Abstract
Physical frailty is a syndrome that typically manifests in later life, although the pathogenic process causing physical frailty likely begins decades earlier. To date, few studies have examined the biological signatures in mid-life associated with physical frailty later in life. Among 4,189 middle-aged participants (57.8 ± 5.0 years, 55.8% women) from the Atherosclerosis Risk in Community (ARIC) study, we evaluated the associations of 4,955 plasma proteins (log 2-transformed and standardized) measured using the SomaScan platform with their frailty status approximately 20 years later. Using multinomial logistic regression models adjusting for demographics, health behaviors, kidney function, total cholesterol, and comorbidities, 12 and 221 proteins were associated with prefrailty and frailty in later life, respectively (FDR p < 0.05). Top frailty-associated proteins included neurocan core protein (NCAN, OR = 0.66), fatty acid-binding protein heart (FABP3, OR = 1.62) and adipocyte (FABP4, OR = 1.65), as well proteins involved in the contactin-1 (CNTN1), toll-like receptor 5 (TLR5), and neurogenic locus notch homolog protein 1 (NOTCH1) signaling pathway relevant to skeletal muscle regeneration, myelination, and inflammation. Pathway analyses suggest midlife dysregulation of inflammation, metabolism, extracellular matrix, angiogenesis, and lysosomal autophagy among those at risk for late-life frailty. After further adjusting for midlife body mass index (BMI) – an established frailty risk factor – only CNTN1 (OR = 0.75) remained significantly associated with frailty. Post-hoc analyses demonstrated that the top 41 midlife frailty-associated proteins mediate 32% of the association between mid-life BMI and late-life frailty. Our findings provide new insights into frailty etiology earlier in the life course, enhancing the potential for prevention.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-024-01219-8.
Keywords: Proteomics, Midlife, Frailty, Aging
Introduction
Physical frailty, a state of reduced reserve and increased vulnerability to stressors, is often considered a geriatric condition. To date, research on frailty, including studies of biological and physiological mechanisms underlying frailty, has largely focused on later life [1], with comparatively less attention given to midlife. However, the biological and physiological changes leading to late-life frailty may start in midlife [2, 3]. For example, the presence of damage repair and clearance mechanisms may compensate for the biological and physiological deterioration and delay the clinical manifestation of frailty until the biological and physiological impairment passes the capacity of the compensatory mechanisms [4, 5]. In support of this hypothesis, midlife obesity [6–9], cardiovascular risk factors (including blood pressure, cholesterol, and glucose) [7], and physical inactivity [10] have been associated with a higher risk of frailty in later life. These midlife risk factors may share common biological mechanisms with frailty or contribute to biological changes that lead to frailty. Walker and colleagues found that higher circulating inflammatory markers (C-reactive protein and a composite score of fibrinogen, von Willebrand factor, Factor VIII, and white blood cell count) during mid-life were associated with a higher risk of frailty in older age [3]. Nguyen and colleagues found that among non-frail African-American participants aged 49–65 years, serum progranulin, a lysosomal protein that is elevated with obesity and type 2 diabetes, was associated with frailty status 9 years later [11]. Together, these findings suggest that specific biological alterations occurring during middle age may influence frailty risk in later life. Yet to our knowledge, no proteomic studies of frailty have investigated the midlife proteins contributing to the development of frailty later in life. Such studies are needed to better understand the mechanisms of frailty and inform the development of novel intervention strategies for delaying or reversing the trajectory of frailty.
To this end, we examined nearly 5000 plasma proteins quantified in midlife from 4,189 participants in the Atherosclerosis Risk in Community (ARIC) study. We assessed the associations of these proteins with frailty status defined by the physical frailty phenotype [12] approximately 20 years after protein measurement. This broad assessment of proteins in a proteomic approach allows agnostic exploration of biomarkers beyond those that are theoretically conceived to be related to frailty.
Methods
Study population
The ARIC study is an ongoing community-based cohort study originally designed to understand the etiology of atherosclerosis and its clinical consequences starting in midlife [13, 14]. The participants were enrolled from 4 communities across the United States: Washington County, MD; Forsyth County, NC; northwestern suburbs of Minneapolis, MN; and Jackson, MS [13]. We used Visit 3 (1993–1995) of the ARIC study as baseline when 11,478 participants had plasma proteomics measured. Late-life frailty status was ascertained in Visit 5 (2011–2013), Visit 6 (2016–2017), and Visit 7 (2018–2019).
Among the 11,478 participants at baseline, we excluded self-identified non-Black and non-White participants or self-identified Black participants at the Washington County and Minneapolis study sites due to small sample sizes (n = 70) and participants who had missing covariates (n = 1,370). Among the remaining participants, 4,189 (42%) participants had at least one frailty assessment in Visits 5–7. The mean follow-up was 19.9 years (SD = 2.9 years). These participants were included in the main analysis. These participants aged 50–71 years at baseline (Fig. S1), among whom 3,698 participants were below 65 years (88%).
Proteomics measurement
Relative abundances of the plasma proteins and protein complexes were measured by the SomaScan platform (Version 4.0, Somalogic, Inc., Boulder, Colorado). The SomaScan platform uses single strands of DNA with chemically modified nucleotides, called modified aptamers or “SOMAmers”, which act as protein-binding reagents with defined three-dimensional structures and unique nucleotide sequences [15]. The abundances of the SOMAmers were quantified using dynamic DNA detection technology and represented the levels of the proteins in plasma. The assay was shown to have a sensitivity comparable to the conventional immunoassay approaches [16] and good reproducibility [17, 18]. A total of 4,995 SOMAmers of 4,712 unique proteins passed the quality control in ARIC and were used in the present study.
Frailty assessment
Frailty was operationalized in the ARIC study using the five criteria of the physical frailty phenotype: weight loss, weakness, slowness, exhaustion, and low physical activity [19]. Weight loss at Visit 5 was defined as > 10% decrease in measured weight from Visit 4 (1996–1998) or having a current BMI < 18.5 kg/m2. At Visits 6 and 7, > 5% lower weight from the previous visit or current BMI < 18.5 kg/m2 was used. The higher weight loss threshold was used at Visit 5 due to the longer time intervals between Visits 4 and 5 compared to the subsequent visits, and the time interval may have overlapped with midlife [20]. Weakness was defined as grip strength below the cut-points established in the Cardiovascular Health Study (CHS, Table 1) [12]. Slowness was defined as the usual gait speed below the CHS cut-points. Exhaustion was defined as responding “some of the time” or “most of the time” to either of the two questions from the CES-D scale: I felt everything I did was an effort, or I could not get “going”. Low physical activity was ascertained as ranking in the lowest quintile of self-reported physical activity.
Table 1.
Definitions and measurements of physical frailty in ARIC
| Components of physical frailty | ||
| Criteria | Definition and measurements | |
| Weight loss |
Visit 5: > 10% weight loss from Visit 4 by direct measure of weight, or BMI < 18.5 kg/m2 at current visit Visit 6/7: > 5% weight loss from Visit 5/6 by direct measure of weight, or BMI < 18.5 kg/m2 at current visit |
|
| Weakness | Grip strength in the preferred hand (better of two measurements) below the following gender and BMI-specific cut-points: | |
|
Men: • BMI ≤ 24 kg/m2: ≤ 29 kg • BMI 24.1–26 kg/m2: ≤ 30 kg • BMI 26.1–28 kg/m2: ≤ 30 kg • BMI > 28 kg/m2: ≤ 32 kg |
Women: • BMI ≤ 23 kg/m2: ≤ 17 kg • BMI 23.1–26 kg/m2: ≤ 17.3 kg • BMI 26.1–29 kg/m2: ≤ 18 kg BMI > 29 kg/m2: ≤ 21 kg |
|
| Slowness | Time to walk 4 m at usual pace below the following gender- and height-specific cut-points: | |
|
Men: • Height ≤ 173 cm: ≥ 7 s • Height > 173 cm: ≥ 6 s |
Women: • Height ≤ 159 cm: ≥ 7 s Height > 159 cm: ≥ 6 s |
|
| Exhaustion | Responded “some of the time” or “most of the time” to the following questions from CES-D scale: I felt everything I did was an effort, or I could not get “going” | |
| Low physical activity | Gender-specific lowest 20% of rank based on the Modified Baecke Physical Activity Questionnaire sports and exercise index | |
| Physical frailty status | ||
| # Criteria missing | # Non-missing criteria met | Frailty status |
| 0 | 0 | Robust |
| 0 | 1 – 2 | Prefrail |
| 0 | 3 – 5 | Frail |
| 1 – 2 | 0 | Missing (can be robust or prefrail) |
| 1 | 1 | Prefrail |
| 1 | 2 | Missing (can be prefrail or frail) |
| 2 | 1 – 2 | Missing (can be prefrail or frail) |
| 2 | 3 | Frail |
| 3 – 4 | 0 | Missing (can be robust or prefrail or frail) |
| 3 – 4 | 1 – 2 | Missing (can be prefrail or frail) |
The presence of no criteria was defined as robust; 1–2 criteria as prefrail; and 3–5 criteria as frail. Participants who were missing data components of the frailty definition leading to ambiguous classification were assigned a missing value for frailty (Table 1). The frailty outcome was assessed as the worst frail state across visits 5–7.
