Abstract
Ulcerative colitis (UC) is associated with changed dietary habits and mainly linked with the gut microbiota dysbiosis, necroptosis of epithelial cells, and mucosal ulcerations. Liver dysfunction and abnormal level of liver metabolism indices were identified in UC patients, suggesting a close interaction between gut and liver disorders. Methionine-choline deficient diet (MCD) has been shown to induce persistent alterations of gut microbiota and metabolome during hepatitis. In this study we further explored the disease phenotypes in UC patients and investigated whether MCD functioned as a trigger for UC susceptibility. After assessing 88 serum specimens from UC patients, we found significant liver dysfunction and dyslipidemia including abnormal ALT, AST, TG, TC, LDL-c and HDL-c. Liver dysfunction and dyslipidemia were confirmed in DSS-induced colitis mice. We fed mice with MCD for 14 days to cause mild liver damage, and then treated with DSS for 7 days. We found that MCD intake significantly exacerbated the pathogenesis of mucosal inflammation in DSS-induced acute, progressive, and chronic colitis, referring to promotion of mucosal ulcers, colon shortening, diarrhea, inflammatory immune cell infiltration, cytokines release, and abnormal activation of inflammatory macrophages in colon and liver specimens. Intraperitoneal injection of clodronate liposomes to globally delete macrophages dramatically compromised the pathogenesis of MCD-triggering colitis. In addition, MCD intake markedly changed the production pattern of short-chain fatty acids (SCFAs) in murine stools, colons, and livers. We demonstrated that MCD-induced colitis pathogenesis largely depended on the gut microbes and the disease phenotypes could be transmissible through fecal microbiota transplantation (FMT). In conclusion, this study supports the concept that intake of MCD predisposes to experimental colitis and enhances its pathogenesis via modulating gut microbes and macrophages in mice.
Keywords: ulcerative colitis, dyslipidemia, methionine-choline deficient diet, macrophages, gut microbes, fecal microbiota transplantation
Introduction
With a higher prevalence and disability, inflammatory bowel disease (IBD) represents the multifactorial chronic gut inflammatory disorders, including Crohn’s disease (CD) and ulcerative colitis (UC), which is characterized by the close communication among genetic susceptibility, environmental factors, dysfunction of epithelial barrier, and imbalance of mucosal immune system [1–3]. By contrast to CD patients, the inflammatory symptoms in UC are dominant in the large intestine and mainly across the mucosal and submucosal layer [4]. The incidence of UC with 9 to 20 cases per 100,000 persons per year is increasing gradually, especially in Western countries and China, which has become a great threaten to human health [5]. Though there remains a complicated pathogenesis of UC, accumulating evidence has demonstrated that the changed dietary habits, digestion, and nutrient consumption might be the contributing factors in the initiation and deterioration of UC [6, 7].
Mammals and their commensal microorganisms co-evolved toward mutualism and homeostasis, contributing to the balance between gut lumen and mucosal immune system [8]. However, perturbation of the gut microbiota by some certain factors (such as dietary and antibiotic application) impairs the host-microbe interaction and leads to the abnormal activation of immune responses, coinciding with the initiation of immune-mediated diseases, especially IBD [9, 10]. The role of dietary reconstruction in the composition and function of human microbiota has been extensively discussed with particular interest [11–13]. Previous researches have demonstrated that dietary changes could largely interfere with the disease severity of mucosal inflammation, which shared a close communication between commensal bacteria and activation of mucosal inflammatory cells [14–17]. Such immune responses in IBD depend on the immune modulation by gut-associated lymphoid tissues and gut-resident immune cells, including dendritic cells (DCs), regulatory T cells (Tregs), innate lymphoid cells (ILCs), and macrophages [16, 18].
The recognition of disturbed gut microbial patterns could result in the activation of downstream signaling cascades from pattern recognition receptors [19, 20]. Meanwhile, the metabolites from gut microbes function as the fundamental orchestrators of host pathogenesis through controlling a large range of metabolic and inflammatory events [21, 22]. In the host-microbes interaction, gut microbial metabolites could shape the frequency, function, and epigenetics of immune cells and exert immune-modulatory effects on the initiation of multiple diseases [23–25]. Upon interfering with host-microbe interaction, environmental factors, especially dietary elements, likely play the critical role in the pathological process of UC [14]. Though not a typical metabolic disease, the incidence of IBD has also increased and the epidemiological studies have linked IBD incidence with the consumption of carbohydrate-rich diet, sugar, and fat [14]. In the previous research, it has been demonstrated that high fat diet (HFD), high salt diet, and high sugar diet could dramatically lead to the susceptibility of UC and exacerbate the progression of colitis through destructing mucosal DCs homeostasis, decreasing the production of butyrate, and altering microbial ecology, respectively [14, 16, 17], which highlighted the critical role of dietary consumption in rectifying the gastrointestinal homeostasis and inflammatory disorders.
In addition, by contrast to healthy controls, patients with UC have a lower level of choline and its derivatives in the mucosa [26]. Choline was considered as an indispensable nutrient for the formation of phosphatidylcholine and decreased choline was largely related to the pathological loss of colonic type II natural killer (NKT) cells and inflammation [27]. Moreover, in our in-house study, liver dysfunction and abnormal level of liver metabolism indices were identified in UC patients, suggesting a close interaction between gut and liver disorders. To test our hypothesis, methionine/choline deficient diet (MCD), a conventional dietary strategy for inducing liver disorders, was included in the present study and could further aggravate the disease severity, as evidenced by mucosal ulcers, diarrhea, and hematochezia, mainly due to the recruitment of macrophages and disturbance of gut microbes.
Materials and methods
Human samples collection
After obtaining written informed consent, the blood samples were collected and subjected to further analysis according to the approval of the Ethics Committee of the Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (Approval No: 2020386, Shanghai, China). The sera from healthy individuals and UC patients were obtained strictly according to the criteria that the volunteers shouldn’t be diagnosed with background diseases, including primary sclerosing cholangitis (PSC), diabetes, and obesity, and acquired drugs that might affect the serum level of biochemical indices of liver function.
Animals and diets
All animal care and experimental procedures were conducted in accordance with the ARRIVE guidelines, National Institutes of Health (NIH) Guide for Care and Use of Laboratory Animals, and were approved by the Bioethics Committee of the Shanghai Institute of Materia Medica, Chinese Academy of Sciences (IACUC: 2020-06-TW-51). Wild-type male C57BL/6 mice (8 weeks, 22–24 g) were purchased from Shanghai Laboratory Animal Center of the Chinese Academy of Sciences. The mice were housed under specific pathogen-free (SPF) conditions with a cycle of 12 h light/12 h dark, a temperature range of 22 ± 1 °C and 55% ± 5% of relative humidity at Shanghai Institute of Materia Medica, Chinese Academy of Sciences. All mice were allowed ad libitum access to food and water and habituated in the animal facility for 1 weeks before experiments. After being fed with normal chow diet for 3 weeks, mice were randomly grouped and fed a normal chow diet (NCD, Shilin Biotechnology, Shanghai, China) or given a normal chow diet and methionine and choline-deficient diet (MCD, A02082002B, Research Diets, New Brunswick, USA) alternately at 24-h intervals.
