Abstract
Aim of the study
Our hypothesis is that nirmatrelvir can penetrate the blood‒brain barrier and reach effective concentrations in the brain. Furthermore, herbal formulations can help maintain nirmatrelvir levels in the body, suggesting potential interactions between these medications.
Materials and methods
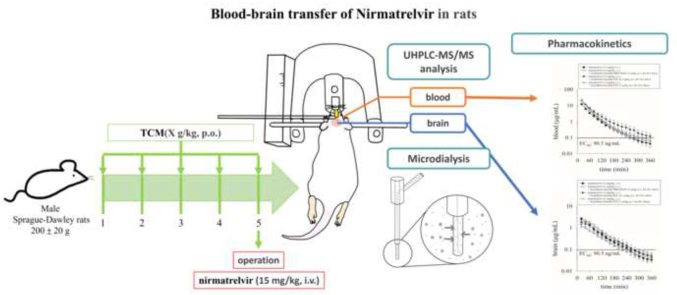
To investigate this hypothesis, an animal model combining multisite microdialysis, ultrahigh-performance liquid chromatography and tandem mass spectrometry (UHPLC-MS/MS) methods was developed to monitor nirmatrelvir levels in the blood and brain of rats.
Results
The pharmacokinetic results showed that the area under the curve (AUC) of nirmatrelvir in the blood and brain was 798.3 ± 58.56 and 187.2 ± 23.46 min μg/mL, respectively, after the administration of nirmatrelvir alone (15 mg/kg, iv). When the Scutellaria baicalensis formulations were administered for five consecutive days prior to drug administration, the AUC of nirmatrelvir in the blood increased.
Conclusions
These results provide constructive preclinical information that the concentrations of nirmatrelvir in the blood and brain were greater than the effective concentration (EC90) for more than 6 h, and the Scutellaria baicalensis formulations had synergistic pharmacokinetic effects by increasing the concentration of nirmatrelvir in the blood.
Keywords: Nirmatrelvir, Microdialysis, Herb–drug interaction, Pharmacokinetics, NRICM101
Graphical abstract
Highlights
-
•
Nirmatrelvir plus with ritonavir (Paxlovid, Pfizer) is used for Covid-19.
-
•
Nirmatrelvir can penetrate the blood‒brain barrier with effective concentration.
-
•
Microdialysis and LC-MS/MS was developed to monitor nirmatrelvir in brain.
-
•
The herbal extract of S. baicalensis had synergistic effects on the nirmatrelvir.
1. Introduction
As of June 2024, COVID-19 has caused nearly 7 million deaths worldwide (WHO, https://covid19.who.int/). Despite progress in COVID-19 research and the promising therapeutic effects of many antiviral drugs against COVID-19, clinical data on these antiviral drugs are still incomplete. Our previous studies demonstrated that both remdesivir and molnupiravir are able to cross biological barriers between the blood and placenta [1,2], as well as between the blood and brain [3], while also reaching the inhibitory antiviral concentration (IC50). Our latest research shows that nirmatrelvir can cross the biological blood–placenta barrier and can also cross the biological blood–brain barrier [4]. This research prompted us to study the interaction between Chinese herbal medicines and nirmatrelvir.
Traditional Chinese medicine (TCM) boasts a rich history and has emerged as a pivotal force in addressing the global challenge presented by the pandemic [5]. The combination of TCM treatments with Western medicine has made significant and enduring contributions to the fight against COVID-19 in Asian countries [6].
Previous research has indicated that certain natural compounds may possess the potential to inhibit SARS-CoV-2 [7]. The literature has documented that these TCM components have anti-SARS-CoV-2 viral properties, suggesting their potential in combating the virus [8]. Among these components, baicalin has attracted our attention. Baicalin is one of the key bioactive ingredients of Scutellaria baicalensis Georgi (Chinese herbal name: huangqin), which features prominently among the three herbal formulas we explored in this study, namely, the Scutellaria formula NRICM101, the Scutellaria formula Jing Guan Fang (JGF), and the Scutellaria formula Zheng Yi Fang (ZYF), and there is evidence in the literature supporting the ability of baicalin to target angiotensin-converting enzyme 2 (ACE2) to prevent SARS-CoV-2 infection [9].
The following three herbal medicines were used in this study: (1) Scutellaria formula NRICM101 (Taiwan Qing Guan No. 1). This traditional Chinese medicine includes 10 herbs, such as Scutellaria root and Heartleaf Houttuynia, which are used for their antiviral and anti-inflammatory properties. It is specifically designed to treat respiratory infections. (2) The Scutellaria formula Jing Guan Fang (JGF), developed by Dr. Chung-Hua Hsu, contains five herbs, namely, Forsythia and Scutellaria, which are used to alleviate symptoms of viral respiratory infections by reducing inflammation and clearing lung heat. (3) Scutellaria formula Zheng Yi Fang (ZYF) - This formula consists of herbs such as Pogostemon and Houttuynia, which are known for improving the immune response and alleviating symptoms such as nasal congestion and cough in respiratory infections, including COVID-19. For a more detailed introduction and references, please refer to Supplementary Material S2.1. Preparation of S. baicalensis formulations.
Because the protein-bound form of the drug does not cross the blood‒brain barrier, only the unbound drug will equilibrate between the blood and the brain. To monitor the level of unbound drug, a multisite microdialysis system was developed to collect blood and brain dialysates. This study aimed to explore the transport of nirmatrelvir across the blood‒brain barrier and the pharmacokinetic interactions between S. baicalensis formulations and nirmatrelvir. Our results suggest that nirmatrelvir crosses the blood‒brain barrier, with a blood‒brain distribution ratio (area under the curve (AUC)tissue/AUCblood) of 0.24 ± 0.02, suggesting that approximately 24 % of nirmatrelvir crosses the barrier. The formulations containing the herbal extract of S. baicalensis had synergistic effects on the pharmacokinetics of nirmatrelvir.