Protein association analysis
We used multinomial logistic regression models with the three categories of final frailty status as the dependent variable and the relative abundances of the SOMAmers as the main independent variable. The relative abundances were first log 2-transformed and then further standardized to mean zero and standard deviation of one. Each multinomial logistic model produced two sets of coefficients: one estimated the odds ratio (OR) of being frail relative to robust per 1 standard deviation (SD) higher abundance of the SOMAmers, and the other estimated the OR of being prefrail relative to robust per 1 SD higher abundance of the SOMAmers.
We progressively adjusted for covariates as follows: Model 1 (adjusted for self-reported age at Visit 3, sex, race-center, education, and family income), Model 2 (additionally adjusted for drinking and smoking status, dietary protein intake, total cholesterol, estimated glomerular filtration rate [eGFR], history of hypertension, diabetes, coronary heart disease, heart failure, cancer, and chronic lung disease, and functional limitation); and Model 3 (additionally adjusted for body mass index [BMI]). Functional limitation was measured during annual phone follow-up as reported inability to do any of the following activities: usual activities such as work around the house or recreation, walking up and down stairs to the second floor without help, doing heavy work around the house without help, and walking half a mile without help. The status reported closest to the date of Visit 3 was used (96% reported status within 180 days before and 30 days after Visit 3). The detailed definitions of other covariates are summarized in Table S1. Chronic conditions and BMI were chosen for adjustment due to their reported associations with the plasma proteins and with frailty to control for potential confounding effects [6–9, 21–24]. Because BMI is related (albeit not deterministically) to the weight loss criteria, we performed separate sets of analyses excluding BMI (Model 2) and including BMI (Model 3) as a covariate. In the main text, we focus on the results from Model 2 (BMI-unadjusted model) and Model 3 (BMI-adjusted model). The results from Model 1 can be found in Table S14 in the Supplemental Material.
SOMAmers were considered significant at the Bonferroni level, the most rigorous and conservative threshold, if p-value < 1 × 10–5 (0.05/4955). We also reported SOMAmers that passed a false discovery rate (FDR) level (Benjamini–Hochberg FDR adjusted p-value < 0.05) to include potentially important proteins.
Due to the number of participants excluded in the main analysis due to death over the 18 years between Visit 3 and Visit 5, lack of an in-person exam during Visits 5–7, or incomplete frailty assessments, we included 5,849 such participants with complete covariates as a separate category, “loss to follow-up”, in addition to the final frailty status (Fig. S1) and used multinomial logistic regression models with four categories of the outcome, i.e., robust, prefrail, frail, and loss to follow-up, as a sensitivity analysis. As frailty assessment was not implemented at baseline, we performed another sensitivity analysis excluding participants with functional limitations (n = 394) to reduce the impact of unknown frailty status at baseline (Fig. S1). Lastly, to address concerns that BMI was both an adjustment variable and a criterion for frailty, we performed two additional sensitivity analyses: first, we excluded 13 participants whose BMI < 18.5 kg/m2 at Visit 3; second, we removed weight loss from our frailty definition and categorized participants meeting 3–4 criteria as frail.
Post-hoc analyses of frailty-associated proteins and BMI
As strong attenuations of protein-prefrailty/frailty associations were observed with BMI adjustment, we examined the bidirectional associations between BMI and protein levels of the 221 proteins associated with frailty in the primary analysis (without BMI adjustment) to better understand whether BMI was a confounder or mediator. Among 3,645 participants who had complete measures of proteomics, BMI, and covariates at both Visit 2 (1990–1992) and Visit 3 (1993–1995, Fig. S1), we used two sets of models to examine the associations of the proteins and BMI with the level of the other variable at a later visit:
where the subscripts of the variables denoted the visit when the variable was measured. Here, can be interpreted as the association between protein at Visit 2 and BMI at Visit 3 among participants with the same BMI at Visit 2, informing the associations on the BMI to protein level direction. Conversely, can be interpreted as the association between BMI at Visit 2 and the protein at Visit 3 among participants with the same protein level at Visit 2, informing the associations on the protein level to BMI direction. To compare and , we scaled the protein levels and BMI at both visits using their respective standard deviations at Visit 2. Pathway analysis was performed for proteins associated with BMI change or whose changes were associated with BMI (FDR p < 0.05) using IPA. Based on the results of these two sets of models, we performed mediation analysis to understand the proportion of the effect of BMI on frailty/prefrail that was mediated through the proteins associated with each status at the Bonferroni level (see Supplemental Methods for details).
Pathway and upstream regulator analyses
To better interpret the biological and functional pathways represented by the proteins, we performed canonical pathway analysis and upstream regulator analysis using QIAGEN’s Ingenuity Pathway Analysis (IPA). Ingenuity Knowledge Base and the 4,955 proteins in our data (user database) were used as separate reference sets and direct and indirect experimentally confirmed relationships from humans were included in the analysis. For Model 2, 150 unique proteins associated with prefrailty, and 372 unique proteins associated with frailty with nominal p < 0.01 were mapped to the IPA database and included in the analyses. For Model 3, 199 prefrailty-associated proteins and 316 frailty-associated proteins with nominal p-value < 0.05 were mapped and included. As QIAGEN recommends approximately 100 proteins for the analyses, a less stringent p-value threshold was chosen to achieve a sufficient number of proteins. The IPA uses a right-tailed Fisher’s exact test to quantify the probability of overlap due to random chance between our list of proteins and a set of proteins known to exist within a specific pathway or being regulated by an upstream regulator [25]. We reported pathways and upstream regulators that have p-values for enrichment < 0.05 after the Benjamini–Hochberg FDR adjustment.
Results
Participant characteristics
The baseline characteristics of the 11,478 participants who had proteomics measurements are summarized in Table 2, stratified by their late-life frailty status or loss to follow-up. Of the 4,189 participants included in the main analysis, 557 participants (13.3%) were frail in later life, 2,586 participants (61.7%) were prefrail in late life, and 1,046 participants (25.0%) were robust in later life. Compared to participants who were robust in late life, frail/prefrail participants were older, more likely to be women, current smokers, self-identify as Black, and have lower education and family income. They also had lower eGFR and higher BMI, and were more likely to have functional limitations, and chronic diseases.
Table 2.
Baseline participant characteristics
| Robust | Prefrail | Frail | Loss to Follow-upa | |
|---|---|---|---|---|
| Mean (SD) / N (%) | n = 1046 | n = 2586 | n = 557 | n = 5849 |
| Age, years | 56.6 (4.5) | 57.9 (5.0) | 59.2 (5.1) | 61.8 (5.6) |
| Women | 546 (52.2%) | 1440 (55.7%) | 350 (62.8%) | 3104 (53.1%) |
| Race center | ||||
| Minneapolis Whites | 386 (36.9%) | 838 (32.4%) | 137 (24.6%) | 1486 (25.4%) |
| Jackson Blacks | 123 (11.8%) | 386 (14.9%) | 101 (18.1%) | 1074 (18.4%) |
| Washington Whites | 288 (27.5%) | 747 (28.9%) | 197 (35.4%) | 1558 (26.6%) |
| Forsyth Blacks | 11 (1.1%) | 40 (1.5%) | 11 (2.0%) | 212 (3.6%) |
| Forsyth Whites | 238 (22.8%) | 575 (22.2%) | 111 (19.9%) | 1519 (26.0%) |
| Education | ||||
| Less than high school | 83 (7.9%) | 290 (11.2%) | 116 (20.8%) | 1483 (25.4%) |
| High school/GED/vocational school | 412 (39.4%) | 1144 (44.2%) | 253 (45.4%) | 2467 (42.2%) |
| Any College | 551 (52.7%) | 1152 (44.5%) | 188 (33.8%) | 1899 (32.5%) |
| Family income | ||||
| Family Income < $25,000 per year | 162 (15.5%) | 592 (22.9%) | 179 (32.1%) | 2426 (41.5%) |
| Family Income $25,000 to < $50,000 per year | 421 (40.2%) | 1082 (41.8%) | 234 (42.0%) | 2216 (37.9%) |
| Family Income ≥ $50,000 per year | 463 (44.3%) | 912 (35.3%) | 144 (25.9%) | 1207 (20.6%) |
| Dietary protein intake, g/day | 75.6 (31.9) | 73.9 (31.4) | 76.6 (35.9) | 77.7 (47.9) |
| Drinking status | ||||
| Current Drinker | 689 (65.9%) | 1564 (60.5%) | 300 (53.9%) | 2825 (48.3%) |
| Former Drinker | 153 (14.6%) | 476 (18.4%) | 109 (19.6%) | 1517 (25.9%) |
| Never Drinker | 204 (19.5%) | 546 (21.1%) | 148 (26.6%) | 1507 (25.8%) |
| Smoking status | ||||
| Never Smoker | 490 (46.8%) | 1173 (45.4%) | 241 (43.3%) | 2159 (36.9%) |
| Former Smoker | 449 (42.9%) | 1095 (42.3%) | 227 (40.8%) | 2427 (41.5%) |
| Current Smoker | 107 (10.2%) | 318 (12.3%) | 89 (16.0%) | 1263 (21.6%) |
| Total cholesterol, mmol/L | 5.3 (0.9) | 5.3 (0.9) | 5.4 (1.0) | 5.4 (1.0) |
| eGFR, ml/min/1.73 m2 | 93.5 (13.5) | 91.8 (14.0) | 87.1 (14.8) | 85.1 (17.7) |
| Functional limitation | 73 (7.0%) | 230 (8.9%) | 91 (16.3%) | 1265 (21.6%) |
| Hypertension | 266 (25.4%) | 784 (30.3%) | 249 (44.7%) | 2797 (47.8%) |
| Diabetes | 60 (5.7%) | 224 (8.7%) | 79 (14.2%) | 1202 (20.6%) |
| Coronary heart disease | 32 (3.1%) | 102 (3.9%) | 25 (4.5%) | 582 (10.0%) |
| Heart failure | 14 (1.3%) | 74 (2.9%) | 24 (4.3%) | 429 (7.3%) |
| Stroke | 3 (0.3%) | 19 (0.7%) | 8 (1.4%) | 164 (2.8%) |
| Cancer | 59 (5.6%) | 178 (6.9%) | 37 (6.6%) | 590 (10.1%) |
| Lung disease | 41 (3.9%) | 138 (5.3%) | 40 (7.2%) | 556 (9.5%) |
| BMI | 26.6 (4.1) | 28.0 (4.8) | 30.7 (6.2) | 28.7 (5.8) |
| Frailty component | ||||
| Weight loss | 565 (22.3%) | 195 (36.1%) | ||
| Low grip strength | 788 (31.8%) | 309 (59.2%) | ||
| Slow walking | 317 (12.8%) | 268 (51.3%) | ||
| Exhaustion | 186 (7.4%) | 145 (26.9%) | ||
| Low physical activity | 357 (14.4%) | 234 (44.1%) | ||
eGFR = estimated glomerular filtration rate. BMI = body mass index. a Loss to follow-up included death over the 18 years between Visit 3 and Visit 5, no in-person exam during Visits 5–7, or incomplete frailty assessments at Visits 5–7
Compared to participants included in the main analysis, participants lost to follow-up were older, more likely to self-identify as Black race, be current smokers, have lower education and family income, lower eGFR, higher BMI, and higher prevalence of functional limitation, and chronic diseases.
