Abstract
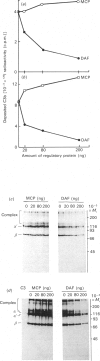
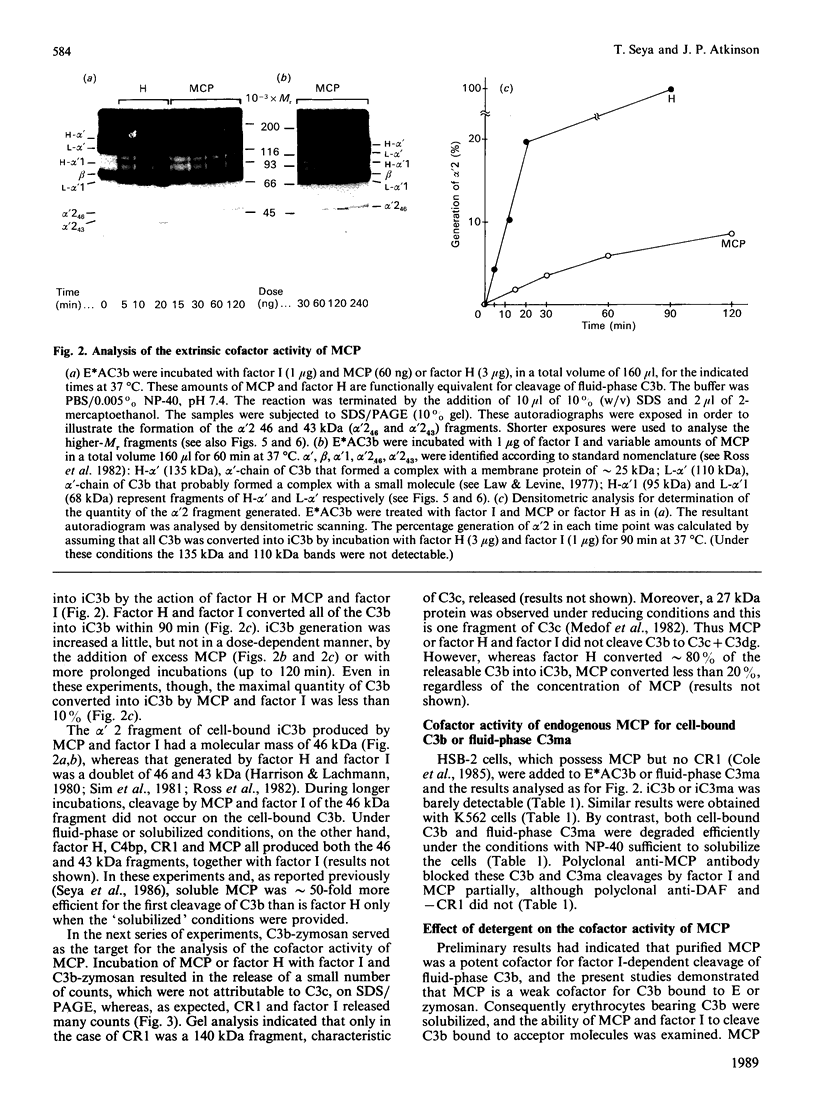
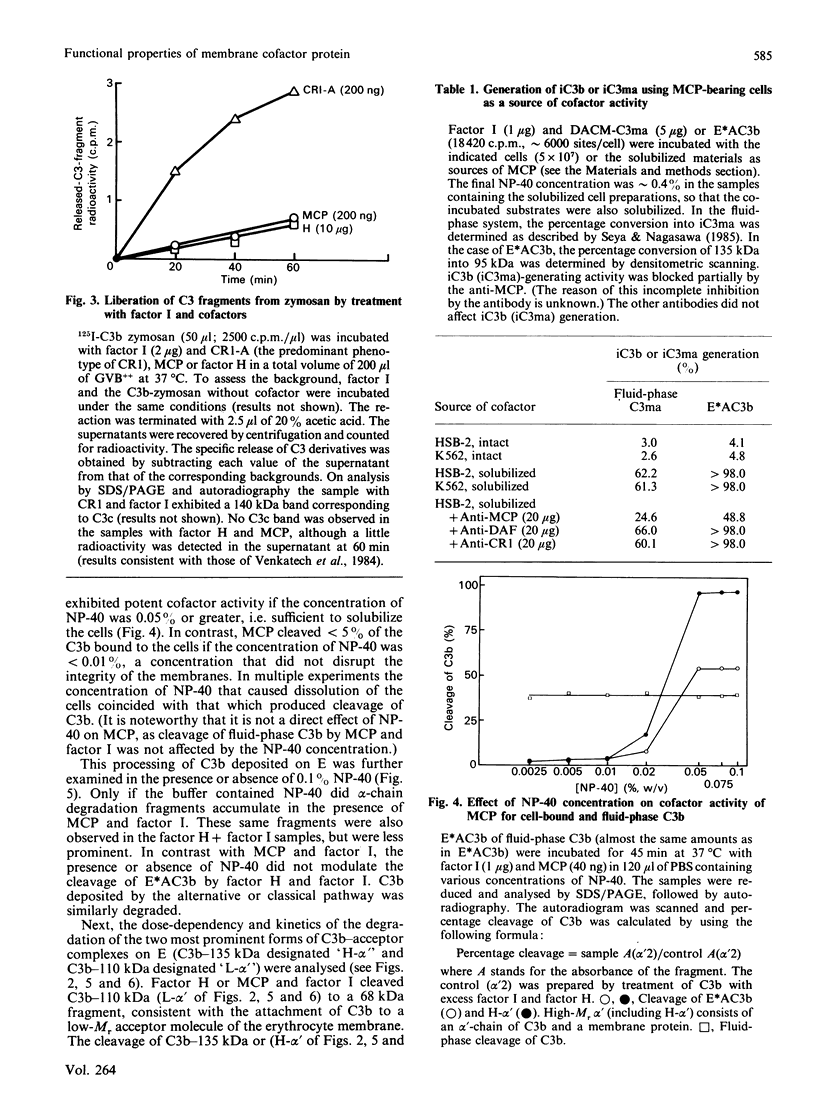
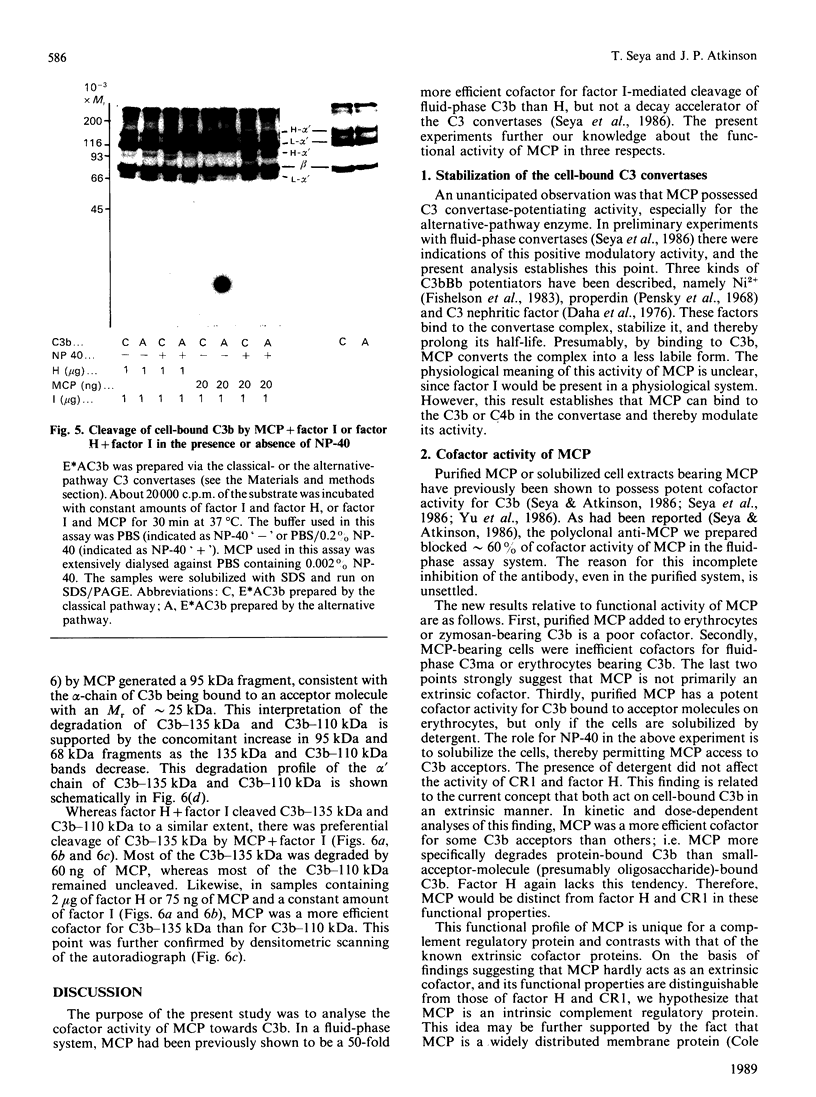
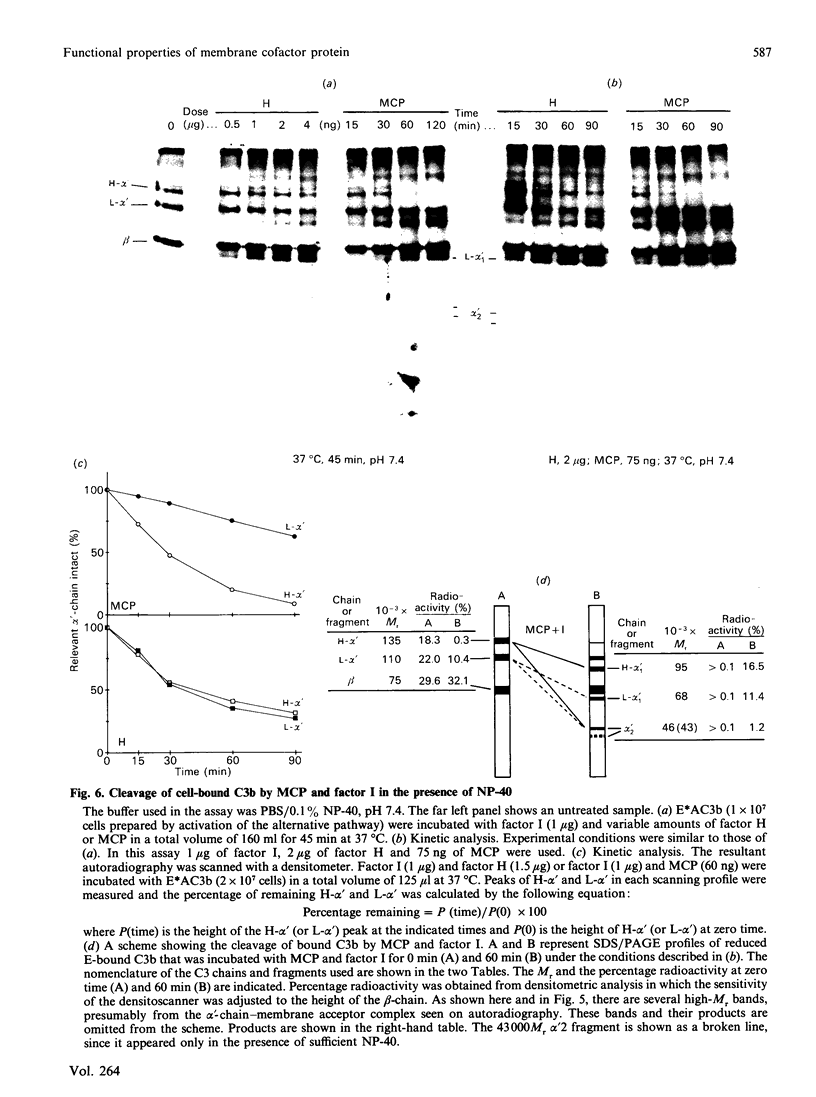
Membrane cofactor protein (MCP or gp45-70) of the complement system is a cofactor for factor I-mediated cleavage of fluid-phase C3b and C3b-like C3, which opens the thioester bond. In the present study the activity of MCP was further characterized. Unexpectedly, in the absence of factor I, MCP stabilized the alternative- and, to a lesser extent, the classical-pathway cell-bound C3 convertases and thereby enhanced C3b deposition. Soluble MCP, if added exogenously, hardly functioned as cofactor for the cleavage of erythrocyte-bound C3b to iC3b; i.e. its activity, compared with the cofactor activity of factor H, was inefficient, since less than 10% of the bound C3b was MCP-sensitive. Further, exogenously added soluble MCP was also a weak cofactor for the cleavage of C3b bound to zymosan. Likewise, factor I, in the presence of cells bearing MCP, cleaved fluid-phase C3b inefficiently. These results imply that MCP has very little extrinsic cofactor activity for factor I. In contrast, exogenously added MCP and factor I mediated efficient cleavage of erythrocyte-bound C3b if the concentration of Nonidet P40 was sufficient to solubilize the cells. Interestingly, soluble MCP and factor I degraded C3b attached to certain solubilized acceptor membrane molecules more readily than others. The cleavage reaction of fluid-phase and cell-bound C3b by soluble MCP and factor I produced iC3b, but no C3c and C3dg. These and prior data indicate that soluble MCP has potent cofactor activity for fluid-phase C3b or C3b bound to solubilized molecules, but acts inefficiently towards C3b on other cells. This functional profile is unique for a C3b/C4b binding protein and, taken together with its wide tissue distribution, suggests an important role for MCP in the regulation of the complement system.