Abstract
Acceptance and reappraisal are considered adaptive emotion regulation strategies. While previous studies have explored the neural underpinnings of these strategies using task-based fMRI and sMRI, a gap exists in the literature concerning resting-state functional brain networks’ contributions to these abilities, especially regarding acceptance. Another intriguing question is whether these strategies rely on similar or different neural mechanisms. Building on the well-known improved emotion regulation and increased cognitive flexibility of individuals who rely on acceptance, we expected to find decreased activity inside the affective network and increased activity inside the executive and sensorimotor networks to be predictive of acceptance. We also expect that these networks may be associated at least in part with reappraisal, indicating a common mechanism behind different strategies. To test these hypotheses, we conducted a functional connectivity analysis of resting-state data from 134 individuals (95 females; mean age: 30.09 ± 12.87 years, mean education: 12.62 ± 1.41 years). To assess acceptance and reappraisal abilities, we used the Cognitive Emotion Regulation Questionnaire (CERQ) and a group-ICA unsupervised machine learning approach to identify resting-state networks. Subsequently, we conducted backward regression to predict acceptance and reappraisal abilities. As expected, results indicated that acceptance was predicted by decreased affective, and executive, and increased sensorimotor networks, while reappraisal was predicted by an increase in the sensorimotor network only. Notably, these findings suggest both distinct and overlapping brain contributions to acceptance and reappraisal strategies, with the sensorimotor network potentially serving as a core common mechanism. These results not only align with previous findings but also expand upon them, illustrating the complex interplay of cognitive, affective, and sensory abilities in emotion regulation.
Keywords: Acceptance, Reappraisal, Emotion regulation, Resting state, Functional connectivity
Subject terms: Neuroscience, Psychology
Introduction
The ability to regulate emotions is considered fundamental to mental health and well-being, and difficulties in regulating emotions have been associated with a wide range of psychological conditions1–5. For example, anxiety, depression, and personality disorders have all been linked to emotion dysregulation6,7. Due to the prevalence of emotion regulation challenges across various psychological disorders, clinicians have started integrating different emotion regulation strategies into their therapeutic approaches3,4,8,9.
Acceptance is characterized by open curiosity towards ongoing mental and sensory experiences10,11. It is considered a fundamental concept in third-wave behavioral therapies12,13 and experiential-dynamic approaches4,5,14,15. Within these frameworks, acceptance is defined as “the active and aware embrace of private experiences without unnecessary attempts to change their frequency or form”16. Reappraisal and acceptance are considered two highly effective strategies frequently used in psychotherapy5,17,18. Reappraisal refers to a voluntary effort to reinterpret the significance of a situation to change its emotional effect19. This process is defined as “construing a potentially emotion-eliciting situation in non-emotional terms”20. Reappraisal is an antecedent-focused regulation strategy that alters emotion before the complete onset of emotional response.
Reappraisal and acceptance are commonly regarded as adaptive strategies due to their positive associations with well-being and mental health21,22. In a study conducted by Dan-Glauser and Gross23, when compared to no regulation, acceptance was found to lead to an increase in positive emotions and a decrease in respiration rate. Additionally, reappraisal is believed to be negatively correlated with anxiety24–26. Uchida et al.27 showed individuals who were more successful in reappraisal had lower levels of trait anxiety and experienced more positive emotions in their daily lives. Hofmann et al.28 reported effectiveness for both strategies in reducing heart rate when compared to suppression. Goldin et al.11 found no difference in respiration rate and skin conductance between both strategies but observed a higher heart rate during reappraisal compared to acceptance.
For what concerns the neural bases of acceptance, just a few task-based fMRI studies inquired into its nature. Traditional models of emotion regulation are based on top-down control processes29. However, neuroimaging studies exploring the neural correlates of acceptance show inconsistent findings. Some of these studies align with traditional models by demonstrating prefrontal activations within the dorsal attention network during acceptance11,30. Other studies report either less activity in prefrontal areas or activations that are more medially located compared to traditional strategies31–33. Furthermore, several studies reveal that neural correlates of acceptance may depend on bottom–up mechanisms and occur without the involvement of prefrontal cortical areas34–38. In an effort to provide a synthetic view of the neural contributions to acceptance, Messina et al.38 performed a meta-analysis of 13 fMRI studies that revealed a consistent association between acceptance and decreased brain activity in emotion related regions such as the posterior cingulate cortex (PCC)/precuneus, insula, and limbic subcortical regions such as the thalamus and the parahippocampal gyrus (PHG), regardless of the control condition. In another study, Sezer et al.39 showed that mindfulness, an ability strongly correlated with acceptance, is correlated with increased functional connectivity between the PCC, a central part of the default mode network (DMN), and the dorsolateral prefrontal cortex, potentially enhancing attention control. They further found that mindfulness is associated with increased connectivity in areas implicated in pain relief and self-awareness, pointing to the multifaceted nature of these cognitive strategies. Of note, the studies included in these meta-analyses did not include information on the individual differences in the abilities to use acceptance, as they were task-based fMRI studies. Besides task based functional studies, to our knowledge, only one study tried to understand the abilities in acceptance measured via dedicated questionnaires40. In this study, a data fusion machine learning approach was used to identify joint gray and white matter contributions to high and low acceptance abilities. Results revealed individuals with higher acceptance trait showed reduced gray and white matter concentrations within the DMN, particularly in posterior and anterior midline structures, anterior temporal regions, the angular gyrus, the dorsal anterior cingulate cortex (dACC), and the right insula. Additionally, these individuals exhibited increased gray and white matter concentrations in the cognitive and attention networks, especially within prefrontal and superior parietal regions. However, this study was limited to the structural properties of the brain and the question of whether similar networks, but at a functional level, may contribute to acceptance remain unaddressed.
Regarding reappraisal, Buhle et al.41 conducted a meta-analysis of 48 task-related fMRI studies and reported that both downregulation and upregulation of emotion are associated with increased activation in the bilateral dorsolateral and ventrolateral prefrontal cortex (dlPFC, vlPFC), dACC, supplementary motor area (SMA), and the inferior/superior parietal cortex. Besides task-based fMRI studies of reappraisal, a few studies inquired into the nature of reappraisal abilities and how they can be predicted by resting state and structural networks. For example, Uchida et al.27 found that effective reappraisal correlates with decreased connectivity between the right amygdala and both the medial prefrontal and posterior cingulate cortices, as well as between the bilateral dorsolateral prefrontal cortex and posterior visual regions during resting-state functional connectivity. Additionally, Morawetz et al.42 reported that the reappraisal usage is linked to stronger functional connectivity between the vlPFC and the amygdala. In another study, Zanella et al.43 found that cognitive and positive reappraisal were predicted by sensorimotor networks. Of note, positive reappraisal, different from cognitive reappraisal, can be seen as a hybrid form of reappraisal and acceptance, wherein someone can attach a positive meaning to the event in terms of personal growth44. Further, from a structural point of view, Ghomroudi et al.45 applied machine learning methods to gray matter structural data and found that a temporo-parahippocampal-orbitofrontal network, which includes regions such as the thalamus, superior temporal gyrus, lentiform nucleus, uncus, and cerebellar tonsil, predicts the use of reappraisal.
These studies point toward the direction of different mechanisms behind acceptance and reappraisal with a possible partial overlap in the insula and frontal regions of the brain, such as the dorsolateral prefrontal cortex, but also in regions of the sensorimotor network43. To test this hypothesis, a recent meta-analysis of 42 fMRI studies conducted by Monachesi et al.46 was run and it was found that reappraisal was associated with increased activity in the superior frontal gyrus and the middle frontal gyrus, while reducing activity in sublobar regions like the globus pallidus and putamen. Conversely, acceptance was associated with increased activity in the claustrum and decreased activity in various limbic structures, including the PCC/precuneus, the PHG, and the thalamus (pulvinar). Interestingly, the conjunction analysis revealed that both acceptance and reappraisal engage the vlPFC and the insula, indicating shared neural pathways in these emotional regulation strategies.
