Abstract
Medicinal plants Highly aromatic crude materials are utilized for treating warts as an alternative medicine to surgical treatment because they can be permanently removed from the body. Thus, this investigation aimed to extract plant material from Calotropis procera leaves, describe the phytochemical screening, analyze anti-microbial activities, determine the functional groups in FTIR, and identify the chemical compounds in GC-MS. The PH, specific gravity, and viscosity of the crude extracts of Calotropis procera were determined at 4.5, 0.79, and 0.49, respectively. Analyze the solubility of crude extracts; ethanol can dissolve while water does not. Flavonoids, alkaloids, phenols, tannins, and saponins were also present in the phytochemical screening tests of the Calotropis procera extracts, triterpenoids, terpenoids, and steroids were not present in the crude extract. Flavonoids, alkaloids, phenols, tannins, and saponins are the primary phytochemical components found in therapeutic plant material. The Calotropis procera crude extracts analyzed for functional groups by FT-IR contained a hydroxyl group, alkane, carbonyl, aldehyde, ketone, phenols, ester, alcohol, and methylene. The chemical compounds analyzed by GC-MS of Calotropis procera crude material were found to have 22 main compounds. Of 22 compounds, 5 compounds are active ingredients for the applications of medical purposes. The bioactive compounds found in the Calotropis procera plant extract are neophytadiene, hexahydrofarnesyl, lanosterol, 2,4-dimethylbenzo [H]quinolone, and squalene. Those bioactive compounds have anti-bacterial, analgesic, antipyretic, anti-inflammatory, antimicrobial, antioxidant, antiviral, and anti-cancer properties. In an in vitro antimicrobial activity test, the crude extract effectively inhibited more gram-positive bacteria than gram-negative bacteria. This collective reason is why the traditional therapist uses this Calotropis procera plant for the treatment of warts.
Keywords: Calotropis procera, Squalene, Anti-microbial, Anti-cancer, Wart, HPV, Gc-ms, FTIR, Invitro ant-microbial, Anti-inflammatory
1. Introduction
In addition to being vital for medical purposes, medicinal plants provide numerous ecological, economic, and cultural functions. Around the world, 64 % of people get their medical care from conventional practitioners [1]. Approximately 3.5 million people in non-developed countries, including Ethiopia, regularly use and believe in the effectiveness of herbal remedies, according to estimates from the World Health Organization (WHO). WHO defines traditional medicine as “Health practices, attitudes, knowledge, and beliefs include manual techniques, exercises, medication derived from plants, animals, and minerals, spiritual therapies, and manual techniques used alone or in combination to treat, diagnose, prevent, and maintain wellness” [2] (see Fig. 1).
Fig. 1.

Calotropis procera plant.
Ethiopian traditional healers use herbal medicines to treat many diseases. Ethiopia has around 800 various kinds of medicinal plants, which are used to cure about 300 diseases. in our country Ethiopia, traditional medicine is still normally practiced. Most human and animal illnesses in Ethiopia are treated with herbal medicinal plants. They are used to treat diarrhea, stomachaches, asthma, sore throats and gums, earaches, and coughs. Certain medicinal plants can be used for therapeutic purposes; leaves and roots make up most of these parts. The most effective method for discovering new medications is said to be using the method of researching naturally occurring compounds [2,3].
Calotropis procera is a shrub that flowers and belongs to the Asclepediaceae family. According to its habitat, it is known by many different names. In Arabic, it is known as dead sea plant, Kisher, and Usher; in England, it has the names Calotropis, Calotrope, dead sea fruit, desert wick, Giant milkweed, mudarfiber, Rubber tree, Swallow-wort, sodom apple; in Hindi, it is described as Akdo, Akada, and Madar; in Amharic, it is known as Koba [4].
Although warts, which are tiny skin growths brought on by the human papillomavirus, are not fatal, they can be unattractive, unpleasant, embarrassing, and spread. They might gradually deteriorate if they do so naturally. People with warts, therefore, look for over-the-counter or prescription treatments. There are a few of the countless commercially available anti-wart drugs that work.