2. Materials and methods
2.1. Experimental animals
The animal experiments in this study were conducted in accordance with the ethical guidelines approved by the Institutional Animal Care and Use Committee of National Yang Ming Chiao Tung University (IACUC no. 1120322 and 1120617). Adult male Sprague‒Dawley rats (6 weeks old, weighing 200 ± 20 g) were obtained from the Laboratory Animal Center at National Yang Ming Chiao Tung University (Taipei, Taiwan). The rats were individually housed under controlled lighting conditions (12-h light-dark cycle) at a temperature of 24 ± 1 °C. The rats were provided ad libitum access to food (diet 5001, PMI Feeds, Richmond, IN) and water.
2.2. Chemicals and reagents
Nirmatrelvir was obtained from MedChemExpress (Monmouth Junction, NJ, USA). Ethanol was acquired from ECHO Chemical Co., Ltd. (Miaoli County, Taiwan). MS-grade acetonitrile was obtained from J.T. Baker, Inc. (Phillipsburg, NJ), while HPLC-grade acetonitrile was obtained from Spectrum (NJ, USA). Dimethyl sulfoxide (DMSO), polyethylene glycol 400 (PEG400), heparin sodium salt, and urethane were purchased from Sigma‒Aldrich Chemicals (St. Louis, MO, USA). Triple deionized water produced by a Millipore purification system (Bedford, MA, USA) was used for preparing all aqueous samples and the mobile phase. A standard stock solution of nirmatrelvir (1 mg/mL) was prepared by dissolving it in ethanol and subsequently stored at −20 °C for experimental purposes. The Scutellaria formula JGF was developed by Dr. Chung-Hua Hsu [10]. The preparation process of the above ZYF is described in the literature [11]. The preparation of S. baicalensis formulations of NRICM101, JGF and ZYF is described in Supplementary Material S2.1. Preparation of S. baicalensis formulations. The UHPLC-MS/MS method optimization and method validation are described in Supplementary Material S2.2. Optimized UHPLC‒MS/MS equipment and conditions and S2.3. Method validation.
2.3. Microdialysis animal experiment and drug administration
2.3.1. Microdialysis sampling system
The experiment utilized a microdialysis system consisting of a microinjection pump (CMA/400, Solna, Sweden) and a microfraction collector (CMA/142). The perfusate used was an anticoagulant citrate dextrose (ACD) solution containing 3.5 mM calcium, 7.5 mM sodium calcium, and 13.6 mM D-(+)-glucose, which was delivered at a constant flow rate of 2 μL/min. To collect dialysate samples from the blood and brain, two probe designs were used in this experiment. A blood microdialysis probe with a length of 11 mm was positioned in the jugular vein of the rats. A brain microdialysis probe with a length of 6 mm was placed in the striatum region of the rat brain. Both probes were constructed using concentric silica capillaries, incorporating a semipermeable dialysis membrane at the tip. The semipermeable membrane was composed of cellulose (Spectrum, New Brunswick, NJ, USA) with a molecular weight cutoff of 13,000 Da. By leveraging the concentration difference between the tissue and the probe, the semipermeable membrane facilitated the measurement of drug concentrations at different time intervals. The in vitro recovery of the microdialysis probes was determined using the following formula: recovery = dialysate concentration/concentration of the standard solution.
2.3.2. Animal experiment
Urethane anesthesia (1 g/kg, i.p.) induced prolonged unconsciousness. A PE 50 polyethylene tube was inserted into the left femoral vein for drug delivery, and a blood microdialysis probe was positioned in the right jugular vein to collect samples. The animal was then prepared for brain surgery, targeting the striatum. The surgical procedure involved marking the striatum at +0.2 mm anteroposterior, +3.2 mm mediolateral, and −7.5 mm dorsoventral to the bregma. A pen drill was then used to create a hole for the placement of the brain microdialysis probe. Nirmatrelvir alone (15 mg/kg, i.v.) or in combination with ritonavir (7 mg/kg, i.v.) was administered after stabilization. Blood and brain dialysates were collected every 20 min for 6 h using a microfraction collector (CMA/142) for subsequent UHPLC MS/MS analysis.
2.3.3. Herbal administration
Prior to conducting experiments involving traditional Chinese medicine interactions, the experimental animals were orally administered the herbal formula for five consecutive days. On the fifth day, the animals were anesthetized 1 h after oral administration of the herbal formula, followed by intravenous administration of the Western medicine to observe the interactions between the traditional Chinese and Western medicines. In terms of dosage selection for this experiment, three different herbal formulations were retrospectively compared to determine the extraction ratios of the herbal ingredients. Subsequently, the translation of dosages between humans and rats was calculated to determine the most appropriate dosage for rats.
NRICM101: The recommended dosage of NRICM101 is 1–3 times a day, with each packet containing a total herbal weight of 135 g [12,13], and the herbal extraction rate is 9.2 %. With human dosages as a reference [14], the recommended human dosage was converted to an equivalent dosage for rats. The animal equivalent dose (AED) (g/kg) = Human does (g/kg) × km ratio. The weight of the raw herbal materials used in NRICM101 was 135 g, while the extraction percentage of 9.2 % was attributed to the efficiency of the NRICM101 extraction process. The recommended daily dose for NRICM101 in this experiment was 3 g/kg.
