Abstract
The study evaluated how ingestion of nicotinamide mononucleotide (NMN) for 12 weeks by older adults affected blood nicotinamide adenine dinucleotide (NAD +) levels and physical function, particularly walking function. Information concerning sleep, and stress was also collected as secondary endpoints. In this randomized, placebo-controlled, double-blind, parallel-group comparison study, 60 participants were randomly allocated into a placebo group or NMN group. Members of the NMN group consumed 250 mg/day NMN for 12 weeks. Motor function tests, blood NAD metabolite analysis, and questionnaires were conducted at the start of the study and 4 and 12 weeks after intake. This trial was registered at umin.ac.jp/ctr as UMIN000047871 on June 22nd, 2022.
At primary outcome, at both 4 weeks and 12 weeks, the NMN and placebo groups had no significant differences in a stepping test. At secondary outcomes, after 12 weeks of NMN intake, the NMN group had a significantly shorter 4-m walking time than the placebo group as well as significantly higher blood levels of NAD + and its metabolites. A significant negative correlation was observed between the change in the 4-m walking time and the change in blood NAD + , N1-methyl-2-pridone-5-carboxamide (2-PY), and N1-methyl-4-pridone-3-carboxamide (4-PY) at 12 weeks. The NMN group had improved sleep quality at 12 weeks relative to the placebo group as evidenced by lower scores for “Daytime dysfunction” and “Global PSQI” on the Pittsburgh Sleep Questionnaire. No adverse effects related to test substance consumption were observed. Together, these results indicate that NMN intake could increase blood NAD + levels, maintain walking speed, and improve sleep quality in older adults. Interventions involving NMN aimed at maintaining walking speed could contribute to extended healthy life expectancy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-024-01204-1.
Keywords: Nicotinamide mononucleotide, Walking speed, Blood NAD +, Older adults
Introduction
As the elderly age, physiological aging progresses due to a decrease in the number of cells that comprise each organ of the body and decreases in the volume of cells [1, 2]. Physiological aging can also be accompanied by various physical characteristics such as decreased organ function and homeostasis function and development of disease. In particular, decreased physical performance, markedly increased movement time, and decreased postural balance function are seen with increasing age [2–4]. Walking is an important function for activities of daily living, which can be negatively impacted by decreased walking ability. Among walking ability parameters, walking speed in particular is strongly associated with mortality risk and is an indicator of physical function and daily living function in older adults. Faster walking speeds, which are related to the function of the musculoskeletal, nervous, cardiovascular, and other systems [5–7], facilitate maintenance of life functions and increase life expectancy. After the age of 65, walking speed gradually declines and can become an obstacle for daily life after the age of 80 years old and 75 years old for men and women, respectively. The loss of walking ability brings a concomitant decline in life function [7]. Therefore, exercise programs and medical interventions aimed at maintaining or increasing walking speed could extend healthy life expectancy [8–10].
Nicotinamide adenine dinucleotide (NAD +) is an essential pyridine nucleotide that functions as a cofactor and substrate for enzymes involved in various biological processes, including energy metabolism, gene expression regulation, DNA repair, mitochondrial function, immune function, calcium homeostasis, and cell death [11–13]. Decreased NAD + levels in vivo have been associated with cardiovascular and metabolic diseases, neurodegenerative diseases, cancer, and aging [14–16]. Under these specific disease conditions and in older adults, supplementation with NAD + precursors may be beneficial [12, 17, 18]. In addition to nicotinamide (NAM) and nicotinic acid (NA), which have been studied for many years, nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN), have recently attracted attention as NAD + precursors. Long-term intake of NMN has been reported to improve physical function in humans, but the number of reports describing the effect of NMN is limited [19].
There is a close relationship between aging and sleep disorders [20, 21]. As people age, sleep quality declines; sleep duration decreases; and sleep disorders, such as insomnia, sleep apnea, and restless legs syndrome, increase. The quality and quantity of sleep declines lead not only to poor physical function, but to cognitive decline and psychological problems. It has been reported that long-term intake of NMN showed a significant interaction between the results of a 5-time sit-to-stand test and drowsiness [19]. This is little information, and further studies are needed to convince us of the validity of NMN.
The purpose of this study was to evaluate how NMN supplementation for older adults for a 12-week period affected blood NAD + levels and physical function, especially walking function. Information related to sleep, and stress was collected as secondary endpoints.
Methods
Ethical approval
The study was approved by the Ethical Review Committee of Chiyoda Paramedical Care Clinic, which is a third party entity that was not involved in the study (No.15000088; date of approval: March 18, 2022). The study was conducted in compliance with ethical principles based on the Declaration of Helsinki and ethical guidelines for medical research involving human subjects (notification of the Ministry of Health, Labor, and Welfare, and Ministry of Education, Culture, Sports, Science and Technology). The trial is registered with the UMIN Clinical Trials Registry (UMIN000047871). The study was conducted at the CPCC Corporation, which performed motor function measurements, blood sampling, and medical examination of study participants at the initial screening and during the study. The initial screening tests for the study were conducted between June 1 and June 7, 2022, and the test substance consumption period was between July 1 and September 25, 2022.
Selection of participants
Before enrolling, the study investigator provided study participants with sufficient explanation of the study as well as the most recent consent and explanation documents and consent forms that were approved by the ethical review committee. Study participants were given the opportunity to ask questions and sufficient time to make a decision about their participation in the study. Participants were included in the study only after their own free and voluntary consent was obtained in writing. Selection and exclusion criteria for study participants are listed in Table 1.
Table 1.
Study selection and exclusion criteria
| Selection criteria |
|---|
|
(1) Men and women who were between 65 and 75 years old when consent was obtained (2) Subjects who received a full explanation of the study, were able to understand its contents, and were able to give their written consent |
| Exclusion criteria |
|
(1) Subjects who were consuming foods that could affect the study, including specified health foods, functional foods, or general health foods (including supplements), at least three times a week and an inability to discontinue consumption of the foods at the time consent was obtained (2) Subjects who were taking drugs that could affect the test (e.g., anabolic proteins, peptide hormones, growth factors, beta2 agonists, hormone modulators, and metabolic modulators listed in the International Standard of the World Anti-Doping Code 2022 Prohibition Table) at least three times a week and who could not limit use during the study period (3) Subjects who had a daily exercise habit of moderate intensity or higher for resistance exercise and high intensity or higher for other exercise (4) Heavy alcohol use (5) Subjects who had sustained severe injuries to their locomotory organs (e.g., broken bones, ruptured tendons, or separated muscles) within the previous year (6) Subjects with motor dysfunction (7) Subjects using implanted medical electronic devices such as pacemakers (8) Subjects who had been stopped from exercising by a physician (9) Subjects with a history or current history of serious diseases of the heart, liver, kidney, lungs, digestive organs, blood, endocrine system, nervous system, and metabolic system (10) Subjects who were allergic to medicines and food (11) Subjects who were currently participating in a clinical trial of another drug or health food or who were scheduled to participate in another clinical trial within 4 weeks of trial completion or after consent to participate in such a trial (12) Subjects who had donated component blood or 200 mL of whole blood in the month prior to the start of the relevant test (13) Men who had donated 400 mL of whole blood during the 3 months prior to the start of the study (14) Women who had donated 400 mL of whole blood during the 4 months prior to the start of the study (15) Men who had a blood collection volume during the 12 months prior to the start of the study plus a total planned blood collection volume for the study that exceeded 1200 mL (16) Women who had a blood collection volume during the 12 months prior to the start of the study plus a total planned blood collection volume for the study that exceeded 800 mL (17) Subjects whose participation in the study was deemed inappropriate by the investigator or sub-investigator |
Screening of participants
The 136 study participants for whom consent was obtained underwent screening tests in a background investigation that included a medical history, physiological examination, medical examination, and stepping test (standing and sitting), as well as blood and urine tests. Based on the overall results of the screening test, 60 study participants were selected to begin consumption of the test substance.
Sample size
The sample size was calculated based on reports of improved gait speed after 12 weeks of NMN intake [22]. The between-group difference in change in gait speed after 12 weeks of NMN intake was 1.00 (NMN group; N = 10, 0.09 ± 0.13, placebo group; N = 10,—0.01 ± 0.10). Based on these factors, the Cohen’s d of the effect size between groups was estimated to be—0.91. The statistical significance level (α) was 5% two-sided, the statistical power (1-β) was 80%, and the sample size for the t-test was calculated to be 20 subjects in each group. To account for a 30% dropout rate, the number of subjects in each group was set to N = 30 for each group.
Study design
Participants were restricted from eating after 9 p.m. the day before their visit. Water intake was permitted. After the visit, a medical interview, physiological tests, blood collection, and questionnaires were conducted. Afterwards, a light meal was provided, and motor function tests were administrated.