Their effectiveness rates are also hampered by the need for many applications over several months and certain unwanted side effects. There are also known recurrences. Thus, the quest for a rapid, inexpensive, safe, effective, and simple-to-use medication continues. In the past few years, several patent applications for anti-wart treatments have been made [5].
The human papillomavirus (HPV) is the cause of warts. HPV is a cancer case that typically has no effective treatment. Throughout human history, many theories and remedies for warts have been developed. These include the use of plants, animal secretions, precious stones, metals, and minerals. Nowadays, natural products are a prominent way to treat warts. Natural products have antiviral and anti-carcinogenic properties [6]. In Ethiopia, numerous plants are essential for traditional herbal medicine, with over 800 medicinal herbs used to treat more than 300 diseases [7]. Some of these plants include Dorstenia barnimiana Schweenf, Ficus ficifolius A. Rich., Croton macrostachyus, Salvadora persica L., Gladiolus schweinfurthii, and Calotropis procera (qoba). These indigenous plants are traditionally used to treat warts [8,9]. Although the plant's pharmaceutical and industrial applications have received sufficient attention, its general biological and chemical properties have not been investigated in depth; additionally, the relationship between C. procera's toxicity and bioactivity, which is essential to supporting the plant's therapeutic claims, has not received enough attention. Lastly, by completing these knowledge gaps, we can better understand how invasive the plant is and what potential risks it may pose to the environment or biodiversity [7]. C. procera of the plants did not investigate the chemical constituents and phytochemical screenings by different solvent extracts and did not identify which compounds have anti-viral or anti-microbial properties [1].
The other limitation is the plants have different geographical locations and there is no scientific study report about calotropis procera leaf extract in Ethiopia.
Calotropis procera plants have medicinal uses traditionally in Ethiopia for wart treatment; however, Calotropis procera requires extra investigation of the plant which is phytochemical screening, functional groups, and chemical compound analysis in the crude extracts by the method of phytochemical screening test, FTIR AND GC-MS to determine the bioactive compounds in the crude extracts for the treatment of warts that are caused by the human papillomavirus (HPV) and inflammations of human body.
2. Material and methods
2.1. Materials
The main raw material, Calotropis procera plant leaves was obtained in Mersa, also known as Mersa-Abagetye, in North Wollo, Ethiopia. The location of the plant was 1600 m above sea level and located at 11°40′N latitude and 39°39.5′E longitude. Two pathogenic bacteria, Escherichia coli (gram-negative) and Staphylococcus aureus (gram-positive), were selected for the antibacterial efficacy assay. These bacteria were sourced from the Clinical Bacteriology and Mycology Laboratory at the Amhara Public Health Institute, Dessie Branch, in Dessie City, Ethiopia. The investigation employed N-hexane (99.9 %) and ethanol (97 %) as extractive solvents and utilized Muler-Hinton agar medium (MHA) for the antibacterial efficacy test. Key analytical tools included FTIR spectroscopy, GC-MS analysis, and a Soxhlet extractor. The supplementary material includes lists of all other chemicals and equipment.
2.2. Methods
2.2.1. Extraction procedure
The Calotropis procera plant leaves were dried in a hot air dryer at a temperature of 110 °C within 4 h to facilitate size reduction. The dried sample was crushed by a jaw crusher and sieved using different sieve sizes (0.5–1 mm and 1–1.8 mm). The crushed sample was extracted through solvent extraction technique via a soxhlet extractor apparatus with ethanol and n-hexane solvents. The ratio of solvent to solid was used 20 mL:3 g. Once the extraction process is completed, complete removal of the solvent is performed by using a rotary evaporator at 50 0c. the graphical representations of the extraction procedure are shown in supplementary material figure S1. Finally, the amount of crude extract for each run was determined by Equation (1), [8].
| (1) |
2.3. Property characterization of crude oil
The characterization of crude material properties was analyzed by ASTM and AOAC standard testing techniques; specific gravity (ASTM D1298), kinematic viscosity (ASTM D445), pH, and solubility [8].
