Abstract
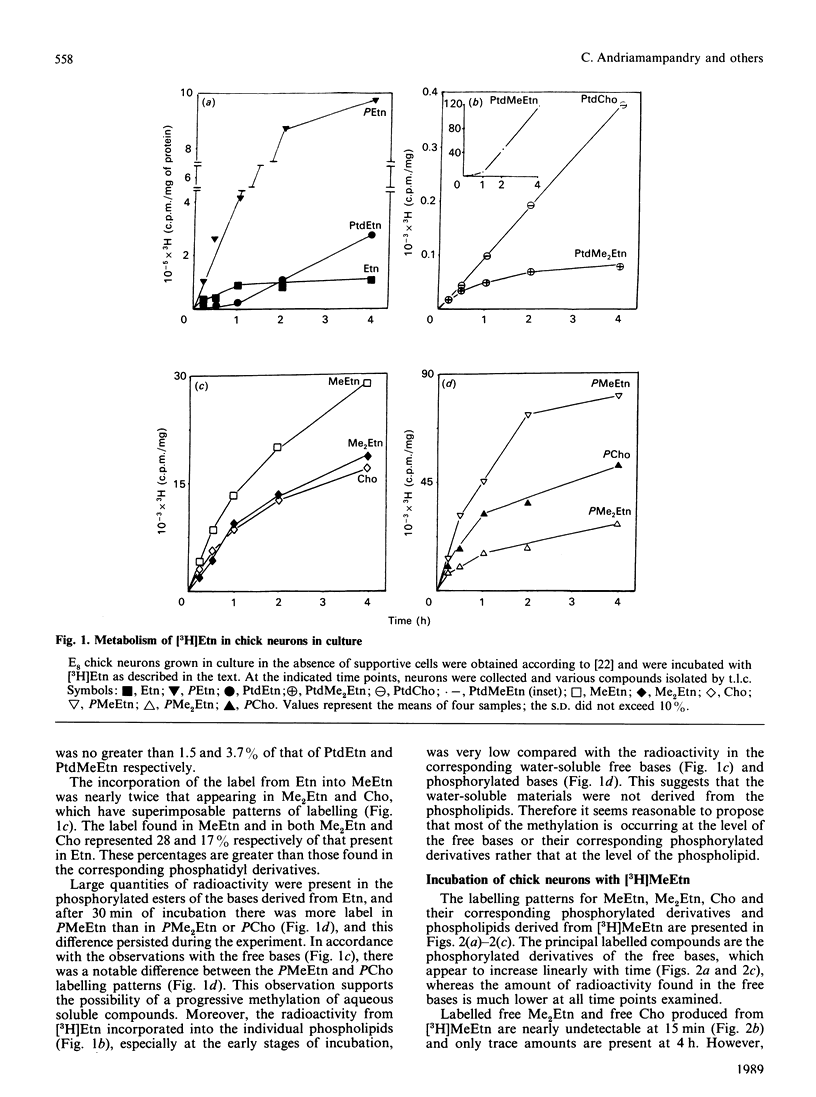
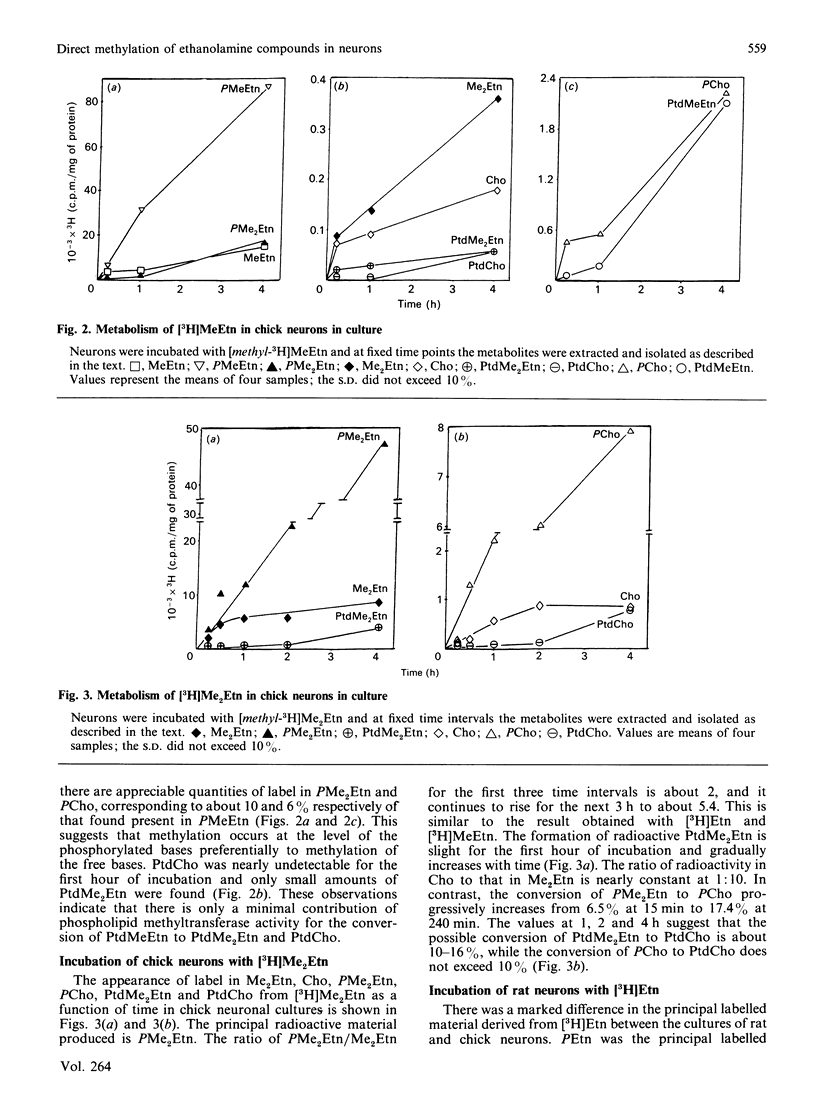
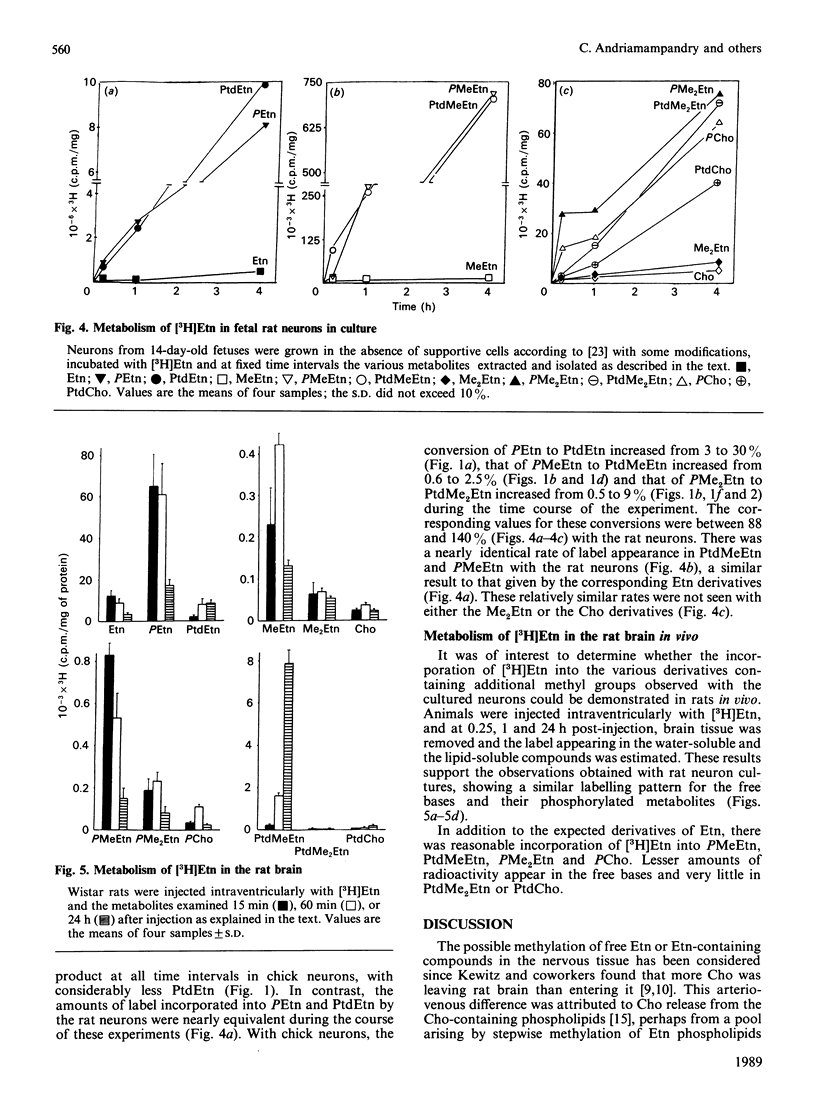
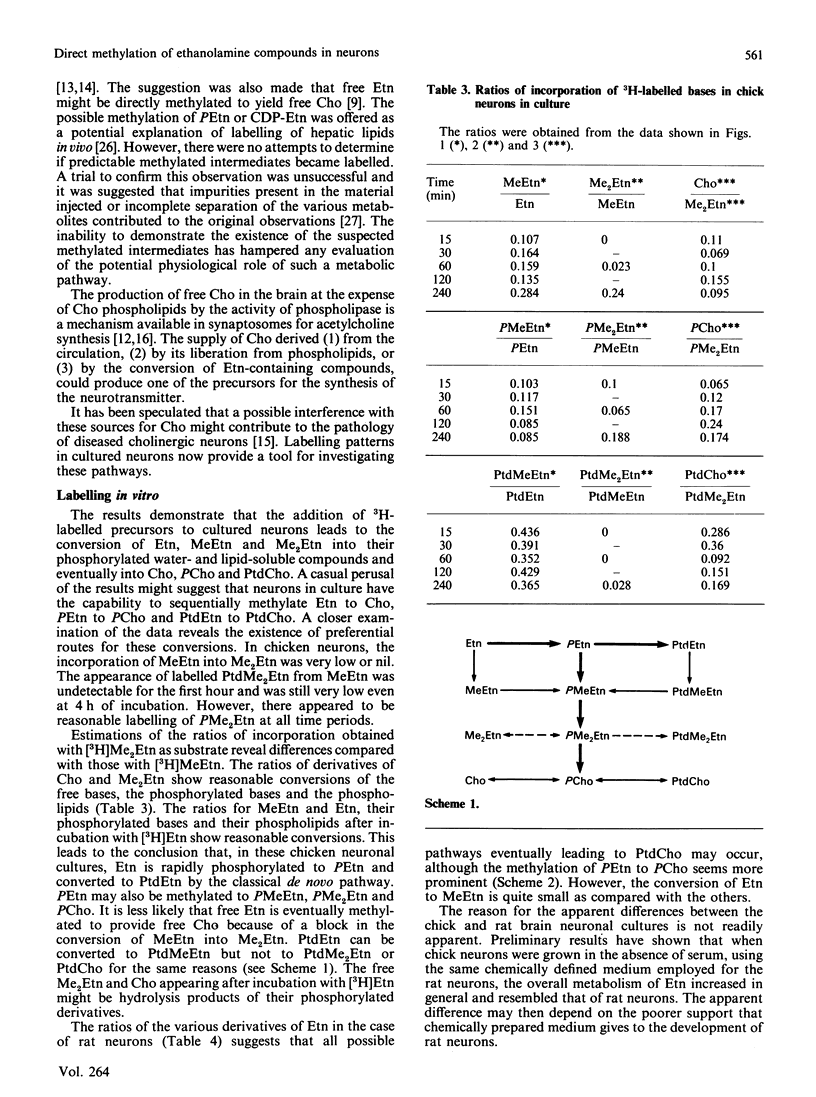
The incubation of neurons from chick embryos in primary culture with [3H]ethanolamine revealed the conversion of this base into monomethyl, dimethyl and choline derivatives, including the corresponding free bases. Labelling with [methyl-3H]monomethylethanolamine and [methyl-3H]dimethylethanolamine supported the conclusion that in chick neuron cultures, phosphoethanolamine appears to be the preferential substrate for methylation, rather than ethanolamine or phosphatidylethanolamine. The methylation of the latter two compounds, in particular that of phosphatidylethanolamine, was seemingly stopped at the level of their monomethyl derivatives. Fetal rat neurons in primary culture incubated with [3H]ethanolamine showed similar results to those observed with chick neurones. However, phosphoethanolamine and phosphatidylethanolamine and, to a lesser extent, free ethanolamine, appeared to be possible substrates for methylation reactions. The methylation of water-soluble ethanolamine compounds de novo was further confirmed by experiments performed in vivo by intraventricular injection of [3H]ethanolamine. Phosphocholine and the monomethyl and dimethyl derivatives of ethanolamine were detected in the brain 15 min after injection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blusztajn J. K., Liscovitch M., Richardson U. I. Synthesis of acetylcholine from choline derived from phosphatidylcholine in a human neuronal cell line. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5474–5477. doi: 10.1073/pnas.84.15.