Abstract
We conducted this cross-sectional study to investigate the independent associations between lipid metabolites and osteoporotic fractures among participants aged 40–69 years from the UK Biobank. Serum lipid, lipoprotein levels and nuclear magnetic resonance (NMR) based metabolic biomarkers were measured at the baseline. We conducted multivariable logistic analyses to investigate potential independent associations between concentrations of lipid metabolites and osteoporotic fractures in both men and women. The odds ratios (ORs) for lipid metabolites were calculated based on their lowest tertile. Over a median follow-up period of 15 years, a total of 978 men and 4515 women were diagnosed with osteoporosis, whereas 138 men and 327 women encountered incident fractures. Statistically significant disparities were identified in NMR-based metabolic biomarkers among men and women with incident fractures compared to those without. Out of the 144 distinct lipid metabolites known, 35 exhibited significant associations with incident fractures in patients diagnosed with osteoporosis. Following the adjustment for confounding factors, degree of unsaturation (p = 0.0066) and docosahexaenoic acids (p = 0.0011) in male patients increased the risk of incident fractures. And high level of different metabolites of HDL (p = 0.0153), 3-Hydroxybutyrate (p = 0.0012) and Sphingomyelins (p = 0.0036) decreased the risk of incident fractures in female patients. This outcome indicates that these identified lipid metabolites may potentially have unique roles in independently contributing to the occurrence of osteoporotic fractures.
Keywords: Lipid metabolites, Osteoporotic fractures, NMR-based metabolic biomarkers, Osteoporosis, UK biobank
Subject terms: Endocrinology, Risk factors
Introduction
Osteoporosis is a systemic skeletal disease characterized by reduced bone mass and the deterioration of bone microarchitecture1. This results in increased bone fragility and a higher risk of fractures compared to the general population, and numerous guidelines specifically address the heightened fracture risk associated with osteoporosis2. Osteoporosis-related fractures are a significant health concern, particularly among the elderly, especially post-menopausal women. Aside from imposing substantial social and economic burdens, these fractures are associated with an increased risk of morbidity and mortality3,4. it's important to note that not all osteoporosis patients will experience fractures. Various factors, including genetics, hormones, and environmental elements, may contribute to the occurrence of osteoporotic fractures5–7.
Lipid metabolites, widely distributed throughout the human body, play a crucial role in numerous metabolic pathways, including bone metabolism8. Serving as an essential component of bone by providing cellular structure and regulating signal pathways in bone remodeling9, alterations in lipid metabolism can lead to significant disruptions in the bone microenvironment10,11. Cholesterol and fatty acids interact with osteoclasts in various ways, including its dysregulation of osteoclast differentiation and inhibition of apoptosis at high concentrations12,13. Previous studies have reported that glucocorticoid stimulation leads to adipocyte aggregation, increased cholesterol levels and decreased bone mineral density in Osteoporosis mice14. Another study found that bone mineral density and serum osteogenic markers significantly decreased and bone resorption markers increased in mice fed with high cholesterol diet15. Increasing evidence suggests a connection between lipid metabolism and osteoporosis, as well as bone fractures. Several studies have indicated a positive correlation between total cholesterol (TC)16,17, low-density lipoprotein (LDL)18, triglyceride (TG)19, polyunsaturated fatty acids20, and risk of fracture. Lipid metabolism reprogramming may change the composition of bone microenvironment in patients with osteoporosis, leading to the occurrence of fractures. Previous studies on lipid metabolites and osteoporotic fractures have small sample size, or highly selected populations, such as postmenopausal women, or lack of comprehensive lipid metabolites. More importantly, some reports show that lipid metabolites do not perform clinical significance in osteoporotic fractures21–23. Therefore, the current evidence from studies remains inconclusive, further studies are needed to clarify the relationship between lipid metabolites (especially serum lipid and lipoprotein) and osteoporotic fractures.
However, there is no lipid metabolism that can well predict the risk of fracture in patients with osteoporosis. To address this, we conducted this study using the detailed serum lipid measurements data from 502,490 participants in the UK Biobank. This study aimed to investigate the associations between lipid metabolites and the risk of osteoporotic fractures within the osteoporosis population.
Methods
Setting and recruitment
The UK Biobank is a prospective population-based cohort study of more than 502,639 participants aged 40–69 years, recruited at 22 assessment centers across the UK between 2006 and 201024. During the baseline visit, participants who consented to participate completed self-administered touch-screen questionnaires covering various aspects such as sociodemographic factors, family history, early life exposure, psychosocial factors, environmental factors, lifestyle, and health status. Additionally, physical measurements were taken, and blood samples were collected at the assessment centers25.