2.4. Characterization of the crude extract's secondary metabolites
Using established methodologies, the crude extract was subjected to a qualitative phytochemical test to determine whether important classes of chemicals, including phenols, tannins, terpenoids, triterpenoids, flavonoids, and saponins, were present or absent. These components are relevant to anti-microbial properties. The plant crude extracts were analyzed using the previously reported analytical procedures to identify secondary metabolites [[8], [9], [10], [11], [12]].
2.4.1. Terpenoids test
The 0.5 mg of crude extract was combined with 1 ml of Chloroform in a test tube, followed by the careful addition of 3 ml of 98 % concentrated Sulfuric acid. The presence of terpenoids was confirmed by observing the formation of a reddish-brown color.
2.4.2. Flavonoids test
Lead acetate test: by adding 0.5 ml of crude extract and treated with 5 drops of 10 % solution of lead acetate. The formation of a yellow precipitate indicated that the presence of flavonoids in the crude sample.
2.4.3. Tannins test
FeCl3 test: Tannin presence was detected by mixing 0.5 mL of crude extracts with a few drops of 5 % FeCl3, resulting in the observation of blue-green, bluish-black, and greenish colors.
2.4.4. Acid anhydride test
The presence of tannin was examined by mixing 3 mL of crude extract with 1 mL of acid anhydride and 1 mL of concentrated H2SO4, resulting in the formation of a green color.
2.4.5. Alkaloids test
For the Hager's Test, 3 drops of crude extract were combined with 5 drops of Hager's reagent. Yellow precipitate formation indicated the presence of alkaloids.
In the Dragendorff test, 0.5 g of crude extract was mixed with 5 g of Dragendorff reagent. The presence of alkaloids was confirmed by the appearance of a reddish-brown precipitate or turbidity.
For Mayer's test, 0.5 g of the crude extract with 5 g of Mayer's reagent was mixed. The reaction caused a color change to cream, opalescent, or yellow, indicating alkaloid presence.
2.4.6. Saponins test
The presence of saponins was determined by mixing 0.5 mg of crude extract with 5 ml of distilled water and shaking the mixture for 15 min. The presence of saponins was indicated by the formation of foam.
2.4.7. Steroids and triterpenoids test
3 drops of crude extract were mixed with 5 mL of Chloroform and filtered. The filtrate was then vigorously shaken after adding 5 drops of 98 % concentrated Sulfuric acid and left to stand for 5 min. The presence of steroids was indicated by a red color observed in the lower layer. Triterpenoids were identified by the formation of a reddish-brown color at the interface upon the addition of H2SO4 without agitation.
2.4.8. Phenol test
Upon adding a few drops of 5 % Ferric chloride solution to 2 mL of the crude extract, a bluish-black or dark green color developed, confirming the existence of phenols.
2.5. Gas chromatograph -mass spectroscopy (GC-MS)
The compounds in the extract were analyzed using GC-MS (Gas Chromatography-Mass Spectrometry) analysis. Dissolve 50 ml oil in 2 ml dichloromethane (DCM). Sample analysis was performed using a medium polarity capillary column. (BPX-5) (29.4 m × 0.25 mm), and the film thickness was determined by a Hewlett-packed mass Hunter GC-MS system model 5977 as carrier gas. Inject 1 μl of the sample using splitless injection and an injector temperature of 300 °C. The GC-MS was run at 50 °C for 2 min and then every minute from 10 °C to 300 °C. We maintain the final temperature for 10 min. Each sample was run for 28 min. Electron ionization at 70 eV using mass spectrometry revealed mass fragments between 40 and 500 m/z. The ion source temperature was 200 °C and the transfer line temperature was 300 °C. Observe that the detector activates after 5 min [13,14].