Mid-life proteins associated with late-life prefrailty and frailty
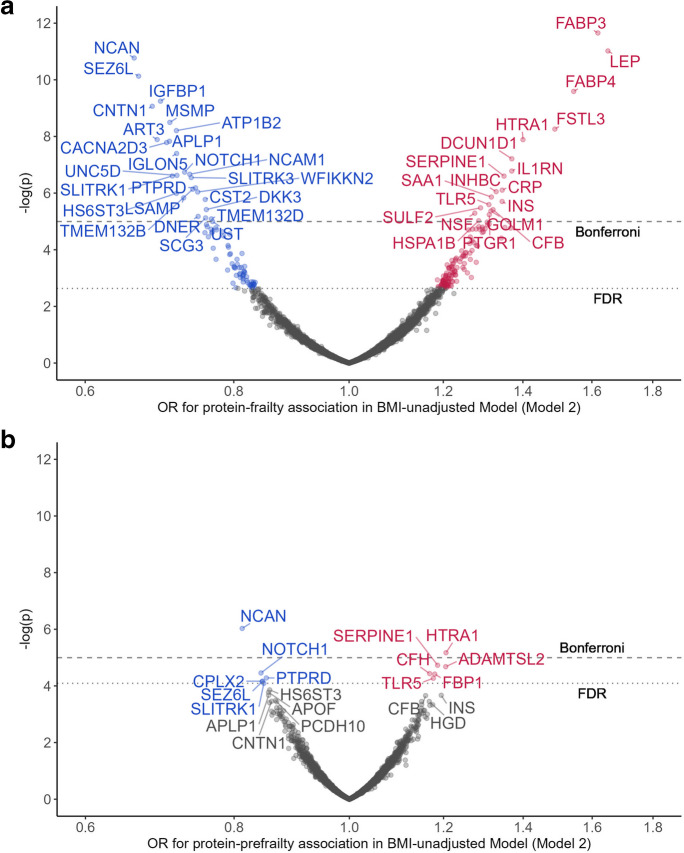
After adjusting for demographic characteristics, health behaviors, physiological variables, and chronic conditions (Model 2), 45 and 221 proteins were associated with frailty at the Bonferroni level and the FDR level, respectively. The top proteins associated with lower risk of frailty included neurocan core protein (NCAN, OR = 0.66), seizure 6-like protein (SEZ6L, OR = 0.67), insulin-like growth factor-binding protein 1 (IGFBP1, OR = 0.69), contactin-1 (CNTN1, OR = 0.68), and prostate-associated microseminoprotein (MSMP, OR = 0.71). The top proteins associated with a higher risk of frailty included fatty acid-binding protein heart (FABP3, OR = 1.62), leptin (LEP, OR = 1.65), fatty acid-binding protein adipocyte (FABP4, OR = 1.54), follistatin-related protein 3 (FSTL3, OR = 1.49), and high-temperature requirement serine protease A1 (HTRA1, OR = 1.40, Fig. 1a, Table S2). NCAN showed the strongest association with a lower risk of prefrailty (OR = 0.81), whereas HTRA1 (OR = 1.21) was most strongly associated with a higher risk of prefrailty, both significant at the Bonferroni level. Another 10 proteins were associated with prefrailty at an FDR-corrected level, including neurogenic locus notch homolog protein 1 (NOTCH1, OR = 0.83), receptor-type tyrosine-protein phosphatase delta (PTPRD, OR = 0.843), plasminogen activator inhibitor 1 (SERPINE1, OR = 1.19), and ADAMTS-like protein 2 (ADAMTSL2, OR = 1.21, Fig. 1b, Table S2). A comparison of protein-specific associations with prefrailty versus frailty (Fig. S2) indicates a general consistency for protein associations across both the prodromal and overt frailty phenotypes (Spearman rho = 0.62).
Fig. 1.
Proteins associated with prefrailty (a) and frailty (b) adjusted for age, sex, race-center, education, family income, dietary protein intake, drinking status, smoking status, total cholesterol, estimated glomerular filtration rate, functional limitation, and history of hypertension, diabetes, coronary heart disease, heart failure, stroke, cancer, and lung disease. Top 20 prefrailty-associated proteins were annotated with entrez gene symbol
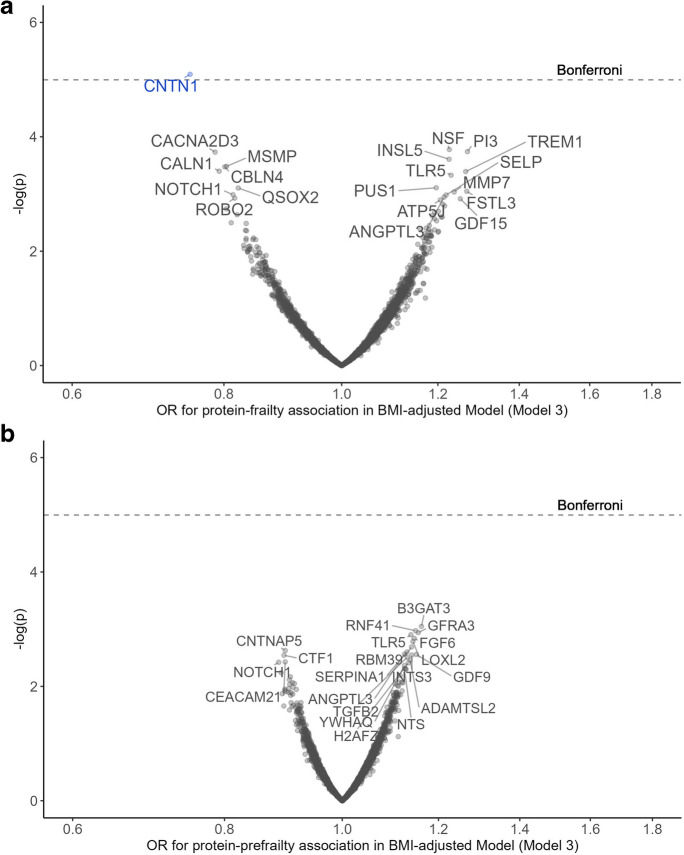
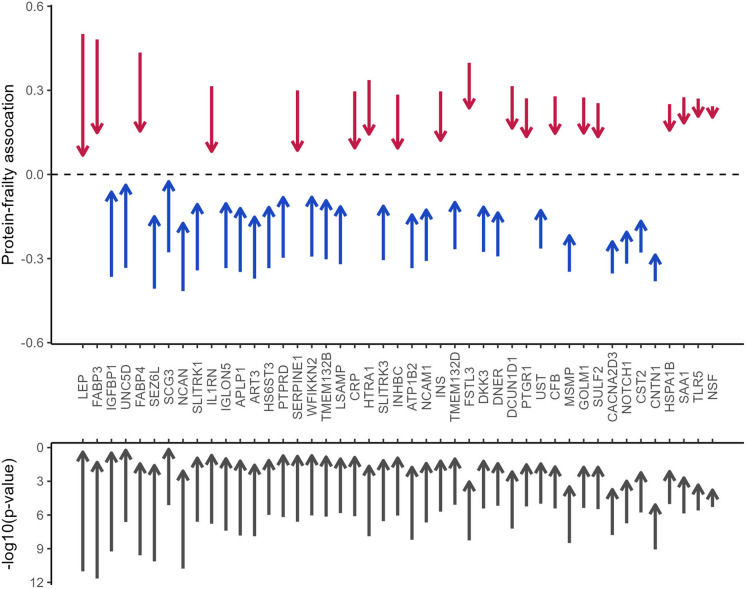
After further adjustment for midlife BMI (Model 3), an established frailty risk factor, only CNTN1 (OR = 0.75) remained significantly associated with frailty at the Bonferroni-corrected threshold. No protein remained significantly associated with prefrailty at either the Bonferroni or the FDR level (Fig. 2, Table S3). Figure 3 summarizes the attenuation of the protein-frailty associations after BMI adjustment for the 45 proteins that passed the Bonferroni correction. The largest effect size attenuation was observed for proteins involved in metabolism, including IGFBP1, LEP, FABP3, and FABP4, whereas other proteins showed minimal effect size and p-value attenuations, e.g., CNTN1, toll-like receptor 5 (TLR5), and vesicle-fusing ATPase (NSF). Notably, several proteins associated with frailty at FDR level in Model 2, including triggering receptor expressed on myeloid cells 1 (TREM1) and elafin (PI3), showed comparatively stronger associations with frailty after BMI adjustment (Fig. S3).