It was suggested that emotion regulation may rely on a core inhibitory (vlPFC)/sensorial awareness (insula) and on strategy-specific top-down and bottom-up processes distinct for different strategies. Although this study suggests different and only partially overlapping mechanisms behind acceptance and reappraisal usage (task-based fMRI studies), we still do not know whether the same applies to the resting state networks associated with the ability to apply these strategies. The aim of this study is to test for the first time the possibility of detecting resting state macro networks contributions to acceptance and reappraisal abilities and to test the hypothesis that they rely on different mechanisms with a common core behind them. If demonstrated, this result may support the model of common and specific neural mechanisms of emotion regulation that expand the previous simplistic dual-routes models5.
While previous studies have predominantly used task-based fMRI to investigate the neural correlates of acceptance5,22,47,48 and structural MRI to examine both reappraisal and acceptance5,22,47,48, there is a noticeable gap in the literature concerning the use of resting state fMRI to study acceptance. Furthermore, as far as we know, there has been no direct comparison between the neural mechanisms underlying acceptance and reappraisal using resting state fMRI. Resting state fMRI is a valuable tool for capturing intrinsic connectivity patterns that underpin a wide range of behaviors and traits. Unlike task-based fMRI, which requires participants to follow specific instructions, resting state fMRI is acquired in the absence of a stimulus or task. This approach simplifies the acquisition process and reduces the variability introduced by task performance. Functional connectivity patterns observed in resting state fMRI are unique to each individual, relatively stable across different mental states, and sensitive to phenotypic differences including age, cognitive abilities, and mental health outcomes. This stability makes resting state fMRI particularly useful for studying individual differences in brain function and behavior49. The lack of task requirements makes resting state fMRI especially advantageous for studying populations that may have difficulty with task instructions, such as individuals with neurologic, neurosurgical, and psychiatric conditions, as well as pediatric patients. This broad applicability enhances the potential for resting state fMRI to contribute to diverse areas of cognitive neuroscience49.
By using resting state fMRI to predict reappraisal and acceptance, our study aims to fill the existing gaps in the literature and provide a more comprehensive understanding of the neural mechanisms underlying these important emotion regulation strategies. The intrinsic connectivity patterns captured by resting state fMRI may reveal fundamental brain-behavior relationships that are not easily discernible through task-based approaches. Also, these findings can generate neuro-predictive models of emotion regulation strategies that can be used in the near future to design pharmacological or neurostimulation interventions to increase their usage by increasing the activity of these areas. Additionally, comparing the neural predictors of acceptance and reappraisal within the same framework will offer novel insights to understand distinct and shared neural substrates. Use of resting state fMRI allows us to advance the understanding of emotion regulation strategies, thereby contributing significantly to the field of emotion regulation.
Of note, in this study we used a different approach to the previous resting-state studies on emotion regulation. Resting-state functional connectivity has been mainly estimated using seed-based correlation50 in the field of emotion27,51. Seed-based functional connectivity analysis, also known as region of interest (ROI)-based functional connectivity, involves identifying brain regions that exhibit correlations with the activity in a predefined seed region. This analysis calculates cross-correlations between the time-series of the seed region and the rest of the brain. Seed-based analysis requires the a priori selection of seed regions. One advantage of seed analysis is its simplicity and intuitive interpretation. However, a drawback is its sensitivity to seed selection, as changing the seed region can significantly alter the results, making it susceptible to bias. The ICA method52,53 is a whole-brain, model-free method that provides a more data-driven approach to quantifying functional connectivity. ICA is a computational approach that decomposes BOLD fMRI signal time courses from the entire brain into spatially and temporally independent components. Several resting-state networks typically emerge from ICA analysis in resting-state fMRI studies, including the DMN, auditory network, salience network, executive control network, medial visual network, lateral visual network, sensorimotor cortex, dorsal visual stream (frontoparietal attention network), basal ganglia network, limbic network, and precuneus network. Unlike seed-based analysis, which relies on a priori assumptions and the selection of regions of interest, ICA is a data-driven method and can be executed without predefined assumptions, except for specifying the number of independent components to identify. In this study, resting-state macro-networks were determined using an ICA approach. To the best of our knowledge, all previous research on emotion regulation involving resting-state analysis has used seed-based analysis27,51.
Based on previous research11,30,38, we expect modulation in BOLD temporal variability across the following networks to predict acceptance strategy. Firstly, we predict a decrease in BOLD temporal variability within a network that includes subcortical regions such as the thalamus and the hippocampus/parahippocampal region, which can be considered part of an affective network. Furthermore, an increase in BOLD temporal variability is expected within the executive network, encompassing cognitive control regions such as the dlPFC, vlPFC, and the dACC. Additionally, based on Zanella et al.43 resting state study and Monachesi et al.46 meta-analysis, we expect increased BOLD temporal variability within the somatosensory network, particularly within the insula and SMA, to predict acceptance strategy. Building upon previous research43,54, we predict that increased temporal variability within the sensorimotor network is predictive of reappraisal. We hypothesize that regions such as the precentral and postcentral gyrus, along with the SMA, which play a pivotal role in the execution of regulatory functions, form a network predictive of reappraisal. Based on the meta-analysis conducted by Monachesi et al.46, we expect that the sensorimotor network, particularly regions such as the insula, may act as a fundamental network underlying both reappraisal and acceptance strategies.
Results
Macro networks contributions to acceptance
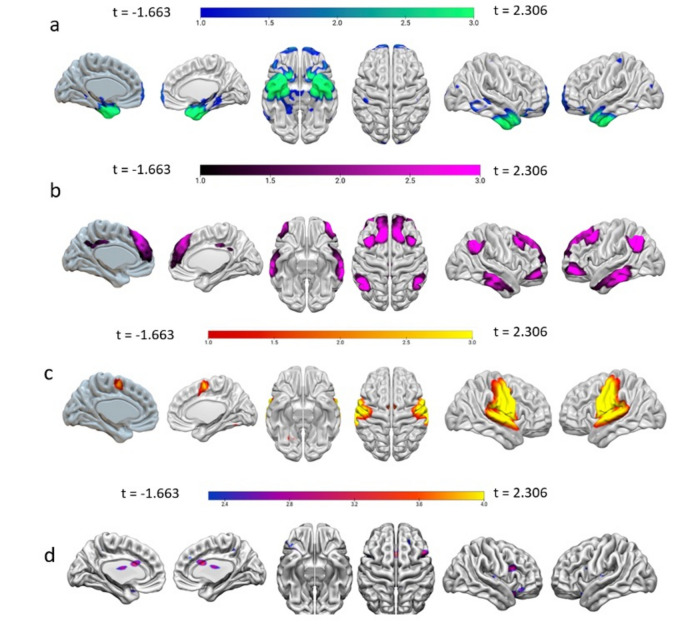
The backward multiple regression analysis returned a significant winning model (F(4, 130) = 10.603, R2 = 0.246, p < 0.001) in which the BOLD variability across four networks, IC2 (β = − 60.85, p = 0.001), IC11 (β = − 67.89, p = 0.001), IC13 (β = 80.16, p < 0.001), and IC18 (β = 24.42, p = 0.022) predicted acceptance, thereby yielding an overall significant model. The corresponding regression equation was as follows: Acceptance strategy = 8.765–60.858 IC2 – 67.899 IC11 + 80.167 IC13 + 24.429 IC18. The identified ICs encompass clusters of both cortical and subcortical regions at a cluster statistical significance level of p < 0.05 (pFDR corrected) and at the voxel significance level p < 0.001 (pFDR corrected) (Tables 1 and 2). These networks correspond to well-established resting state networks, specifically: IC2: associated with the affective system, IC11: corresponding to the executive network, IC13: representative of the sensorimotor network and IC18: aligned partly with the language network (Fig. 1).
Table 1.
Result of backward regression.
| ICs | Emotion regulation strategy | β | P |
|---|---|---|---|
| IC2 | Acceptance | − 60.85 | 0.001 |
| IC11 | − 67.89 | 0.001 | |
| IC13 | 80.16 | < 0.001 | |
| 1C18 | 24.42 | 0.022 | |
| IC20 | Reapprasial | 0.339 | < 0.001 |
Beta (β) and corrected p-value for the significant relationships between the BOLD temporal variability and both ER strategies.
Table 2.
