the content of acetic acid in the intestine of liver fibrosis mice, revealing that its function of improving the intestinal mucosal barrier may be related to the increase of its regulated microbial metabolite acetic acid.
In the liver, Stat3 mediates the signaling of various inflammatory cytokines, and studies had shown that gp130/Stat3 deficiency in hepatocytes exacerbates liver injury and the inflammatory response by increasing TNF-α expression, further promoting hepatic stellate cell activation and accelerating the development of liver fibrosis34,35. A study by Su et al. showed that the Stat3 inhibitor sorafenib and its derivative SC-1 downregulated Stat3 phosphorylation levels in HSC and liver tissues and reduced α-SMA expression, thereby preventing liver fibrosis36. This is consistent with our findings that Lactucin in this study not only significantly promoted apoptosis of HSC-T6 directly, but also significantly downregulated Stat3 phosphorylation levels in HSC-T6 and mouse liver tissues, thereby reducing the production of inflammatory factors TNF-α and IL-6. In addition, it has been shown that butyric acid can also exert anti-inflammatory effects by regulating Stat3, thereby upregulating the Blymyhocyte inducible maturation protein Blimp-1, which in turn induces the production of the anti-inflammatory factor IL-1037. In the present study, Lactucin also significantly increased the content of butyric acid in the intestine of mice, so we speculate that the regulatory effect of Lactucin on Stat3 signaling pathway may be the result of a combination of direct regulation and indirect regulation by increasing butyric acid content (Supplementary Information).
As is well known, TGF-β Mainly through the activation of downstream mediators Smad2 and Smad3, their biological effects are exerted and negatively regulated by inhibitory Smad738,39. After knockdown of the Smad3 gene in mice, RNA expressing type I collagen was significantly reduced in the liver, while Smad3 expression required for activation of HSCs was significantly increased40. And Hu et al. showed that by decreasing Smad3, Smad4 protein expression and increasing Smad7 protein expression, the TGF-β1/Smads signaling pathway could be modulated to inhibit radioactive liver fibrosis41. Lactucin was the main active ingredient in CGEA. Our results showed that Lactucin significantly down-regulated Smad3 and TGF-β1 protein expression and up-regulated Smad7 protein expression, thereby ameliorating CCl4-induced liver fibrosis in mice, which is consistent with our previous findings25,42 that CGEA reduced carbon tetrachloride and thioacetamide-induced liver fibrosis in rats via the TGF-β1/Smads signaling pathway. Therefore, the present experiment again demonstrated that the development of liver fibrosis is closely related to the TGF-β signaling pathway, and Lactucin can inhibit liver fibrosis in mice by regulating this signaling pathway.
Conclusion
The pathogenesis of hepatic fibrosis has been relatively well-defined, and previous studies have demonstrated that the development of hepatic fibrosis is closely related to the TGF-β signaling pathway. In the present study, we demonstrated that Lactucin can affect short-chain fatty acids, which are metabolites of intestinal microorganisms, and then further reduce hepatic inflammatory responses through the enterohepatic axis circulation, and ultimately ameliorate CCl4-induced hepatic fibrosis in mice. In conclusion, our study suggests that Lactucin ameliorates CCl4-induced hepatic fibrosis in mice, and its mechanism of action may be related to the increased content of acetate and butyrate in SCFAs, which modulate inflammatory responses and further act on the TGF-β1/STAT3 signaling pathway. Although this study provides a possibility for the treatment of liver fibrosis with Lactucin, further studies are needed to validate it.
Supplementary Information
Author contributions
The study conception and design was prepared by QD and HC. Material preparation and data collection were performed by HC, GY and LH. The biochemical and molecular analyses were done by QD, HC and LH, and the histological studies were done by QD, ZL and GY, The first draft of the manuscript was written by HC and GY, and the final version was read and finalized by QD, HC and ZL. All authors reviewed and approved the final manuscript.
Funding
This study was funded by grants from the National Natural Science Foundation of China (NO. 82160742) and the Corps key areas of scientific and technological research program (2023CB008-25) for financial.
Data availability
The data used to support the findings of this study are available in Science Data Bank at https://www.scidb.cn/en/s/iYNRF3 (access link). Data DOI: 10.57760/sciencedb.06826registering.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-70253-5.