Prior to the start of the supplement intake, a medical interview, physiological tests, motor function tests, blood and urine tests, NAD-related metabolite blood analysis, and questionnaires were administered. Consumption of the test substance began on the day of the pre-commencement examination and thereafter two capsules (125 mg NMN/capsule) per day were consumed for 12 weeks. During the study period, the study participants were asked to make their own entries in a daily life diary regarding the intake of the test substances, subjective symptoms, and confirmation of their intake of medicines and health foods. At 4 weeks and 12 weeks after beginning intake, physiological tests, motor function tests, blood and urine tests (12 weeks only), NAD metabolite blood analysis, questionnaires, and a medical interview were again administered.
Restrictions during the study period
During the study period, study participants made daily entries of necessary information in a daily life diary and observed the following activities restrictions and prohibitions: (1) avoiding introduction of major changes in lifestyle relative to those before participation in the study; (2) avoiding intake of new foods for specified health use, functional foods, or health foods (including supplements); and (3) immediately notifying the study consultation desk and noting in the daily life diary if the participant felt unwell or began taking any new medication.
Preparation of test substance
Hard capsules containing 125 mg β-NMN per capsule with crystalline cellulose, lubricant (calcium stearate), and anti-caking agent (silicon dioxide) as additives were prepared. For the placebo, NMN was replaced with crystalline cellulose. The NMN content was assessed using HPLC. A third party entity that was not involved in the conduct of the study confirmed that the test and placebo substances were indistinguishable.
Allocation, blinding of test substance
The study was a randomized, placebo-controlled, double-blind, parallel-group comparison. The 60 selected participants were randomly allocated into two groups (NMN group and placebo group). The number of steps in the stepping test (see below) was used as an allocation factor. Blinding throughout the study was maintained by all parties except the study substance allocation manager.
Case fixation and key opening
After the 12-week post-intake examination, discontinuations, presence or absence of adverse events, intake rate of study substances, and records of intake of pharmaceuticals, quasi-drugs, health foods, supplements, and foods for specified health uses were checked for noncompliance, and cases for analysis were identified. After adjusting the measurement data obtained from the test (data fixation), key opening of the sealed test substance allocation table was performed.
Evaluation of outcomes
Primary outcome
Motor function test: stepping test
A stepping test was carried out using a TKK5301 instrument (Takei Scientific Instruments Co. Ltd., Niigata, Japan). The patients performed two 5-s periods of stepping in place while standing, and the number of steps taken was recorded. Using a chair with a seat height adjusted so that the knee angle was 90°, subjects performed two 5-s periods of stepping while seated, with both hands holding the handles next to the seat surface, and the number of steps was recorded. Maximum values were used as the data.
Secondary outcomes
Physiological tests
Body composition was measured using a direct segment multi-frequency bioelectrical impedance analyzer (In body 570, In body Japan Co., Ltd.). BMI (body mass index) and SMI (skeletal muscle mass index) were calculated based on measurements of body weight, skeletal muscle weight, and height. Systolic and diastolic blood pressure and pulse rate were measured.
Motor function tests
Selection reaction time was evaluated using Reaction MR (TKK1264, Takei Scientific Instruments Co., Ltd.). Participants stood in the center panel and received a visual signal indicating that the subject should move forward, backward, left, or right. Directions were given eight times in a random order. The Motion Initiation time (the time between the visual cue and movement of one foot away from the other to move to the indicated panel) and the Reaction Time (the time between receiving the cue and moving both feet) were measured, and the average time for eight tests was calculated.
Muscle strength was measured using a motor function analyzer (zaRitz BM-220 Tanita Corporation, Tokyo, Japan) [23]. The subject was seated in a chair and, upon receiving instruction from the measuring device, was asked to stand up as fast as possible, remain still while standing for approximately 3 s, and then sit down. This movement was repeated three times, and the power, speed, balance score, and muscle strength score were measured.
Grip strength was measured using a Jaymar-type hydraulic grip strength tester (SH5001 Sakai Medical Co., Ltd.). Two measurements were taken for each hand, and the higher value was used.
The Japanese version of Short Physical Performance Battery (SPPB) was conducted to assess physical performance. This test consists of three tests to measure balance, muscle strength, and gait: standing static balance in three postures, lower extremity muscle strength and power associated with rising from and sitting on a chair, and the 4-m walking speed at a normal pace. SPPB was evaluated in terms of total scores and measurements. The walk and rise tests were repeated twice, and data from the trial in which the speed was highest was used [24].
From the day after the 0 week visit to bedtime the day before the 12 week visit, the study subjects wore a pedometer from the time they woke up to bedtime (except when bathing) and entered the number of daily steps in their daily diary.
Blood and urine analysis
First-morning urine and blood samples from the median cubital vein were collected. Hematological tests, including counts of white and red blood cells and platelets, hemoglobin, and hematocrit, were performed. Blood biochemical tests, including total protein, albumin, total bilirubin, alkaline phosphatase, lactate dehydrogenase, aspartate aminotransferase, alanine aminotransferase, gamma-glutamyl transpeptidase, creatine kinase, total cholesterol, triglycerides, HDL cholesterol, LDL cholesterol, urea nitrogen, creatinine, uric acid, sodium, potassium, chloride, calcium, glucose, and HbA1c (NGSP), were also carried out. Urine was tested for protein, glucose, urobilinogen, bilirubin, and occult blood. All blood and urine analyses were performed by BML, Inc. (Tokyo, Japan).
Analysis of blood NAD related metabolites
Levels of NAD-related metabolites (NAD + , NMN, NADP + , NAM, N1-methyl-2-pridone-5-carboxamide (2-PY), and N1-methyl-4-pridone-3-carboxamide (4-PY), NaMN, NaR) were assessed using high-performance liquid chromatography-tandem mass spectrometry (HPLC–MS/MS, HPLC; ACQUITY H-class Bio Binary system, Waters Corporation, Milford, MA, USA, MS/MS; TQ-XS, Waters Corporation). Immediately after collecting blood samples, 800 μL methanol was added to 200 μL whole blood. The samples were mixed thoroughly and stored at—80 °C until analysis. For analysis, mixtures were centrifuged for 10 min at 4 °C, 15,000xg. A 40 μL aliquot was mixed with 2 μL internal standard solution and evaporated using a miVac concentrator (Genevac Limited, Gothenburg, Sweden) at 30 °C. The dried extract was dissolved with 420 μL citric acid (0.1 mg/mL), and the suspension was filtered through a 0.2 μm filter before HPLC–MS/MS analysis.
All analyses were performed on a 2.1 × 150 mm column with a 1.8 µm particle size (ACQUITY UPLC Premier HSS T3, Waters Corporation). The mobile phase A and B was 5 mM ammonium formate and acetonitrile, respectively. The initial eluent composition was 100% A with an increase to 10% B over 3 min. The 10% B concentration was maintained for 0.5 min and then increased to 90% B over 1 min, held at 90% B for 3.5 min, and then reduced to 0% B over 3 min. The total run time was 11 min. The eluent flow was 0.3 mL/min, and the column was maintained at 45 °C. Analytes were detected using electrospray ionization in the positive mode. Multiple-reaction-monitoring (MRM) was performed using characteristic fragmentation ions (NAD + , m/z = 664.2/428.1, 13C5 NAD + _IS, m/z = 669.1/428.0; NMN, m/z = 335.2/123.0; NaMN, m/z = 336.2/124.0, NADP + , m/z = 744.0/136.0, D4 NMN_IS, m/z = 339.1/127.0, NAM, m/z = 123.2/79.9; 2-PY, m/z = 153.1/110.0, 4-PY, m/z = 153.1/136.0, 13C6 NAM_IS, m/z = 129.0/85.4, NaR, m/z = 256.1/124.0, 13C6 NA_IS, m/z = 130.1/83.0).
Questionnaire
Mood profiles of all study subjects were analyzed using a shortened version of POMS2, a psychological rating scale to assess transient, distinct mood states [25]. The shortened POMS2 is a mood inventory containing 35 items to assess the following seven different subscales (moods): Anger-Hostility (AH), Confusion-Bewilderment (CB), Depression-Dejection (DD), Fatigue-Inertia (FI), Tension-Anxiety (TA), Vigor-Activity (VA), and Friendliness (F). Subjects were asked to indicate mood states during the previous 1-week period using a 5-point score ranging from 0 (“not-at-all”) to 4 (“extremely”). The sum of the scores was calculated for each subscale. A total mood disorder (TMD) score was calculated based on the individual total score of six subscales (excluding subscale F) using the formula TMD = (AH + CB + DD + FI + TA)—VA. Evaluation of individual subscales and TMDs was performed using standardized scores (T-scores) that were converted from the individual total subscale scores and TMD scores using a conversion table.
Subjective sleep quality was measured using the Pittsburgh Sleep Quality Index (PSQI) [26]. The PSQI is a subscale score ranging from 0 to 3 that assesses subjective sleep quality over the previous month and consists of seven items (sleep duration, sleep latency, sleep medications, sleep disturbances, daytime dysfunction, sleep quality, and sleep efficiency). Lower scores correspond to better subjective sleep quality.