Ethics
This analysis obtained approval from the National Information Governance Board for Health and Social Care as well as the National Health Service North West Multicenter Research Ethics Committee (reference 13/NW/0382) and all methods were performed in accordance with the relevant guidelines26,27. All participants provided informed consent by electronically signing at the initial assessment. The study protocol is available online (http://www.ukbiobank.ac.uk/).
Assessment of population characteristics
Characteristics of study participants were collected at baseline, including sociodemographic variables such as age, sex, race, education level, employment status, and income. Physical examination variables such as body mass index (BMI), systolic and diastolic pressure were also recorded, along with lifestyle factors such as physical activity, diet, alcohol consumption, and smoking status. The gender data included both information from the National Health Service records and self-reported gender from the participants. Age was calculated based on the date of birth and the date of the initial assessment. Ethnicity, education level, and lifestyle data were obtained through a touch-screen questionnaire.
Assessment of exposures
Serum lipid and lipoprotein levels (mmol/L), including TC (Total Cholesterol), TG (Triglyceride), HDL (High-density Lipoprotein), LDL (Low-density Lipoprotein), ApoA (Apolipoprotein A), ApoB (Apolipoprotein B), as well as fasting blood glucose, Hb, Ca, P, Vitamin D, Oestradiol, SHBG (Sex Hormone Binding Globulin), and testosterone, were considered as the lipid metabolites and measured through biochemical assays from blood samples collected at the baseline, utilizing the Bechman Coulter AU5800 platform. The data obtained were stored and made available in the UK Biobank for further analysis. The handling and storage protocols for the biological samples underwent rigorous development, involving extensive consultation and peer review within the scientific community, followed by thorough validation28. Moreover, NMR (nuclear magnetic resonance)-based metabolic biomarkers were measured by nuclear magnetic resonance spectroscopy which included 118,461 baseline plasma samples, generated by Nightingale Health Plc, NMR-based metabolic biomarker comprises 249 measures of lipids and metabolites29,30. These data are now available to approved researchers through the UK Biobank for use in all aspects of public health31,32.
Assessment of incident osteoporotic fracture
Osteoporosis was defined using the International Classification of Diseases, Tenth Revision (ICD-10) code M80, M81 and M82. The inclusion criteria for incident osteoporotic fractures were based on hospital admissions and primary care diagnoses as their initial nontraumatic fracture. To ensure adequate statistical power, any fracture occurring in skeletal locations, excluding the toe, digit, or face, was recorded as an outcome measure. Exclusion criteria for incident osteoporotic fractures involved fractures attributed to cancer (ICD-10 code C00-D49) or accidents (ICD-10 code Z87.81), such as motor vehicle accidents, which were considered non-osteoporotic and thus excluded from the analysis.
The follow-up period was calculated from the time of enrollment until the occurrence of the first fracture (no prior fractures at baseline), death, or the planned end of the follow-up period (2019), whichever happened first. In cases where subjects were lost to the study during the follow-up, we determined the follow-up period as the time from enrollment to the date of their last contact with the subject. This approach allowed us to accurately assess the occurrence of osteoporotic fractures and ensure the validity of our findings.
Statistical analysis
Baseline characteristics were delineated for the entire population, as well as separately for individuals with osteoporotic fractures (OPF cases) and those with non-osteoporotic fractures (non-OPF cases). Continuous data were expressed as mean (standard deviation, SD). For normally distributed variables, significance between two groups was assessed using Student's t-tests, while log-transformed Student's t-tests were employed for non-normally distributed variables. Marginal means (95% CIs) were calculated using a generalized linear model adjusted for age, sex, and BMI. The distribution of lipid metabolite concentrations was divided into tertiles, categorizing subjects into low (tertile 1), median (tertile 2), and high (tertile 3) levels. Multivariable logistic analyses were conducted to determine whether lipid metabolite concentrations were independently associated with osteoporotic fractures among men and women, respectively. Several models were constructed to adjust for confounding factors (age, ethnicity background, education, average total household income, employment status, smoking status, alcohol consumption and physical activity), and odds ratios (ORs) for lipid metabolites were calculated with the lowest tertile as the reference. All analyses were performed using R software, version 4.0.3, and a two-tailed P-value less than 0.01 was considered statistically significant.
Results
Over an average follow-up period of 15 years (1297 participants lost to follow-up), 978 men and 4515 women developed osteoporosis, while incident fractures occurred in 138 men and 327 women. Table 1 presents the baseline characteristics of the participants. Supplementary table showed multi-collinearity for confounding factors. VIF of the covariates less than 10 or more strictly less than 4 means that there is no multicollinearity.