Fig. 2.
Proteins associated with prefrailty (a) and frailty (b) adjusted for age, sex, race-center, education, family income, dietary protein intake, drinking status, smoking status, total cholesterol, estimated glomerular filtration rate, functional limitation, history of hypertension, diabetes, coronary heart disease, heart failure, stroke, cancer, and lung disease, and BMI. Top 20 proteins were annotated with entrez gene symbol
Fig. 3.
The attenuation of protein associations (upper plot) and p-values (lower plot) with frailty after BMI adjustment for the 45 proteins associated with frailty at Bonferroni level in BMI-unadjusted model. The starting positions of the arrows denote the coefficients and p-values in the BMI-unadjusted model (Model 2). The end positions of the arrows denote the coefficients and p-values in BMI-adjusted model (Model 3). The directions of the arrows denote direction of change. Proteins were arranged by the magnitude of the attenuation in coefficients
Sensitivity analyses including participants lost to follow-up as a separate outcome category and excluding participants with functional limitation at Visit 3 in Model 2 produced consistent findings with minimal changes in the magnitudes of the protein-frailty/protein-prefrailty associations (Figs S4-S7, Tables S4-S5). BMI adjustment still greatly attenuated the associations between proteins and prefrail/frail state in the analyses excluding the 13 participants with BMI < 18.5 kg/m2 at Visit 3 or excluding weight loss from our frailty definition (data not shown).
Characterizing the link between BMI and frailty-associated proteins
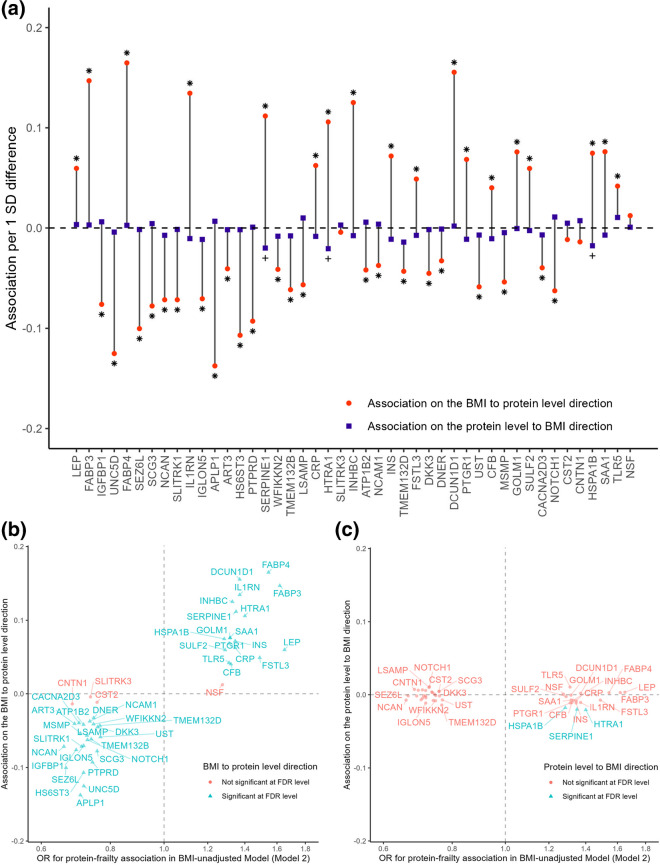
With few exceptions, the 221 proteins associated with frailty in the primary analysis without BMI adjustment had stronger standardized associations on the BMI to protein level direction than the protein level to BMI directions over a three-year follow-up period during midlife (Visit 2 to Visit 3). Moreover, 155 proteins (70%) were significant on the BMI to protein level direction (FDR p < 0.05) whereas only 24 proteins (11%) were significant on the protein level to BMI direction (FDR p < 0.05, Fig. 4a and Fig. S8A). Lastly, the associations on the BMI to protein level direction were consistent with the protein-frailty associations from the primary analysis for most proteins (Fig. 4b and Fig. S8B). Given the small number of underweight participants (BMI < 18.5 kg/m2) at Visit 2 (n = 10) and Visit 3 (n = 13), the BMI to protein level direction suggested that higher (less healthy) BMI was associated with worse protein profile at a later visit. In contrast, the protein level to BMI associations were weaker and did not explain the protein-frailty associations in the primary analysis (Fig. 4c and Fig. S8C). These results suggest that although the protein-BMI association is possibly bidirectional, at this stage, BMI appears to be a stronger driver of protein levels than proteins are of BMI.
Fig. 4.
(a) The associations between BMI at Visit 2 and change in protein levels between Visit 2 and Visit 3 and the associations between protein at Visit 2 and change in BMI between Visit 2 and Visit 3 for the 45 proteins associated with frailty at the Bonferroni level in the BMI-unadjusted model. (b) The comparison of association between BMI at Visit 2 and change in protein levels between Visit 2 and Visit 3 and the association between protein levels at Visit 3 and late-life frailty in BMI-unadjusted models. (c) The comparison of association between protein at Visit 2 and change in BMI between Visit 2 and Visit 3 and the association between protein levels at Visit 3 and late-life frailty in BMI-unadjusted models
The 155 proteins on the BMI to protein level direction were enriched for inflammation and metabolisms with the strongest activation predicted for acute phase response and cachexia signaling pathways Fig. S9). Based on the stronger association between Visit 2 BMI and Visit 3 proteins than the other way around, we performed a mediation analysis to determine whether frailty-associated proteins mediated the association between midlife BMI and frailty risk in later life. The analysis revealed that the 41 midlife proteins associated with late-life frailty at the Bonferroni level mediated 31.6% of the effect of midlife BMI at Visit 3 on late-life frailty on the relative risk scale (95% CI: 13.5, 50.3). The 2 proteins that were associated with prefrailty at the Bonferroni level mediated 14.4% of the effect of midlife BMI and late-life prefrailty on the relative risk scale (95% CI: 6.0, 24.0).
Enriched pathways and upstream regulators of the discovered proteins
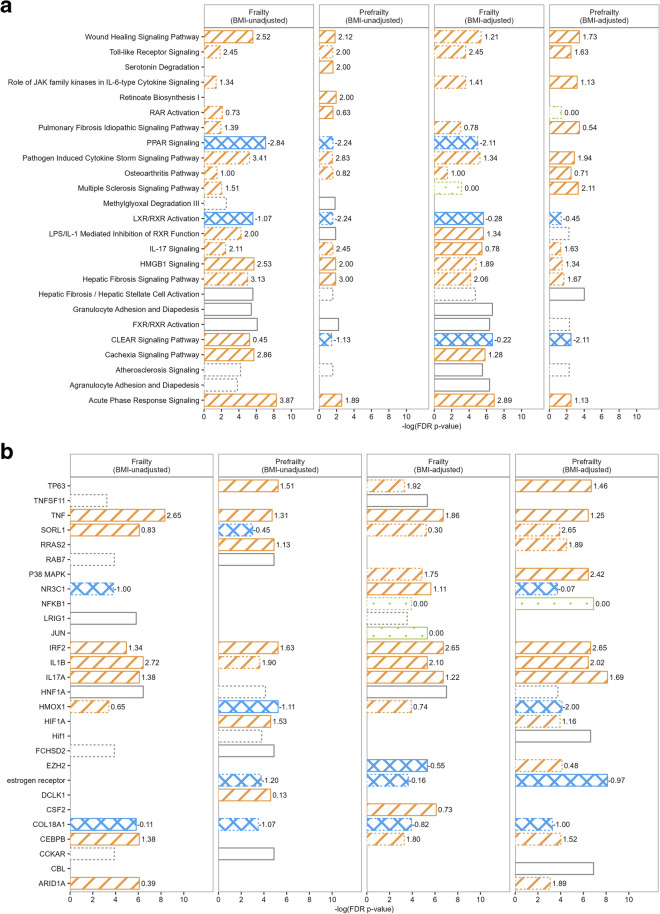
The top 10 enriched pathways among the prefrailty- or frailty-associated proteins using Ingenuity Knowledge Base as the reference set are shown in Fig. 5a. Several pathways related to inflammation were among the top activated pathways, e.g., acute phase response, wound healing, and high mobility group-B1 (HMGB1) signaling pathways. Metabolic pathways, including peroxisome proliferator-activated receptor (PPAR) signaling and liver X receptors/retinoid X receptors (LXR/RXR) activation pathways, showed the strongest evidence for inhibition. Results also suggest that LPS/IL-1 mediated inhibition of RXR function may be activated, particularly among those at risk for frailty, potentially linking inflammation with impaired metabolism. When pathway analyses were applied to prefrailty/frailty-associated proteins from BMI-adjusted models, the metabolism-related pathways showed comparatively lower enrichment, whereas the inflammation-related pathways, such as toll-like receptor signaling and pathogen induced cytokine storm signaling, were more prominently enriched among prefrailty-associated proteins. The complete lists of significantly enriched pathways (FDR p-value for enrichment < 0.05) are summarized in Tables S6-S9. Using the user database as the reference set, no pathways reached significance at an FDR-corrected threshold. However, the farnesoid X receptor/liver X receptor (FXR/RXR) activation, the LPS/IL-1 mediated inhibition of RXR function pathway, and the CLEAR signaling pathway remained as top enriched pathways across models at a nominal p < 0.05 (Fig. S10).