JGF: The recommended dosage of JGF is once per day, with each packet containing 33 g of herbal ingredients (yielding 100 mL/bag of decoction) [10]. The extraction rate for the formula was 6 %. By employing a conversion reference, the human-recommended dosage has been translated into an equivalent dose for rats [14]. The recommended daily dose for JGF in this experiment was 0.3 g/kg.
ZYF: The recommended dosage of ZYF is once per day, with each packet containing 100 g of herbal ingredients, and the extraction rate for the formula is 25 % [11]. By utilizing a reference guide, the human-recommended dosage has been converted into an equivalent dose for rats [14]. The recommended daily dose for ZYF in this experiment was 3 g/kg.
2.4. Pharmacokinetic analysis and statistics
Pharmacokinetic analysis was performed using WinNonlin software (version 1.0, Pharsight Corp., Mountain View, CA, USA). Blood parameters for nirmatrelvir were analyzed with a two-compartment model, while brain parameters were analyzed with a noncompartmental model. The blood concentration equation followed a biexponential decline (Cp = Ae−αt + Be−βt). The AUC ratio of brain to blood (AUCbrain/AUCblood) × 100 % indicated drug transfer across the blood‒brain barrier. The data were plotted using SigmaPlot software (version 12.0, Systat Software Inc., San Jose, CA). Statistical analysis included one-way ANOVA with Tukey's HSD post hoc test (p < 0.05). The results are presented as the mean ± S.E.M. (n = 6).
3. Results
3.1. Optimization of UHPLC‒MS/MS conditions
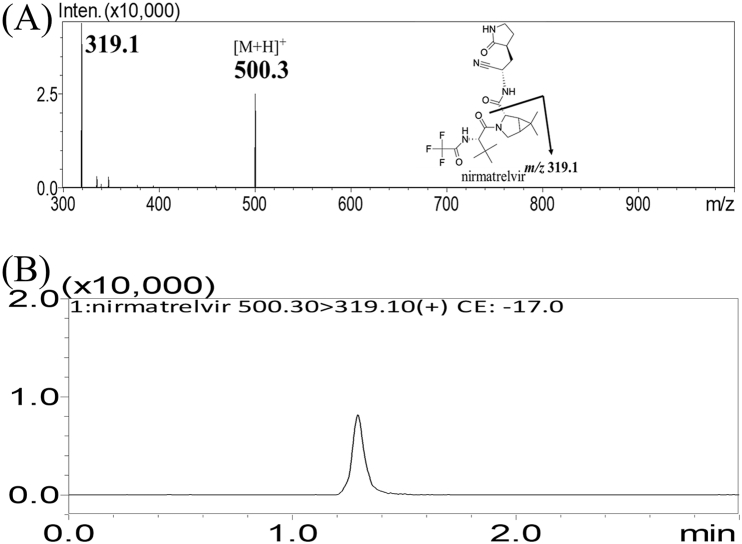
Nirmatrelvir, a moderately polar molecule, was efficiently separated using a reversed-phase C18 column with acetonitrile as the organic phase for elution. The addition of 0.1 % formic acid to the aqueous phase improved the peak shape and prevented tailing. This method enabled precise quantification of nirmatrelvir in dialysate samples with a limit of quantitation of 2 ng/mL. The absence of any peaks of endogenous substances confirmed the selectivity of the observed ion traces for each analyte (Fig. 1A). The method validation results are presented in Supplementary Material S3.1. Method validation [15] (see Fig. 2).
Fig. 1.
(A) MRM product ion mass spectra of nirmatrelvir at m/z 500.3 → 319.1. (B) Representative MRM chromatograms of nirmatrelvir; the retention time was 1.30 min. Peak 1 = nirmatrelvir.
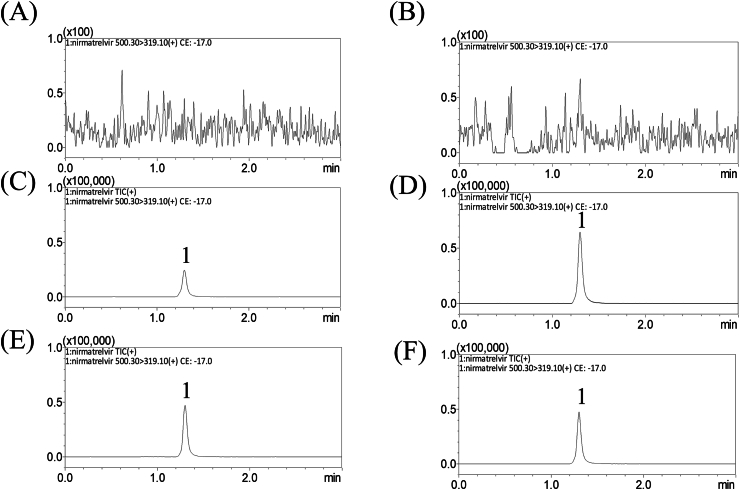
Fig. 2.
Representative MRM chromatograms of (A) blank blood dialysate, (B) blank brain dialysate, (C) blank blood dialysate spiked with nirmatrelvir (50 ng/mL), (D) blank brain dialysate spiked with nirmatrelvir (50 ng/mL), (E) blood dialysate sample containing nirmatrelvir (88.48 ng/mL), and (F) brain dialysate sample containing nirmatrelvir (39.14 ng/mL) collected 60 min after nirmatrelvir administration (15 mg/kg, i.v.); peak 1 = nirmatrelvir.