Table 1.
Baseline characteristics of the patients with osteoporosis divided by gender.
| Male | Female | |
|---|---|---|
| N = 978 | N = 4515 | |
| Age | 61.59 (6.78) | 61.71 (6.12) |
| Ethnic | ||
| White (%) | 932 (95.3%) | 4216 (93.4%) |
| Non-white (%) | 46 (4.7%) | 299 (6.6%) |
| Edu: | ||
| College or University degree | 247 (25.2%) | 1181 (26.2%) |
| A levels/AS levels or equivalent | 98 (10.0%) | 443 (9.8%) |
| O levels/GCSEs or equivalent | 164 (16.8%) | 995 (22.0%) |
| Others | 469 (48.0%) | 1896 (42.0%) |
| Income | ||
| Less than 18,000 | 378 (38.7%) | 1580 (35.0%) |
| 18,000 to 30,999 | 266 (27.2%) | 1291 (28.6%) |
| 31,000 to 51,999 | 182 (18.6%) | 955 (21.2%) |
| 52,000 to 100,000 | 123 (12.5%) | 550 (12.2%) |
| Greater than 100,000 | 29 (3.0%) | 139 (3.0%) |
| Employ status | ||
| Paid job | 312 (31.9%) | 1488 (33.0%) |
| Retired | 483 (49.4%) | 2533 (56.1%) |
| Unpaid job | 183 (18.7%) | 494 (10.9%) |
| Smoking status | ||
| Never | 361 (36.9%) | 2551 (56.5%) |
| Previous | 441 (45.1%) | 1528 (33.8%) |
| Current | 176 (18.0%) | 436 (9.7%) |
| Alcohol frequency | ||
| Daily or almost daily | 265 (27.1%) | 735 (16.3%) |
| Three or four times a week | 192 (19.6%) | 783 (17.3%) |
| Once or twice a week | 204 (20.9%) | 1088 (24.1%) |
| One to three times a month | 91 (9.3%) | 509 (11.3%) |
| Special occasions only | 98 (10.0%) | 763 (16.9%) |
| Never | 128 (13.1%) | 637 (14.1%) |
| Physical activity | ||
| Low | 238 (24.3%) | 882 (19.5%) |
| Moderate | 384 (39.3%) | 1887 (41.8%) |
| High | 356 (36.4%) | 1746 (38.7%) |
| BMI (kg/m2) | 27.09 (5.02) | 25.97 (4.87) |
| SBP (mmHg) | 140.95 (18.51) | 137.36 (18.86) |
| DBP (mmHg) | 82.53 (10.42) | 80.00 (9.80) |
| FBG (mmol/L) | 5.28 (1.66) | 5.11 (1.08) |
| HbA1C, % | 5.55 (0.75) | 5.48 (0.56) |
| TC (mmol/L) | 5.26 (1.12) | 5.92 (1.13) |
| TG (mmol/L) | 1.85 (1.15) | 1.56 (0.81) |
| HDL cholesterol (mmol/L) | 1.33 (0.35) | 1.63 (0.39) |
| LDL cholesterol (mmol/L) | 3.26 (0.85) | 3.64 (0.88) |
| Ca (mmol/L) | 2.36 (0.11) | 2.40 (0.11) |
| P (mmol/L) | 1.13 (0.17) | 1.21 (0.15) |
| VitaminD (nmol/L) | 49.23 (22.96) | 52.70 (22.69) |
| Oestradiol (pmol/L) | 263.83 (159.81) | 428.54 (357.75) |
| SHBG (nmol/L) | 48.40 (24.19) | 65.94 (31.87) |
| Testosterone (nmol/L) | 11.70 (4.88) | 1.64 (2.65) |
Table 2 presents the concentration of NMR-based metabolic biomarkers in osteoporosis patients, both with incident fracture and free of fracture, categorized by sex. Of the 144 kinds of known lipid metabolites, 35 showed significant associations with incident fractures in patients with osteoporosis, but there were notable differences between male and female patients.
Table 2.