Dextran sodium sulfate (DSS)-induced colitis and disease severity evaluation
Ulcerative colitis was induced as described previously [28, 29]. In brief, mice were given 2% (w/v) DSS (MP Biomedicals, Irvine, CA, USA. MW. 36–50 kDa) in drinking water ad libitum for 7 days. During DSS treatment, weight loss, stool consistency, and fecal blood, as indicators of disease activity index (DAI), were determined daily. The criteria of the calculated scores of DAI were defined as follows: body weight loss (0, none; 1, 1%–5%; 2, 6%–10%; 3, 11%–20%; 4, >20%); stool consistency (0, normal; 1, soft but still formed; 2, soft; 3, very soft and wet; 4, watery diarrhea); fecal blood (0, negative hemoccult; 1, weakly positive hemoccult; 2, positive hemoccult; 3, blood traces in stool visible; 4, gross rectal bleeding). The value of DAI was summed by the score of body weight loss, stool consistency, and fecal blood, ranging from 0–12. At the end point of sacrifice, the serums, livers, spleens, and entire colonic samples were collected for further biochemical and flow cytometry analysis, as described below.
Depletion of commensal bacteria
To deplete the gut microbiota, mice were given an antibiotic cocktail supplement in the drinking water containing 1 g/L metronidazole (Sigma-Aldrich, St. Louis, MO, USA), 1 g/L neomycin (Sigma-Aldrich), 500 mg/L vancomycin (Sigma-Aldrich), and 1 g/L ampicillin (Sigma-Aldrich) for 2 weeks. 3% (w/v) sucrose was added into the antibiotics (Abx) water as artificial sweetener.
Fecal microbiota transplantation (FMT)
In the fecal microbiota transplantation study, the microbiota donors were mice treated with NCD or MCD for 2 weeks. Feces of the donors were collected at the end of week 2, and a pooled sample in each group was used in the following experiment. Pooled feces sample was dispersed in sterilized PBS (50 mg/mL). The solution was suspended by vortex and then centrifuged at 1000 r/min for 10 min. The supernatant was transferred to new sterile tubes and administered into recipients by oral gavage (10 mL/kg) one day before DSS administration.
Macroscopic and histopathological examination
The length of the colon from anus to appendix was measured by a ruler. The colons were washed thoroughly with cold phosphate-buffered saline (PBS), and were divided in portions to be fixed in 4% paraformaldehyde, embedded in paraffin for histological analyses. The weight of livers and spleens were measured. The liver samples were cut in several parts by sterile scissors respectively to be fixed in 4% paraformaldehyde, then embedded in paraffin, snap-frozen, shaken thoroughly in ethanol and prepared for single cell suspension. For histopathological analysis, colonic, and liver tissue sections (5 μm) were stained with hematoxylin and eosin (H&E) according to the standard protocol. Histological evaluation of H&E-stained tissue sections was achieved by two independent observers blinded to the experimental conditions and graded as previously described [30].
Serum biochemical indices assay
The serum samples were collected for biochemical assays, using a HITACHI-7080 automatic biochemical analyzer (Hitachi High Technologies Corporation, Tokyo, Japan).
Single cell suspensions preparation from colonic mucosa and liver
Colonic lamina propria (LP) cells were prepared as described previously [31]. In brief, colons were cut into small pieces after removing intestinal contents and residual fat tissues. Cleaned colonic pieces were firstly incubated in Hank’s balanced salt solution (HBSS, Gibco, Grand Island, NY, USA) containing 5 mM EDTA for 15 min in a shaking incubator at 37 °C for three times to dissociate epithelial cells and mucus. Then the remaining tissues were minced and digested with HBSS solution containing Type IV collagenase (0.5 mg/mL, Sigma-Aldrich), Dispase II (3 mg/mL, Sigma-Aldrich), and DNase I (0.1 mg/mL, Sigma-Aldrich) for 30 min in a 37 °C shaking incubator. Digested tissue solutions were then centrifugated and filtered through 70 μm cell trainers to collect the colonic single cell suspensions. Liver tissues were disaggregated by mechanical mincing with blades initially and suspensions were filtered with 100 μm nylon mesh strainers to remove clumps and debris. Then ammonium chloride buffer solution was used to deplete erythrocytes and then washed by clean medium. Single cells were finally captured by filtering suspensions through 40 μm cell trainers.
Flow cytometry assay
All single cell suspensions were washed with PBS and stained with fixable viability dye eFluor™ 780 (eBioscience, San Diego, CA, USA) for 30 min at 4 °C to identify the viable cells. Cells were then blocked with anti-mCD16/CD32 (2.4G2, Thermo Fisher Scientific, Waltham, MA, USA) and stained with appropriate antibody-fluorophore conjugates in dark. Subsequently, cells were labeled intracellularly with antibody-fluorophore conjugates and then analyzed on BD LSRFortessa instruments (BD Biosciences, San Jose, CA, USA). All data were further analyzed by gating CD45+FVDint viable and single cells on FlowJo software (Tree Star, Ashland, OR, USA).
Macrophage depletion
Mice were intraperitoneally administered 100 μL of Clodronate Liposomes (YEASEN, Shanghai, China) to globally delete macrophages. After macrophage depletion, these mice were started to have DSS water for 7 days.
Cytokines detection by ELISA
Quantification of cytokines in the tissue homogenates was performed using mouse TNF-α, IL-6, IL-12p40, IL-4, IL-17A, and IL-1β ELISA kit (BD Pharmingen, San Diego, CA, USA) according to the manufacturer’s instructions. The total protein concentration was determined by the Pierce BCA protein assay kit (Thermo Fisher Scientific).
RNA extraction and quantitative real-time PCR analysis
RNA samples were prepared using the RNA simple total RNA kit (Tiangen, Beijing, China) and cDNA was synthesized using an All-in-One cDNA Synthesis SuperMix (Biotool, Houston, TX, USA). Real-time (RT)-qPCR was performed using the SYBR® Green Realtime PCR Master Mix (TOYOBO, Osaka, Japan) and a 7500 Fast Real-Time PCR System (Applied Biosystems. Foster city, CA, USA).
Immunofluorescent analysis
Paraffin-embedded colon and liver sections were dewaxed, blocked with 3% H2O2, and then buffered in citrate buffer solution for epitope retrieval. Subsequently, the sections were stained at 4 °C overnight with anti-F4/80 antibodies (Abcam, Cambridge, MA, USA), counterstained with DAPI (Abcam) to visualize the nuclei and the fluorescent signals were visualized by the Leica TCS SPS microscope (Wetzlar, Germany).
Statistical analysis
All data were expressed as mean ± SEM. Group comparisons were performed using Student’s t-test or one-way ANOVA followed by multiple comparison test with GraphPad Prism 8.0 (San Diego, CA, USA) statistical software to determine significance. P-value < 0.05 was considered statistically significant.
Results
Dyslipidemia and liver inflammatory infiltrations were observed in UC
Previously, gut microbiota dysbiosis and abnormal transduction of inflammatory signaling have been identified in DSS-induced murine colitis models and UC patients [31]. In our continuous research on UC, we surprisingly found that patients with UC were often associated with dyslipidemia, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglyceride (TG), total cholesterol (TC), low-density lipoproteins (LDL-c), and high-density lipoproteins (HDL-c) (Fig. 1a), which displayed little-to-moderate gender difference (Fig. 1b). To confirm this phenomenon, we examined the corresponding serum biochemical indicators on DSS-induced murine colitis model, and it turned out that compared with healthy mice, increased levels of TG, TC, and LDL-c were observed in colitis mice (Fig. 1c). Consistently, to further probe into the gut and liver inflammatory conditions, gene expression of inflammatory mediators in liver was detected. As illustrated in Fig. 1d, the levels of inflammatory cytokines in liver homogenates of colitis mice were much higher than that in healthy mice, such as TNF-α, IL-1β, IL-6, IL-12, IL-4, and IL-17A. Likewise, the mRNA expression levels of genes associated with cell adhesion, inflammation, immune disorders, and cell metabolism were increased in liver homogenates of murine colitis (Fig. 1e), which collectively indicated a close interaction between gut and liver disorders in UC. Moreover, increased infiltration of inflammatory cells was also observed in livers from DSS-induced colitis mice (Supplementary Fig. S1). Given the previous study that HFD, inducing hepatic disorders, could promote the disease severity of colitis, we then reasonably included MCD to further confirm the relationship between dietary intervention and colitis etiology.