3.2. Pharmacokinetics of nirmatrelvir administration alone
For the group receiving nirmatrelvir alone (15 mg/kg, i.v.), where no herbal medicine treatment was given, dialysates were collected every 20 min over a span of 6 h. This continuous monitoring allowed us to assess the concentration of nirmatrelvir in both blood and brain tissues. By utilizing the Akaike information criterion (AIC) values, we identified a suitable pharmacokinetic compartment model. Within the one-compartment model, nirmatrelvir exhibited an AIC value of −4.33 ± 8.05 in blood, whereas in the two-compartment model, the AIC value was −60.43 ± 11.8. The lower the AIC value is, the better the fit of the model. Consequently, for the group receiving nirmatrelvir alone, we selected the two-compartment model as the most suitable model for calculating the pharmacokinetics. The resulting concentration equation for nirmatrelvir was CP = 20.01e−0.05t + 7.32e−0.02t, where CP represents the blood dialysate concentration of nirmatrelvir and t denotes time.
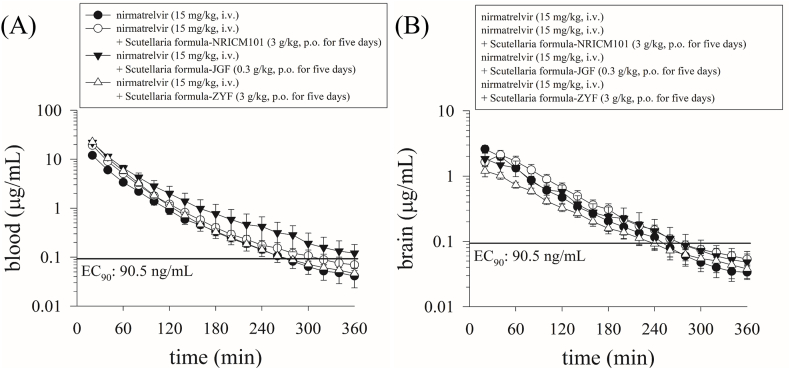
In the nirmatrelvir alone group, the AUC for nirmatrelvir in the blood was 798.3 ± 58.56 min μg/mL, while the AUC in the brain tissue was 187.2 ± 23.46 min μg/mL. Furthermore, the blood‒brain transfer ratio (AUCbrain/AUCblood) was approximately 24 %, indicating that approximately 24 % of the drug could enter the brain from the blood. The maximum concentrations (Cmax) in the blood and brain were 27.39 ± 4.03 and 2.60 ± 0.36 μg/mL, respectively (Fig. 3; Table 1).
Fig. 3.
Concentration‒time curves of protein-unbound nirmatrelvir in the blood and brain of male rats after the administration of nirmatrelvir (15 mg/kg, i.v.) or nirmatrelvir (15 mg/kg, i.v.) plus three Scutellaria formula; n = 6 for each group. The EC90 of the line was 90.5 ng/mL (total EC90 = 292 ng/mL, unbound EC90 = 90.5 ng/mL, 181 nM), which is the concentration at which 90 % inhibition of viral replication is observed [16].
Table 1.
Pharmacokinetic parameters of nirmatrelvir in the blood and brain after nirmatrelvir (15 mg/kg, i.v.) administration compared with those after nirmatrelvir (15 mg/kg, i.v.) and nirmatrelvir (15 mg/kg, i.v.) + Scutellaria formula NRICM101 (3 g/kg, p.o. for five days) (n = 6), nirmatrelvir (15 mg/kg, i.v.) + Scutellaria formula JGF (0.3 g/kg, p.o. for five days) (n = 6), and nirmatrelvir (15 mg/kg, i.v.) + Scutellaria formula ZYF (3 g/kg, p.o. for five days) (n = 6) administration in male rats.
| Parameter | nirmatrelvir (15 mg/kg, i.v.) |
nirmatrelvir (15 mg/kg, i.v.) + NRICM101 (3 g/kg, p.o. for five days) |
nirmatrelvir (15 mg/kg, i.v.) + JGF (0.3 g/kg, p.o. for five days) |
nirmatrelvir (15 mg/kg, i.v.) + ZYF (3 g/kg, p.o. for five days) |
||||
|---|---|---|---|---|---|---|---|---|
| blood | brain | blood | brain | blood | brain | blood | brain | |
| AUClast (min μg/mL) | 798.3 ± 58.56 | 187.2 ± 23.46 | 1297 ± 91.80a | 207.7 ± 32.38 | 1543 ± 153.0a | 171.7 ± 45.41 | 1467 ± 61.83a | 111.6 ± 6.50 |
| Cmax (μg/mL) | 27.39 ± 4.03 | 2.60 ± 0.36 | 57.90 ± 9.14 | 2.32 ± 0.32 | 53.38 ± 9.59 | 1.95 ± 0.43 | 60.74 ± 7.52 | 1.30 ± 0.21 |
| t1/2, α (min) | 16 ± 3 | – | 12 ± 2 | – | 13 ± 2 | – | 14 ± 3 | – |
| t1/2, β (min) | 47 ± 7 | – | 38 ± 4 | – | 62 ± 23 | – | 47 ± 15 | – |
| t1/2 (min) | 23 ± 4 | 55 ± 5 | 17 ± 2 | 71 ± 4 | 23 ± 5 | 76 ± 9 | 18 ± 3 | 83 ± 6 |
| Tmax (min) | – | 20 ± 0 | – | 36 ± 3 | – | 27 ± 6 | – | 30 ± 4 |
| CL (mL/min/kg) | 2.64 ± 2.38 | – | 11.98 ± 1.04a | – | 10.25 ± 0.91a | – | 10.33 ± 0.44a | – |
| MRT (min) | 42 ± 6 | 79 ± 7 | 34 ± 3 | 96 ± 3 | 42 ± 9 | 98 ± 10 | 30 ± 3 | 107 ± 11 |
| AUCtissue/AUCblood | – | 0.24 ± 0.02 | – | 0.17 ± 0.01b | – | 0.13 ± 0.02b | – | 0.08 ± 0.002b |
| AUCherb/AUCnirmatrelvir | – | – | 1.62 ± 0.12 | 1.1 ± 0.17 | 1.93 ± 0.19 | 0.92 ± 0.24 | 1.84 ± 0.15 | 0.60 ± 0.03 |
The data are expressed as the means ± S.E.M.s (n = 6). NRICM101, JGF and ZYF represent the herbal formulas Scutellaria formula JGF, Scutellaria formula JGF and Scutellaria formula ZYF, respectively. The AUCbrain/AUCblood represents the maternal blood-to-tissue transfer ratio. AUCherb/AUCnirmatrelvir represents nirmatrelvir (15 mg/kg, i.v.) administered alone compared with nirmatrelvir (15 mg/kg, i.v.) plus three Scutellaria formula group ratios. a: p < 0.05 compared with blood in the nirmatrelvir (15 mg/kg, i.v.) group, as determined by one-way ANOVA with Tukey's HSD post hoc test. b: p < 0.05 compared with brain tissue in the nirmatrelvir (15 mg/kg, i.v.) group, as determined by one-way ANOVA with Tukey's HSD post hoc test.