Safety assessment
Symptoms, severity, outcome, and frequency of adverse events and side effects that occurred from the start to the end of the study period by all study subjects who had consumed the study substance at least once were recorded, and the association with the study substance was evaluated for all events.
Statistical analysis
Test data are presented as mean ± standard deviation. Measurement data at 12 weeks were used as the primary outcome. Differences among the groups at each time point were analyzed by ANCOVA with the 0 week measurement data as a covariate. Analysis of data for the 4-week time point was carried out only for those parameters that showed significant differences at 12 weeks. All statistical analyses were performed using SPSS 22.0 (SPSS Inc., Chicago, IL, USA).
Results
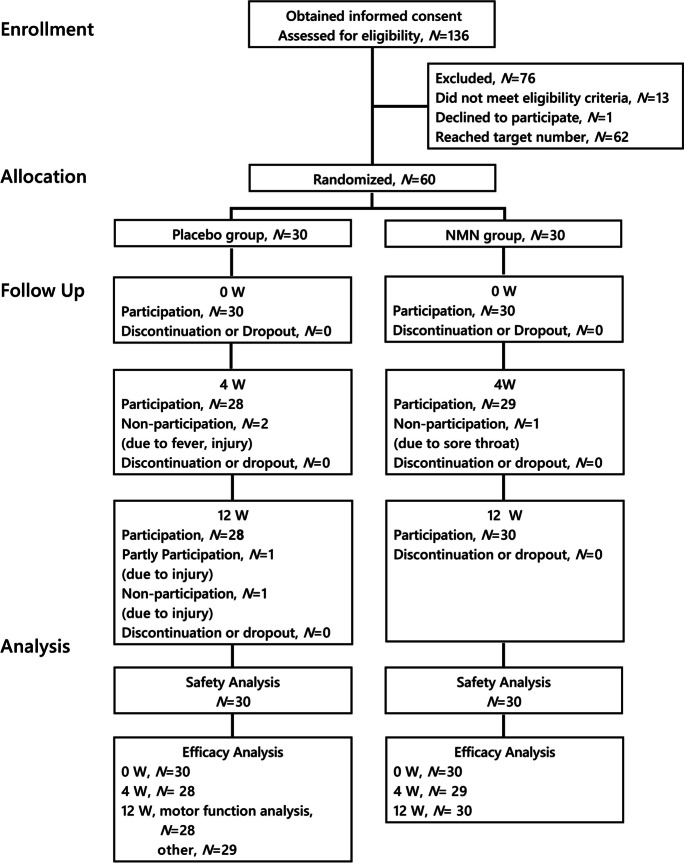
The screening study included 136 participants (Fig. 1). A total of 60 participants who met the selection criteria and did not violate the exclusion criteria were selected and randomly assigned into one of two groups. The efficacy and safety analyses were conducted for all 60 subjects (the placebo group 30 subjects, the NMN group 30 subjects), since none of the study subjects met the criteria for exclusion. Data were not collected for five of the subjects because they were unable to participate in the study due to injury or illness (placebo group, fever (N = 1), injury (N = 1) at 4 weeks, injury (N = 2) at 12 weeks; NMN group, sore throat (N = 1) at 4 weeks).
Fig. 1.
Subject enrollment in the clinical study
There were no significant differences between the age, height, weight, BMI, blood pressure, pulse, and blood test parameters for the control and NMN groups (Table 2).
Table 2.
Study participant parameters before (0 W) NMN intervention
| Unit | Placebo group | NMN group | |||||
|---|---|---|---|---|---|---|---|
| Number of participants | N | 30 | 30 | ||||
| Male | 18 | 18 | |||||
| Female | 12 | 12 | |||||
| Age | Year | 69 | ± | 3 | 69 | ± | 3 |
| Height | cm | 162.1 | ± | 7.4 | 161.4 | ± | 8.3 |
| Body weight | kg | 59.6 | ± | 11.5 | 58.7 | ± | 10.3 |
| Body mass index | kg/m2 | 22.6 | ± | 3.6 | 22.4 | ± | 2.6 |
| Systolic blood pressure | mmHg | 125 | ± | 18 | 124 | ± | 12 |
| Diastolic blood pressure | mmHg | 77 | ± | 13 | 75 | ± | 10 |
| Pulse rate | bpm | 71 | ± | 9 | 69 | ± | 10 |
| White blood cells | /μL | 5363.7 | ± | 1066.1 | 5339.3 | ± | 1571.8 |
| Red blood cells | × 104/μL | 445.4 | ± | 33.5 | 458.5 | ± | 37.1 |
| Hemoglobin | g/L | 137.3 | ± | 11.0 | 140.1 | ± | 10.5 |
| Hematocrit | 0.43 | ± | 0.03 | 0.44 | ± | 0.03 | |
| Platelets | × 104/μL | 24.4 | ± | 4.9 | 25.0 | ± | 4.6 |
| Total protein | g/L | 70.37 | ± | 2.41 | 71.73 | ± | 3.49 |
| Albumin | g/d | 43.23 | ± | 2.78 | 43.77 | ± | 2.25 |
| Total bilirubin | mg/dL | 0.80 | ± | 0.26 | 0.73 | ± | 0.22 |
| Alkaline phosphatase | IU/L | 74.2 | ± | 18.2 | 64.1 | ± | 19.7 |
| Lactate dehydrogenase | IU/L | 179.9 | ± | 20.8 | 180.5 | ± | 30.4 |
| Aspartate aminotransferase | IU/L | 22.1 | ± | 3.7 | 23.7 | ± | 4.8 |
| Alanine aminotransferase | IU/L | 16.7 | ± | 4.4 | 19.7 | ± | 7.2 |
| Gamma-glutamyl transpeptidase | IU/L | 29.9 | ± | 20.1 | 23.8 | ± | 13.3 |
| Creatine kinase | IU/L | 157.2 | ± | 178.4 | 113.9 | ± | 58.8 |
| Total cholesterol | mmol/L | 5.74 | ± | 0.95 | 5.56 | ± | 0.88 |
| Triglycerides | mmol/L | 1.07 | ± | 0.60 | 0.94 | ± | 0.47 |
| HDL cholesterol | mmol/L | 1.75 | ± | 0.51 | 1.76 | ± | 0.38 |
| LDL cholesterol | mmol/L | 3.32 | ± | 0.65 | 3.15 | ± | 0.72 |
| Urea nitrogen | mmol/L | 5.61 | ± | 1.41 | 6.04 | ± | 1.28 |
| Creatinine | μmol/L | 74.31 | ± | 14.29 | 72.28 | ± | 14.65 |
| Uric acid | μmol/L | 338.4 | ± | 73.0 | 320.4 | ± | 75.2 |
| Sodium | mmol/L | 140.9 | ± | 1.6 | 142.0 | ± | 1.5 |
| Potassium | mmol/L | 4.36 | ± | 0.35 | 4.52 | ± | 0.27 |
| Chloride | mmol/L | 104.4 | ± | 1.6 | 104.5 | ± | 2.2 |
| Calcium | mmol/L | 2.30 | ± | 0.07 | 2.35 | ± | 0.06 |
| Glucose | mmol/L | 5.29 | ± | 0.46 | 5.45 | ± | 0.46 |
| HbA1c (NGSP) | % | 5.48 | ± | 0.31 | 5.45 | ± | 0.26 |
Means ± standard deviation are shown
N = 60 (placebo group N = 30, NMN group N = 30)
Primary outcome: stepping test
The stepping test (sitting and standing) showed no significant difference between the placebo and NMN groups at either 4 or 12 weeks of intake (Table 4, Supplementary Table S2).
Table 4.