Fig. 5.
The top 10 enriched pathway (a) and top 10 upstream regulators (b) among proteins associated with prefrailty and frailty in BMI-unadjusted model (Model 2) and BMI-adjusted model (Model 3) using the IPA Ingenuity Knowledge Base as the reference set. Bars with solid border denote the top 10 pathways or regulators for each analysis. Bars with dashed border denote pathways or regulators that are significantly enriched after FDR correction but are not among the top 10 pathways or regulators for the specific analysis. The length of the bar denotes the -log(p-values after FDR correction). Strip pattern denotes pathways or regulators that are predicted to be activated (z-score labeled at the end of the bar > 0). Crosshatched pattern denotes pathways or regulators that are predicted to be inhibited (z-score < 0). Dot pattern denotes pathways or regulators that are predicted to be neither activated nor inhibited (z-score = 0). Blank pattern denotes pathways or regulators whose activation/inhibition states are not predicted
The top 10 enriched upstream regulators associated with prefrailty and frailty using Ingenuity Knowledge Base as the reference set are shown in Fig. 5b. Inflammatory cytokines including the tumor necrosis factor (TNF) superfamily, interferon regulator factor 2 (IRF2), IL-1 beta, and IL-17A were among the enriched upstream regulators for proteins associated with prefrailty or frailty (both with and without BMI adjustment). Collagen type XVIII alpha 1 chain (COL18A1) and estrogen receptor that regulate proteins involved in angiogenesis, and Sortilin related receptor 1 (SORL1) related to lipid and beta-amyloid metabolism were another three upstream regulators that were enriched for both frailty status in both models. The full lists of significant upstream regulators (FDR p-value for enrichment < 0.05) and their target proteins can be found in Tables S10-S13. Using user database as the reference set, less enrichment was observed among upstream regulators. However, IRF2 remained a top activated upstream regulator, and several regulators related to metabolism (e.g., hepatocyte nuclear factor 1 homeobox A [NHF1A], delta like non-canonical Notch ligand 1 [DLK1], and anoctamin 1 [ANO1]) emerged as consistent enriched upstream regulators across models (nominal p < 0.05, Fig. S10).
Discussion
Our proteome-wide analyses discovered a large set of plasma proteins measured in mid-life that were associated with prefrailty and/or frailty in late-life after adjusting for demographic factors, health behaviors, health indicators excluding BMI, and history of various chronic conditions. Inflammation, metabolism, extracellular matrix, angiogenesis, and lysosomal autophagy are biological mechanisms implicated by the discovered proteins using pathway and upstream regulator analyses. To our knowledge, this is the first study of midlife proteins and late-life frailty in a large sample mostly consisting of community-dwelling middle-aged adults. Our findings extend the understanding of frailty pathogenesis beyond older adulthood into midlife and provide a set of candidate proteins to further examine their mechanistic contribution to frailty earlier in the life course.
Some of the top proteins in the present study, including NCAN, CNTN1, voltage-dependent calcium channel subunit alpha-2/delta-3 (CACNA2D3), FABP, FABPA, LEP, FSTL3, RNASE1, and TREM1, have been shown to be associated with frailty trajectories [26] and incident frailty when measured in late-life [25]. Importantly, our findings suggest that the role of these proteins in frailty development may start well before older adulthood, advocating the need for preventive efforts with the onset of midlife health conditions. Additionally, our discovery included many novel proteins measured in mid-life that have not previously been associated with incident late-life frailty when proteins were also measured in older age [25], e.g., NOTCH1, TLR5, PTPRD, and SERPINE1, suggesting that different proteins may be important at different stages of life. The common and unique protein signatures of frailty in mid-life and in our previous late-life proteomics study of frailty [25] can be found in Table S15. The large sample size and the focus on the early stage of frailty development are two strengths of the current study that have likely facilitated the detection of novel frailty-associated proteins.
Our findings of protein-frailty associations were mostly consistent with the current understanding of the roles of these proteins in age-related conditions. Notably, midlife NCAN, the strongest protective protein for late-life prefrailty and frailty in our analyses, is a chondroitin sulfate proteoglycan of the lectican family that modulates neuronal adhesion and neurite growth in brain development by binding to neural cell adhesion molecule 1 (NCAM1), another frailty-associated protein [27]. Previous studies demonstrating an association between a higher level of plasma NCAN and greater whole brain and grey matter volume [28], and a downregulation of NCAN expression in aged skeletal muscle support the protective association between NCAN and the frailty phenotype in the present study [29]. Although NCAN has a role in brain development, genetic variants in or near the NCAN gene have been associated with triglycerides, LDL cholesterol, and body composition, suggesting a pleiotropic role of this protein in brain and metabolic health and a potential link between brain health and physical health [30].
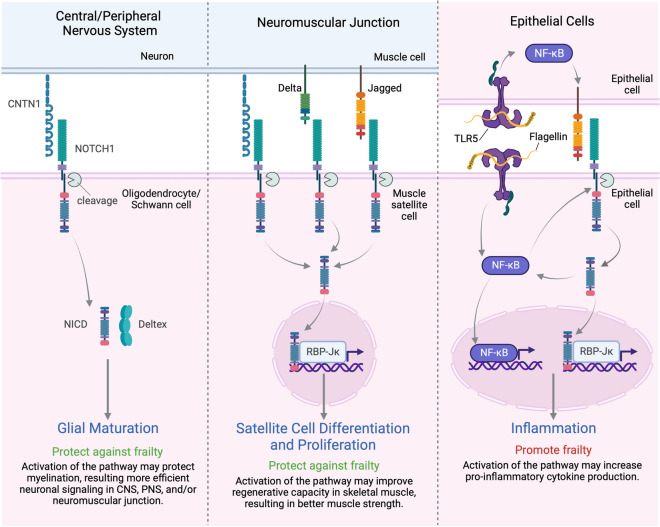
CNTN1 was the only protective protein associated with frailty after adjusting for BMI. Like NCAN, it is a cell adhesion molecule expressed primarily in the central and peripheral nervous system [31, 32], though it is also expressed in the neuromuscular junction in the skeletal muscle [33]. Genetic variants in the CNTN1 gene, gene expression level in the neuromuscular junction, and plasma protein level have been associated with muscle strength and mobility disability [33–35]. Also, loss of the CNTN1 gene in a mouse model showed intestinal dysfunction and wasting [36]. All this evidence supports that CNTN1 may play a protective role in frailty development. Some of the effects of CNTN1 may be mediated by NOTCH1, another top protective prefrailty- and frailty-associated protein (Fig. 6). In the central nervous system, CNTN1 binds to NOTCH1 which leads to the cleavage of Notch intracellular domain (NICD). NICD binds to Deltex to induce Oligodendrocyte differentiation [31, 37]. This interaction may also play a role in the maturation of Schwann cells in the peripheral nervous system as CNTN1 null mice showed myelination defects in the peripheral nervous system [32]. Schwann cells are important for synaptic development and maintenance at the neuromuscular junction [38]. In the neuromuscular junction, the NICD cleaved from CNTN1-NOTCH1 binding may also activate proliferation and differentiation in muscle satellite cells through the canonical pathway via the recombinant signal binding protein 1 for Jκ (JBP-Jκ) [37]. Taken together, disrupted CNTN1 and NOTCH1 interactions may disrupt neuromuscular communication and lead to muscle weakness [33, 39].
Fig. 6.