Fig. 1. Dyslipidemia and liver inflammation were observed in UC patients and mice.
a The serum biochemical indices, including ALT, AST, TG, TC, LDL-c, and HDL-c, of healthy individuals (n = 88) and UC patients (n = 88) were determined; b The serum level of biochemical indices TG, TC, LDL-c, and HDL-c was compared in male and female healthy controls and UC patients; c The serum biochemical indices TG, TC, and LDL-c of DSS-induced colitis mice were determined (n = 15); d The level of inflammatory cytokines in DSS-induced murine liver homogenates, assayed by ELISA; e The mRNA expression level in DSS-induced murine colons, assayed by RT-PCR. Data were shown as mean ± SEM. n = 6 mice per group. *P < 0.05, **P < 0.01, and ***P < 0.001.
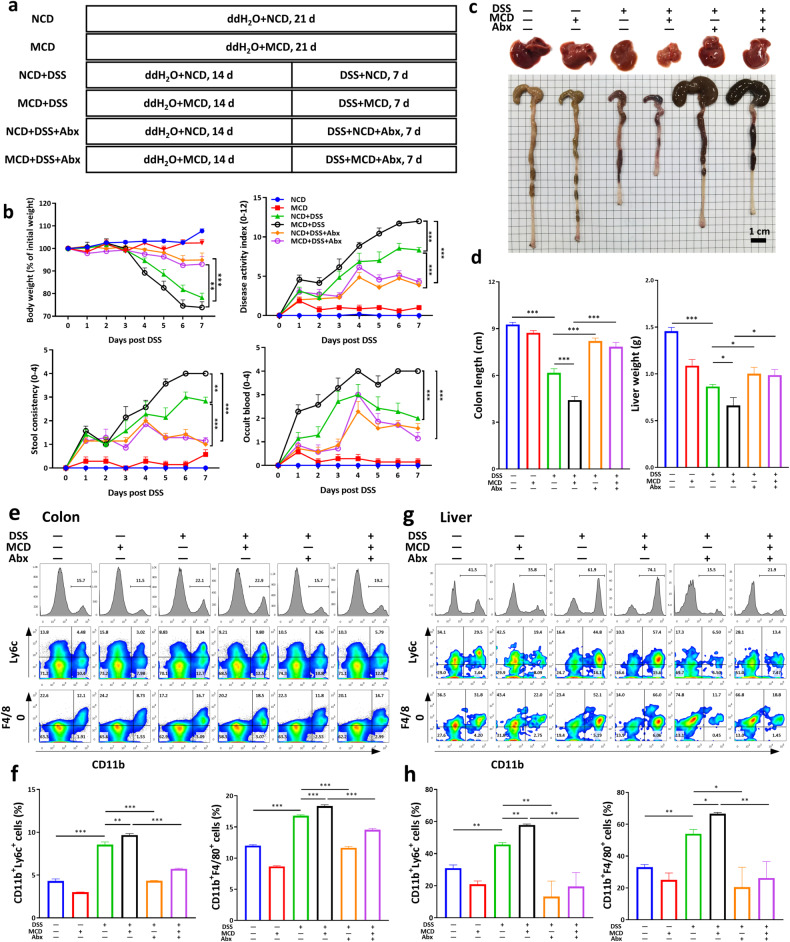
DSS-induced murine acute colitis was aggravated by MCD
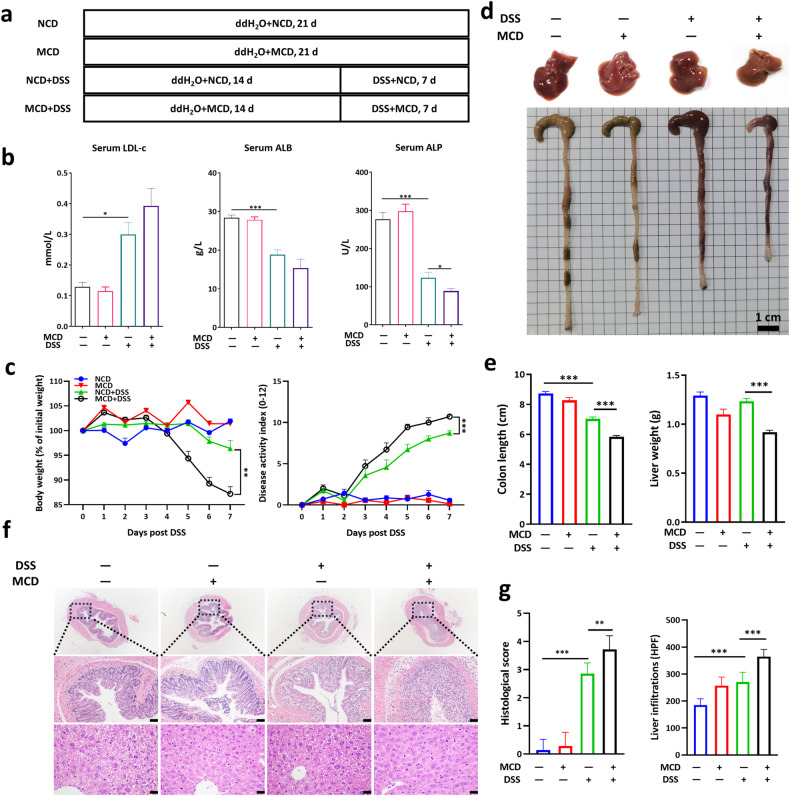
To test the hypothesis that MCD-induced liver dysfunction might be a contributing factor in the incidence of UC, mice were fed with a MCD for 14 days to cause mild liver damage and then treated with DSS for 7 days (Fig. 2a). In keeping with the results in Fig. 1, MCD could further alter the serum biochemical indices, refereeing to increased level of LDL-c and decreased level of albumin (ALB) and alkaline phosphatase (ALP) in DSS-induced acute colitis (Fig. 2b). Interestingly, mice in MCD + DSS group showed greater sensitivity to DSS treatment, as evidenced by a greater loss of body weight, bloody diarrhea, and higher disease activity index (DAI) scores than mice in NCD + DSS group (Fig. 2c). The colon shortening upon DSS administration was further deteriorated by MCD application (Fig. 2d, e). Additionally, dramatic changes on the phenotype of livers were monitored in DSS-treated mice, which suggested that the livers of NCD-fed mice were normal in size and shape, with sharp edges, smooth membranes, and deep red in color, whereas colitis mice livers had reduced volume, soft texture, and lighter color, further aggravated by the intervention of MCD (Fig. 2d). Meanwhile, the liver weights and colonic lengths in MCD-fed mice also were lower than those in the NCD-fed mice (Fig. 2e). Moreover, the histopathological examinations demonstrated that broader damage in colonic epithelial cells, mucosal ulcerations, and more inflammatory infiltrations in liver were observed in MCD + DSS group (Fig. 2f, g), revealing that pre-application of MCD aggravated the disease severity of DSS-induced acute colitis.