Summary of the main neural results for acceptance.
| Strategy | Network | Brain Region | Number of Voxels | Peak (x, y, z) |
|---|---|---|---|---|
| Acceptance | IC 2 | R Temporal Fusiform Cortex | 654 | (36, − 24, − 28) |
| L Temporal Fusiform Cortex | 654 | (− 34, − 28, − 26) | ||
| R Inferior Temporal Gyrus | 647 | (52, − 20, − 32) | ||
| L Inferior Temporal Gyrus | 647 | (− 52, − 20, − 30) | ||
| R Parahippocampal Gyrus | 649 | (22, − 8, − 30) | ||
| L Parahippocampal Gyrus | 649 | ( − 22, − 10, − 30) | ||
| R Hippocampus | 668 | (26, − 20, − 14) | ||
| L Hippocampus | 668 | (− 24, − 22, − 14) | ||
| L Middle Temporal Gyrus | 377 | (− 56, − 4, − 24) | ||
| R Amygdala | 296 | (24, − 4, − 18) | ||
| L Amygdala | 296 | ( − 24, − 6, − 18) | ||
| R Frontal Orbital Cortex | 899 | (24, 22, − 20) | ||
| L Frontal Orbital Cortex | 899 | (− 26, 22, − 20) | ||
| R R Superior Temporal Gyrus | 193 | (56, − 2, − 12) | ||
| R R Thalamus | 444 | (14, − 26, 2) | ||
| Acceptance | IC 11 | R Angular Gyrus | 724 | (52, − 52, 38) |
| L Angular Gyrus | 724 | (− 50, − 56, 34) | ||
| L L Supramarginal Gyrus | 636 | (− 54, − 46, 42) | ||
| R Middle Temporal Gyrus | 1212 | (64, − 22, − 14) | ||
| L Middle Temporal Gyrus | 1212 | (− 62, − 28, − 12) | ||
| R Superior Frontal Gyrus | 1643 | (14, 30, 52) | ||
| L Superior Frontal Gyrus | 1643 | (− 14, 30, 50) | ||
| R Middle Frontal Gyrus | 1513 | (38, 22, 46) | ||
| L Middle Frontal Gyrus | 1513 | (− 36, 18, 46) | ||
| L Paracingulate Gyrus Left | 803 | (− 6, 44, 16) | ||
| R Amygdala | 327 | (24, − 4, − 18) | ||
| L Amygdala | 327 | (− 22, − 4, − 18) | ||
| R Putamen | 761 | (24, 4, 0) | ||
| L Putamen | 761 | (− 24, 2, 0) | ||
| L L Frontal Orbital CorteX | 623 | (− 28, 24, − 12) | ||
| L L Insular Cortex | 875 | (− 36, 8, − 2) | ||
| Acceptance | IC13 | R Precentral Gyrus | 2219 | (50, − 4, 40) |
| L Precentral Gyrus | 2219 | (− 48, − 6, 40) | ||
| R Postcentral Gyrus | 1898 | (50, − 20, 42) | ||
| L Postcentral Gyrus | 1898 | (− 52, − 20, 42) | ||
| R Superior Temporal Gyrus | 261 | (60, − 24, 2) | ||
| L Superior Temporal Gyrus | 261 | (− 62, − 28, 4) | ||
| R Insular Cortex | 1117 | (38, 0, 2) | ||
| L Insular Cortex | 1117 | (− 38, − 4, 2) | ||
| R R/L Parietal Operculum Cortex | 533 | (48, − 28, 22) | ||
| L R/L Parietal Operculum Cortex | 533 | (− 48, − 32, 20) | ||
| R Supramarginal Gyrus | 678 | (58, − 26, 38) | ||
| L Supramarginal Gyrus | 678 | (− 58, − 30, 34) | ||
| Acceptance | IC18 | R Caudate r | 213 | (14, 2, 18) |
| L Caudate r | 213 | (− 14, 2, 18) | ||
| R Thalamus r | 843 | (12, − 20, 10) | ||
| L Thalamus r | 843 | (− 10, − 20, 10) | ||
| R Inferior Frontal Gyrus | 464 | (52, 16, 20) | ||
| R Frontal Orbital Cortex | 1356 | (30, 22, − 16) | ||
| L Frontal Orbital Cortex | 1356 | (− 30, 24, − 16) | ||
| R R Middle Frontal Gyrus | 1672 | (40, 20, 40) | ||
| R Insular Cortex | 649 | (38, 4, − 2) | ||
| L Insular Cortex | 649 | (− 38, 4, − 2) | ||
| R Precentral Gyrus | 2493 | (36, − 10, 52) | ||
| L Precentral Gyrus | 2493 | (− 34, − 12, 50) | ||
| R R Posterior Parahippocampal Gyrus | 274 | (22, − 32, − 16) | ||
| R R Middle Temporal Gyrus | 1064 | (58, − 50, 2) |
Figure 1.
(a) Resting-state BOLD temporal variability predicting acceptance in the affective network (IC2). (b) Resting-state BOLD temporal variability predicting acceptance in the frontoparietal network (IC11). (c) Resting-state BOLD temporal variability predicting acceptance in the sensorimotor network (IC13). (d) Resting-state BOLD temporal variability predicting acceptance in the executive network (IC18). The color bar in this figure represents t-values. Lower values indicate negative t-values, corresponding to areas with significantly lower brain activity. Higher values represent positive t-values, indicating areas with significantly higher brain activity.
Macro networks contributions to reappraisal
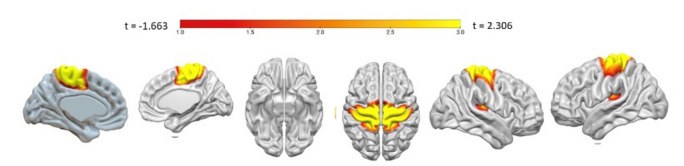
Backward multiple regression analysis returned a significant winning model (F (1, 133) = 28.59, R2 = 0.421, p < 0.001) in which the BOLD variability of IC20 (β = 0.339, p < 0.001), predicted reappraisal usage. The corresponding regression equation was as follows: Reappraisal usage = 6.757 + 0.339 IC20. IC20 encompasses a cluster of sensory motor regions at a cluster statistical significance level of p < 0.05 (pFDR corrected) and at the voxel significant level p < 0.001 (pFDR corrected). This network corresponds to the well-established sensory motor resting-state network (Table 3, Fig. 2). The level of variability observed in a specific brain region is positively correlated with the degree of functional connectivity55. In other words, when a brain area is highly interconnected with other brain regions, it tends to exhibit increased variability in its BOLD signal. Greater BOLD variability corresponds to an increased frequency of both reappraisal and acceptance usage. Conversely, lower BOLD variability corresponds to a decreased frequency of both acceptance and reappraisal usage.
Table 3.
Summary of the main neural results for reappraisal.
| Strategy | Network | Brain Region | Number of Voxels | Peak (x, y, z) |
|---|---|---|---|---|
| Reappraisal | IC20 | R Precentral Gyrus Left | 2784 | (26, − 18, 60) |
| L Precentral Gyrus Left | 2784 | (− 24, − 18, 60) | ||
| R Postcentral Gyrus Left | 2492 | (30, − 30, 60) | ||
| L Postcentral Gyrus Left | 2492 | (− 30, − 32, 60) | ||
| R Supplementary Motor Cortex | 587 | (6, − 4, 58) | ||
| L Supplementary Motor Cortex | 587 | (− 6, − 2, 56) | ||
| R Precuneous | 1059 | (8, − 46, 48) | ||
| R Superior Frontal Gyrus | 699 | (16, − 4, 68) | ||
| L Superior Frontal Gyrus | 699 | (− 14, 0, 66) | ||
| R Cingulate Gyrus AC/PC | 468 | (4, − 28, 42) | ||
| R Insular Cortex | 204 | (36, − 18, 10) | ||
| L Insular Cortex | 204 | (− 36, − 20, 10) | ||
| R Parietal Operculum Cortex | 304 | (44, − 26, 20) | ||
| L Parietal Operculum Cortex | 304 | (− 44, − 30, 20) | ||
| R Central Opercular Cortex | 240 | (44, − 14, 16) | ||
| L Central Opercular Cortex | 240 | (− 46, − 18, 16) | ||
| R Superior Parietal Lobule | 469 | (22, − 46, 66) | ||
| L Superior Parietal Lobule | 469 | (− 24, − 48, 64) | ||
| R Central Opercular Cortex | 155 | (44, − 14, 16) | ||
| R Heschl’s Gyrus | 204 | (46, − 20, 8) |
Figure 2.