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bora N. S., Lublin D. M., Kumar B. V., Hockett R. D., Holers V. M., Atkinson J. P. Structural gene for human membrane cofactor protein (MCP) of complement maps to within 100 kb of the 3' end of the C3b/C4b receptor gene. J Exp Med. 1989 Feb 1;169(2):597–602. doi: 10.1084/jem.169.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J. L., Housley G. A., Jr, Dykman T. R., MacDermott R. P., Atkinson J. P. Identification of an additional class of C3-binding membrane proteins of human peripheral blood leukocytes and cell lines. Proc Natl Acad Sci U S A. 1985 Feb;82(3):859–863. doi: 10.1073/pnas.82.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daha M. R., Fearon D. T., Austen K. F. C3 nephritic factor (C3NeF): stabilization of fluid phase and cell-bound alternative pathway convertase. J Immunol. 1976 Jan;116(1):1–7. [PubMed] [Google Scholar]
- Ensky J., Hinz C. F., Jr, Todd E. W., Wedgwood R. J., Boyer J. T., Lepow I. H. Properties of highly purified human properdin. J Immunol. 1968 Jan;100(1):142–158. [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F. Activation of the alternative complement pathway due to resistance of zymosan-bound amplification convertase to endogenous regulatory mechanisms. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1683–1687. doi: 10.1073/pnas.74.4.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Regulation of the amplification C3 convertase of human complement by an inhibitory protein isolated from human erythrocyte membrane. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5867–5871. doi: 10.1073/pnas.76.11.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishelson Z., Pangburn M. K., Müller-Eberhard H. J. C3 convertase of the alternative complement pathway. Demonstration of an active, stable C3b, Bb (Ni) complex. J Biol Chem. 1983 Jun 25;258(12):7411–7415. [PubMed] [Google Scholar]
- Harrison R. A., Lachmann P. J. Novel cleavage products of the third component of human complement. Mol Immunol. 1980 Feb;17(2):219–228. doi: 10.1016/0161-5890(80)90074-7. [DOI] [PubMed] [Google Scholar]