2.6. Fourier-transforms infrared spectroscopy (FTIR)
Functional groups were identified using Fourier transform infrared spectroscopy. An ATR-FTIR from Thermo Scientific (Thermo Nicolet Analytical Instruments, Madison, WI) was used to record the spectra. The spectra were recorded in the range 4000–450 cm−1 with a resolution of 4 cm−1. Each spectrum is compared to a completely new background spectrum recorded from a common ATR crystal. To clean the ATR crystals, pure ethanol was used to remove any residue before collecting each background spectrum. Scan each sample 3 times [14].
2.7. In vitro ant-bacterial activities of Calotropis procera leaves extract
The agar-well diffusion method for the antibacterial assay was employed. MHA medium was prepared according to the manufacturer's instructions and inoculated with the bacterial solution. The selected pathogenic bacteria were exposed to a 1 mg/mL concentration of crude extract. Wells was created in the agar medium using a sterile cork borer with a 6 mm diameter. A positive control (ciprofloxacin, 5 μg) was placed in the center of the plate in a predetermined arrangement. Subsequently, 50 μL of the 1 mg/mL extract and 50 μL of the negative control (DMSO) were carefully added to the wells. To ensure adequate diffusion, a 30-min incubation period was included. After incubation at 37 °C for 24 h, the diameter of the clear zone of growth inhibition around each well was measured in millimeters. Each experiment was performed in triplicate [12,15].
3. Results and discussion
3.1. Moisture content determination
The moisture content of the Calotropis procera leaves has been determined and tabulated in supplementary material Table S1. The moisture content was analyzed at a temperature of 110 oc and measured at different hours until it reached a constant moisture content. Finally, at a temperature of 110 oc and for 4 h, the final moisture content was 25 %.
3.2. The physical properties of the crude extract
The pH, kinematic viscosity, and specific gravity of the Calotropis procera leaf crude extract are 4.5, 0.49, and 0.79, respectively. The solubility of Calotropis procera leaf crude extract is soluble in alcohol (ethanol) and insoluble in water.
3.3. Determination of crude extract yield
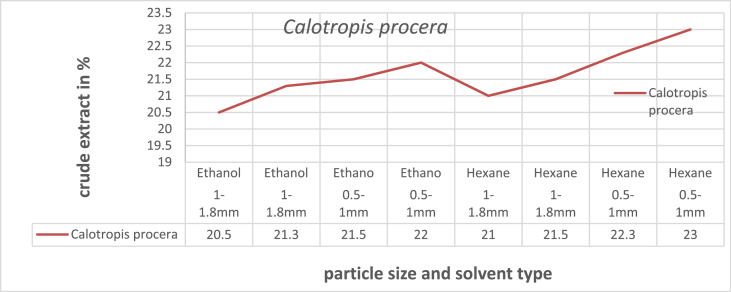
The maximum crude yield is 23.5 % at 0.5–1 mm particle size, and solvent-based n-hexane is used. The minimum oil yield is 20.5 at 1–1.8 mm particle size and solvent-based ethanol. All factors, levels, and oil yields are tabulated in supplementary material Table S2. Fig. 2, shows the graphical representations of the oil yield investigation. When compared to ethanol and n-hexane as a solvent and fine and coarse particle size, n-hexane is better than ethanol, and fine particles are more extractable than coarse particles [8]. The optimum extraction solvent is hexane, and the particle size is 0.5–1 mm. Take these optimum crude extracts for further investigation and analysis.
Fig. 2.
Determination of crude extract yield.
3.4. Characterization of the crude extract's secondary metabolites
The preliminary qualitative phytochemical screening of the Calotropis procera leaf crude extract was to assess the presence of bioactive components. The absence and presence of flavonoids, tannins, alkaloids, phenols, steroids, terpenoids, and saponins were determined. The results obtained in phytochemical screening tests of the extract were summarized in Table 1.
Table 1.