Marginal means of NMR-based metabolic biomarkers in osteoporosis patients with and without incident fracture, divided by gender.
| Male | Female | |||||
|---|---|---|---|---|---|---|
| Osteoporosis without fracture N = 840 | Osteoporosis with fracture N = 138 | p value | Osteoporosis without fracture N = 4188 | Osteoporosis with fracture N = 327 | p value | |
| Free Cholesterol in HDL | 0.25 (0.22, 0.30) | 0.25 (0.23, 0.30) | 0.576 | 0.32 (0.27, 0.37) | 0.32 (0.28, 0.38) | 0.149 |
| Total Lipids in HDL | 2.69 (2.36, 3.12) | 2.67 (2.41, 3.12) | 0.510 | 3.23 (2.83, 3.66) | 3.22 (2.86, 3.81) | 0.199 |
| Total Cholines | 2.34 (2.06, 2.60) | 2.38 (2.15, 2.65) | 0.185 | 2.68 (2.43, 2.93) | 2.69 (2.44, 2.98) | 0.469 |
| Sphingomyelins | 0.41 (0.36, 0.45) | 0.41(0.37, 0.46) | 0.238 | 0.47 (0.42, 0.52) | 0.47 (0.43, 0.52) | 0.270 |
| Degree of Unsaturation | 1.33 (1.28, 1.39) | 1.32 (1.27, 1.37) | 0.118 | 1.39 (1.34, 1.44) | 1.38 (1.33, 1.43) | 0.280 |
| Polyunsaturated Fatty Acids to Total Fatty Acids percentage | 42.27 (38.86, 44.64) | 41.32 (38.19, 44.05) | 0.142 | 43.59 (41.17, 45.55) | 43.29 (41.07, 45.23) | 0.138 |
| Histidine | 0.06 (0.06, 0.07) | 0.06 (0.06, 0.07) | 0.400 | 0.06 (0.06, 0.07) | 0.06 (0.06, 0.07) | 0.114 |
| 3-Hydroxybutyrate | 0.04 (0.03, 0.07) | 0.05 (0.03, 0.08) | 0.598 | 0.04 (0.03, 0.08) | 0.05 (0.03, 0.09) | 0.001 |
| Acetone | 0.01 (0.01, 0.02) | 0.01 (0.01, 0.02) | 0.408 | 0.01 (0.01, 0.02) | 0.01 (0.01, 0.02) | 0.003 |
| Total Lipids in Medium HDL | 0.93 (0.81, 1.08) | 0.94 (0.82, 1.08) | 0.352 | 1.10 (0.96, 1.24) | 1.10 (0.97, 1.27) | 0.268 |
| Phospholipids in Medium HDL | 0.44 (0.39, 0.51) | 0.45 (0.40, 0.51) | 0.293 | 0.51 (0.45, 0.58) | 0.51 (0.458, 0.60) | 0.273 |
| Cholesteryl Esters in Large HDL | 0.17 (0.11, 0.26) | 0.16 (0.12, 0.24) | 0.844 | 0.27 (0.19, 0.38) | 0.28 (0.18, 0.39) | 0.273 |
| Triglycerides in Small LDL | 0.01 (0.01, 0.02) | 0.01 (0.01, 0.02) | 0.470 | 0.01 (0.01, 0.02) | 0.01 (0.01, 0.02) | 0.558 |
| Phospholipids in Very Large HDL | 0.06 (0.04, 0.09) | 0.06 (0.05, 0.08) | 0.604 | 0.09 (0.07, 0.13) | 0.09 (0.07, 0.13) | 0.476 |
| Concentration of Large HDL Particles | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.970 | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.248 |
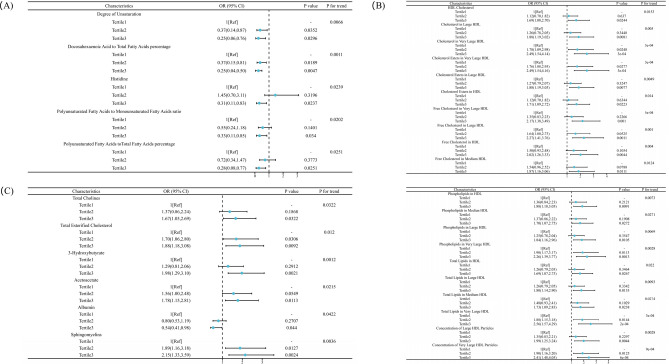
From the logistic regression model, which was adjusted for age, ethnicity background, education, average total household income, employment status, smoking incident, alcohol frequency, and physical activity, 2 metabolites were found to be inversely associated with the risk of incident fractures in male patients. These metabolites include Degree of Unsaturation (0.25 (0.06, 0.76)), Docosahexaenoic Acids to Total Fatty Acids percentage (0.17 (0.04, 0.50)) (Fig. 1A). In contrast, no metabolites were positively associated in male patients. In female patients, 16 lipid metabolites were found to be positively associated with the risk of incident fractures, include 14 different metabolites of HDL, 3-Hydroxybutyrate (1.98 (1.29, 3.10)) and Sphingomyelins (2.15 (1.33, 3.59)) (Fig. 1B,C). Conversely, no metabolites were inversely associated with the risk of incident fractures in female patients. Furthermore, there was no correlation found between total cholesterol and the risk of fractures.