Fig. 2. MCD exacerbated the disease progression and aggravated the colonic mucosal and liver inflammation in DSS-induced acute colitis.
a The flow diagram of DSS-induced acute colitis that mice were pre-treated with dietary intervention and then given with DSS; b Serum level of LDL-c (low density lipoprotein), ALB (albumin), and ALP (alkaline phosphatase); c The body weight changes and disease activity index (DAI) were monitored daily upon DSS treatment; d The representative images of murine livers and colons; e The colonic lengths and liver weights were measured; f The representative images of H&E staining of colon and liver sections; g The colonic histological scores and liver inflammatory infiltrations (Scale bar, 50 μm). NCD normal chow diet, HPF high power field. Data were shown as mean ± SEM. n = 6–7 mice per group. *P < 0.05, **P < 0.01, and ***P < 0.001.
Intake of MCD aggravated the disease severity of progressive chronic UC
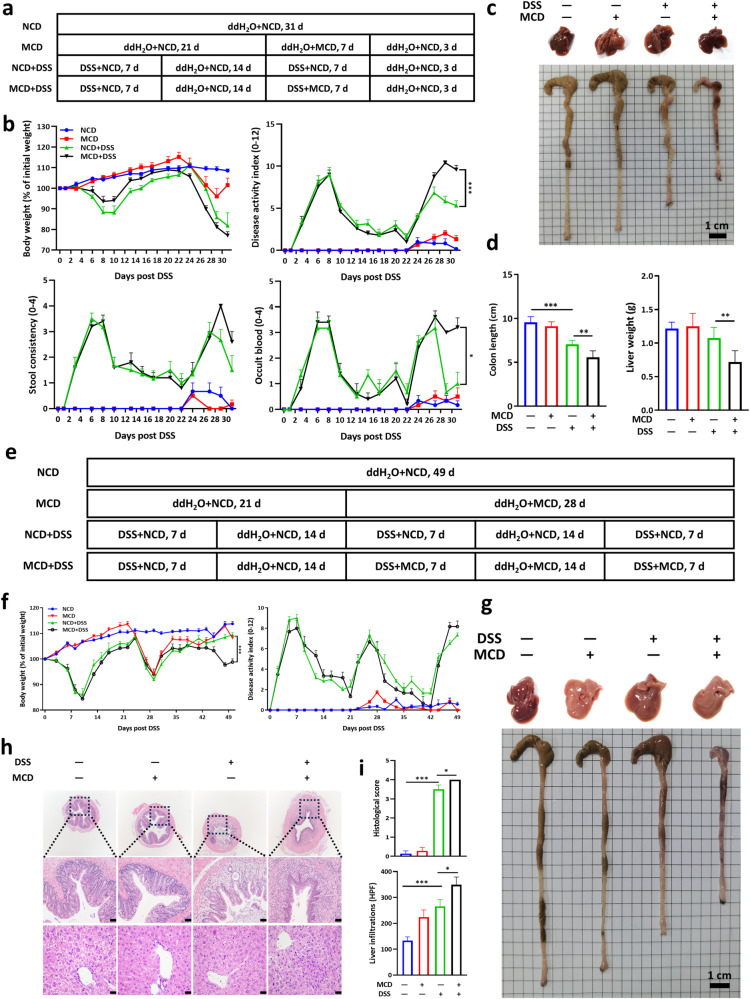
Accelerated acute colitis development in MCD-fed mice drives us to figure out whether MCD aggravated chronic colitis. To figure out whether post-treatment of MCD exerted similar effects on the disease progression of UC, mice were administered 2% DSS for 7 days followed by 14 days of regular drinking water, then the second round of DSS treatment was given along with the MCD followed by 3 days of normal water and normal diet (Fig. 3a). Consistently, MCD could also accelerate the disease progression, including body weight loss, bloody diarrhea, colon shortening, and decreased liver weights (Fig. 3b–d), which were in line with the findings from DSS-induced acute colitis. Furthermore, experimental chronic colitis was achieved by giving mice three cycles of 2% DSS for 7 days followed by sterile water for 14 days, and MCD was administered starting from the second cycle (Fig. 3e). Consistent with the above results, MCD still played a pathological role in aggravating the severity of chronic colitis, mainly in terms of body weight loss and colon shortening (Fig. 3f, g). Similarly, the pathological changes in the liver of colitis mice were further aggravated by the MCD, which was manifested in the volume and soft texture of the liver (Fig. 3f, g). Further analysis by H&E staining suggested that more severe pathological damages occurred in colon and there was more inflammatory infiltration in liver of colitis mice fed MCD, compared with those fed normal diet (Fig. 3h, i). In short, these findings proved that colitis development is accelerated by MCD.
Fig. 3. MCD exacerbated DSS-induced murine progressive and chronic colitis.
a The flow diagram of DSS-induced progressive colitis that mice were pre-treated with DSS and then with dietary intervention; b The body weight changes and DAI were monitored daily; c The representative images of livers and colons; d The colonic lengths and liver weights; e The flow diagram of DSS-induced chronic colitis that mice were treated with MCD in the presence or absence of DSS; f The body weight changes and DAI were monitored; g The representative images of livers and colons; h The representative images and i histological scores of H&E staining of colon and liver sections (Scale bar, 50 μm). NCD, normal chow diet. Data were shown as mean ± SEM. n = 6–7 mice per group. *P < 0.05, **P < 0.01, and ***P < 0.001.
MCD aggravated the colonic mucosal and liver inflammatory responses in macrophages in murine colitis
Given the morphological and pathological manifestations, we further uncovered the inflammatory microenvironments in colon and liver by flow cytometry analysis. As illustrated in Fig. 4a, b and Supplementary Fig. S2, compared to control mice, inflammatory immune cells infiltration in colonic LP and liver were significantly increased in DSS-induced colitis, including CD11b+ myeloid cells, CD11b+F4/80+ macrophages, CD11b+Ly6C+ monocytes, CD11b+CCR2+ cells, CD11b+CCR5+ cells, and CD11b+CX3CR1+ cells. By contrast, intake of MCD could obviously increase the population of inflammatory macrophages in colon, which were verified by further immunofluorescent staining of F4/80 in colonic sections (Fig. 4c). For a more comprehensive understanding of the inflammatory status of the colitis, we examined the gene expression of inflammatory mediators in colonic and liver homogenates. As illustrated in Fig. 4d, e, the results suggested that by contrast to NCD-treated mice, MCD intervention promoted the upregulation of inflammatory cytokines, IL-6, TNF-α, and MIP-1α, and increased the expression of S100A8 and S100A9 both in colon and liver homogenates, which were mainly derived from inflammatory macrophages (Supplementary Fig. S3).
Fig. 4. MCD exacerbated infiltration of inflammatory macrophages in DSS-induced murine UC.
Flow cytometry analysis of myeloid cells, macrophages, monocytes, and the expression of CCR2, CCR5, and CX3CR1 on CD11b+ cells (gated on FVDint viable single cells) in the colonic lamina propria (a) and livers (b); c Immunofluorescent analysis of macrophages in colonic sections (Scale bar, 100 μm); d The mRNA expression level of inflammatory mediators in colonic homogenates, assayed by RT-PCR; e The mRNA expression level of inflammatory mediators in liver homogenates, assayed by RT-PCR. Data were shown as mean ± SEM. n = 6–7 mice per group. *P < 0.05, **P < 0.01, and ***P < 0.001.