Phytochemical Screening tests of Calotropis procera extracts.
| Test | Method | Test result | Sign indicates |
|---|---|---|---|
| Saponins | Foam | ++ | high presence |
| Terpenoids | salkowaski test | – | Absent |
| Triterpenoids | salkowaski test | – | Absent |
| Steroids | salkowaski test | – | Absent |
| Alkaloids | Mayer's | + | Presence |
| Dragondrof's | +++ | Extremely High Presence | |
| Hager's | + | Presence | |
| Flavonoids | Alkaline Reagent | ++ | High Presence |
| Phenols | FeCl3 | + | Presence |
| Tannins | FeCl3 | ++ | high presence |
| Anhydride | +++ | Extremely high presence |
The pictorial representation of phytochemical screening was shown in supplementary material Figure S2. The crude extract of Calotropis procera leaves has been phenol in insignificant amounts, while alkaloids by Dragondrof's test, flavonoids, and tannins were all significantly present. The crude extract of leaves from Calotropis procera did not contain terpenoids, triterpenoids, or steroids. Satvabrata Kundu 2021 reported that numerous naturally occurring chemical components, such as cardenolides, tannins, glycosides, phenols, terpenoids, flavonoids, alkaloids, and saponins, are present in the powdered leaves of Calotropis procera [4]. In our case, the absence of terpenoid, triterpenoid, and steroid may be, due to a geographical influence and solvent type. The main secondary metabolites that give the crude extract medicinal benefits are flavonoids, alkaloids, and phenols. Terpenoids and tannins have analgesic and anti-inflammatory properties. In addition, tannins support the astringent quality, which promotes quicker healing of wounds and irritated mucous membranes [13,16].
The bioactivity of Calotropis procera leaf crude extract is influenced by interactions among its phytochemicals and their solubility in n-hexane. Alkaloids can dissolve in n-hexane if they have non-polar side chains. Flavonoids and phenols have limited solubility unless methylated or glycosylated, while tannins are poorly soluble. Steroids and terpenoids dissolve well in n-hexane, and saponins are less soluble due to their polar regions. In n-hexane, non-polar compounds like steroids and terpenoids enhance the permeability and stability of other components. Hydrophobic interactions stabilize non-polar molecules like steroids and terpenoids in n-hexane, while hydrogen bonding among flavonoids, phenols, and tannins can reduce their solubility but alter bioactivity. Tannins can form complexes with alkaloids, affecting solubility and bioavailability, and flavonoids and phenols also form hydrogen-bonded complexes. Synergistic effects, such as terpenoids enhancing cell membrane permeability, increase the bioavailability of other compounds, while tannins can reduce bioavailability by forming insoluble complexes.
3.5. Chemical composition analysis by GC-MS of Calotropis procera leave extracts
The graphical representations of the GC-MS result show that in supplementary material Figure S3 and Table 2. Neophytadiene is used for analgesic, antipyretic, anti-inflammatory, antimicrobial, and antioxidant compounds [17]. Hexahydrofarnesyl acetone has anti-bacterial, anti-nociceptive, and anti-inflammatory activities [18]. One type of tetracyclic triterpenoid is lanosterol. It is the substance from which all steroids generated lanosterol [19]. 2, 4-dimethylbenzo [H]quinoline is a chemical compound that has been isolated from the leaves of Lasiocarpa Americana. It is an antibacterial agent that prevents bacterial growth by inhibiting protein synthesis and antiprotozoal activity [20]. Squalene is used as an antimicrobial, antitumor agent, supplement, antioxidant, anti-cancer, and repellent [21]. Table S3 shows a detailed explanation of each compound's fragmental pattern.
Table 2.
list of phytochemicals found in Calotropis procera leaves.