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blusztajn J. K., Wurtman R. J. Choline biosynthesis by a preparation enriched in synaptosomes from rat brain. Nature. 1981 Apr 2;290(5805):417–418. doi: 10.1038/290417a0. [DOI] [PubMed] [Google Scholar]
- Blusztajn J. K., Zeisel S. H., Wurtman R. J. Synthesis of lecithin (phosphatidylcholine) from phosphatidylethanolamine in bovine brain. Brain Res. 1979 Dec 28;179(2):319–327. doi: 10.1016/0006-8993(79)90447-5. [DOI] [PubMed] [Google Scholar]
- Cornford E. M., Braun L. D., Oldendorf W. H. Carrier mediated blood-brain barrier transport of choline and certain choline analogs. J Neurochem. 1978 Feb;30(2):299–308. doi: 10.1111/j.1471-4159.1978.tb06530.x. [DOI] [PubMed] [Google Scholar]
- Crews F. T., Hirata F., Axelrod J. Identification and properties of methyltransferases that synthesize phosphatidylcholine in rat brain synaptosomes. J Neurochem. 1980 Jun;34(6):1491–1498. doi: 10.1111/j.1471-4159.1980.tb11229.x. [DOI] [PubMed] [Google Scholar]
- Dainous F., Freysz L., Mozzi R., Dreyfus H., Louis J. C., Porcellati G., Massarelli R. Synthesis of choline phospholipids in neuronal and glial cell cultures by the methylation pathway. FEBS Lett. 1982 Sep 6;146(1):221–223. doi: 10.1016/0014-5793(82)80740-0. [DOI] [PubMed] [Google Scholar]
- Dainous F., Kanfer J. N. The incorporation of monomethylethanolamine and dimethylethanolamine in fetal brain aggregating cell culture. Neurochem Res. 1988 Jan;13(1):1–8. doi: 10.1007/BF00971848. [DOI] [PubMed] [Google Scholar]