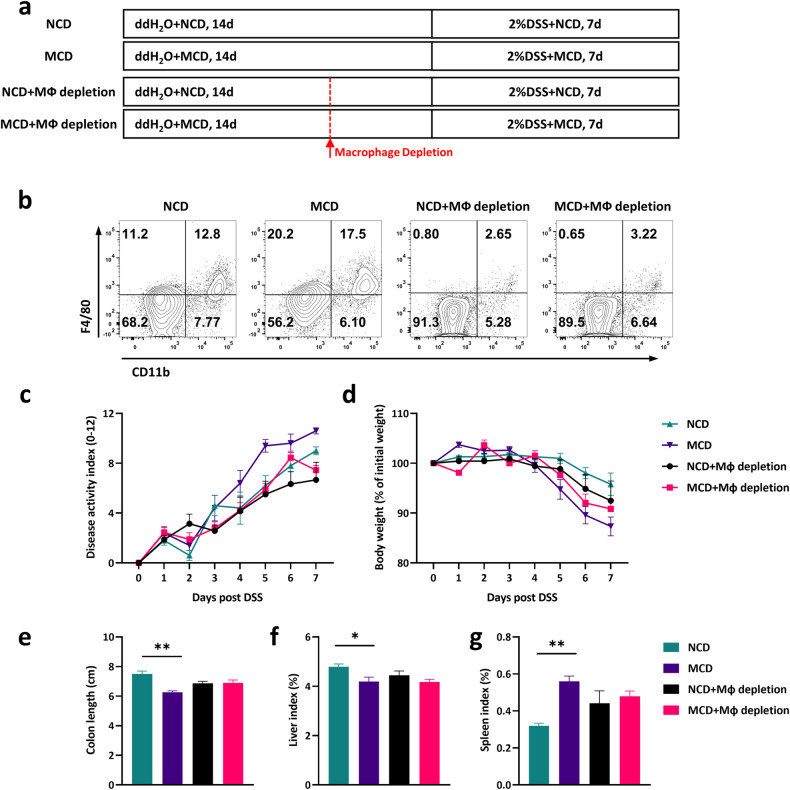
Macrophage depletion compromised the pathology of MCD-induced colitis susceptibility
To illustrate whether macrophages functioned as the indispensable part in MCD-triggered colitis pathogenesis, clodronate liposomes were conducted in the present research to deplete the macrophages (Fig. 5a). Clodronate liposomes injection could largely deplete the population of CD11b+F4/80+ macrophages in colonic tissues (Fig. 5b). As demonstrated in Fig. 5c, d, macrophage depletion could compromise the pathological changes between NCD and MCD-treated mice to some extent, including body weight loss, diarrhea, and disease activity index. Meanwhile, macrophage depletion possessed the capacity to diminish the colon shortening and liver lesions in MCD-treated mice (Fig. 5e–g), which were consistently confirmed in DSS-induced acute and chronic colitis (Figs. 2 and 3). In brief, the findings suggested that macrophages played an indispensable part in the pathogenesis of MCD-aggravated colitis.
Fig. 5. Macrophage depletion compromised the pathological process of MCD-induced colitis susceptibility.
a The flow diagram of experiment that mice were intraperitoneally injected with Clodronate Liposomes to globally delete macrophages. After macrophage depletion, these mice were started to have DSS water for 7 days; b Flow cytometry assay of macrophage depletion efficiency in colonic tissues; c The desease severity index (DAI) and d body weight changes were monitored daily; e The colonic lengths, f liver index, and g spleen index were measured. Data were shown as mean ± SEM. n = 8–10 mice per group. *P < 0.05, **P < 0.01.
MCD-induced susceptibility of colitis is mediated by gut microbiota
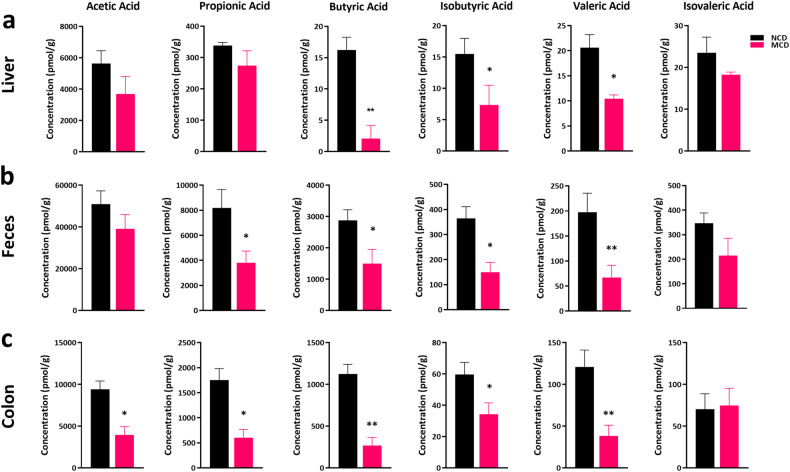
Previous study implicated that dietary changes could rectify the gut microbiota, which led to the hypothesis that MCD might exert effects on bacterial metabolites through modulating the differentiation and phenotype of gut immune cells [14, 17, 18]. Accordingly, the short-chain fatty acids (SCFAs), derived from intestinal microorganisms, played a critical part in maintaining mucosal immunity and homeostasis [32, 33]. In the present study, disturbance of the level of SCFAs from liver homogenates, colonic homogenates, and feces was displayed upon MCD-treated colitis mice (Fig. 6a–c). MCD intervention could largely decrease the production of several SCFAs, such as propionic acid, butyric acid, and valeric acid (Fig. 6), which further highlighted the protective role of SCFAs in the development of UC and confirmed that MCD might be a contributing factor to promoting intestinal inflammation and rectifying the gut microbiota during colitis.
Fig. 6. Alteration of the metabolites from gut microbes was observed in MCD-treated colitis.
Determination of SCFAs in murine liver homogenates (a), murine feces (b), and colonic homogenates (c) in DSS/MCD-treated acute colitic mice. Data were shown as mean ± SEM. n = 6 mice per group. *P < 0.05, **P < 0.01.
To further explore the correlation between the gut microbiota and the pathogenesis of worsened-colitis by MCD, we depleted the intestinal symbiotic bacteria with antibiotics by dissolving antibiotics in drinking water. Mice were fed with MCD for 14 days, followed by DSS treatment for 7 days, and then subjected to further observations (Fig. 7a). Compared with untreated controls, antibiotics (Abx) treatment ameliorated the body weight loss, DAI, and bloody diarrhea in both MCD-fed or unfed mice (Fig. 7b). Meanwhile, there remained little difference on the disease severity, colon length, and liver weight between NCD + DSS and MCD + DSS group upon Abx treatment (Fig. 7b–d). Moreover, lower amounts of inflammatory monocytes, together with reduced level of IL-6 from liver and colonic cells, were observed in the colons and livers of bacteria-cleared mice (Fig. 7e–h and Supplementary Fig. S4). As expected, these data supported that gut microbiota was extensively involved in the exacerbation of MCD-induced UC.
Fig. 7. MCD-induced colitis pathogenesis was largely dependent on gut microbes.
a The flow diagram of experiment that mice were orally treated with or without DSS, MCD, and antibiotic cocktail (Abx, antibiotics); b The body weight changes and DAI were monitored daily after DSS administration; c The representative images of livers and colons; d The colonic lengths and liver weights; Flow cytometry analysis (e) and quantification analysis (f) of myeloid cells, macrophages, and monocytes (gated on FVDint viable single cells) in colonic lamina propria; Flow cytometry analysis (g) and quantification analysis (h) of myeloid cells, macrophages, and monocytes (gated on FVDint viable single cells) in livers. Data were shown as mean ± SEM. n = 7 mice per group. *P < 0.05, **P < 0.01, and ***P < 0.001.