Figure 1.
(A) Association between lipid metabolites as measured by nuclear magnetic resonance spectroscopy and the incident fracture in male patients with osteoporosis. Association between lipid metabolites as measured by nuclear magnetic resonance spectroscopy and the incident fracture in female patients with osteoporosis. (B) Adjusted odds ratios (95% confidence interval) for HDL in female patients. (C) Adjusted odds ratios (95% confidence interval) for other lipid metabolites in female patients.
Discussion
Our study conducted within the osteoporosis population using data from the UK Biobank, identified 35 out of 144 lipid metabolites associated with fractures related to osteoporosis. We adjust for age, ethnic background, education, average total household income, employment status, smoking events, alcohol consumption frequency, and physical activity. Abnormal lipid metabolism can cause osteoporosis by changing physiological hormone secretion, making the bony microenvironment of obese patients more disordered. In this study, the lipid composition profiles of different sexes were identified. Importantly, unsaturation and docosahexaenoic acids in osteoporotic male patients increased the risk of incident fractures. Conversely, HDL, 3-Hydroxybutyrate and Sphingomyelins can prevent fractures in osteoporotic female patients. These results suggested that these lipid metabolites may be involved in the occurrence of osteoporotic fractures independently.
The association between lipid metabolites and the risk of osteoporotic fractures has been investigated in various population-based studies. However, the results from these studies have been highly inconsistent. According to a 20-year follow-up population-based random study involving 1,396 participants (53% women), the findings indicate that serum total cholesterol is an autonomous risk factor for osteoporotic fractures, and its predictive ability strengthens over time. Elevated serum total cholesterol levels are associated with a long-term risk of osteoporotic fractures17. A Retrospective Study involving 1158 older patients with T2DM revealed that HDL-C is a protective factor for osteoporosis (OP) in both men and women, and LDL-C was found to be an independent predictor of OP specifically in postmenopausal women33. In a 13-year prospective, longitudinal study involving multiethnic women, it was observed that midlife women with high fasting plasma triglycerides (TG) faced an elevated risk of incident nontraumatic fractures. Specifically, midlife women with fasting plasma TG levels of at least 300 mg/dl had a 2.5-fold greater risk of fracture starting from 2 years later and onward, compared to those with TG levels below 150 mg/dl, within the multiethnic cohort. Time-varying analyses yielded similar outcomes19. Part of polyunsaturated fatty acids were typically pro-inflammatory and have been associated with an increased fracture risk34. Histidine metabolism and biosynthesis of unsaturated fatty acids were the most common metabolic pathways dysregulated in low bone mineral density patient, resulting in decreased bone mineral density (BMD) and a subsequent increase in fracture risk35. Additionally, several studies have also found the association between lipid metabolites and osteoporotic fractures18,36–38. As far as we know, previous observational studies have been limited to the impact of certain types of fatty acid metabolites on the occurrence of fractures in patients with osteoporosis, or to specific subtypes of people, such as postmenopausal women and elderly patients with type 2 diabetes. Due to this, it is not surprising that the previous studies are controversial.
Conversely, some studies vowed that there was no or even negative correlation between blood lipids and the risk of fracture. A Systematic Review and Meta-Analysis showed plasma levels of total cholesterol were positively associated with bone fractures. However, no significant association was found between plasma level of TG and LDL with the risk of bone fractures in either prospective or cross-sectional studies16. A South Korean study included 107 postmenopausal women aged 45 to 79 and found no association between blood lipid profiles and BMD22. Another research covered 958 postmenopausal Korean women showed patients with vertebrae fractures had lower levels of TC, TG, LDL-C than the patients without vertebrae fractures39. Docosahexaenoic acids could improve bone quality probably by preventing bone decay and augmenting bone mineralization40. Possible reasons for these differences could be as follows: (1) studies may have been limited by its research methods, potentially failing to encompass a comprehensive metabolomic analysis of lipid metabolites, consequently leaving numerous lipid components not fully explicated. (2) Several studies might have relied on small sample sizes. (3) Studies based on lipid metabolites and the risk of osteoporotic fractures are usually retrospective and have methodological limitation.