Motor function of study subjects before (0 W) and after (12 W) NMN intervention
| Unit | Placebo group | NMN group | ANCOVA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Stepping test | |||||||||
| Standing |
step count |
0 W | 30.4 | ± | 4.4 | 31.6 | ± | 3.4 | |
| 12 W | 32.6 | ± | 4.6 | 33.3 | ± | 3.1 | 0.579 | ||
| Seated |
step count |
0 W | 41.0 | ± | 4.6 | 41.9 | ± | 4.0 | |
| 12 W | 42.3 | ± | 5.3 | 42.6 | ± | 4.4 | 0.534 | ||
| Selective response time | |||||||||
| Movement start time | sec | 0 W | 0.65 | ± | 0.14 | 0.64 | ± | 0.11 | |
| 12 W | 0.61 | ± | 0.13 | 0.64 | ± | 0.14 | 0.147 | ||
| Reaction time | sec | 0 W | 1.26 | ± | 0.21 | 1.30 | ± | 0.16 | |
| 12 W | 1.25 | ± | 0.21 | 1.28 | ± | 0.18 | 0.968 | ||
| Grip strength | |||||||||
| Left hand | kg | 0 W | 26.8 | ± | 7.2 | 28.1 | ± | 7.8 | |
| 12 W | 26.6 | ± | 6.7 | 27.1 | ± | 7.8 | 0.374 | ||
| Right hand | kg | 0 W | 28.9 | ± | 7.6 | 29.8 | ± | 7.5 | |
| 12 W | 27.8 | ± | 7.3 | 27.9 | ± | 7.0 | 0.538 | ||
| Dominant hand | kg | 0 W | 28.8 | ± | 7.6 | 29.8 | ± | 7.5 | |
| 12 W | 27.7 | ± | 7.3 | 27.9 | ± | 7.0 | 0.571 | ||
| Short physical performance battery | |||||||||
| Score | - | 0 W | 11.7 | ± | 0.6 | 11.9 | ± | 0.3 | |
| 12 W | 11.7 | ± | 0.8 | 11.8 | ± | 0.6 | 0.820 | ||
| Chair stand test | sec | 0 W | 9.34 | ± | 2.16 | 9.13 | ± | 1.38 | |
| 12 W | 8.62 | ± | 1.57 | 8.50 | ± | 1.42 | 0.977 | ||
| Muscle function analysis | |||||||||
| Power score | - | 0 W | 1.35 | ± | 0.10 | 1.33 | ± | 0.11 | |
| 12 W | 1.35 | ± | 0.11 | 1.33 | ± | 0.10 | 0.812 | ||
| Speed score | - | 0 W | 9.94 | ± | 1.57 | 9.60 | ± | 1.65 | |
| 12 W | 10.03 | ± | 1.73 | 9.65 | ± | 1.74 | 0.776 | ||
| Balance score | - | 0 W | 49.2 | ± | 9.6 | 48.9 | ± | 7.5 | |
| 12 W | 49.8 | ± | 7.7 | 50.0 | ± | 6.7 | 0.900 | ||
| Total score | - | 0 W | 49.0 | ± | 10.0 | 46.0 | ± | 8.6 | |
| 12 W | 49.1 | ± | 10.6 | 46.4 | ± | 8.3 | 0.959 | ||
Means ± standard deviation are shown
Efficacy analysis population: N = 58 (placebo group N = 28, NMN group N = 30)
Secondary outcomes
Body composition
The body composition at 0 week and at 4 and 12 weeks of the study was similar between the placebo and NMN groups (Supplementary Table S1 and Table 3).
Table 3.
Body composition of study subjects before (0 W) and after (12 W) NMN intervention
| Unit | Placebo group | NMN group | ANCOVA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Body weight | kg | 0 W | 59.6 | ± | 11.7 | 58.7 | ± | 10.3 | |
| 12 W | 59.4 | ± | 11.9 | 58.2 | ± | 10.6 | 0.254 | ||
| Body fat mass | kg | 0 W | 15.7 | ± | 6.1 | 15.7 | ± | 4.8 | |
| 12 W | 17.1 | ± | 6.4 | 16.6 | ± | 5.0 | 0.068 | ||
| Skeletal muscle mass | kg | 0 W | 23.7 | ± | 5.1 | 23.2 | ± | 4.8 | |
| 12 W | 22.7 | ± | 4.9 | 22.3 | ± | 4.7 | 0.703 | ||
| Body mass index | kg/m2 | 0 W | 22.6 | ± | 3.6 | 22.4 | ± | 2.6 | |
| 12 W | 22.6 | ± | 3.7 | 22.2 | ± | 2.7 | 0.262 | ||
| Skeletal muscle mass index | kg/m2 | 0 W | 6.9 | ± | 1.0 | 6.7 | ± | 1.0 | |
| 12 W | 6.5 | ± | 1.0 | 6.4 | ± | 1.1 | 0.701 | ||
Means ± standard deviation are shown
Efficacy analysis population: N = 59 (placebo group N = 29, NMN group N = 30)
Motor function
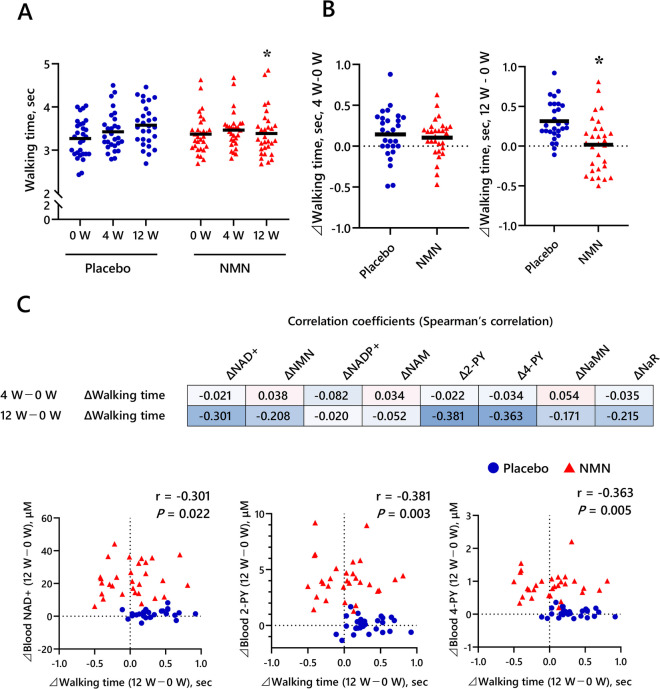
At both 4 weeks and 12 weeks, the NMN and placebo groups had no significant differences in a selective response time, grip strength, or SPPB scores (Table 4, Fig. 2 A and B, Supplementary Table S2). However, at 12 weeks, the NMN group had a significantly faster 4-m walking time than the placebo group.
Fig. 2.
Effect of NMN supplementation on 4-m walk time and NAD + metabolites. A Results of 4-m walk test for placebo and NMN groups at week 0 (0W) and at weeks 4 (4W) and 12 (12W) of the study period. B Change in the 4-m walk time from 0 to 4W and 12W of the study period. Black horizontal lines indicate average values. *P < 0.05 vs. Placebo group. Results of ANCOVA with 0 week as the covariate. C Upper diagram: Spearman’s correlation between change in 4-m walk time and change in NAD + metabolites from 0W and at 4W and 12W of the study period. The numbers indicate the correlation coefficients. Lower diagram: Spearman’s correlation between change in 4-m walk time and change in NAD + , 2-PY, and 4-PY from 0W and at 12W of the study period. NAD + nicotinamide adenine dinucleotide, NMN nicotinamide mononucleotide, NADP + nicotinamide adenine dinucleotide phosphate, NAM nicotinamide, 2-PY N1-methyl-2-pyridone-5-carboxamide, 4-PY N1-methyl-4-pyridone-3-carboxamide, NaMN nicotinic acid mononucleotide, NaR nicotinic acid riboside
Blood chemistry and concentrations of NAD-related metabolites in blood samples
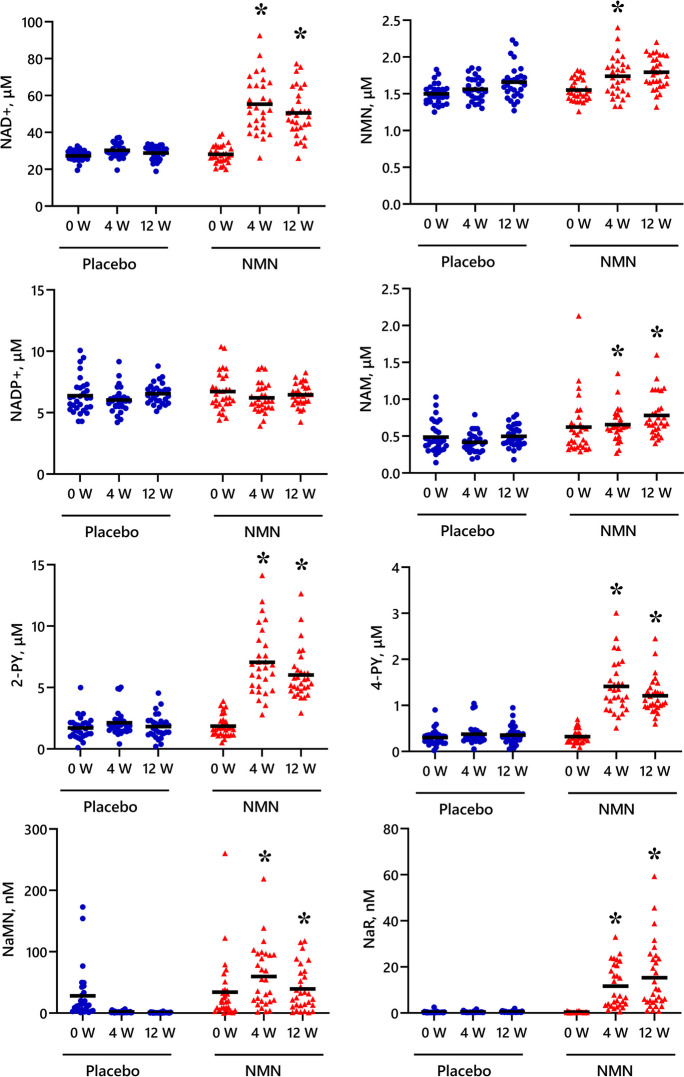
At both 4 weeks and 12 weeks, the blood concentration of NAD + , NAM, 2-PY, 4-PY, NaMN, and NaR was significantly higher in the NMN group compared with the placebo group (Fig. 3). Moreover, a significant negative correlation was observed between the change in the 4-m walking time and the change in blood NAD + , 2-PY, and 4-PY at 12 weeks, but not at 4 weeks (Fig. 2C).