Resting-state BOLD temporal variability predicting reappraisal in the sensorimotor network (IC20). The color bar in this figure represents t-values. Lower values indicate negative t-values, corresponding to areas with significantly lower brain activity. Higher values represent positive t-values, indicating areas with significantly higher brain activity.
Discussion
In this study, we applied group-ICA, an unsupervised machine learning technique, to identify the resting state networks that predict acceptance and reappraisal, and eventually the commonalities between the two strategies. The findings indicated that modulations in four BOLD temporal variability networks were predictive of acceptance, while one network was predictive of reappraisal. Specifically, reduced BOLD temporal variability in the affective (IC2) and executive (IC11) networks, and increased BOLD temporal variability in the sensorimotor (IC13) and part of the language (IC18) networks, were associated with predicting acceptance. Furthermore, reappraisal strategy was predicted by an increase in variability inside the sensorimotor network (IC20). Of note, the sensorimotor network was associated with both acceptance and reappraisal, demonstrating a commonality between the two.
The results indicated that decreased BOLD temporal variability in the IC2 network is associated with acceptance strategy. Notably, several regions within the IC2 brain area overlap with the affective network, which is crucial for emotional processing. Key regions in IC2 encompass the PHG, hippocampus, amygdala, and thalamus. Previous studies37,38,46 have found a correlation between reduced activity in the PHG and acceptance. The PHG is significant in the early and automatic assessment of emotional significance during emotion regulation56. Additionally, decreased connectivity in the PHG has been noted during mindfulness and meditation practices57. This suggests that diminished PHG activity during acceptance may reflect a reduced impact of emotional events on the individual, potentially influencing memory associations or the retrieval of stimuli46,58. The thalamus characterized as a critical relay hub in the brain for processing sensory information59, might signify a sensory filtering process upon its deactivation, leading to enhanced openness and a non-judgmental attitude inherent in acceptance60. Furthermore, the thalamus, along with the hippocampus and PHG, contributes to regulation efficacy38,61,62. Overall, these results align with the observation that individuals who rely on acceptance have better emotion regulation63,64.
The findings showed a reduction in BOLD temporal variability within the frontoparietal network, also named the central executive network (IC11), which correlates with the acceptance strategy. This network includes the superior frontal gyrus and the middle frontal gyrus. This result aligns with previous studies36,65. The decreased activity in the middle frontal gyrus might indicate an enhanced capacity to maintain attention and regulate impulses during acceptance40,65,66.
The results revealed that increased BOLD temporal variability in the somatosensory networks (IC13) is associated with acceptance strategy. This network consists of the precentral gyrus and postcentral gyrus. The postcentral gyrus and the insula are associated with interoceptive awareness66–68. In addition, staying in the present moment a key aspect of mindfulness, is linked to a brain network that includes the thalamus, insula, and sensorimotor regions such as the postcentral and precentral gyri69,70. This result is consistent with the fact that individuals using acceptance may have a coherent perception of their emotional states, fostering a non-judgmental attitude towards emotional experiences. Moreover, it highlights the significance of interoceptive awareness, emphasizing the importance of remaining present, a fundamental aspect of acceptance38,40,65.
Lastly, results revealed that increased BOLD temporal variability within IC18, recognized by CONN as the language network, which includes the vlPFC and the insula, is associated with acceptance strategy. The vlPFC plays a role in various functions, such as response selection and inhibition, as well as language processing71–73. Several studies suggest that both the insula and the vlPFC are consistently linked to effective emotion regulation across different strategies, including acceptance38,62,74. The insula integrates sensory information from both internal and external sources, helps form awareness of the body’s emotional state, and labels emotions75,76, as found in previous meta-analyses on this topic38. In addition to language-related regions, this network includes the dlPFC and the anterior cingulate cortex (ACC), which are more associated with control mechanisms. The dlPFC is a crucial area in the central executive network, playing significant roles in attention, decision-making, working memory, and cognitive control77–79. Moreover, the dACC is related to the regulation of cognitive processes80–82 and is also linked to increased connectivity in mindful individuals70,83. A few studies examining resting state connectivity indicate that dlPFC and vlPFC are effectively interconnected during emotion regulation42,84. Increased activity in executive network regions such as the dlPFC, vlPFC, and ACC, along with decreased activity in affective network regions like the amygdala, supports a dual-process model29,71,73.
The result indicates an increase in BOLD variability in another somatosensory network (IC 20) is predictive of reappraisal. The somatosensory network includes regions such as the precentral gyrus, the postcentral gyrus, SMA and insula. The somatosensory cortex is involved in the recognition of emotions and the understanding of the emotional states of others85. Picó-Pérez et al.46,54 revealed the significant role of the SMA in cognitive reappraisal. The key role of the SMA is top-down inhibitory control over the amygdala, whose role is in the initial processing and linking of sensory and affective input86. The insula plays a crucial role in integrating sensory information from both internal and external environments. This integration is essential for forming a coherent and conscious representation of one’s internal emotional state64,76. Moreover, functional connectivity of the left insula and SMA is associated with the frequency of use of reappraisal54. The dACC and the superior parietal cortex are linked to goal-oriented attention87,88. Specifically, the superior parietal cortex is involved in detecting salience and directing attention89, and the dACC plays a role in control allocation90. The results of this study suggest that the sensorimotor network functions as the common core network underlying both acceptance and reappraisal strategies. Considering that emotion regulation involves paying attention to and being aware of one’s emotional state, it is plausible to associate this process with the awareness of bodily states91. The somatosensory cortex is pivotal in emotional processing, the generation of emotional states, and interoceptive awareness92–94. Since regulating emotions requires an awareness of both emotional and bodily states, it follows that increased interoceptive awareness could enhance emotion regulation. This enhancement could be through the improved detection of early bodily reactions to emotional stimuli91. Therefore, due to the role of the somatosensory network in awareness, it could be a common core in emotion regulation, regardless of the specific strategy used.
To conclude, this study aimed to identify specific resting state functional brain networks that are predictive of acceptance and reappraisal capabilities. Acceptance and reappraisal are both recognized as effective emotion regulation strategies, as highlighted in several research studies5,22,47,48. These strategies are frequently used in psychotherapy17. While numerous studies have explored the brain mechanisms behind acceptance and reappraisal in task-based fMRI and sMRI techniques, there remains a lack of understanding about the resting state functional brain networks that underlie these strategies. Our findings indicate that acceptance was predicted by the executive, the affective and the sensorimotor networks, whereas reappraisal was predicted by the sensorimotor network. These findings not only are in line, but also extend previous findings, revealing interactions among cognitive, emotional, and sensory processes in emotion regulation. While our earlier work43 identified general associations between trait emotional intelligence and different emotion regulation strategies, this study reveals distinct neural predictors for acceptance and reappraisal. Acceptance was associated with decreased activity in the affective and executive networks and increased activity in the sensorimotor network, whereas reappraisal was linked to increased sensorimotor network activity. These findings underscore the critical role of the sensorimotor network in both strategies while highlighting the involvement of other networks in differentiating these strategies.
This novel insight into the specific neural mechanisms underlying acceptance and reappraisal contributes significantly to the field by illustrating the complex interplay of cognitive, affective, and sensory processes in emotion regulation. From a translational point of view, these findings can be used in the future to design specific protocols that increase the usage of these strategies through pharmacological stimulation or non-invasive neurostimulation.
Method
Participants
Participants included in this study comprised 134 (95 female) native German-speaking individuals, with a mean age of 30.09 ± 12.87 years and an average of 12.62 ± 1.41 years of education. The participants’ mean acceptance score was 7.06 ± 2.85, and the mean reappraisal score was 6.78 ± 2.84. The data was drawn from “Leipzig study for mind–body–emotion interactions” (OpenNeuro database, accession number ds000221) (LEMON). The data collection was conducted at the Max Planck Institute for Human Cognitive and Brain Sciences (MPI CBS) in Leipzig95. The data collection for this study was conducted in accordance with the Declaration of Helsinki principles. The study protocol received approval from the Ethics Committee of the University of Leipzig Medical Faculty (reference number 154/13-ff). The inclusion criteria were, absence of cardiovascular, psychiatric, or neurological disorders, and malignant diseases, as well as non-use of certain medications. Individuals reporting drug use or excessive alcohol use were excluded. Participants provided written informed consent and agreed to anonymous data sharing. Compensation was provided upon completion of all assessments. A power analysis conducted in R aimed to determine the required sample size for the multiple regression analysis. This analysis was conducted based on the following parameters: a number of predictors of 20, an effect size of 0.2 (Cohen’s d), a significance level set at 0.05, and a desired statistical power of 0.85. The results of this analysis yielded a recommended sample size of 133 participants.