3.3. Pharmacokinetic interaction between nirmatrelvir and herbal medicines in rat blood
The pharmacokinetic compartmental model with the best AIC was used to evaluate the pharmacokinetic behavior. The smaller the AIC is, the greater the degree of alignment between the model and the actual data. Based on the results of the aforementioned experiment, the pharmacokinetic interactions of nirmatrelvir (15 mg/kg, i.v.) and the herbal combination groups of NRICM101 (3 g/kg, p.o. for five consecutive days), JGF (0.3 g/kg, p.o. for five consecutive days), and ZYF (3 g/kg, p.o. for five consecutive days) were all best fitted with two-compartment models of CP = 45.26e−0.07t + 12.63e−0.02t, CP = 37.11e−0.06t + 16.27e−0.02t, and CP = 46.82e−0.06t + 16.68e−0.02t, respectively, where CP represents the blood dialysate concentration of nirmatrelvir, and t denotes time.
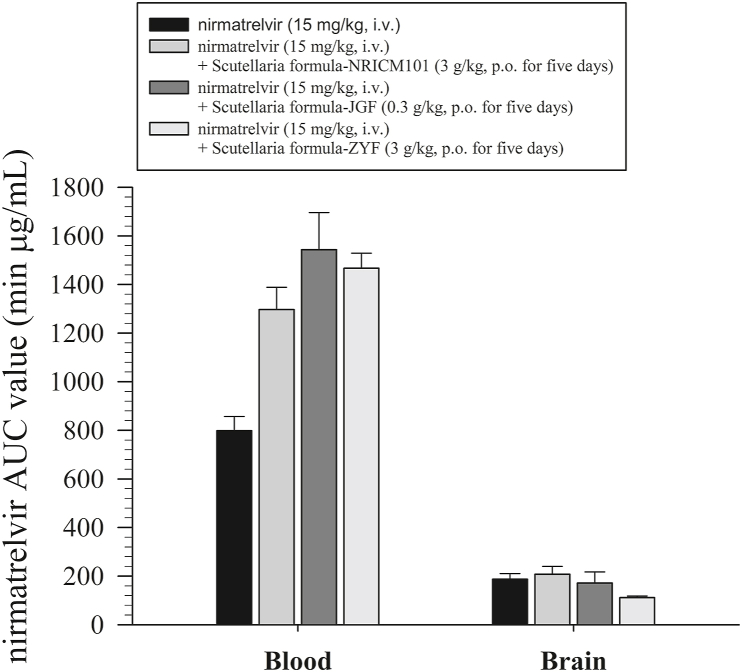
Following the combined administration of nirmatrelvir alone (15 mg/kg, i.v.), nirmatrelvir (15 mg/kg, i.v.) + Scutellaria formula NRICM101 (3 g/kg, p.o. for five days), nirmatrelvir (15 mg/kg, i.v.) + Scutellaria formula JGF (0.3 g/kg, p.o. for five days), and nirmatrelvir (15 mg/kg, i.v.) + Scutellaria formula ZYF (3 g/kg, p.o. for five days), the AUCs of nirmatrelvir were 798.3 ± 58.56, 1297 ± 91.80, 1543 ± 153, and 1467 ± 61.83 min μg/mL, respectively (Table 1). The AUC ratios of the combination of the herbal medicine and nirmatrelvir alone (AUCherb + nirmatrelvir/AUCnirmatrelvir) were 1.62 ± 0.12, 1.93 ± 0.19, and 1.84 ± 0.15 for nirmatrelvir (15 mg/kg, i.v.) + Scutellaria formula NRICM101 (3 g/kg, p.o. for five days), nirmatrelvir (15 mg/kg, i.v.) + Scutellaria formula JGF (0.3 g/kg, p.o. for five days), and nirmatrelvir (15 mg/kg, i.v.) + Scutellaria formula ZYF (3 g/kg, p.o. for five days), respectively. These results suggested that compared with nirmatrelvir alone (15 mg/kg, i.v.), its combination with the three Scutellaria formulations increased the blood nirmatrelvir concentration by more than 1.6-fold (Table 1).