Fig. 3.
Blood concentration of NAD-related metabolites before (0W) and at week 4 (4W) and week 12 (12W) of the study period. Black horizontal lines indicate average values. *P < 0.05 vs. Placebo group. Results of ANCOVA with 0 week as the covariate. NAD + nicotinamide adenine dinucleotide, NMN nicotinamide mononucleotide, NADP + nicotinamide adenine dinucleotide phosphate, NAM nicotinamide, 2-PY N1-methyl-2-pyridone-5-carboxamide, 4-PY N1-methyl-4-pyridone-3-carboxamide, NaMN nicotinic acid mononucleotide, NaR nicotinic acid riboside
Questionnaire responses
Mood was assessed using a shortened version of the POMS2; a psychological rating scale to assess transient, distinct mood states, and subjective sleep quality was assessed using Pittsburgh Sleep Questionnaire (PSQI Table 5). There were no significant differences between the groups for responses to any questions in the POMS2 questionnaire at 12 weeks. For the PSQI, scores for “Daytime dysfunction” and “Global PSQI” were significantly lower for the NMN group than the placebo group at 12 weeks, whereas at 4 weeks, the NMN score for “Global PSQI” was also significantly lower than that for the placebo group (Supplementary Table S3). These lower PSQI scores correspond to improved sleep quality.
Table 5.
Questionnaire responses for study subjects before (0 W) and after (12 W) NMN intervention
| Unit | Placebo group | NMN group | ANCOVA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Profile of Mood States 2 (POMS2) | |||||||||
| Anger-Hostility (AH) | Point | 0 W | 43.2 | ± | 6.1 | 41.7 | ± | 4.7 | |
| 12 W | 41.8 | ± | 4.6 | 41.5 | ± | 4.2 | 0.506 | ||
| Confusion-Bewilderment (CB) | Point | 0 W | 44.4 | ± | 6.7 | 43.3 | ± | 5.1 | |
| 12 W | 43.5 | ± | 4.3 | 42.5 | ± | 4.1 | 0.559 | ||
| Depression-Dejection (DD) | Point | 0 W | 44.7 | ± | 5.3 | 44.4 | ± | 6.1 | |
| 12 W | 43.4 | ± | 3.3 | 43.6 | ± | 4.1 | 0.660 | ||
| Fatigue-Inertia (FI) | Point | 0 W | 42.1 | ± | 6.4 | 39.3 | ± | 3.7 | |
| 12 W | 39.9 | ± | 4.5 | 38.8 | ± | 2.8 | 0.961 | ||
| Tension-Anxiety (TA) | Point | 0 W | 42.4 | ± | 6.1 | 41.3 | ± | 5.8 | |
| 12 W | 40.9 | ± | 5.7 | 41.1 | ± | 5.3 | 0.352 | ||
| Vigor-Activity (VA) | Point | 0 W | 58.7 | ± | 7.1 | 51.7 | ± | 8.4 | |
| 12 W | 58.8 | ± | 7.7 | 55.3 | ± | 10.0 | 0.355 | ||
| Friendliness (F) | Point | 0 W | 56.6 | ± | 7.4 | 47.8 | ± | 7.6 | |
| 12 W | 56.8 | ± | 7.6 | 50.4 | ± | 9.4 | 0.420 | ||
| Total score (TMD) | Point | 0 W | 41.1 | ± | 6.3 | 41.3 | ± | 5.1 | |
| 12 W | 39.5 | ± | 4.9 | 39.9 | ± | 4.5 | 0.712 | ||
| Pittsburgh Sleep Quality Index (PSQI) | |||||||||
| Sleep quality | Score | 0 W | 1.0 | ± | 0.6 | 1.0 | ± | 0.4 | |
| 12 W | 1.1 | ± | 0.4 | 0.9 | ± | 0.4 | 0.062 | ||
| Sleep latency | Score | 0 W | 0.7 | ± | 0.6 | 0.4 | ± | 0.6 | |
| 12 W | 0.9 | ± | 0.9 | 0.4 | ± | 0.7 | 0.195 | ||
| Sleep duration | Score | 0 W | 0.8 | ± | 0.7 | 1.0 | ± | 0.7 | |
| 12 W | 1.0 | ± | 0.7 | 1.0 | ± | 0.6 | 0.337 | ||
| Habitual sleep efficiency | Score | 0 W | 0.0 | ± | 0.0 | 0.0 | ± | 0.0 | |
| 12 W | 0.0 | ± | 0.2 | 0.0 | ± | 0.2 | 0.981 | ||
| Sleep disturbance | Score | 0 W | 1.0 | ± | 0.0 | 0.9 | ± | 0.4 | |
| 12 W | 0.9 | ± | 0.4 | 0.8 | ± | 0.4 | 0.664 | ||
| Use of sleep medications | Score | 0 W | 0.0 | ± | 0.0 | 0.0 | ± | 0.0 | |
| 12 W | 0.0 | ± | 0.0 | 0.0 | ± | 0.0 | - | ||
| Daytime dysfunction | Score | 0 W | 0.4 | ± | 0.6 | 0.2 | ± | 0.4 | |
| 12 W | 0.4 | ± | 0.6 | 0.0 | ± | 0.2 | 0.010 | ||
| Global PSQI | Score | 0 W | 3.9 | ± | 1.3 | 3.5 | ± | 1.3 | |
| 12 W | 4.3 | ± | 1.5 | 3.2 | ± | 1.4 | 0.013 | ||
Means ± standard deviation are shown
Efficacy analysis population: N = 59 (Placebo group N = 29; NMN group N = 30)
Safety analysis
The target population for the safety analysis was all study subjects who initiated the study and consumed the test substance at least once. During the study period, no adverse effects related to the consumption of the test substance were observed. There were 23 cases of adverse events during the test substance intake period experienced by 12 of 30 participants in the placebo group and 20 cases for 13 of 30 participants in the NMN group; all were minor events. A comparison of the occurrence of adverse events between the test substance intake groups showed no significant difference between the type of test substance consumed and the occurrence of events. There were no significant changes in blood hematological and biological parameters between groups (Table 6). Furthermore, there were no significant differences in food intake (energy) or physical activity (steps) between the groups (data not shown).
Table 6.