- Iida K., Nussenzweig V. Complement receptor is an inhibitor of the complement cascade. J Exp Med. 1981 May 1;153(5):1138–1150. doi: 10.1084/jem.153.5.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Law S. K., Levine R. P. Interaction between the third complement protein and cell surface macromolecules. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2701–2705. doi: 10.1073/pnas.74.7.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law S. K., Lichtenberg N. A., Levine R. P. Evidence for an ester linkage between the labile binding site of C3b and receptive surfaces. J Immunol. 1979 Sep;123(3):1388–1394. [PubMed] [Google Scholar]
- Lublin D. M., Liszewski M. K., Post T. W., Arce M. A., Le Beau M. M., Rebentisch M. B., Lemons L. S., Seya T., Atkinson J. P. Molecular cloning and chromosomal localization of human membrane cofactor protein (MCP). Evidence for inclusion in the multigene family of complement-regulatory proteins. J Exp Med. 1988 Jul 1;168(1):181–194. doi: 10.1084/jem.168.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Iida K., Mold C., Nussenzweig V. Unique role of the complement receptor CR1 in the degradation of C3b associated with immune complexes. J Exp Med. 1982 Dec 1;156(6):1739–1754. doi: 10.1084/jem.156.6.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Kinoshita T., Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984 Nov 1;160(5):1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa S., Ichihara C., Stroud R. M. Cleavage of C4b by C3b inactivator: production of a nicked form of C4b, C4b', as an intermediate cleavage product of C4b by C3b inactivator. J Immunol. 1980 Aug;125(2):578–582. [PubMed] [Google Scholar]
- Nagasawa S., Mizuguchi K., Ichihara C., Koyama J. Limited chymotryptic cleavage of human C4-binding protein: isolation of a carbohydrate-containing core domain and an active fragment. J Biochem. 1982 Oct;92(4):1329–1332. doi: 10.1093/oxfordjournals.jbchem.a134052. [DOI] [PubMed] [Google Scholar]
- Nagasawa S., Stroud R. M. Cleavage of C2 by C1s into the antigenically distinct fragments C2a and C2b: demonstration of binding of C2b to C4b. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2998–3001. doi: 10.1073/pnas.74.7.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa S., Stroud R. M. Mechanism of action of the C3b inactivator: requirement for a high molecular weight cofactor (C3b-C4bINA cofactor) and production of a new C3b derivative (C3b'). Immunochemistry. 1977 Nov-Dec;14(11-12):749–756. doi: 10.1016/0019-2791(77)90345-7. [DOI] [PubMed] [Google Scholar]