Behavioural data
The German version of Cognitive Emotion Regulation Questionnaire (CERQ)44,96 was included in this study. This questionnaire assesses nine cognitive coping strategies, which encompass self-blame, acceptance, rumination, positive refocusing, refocus on planning, positive reappraisal, putting into perspective, catastrophizing, and blaming others. The German version of the questionnaire comprises 36 items, with each strategy measured through four questions. Participants responded using a 5-point Likert-type scale ranging from 1 (almost never) to 5 (almost always). In this study the investigation of functional connectivity associated with acceptance and positive reappraisal scales were mainly focused.
Image acquisition
Structural and functional MRI was conducted using a 3 Tesla scanner (MAGNETOM Verio, Siemens Healthcare GmbH, Erlangen, Germany) equipped with a 32-channel head coil. Throughout the MRI data acquisition process, no significant maintenance or updates were carried out that could have impacted the data quality. Our analyses focused on a BOLD resting state fMRI scan using a T2-weighted multiband EPI sequence (TR = 1400 ms, TE = 30 ms, flip angle = 69°, echo spacing = 0.67 ms, number of volumes = 657, voxel size = 2.3 mm), with a total acquisition time of 15 min and 30 s. Additionally, T1-weighted structural volumes were obtained using the MP2RAGE sequence (TR = 5000 ms, TE = 2.92 ms, TI1 = 700 ms, TI2 = 2500 ms, FOV = 256 mm, voxel size = 1 mm isotropic). The acquisition of the structural volumes contained 176 slices acquired interspersed over a scanning duration of 8 min and 22 s. During the image acquisition, participants were instructed to maintain wakefulness, keep still, and gaze at a low-contrast fixation cross while keeping their eyes open.
Data analysis
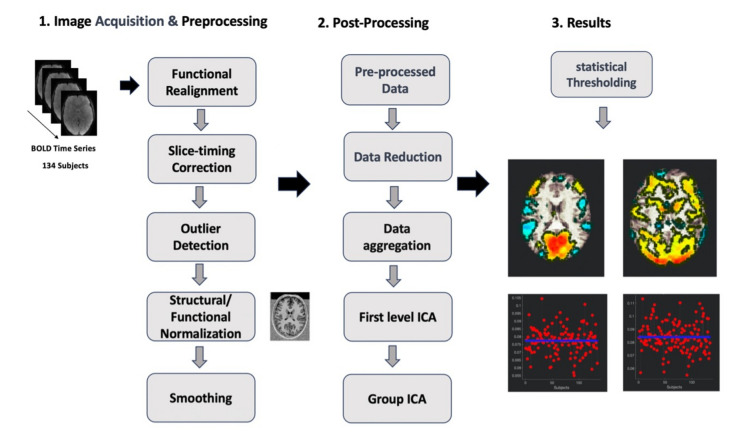
Pre-processsing
Data pre-processing was performed using CONN (version 2022), SPM12, and the MATLAB Toolbox (version 2021b). First, CONN’s default pre-processing pipeline using SMP12’s default parameters was used. This pipeline encompassed several stages: functional realignment and unwarping, translation and centering, conservative functional outlier detection, direct segmentation and normalization of functional data (1 mm resolution), translation and centering of structural data, segmentation and normalization of structural data (2.4 mm resolution), and lastly, spatial smoothing of functional and structural data using an 8 mm Gaussian kernel.
Subsequently, the denoising phase was conducted. The aim of this phase was to pinpoint and eliminate confounding variables and artifacts from the estimated BOLD signal. These factors arise from three distinct sources: the BOLD signal originating from masks of white matter or cerebrospinal fluid (CSF), parameters and outliers defined during the pre-processing step, and an estimation of the subjects’ motion parameters. Following identification, these factors were included in a regression model (using Ordinary Least Squares) as covariates. Finally, the time series underwent temporal band-pass filtering within the 0.0008 Hz to infinity range, a standard procedure for resting-state connectivity analyses.
Resting state analysis
For functional connectivity analysis in this study, the data-driven group-independent component approach (group-ICA) was performed using CONN. The CONN group-ICA consists of several steps: pre-conditioning variance normalization, concatenation of BOLD signal temporally, group-level dimensionality reduction, fast-ICA for spatial component estimation, and back-projection for individual spatial estimation. The analysis aimed to identify 20 independent components, aligning with earlier research adopting low model order analysis43,97,98. To differentiate noise components from underlying resting-state networks, each identified independent component (IC) underwent visual inspection and was compared with CONN’s networks atlas via a spatial match-to-template function. This function gauged the overlap between individual IC’s spatial maps and eight predefined brain networks (Default Mode, Sensorimotor, Visual, Salience, Dorsal Attention, Frontoparietal, Language, Cerebellar), as defined by CONN’s ICA analyses of the HCP dataset (497 subjects). Subsequently, the temporal variability and frequency of each IC were quantified using CONN, calculated as the standard deviation of BOLD time-series. To control for Type I errors, cluster-size-based false discovery rate (FDR) correction was applied (p < 0.05, voxel thresholded at p < 0.001 within each analysis). Figure 3
Figure 3.
Schematic diagram of the methodology. First the resting state data was preprocessed. Then 20 independent components were extracted using an unsupervised machine learning Group ICA approach.
To determine which of the 20 identified ICs were predictive of the use of acceptance and reappraisal strategies, we examined the impact of each IC’s BOLD signal variability on these emotion regulation strategies. This was achieved by conducting a multiple linear regression model (Ordinary Least Squares) with a backward elimination approach. The analyses were conducted separately for the dependent variables of acceptance and reappraisal, incorporating gender as a categorical fixed factor to assess its influence within the regression framework.
Author contributions
P.AG. Contributed to the writing of the manuscript, and performed data analysis. R.S. Provided supervision. I.M. Reviewed the manuscript. A.G. Conceived the work, reviewed the manuscript, and provided supervision. All the co-authors have read and approved the content of the final version of the manuscript.