- Dross K., Kewitz H. Concentration and origin of choline in the rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1972;274(1):91–106. doi: 10.1007/BF00501010. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Gensburger C., Labourdette G., Sensenbrenner M. Influence of meningeal cells on the proliferation and maturation of rat neuroblasts in culture. Exp Brain Res. 1986;63(2):321–330. doi: 10.1007/BF00236849. [DOI] [PubMed] [Google Scholar]
- HANAHAN D. J., BROCKERHOFF H., BARRON E. J. The site of attack of phospholipase (lecithinase) A on lecithin: a re-evaluation. Position of fatty acids on lecithins and triglycerides. J Biol Chem. 1960 Jul;235:1917–1923. [PubMed] [Google Scholar]
- Hattori H., Kanfer J. N. Synaptosomal phospholipase D: potential role in providing choline for acetylcholine synthesis. Biochem Biophys Res Commun. 1984 Nov 14;124(3):945–949. doi: 10.1016/0006-291x(84)91049-0. [DOI] [PubMed] [Google Scholar]
- Jope R. S. High affinity choline transport and acetylCoA production in brain and their roles in the regulation of acetylcholine synthesis. Brain Res. 1979 Dec;180(3):313–344. doi: 10.1016/0165-0173(79)90009-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mozzi R., Porcellati G. Conversion of phosphatidylethanolamine to phosphatidylcholine in rat brain by the methylation pathway. FEBS Lett. 1979 Apr 15;100(2):363–366. doi: 10.1016/0014-5793(79)80370-1. [DOI] [PubMed] [Google Scholar]
- Nehme D., Rondeau E., Paillard F., Moreau J. F., Nussaume O., Kanfer A., Sraer J. D. Aseptic necrosis of bone following renal transplantation: relation with hyperparathyroidism. Nephrol Dial Transplant. 1989;4(2):123–128. [PubMed] [Google Scholar]