| PK | Library/ID | RT | Area Pct |
|---|---|---|---|
| 1 | Methyl tetradecanoate | 9.9936 | 0.4998 |
| 2 | Neophytadiene | 10.5971 | 2.4071 |
| 3 | 1-Hexadecyne | 10.8479 | 0.3394 |
| 4 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | 11.1081 | 0.7881 |
| 5 | 2-Pentadecanone, 6,10,14-trimethyl- | 11.589 | 0.816 |
| 6 | Hexadecanoic acid, methyl ester | 12.2122 | 6.6098 |
| 7 | 9-Octadecenoic acid (Z)-, methyl ester | 14.9683 | 1.2897 |
| 8 | 9,12-Octadecadienoic acid (Z,Z)-, methyl ester | 15.0561 | 5.0601 |
| 9 | Methyl stearate | 15.2025 | 0.9388 |
| 10 | 9,12,15-Octadecatrienoic acid, methyl ester, (Z,Z,Z)- | 15.3426 | 7.2336 |
| 11 | Neophytadiene | 15.5722 | 8.914 |
| 12 | Stigmasta-7,16-dien-3-ol, (3.beta.,5.alpha.)- | 21.4033 | 20.4337 |
| 13 | 1,1,6-trimethyl-3-methylene-2-(3,6,9,13-tetramethyl-6-ethenye-10,14-dimethylene-pentadec-4-enyl)cyclohexane | 22.1262 | 1.507 |
| 14 | 9,19-Cycloergost-24 (28)-en-3-ol, 4,14-dimethyl-, acetate, (3.beta.,4.alpha.,5.alpha.)- | 22.6228 | 9.7416 |
| 15 | 9,19-Cyclolanost-25-en-3-ol, 24-methyl-, (3.beta.,24S)- | 22.8563 | 9.1863 |
| 16 | 9,19-Cyclolanostan-3-ol, 24-methylene-, (3.beta.)- | 22.9162 | 4.7309 |
| 17 | Ergost-25-ene-3,5,6,12-tetrol, (3.beta.,5.alpha.,6.beta.,12.beta.)- | 23.0291 | 6.6599 |
| 18 | Eicosane | 24.7196 | 0.528 |
| 19 | Lanosterol | 25.577 | 2.2758 |
| 20 | Benzo [h]quinoline, 2,4-dimethyl- | 26.2479 | 0.7771 |
| 21 | Tetracosanoic acid, methyl ester | 26.7738 | 0.4189 |
| 22 | Squalene | 27.8659 | 8.8444 |
The chemical structures and functional groups of neophytadiene, hexahydrofarnesyl acetone, lanosterol, 2,4-dimethylbenzo [h]quinoline, and squalene significantly influence their biological activities, and their potential therapeutic applications are discussed below in depth.
3.5.1. Neophytadiene
Neophytadiene, a diterpene with specific molecular features, interacts with GABA receptors, leading to its anxiolytic-like, antidepressant-like, anticonvulsant, and sedative effects [22]. The prenyl groups in neophyte diene are crucial for binding to GABA receptors, thereby mediating its neuropharmacological activity [22,23].
3.5.2. Hexahydrofarnesyl acetone
Hexahydrofarnesyl acetone, a derivative of farnesol and similar in structure to squalene, possesses antioxidant and cytoprotective properties. The prenyl groups present in its structure are responsible for these beneficial effects, making it a candidate for treating diseases such as cancer, cardiovascular disease, and neurodegenerative disorders [24].
3.5.3. Lanosterol
Lanosterol is a sterol that interacts with cholesterol to regulate cell membrane fluidity and permeability. Its hydroxyl and methyl groups facilitate these interactions, suggesting its potential in treating conditions related to cholesterol imbalance, such as atherosclerosis [24].
3.5.4. 2,4-Dimethylbenzo [h]quinolone
2,4-Dimethylbenzo [h]quinoline is a quinoline derivative with antimicrobial and antifungal activities. The methyl and hydroxyl groups in its structure contribute to these properties, making it a potential treatment for bacterial and fungal infections [24].
3.5.5. Squalene
Squalene, an isoprenoid antioxidant, exhibits significant antioxidant and cytoprotective properties due to its unique molecular structure and prenyl groups. These characteristics make it useful in treating diseases like cancer, cardiovascular disease, and neurodegenerative disorders [24,25].
The therapeutic implications of these compounds are significant. Neophytadiene shows potential as a treatment for anxiety disorders, depression, and seizures. Hexahydrofarnesyl acetone is useful in combating cancer, and cardiovascular, and neurodegenerative diseases. Lanosterol may help manage cholesterol-related diseases such as atherosclerosis. 2,4-Dimethylbenzo [h]quinoline is effective against bacterial and fungal infections. Squalene is valuable in treating cancer, cardiovascular, and neurodegenerative diseases [22,24,25]. Thus, these compounds demonstrate promising biological activities and therapeutic potential.