Data availability
The complete Data of this study is accessible through Gesellschaft für wissenschaftliche Datenverarbeitung mbH Göttingen (GWDG) at https://www.gwdg.de/. Both raw and preprocessed data can be accessed via web browser (https://ftp.gwdg.de/pub/misc/MPI-Leipzig_Mind-Brain-Body-LEMON/) or through a fast FTP connection (ftp://ftp.gwdg.de/pub/misc/MPI-Leipzig_Mind-Brain-Body-LEMON/). In the event of any future changes in the data’s location, the dataset can be located using PID 21.11101/0000-0007-C379-5 (e.g., http://hdl.handle.net/21.11101/0000-0007-C379-5).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kring, A. M. & Sloan, D. M. Emotion Regulation and Psychopathology: A Transdiagnostic Approach to Etiology and Treatment (Guliford, 2009). [Google Scholar]
- 2.Sheppes, G., Suri, G. & Gross, J. J. Emotion regulation and psychopathology. Annu. Rev. Clin. Psychol.11, 379–405 (2015). 10.1146/annurev-clinpsy-032814-112739 [DOI] [PubMed] [Google Scholar]
- 3.Dadomo, H., Panzeri, M., Caponcello, D., Carmelita, A. & Grecucci, A. Schema therapy for emotional dysregulation in personality disorders: A review. Curr. Opin. Psychiatry31, 43–49. 10.1097/YCO.0000000000000380 (2018). 10.1097/YCO.0000000000000380 [DOI] [PubMed] [Google Scholar]
- 4.Frederickson, J. J., Messina, I. & Grecucci, A. Dysregulated anxiety and dysregulating defenses: Toward an emotion regulation informed dynamic psychotherapy. Front. Psychol.9, 2054 (2018). 10.3389/fpsyg.2018.02054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grecucci, A. et al. A dual route model for regulating emotions: Comparing models, techniques and biological mechanisms. Front. Psychol.11, 1–13 (2020). 10.3389/fpsyg.2020.00930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mennin, D. S., McLaughlin, K. A. & Flanagan, T. J. Emotion regulation deficits in generalized anxiety disorder, social anxiety disorder, and their co-occurrence. J. Anxiety Disord.23, 866 (2009). 10.1016/j.janxdis.2009.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulze, L. et al. Neuronal correlates of cognitive reappraisal in borderline patients with affective instability. Biol. Psychiatry69, 564–573 (2011). 10.1016/j.biopsych.2010.10.025 [DOI] [PubMed] [Google Scholar]
- 8.Leahy, R. L., Tirch, D. & Napolitano, L. A. Emotion Regulation in Psychotherapy: A Practitioner’s Guide (Guilford Press, 2011). [Google Scholar]
- 9.Messina, I., Sambin, M., Palmieri, A. & Viviani, R. Neural correlates of psychotherapy in anxiety and depression: A meta-analysis. PLoS One8, e74657 (2013). 10.1371/journal.pone.0074657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grecucci, A. et al. Baseline and strategic effects behind mindful emotion regulation: Behavioral and physiological investigation. PLoS One10, e0116541 (2015). 10.1371/journal.pone.0116541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldin, P. R., Moodie, C. A. & Gross, J. J. Acceptance versus reappraisal: Behavioral, autonomic, and neural effects. Cogn. Affect. Behav. Neurosci.19, 927–944 (2019). 10.3758/s13415-019-00690-7 [DOI] [PubMed] [Google Scholar]
- 12.Hayes, S. C. Integrative Behavioral Couples Therapy (IBCT; Jacobson & Christensen, 1996), and Mindfulness-Based Cognitive Therapy (MBCT. Borkovec & Roemer vol. 35 (2004).
- 13.Kahl, K. G., Winter, L., Schweiger, U. & Sipos, V. The third wave of cognitive-behavioural psychotherapies: Concepts and efficacy. Fortschr. Neurol. Psychiatr.79, 330–339 (2011). 10.1055/s-0029-1245963 [DOI] [PubMed] [Google Scholar]
- 14.Messina, I., Sambin, M., Beschoner, P. & Viviani, R. Changing views of emotion regulation and neurobiological models of the mechanism of action of psychotherapy. Cogn. Affect. Behav. Neurosci.16, 571–587. 10.3758/s13415-016-0440-5 (2016). 10.3758/s13415-016-0440-5 [DOI] [PubMed] [Google Scholar]
- 15.Messina, I., Grecucci, A., Marogna, C. & Calvo, V. Relational exposure and semantic processes as mechanisms of change in psychodynamic psychotherapy: Convergences between psychotherapy research and affective neuroscience. TPM Test. Psychom. Methodol. Appl. Psychol.27, 43–56 (2020). [Google Scholar]
- 16.Hayes, S. C., Pistorello, J. & Levin, M. E. acceptance and commitment therapy as a unified model of behavior change. Couns. Psychol.40, 976–1002 (2012). 10.1177/0011000012460836 [DOI] [Google Scholar]
- 17.Wolgast, M., Lundh, L. G. & Viborg, G. Cognitive reappraisal and acceptance: An experimental comparison of two emotion regulation strategies. Behav. Res. Ther.49, 858–866 (2011). 10.1016/j.brat.2011.09.011 [DOI] [PubMed] [Google Scholar]
- 18.Spencer, S. D., Buchanan, J. A. & Masuda, A. Effects of brief acceptance and cognitive reappraisal interventions on experiential avoidance in socially anxious individuals: A preliminary investigation. Behav. Modif.44, 841–864 (2020). 10.1177/0145445519854321 [DOI] [PubMed] [Google Scholar]
- 19.Gross, J. The emerging field of emotion regulation: An integrative review. Rev. Gen. Psychol.2, 271–299 (1998). 10.1037/1089-2680.2.3.271 [DOI] [Google Scholar]
- 20.Gross, J. Emotion regulation: Affective, cognitive, and social consequences. Psychophysiology39, 281–291 (2002). 10.1017/S0048577201393198 [DOI] [PubMed] [Google Scholar]
- 21.Aldao, A. & Nolen-Hoeksema, S. When are adaptive strategies most predictive of psychopathology?. J. Abnorm. Psychol.121, 276–281 (2012). 10.1037/a0023598 [DOI] [PubMed] [Google Scholar]
- 22.Aldao, A., Nolen-Hoeksema, S. & Schweizer, S. Emotion-regulation strategies across psychopathology: A meta-analytic review. Clin. Psychol. Rev.30, 217–237. 10.1016/j.cpr.2009.11.004 (2010). 10.1016/j.cpr.2009.11.004 [DOI] [PubMed] [Google Scholar]
- 23.Dan-Glauser, E. S. & Gross, J. J. The temporal dynamics of emotional acceptance: Experience, expression, and physiology. Biol. Psychol.108, 1–12 (2015). 10.1016/j.biopsycho.2015.03.005 [DOI] [PubMed] [Google Scholar]
- 24.Garland, E. L., Gaylord, S. A. & Fredrickson, B. L. Positive reappraisal mediates the stress-reductive effects of mindfulness: An upward spiral process. Mindfulness (N. Y.)2, 59–67 (2011). 10.1007/s12671-011-0043-8 [DOI] [Google Scholar]
- 25.Desrosiers, A., Vine, V., Klemanski, D. H. & Nolen-Hoeksema, S. Mindfulness and emotion regulation in depression and anxiety: Common and distinct mechanisms of action. Depress. Anxiety30, 654–661 (2013). 10.1002/da.22124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peh, C. X. et al. Emotion regulation and emotional distress: The mediating role of hope on reappraisal and anxiety/depression in newly diagnosed cancer patients. Psychooncology26, 1191–1197 (2017). 10.1002/pon.4297 [DOI] [PubMed] [Google Scholar]
- 27.Uchida, M. et al. Emotion regulation ability varies in relation to intrinsic functional brain architecture. Soc. Cogn. Affect. Neurosci.10, 1738–1748 (2014). 10.1093/scan/nsv059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hofmann, S. G., Heering, S., Sawyer, A. T. & Asnaani, A. How to handle anxiety: The effects of reappraisal, acceptance, and suppression strategies on anxious arousal. Behav. Res. Ther.47, 389–394 (2009). 10.1016/j.brat.2009.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ochsner, K. N. & Gross, J. J. The cognitive control of emotion. Trends Cogn Sci9, 242–249. 10.1016/j.tics.2005.03.010 (2005). 10.1016/j.tics.2005.03.010 [DOI] [PubMed] [Google Scholar]
- 30.Lebois, L. A. M. et al. A shift in perspective: Decentering through mindful attention to imagined stressful events. Neuropsychologia75, 505–524 (2015). 10.1016/j.neuropsychologia.2015.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murakami, H. et al. Neural networks for mindfulness and emotion suppression. PLoS One10, e0128005 (2015). 10.1371/journal.pone.0128005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smoski, M. J. et al. Neural indicators of emotion regulation via acceptance vs reappraisal in remitted major depressive disorder. Soc. Cogn. Affect. Neurosci.10, 1187–1194 (2014). 10.1093/scan/nsv003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellard, K. K., Barlow, D. H., Whitfield-Gabrieli, S., Gabrieli, J. D. E. & Deckersbach, T. Neural correlates of emotion acceptance vs worry or suppression in generalized anxiety disorder. Soc. Cogn. Affect. Neurosci.12, 1009–1021 (2017). 10.1093/scan/nsx025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kross, E., Davidson, M., Weber, J. & Ochsner, K. Coping with emotions past: The neural bases of regulating affect associated with negative autobiographical memories. Biol. Psychiatry65, 361–366 (2009). 10.1016/j.biopsych.2008.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Westbrook, C. et al. Mindful attention reduces neural and self-reported cue-induced craving in smokers. Soc. Cogn. Affect. Neurosci.8, 73–84 (2013). 10.1093/scan/nsr076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kober, H., Buhle, J., Weber, J., Ochsner, K. N. & Wager, T. D. Let it be: Mindful acceptance down-regulates pain and negative emotion. Soc. Cogn. Affect. Neurosci.14, 1147–1158 (2019). 10.1093/scan/nsz104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dixon, M. L. et al. Emotion regulation in social anxiety disorder: Reappraisal and acceptance of negative self-beliefs. Biol. Psychiatry Cogn. Neurosci. Neuroimaging5, 119–129 (2020). [DOI] [PubMed] [Google Scholar]
- 38.Messina, I., Grecucci, A. & Viviani, R. Neurobiological models of emotion regulation: A meta-analysis of neuroimaging studies of acceptance as an emotion regulation strategy. Soc. Cogn. Affect. Neurosci.16, 257–267 (2021). 10.1093/scan/nsab007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sezer, I., Pizzagalli, D. A. & Sacchet, M. D. Resting-state fMRI functional connectivity and mindfulness in clinical and non-clinical contexts: A review and synthesis. Neurosci. Biobehav. Rev.135, 104583 (2022). 10.1016/j.neubiorev.2022.104583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grecucci, A., Ghomroudi, P. A., Monachesi, B. & Messina, I. The Neural Signature of Inner Peace: Morphometric Differences between High and Low Accepters.