- Nagasawa S., Stroud R. M. Purification and characterization of a macromolecular weight cofactor for C3b-inactivator, C4bC3bINA-cofactor, of human plasma. Mol Immunol. 1980 Nov;17(11):1365–1372. doi: 10.1016/0161-5890(80)90005-x. [DOI] [PubMed] [Google Scholar]
- Nicholson-Weller A., Burge J., Fearon D. T., Weller P. F., Austen K. F. Isolation of a human erythrocyte membrane glycoprotein with decay-accelerating activity for C3 convertases of the complement system. J Immunol. 1982 Jul;129(1):184–189. [PubMed] [Google Scholar]
- Nicholson-Weller A., March J. P., Rosenfeld S. I., Austen K. F. Affected erythrocytes of patients with paroxysmal nocturnal hemoglobinuria are deficient in the complement regulatory protein, decay accelerating factor. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5066–5070. doi: 10.1073/pnas.80.16.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Deficiency of an erythrocyte membrane protein with complement regulatory activity in paroxysmal nocturnal hemoglobinuria. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5430–5434. doi: 10.1073/pnas.80.17.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G. D., Lambris J. D., Cain J. A., Newman S. L. Generation of three different fragments of bound C3 with purified factor I or serum. I. Requirements for factor H vs CR1 cofactor activity. J Immunol. 1982 Nov;129(5):2051–2060. [PubMed] [Google Scholar]
- Seya T., Ballard L. L., Bora N. S., Kumar V., Cui W., Atkinson J. P. Distribution of membrane cofactor protein of complement on human peripheral blood cells. An altered form is found on granulocytes. Eur J Immunol. 1988 Aug;18(8):1289–1294. doi: 10.1002/eji.1830180821. [DOI] [PubMed] [Google Scholar]
- Seya T., Farries T., Nickells M., Atkinson J. P. Additional forms of human decay-accelerating factor (DAF). J Immunol. 1987 Aug 15;139(4):1260–1267. [PubMed] [Google Scholar]