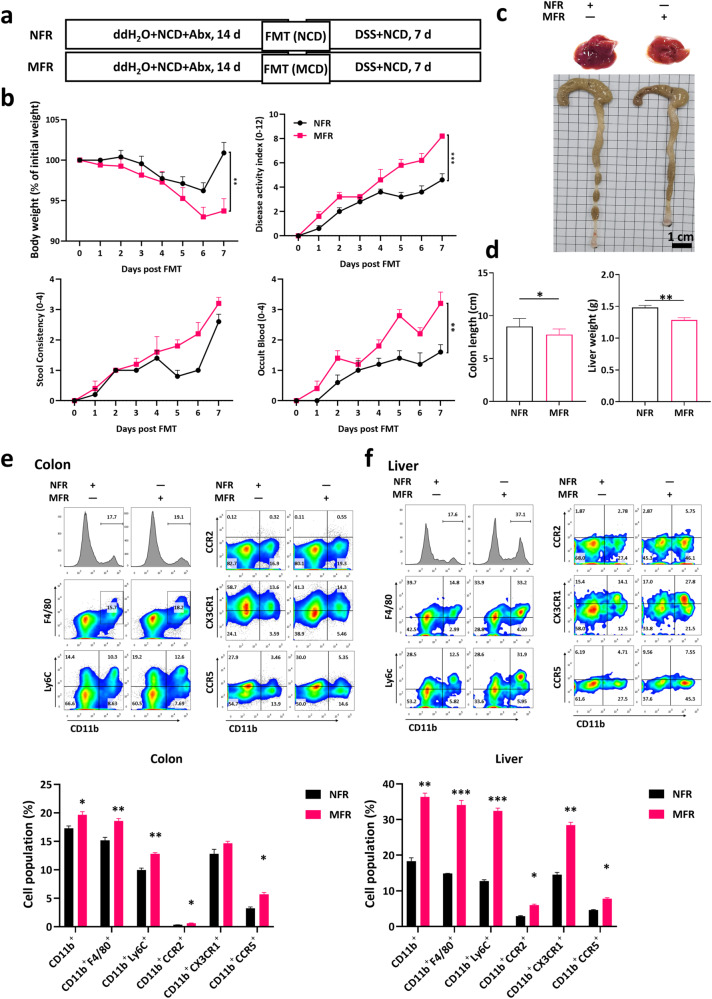
MCD-induced susceptibility of colitis was transmissible
Subsequently, we wondered whether the microbiota alteration played a critical role in the exacerbation caused by MCD. We fed mice with an NCD/MCD for 14 days as the donors and then collected their feces, respectively. Recipient mice, pretreated with a cocktail of broad-spectrum antibiotics in advance for 14 days to deplete the gut commensal bacteria, acquired the feces from the donor mice (Fig. 8a). During DSS administration, normal fecal recipients (NFR) that received NCD-fed mouse feces, displayed less body weight loss and lower DAI scores than MCD fecal recipients (MFR) receiving MCD-fed mouse feces (Fig. 8b). Correspondingly, it could be seen from the morphological pictures that both the colon length and liver volume was decreased in MFR group (Fig. 8c, d). Consistently, mice receiving feces transplantation from mice fed MCD were able to largely mimic the increase of the immunocytes abundance in LP and liver (Fig. 8e, f). In sum, the aggravating effect on colitis of MCD could be transmitted by FMT.
Fig. 8. MCD-induced colitis pathogenesis was reproducible by FMT.
a The flow diagram of FMT with NCD/MCD-treated feces that mice with an NCD/MCD for 14 days were set as the donors. Recipient mice, pretreated with a cocktail of broad-spectrum antibiotics in advance for 14 days to deplete the gut commensal bacteria, acquired the feces from the donor mice; b The body weight changes and DAI were monitored daily in fecal microbiota transplantation (FMT) in DSS-induced colitis; c The representative images of livers and colons; d The colonic lengths and liver weights; Flow cytometry analysis and quantification assay of myeloid cells, macrophages, monocytes, and the expression of CCR2, CCR5, and CX3CR1 on CD11b+ cells (gated on FVDint viable single cells) in colonic lamina propria (e) and livers (f) in FMT-treated mice. Data were shown as mean ± SEM. n = 7 mice per group. *P < 0.05, **P < 0.01, and ***P < 0.001.
Discussion
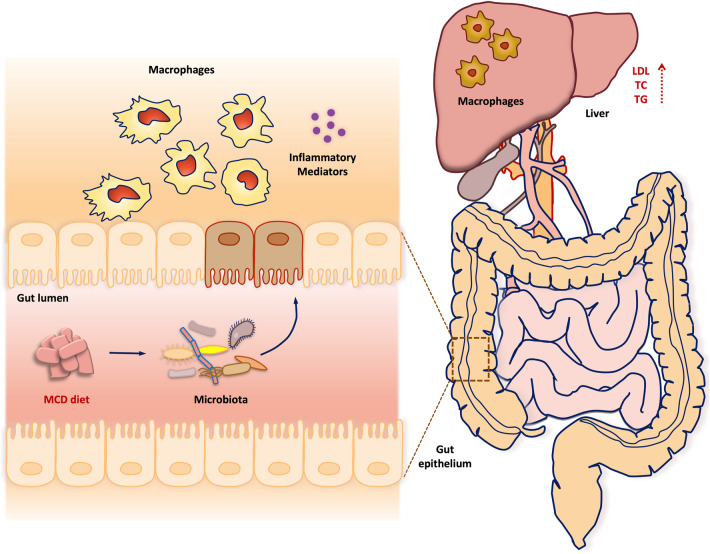
It is well-defined that IBD represents a chronic, relapsing, and inflammatory disorder with a tremendously complicated etiology, which is largely associated with the imbalance between gut lumen and mucosal immune system [9, 10, 34]. Dysregulation of immune response to the gut microbiota and their corresponding metabolites contributes to the initiation of epithelial damage in UC [14]. In addition to host genetic make-up, the close communication between environmental factors, such as microbiota composition and diet, and immune responses has been shown to play a critical role in the development of multiple inflammatory diseases [35]. Although recent experimental studies demonstrated that HFD is a pathological trigger for IBD, the role of MCD, a conventional diet for inducing hepatic inflammation, in UC pathogenesis remains controversial. Consistently, the present study collectively suggested that intake of MCD exhibited an increased susceptibility of UC in DSS-induced murine acute, progressive, and chronic colitis models. To uncover the underlying mechanisms, clodronate liposomes, FMT and antibiotic therapy were conducted in the research and demonstrated that MCD-induced colitis pathogenesis was largely dependent on the alteration of gut microbes and propagation of macrophages (Fig. 9).
Fig. 9.
The schematic diagram of MCD in promoting the severity of UC depending on gut microbes and propagation of inflammatory macrophages.
The close physical and chemical connection between gut and liver in the process of digestion and absorption resulted in the fact that liver disorders represented as the most common extraintestinal complication in UC patients [36, 37]. Previous studies have shown that abnormal bile acid metabolism and transport, imbalance of gut microbiota, and lymphocyte homing disorders may participate in the occurrence and development of UC through the gut-liver axis system, which induce and aggravate intestinal inflammation [38–40]. Moreover, it has been suggested that colitis might act as a pathological trigger for liver dysfunction and imbalance between Th17 cells and Tregs played an imported part in modulating colitis-related liver inflammatory response [41]. During the treatment of UC, early diagnosis of liver injury or abnormal hepatic biochemistries is of great importance for the further managements [36]. Epidemiological investigations suggested that fat rich western diet largely led to a higher incidence of IBD and exerted impacts on the composition and metabolism of gut microbiota [14, 16]. Consistently, the present research collectively proved that UC patients and murine colitis models both exhibited liver inflammatory infiltrations, dyslipidemia, and gut microbiota disturbance, which indicated a close interaction between gut and liver during the development of UC.