- 41.Buhle, J. T. et al. Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cereb. Cortex24, 2981–2990 (2014). 10.1093/cercor/bht154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morawetz, C., Bode, S., Baudewig, J., Kirilina, E. & Heekeren, H. R. Changes in effective connectivity between dorsal and ventral prefrontal regions moderate emotion regulation. Cereb. Cortex26, 1923–1937 (2016). 10.1093/cercor/bhv005 [DOI] [PubMed] [Google Scholar]
- 43.Zanella, F., Monachesi, B. & Grecucci, A. Resting-state BOLD temporal variability in sensorimotor and salience networks underlies trait emotional intelligence and explains differences in emotion regulation strategies. Sci. Rep.12, 15163 (2022). 10.1038/s41598-022-19477-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garnefski, N., Kraaij, V. & Spinhoven, P. Negative life events, cognitive emotion regulation and emotional problems. Pers. Individ. Differ.30(8), 1311–1327 (2001). 10.1016/S0191-8869(00)00113-6 [DOI] [Google Scholar]
- 45.Ghomroudi, P. A., Scaltritti, M. & Grecucci, A. Decoding reappraisal and suppression from neural circuits: A combined supervised and unsupervised machine learning approach. Cogn. Affect. Behav. Neurosci.10.3758/s13415-023-01076-6 (2023). 10.3758/s13415-023-01076-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Monachesi, B., Grecucci, A., Ahmadi Ghomroudi, P. & Messina, I. Comparing reappraisal and acceptance strategies to understand the neural architecture of emotion regulation: A meta-analytic approach. Front. Psychol.10.3389/fpsyg.2023.1187092 (2023). 10.3389/fpsyg.2023.1187092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kohl, A., Rief, W. & Glombiewski, J. A. How effective are acceptance strategies? A meta-analytic review of experimental results. J. Behav. Ther. Exp. Psychiatry43, 988–1001 (2012). 10.1016/j.jbtep.2012.03.004 [DOI] [PubMed] [Google Scholar]
- 48.McRae, K. Cognitive emotion regulation: A review of theory and scientific findings. Curr. Opin. Behav. Sci.10, 119–124. 10.1016/j.cobeha.2016.06.004 (2016). 10.1016/j.cobeha.2016.06.004 [DOI] [Google Scholar]
- 49.Lv, H. et al. Resting-state functional MRI: Everything that nonexperts have always wanted to know. Am. J. Neuroradiol.39, 1390–1399 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Biswal, B., Yetkin, F. Z., Haughton, V. M. & Hyde, J. S. Functional connectivity Echo-Planar MRI. Magn. Reson. Med.34, 537–541 (1995). 10.1002/mrm.1910340409 [DOI] [PubMed] [Google Scholar]
- 51.Dörfel, D., Gärtner, A. & Scheffel, C. Resting state cortico-limbic functional connectivity and dispositional use of emotion regulation strategies: A replication and extension study. Front. Behav. Neurosci.14, 128 (2020). 10.3389/fnbeh.2020.00128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bell, A. J. & Sejnowski, T. J. An information-maximisation approach to blind separation and blind deconvolution. Neural Comput.7(6), 1129–1159 (1995). 10.1162/neco.1995.7.6.1129 [DOI] [PubMed] [Google Scholar]
- 53.Calhoun, V. D., Adali, T., Pearlson, G. D. & Pekar, J. J. A method for making group inferences from functional MRI data using independent component analysis. Hum. Brain Mapp.14(3), 140–151. 10.1002/hbm (2001). 10.1002/hbm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Picó-Pérez, M. et al. Dispositional use of emotion regulation strategies and resting-state cortico-limbic functional connectivity. Brain Imaging Behav.12, 1022–1031 (2018). 10.1007/s11682-017-9762-3 [DOI] [PubMed] [Google Scholar]
- 55.Garrett, D. D., Epp, S. M., Perry, A. & Lindenberger, U. Local temporal variability reflects functional integration in the human brain. Neuroimage183, 776–787 (2018). 10.1016/j.neuroimage.2018.08.019 [DOI] [PubMed] [Google Scholar]
- 56.Phillips, M. L., Ladouceur, C. & Drevets, W. C. Automatic and volontary regulation of emotion. Mol. Psychiatry13, 829–857 (2008). 10.1038/mp.2008.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hernández, S. E., Barros-Loscertales, A., Xiao, Y., González-Mora, J. L. & Rubia, K. Gray matter and functional connectivity in anterior cingulate cortex are associated with the state of mental silence during sahaja yoga meditation. Neuroscience371, 395–406 (2018). 10.1016/j.neuroscience.2017.12.017 [DOI] [PubMed] [Google Scholar]
- 58.Yang, L. Z. et al. Electrical stimulation reduces smokers’ craving by modulating the coupling between dorsal lateral prefrontal cortex and parahippocampal gyrus. Soc. Cogn. Affect. Neurosci.12, 1296–1302 (2017). 10.1093/scan/nsx055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Katz, B., Adams, R. D. & Victor, M. Principles of neurology. J. Neuro-Ophthalmol.16, 75 (1996). 10.1097/00041327-199603000-00095 [DOI] [Google Scholar]
- 60.Zeidan, F. et al. Brain mechanisms supporting the modulation of pain by mindfulness meditation. J. Neurosci.31, 5540–5548 (2011). 10.1523/JNEUROSCI.5791-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Frank, D. W. et al. Emotion regulation: Quantitative meta-analysis of functional activation and deactivation. Neurosci. Biobehav. Rev.45, 202–211. 10.1016/j.neubiorev.2014.06.010 (2014). 10.1016/j.neubiorev.2014.06.010 [DOI] [PubMed] [Google Scholar]
- 62.Morawetz, C., Bode, S., Derntl, B. & Heekeren, H. R. The effect of strategies, goals and stimulus material on the neural mechanisms of emotion regulation: A meta-analysis of fMRI studies. Neurosci. Biobehav. Rev.72, 111–128. 10.1016/j.neubiorev.2016.11.014 (2017). 10.1016/j.neubiorev.2016.11.014 [DOI] [PubMed] [Google Scholar]
- 63.Grecucci, A., Giorgetta, C., Bonini, N. & Sanfey, A. G. Living emotions, avoiding emotions: Behavioral investigation of the regulation of socially driven emotions. Front. Psychol.3, 1–14 (2013). 10.3389/fpsyg.2012.00616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grecucci, A., Giorgetta, C., Van’tWout, M., Bonini, N. & Sanfey, A. G. Reappraising the ultimatum: An fMRI study of emotion regulation and decision making. Cereb. Cortex23, 399–410 (2013). 10.1093/cercor/bhs028 [DOI] [PubMed] [Google Scholar]
- 65.Kozasa, E. H. et al. Meditation training increases brain efficiency in an attention task. Neuroimage59, 745–749 (2012). 10.1016/j.neuroimage.2011.06.088 [DOI] [PubMed] [Google Scholar]
- 66.Craig, A. D. How do you feel? Interoception: The sense of the physiological condition of the body. Nat. Rev. Neurosci.3, 655–656 (2002). 10.1038/nrn894 [DOI] [PubMed] [Google Scholar]