3.4. Pharmacokinetic interaction between nirmatrelvir and herbal medicines in the rat brain
The experimental results demonstrated that nirmatrelvir crossed the blood‒brain barrier at 20 min with maximum concentration (Cmax) values of 2.60 ± 0.36, 2.32 ± 0.32, 1.95 ± 0.43, and 1.30 ± 0.21 μg/mL, respectively, and the AUC values were 187.2 ± 23.46, 207.7 ± 32.38, 171.7 ± 45.41, and 116.6 ± 6.50 min μg/mL for nirmatrelvir alone (15 mg/kg, i.v.), nirmatrelvir (15 mg/kg, i.v.) + Scutellaria formula NRICM101 (3 g/kg, p.o. for five days), nirmatrelvir (15 mg/kg, i.v.) + Scutellaria formula JGF (0.3 g/kg, p.o. for five days), and nirmatrelvir (15 mg/kg, i.v.) + Scutellaria formula ZYF (3 g/kg, p.o. for five days), respectively (Fig. 3; Table 1 Moreover, in comparison to that in the nirmatrelvir alone group, the concentration of nirmatrelvir in the brain slightly increased to 207.7 ± 32.38 min μg/mL in the nirmatrelvir (15 mg/kg, i.v.) + Scutellaria formula NRICM101 (3 g/kg, p.o. for five days) group, but this increase was not as pronounced as that in the bloodstream. These findings suggest that treatment with the Scutellaria formula NRICM101 (3 g/kg, p.o. for five days) modestly increased the blood concentration of nirmatrelvir, leading to a slight increase in brain tissue concentration. Moreover, there was a slight decrease in the other two herbal medicine groups compared to the group treated with nirmatrelvir alone. However, this difference in brain concentration did not reach statistical significance.
Furthermore, we evaluated the blood‒brain barrier transfer ratio (AUCbrain/AUCblood) to estimate drug passage from the bloodstream to brain tissues. The blood‒brain transfer ratios (AUCbrain/AUCblood) of nirmatrelvir were 0.24 ± 0.02, 0.17 ± 0.01, 0.13 ± 0.02, and 0.08 ± 0.002 for nirmatrelvir alone (15 mg/kg, i.v.), nirmatrelvir (15 mg/kg, i.v.) + Scutellaria formula NRICM101 (3 g/kg, p.o. for five days), nirmatrelvir (15 mg/kg, i.v.) + Scutellaria formula JGF (0.3 g/kg, p.o. for five days), and nirmatrelvir (15 mg/kg, i.v.) + Scutellaria formula ZYF (3 g/kg, p.o. for five days), respectively (Table 1 and Fig. 4).
Fig. 4.
Concentration bar chart of protein-unbound nirmatrelvir in the blood and brain after treatment with nirmatrelvir (15 mg/kg, i.v.) or nirmatrelvir (15 mg/kg, i.v.) plus three Scutellaria formulas; n = 6 for each group.
*: p < 0.05 compared with pregnant female blood within groups by one-way ANOVA with Tukey's HSD post hoc test.
4. Discussion
Multisite microdialysis is a precise sampling method that offers high temporal accuracy and resolution for the collection of unbound protein dialysate. This approach reduces the number of experimental animals needed and allows continuous sampling from the same animal, enhancing the understanding of pharmacokinetics. In this study, only six rats per group were used. Our study optimized the use of microdialysis sampling combined with UHPLC-MS/MS, which, while built on established techniques, provided robust and precise analysis suitable for investigating drug interactions in complex biological matrices. This methodological refinement adds value by offering reliable tools for similar pharmacokinetic studies [17].
LC‒MS/MS combines chromatographic separation and sensitive triple-quadrupole mass spectrometry to monitor analytes from biological samples. A previous report demonstrated that remdesivir was used as an internal standard and that acetonitrile was used for protein precipitation to determine nirmatrelvir in human plasma by LC‒MS/MS with a concentration range of 50–5000 ng/mL [18]. Another analytical method with a linear range of 10–10000 ng/mL was developed to measure nirmatrelvir in the plasma of patients with COVID-19 treated with regular-dose Paxlovid [19,20]. Because a UHPLC‒MS/MS separation column was used in this study, the range of nirmatrelvir concentrations obtained using the calibration curve was 2–500 ng/mL for blood and brain samples from the various dialysates.
In this study, we investigated the pharmacokinetic interactions between nirmatrelvir and three Chinese herbal medicines: Scutellaria formula NRICM101, Scutellaria formula JGF, and Scutellaria formula ZYF. These interactions were examined in both the blood and brain (Fig. 4). The bar chart in Fig. 4 provides a clear and quantified comparison, showing a significant increase in the concentration of nirmatrelvir in the blood after the administration of these herbal medicines. The results demonstrated that the use of these herbal formulations can significantly improve blood levels of nirmatrelvir. The literature suggests that the simultaneous use of some herbal remedies and prescription drugs can lead to an increase in the plasma concentration of prescription drugs. In contrast, prolonged intake of herbal supplements followed by prescription drug use can result in plasma concentrations decreasing below therapeutic levels [21]. Furthermore, in 2000, the Lancet published an article highlighting the interactions between various herbal remedies and medications [22]. Herbal drug interactions may depend on a variety of factors, such as enzymatic inhibitors or inducers, transporter interactions, and therapeutic ranges [21].
In this study, the three TCM preparations used were S. baicalensis (Chinese herbal name, huangqin), with S. baicalensis comprising approximately 14 % of the Scutellaria formula NRICM101, 24 % of the Scutellaria formula JGF, and 14 % of the Scutellaria formula ZYF. The primary active component in S. baicalensis is baicalin, which can be converted to baicalein by human intestinal bacteria [23]. Previous reports have confirmed that baicalin has the potential to modulate host inflammatory responses, limit pathological immune damage, and improve clinical and survival outcomes [24]. Baicalin also possesses notable antibacterial properties, as well as anti-inflammatory and anti-apoptotic effects [25]. Furthermore, the metabolite baicalein has been shown to inhibit the activity of CYP3A4 and P-glycoprotein [26].