Safety evaluation of study subjects before (0 W) and after (12 W) NMN intervention
| Unit | Placebo group | NMN group | ANCOVA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Systolic blood pressure | mmHg | 0 W | 124 | ± | 19 | 124 | ± | 12 | |
| 12 W | 124 | ± | 19 | 122 | ± | 15 | 0.494 | ||
| Diastolic blood pressure | mmHg | 0 W | 77 | ± | 13 | 75 | ± | 10 | |
| 12 W | 77 | ± | 13 | 74 | ± | 11 | 0.557 | ||
| Pulse rate | bpm | 0 W | 71 | ± | 9 | 69 | ± | 10 | |
| 12 W | 73 | ± | 9 | 69 | ± | 9 | 0.157 | ||
| White blood cells | /μL | 0 W | 5356.2 | ± | 1084.1 | 5339.3 | ± | 1571.8 | |
| 12 W | 5166.9 | ± | 1257.3 | 5086.7 | ± | 1819.7 | 0.749 | ||
| Red blood cells | × 104/μL | 0 W | 446.6 | ± | 33.5 | 458.5 | ± | 37.1 | |
| 12 W | 441.4 | ± | 37.6 | 451.4 | ± | 41.2 | 0.631 | ||
| Hemoglobin | g/L | 0 W | 137.5 | ± | 11.1 | 140.1 | ± | 10.6 | |
| 12 W | 137.1 | ± | 12.2 | 140.0 | ± | 11.6 | 0.470 | ||
| Hematocrit | % | 0 W | 0.43 | ± | 0.03 | 0.44 | ± | 0.03 | |
| 12 W | 0.42 | ± | 0.03 | 0.42 | ± | 0.03 | 0.582 | ||
| Platelets | × 104/μL | 0 W | 24.5 | ± | 5.0 | 25.0 | ± | 4.6 | |
| 12 W | 24.2 | ± | 4.8 | 25.0 | ± | 4.3 | 0.568 | ||
| Total protein | g/L | 0 W | 70.41 | ± | 2.44 | 71.73 | ± | 3.49 | |
| 12 W | 70.86 | ± | 2.76 | 72.27 | ± | 3.60 | 0.521 | ||
| Albumin | g/L | 0 W | 43.17 | ± | 2.80 | 43.77 | ± | 2.25 | |
| 12 W | 42.90 | ± | 2.76 | 43.73 | ± | 2.03 | 0.333 | ||
| Total bilirubin | mg/dL | 0 W | 0.79 | ± | 0.26 | 0.73 | ± | 0.22 | |
| 12 W | 0.79 | ± | 0.31 | 0.77 | ± | 0.25 | 0.477 | ||
| Alkaline phosphatase | IU/L | 0 W | 73.4 | ± | 18.0 | 64.1 | ± | 19.7 | |
| 12 W | 73.3 | ± | 17.0 | 63.2 | ± | 18.8 | 0.214 | ||
| Lactate dehydrogenase | IU/L | 0 W | 178.9 | ± | 20.5 | 180.5 | ± | 30.4 | |
| 12 W | 170.2 | ± | 15.8 | 175.5 | ± | 28.3 | 0.999 | ||
| Aspartate aminotransferase | IU/L | 0 W | 22.2 | ± | 3.7 | 23.7 | ± | 4.8 | |
| 12 W | 22.1 | ± | 5.0 | 23.3 | ± | 5.1 | 0.858 | ||
| Alanine aminotransferase | IU/L | 0 W | 16.9 | ± | 4.4 | 19.7 | ± | 7.2 | |
| 12 W | 16.4 | ± | 5.3 | 18.4 | ± | 5.6 | 0.771 | ||
| Gamma-glutamyl transpeptidase | IU/L | 0 W | 30.6 | ± | 20.1 | 23.8 | ± | 13.3 | |
| 12 W | 29.9 | ± | 20.6 | 23.9 | ± | 17.2 | 0.908 | ||
| Creatine kinase | IU/L | 0 W | 158.1 | ± | 181.5 | 113.9 | ± | 58.8 | |
| 12 W | 114.6 | ± | 51.7 | 111.1 | ± | 56.9 | 0.233 | ||
| Total cholesterol | mmol/L | 0 W | 5.79 | ± | 0.93 | 5.56 | ± | 0.88 | |
| 12 W | 5.69 | ± | 0.88 | 5.66 | ± | 1.00 | 0.194 | ||
| Triglycerides | mmol/L | 0 W | 1.08 | ± | 0.61 | 0.94 | ± | 0.47 | |
| 12 W | 1.26 | ± | 0.74 | 1.04 | ± | 0.55 | 0.390 | ||
| HDL cholesterol | mmol/L | 0 W | 1.77 | ± | 0.51 | 1.76 | ± | 0.38 | |
| 12 W | 1.68 | ± | 0.52 | 1.72 | ± | 0.38 | 0.194 | ||
| LDL cholesterol | mmol/L | 0 W | 3.34 | ± | 0.65 | 3.15 | ± | 0.72 | |
| 12 W | 3.19 | ± | 0.71 | 3.21 | ± | 0.76 | 0.146 | ||
| Urea nitrogen | mmol/L | 0 W | 5.60 | ± | 1.44 | 6.04 | ± | 1.28 | |
| 12 W | 5.38 | ± | 1.00 | 5.69 | ± | 1.13 | 0.650 | ||
| Creatinine | μmol/L | 0 W | 73.55 | ± | 13.91 | 72.28 | ± | 14.65 | |
| 12 W | 67.58 | ± | 14.32 | 67.66 | ± | 14.96 | 0.354 | ||
| Uric acid | μmol/L | 0 W | 340.9 | ± | 73.0 | 320.4 | ± | 75.2 | |
| 12 W | 343.3 | ± | 83.6 | 326.0 | ± | 82.5 | 0.797 | ||
| Sodium | mmol/L | 0 W | 140.8 | ± | 1.6 | 142.0 | ± | 1.5 | |
| 12 W | 139.2 | ± | 1.7 | 139.5 | ± | 1.6 | 0.897 | ||
| Potassium | mmol/L | 0 W | 4.38 | ± | 0.35 | 4.52 | ± | 0.27 | |
| 12 W | 4.36 | ± | 0.25 | 4.50 | ± | 0.36 | 0.219 | ||
| Chloride | mmol/L | 0 W | 104.4 | ± | 1.7 | 104.5 | ± | 2.2 | |
| 12 W | 103.9 | ± | 1.8 | 103.4 | ± | 2.1 | 0.204 | ||
| Calcium | mmol/L | 0 W | 2.30 | ± | 0.07 | 2.35 | ± | 0.06 | |
| 12 W | 2.33 | ± | 0.07 | 2.37 | ± | 0.09 | 0.545 | ||
| Glucose | mmol/L | 0 W | 5.28 | ± | 0.47 | 5.45 | ± | 0.46 | |
| 12 W | 5.19 | ± | 0.36 | 5.35 | ± | 0.40 | 0.443 | ||
| HbA1c(NGSP) | % | 0 W | 5.49 | ± | 0.31 | 5.45 | ± | 0.26 | |
| 12 W | 5.47 | ± | 0.30 | 5.46 | ± | 0.27 | 0.616 | ||
Means ± standard deviation are shown
Safety analysis population: N = 59 (placebo group N = 29, NMN group N = 30)
Discussion
This study describes the results of a randomized control trial to examine the effect of 12 weeks of NMN intake. Consumption of NMN was associated with increased blood NAD + levels and maintenance of walking time “walking speed test” as part of the SPPB. Moreover, those who consumed NMN reported increased subjective sleep quality, as evidenced by lower scores for the PSQI test compared with the placebo group.
Several earlier studies examined how NMN affects human physical functions. Similar to the results of the present study, a study by Igarashi et al. in which older adults also consumed 250 mg of β-NMN continuously for 12 weeks reported improvements in average walking speed (10 m walk test) and grip strength (left hand) motor function, but there was no significant difference for results of the chair stand test [22]. Meanwhile, Kim et al. reported that consumption of 250 mg of β-NMN for 12 weeks significantly improved performance on a sit-to-stand test repeated 5 times, but observed no difference in walking speed or grip strength [19], whereas a study by Yi et al. showed that individuals who consumed 600 and 900 mg/day of NMN had significantly longer six-minute walking distances on days 30 and 60 of the study period compared to those who were in the placebo group [27].
In our study, daily intake of 250 mg of NMN for 12 weeks was associated with a significant effect on gait function, but there were no differences between the NMN and placebo groups on other motor function tests. The lack of observed differences between groups for the stepping test as well as the reaction time and rise and fall tests may have been because the movements of these tests are not closely related to everyday activities. As such, participants’ understanding and unfamiliarity with the movements may have prevented them from noticing differences that could have been associated with the substance intervention. Previous studies have reported some effects on physical function, especially walking function, of long-term NMN intake, but additional studies are needed to further define ways that NMN affects physical function over the long term.
In our study, the placebo group exhibited a significant decrease in walking speed over the 12-week study period (paired t test, P < 0.001). Notably, the study was conducted in June through September 2022, which coincided with an outbreak of COVID-19. Measures to address this COVID-19 outbreak may have reduced opportunities to go outside and walk. In this study, the amount of physical activity was measured using the number of steps. No differences were observed between groups at all time periods. Therefore, the maintenance of walking speed was thought to be due to the effect of NMN intake, rather than differences in the amount of physical activity.
In the present study levels of NMN, NAD + , NAM, 2-PY, 4-PY, NaMN and NaR in the blood were all higher for the NMN group than for the placebo group at 12 weeks. These findings are consistent with a study by Yoshino et al., which reported that individuals who consumed 10 mg/day NMN for 41 weeks had elevated NAD + levels and plasma 2-PY and 4-PY in PBMCs [28]. Okabe et al. showed that administration of 250 mg/day NMN for 12 weeks resulted in ~ twofold increases in NAD levels in whole blood by week 4 [29]. These results are highly consistent with our results. There were large individual differences in the changes in NAD metabolites after the NMN intake. This is thought to be due to differences in the body pool of NAD + and metabolic rate of niacin.
We also observed a dramatic increase in NaMN levels after NMN administration, which is similar to significant increases in deamidated NAD-related metabolites like NAAD and NAR that were reported in other studies in which NR was administered to human subjects [30–32]. Orally administered NAM is reported to be converted to NA by deamidation mediated by the intestinal microflora, and this NA is absorbed from the colon [33]. We recently demonstrated that orally administered NR is cleaved to NAM by bone marrow stromal cell antigen 1 (BST1) and subsequently converted to NA by the intestinal microflora [34]. The absorbed NA may contribute to NAD synthesis via the Preiss-Handler pathway, which generates NaMN as an intermediate.