- 67.Khalsa, S. S., Rudrauf, D., Feinstein, J. S. & Tranel, D. The pathways of interoceptive awareness. Nat. Neurosci.12, 1494–1496 (2009). 10.1038/nn.2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Min, J. et al. Emotion downregulation targets interoceptive brain regions while emotion upregulation targets other affective brain regions. J. Neurosci.42, 2973–2985 (2022). 10.1523/JNEUROSCI.1865-21.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Farb, N. A. S., Anderson, A. K. & Segal, Z. V. The mindful brain and emotion regulation in mood disorders. Can. J. Psychiatry57, 70–77 (2012). 10.1177/070674371205700203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parkinson, T. D., Kornelsen, J. & Smith, S. D. Trait mindfulness and functional connectivity in cognitive and attentional resting state networks. Front. Hum. Neurosci.13, 112 (2019). 10.3389/fnhum.2019.00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ochsner, K. N., Silvers, J. A. & Buhle, J. T. Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Ann. N. Y. Acad. Sci.1251, E1–E24. 10.1111/j.1749-6632.2012.06751.x (2012). 10.1111/j.1749-6632.2012.06751.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kohn, N. et al. Neural network of cognitive emotion regulation—An ALE meta-analysis and MACM analysis. Neuroimage87, 345–355 (2014). 10.1016/j.neuroimage.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Messina, I., Bianco, S., Sambin, M. & Viviani, R. Executive and semantic processes in reappraisal of negative stimuli: Insights from a meta-analysis of neuroimaging studies. Front. Psychol.6, 956 (2015). 10.3389/fpsyg.2015.00956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li, W. et al. Differential involvement of frontoparietal network and insula cortex in emotion regulation. Neuropsychologia161, 107991 (2021). 10.1016/j.neuropsychologia.2021.107991 [DOI] [PubMed] [Google Scholar]
- 75.Craig, A. D. Emotional moments across time: A possible neural basis for time perception in the anterior insula. Philos. Trans. R. Soc. B Biol. Sci.364, 1933–1942. 10.1098/rstb.2009.0008 (2009). 10.1098/rstb.2009.0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zaki, J., Davis, J. I. & Ochsner, K. N. Overlapping activity in anterior insula during interoception and emotional experience. Neuroimage62, 493–499 (2012). 10.1016/j.neuroimage.2012.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.MacDonald, D. D., Ingersoll, C. G. & Berger, T. A. Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch. Environ. Contam. Toxicol.39, 20–31 (2000). 10.1007/s002440010075 [DOI] [PubMed] [Google Scholar]
- 78.Curtis, C. E. & D’Esposito, M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn. Sci.7, 415–423 (2003). 10.1016/S1364-6613(03)00197-9 [DOI] [PubMed] [Google Scholar]
- 79.Hare, T. A., Camerer, C. F. & Rangel, A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science 324, 646–648 (2009). 10.1126/science.1168450 [DOI] [PubMed] [Google Scholar]
- 80.Bush, G., Luu, P. & Posner, M. I. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci.10.1016/S1364-6613(00)01483-2 (2000). 10.1016/S1364-6613(00)01483-2 [DOI] [PubMed] [Google Scholar]
- 81.Stevens, F. L., Hurley, R. A. & Taber, K. H. Anterior cingulate cortex: Unique role in cognition and emotion. J. Neuropsychiatry Clin. Neurosci.s23, 121–125 (2011). 10.1176/jnp.23.2.jnp121 [DOI] [PubMed] [Google Scholar]
- 82.Piretti, L. et al. Impaired processing of conspecifics in Parkinson’s disease. Appl. Neuropsychol. Adult10.1080/23279095.2022.2074299 (2022). 10.1080/23279095.2022.2074299 [DOI] [PubMed] [Google Scholar]
- 83.Bilevicius, E., Smith, S. D. & Kornelsen, J. Resting-state network functional connectivity patterns associated with the mindful attention awareness scale. Brain Connect.8, 40–48 (2018). 10.1089/brain.2017.0520 [DOI] [PubMed] [Google Scholar]
- 84.Goulas, A., Uylings, H. B. M. & Stiers, P. Unravelling the intrinsic functional organization of the human lateral frontal cortex: A parcellation scheme based on resting state fMRI. J. Neurosci.32, 10238–10252 (2012). 10.1523/JNEUROSCI.5852-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grecucci, A., Rubicondo, D., Siugzdaite, R., Surian, L. & Job, R. Uncovering the social deficits in the autistic brain. A source-based morphometric study. Front. Neurosci.10, 388 (2016). 10.3389/fnins.2016.00388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bzdok, D., Laird, A. R., Zilles, K., Fox, P. T. & Eickhoff, S. B. An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Hum. Brain Mapp.34, 3247–3266 (2013). 10.1002/hbm.22138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cole, M. W. & Schneider, W. The cognitive control network: Integrated cortical regions with dissociable functions. Neuroimage37, 343–360 (2007). 10.1016/j.neuroimage.2007.03.071 [DOI] [PubMed] [Google Scholar]
- 88.Lückmann, H. C., Jacobs, H. I. L. & Sack, A. T. The cross-functional role of frontoparietal regions in cognition: Internal attention as the overarching mechanism. Prog. Neurobiol.10.1016/j.pneurobio.2014.02.002 (2014). 10.1016/j.pneurobio.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 89.Corbetta, M. & Shulman, G. L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci.3, 201–215 (2002). 10.1038/nrn755 [DOI] [PubMed] [Google Scholar]
- 90.Shenhav, A., Botvinick, M. M. & Cohen, J. D. The expected value of control: An integrative theory of anterior cingulate cortex function. Neuron10.1016/j.neuron.2013.07.007 (2013). 10.1016/j.neuron.2013.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Füstös, J., Gramann, K., Herbert, B. M. & Pollatos, O. On the embodiment of emotion regulation: Interoceptive awareness facilitates reappraisal. Soc. Cogn. Affect. Neurosci.8, 911–917 (2013). 10.1093/scan/nss089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kropf, E., Syan, S. K., Minuzzi, L. & Frey, B. N. From anatomy to function: The role of the somatosensory cortex in emotional regulation. Braz. J. Psychiatry41, 261–269 (2019). 10.1590/1516-4446-2018-0183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Critchley, H. D., Wiens, S., Rotshtein, P., Öhman, A. & Dolan, R. J. Neural systems supporting interoceptive awareness. Nat. Neurosci.7, 189–195 (2004). 10.1038/nn1176 [DOI] [PubMed] [Google Scholar]
- 94.Damasio, A. R. et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat. Neurosci.3, 1049–1056 (2000). 10.1038/79871 [DOI] [PubMed] [Google Scholar]
- 95.Babayan, A. et al. Data descriptor: A mind-brain-body dataset of MRI, EEG, cognition, emotion, and peripheral physiology in young and old adults. Sci. Data6, 1–21 (2019). 10.1038/sdata.2018.308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Loch, N., Hiller, W. & Witthöft, M. D. cognitive emotion regulation questionnaire (CERQ) erste teststatistische überprüfung einer deutschen adaption. Z Klin Psychol Psychother40, 94–106 (2011). 10.1026/1616-3443/a000079 [DOI] [Google Scholar]
- 97.Elseoud, A. A. et al. Group-ICA model order highlights patterns of functional brain connectivity. Front. Syst. Neurosci.10.3389/fnsys.2011.00037 (2011). 10.3389/fnsys.2011.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Saviola, F. et al. Trait and state anxiety are mapped differently in the human brain. Sci. Rep.10, 1–11 (2020). 10.1038/s41598-020-68008-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The complete Data of this study is accessible through Gesellschaft für wissenschaftliche Datenverarbeitung mbH Göttingen (GWDG) at https://www.gwdg.de/. Both raw and preprocessed data can be accessed via web browser (https://ftp.gwdg.de/pub/misc/MPI-Leipzig_Mind-Brain-Body-LEMON/) or through a fast FTP connection (ftp://ftp.gwdg.de/pub/misc/MPI-Leipzig_Mind-Brain-Body-LEMON/). In the event of any future changes in the data’s location, the dataset can be located using PID 21.11101/0000-0007-C379-5 (e.g., http://hdl.handle.net/21.11101/0000-0007-C379-5).