The vast discrepancy of various studies may be attributed to multiple complex confounders such as sex and gender, etc. We employed NMR metabolomics and identified lipid metabolites associated with osteoporotic fractures occurrence in both male and female patients. The risk of incident fractures in males increased with the degree of unsaturation, histidine, and polyunsaturated fatty acids, while these metabolites decreased the risk of incident fractures in females. In females, lipid metabolism molecules, primarily HDL, play a protective role in osteoporotic fracture. Interestingly, docosahexaenoic acids were found to increase the risk of fractures in males, which contrasts with existing literature.
The precise mechanisms through which these molecules contribute to fractures in osteoporosis remain unclear. Prolonged osteoporosis leads to the accumulation of triglycerides, diminished levels of arachidonic and docosahexaenoic acids, elevated stearoyl-CoA desaturase indices, and decreased sphingomyelin in the mineralized tissue. This disrupts the equilibrium between bone resorption and formation, with bone resorption surpassing bone formation. Potential pathways for this phenomenon include interactions with the Wnt signaling pathway via lipoprotein receptors, involvement of PPARγ2, and modulation of the RANKL/RANK/OPG pathway41–43. However, we are still at an early stage of understanding the roles of lipids in the OP development and more investigations will be necessary.
The study had limitations due to its cross-sectional design, making it challenging to establish causal relationships. Second, the samples were not further substratified according to age, which was believed to be an important risk factor of osteoporotic fractures based on previous studies. However, the effect on our analyses could be small due to the strength of the large sample size. Additionally, the majority of participants were of Caucasian ethnicity, necessitating further exploration in other ethnic groups. Lastly, the exact mechanism underlying the causality between them was not explored in-depth. Therefore, a mechanistic research should be carried out in the future.
Conclusion
Our research highlights that metabolomic profiles derived from NMR can reveal numerous potential small lipid metabolites linked to osteoporotic fractures. We found that an increased degree of unsaturation and docosahexaenoic acids in male patients raised the risk of incident fractures. Conversely, various metabolites of HDL, 3-Hydroxybutyrate and Sphingomyelins decreased the risk of incident fractures in female patients. This finding suggests that the molecules mentioned above might play distinct roles in contributing to the occurrence of osteoporotic fractures independently.
Supplementary Information
Acknowledgements
We extend our sincere appreciation to Dr. Jinbo Hu from the Department of Endocrinology at the First Affiliated Hospital of Chongqing Medical University for their significant contributions to data curation and formal analysis.
Author contributions
Lan Shao: Conceptualization; investigation; methodology; project administration; writing-original draft; writing—review and editing. Shengjun Luo : Conceptualization; funding acquisition; methodology; project administration; supervision; writing-original draft. Zenghui Zhao: Data curation; formal analysis; funding acquisition; investigation; methodology; writing-original draft; writing- review and editing.
Funding
This research received foundation of Chongqing Bureau of Science and Technology (China), Number: CSTB2022BSXM-JCX0040.
Data availability
All data are available via an access application to UK Biobank. https://www.ukbiobank.ac.uk/enable-your-research.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-69594-y.
References
- 1.Gregson, C. L. et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch. Osteoporos.17(1), 58 (2022). 10.1007/s11657-022-01061-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rentzeperi, E. et al. Diagnosis and management of osteoporosis: A comprehensive review of guidelines. Obstet. Gynecol. Surv.78(11), 657–681 (2023). 10.1097/OGX.0000000000001181 [DOI] [PubMed] [Google Scholar]
- 3.Bliuc, D. et al. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. Jama301(5), 513–521 (2009). 10.1001/jama.2009.50 [DOI] [PubMed] [Google Scholar]
- 4.Melton, L. J. 3rd. Adverse outcomes of osteoporotic fractures in the general population. J. Bone Miner. Res.18(6), 1139–1141 (2003). 10.1359/jbmr.2003.18.6.1139 [DOI] [PubMed] [Google Scholar]