The pleiotropic effects of NOTCH1 pathways involving CNTN1 and TLR5. In the central and peripheral nervous system, CNTN1-NOTCH1 binding leads to the cleavage of Notch intracellular domain (NICD). NICD binds to Deltex to induce maturation of Oligodendrocytes Schwann cells. Disrupted CNTN1 and NOTCH1 interaction may therefore disrupt neuromuscular communication and lead to muscle weakness. In the neuromuscular junction, NICD cleaved from CNTN1-NOTCH1 binding – as well as from the binding of canonical ligands of NOTCH1 (delta-like and Jagged families) – translocates to the nucleus, binds to the recombinant signal binding protein 1 for Jκ (JBP-Jκ), and activates many cell functions including proliferation and differentiation in muscle satellite cells. In intestinal epithelial cell, flagellin-induced TLR5 signaling activates NF-κB and increases the expression of Jagged 1 and NOTCH1. NICD cleaved from the enhanced NOTCH1 signaling augment the NF-κB activation which leads to pro-inflammatory cytokine production (e.g., IL6). Figure created with Biorender.com
However, the role of NOTCH1 in frailty development may be pleiotropic. Though exception exists [40], many studies have found that the activation of the NOTCH1 pathway promoted inflammation [37, 41, 42]. Notably, one study showed that the NOTCH1 pathway is activated by inflammation mediated by TLR5 in the epithelial cells [43], a top protein associated with higher odds of prefrailty and frailty. TLR5 is a pattern recognition receptor that promotes nuclear factor-κB (NF-κB) activation, cytokine secretion, and inflammation response (Fig. 6) [43, 44]. In our study, a higher plasma level of NOTCH1 was associated with a lower risk of frailty and prefrailty, which may be driven by the protective functions in the nervous system and skeletal muscle system.
Other top proteins we discovered also have roles in glucose and lipid metabolism (e.g., FSTL3 [45], FABP3 [46], FABP4 [46], and leptin [47]), inflammation (e.g., TREM1 [48] and HTRA1 [49]), and cellular senescence (e.g., SERPINE1 [50]). Metabolism and inflammation were also strongly implicated by enriched pathways and upstream regulators. The overexpression of upstream regulator SORL1 in adipose tissue has been shown to enhance fat deposition [51]. PPAR signaling, LXR/RXR, and FXR/RXR pathways function by activating genes that regulate lipid and/or glucose metabolism [52–54]; these same pathways have also been shown to inhibit inflammation [53, 55, 56]. Metabolic and inflammatory pathways have been consistently associated with physical frailty [3, 4, 57]. Our results predicted inhibition of the PPAR signaling and LXR/RXR activation pathways among those at risk for incident frailty in a manner that would inhibit metabolism and enhance inflammation during middle adulthood. LPS/IL-1 mediated inhibition of RXR function pathway implicated in this study may be just one pathway through which inflammation can also impair metabolism [58].
Extracellular matrix (ECM) is implicated by the enrichment of hepatic fibrosis/hepatic stellate cell activation, pulmonary fibrosis idiopathic signaling, and osteoarthritis pathways. Age-related alteration in ECM has been suggested to induce cell senescence [59], and have an impact on skeletal muscle, cartilage, and bone health [60, 61]. Some of the ECM components such as matrix metallopeptidases (MMPs) and tissue inhibitors of metallopeptidase (TIMPs) are also involved in angiogenesis. Fibroblast growth factors (FGFRs), epidermal growth factor receptor (EGFR), and vascular endothelial growth factor A (VEGFA) are other players in angiogenesis. These proteins were part of the enriched pathways of wound healing signaling and were targets of two enriched upstream regulators, COL18A1 and estrogen receptors. COL18A1 and estrogen receptors inhibit and promote angiogenesis, respectively [62, 63]. The role of angiogenesis in aging is complex. Promoting angiogenesis is beneficial for skeletal muscle, and recovery from myocardial infarction and stroke, but it can also contribute to cancer and atherosclerosis [64–66]. Angiogenesis has also been shown to have diverging effects on Alzheimer’s disease and cognitive health [67]. The CLEAR signaling pathway suggested lysosomal autophagy as a biological mechanism of frailty. CLEAR network refers to genes that carry a CLEAR motif (TCACG) and have a variety of functions in the lysosomal-autophagic process [68]. Lysosomal dysfunction and decreased autophagy have been linked to aging muscle [69]. This is consistent with the predicted inhibition of the CLEAR signaling pathway in our analysis.
A body of literature has linked obesity in mid-life with frailty in later life [6–9], but the finding that mid-life BMI alone explained the associations of many proteins with frailty was surprising. We do not believe these attenuations resulted from the use of BMI < 18.5 kg/m2 as part of the weight loss definition because (i) BMI adjustment similarly attenuated the protein-frailty associations in sensitivity analyses excluding participants with BMI < 18.5 kg/m2 at baseline and (ii) results remained similar after excluding weight loss from our frailty definition. Moreover, we observed smaller attenuations of the associations between late-life proteomics and incident frailty in our previous work, even though BMI was measured much closer to when incident frailty was ascertained [25]. Our post-hoc analyses suggested that the effect of mid-life obesity and late-life frailty reported in the previous studies may be mediated by a number of the proteins discovered in this study that are involved in inflammation and metabolism, together accounting for as much as 31.6% of the BMI-frailty association. However, we could not rule out the possibility that BMI could itself mediate the association between a portion of the midlife proteome and frailty in late life, or bidirectional relationships existed between proteins and BMI (e.g., SERPEIN1). Due to the small number of underweight participants in our sample (BMI < 18.5 kg/m2; n = 10 at Visit 2, and 13 at Visit 3), we did not consider a nonlinear relationship between BMI and proteins. We also assumed no interactions between BMI and the proteins and among the proteins in the mediation analysis and excluded many proteins associated with frailty in the analysis due to high dimensionality. Future studies are needed to further investigate the potential nonlinear, bidirectional, and mediating relationships between circulating midlife proteins, BMI, and frailty.
Though we only found one protein, CNTN1, to be associated with late-life frail status after mid-life BMI adjustment, a single protein is unlikely to explain the complex etiology of frailty. Our results suggest that an even larger sample size may be required to understand the mid-life protein signatures and potential mechanisms of late-life frailty because of the long intervening period and heterogeneity of aging. Future studies may consider using meta-analysis of multiple middle-aged cohorts to further examine the associations of proteins in our study that did not reach significance in our study.
Strengths of our study include a larger number of community-dwelling White and Black participants, a broad assessment of the plasma proteome using a highly reliable state-of-the-art proteomic platform, and the separation of prefrail and frail states. Our study also has limitations. First, frailty status in mid-life was unknown. Therefore, our results cannot be interpreted as proteins associated with incident prefrailty or frailty. Previous studies have found that the prevalence of frailty is low in mid-life but the prevalence of prefrailty is considerable [70]. This may explain why we found a larger number of proteins associated with frailty than with prefrailty. We excluded participants with functional limitations at baseline as a sensitivity analysis and found similar associations between the proteins and frailty status. The unknown frailty status in mid-life also limited our ability to perform time-to-event analysis and hence account for when participants became prefrail or frail. However, the variability of follow-up time in our sample was small (SD = 2.9 years), so the impact should be minimal. Second, many participants were lost due to death, no return to study visit, or missing frailty assessments during the decades between mid-life and later-life. Though we found similar results including these lost participants as a separate outcome category, this loss could still bias our results. Third, we focused on the worst frailty status in late-life and did not examine all possible transitions among frailty states such as recovering to a better state. Future studies should follow robust participants in mid-life for incident prefrailty and frailty and explore different transitions among frailty states across time. Lastly, our findings warrant replication in an independent cohort of middle-aged adults with late-life follow-up. However, the consistency of the findings with previously published proteomic studies of frailty in later life provides support to our results.
Despite these limitations, our study expands the current knowledge of biological alterations contributing to physical frailty by identifying a set of novel proteins of which the expression levels in mid-life were associated with frailty status in later life. The top proteins, pathways, and upstream regulators suggest that inflammation, lipid metabolism, extracellular matrix, angiogenesis, and lysosomal autophagy may be important mechanisms to consider for understanding the etiology of frailty and designing interventions to prevent frailty in mid-life. Furthermore, the work elucidates some of the biology mediating the association of high BMI with frailty risk. Future efforts should focus on investigating the potential causal role of these proteins/pathways in frailty development and effective interventions to regulate these biological mechanisms.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (75N92022D00001, 75N92022D00002, 75N92022D00003, 75N92022D00004, 75N92022D00005). The ARIC Neurocognitive Study is supported by U01HL096812, U01HL096814, U01HL096899, U01HL096902, and U01HL096917 from the NIH (NHLBI, NINDS, NIA, and NIDCD). The authors thank the staff and participants of the ARIC study for their important contributions. SomaLogic Inc. conducted the SomaScan assays in exchange for the use of ARIC data. This work was supported in part by NIH/NHLBI grant R01 HL134320, NIA Intramural Research Program (KAW), K24 HL15561 (MG) and R01 DK124399 (MG, JC). Dr. Walker was supported by the NIA Intramural Research Program.
Data availability
Pre-existing data access policies for each of the parent cohort studies specify that research data requests can be submitted to each steering committee; these will be promptly reviewed for confidentiality or intellectual property restrictions and will not unreasonably be refused. Please refer to the data sharing policies of these studies. Individual level patient or protein data may further be restricted by consent, confidentiality or privacy laws/considerations. These policies apply to both clinical and proteomic data.