Motor skills are involved in nearly all movements needed for everyday life. Especially regarding short-duration physical activities such as a 4-m walk, it is important that the signal from the brain to “move” is transmitted to the muscles via motor nerves that stimulate muscle contraction and body movement. When the neurotransmitter acetylcholine is released from motor nerve terminals and received by acetylcholine receptors on muscle fibers, muscle contraction occurs. Motor nerves connect to muscle fibers at neuromuscular junctions. With aging, innervation of muscle fibers is thought to be lost (denervation), resulting in atrophy of inactive muscles and subsequent decrease in muscle mass [35]. NAD is associated with the structure and function of neuromuscular junctions and muscles [36, 37], while nicotinamide phosphoribosyltransferase (NAMPT), which is involved in NAD synthesis in vivo, is associated with neural stem cell proliferation. Mice having NAMPT knockout in muscle tissue show faster loss of neuromuscular junctions than normal mice and consequently impaired motor function. NMN administration in these NAMPT knockout mice is reported to improve locomotor function through the accumulation of NAD in muscle [38]. Meanwhile, Ito et al. reported decreased levels of β2-adrenergic receptors and reduced sympathetic signaling in mice with hypothalamus-specific knockdown of Slc12a8, which is a known transporter for NMN [39]. In humans, NAD is one of the most prominent metabolites in muscle, and its concentration decreases with age. These lower levels are even more pronounced in older individuals with physical impairments, while exercise-trained older individuals maintained NAD levels that were more similar to those found in younger individuals. NAD abundance also positively correlated with the average number of steps per day as well as with mitochondrial and muscle function [40]. In this study, we observed a significant negative correlation between the change in the 4-m walking time and changes in blood NAD + , 2-PY, and 4-PY at 12 weeks of NMN intake. These results indicate that the higher the NAD + level in the blood, the faster the walking speed is maintained in older adults. Thus, accumulation of NAD may maintain short-term locomotor performance such as 4-m walking speed, and this improvement could occur via neuromodulation.
Here, we observed significant correlation between the degree of change in gait function and the change in blood NAD + levels at 12 weeks but not at 4 weeks. Thus, increased NAD + levels could positively affect the gait, but the lag observed for the physiological effects to appear suggests that the positive changes require that a threshold for NAD + levels be reached.
We also observed improved sleep quality scores for the NMN group relative to the placebo group. Although the mechanism underlying the effect of NMN or NAD + on sleep-related factors has not been fully understood, Shen et al. showed in mice that consumption of NMN was associated with alleviated p-chlorophenylalanine-induced sleep disorders by simultaneous regulation of oxidative stress and the silent mating type information regulation 2 homolog 1 (SIRT1) pathway, as well as by the 5-hydroxytryptamine-ergic, γ-aminobutyric acid-ergic, and immune system pathways [41]. Furthermore, the abovementioned study by Kim et al. showed a significant interaction between results of a 5-time sit-to-stand test and drowsiness in individuals who consumed NMN for 12 weeks [19]. Lack of exercise and decline in physical function due to aging can cause fatigue, which is strongly associated not only with lethargy and depression, but also with sleep disturbances [42, 43]. A population-based study by Hishikawa et al. indicated that over a third (36.2%) of older adults surveyed complained of insomnia [44]. The ability of NMN intake to improve physical functions, including walking, could likely also improve sleep quality as evidenced by the improved sleep scores we observed for the group that consumed NMN. However, this study was not designed for specific inclusion of people with sleep difficulties, so validation of the effectiveness of NMN for sleep will require a study that selects subjects who report difficulties with sleep.
In conclusion, these results show that 12 weeks of NMN intake helped older adults maintain walking speed and improved sleep quality. This is an important report that supports previous findings and provides confidence that long-term intake of NMN has a beneficial effect on walking speed in older adults. Walking speed is strongly associated with mortality risk and is an indicator of physical function and daily living function in the older adults. Individuals who maintain faster walking speed will better maintain life functions and in turn may have longer life expectancy. Exercise programs and medical interventions like NMN that can help maintain walking speed could contribute to extending the length of healthy life.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank CPCC Company Limited for the clinical management and analysis of the results as the practitioners of this study.
Author contribution
MM, SH, and MN contributed to the trial design. MM and SH wrote the report and conducted the data analysis. SE acted as a principal investigator for the clinical trial.
Funding
The clinical trial was fully funded by Meiji Holdings Co., Ltd.
Data availability
All data will be made available on reasonable request to the corresponding author. A proposal will be needed for assessment of the request.
Declarations
Conflict of interest
MM, SH, and MN are employees of Meiji Holding Co., Ltd.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cesari M, Vellas B, Gambassi G. The stress of aging. Exp Gerontol. 2013;48:451–6. 10.1016/j.exger.2012.10.004. 10.1016/j.exger.2012.10.004 [DOI] [PubMed] [Google Scholar]
- 2.Sieber CC. Frailty – from concept to clinical practice. Exp Gerontol. 2017;87:160–7. 10.1016/j.exger.2016.05.004. 10.1016/j.exger.2016.05.004 [DOI] [PubMed] [Google Scholar]
- 3.Chang S-F, Lin P-L. Frail phenotype and mortality prediction: a systematic review and meta-analysis of prospective cohort studies. Int J Nurs Stud. 2015;52:1362–74. 10.1016/j.ijnurstu.2015.04.005. 10.1016/j.ijnurstu.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 4.Nascimento CM, Ingles M, Salvador-Pascual A, Cominetti MR, Gomez-Cabrera MC, Viña J. Sarcopenia, frailty and their prevention by exercise. Free Radic Biol Med. 2019;132:42–9. 10.1016/j.freeradbiomed.2018.08.035. 10.1016/j.freeradbiomed.2018.08.035 [DOI] [PubMed] [Google Scholar]
- 5.Hardy SE, Perera S, Roumani YF, Chandler JM, Studenski SA. Improvement in usual gait speed predicts better survival in older adults. J Am Geriatr Soc. 2007;55:1727–34. 10.1111/j.1532-5415.2007.01413.x. 10.1111/j.1532-5415.2007.01413.x [DOI] [PubMed] [Google Scholar]
- 6.Himann JE, Cunningham DA, Rechnitzer PA, Paterson DH. Age-related changes in speed of walking. Med Sci Sports Exerc. 1988;20:161–6. 10.1249/00005768-198820020-00010. 10.1249/00005768-198820020-00010 [DOI] [PubMed] [Google Scholar]
- 7.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–8. 10.1001/jama.2010.1923. 10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Vries AW, Faber G, Jonkers I, Van Dieen JH, Verschueren SMP. Virtual reality balance training for elderly: similar skiing games elicit different challenges in balance training. Gait Posture. 2018;59:111–6. 10.1016/j.gaitpost.2017.10.006. 10.1016/j.gaitpost.2017.10.006 [DOI] [PubMed] [Google Scholar]
- 9.de Vries NM, van Ravensberg CD, Hobbelen JSM, Olde Rikkert MGM, Staal JB, Nijhuis-van der Sanden MWG. Effects of physical exercise therapy on mobility, physical functioning, physical activity and quality of life in community-dwelling older adults with impaired mobility, physical disability and/or multi-morbidity: a meta-analysis. Ageing Res Rev. 2012;11:136–149. 10.1016/j.arr.2011.11.002 [DOI] [PubMed]
- 10.Liu-Ambrose T, Khan KM, Eng JJ, Janssen PA, Lord SR, Mckay HA. Resistance and agility training reduce fall risk in women aged 75 to 85 with low bone mass: a 6-month randomized, controlled trial. J Am Geriatr Soc. 2004;52:657–65. 10.1111/j.1532-5415.2004.52200.x. 10.1111/j.1532-5415.2004.52200.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peluso A, Damgaard MV, Mori MAS, Treebak JT. Age-dependent decline of nad(+)-universal truth or confounded consensus? Nutrients. 2021;14:101. 10.3390/nu14010101. 10.3390/nu14010101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.She J, Sheng R, Qin ZH. Pharmacology and potential implications of nicotinamide adenine dinucleotide precursors. Aging Dis. 2021;12:1879–97. 10.14336/ad.2021.0523. 10.14336/ad.2021.0523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu X, Raju RP. Regulation of nad(+) metabolism in aging and disease. Metabolism. 2022;126:154923. 10.1016/j.metabol.2021.154923. 10.1016/j.metabol.2021.154923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang EF, Lautrup S, Hou Y, Demarest TG, Croteau DL, Mattson MP, et al. Nad(+) in aging: molecular mechanisms and translational implications. Trends Mol Med. 2017;23:899–916. 10.1016/j.molmed.2017.08.001. 10.1016/j.molmed.2017.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radenkovic D, Reason, Verdin E. Clinical evidence for targeting NAD therapeutically. Pharmaceuticals (Basel). 2020;13:247. 10.3390/ph13090247 [DOI] [PMC free article] [PubMed]