- Pardridge W. M., Oldendorf W. H. Transport of metabolic substrates through the blood-brain barrier. J Neurochem. 1977 Jan;28(1):5–12. doi: 10.1111/j.1471-4159.1977.tb07702.x. [DOI] [PubMed] [Google Scholar]
- Pettmann B., Louis J. C., Sensenbrenner M. Morphological and biochemical maturation of neurones cultured in the absence of glial cells. Nature. 1979 Oct 4;281(5730):378–380. doi: 10.1038/281378a0. [DOI] [PubMed] [Google Scholar]
- Rouser G., Fkeischer S., Yamamoto A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970 May;5(5):494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- Salerno D. M., Beeler D. A. The biosynthesis of phospholipids and their precursors in rat liver involving de novo methylation, and base-exchange pathways, in vivo. Biochim Biophys Acta. 1973 Dec 20;326(3):325–338. doi: 10.1016/0005-2760(73)90134-3. [DOI] [PubMed] [Google Scholar]
- Sundler R., Akesson B. Biosynthesis of phosphatidylethanolamines and phosphatidylcholines from ethanolamine and choline in rat liver. Biochem J. 1975 Feb;146(2):309–315. doi: 10.1042/bj1460309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucek S. Problems in the organization and control of acetylcholine synthesis in brain neurons. Prog Biophys Mol Biol. 1984;44(1):1–46. doi: 10.1016/0079-6107(84)90011-7. [DOI] [PubMed] [Google Scholar]
- Wecker L., Trommer B. A. Effects of chronic (dietary) choline availability on the transport of choline across the blood-brain barrier. J Neurochem. 1984 Dec;43(6):1762–1765. doi: 10.1111/j.1471-4159.1984.tb06107.x. [DOI] [PubMed] [Google Scholar]
- Zeisel S. H. Formation of unesterified choline by rat brain. Biochim Biophys Acta. 1985 Jul 9;835(2):331–343. doi: 10.1016/0005-2760(85)90289-9. [DOI] [PubMed] [Google Scholar]