Declarations
Competing interests
Dr. Josef Coresh is a scientific advisor to Soma Logic.
Footnotes
Josef Coresh and Keenan A. Walker joint last author with equal contributions.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang X, Strizich G, Hua S, Sotres-Alvarez D, Buelna C, Gallo LC, et al. Objectively Measured Sedentary Time and Cardiovascular Risk Factor Control in US Hispanics/Latinos With Diabetes Mellitus: Results From the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). J Am Heart Assoc. 2017;6:e004324 [DOI] [PMC free article] [PubMed]
- 2.Elliott ML, Caspi A, Houts RM, Ambler A, Broadbent JM, Hancox RJ, et al. Disparities in the pace of biological aging among midlife adults of the same chronological age have implications for future frailty risk and policy. Nat Aging. 2021;1:295–308. 10.1038/s43587-021-00044-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker KA, Walston J, Gottesman RF, Kucharska-Newton A, Palta P, Windham BG. Midlife systemic inflammation is associated with frailty in later life: The ARIC study. J Gerontol A Biol Sci Med Sci. 2019;74:343–9. 10.1093/gerona/gly045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fried LP, Cohen AA, Xue Q-L, Walston J, Bandeen-Roche K, Varadhan R. The physical frailty syndrome as a transition from homeostatic symphony to cacophony. Nat Aging. 2021;1:36–46. 10.1038/s43587-020-00017-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrucci L, Levine ME, Kuo P-L, Simonsick EM. Time and the Metrics of Aging. Circ Res. 2018;123:740–4. 10.1161/CIRCRESAHA.118.312816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stenholm S, Strandberg TE, Pitkälä K, Sainio P, Heliövaara M, Koskinen S. Midlife obesity and risk of frailty in old age during a 22-year follow-up in men and women: the Mini-Finland Follow-up Survey. J Gerontol A Biol Sci Med Sci. 2014;69:73–8. 10.1093/gerona/glt052 [DOI] [PubMed] [Google Scholar]
- 7.Strandberg TE, Sirola J, Pitkälä KH, Tilvis RS, Strandberg AY, Stenholm S. Association of midlife obesity and cardiovascular risk with old age frailty: a 26-year follow-up of initially healthy men. Int J Obes. 2005;2012(36):1153–7. [DOI] [PubMed] [Google Scholar]
- 8.Ho H-E, Yeh C-J, Chu W-M, Lee M-C. Midlife Body Mass Index Trajectory and Risk of Frailty 8 Years Later in Taiwan. J Nutr Health Aging. 2019;23:849–55. 10.1007/s12603-019-1226-6 [DOI] [PubMed] [Google Scholar]
- 9.Uchai S, Andersen LF, Hopstock LA, Hjartåker A. Body mass index, waist circumference and pre-frailty/frailty: the Tromsø study 1994–2016. BMJ Open. 2023;13:e065707. 10.1136/bmjopen-2022-065707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savela SL, Koistinen P, Stenholm S, Tilvis RS, Strandberg AY, Pitkälä KH, et al. Leisure-time physical activity in midlife is related to old age frailty. J Gerontol A Biol Sci Med Sci. 2013;68:1433–8. 10.1093/gerona/glt029 [DOI] [PubMed] [Google Scholar]
- 11.Nguyen AD, Malmstrom TK, Niehoff ML, Aziz A, Miller DK, Morley JE. Serum progranulin levels are associated with frailty in middle-aged individuals. PLoS ONE. 2020;15:e0238877. 10.1371/journal.pone.0238877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146-156. 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 13.The ARIC investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. 10.1093/oxfordjournals.aje.a115184 [DOI] [PubMed] [Google Scholar]
- 14.Wright JD, Folsom AR, Coresh J, Sharrett AR, Couper D, Wagenknecht LE, et al. The ARIC (Atherosclerosis Risk In Communities) Study: JACC Focus Seminar 3/8. J Am Coll Cardiol. 2021;77:2939–59. 10.1016/j.jacc.2021.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gold L, Ayers D, Bertino J, Bock C, Bock A, Brody EN, et al. Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS ONE. 2010;5:e15004. 10.1371/journal.pone.0015004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams SA, Kivimaki M, Langenberg C, Hingorani AD, Casas JP, Bouchard C, et al. Plasma protein patterns as comprehensive indicators of health. Nat Med. 2019;25:1851–7. 10.1038/s41591-019-0665-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tin A, Yu B, Ma J, Masushita K, Daya N, Hoogeveen RC, et al. Reproducibility and variability of protein analytes measured using a multiplexed modified aptamer assay. J Appl Lab Med. 2019;4:30–9. 10.1373/jalm.2018.027086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker KA, Chen J, Zhang J, Fornage M, Yang Y, Zhou L, et al. Large-scale plasma proteomic analysis identifies proteins and pathways associated with dementia risk. Nat Aging. 2021;1:473–89. 10.1038/s43587-021-00064-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kucharska-Newton AM, Palta P, Burgard S, Griswold ME, Lund JL, Capistrant BD, et al. Operationalizing Frailty in the Atherosclerosis Risk in Communities Study Cohort. J Gerontol A Biol Sci Med Sci. 2017;72:382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bandeen-Roche K, Xue Q-L, Ferrucci L, Walston J, Guralnik JM, Chaves P, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–6. 10.1093/gerona/61.3.262 [DOI] [PubMed] [Google Scholar]
- 21.Goudswaard LJ, Bell JA, Hughes DA, Corbin LJ, Walter K, Davey Smith G, et al. Effects of adiposity on the human plasma proteome: observational and Mendelian randomisation estimates. Int J Obes. 2005;2021(45):2221–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–63. 10.1093/gerona/59.3.M255 [DOI] [PubMed] [Google Scholar]
- 23.Damluji AA, Chung S-E, Xue Q-L, Hasan RK, Moscucci M, Forman DE, et al. Frailty and cardiovascular outcomes in the National Health and Aging Trends Study. Eur Heart J. 2021;42:3856–65. 10.1093/eurheartj/ehab468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu J, Zhou D, Wang J, Yang Y, Chen D, He F, et al. Frailty and cardiometabolic diseases: a bidirectional Mendelian randomisation study. Age Ageing. 2022;51:256. [DOI] [PubMed] [Google Scholar]
- 25.Liu F, Austin TR, Schrack JA, Chen J, Walston J, Mathias RA, et al. Late-life plasma proteins associated with prevalent and incident frailty: A proteomic analysis. Aging Cell. 2023;22:e13975. 10.1111/acel.13975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verghese J, Ayers E, Sathyan S, Lipton RB, Milman S, Barzilai N, et al. Trajectories of frailty in aging: Prospective cohort study. PLoS ONE. 2021;16:e0253976. 10.1371/journal.pone.0253976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rauch U, Feng K, Zhou XH. Neurocan: a brain chondroitin sulfate proteoglycan. Cell Mol Life Sci CMLS. 2001;58:1842–56. 10.1007/PL00000822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gadd DA, Hillary RF, McCartney DL, Shi L, Stolicyn A, Robertson NA, et al. Integrated methylome and phenome study of the circulating proteome reveals markers pertinent to brain health. Nat Commun. 2022;13:4670. 10.1038/s41467-022-32319-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jing Y, Zuo Y, Yu Y, Sun L, Yu Z, Ma S, et al. Single-nucleus profiling unveils a geroprotective role of the FOXO3 in primate skeletal muscle aging. Protein Cell. 2022;14:497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willer CJ, Sanna S, Jackson AU, Scuteri A, Bonnycastle LL, Clarke R, et al. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet. 2008;40:161–9. 10.1038/ng.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bizzoca A, Corsi P, Polizzi A, Pinto MF, Xenaki D, Furley AJW, et al. F3/Contactin acts as a modulator of neurogenesis during cerebral cortex development. Dev Biol. 2012;365:133–51. 10.1016/j.ydbio.2012.02.011 [DOI] [PubMed] [Google Scholar]
- 32.Gennarini G, Bizzoca A, Picocci S, Puzzo D, Corsi P, Furley AJW. The role of Gpi-anchored axonal glycoproteins in neural development and neurological disorders. Mol Cell Neurosci. 2017;81:49–63. 10.1016/j.mcn.2016.11.006 [DOI] [PubMed] [Google Scholar]
- 33.Compton AG, Albrecht DE, Seto JT, Cooper ST, Ilkovski B, Jones KJ, et al. Mutations in contactin-1, a neural adhesion and neuromuscular junction protein, cause a familial form of lethal congenital myopathy. Am J Hum Genet. 2008;83:714–24. 10.1016/j.ajhg.2008.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osawa Y, Semba RD, Fantoni G, Candia J, Biancotto A, Tanaka T, et al. Plasma proteomic signature of the risk of developing mobility disability: A 9-year follow-up. Aging Cell. 2020;19:e13132. 10.1111/acel.13132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Windelinckx A, De Mars G, Huygens W, Peeters MW, Vincent B, Wijmenga C, et al. Comprehensive fine mapping of chr12q12-14 and follow-up replication identify activin receptor 1B (ACVR1B) as a muscle strength gene. Eur J Hum Genet EJHG. 2011;19:208–15. 10.1038/ejhg.2010.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veny M, Grases D, Kucharova K, Lin WW, Nguyen J, Huang S, et al. Contactin-1 Is Required for Peripheral Innervation and Immune Homeostasis Within the Intestinal Mucosa. Front Immunol. 2020;11:1268. 10.3389/fimmu.2020.01268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balistreri CR, Madonna R, Melino G, Caruso C. The emerging role of Notch pathway in ageing: Focus on the related mechanisms in age-related diseases. Ageing Res Rev. 2016;29:50–65. 10.1016/j.arr.2016.06.004 [DOI] [PubMed] [Google Scholar]