- 5.Mozaffari, H., Daneshzad, E. & Azadbakht, L. Dietary carbohydrate intake and risk of bone fracture: A systematic review and meta-analysis of observational studies. Public Health181, 102–109 (2020). 10.1016/j.puhe.2019.12.001 [DOI] [PubMed] [Google Scholar]
- 6.Alghadir, A. H., Gabr, S. A. & Al-Eisa, E. Physical activity and lifestyle effects on bone mineral density among young adults: Sociodemographic and biochemical analysis. J. Phys. Ther. Sci.27(7), 2261–2270 (2015). 10.1589/jpts.27.2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallin, M. et al. Low-level cadmium exposure is associated with decreased bone mineral density and increased risk of incident fractures in elderly men: The MrOS Sweden study. J. Bone Miner. Res.31(4), 732–741 (2016). 10.1002/jbmr.2743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, L. et al. Regulation of glucose and lipid metabolism in health and disease. Sci. China Life Sci.62(11), 1420–1458 (2019). 10.1007/s11427-019-1563-3 [DOI] [PubMed] [Google Scholar]
- 9.Tintut, Y. & Demer, L. L. Effects of bioactive lipids and lipoproteins on bone. Trends Endocrinol. Metab.25(2), 53–59 (2014). 10.1016/j.tem.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang, B., Wang, H., Li, Y. & Song, L. Lipid metabolism within the bone micro-environment is closely associated with bone metabolism in physiological and pathophysiological stages. Lipids Health Dis.21(1), 5 (2022). 10.1186/s12944-021-01615-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim, H., Oh, B. & Park-Min, K. H. Regulation of osteoclast differentiation and activity by lipid metabolism. Cells10(1), 89 (2021). 10.3390/cells10010089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou, C., Luan, L. & Ren, C. Oxidized low-density lipoprotein promotes osteoclast differentiation from CD68 positive mononuclear cells by regulating HMGB1 release. Biochem. Biophys. Res. Commun.495(1), 1356–1362 (2018). 10.1016/j.bbrc.2017.11.083 [DOI] [PubMed] [Google Scholar]
- 13.Yin, W., Li, Z. & Zhang, W. Modulation of bone and marrow niche by cholesterol. Nutrients11(6), 1394 (2019). 10.3390/nu11061394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao, W. et al. Glucocorticoid excess in mice results in early activation of osteoclastogenesis and adipogenesis and prolonged suppression of osteogenesis: A longitudinal study of gene expression in bone tissue from glucocorticoid-treated mice. Arthritis Rheum.58(6), 1674–1686 (2008). 10.1002/art.23454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.You, L. et al. High cholesterol diet increases osteoporosis risk via inhibiting bone formation in rats. Acta Pharmacol. Sin.32(12), 1498–1504 (2011). 10.1038/aps.2011.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghorabi, S. et al. Lipid profile and risk of bone fracture: A systematic review and meta-analysis of observational studies. Endocr. Res.44(4), 168–184 (2019). 10.1080/07435800.2019.1625057 [DOI] [PubMed] [Google Scholar]
- 17.Trimpou, P., Odén, A., Simonsson, T., Wilhelmsen, L. & Landin-Wilhelmsen, K. High serum total cholesterol is a long-term cause of osteoporotic fracture. Osteoporos. Int.22(5), 1615–1620 (2011). 10.1007/s00198-010-1367-2 [DOI] [PubMed] [Google Scholar]
- 18.Yamauchi, M. et al. Increased low-density lipoprotein cholesterol level is associated with non-vertebral fractures in postmenopausal women. Endocrine48(1), 279–286 (2015). 10.1007/s12020-014-0292-0 [DOI] [PubMed] [Google Scholar]
- 19.Chang, P. Y. et al. Triglyceride levels and fracture risk in midlife women: Study of Women’s Health Across the Nation (SWAN). J. Clin. Endocrinol. Metab.101(9), 3297–3305 (2016). 10.1210/jc.2016-1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang, L. et al. Polyunsaturated fatty acids level and bone mineral density: A two-sample Mendelian randomization study. Front. Endocrinol. (Lausanne)13, 858851 (2022). 10.3389/fendo.2022.858851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brownbill, R. A. & Ilich, J. Z. Lipid profile and bone paradox: Higher serum lipids are associated with higher bone mineral density in postmenopausal women. J. Womens Health (Larchmt)15(3), 261–270 (2006). 10.1089/jwh.2006.15.261 [DOI] [PubMed] [Google Scholar]
- 22.Go, J. H., Song, Y. M., Park, J. H., Park, J. Y. & Choi, Y. H. Association between serum cholesterol level and bone mineral density at lumbar spine and femur neck in postmenopausal Korean women. Korean J. Fam. Med.33(3), 166–173 (2012). 10.4082/kjfm.2012.33.3.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loke, S. S., Chang, H. W. & Li, W. C. Association between metabolic syndrome and bone mineral density in a Taiwanese elderly population. J. Bone Miner. Metab.36(2), 200–208 (2018). 10.1007/s00774-017-0826-7 [DOI] [PubMed] [Google Scholar]