- 16.Zhang M, Ying W. Nad(+) deficiency is a common central pathological factor of a number of diseases and aging: mechanisms and therapeutic implications. Antioxid Redox Signal. 2019;30:890–905. 10.1089/ars.2017.7445. 10.1089/ars.2017.7445 [DOI] [PubMed] [Google Scholar]
- 17.Yoshino J, Baur JA, Imai SI. Nad(+) intermediates: the biology and therapeutic potential of nmn and nr. Cell Metab. 2018;27:513–28. 10.1016/j.cmet.2017.11.002. 10.1016/j.cmet.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campagna R, Vignini A. Nad(+) homeostasis and nad(+)-consuming enzymes: Implications for vascular health. Antioxidants (Basel). 2023;12:376. 10.3390/antiox12020376. 10.3390/antiox12020376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim M, Seol J, Sato T, Fukamizu Y, Sakurai T, Okura T. Effect of 12-week intake of nicotinamide mononucleotide on sleep quality, fatigue, and physical performance in older Japanese adults: a randomized, double-blind placebo-controlled study. Nutrients. 2022;14:755. 10.3390/nu14040755. 10.3390/nu14040755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolkove N, Elkholy O, Baltzan M, Palayew M. Sleep and aging: 1. Sleep disorders commonly found in older people. Canadian Med Assoc J. 2007;176:1299–304. 10.1503/cmaj.060792. 10.1503/cmaj.060792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prinz PN, Vitiello MV, Raskind MA, Thorpy MJ. Sleep disorders and aging. N Eng J Med. 1990;323:520–6. 10.1056/NEJM199008233230805. 10.1056/NEJM199008233230805 [DOI] [PubMed] [Google Scholar]
- 22.Igarashi M, Nakagawa-Nagahama Y, Miura M, Kashiwabara K, Yaku K, Sawada M, et al. Chronic nicotinamide mononucleotide supplementation elevates blood nicotinamide adenine dinucleotide levels and alters muscle function in healthy older men. Npj Aging. 2022;8:5. 10.1038/s41514-022-00084-z. 10.1038/s41514-022-00084-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oba K, Ishikawa J, Tamura Y, Fujita Y, Ito M, Iizuka A, et al. Serum growth differentiation factor 15 level is associated with muscle strength and lower extremity function in older patients with cardiometabolic disease. Geriatr Gerontol Int. 2020;20:980–7. 10.1111/ggi.14021. 10.1111/ggi.14021 [DOI] [PubMed] [Google Scholar]
- 24.de Fátima Ribeiro Silva C, Ohara DG, Matos AP, Pinto ACPN, Pegorari MS. Short physical performance battery as a measure of physical performance and mortality predictor in older adults: a comprehensive literature review. Int J Environ Res Public Health. 2021;18:10612. 10.3390/ijerph182010612 [DOI] [PMC free article] [PubMed]
- 25.Konuma H, Hirose H, Yokoyama K. Relationship of the japanese translation of the profile of mood states second edition (POMS 2®) to the First Edition (POMS®). Juntendo Med J. 2015; 61: 517–519.10.14789/jmj.61.517
- 26.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. 10.1016/0165-1781(89)90047-4. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 27.Yi L, Maier AB, Tao R, Lin Z, Vaidya A, Pendse S, et al. The efficacy and safety of β-nicotinamide mononucleotide (nmn) supplementation in healthy middle-aged adults: a randomized, multicenter, double-blind, placebo-controlled, parallel-group, dose-dependent clinical trial. GeroScience. 2023;45:29–43. 10.1007/s11357-022-00705-1. 10.1007/s11357-022-00705-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshino M, Yoshino J, Kayser BD, Patti GJ, Franczyk MP, Mills KF, et al. Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women. Science. 2021;372:1224–9. 10.1126/science.abe9985. 10.1126/science.abe9985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okabe K, Yaku K, Uchida Y, Fukamizu Y, Sato T, Sakurai T, et al. Oral administration of nicotinamide mononucleotide is safe and efficiently increases blood nicotinamide adenine dinucleotide levels in healthy subjects. Front Nutr. 2022;9:868640. 10.3389/fnut.2022.868640. 10.3389/fnut.2022.868640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trammell SAJ, Schmidt MS, Weidemann BJ, Redpath P, Jaksch F, Dellinger RW, et al. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun. 2016;7:12948. 10.1038/ncomms12948. 10.1038/ncomms12948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martens CR, Denman BA, Mazzo MR, Armstrong ML, Reisdorph N, McQueen MB, et al. Chronic nicotinamide riboside supplementation is well-tolerated and elevates nad+ in healthy middle-aged and older adults. Nat Commun. 2018;9:1286. 10.1038/s41467-018-03421-7. 10.1038/s41467-018-03421-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dollerup OL, Christensen B, Svart M, Schmidt MS, Sulek K, Ringgaard S, et al. A randomized placebo-controlled clinical trial of nicotinamide riboside in obese men: Safety, insulin-sensitivity, and lipid-mobilizing effects. Am J Clin Nutr. 2018;108:343–53. 10.1093/ajcn/nqy132. 10.1093/ajcn/nqy132 [DOI] [PubMed] [Google Scholar]
- 33.Shats I, Williams JG, Liu J, Makarov MV, Wu X, Lih FB, et al. Bacteria boost mammalian host NAD metabolism by engaging the deamidated biosynthesis pathway. Cell Metab. 2020;31:564-579.e567. 10.1016/j.cmet.2020.02.001. 10.1016/j.cmet.2020.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yaku K, Palikhe S, Izumi H, Yoshida T, Hikosaka K, Hayat F, et al. Bst1 regulates nicotinamide riboside metabolism via its glycohydrolase and base-exchange activities. Nat Commun. 2021;12:6767. 10.1038/s41467-021-27080-3. 10.1038/s41467-021-27080-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faulkner JA, Larkin LM, Claflin DR, Brooks SV. Age-related changes in the structure and function of skeletal muscles. Clin Exp Pharmacol Physiol. 2007;34:1091–6. 10.1111/j.1440-1681.2007.04752.x. 10.1111/j.1440-1681.2007.04752.x [DOI] [PubMed] [Google Scholar]
- 36.Bailey EC, Alrowaished SS, Kilroy EA, Crooks ES, Drinkert DM, Karunasiri CM, et al. Nad+ improves neuromuscular development in a zebrafish model of FKRP-associated dystroglycanopathy. Skelet Muscle. 2019;9:21. 10.1186/s13395-019-0206-1. 10.1186/s13395-019-0206-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Zhang Q, Bao R, Zhang N, Wang Y, Polo-Parada L, et al. Deletion of nampt in projection neurons of adult mice leads to motor dysfunction, neurodegeneration, and death. Cell Rep. 2017;20:2184–200. 10.1016/j.celrep.2017.08.022. 10.1016/j.celrep.2017.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lundt S, Zhang N, Wang X, Polo-Parada L, Ding S. The effect of NAMPT deletion in projection neurons on the function and structure of neuromuscular junction (NMJ) in mice. Sci Rep. 2020;10:99. 10.1038/s41598-019-57085-4. 10.1038/s41598-019-57085-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ito N, Takatsu A, Ito H, Koike Y, Yoshioka K, Kamei Y, et al. Slc12a8 in the lateral hypothalamus maintains energy metabolism and skeletal muscle functions during aging. Cell Rep. 2022;40:111131. 10.1016/j.celrep.2022.111131. 10.1016/j.celrep.2022.111131 [DOI] [PubMed] [Google Scholar]
- 40.Janssens GE, Grevendonk L, Perez RZ, Schomakers BV, de Vogel-van den Bosch J, Geurts JMW, et al. Healthy aging and muscle function are positively associated with nad+ abundance in humans. Nature Aging. 2022; 2: 254–263. 10.1038/s43587-022-00174-3 [DOI] [PubMed]
- 41.Shen C-Y, Li X-Y, Ma P-Y, Li H-L, Xiao B, Cai W-F, et al. Nicotinamide mononucleotide (NMN) and NMN-rich product supplementation alleviate p-chlorophenylalanine-induced sleep disorders. Journal of Functional Foods. 2022;91:105031. 10.1016/j.jff.2022.105031. 10.1016/j.jff.2022.105031 [DOI] [Google Scholar]
- 42.Yu DSF, Lee DTF, Man NW. Fatigue among older people: a review of the research literature. Int J Nurs Stud. 2010;47:216–28. 10.1016/j.ijnurstu.2009.05.009. 10.1016/j.ijnurstu.2009.05.009 [DOI] [PubMed] [Google Scholar]
- 43.Wijeratne C, Hickie I, Brodaty H. The characteristics of fatigue in an older primary care sample. J Psychosom Res. 2007;62:153–8. 10.1016/j.jpsychores.2006.09.011. 10.1016/j.jpsychores.2006.09.011 [DOI] [PubMed] [Google Scholar]
- 44.Hishikawa N, Fukui Y, Sato K, Ohta Y, Yamashita T, Abe K. Cognitive and affective functions associated with insomnia: a population-based study. Neurol Res. 2017;39:331–6. 10.1080/01616412.2017.1281200. 10.1080/01616412.2017.1281200 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data will be made available on reasonable request to the corresponding author. A proposal will be needed for assessment of the request.