Given the fact that MCD, a conventional diet for inducing liver damage and non-alcoholic steatohepatitis (NASH), possessed the capacity to promote the inflammatory macrophages infiltrations in UC, which were consistent with the previous study that HFD could further exacerbate the disease severity of colitis. Collectively, dietary interventions with affecting host lipid metabolism contributed to the pathogenesis of colitis. We further identified in the present research that macrophages played a critical part in MCD-induced colitis pathogenesis. Compared to NCD-treated mice, more infiltrations of macrophages and higher level of inflammatory cytokines were identified in MCD-treated colitis mice, which suggested that MCD exacerbated infiltration of inflammatory macrophages in DSS-induced murine UC. To illustrate whether macrophages functioned as the indispensable part in MCD-triggered colitis pathogenesis, clodronate liposomes were conducted in the present research to deplete the macrophages. Macrophage depletion could largely compromise the pathological changes between NCD and MCD-treated mice.
Additionally, accumulated data also confirmed a disarranged pattern of diet could interfere with the progression of mucosal inflammation [14, 15, 17]. Given the physical and physiological association between gut and liver, we held a hypothesis that intervention of liver function exhibited modulatory effects on the mucosal immune system. Due to the destruction of the intestinal epithelial integrity, numerous harmful intestinal pathogens and endotoxins invaded through the portal vein and entered the systemic circulation, which directly induced the up-regulation of pathogen-related molecular patterns (PAMPs) or damage-related molecular patterns (DAMPs) and further activate the macrophages (Kupffer cells) residing in liver tissues [41–45]. In line with the gut microbiota dysbiosis in UC, MCD-induced colitis pathology could be transmissible with a higher severity of colonic mucosal inflammation and liver tissue damage. In keeping with our findings, depletion of symbiotic microbes could largely diminish the difference between NCD and MCD treated mice, which indicated that disturbance of gut microbiota by MCD played an important role in the inflammatory microenvironments. Given the fact that dietary intake played a key part in reprogramming the gut microbiota, barrier function, and mucosal inflammation, several therapeutic strategies have been confirmed their effects on preventing colitis progression. Previously, a ketogenic diet (KD), characterized by high-fat and low carbohydrate, exerted protective effects on UC by reduction of colonic ILC3 and alteration of gut microbiome [18].
In summary, our data suggested that increased liver injury by MCD could exacerbate colitis pathogenesis in DSS-induced acute, progressive, and chronic colitis models. Among such pathological process, MCD shifted the gut microbes and propagation of inflammatory macrophages, which were confirmed by antibiotics treatment, fecal microbiota transplantation, and clodronate liposomes injection, respectively. This study contributed to the understanding on the fact that patients with dyslipidemia might possess a susceptibility of UC incidence in clinic and provided the critical evidence that UC patients with a greater liver dysfunction tended to a worse manifestation of mucosal inflammation. Briefly, the present findings strongly supported the concept that intake of dystrophy might be a potential trigger for UC and MCD promoted colitis depending on gut microbes and inflammatory macrophages.
It has been well-defined that abnormal level of SCFAs and other metabolites from dysbiosis of gut microbiota were uncovered in murine colitis and human patients. In our continuous study and previous investigations, supplement with SCFAs, such as valeric acid and propionic acid, dramatically exerted therapeutic effects on colitis [46]. However, the underlying molecular mechanism should be performed in future to illustrate the signaling cascades by SCFAs and other metabolites. Further investigations could be required for expanding the significance of the current findings and the potential therapeutic strategy based on valeric acid or other SCFAs additives. Moreover, given the gender susceptibility of murine colitis and potential impact from hormone difference between male and female mice, the present research collectively showed the disease outcomes from DSS/MCD-induced colitis in male mice.
Supplementary information
Acknowledgements
This work was granted by National Natural Science Foundation of China (No. 82173822).
Author contributions
MTL and HL designed the research and wrote the manuscript; MTL, CGX, QKL, HML, CF, TY, XHW, CLF, XQY, and HL performed the experiments and data analysis; YZ and DWZ collected the human samples; HL and WT revised the manuscript. DWZ and WT made a substantial contribution to the concept and supervision of the article. All the authors contributed to the interpretation of present data and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Mo-ting Liu, Yao Zhang
Contributor Information
Duo-wu Zou, Email: zdw12125@rjh.com.cn.
Heng Li, Email: cpuliheng@126.com.
Wei Tang, Email: tangwei@simm.ac.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41401-024-01291-y.
References
- 1.Neurath MF, Leppkes M. Resolution of ulcerative colitis. Semin Immunopathol. 2019;41:747–56. 10.1007/s00281-019-00751-6 [DOI] [PubMed] [Google Scholar]
- 2.Neurath MF. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat Immunol. 2019;20:970–9. 10.1038/s41590-019-0415-0 [DOI] [PubMed] [Google Scholar]
- 3.Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390:2769–78. 10.1016/S0140-6736(17)32448-0 [DOI] [PubMed] [Google Scholar]
- 4.Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380:1606–19. 10.1016/S0140-6736(12)60150-0 [DOI] [PubMed] [Google Scholar]
- 5.Pasvol TJ, Horsfall L, Bloom S, Segal AW, Sabin C, Field N, et al. Incidence and prevalence of inflammatory bowel disease in UK primary care: a population-based cohort study. BMJ Open. 2020;10:e036584. 10.1136/bmjopen-2019-036584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owczarek D, Rodacki T, Domagala-Rodacka R, Cibor D, Mach T. Diet and nutritional factors in inflammatory bowel diseases. World J Gastroenterol. 2016;22:895–905. 10.3748/wjg.v22.i3.895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keshteli AH, Madsen KL, Dieleman LA. Diet in the pathogenesis and management of ulcerative colitis; a review of randomized controlled dietary interventions. Nutrients. 2019;11:1498. 10.3390/nu11071498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu HJ, Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes. 2012;3:4–14. 10.4161/gmic.19320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruff WE, Greiling TM, Kriegel MA. Host-microbiota interactions in immune-mediated diseases. Nat Rev Microbiol. 2020;18:521–38. 10.1038/s41579-020-0367-2 [DOI] [PubMed] [Google Scholar]
- 10.Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30:492–506. 10.1038/s41422-020-0332-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexander M, Turnbaugh PJ. Deconstructing mechanisms of diet-microbiome-immune interactions. Immunity. 2020;53:264–76. 10.1016/j.immuni.2020.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tilg H, Moschen AR. Food, immunity, and the microbiome. Gastroenterology. 2015;148:1107–19. 10.1053/j.gastro.2014.12.036 [DOI] [PubMed] [Google Scholar]