- Seya T., Holers V. M., Atkinson J. P. Purification and functional analysis of the polymorphic variants of the C3b/C4b receptor (CR1) and comparison with H, C4b-binding protein (C4bp), and decay accelerating factor (DAF). J Immunol. 1985 Oct;135(4):2661–2667. [PubMed] [Google Scholar]
- Seya T., Nagasawa S. A fluorometric method for determination of C3b inactivator. Clin Chim Acta. 1982 Mar 12;119(3):189–196. doi: 10.1016/0009-8981(82)90331-x. [DOI] [PubMed] [Google Scholar]
- Seya T., Nagasawa S. Limited proteolysis of complement protein C3b by regulatory enzyme C3b inactivator: isolation and characterization of a biologically active fragment, C3d,g. J Biochem. 1985 Jan;97(1):373–382. doi: 10.1093/oxfordjournals.jbchem.a135064. [DOI] [PubMed] [Google Scholar]
- Seya T., Nagasawa S., Matsukura M., Hasegawa H., Atkinson J. P. Generation of C3d,g and C3d by urokinase-treated plasma in association with fibrinolysis. Complement. 1985;2(2-3):165–174. doi: 10.1159/000467857. [DOI] [PubMed] [Google Scholar]
- Seya T., Turner J. R., Atkinson J. P. Purification and characterization of a membrane protein (gp45-70) that is a cofactor for cleavage of C3b and C4b. J Exp Med. 1986 Apr 1;163(4):837–855. doi: 10.1084/jem.163.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim E., Wood A. B., Hsiung L. M., Sim R. B. Pattern of degradation of human complement fragment, C3b. FEBS Lett. 1981 Sep 14;132(1):55–60. doi: 10.1016/0014-5793(81)80426-7. [DOI] [PubMed] [Google Scholar]
- Venkatesh Y. P., Minich T. M., Law S. K., Levine R. P. Natural release of covalently bound C3b from cell surfaces and the study of this phenomenon in the fluid-phase system. J Immunol. 1984 Mar;132(3):1435–1439. [PubMed] [Google Scholar]
- Volanakis J. E., Schrohenloher R. E., Stroud R. M. Human factor D of the alternative complement pathway: purification and characterization. J Immunol. 1977 Jul;119(1):337–342. [PubMed] [Google Scholar]
- Yu G. H., Holers V. M., Seya T., Ballard L., Atkinson J. P. Identification of a third component of complement-binding glycoprotein of human platelets. J Clin Invest. 1986 Aug;78(2):494–501. doi: 10.1172/JCI112601. [DOI] [PMC free article] [PubMed] [Google Scholar]