Based on the literature review, it is evident that the components of S. baicalensis can modulate the body's ability to metabolize CYP enzymes. Paxlovid, which is the subject of this study, has been confirmed to be a substrate for CYP3A4 [27]. Reports have also suggested that when using Paxlovid for the treatment of COVID-19, caution should be exercised to avoid concurrent use of drugs related to CYP3A4 metabolism to prevent potential interactions. The effects of cardiovascular agents, clopidogrel, and the anticonvulsant drug carbamazepine are among those recommended for alternative therapies in the guidelines [28]. Previous literature has also indicated that components in TCM are likely to affect the activity of CYP drug-metabolizing enzymes, thus influencing the metabolism of CYP-metabolized drugs in the body [21,29].
The results of the herbal drug interaction demonstrated that the synergistic pharmacokinetic improvement of the herbal drug may be due to the enzymatic interaction of the active herbal ingredients with nirmatrelvir in the blood (Table 1). No significant changes were observed in the brain among the three traditional Chinese medicines (TCMs), which may be due to the biological barrier of the brain, which minimizes the transfer of nirmatrelvir from the blood to the brain. This disparity resulted in differing blood‒brain transfer ratios (AUCbrain/AUCblood) between the TCM-pretreated group and the nirmatrelvir-alone group, highlighting the impact of TCMs on the pharmacokinetics of nirmatrelvir and suggesting the need to avoid simultaneous administration to prevent potential adverse reactions. However, further studies are needed to validate these findings in clinical settings [16].
5. Conclusions
This study developed a multisite microdialysis sampling method coupled with a validated and optimized UHPLC-MS/MS system to analyze nirmatrelvir, achieving excellent separation and quantification in rat blood and brain tissues. In particular, it was confirmed that nirmatrelvir crosses the blood‒brain barrier and significantly reaches the therapeutic EC90 to treat CNS complications caused by SARS-CoV-2 infection. Our findings also indicate that different herbal formulations affect the pharmacokinetics of nirmatrelvir. In general, in the evaluation of interactions caused by the concurrent use of the Scutellaria formula and nirmatrelvir, based on the data from this study, these three herbal Scutellaria formulations synergistically enhance nirmatrelvir in the blood but do not affect the pharmacokinetics of nirmatrelvir in the brain. However, further clinical studies are needed to validate these interactions in clinical practice.
Ethical approval statement
The animal experiments in this study were conducted in accordance with the ethical guidelines approved by the Institutional Animal Care and Use Committee of National Yang Ming Chiao Tung University, Taipei, Taiwan (IACUC no. 1120322 and 1120617).
Data availability statement
Data are available from the corresponding author upon reasonable request.
CRediT authorship contribution statement
Wan-Hsin Lee: Writing – review & editing, Writing – original draft, Validation, Methodology, Formal analysis, Data curation. Yen-Ying Kung: Resources, Project administration, Conceptualization. Chung-Kai Sun: Methodology, Investigation, Formal analysis, Conceptualization. Chun-Hao Chang: Methodology, Investigation. Wen-Ya Peng: Resources, Investigation. Lie-Chwen Lin: Resources, Project administration, Funding acquisition. Chung-Hua Hsu: Project administration, Funding acquisition. Muh-Hwa Yang: Project administration, Funding acquisition. Tung-Hu Tsai: Writing – review & editing, Writing – original draft, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This paper is a part of the graduate degree thesis of Wan-Hsin Lee. This study was supported in part by a graduate student scholarship from the College of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan. This study was also supported in part by research grants from the National Science and Technology Council of Taiwan (NSTC 1123-2321-B-A49-00511 and NSTC 113-2321-B-A49-011).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e34820.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Yang L., Lin I.H., Lin L.C., Dalley J.W., Tsai T.H. Biotransformation and transplacental transfer of the anti-viral remdesivir and predominant metabolite, GS-441524 in pregnant rats. EBioMedicine. 2022;81 doi: 10.1016/j.ebiom.2022.104095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang C.H., Peng W.Y., Lee W.H., Lin T.Y., Yang M.H., Dalley J.W., et al. Transfer and biotransformation of the COVID-19 prodrug molnupiravir and its metabolite β-D-N4-hydroxycytidine across the blood-placenta barrier. EBioMedicine. 2023;95 doi: 10.1016/j.ebiom.2023.104748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang C.H., Peng W.Y., Lee W.H., Lin T.Y., Yang M.H., Dalley J.W., et al. Biotransformation and brain distribution of the anti-COVID-19 drug molnupiravir and herb-drug pharmacokinetic interactions between the herbal extract Scutellaria formula-NRICM101. J. Pharm. Biomed. Anal. 2023;234 doi: 10.1016/j.jpba.2023.115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee W.-H., Sun C.-K., Chang C.-H., Yang M.-H., Tsai T.-H. The anti-COVID-19 drug Paxlovid crosses biological barriers of the placenta and brain in rats. npj Viruses. 2024;2:4. [Google Scholar]