References
- 1.Mokdad, A. A. et al. Liver cirrhosis mortality in 187 countries between 1980 and 2010: A systematic analysis. BMC Med.12, 145 (2014). 10.1186/s12916-014-0145-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qin, D., Zhang, Y. & Li, L. Progress in research of Chinese herbal medicines with anti-hepatic fibrosis activity. World Chin. J. Digestol.25, 958–965 (2017). 10.11569/wcjd.v25.i11.958 [DOI] [Google Scholar]
- 3.Seo, Y. S. & Shah, V. H. The role of gut-liver axis in the pathogenesis of liver cirrhosis and portal hypertension. Clin. Mol. Hepatol.18, 337–346 (2012). 10.3350/cmh.2012.18.4.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, F. & Stappenbeck, T. S. Microbiome control of innate reactivity. Curr. Opin. Immunol.56, 107–113 (2019). 10.1016/j.coi.2018.12.003 [DOI] [PubMed] [Google Scholar]
- 5.Agus, A., Planchais, J. & Sokol, H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe23, 716–724 (2018). 10.1016/j.chom.2018.05.003 [DOI] [PubMed] [Google Scholar]
- 6.Han, C. et al.Cichoriumpumilum Jacq extract inhibits LPS-induced inflammation via MAPK signaling pathway and protects rats from hepatic fibrosis caused by abnormalities in the gut-liver axis. Front. Pharmacol.12, 683613 (2021). 10.3389/fphar.2021.683613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nie, Y. et al. Ursolic acid reverses liver fibrosis by inhibiting NOX4/NLRP3 inflammasome pathways and bacterial dysbiosis. Gut Microbes13, 1972746 (2021). 10.1080/19490976.2021.1972746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li, H. et al. Clinical significance of the determination of fecal short - chain fatty acids in patients with nonalcoholic fatty liver disease. J. Clin. Hepatol.38, 1299–1306 (2022). [Google Scholar]
- 9.Park, J. W. et al. Short-chain fatty acids inhibit staphylococcal lipoprotein-induced nitric oxide production in murine macrophages. Immune Netw.19, e9 (2019). 10.4110/in.2019.19.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang, T. T. et al. Amelioration of non-alcoholic fatty liver disease by sodium butyrate is linked to the modulation of intestinal tight junctions in db/db mice. Food Funct.11, 01954b (2020). 10.1039/D0FO01954B [DOI] [PubMed] [Google Scholar]
- 11.Takaaki Higashi, S. L. F. & Hoshida, Y. Hepatic stellate cells as key target in liver fibrosis. Adv. Drug Deliv. Rev.121, 27–42 (2017). 10.1016/j.addr.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steven Dooley PtD. TGF-β in progression of liver disease. Cell Tissue Res.347, 245–256 (2012). 10.1007/s00441-011-1246-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dewidar, B., Meyer, C. & Dooley, S. TGF-β in hepatic stellate cell activation and liver fibrogenesis - updated. Cells8, 1419 (2019). 10.3390/cells8111419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duarte, S., Baber, J., Fujii, T. & Coito, A. J. Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Biol.44–46, 147–156 (2015). 10.1016/j.matbio.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roeb, E. Matrix metalloproteinases and liver fibrosis (translational aspects). Matrix Biol.68–69, 463–473 (2018). 10.1016/j.matbio.2017.12.012 [DOI] [PubMed] [Google Scholar]
- 16.Raikhelson, K. L. K. V. & Marchenko, N. V. Role of transforming growth factor-beta in the development of some liver diseases. Terapevticheskii Arkhiv86, 44–48 (2014). [PubMed] [Google Scholar]
- 17.Bissell, D. M., Roulot, D. & George, J. Transforming growth factor beta and the liver. Hepatology34, 859–867 (2001). 10.1053/jhep.2001.28457 [DOI] [PubMed] [Google Scholar]
- 18.Li, T. Research progress of STAT3 on liver disease. J. Pharm. Pract.40, 208–211 (2022). [Google Scholar]
- 19.Hou, G. et al. Long ameliorates hepatic fibrosis by inhibiting PI3K/AKT, Ras/ERK and JAK1/STAT3 signaling pathways in CCl4-induced liver fibrosis rats. Curr. Med. Sci.40, 539–547 (2020). 10.1007/s11596-020-2211-3 [DOI] [PubMed] [Google Scholar]
- 20.Bischoff, T. A. et al. Antimalarial activity of lactucin and lactucopicrin: Sesquiterpene lactones isolated from Cichoriumintybus L. J. Ethnopharmacol.95, 455–457 (2004). 10.1016/j.jep.2004.06.031 [DOI] [PubMed] [Google Scholar]
- 21.Han, C. et al. Study on differences in antibacterial activity of ethanol extracts from chicory roots and stems against Staphylococcusaureus and Enterococcusfaecalis. Chin. Pharmacol. Bull.35, 540–545 (2019). [Google Scholar]
- 22.Wang, X. et al. A potential nutraceutical Candidatelactucin inhibits adipogenesis through downregulation of JAK2/STAT3 signaling pathway-mediated mitotic clonal expansion. Cells9, 331 (2020). 10.3390/cells9020331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dang, T. et al. Sesquiterpenoids with diverse carbon skeletons from the roots of Cichoriumglandulosum and their anti-inflammatory activities. Fitoterapia136, 104170 (2019). 10.1016/j.fitote.2019.104170 [DOI] [PubMed] [Google Scholar]