- 24.Buergel, T. et al. Metabolomic profiles predict individual multidisease outcomes. Nat. Med.28(11), 2309–2320 (2022). 10.1038/s41591-022-01980-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sudlow, C. et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med.12(3), e1001779 (2015). 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins, R. What makes UK Biobank special?. Lancet379(9822), 1173–1174 (2012). 10.1016/S0140-6736(12)60404-8 [DOI] [PubMed] [Google Scholar]
- 27.Palmer, L. J. UK Biobank: Bank on it. Lancet369(9578), 1980–1982 (2007). 10.1016/S0140-6736(07)60924-6 [DOI] [PubMed] [Google Scholar]
- 28.Peakman, T. C. & Elliott, P. The UK Biobank sample handling and storage validation studies. Int. J. Epidemiol.37(Suppl 1), i2-6 (2008). 10.1093/ije/dyn019 [DOI] [PubMed] [Google Scholar]
- 29.Würtz, P. et al. Quantitative serum nuclear magnetic resonance metabolomics in large-scale epidemiology: A primer on -omic technologies. Am. J. Epidemiol.186(9), 1084–1096 (2017). 10.1093/aje/kwx016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pietzner, M. et al. Plasma metabolites to profile pathways in noncommunicable disease multimorbidity. Nat. Med.27(3), 471–479 (2021). 10.1038/s41591-021-01266-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bragg, F. et al. Predictive value of circulating NMR metabolic biomarkers for type 2 diabetes risk in the UK Biobank study. BMC Med.20(1), 159 (2022). 10.1186/s12916-022-02354-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richardson, T. G. et al. Characterising metabolomic signatures of lipid-modifying therapies through drug target Mendelian randomisation. PLoS Biol.20(2), e3001547 (2022). 10.1371/journal.pbio.3001547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao, X., Sun, J., Xin, S. & Zhang, X. Correlation between blood lipid level and osteoporosis in older adults with type 2 diabetes mellitus: A retrospective study based on inpatients in Beijing, China. Biomolecules13(4), 616 (2023). 10.3390/biom13040616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martyniak, K. et al. Do polyunsaturated fatty acids protect against bone loss in our aging and osteoporotic population?. Bone143, 115736 (2021). 10.1016/j.bone.2020.115736 [DOI] [PubMed] [Google Scholar]
- 35.Aleidi, S. M. et al. A distinctive human metabolomics alteration associated with osteopenic and osteoporotic patients. Metabolites11(9), 628 (2021). 10.3390/metabo11090628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim, D., Kim, J. H. & Song, T. J. Total cholesterol variability and the risk of osteoporotic fractures: A nationwide population-based cohort study. J. Pers. Med.13(3), 509 (2023). 10.3390/jpm13030509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kunutsor, S. K. & Laukkanen, J. A. The interplay between circulating high-density lipoprotein, age and fracture risk: A new cohort study and systematic meta-analysis. Geroscience45, 2727–2741 (2023). 10.1007/s11357-023-00801-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang, Y. et al. Association between serum cholesterol level and osteoporotic fractures. Front. Endocrinol. (Lausanne)9, 30 (2018). 10.3389/fendo.2018.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sivas, F., Alemdaroğlu, E., Elverici, E., Kuluğ, T. & Ozoran, K. Serum lipid profile: Its relationship with osteoporotic vertebrae fractures and bone mineral density in Turkish postmenopausal women. Rheumatol. Int.29(8), 885–890 (2009). 10.1007/s00296-008-0784-4 [DOI] [PubMed] [Google Scholar]
- 40.Sharma, T. & Mandal, C. C. Omega-3 fatty acids in pathological calcification and bone health. J. Food Biochem.44(8), e13333 (2020). 10.1111/jfbc.13333 [DOI] [PubMed] [Google Scholar]
- 41.Tanaka, S. & Matsumoto, T. Sclerostin: From bench to bedside. J. Bone Miner. Metab.39(3), 332–340 (2021). 10.1007/s00774-020-01176-0 [DOI] [PubMed] [Google Scholar]
- 42.During, A. Osteoporosis: A role for lipids. Biochimie178, 49–55 (2020). 10.1016/j.biochi.2020.08.004 [DOI] [PubMed] [Google Scholar]
- 43.Tian, L. & Yu, X. Lipid metabolism disorders and bone dysfunction–interrelated and mutually regulated (review). Mol. Med. Rep.12(1), 783–794 (2015). 10.3892/mmr.2015.3472 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available via an access application to UK Biobank. https://www.ukbiobank.ac.uk/enable-your-research.