- 13.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–36. 10.1038/nature10213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan S, Waliullah S, Godfrey V, Khan MAW, Ramachandran RA, Cantarel BL, et al. Dietary simple sugars alter microbial ecology in the gut and promote colitis in mice. Sci Transl Med. 2020;12:eaay6218. 10.1126/scitranslmed.aay6218 [DOI] [PubMed] [Google Scholar]
- 15.He Z, Chen L, Catalan-Dibene J, Bongers G, Faith JJ, Suebsuwong C, et al. Food colorants metabolized by commensal bacteria promote colitis in mice with dysregulated expression of interleukin-23. Cell Metab. 2021;33:1358–71. 10.1016/j.cmet.2021.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng L, Jin H, Qiang Y, Wu S, Yan C, Han M, et al. High fat diet exacerbates dextran sulfate sodium induced colitis through disturbing mucosal dendritic cell homeostasis. Int Immunopharmacol. 2016;40:1–10. 10.1016/j.intimp.2016.08.018 [DOI] [PubMed] [Google Scholar]
- 17.Miranda PM, De Palma G, Serkis V, Lu J, Louis-Auguste MP, McCarville JL, et al. High salt diet exacerbates colitis in mice by decreasing Lactobacillus levels and butyrate production. Microbiome. 2018;6:57. 10.1186/s40168-018-0433-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong C, Yan X, Liu Y, Huang L, Zhu Y, He J, et al. Ketogenic diet alleviates colitis by reduction of colonic group 3 innate lymphoid cells through altering gut microbiome. Signal Transduct Target Ther. 2021;6:154. 10.1038/s41392-021-00549-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol. 2012;9:599–608. 10.1038/nrgastro.2012.152 [DOI] [PubMed] [Google Scholar]
- 20.Xu S, He Y, Lin L, Chen P, Chen M, Zhang S. The emerging role of ferroptosis in intestinal disease. Cell Death Dis. 2021;12:289. 10.1038/s41419-021-03559-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy M, Thaiss CA, Elinav E. Metabolites: messengers between the microbiota and the immune system. Genes Dev. 2016;30:1589–97. 10.1101/gad.284091.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin L, Zhang J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 2017;18:2. 10.1186/s12865-016-0187-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20:159–66. 10.1038/nm.3444 [DOI] [PubMed] [Google Scholar]
- 24.Luu M, Pautz S, Kohl V, Singh R, Romero R, Lucas S, et al. The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nat Commun. 2019;10:760. 10.1038/s41467-019-08711-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duscha A, Gisevius B, Hirschberg S, Yissachar N, Stangl GI, Eilers E, et al. Propionic acid shapes the multiple sclerosis disease course by an immunomodulatory mechanism. Cell. 2020;180:1067–80. 10.1016/j.cell.2020.02.035 [DOI] [PubMed] [Google Scholar]
- 26.Williams HR, Willsmore JD, Cox IJ, Walker DG, Cobbold JF, Taylor-Robinson SD, et al. Serum metabolic profiling in inflammatory bowel disease. Dig Dis Sci. 2012;57:2157–65. 10.1007/s10620-012-2127-2 [DOI] [PubMed] [Google Scholar]
- 27.Sagami S, Ueno Y, Tanaka S, Fujita A, Niitsu H, Hayashi R, et al. Choline deficiency causes colonic type II natural killer T (NKT) cell loss and alleviates murine colitis under type I NKT cell deficiency. PLoS One. 2017;12:e0169681. 10.1371/journal.pone.0169681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H, Feng C, Fan C, Yang Y, Yang X, Lu H, et al. Intervention of oncostatin M-driven mucosal inflammation by berberine exerts therapeutic property in chronic ulcerative colitis. Cell Death Dis. 2020;11:271. 10.1038/s41419-020-2470-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H, Fan C, Feng C, Wu Y, Lu H, He P, et al. Inhibition of phosphodiesterase-4 attenuates murine ulcerative colitis through interference with mucosal immunity. Br J Pharmacol. 2019;176:2209–26. 10.1111/bph.14667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H, Fan C, Lu H, Feng C, He P, Yang X, et al. Protective role of berberine on ulcerative colitis through modulating enteric glial cells–intestinal epithelial cells–immune cells interactions. Acta Pharm Sin B. 2020;10:447–61. 10.1016/j.apsb.2019.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H, Zhang Y, Liu M, Fan C, Feng C, Lu Q, et al. Targeting PDE4 as a promising therapeutic strategy in chronic ulcerative colitis through modulating mucosal homeostasis. Acta Pharm Sin B. 2022;12:228–45. 10.1016/j.apsb.2021.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wardill HR, Da Silva Ferreira AR, Kumar H, Bateman EH, Cross CB, Bowen JM, et al. Whey-based diet containing medium chain triglycerides modulates the gut microbiota and protects the intestinal mucosa from chemotherapy while maintaining therapy efficacy. Cell Death Dis. 2023;14:338. 10.1038/s41419-023-05850-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thoo L, Noti M, Krebs P. Keep calm: the intestinal barrier at the interface of peace and war. Cell Death Dis. 2019;10:849. 10.1038/s41419-019-2086-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16:341–52. 10.1038/nri.2016.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, Yousefi S, Simon H-U. Necroptosis and neutrophil-associated disorders. Cell Death Dis. 2018;9:111. 10.1038/s41419-017-0058-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Restellini S, Chazouilleres O, Frossard JL. Hepatic manifestations of inflammatory bowel diseases. Liver Int. 2017;37:475–89. 10.1111/liv.13265 [DOI] [PubMed] [Google Scholar]
- 37.Mendes FD, Levy C, Enders FB, Loftus EV Jr, Angulo P, Lindor KD. Abnormal hepatic biochemistries in patients with inflammatory bowel disease. Am J Gastroenterol. 2007;102:344–50. 10.1111/j.1572-0241.2006.00947.x [DOI] [PubMed] [Google Scholar]
- 38.Yang X, Lu D, Zhuo J, Lin Z, Yang M, Xu X. The gut-liver axis in immune remodeling: new insight into liver diseases. Int J Biol Sci. 2020;16:2357–66. 10.7150/ijbs.46405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trapecar M, Communal C, Velazquez J, Maass CA, Huang YJ, Schneider K, et al. Gut-liver physiomimetics reveal paradoxical modulation of IBD-related inflammation by short-chain fatty acids. Cell Syst. 2020;10:223–39. 10.1016/j.cels.2020.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uko V, Thangada S, Radhakrishnan K. Liver disorders in inflammatory bowel disease. Gastroenterol Res Pr. 2012;2012:642923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mathies F, Steffens N, Kleinschmidt D, Stuhlmann F, Huber FJ, Roy U, et al. Colitis promotes a pathological condition of the liver in the absence of Foxp3+ regulatory T cells. J Immunol. 2018;201:3558–68. 10.4049/jimmunol.1800711 [DOI] [PubMed] [Google Scholar]
- 42.Kim SH, Lee W, Kwon D, Lee S, Son SW, Seo MS, et al. Metabolomic analysis of the liver of a dextran sodium sulfate-induced acute colitis mouse model: implications of the gut-liver connection. Cells. 2020;9:341. 10.3390/cells9020341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taniki N, Nakamoto N, Chu P-S, Mikami Y, Amiya T, Teratani T, et al. Intestinal barrier regulates immune responses in the liver via IL-10–producing macrophages. JCI Insight. 2018;3:e91980. 10.1172/jci.insight.91980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ong J, Mebarek L, Bath M, Swift C, Javaid B, Patel J, et al. Interference with the lower gut-liver axis induces remission of primary sclerosing cholangitis in a patient with ulcerative colitis. BMJ Open Gastroenterol. 2018;5:e000239. 10.1136/bmjgast-2018-000239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Navaneethan U. Hepatobiliary manifestations of ulcerative colitis: an example of gut-liver crosstalk. Gastroenterol Rep. 2014;2:193–200. 10.1093/gastro/gou036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu M, Zhang Y, Liu J, Xiang C, Lu Q, Lu H, et al. Revisiting the role of valeric acid in manipulating ulcerative colitis. Inflamm Bowel Dis. 2024;30:617–28. 10.1093/ibd/izad187 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.