- 24.Qin, D., Wen, Z., Nie, Y. & Yao, G. Effect of cichorium glandulosum extracts on CCl4-induced hepatic fibrosis. Iran Red Crescent Med. J.15, e10908 (2013). 10.5812/ircmj.10908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin, D. et al. Studies on the protective effect of total flavonoids from Cichoriumglandulosum roots against carbon tetrachloride-induced liver fibrosis in rats. Trop. J. Pharm. Res.18, 311–319 (2019). 10.4314/tjpr.v18i2.13 [DOI] [Google Scholar]
- 26.Hui, D. & Cheng, S. Association between nonalcoholic fatty liver disease and gut microbiota based on the theory of gut-liver axis. J. Clin. Hepatol.36, 1627–1630 (2020). [Google Scholar]
- 27.Sharma, S. P., Suk, K. T. & Kim, D. J. Significance of gut microbiota in alcoholic and non-alcoholic fatty liver diseases. World J. Gastroenterol.27, 6161–6179 (2021). 10.3748/wjg.v27.i37.6161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flint, H. J., Duncan, S. H., Scott, K. P. & Louis, P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc.74, 13–22 (2015). 10.1017/S0029665114001463 [DOI] [PubMed] [Google Scholar]
- 29.Levy, M., Thaiss, C. A. & Elinav, E. Metabolites: Messengers between the microbiota and the immune system. Genes Dev.30, 1589–1597 (2016). 10.1101/gad.284091.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimura, I. et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun.4, 1829 (2013). 10.1038/ncomms2852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ganapathy, V., Thangaraju, M., Prasad, P. D., Martin, P. M. & Singh, N. Transporters and receptors for short-chain fatty acids as the molecular link between colonic bacteria and the host. Curr. Opin. Pharmacol.13, 869–874 (2013). 10.1016/j.coph.2013.08.006 [DOI] [PubMed] [Google Scholar]
- 32.Leung, C., Rivera, L., Furness, J. B. & Angus, P. W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol.13, 412–425 (2016). 10.1038/nrgastro.2016.85 [DOI] [PubMed] [Google Scholar]
- 33.Fachi, J. L. et al. Acetate coordinates neutrophil and ILC3 responses against C. difficile through FFAR2. J. Exp. Med.10.1084/jem.20190489 (2020). 10.1084/jem.20190489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mair, M. et al. Signal transducer and activator of transcription 3 protects from liver injury and fibrosis in a mouse model of sclerosing cholangitis. Gastroenterology138, 2499–2508 (2010). 10.1053/j.gastro.2010.02.049 [DOI] [PubMed] [Google Scholar]
- 35.Kiu, H. & Nicholson, S. E. Biology and significance of the JAK/STAT signalling pathways. Growth Factors30, 88–106 (2012). 10.3109/08977194.2012.660936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su, T. H. et al. Sorafenib and its derivative SC-1 exhibit antifibrotic effects through signal transducer and activator of transcription 3 inhibition. Proc. Natl. Acad. Sci. U. S. A.112, 7243–7248 (2015). 10.1073/pnas.1507499112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun, M. et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat. Commun.9, 3555 (2018). 10.1038/s41467-018-05901-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen, Y., Li, D. G., Wu, J., Chen, Y. & Lu, H. Tetrandrine inhibits activation of rat hepatic stellate cells stimulated by transforming growth factor-beta in vitro via up-regulation of Smad 7. J. Ethnopharmacol.100, 299–305 (2005). 10.1016/j.jep.2005.03.027 [DOI] [PubMed] [Google Scholar]
- 39.Tu, X. et al. MicroRNA-30 protects against carbon tetrachloride-induced liver fibrosis by attenuating transforming growth factor beta signaling in hepatic stellate cells. Toxicol. Sci.146, 157–169 (2015). 10.1093/toxsci/kfv081 [DOI] [PubMed] [Google Scholar]
- 40.Roberts, A. B., Russo, A., Felici, A. & Flanders, K. C. Smad3: A key player in pathogenetic mechanisms dependent on TGF-beta. Ann. N. Y. Acad. Sci.995, 1–10 (2003). 10.1111/j.1749-6632.2003.tb03205.x [DOI] [PubMed] [Google Scholar]
- 41.Hu, Z. et al. Paeoniflorin exerts protective effect on radiation-induced hepatic fibrosis in rats via TGF-beta1/Smads signaling pathway. Am. J. Transl. Res.10, 1012–1021 (2018). [PMC free article] [PubMed] [Google Scholar]
- 42.Qin, D., Nie, Y. & Wen, Z. Protection of rats from thioacetamide-induced hepatic fibrosis by the extracts of a traditional Uighur medicine Cichorium glandulosum, Iran J Basic. Med. Sci.17, 879–885 (2014). [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are available in Science Data Bank at https://www.scidb.cn/en/s/iYNRF3 (access link). Data DOI: 10.57760/sciencedb.06826registering.