- 38.Santosa KB, Keane AM, Jablonka-Shariff A, Vannucci B, Snyder-Warwick AK. Clinical relevance of terminal Schwann cells: An overlooked component of the neuromuscular junction. J Neurosci Res. 2018;96:1125–35. 10.1002/jnr.24231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conboy IH, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–7. 10.1126/science.1087573 [DOI] [PubMed] [Google Scholar]
- 40.Briot A, Civelek M, Seki A, Hoi K, Mack JJ, Lee SD, et al. Endothelial NOTCH1 is suppressed by circulating lipids and antagonizes inflammation during atherosclerosis. J Exp Med. 2015;212:2147–63. 10.1084/jem.20150603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamilton Outtz H, Wu JK, Wang X, Kitajewski J. Notch1 deficiency results in decreased inflammation during wound healing and regulates vascular endothelial growth factor receptor-1 and inflammatory cytokine expression in macrophages. J Immunol Baltim Md 1950. 2010;185:10.4049/jimmunol.1000720. [DOI] [PMC free article] [PubMed]
- 42.la Cour Poulsen L, Edelmann RJ, Krüger S, Diéguez-Hurtado R, Shah A, Stav-Noraas TE, et al. Inhibition of endothelial NOTCH1 signaling attenuates inflammation by reducing cytokine-mediated histone acetylation at inflammatory enhancers. Arterioscler Thromb Vasc Biol. 2018;38:854–69. 10.1161/ATVBAHA.117.310388 [DOI] [PubMed] [Google Scholar]
- 43.Aziz M, Ishihara S, Ansary MU, Sonoyama H, Tada Y, Oka A, et al. Crosstalk between TLR5 and Notch1 signaling in epithelial cells during intestinal inflammation. Int J Mol Med. 2013;32:1051–62. 10.3892/ijmm.2013.1501 [DOI] [PubMed] [Google Scholar]
- 44.Qian F, Wang X, Zhang L, Chen S, Piecychna M, Allore H, et al. Age-associated elevation in TLR5 leads to increased inflammatory responses in the elderly. Aging Cell. 2012;11:104–10. 10.1111/j.1474-9726.2011.00759.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mukherjee A, Sidis Y, Mahan A, Raher MJ, Xia Y, Rosen ED, et al. FSTL3 deletion reveals roles for TGF-β family ligands in glucose and fat homeostasis in adults. Proc Natl Acad Sci. 2007;104:1348–53. 10.1073/pnas.0607966104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov. 2008;7:489–503. 10.1038/nrd2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hubbard RE, O’Mahony MS, Calver BL, Woodhouse KW. Nutrition, inflammation, and leptin levels in aging and frailty. J Am Geriatr Soc. 2008;56:279–84. 10.1111/j.1532-5415.2007.01548.x [DOI] [PubMed] [Google Scholar]
- 48.Tessarz AS, Cerwenka A. The TREM-1/DAP12 pathway. Immunol Lett. 2008;116:111–6. 10.1016/j.imlet.2007.11.021 [DOI] [PubMed] [Google Scholar]
- 49.Lorenzi M, Lorenzi T, Marzetti E, Landi F, Vetrano DL, Settanni S, et al. Association of frailty with the serine protease HtrA1 in older adults. Exp Gerontol. 2016;81:8–12. 10.1016/j.exger.2016.03.019 [DOI] [PubMed] [Google Scholar]
- 50.Vaughan DE, Rai R, Khan SS, Eren M, Ghosh AK. Plasminogen activator inhibitor-1 Is a marker and a mediator of senescence. Arterioscler Thromb Vasc Biol. 2017;37:1446–52. 10.1161/ATVBAHA.117.309451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmidt V, Schulz N, Yan X, et al. SORLA facilitates insulin receptor signaling in adipocytes and exacerbates obesity. J Clin Invest. 2016;126(7):2706–2720. 10.1172/JCI84708 [DOI] [PMC free article] [PubMed]
- 52.Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, et al. PPARγ signaling and metabolism: the good, the bad and the future. Nat Med. 2013;19:10.1038/nm.3159. [DOI] [PMC free article] [PubMed]
- 53.Zelcer N, Tontonoz P. Liver X receptors as integrators of metabolic and inflammatory signaling. J Clin Invest. 2006;116:607–14. 10.1172/JCI27883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Claudel T, Staels B, Kuipers F. The farnesoid X receptor. Arterioscler Thromb Vasc Biol. 2005;25:2020–30. 10.1161/01.ATV.0000178994.21828.a7 [DOI] [PubMed] [Google Scholar]
- 55.Decara J, Rivera P, López-Gambero AJ, Serrano A, Pavón FJ, Baixeras E, et al. Peroxisome Proliferator-Activated Receptors: Experimental Targeting for the Treatment of Inflammatory Bowel Diseases. Front Pharmacol [Internet]. 2020 [cited 2023 Jul 4];11. Available from: https://www.frontiersin.org/articles/10.3389/fphar.2020.00730 [DOI] [PMC free article] [PubMed]
- 56.Wang Y-D, Chen W-D, Wang M, Yu D, Forman BM, Huang W. Farnesoid X receptor antagonizes NF-κB in hepatic inflammatory response. Hepatol Baltim Md. 2008;48:1632–43. 10.1002/hep.22519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the cardiovascular health study. Arch Intern Med. 2002;162:2333–41. 10.1001/archinte.162.20.2333 [DOI] [PubMed] [Google Scholar]
- 58.Zimmerman TL, Thevananther S, Ghose R, Burns AR, Karpen SJ. Nuclear export of retinoid X receptor α in response to interleukin-1β-mediated cell signaling. J Biol Chem. 2006;281:15434–40. 10.1074/jbc.M508277200 [DOI] [PubMed] [Google Scholar]
- 59.De Luca M. The role of the cell-matrix interface in aging and its interaction with the renin-angiotensin system in the aged vasculature. Mech Ageing Dev. 2019;177:66–73. 10.1016/j.mad.2018.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gumpenberger M, Wessner B, Graf A, Narici MV, Fink C, Braun S, et al. Remodeling the skeletal muscle extracellular matrix in older age—effects of acute exercise stimuli on gene expression. Int J Mol Sci. 2020;21:7089. 10.3390/ijms21197089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Romero-Ortuno R, Kenny RA, McManus R. Collagens and elastin genetic variations and their potential role in aging-related diseases and longevity in humans. Exp Gerontol. 2020;129:110781. 10.1016/j.exger.2019.110781 [DOI] [PubMed] [Google Scholar]
- 62.O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–85. 10.1016/S0092-8674(00)81848-6 [DOI] [PubMed] [Google Scholar]
- 63.Losordo DW, Isner JM. Estrogen and angiogenesis: A review. Arterioscler Thromb Vasc Biol. 2001;21:6–12. 10.1161/01.ATV.21.1.6 [DOI] [PubMed] [Google Scholar]
- 64.Gu J-W, Shparago M, Tan W, Bailey AP. Tissue endostatin correlates inversely with capillary network in rat heart and skeletal muscles. Angiogenesis. 2006;9:93–9. 10.1007/s10456-006-9035-z [DOI] [PubMed] [Google Scholar]
- 65.Lähteenvuo J, Rosenzweig A, Sinclair D, North B. Effects of aging on angiogenesis. Circ Res. 2012;110:1252–64. 10.1161/CIRCRESAHA.111.246116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hodges NA, Suarez-Martinez AD, Murfee WL. Understanding angiogenesis during aging: opportunities for discoveries and new models. J Appl Physiol. 2018;125:1843–50. 10.1152/japplphysiol.00112.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kapoor A, Nation DA. Role of Notch signaling in neurovascular aging and Alzheimer’s disease. Semin Cell Dev Biol. 2021;116:90–7. 10.1016/j.semcdb.2020.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao Q, Gao SM, Wang MC. Molecular mechanisms of lysosome and nucleus communication. Trends Biochem Sci. 2020;45:978–91. 10.1016/j.tibs.2020.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Triolo M, Hood DA. Manifestations of age on autophagy, mitophagy and lysosomes in skeletal muscle. Cells. 2021;10:1054. 10.3390/cells10051054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Santos-Eggimann B, Cuénoud P, Spagnoli J, Junod J. Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries. J Gerontol A Biol Sci Med Sci. 2009;64:675–81. 10.1093/gerona/glp012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Pre-existing data access policies for each of the parent cohort studies specify that research data requests can be submitted to each steering committee; these will be promptly reviewed for confidentiality or intellectual property restrictions and will not unreasonably be refused. Please refer to the data sharing policies of these studies. Individual level patient or protein data may further be restricted by consent, confidentiality or privacy laws/considerations. These policies apply to both clinical and proteomic data.