- 5.Ren J.L., Zhang A.H., Wang X.J. Traditional Chinese medicine for COVID-19 treatment. Pharmacol. Res. 2020;155 doi: 10.1016/j.phrs.2020.104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu R., Zhang S., Zhao D., Yuan Z. A systematic review of outcomes in COVID-19 patients treated with western medicine in combination with traditional Chinese medicine versus western medicine alone. Expert Rev Mol Med. 2022;24:e5. doi: 10.1017/erm.2021.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Y., Islam M.S., Wang J., Li Y., Chen X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int. J. Biol. Sci. 2020;16:1708–1717. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng Y.F., Aluko R.E., Jin Q., Zhang Y., Yuan L.J. Inhibitory activities of baicalin against renin and angiotensin-converting enzyme. Pharm. Biol. 2012;50:401–406. doi: 10.3109/13880209.2011.608076. [DOI] [PubMed] [Google Scholar]
- 10.Ping Y.H., Yeh H., Chu L.W., Lin Z.H., Hsu Y.C., Lin L.C., et al. The traditional Chinese medicine formula jing guan Fang for preventing SARS-CoV-2 infection: from clinical observation to basic research. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.744439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang K.W., Lin T.Y., Fu S.L., Ping Y.H., Chen F.P., Kung Y.Y. A Houttuynia cordata-based Chinese herbal formula improved symptoms of allergic rhinitis during the COVID-19 pandemic. J. Chin. Med. Assoc. 2022;85:717–722. doi: 10.1097/JCMA.0000000000000732. [DOI] [PubMed] [Google Scholar]
- 12.Tsai K.C., Huang Y.C., Liaw C.C., Tsai C.I., Chiou C.T., Lin C.J., et al. A traditional Chinese medicine formula NRICM101 to target COVID-19 through multiple pathways: a bedside-to-bench study. Biomed. Pharmacother. 2021;133 doi: 10.1016/j.biopha.2020.111037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su Y.C., Huang G.J., Lin J.G. Chinese herbal prescriptions for COVID-19 management: special reference to Taiwan Chingguan Yihau (NRICM101) Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.928106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nair A.B., Jacob S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016;7:27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.US Food and Drug Administration . 2018. Bioanalytical-Method-Validation-Guidance-for-Industry. [Google Scholar]
- 16.Singh R.S.P., Toussi S.S., Hackman F., Chan P.L., Rao R., Allen R., et al. Innovative randomized phase I study and dosing regimen selection to accelerate and inform pivotal COVID-19 trial of nirmatrelvir. Clin. Pharmacol. Ther. 2022;112:101–111. doi: 10.1002/cpt.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zestos A.G., Kennedy R.T. Microdialysis coupled with LC-MS/MS for in vivo neurochemical monitoring. AAPS J. 2017;19:1284–1293. doi: 10.1208/s12248-017-0114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu C., Zhu M., Cao L., Boucetta H., Song M., Hang T., et al. Simultaneous determination of nirmatrelvir and ritonavir in human plasma using LC–MS/MS and its pharmacokinetic application in healthy Chinese volunteers. Biomed. Chromatogr. 2022;36 doi: 10.1002/bmc.5456. [DOI] [PubMed] [Google Scholar]
- 19.Martens-Lobenhoffer J., Boger C.R., Kielstein J., Bode-Boger S.M. Simultaneous quantification of nirmatrelvir and ritonavir by LC-MS/MS in patients treated for COVID-19. J Chromatogr B Analyt Technol Biomed Life Sci. 2022;1212 doi: 10.1016/j.jchromb.2022.123510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toussi S.S., Neutel J.M., Navarro J., Preston R.A., Shi H., Kavetska O., et al. Pharmacokinetics of oral nirmatrelvir/ritonavir, a protease inhibitor for treatment of COVID‐19, in subjects with renal impairment. Clin. Pharmacol. Ther. 2022;112:892–900. doi: 10.1002/cpt.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pal D., Mitra A.K. MDR- and CYP3A4-mediated drug-herbal interactions. Life Sci. 2006;78:2131–2145. doi: 10.1016/j.lfs.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Fugh-Berman A. Herb-drug interactions. Lancet. 2000;355:134–138. doi: 10.1016/S0140-6736(99)06457-0. [DOI] [PubMed] [Google Scholar]
- 23.Kim D.-H., Jang I.-S., Lee H.-K., Jung E.-A., Lee K.-Y. Metabolism of glycyrrhizin and baicalin by human intestinal bacteria. Arch Pharm. Res. (Seoul) 1996;19:292–296. [Google Scholar]
- 24.Pang P., Zheng K., Wu S., Xu H., Deng L., Shi Y., et al. Baicalin downregulates RLRs signaling pathway to control influenza A virus infection and improve the prognosis. Evid Based Complement Alternat Med. 2018;2018 doi: 10.1155/2018/4923062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu J., Wang J., Sheng Y., Zou Y., Bo L., Wang F., et al. Baicalin improves survival in a murine model of polymicrobial sepsis via suppressing inflammatory response and lymphocyte apoptosis. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho Y.-A., Choi J.-S., Burm J.-P. Effects of the antioxidant baicalein on the pharmacokinetics of nimodipine in rats: a possible role of P-glycoprotein and CYP3A4 inhibition by baicalein. Pharmacol. Rep. 2011;63:1066–1073. doi: 10.1016/s1734-1140(11)70624-7. [DOI] [PubMed] [Google Scholar]
- 27.US Food and Drug Administration . 2023. Fact Sheet for Healthcare Providers: Emergency Use Authorization for Paxlovid. [Google Scholar]
- 28.National Institutes of Health . 2023. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. [PubMed] [Google Scholar]
- 29.Zhang F., Huang J., Liu W., Wang C.R., Liu Y.F., Tu D.Z., et al. Inhibition of drug-metabolizing enzymes by Qingfei Paidu decoction: implication of herb-drug interactions in COVID-19 pharmacotherapy. Food Chem. Toxicol. 2021;149 doi: 10.1016/j.fct.2021.111998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the corresponding author upon reasonable request.