3.6. FTIR analysis of Calotropis procera crude extract
The Calotropis procera crude extract's FTIR analysis result showed distinctive absorption peaks in the supplementary material (Fig. S4 and Table 3). The chemical bond's characteristic wavelength was found in the FTIR spectra. The IR absorption spectra are used to identify these chemical bonds in a molecule. Fourier-transform infrared spectroscopy is used to analyze the functional groups of Calotropis procera plant extract. Calotropis procera extract has a hydroxyl group, alkane, carbonyl, aldehyde, ketone, phenols, ester, alcohol, and methylene. The phytochemical screening test of Calotropis procera extract showed alkaline, and in the FTIR test, most of the peaks were alkaline. Depending on the wave number, express the functional bond, and its groshownhows in Table 3 [26,27].
Table 3.
Fourier-transform infrared spectroscopy peak values of Calotropis procera.
| No | Peak (wave number cm −1) | Intensity | Bond | Functional group assignment | Remark an assignment |
|---|---|---|---|---|---|
| 1 | 3391 | Weak broad | O–H | Hydroxyl group | stretching vibrations of O–H bond in alcohols or phenols |
| 2 | 2917 | Strong | C–H | Alkane | C–H Stretching alkanes |
| 3 | 2849 | Strong | C–H | Alkane | C–H Stretching alkanes |
| 4 | 1736 | medium | C=O | Carbonyl | carbonyl of aldehyde or ketone |
| 5 | 1462 | Medium | C=O | Carbonyl group | carbonyl of aldehyde or ketone |
| 6 | 1378 | Medium | C–H | methylene (–CH2–) | bending vibrations of a methylene (–CH2–) group or a substituted aromatic ring, |
| 7 | 1244 | Weak | C–O | C–O stretching | C–O stretching, bond vibrations from ester groups |
| 8 | 1013 | Weak | C–O | C–O stretching | C–O stretching vibrations of esters, alcohols, or phenols |
| 11 | 824 | Weak | C–H | Alkane | out-of-plane bending vibrations of C–H bonds in aromatic compounds |
| 12 | 718 | Weak | C–H | Methylene | -(CH2)n- Rocking |
The current FT-IR data verified that the leaf extract of the Calotropis procera plant contains alkanes, alcohols, phenols, aldehydes, ketone, esters, and aromatic chemicals. The phytochemical test also showed the presence of tannin, alkane, phenol, and flavonoids. The GC-MS results have exposed the presence of anti-viral, anti-bacterial, and anti-inflammatory compounds. The presence of all these compounds showed that the Calotropis procera leaf extract has medicinal properties.
The FTIR (Fourier Transform Infrared) spectrum of the Calotropis procera crude extract exhibits several characteristic absorption peaks related to the extract's phytochemical composition. These peaks are attributed to specific vibrational modes and molecular interactions involving various functional groups present in the extract that can be shown in Table 3. The peaks are expressed in CH2 Stretching, C–O Bonds, Aromatic Ring, Aldehyde Groups, Hydroxyl (O–H) Groups, C–H Stretching and Bending. These characteristic absorption peaks in the FTIR spectrum of the Calotropis procera crude extract are related to the phytochemical composition of the extract, which includes compounds such as catechin, rutin, p-coumaric acid, caffeic acid, luteolin, and kaempferol. These compounds are known to exhibit various biological activities, including antioxidant, anticancer, and antimicrobial properties [28].
3.7. In vitro anti-bacterial assay
The findings of the assessment of the antibacterial activity revealed that the Calotropis procera plant's leaf extract efficiently suppressed the development of gram-negative bacteria and only slightly inhibited the growth of gram-positive bacteria. According to Table 4, gram-positive and gram-negative bacteria had inhibitory zone diameters of 6.33 and 11.33 mm, respectively. According to this study, the crude extract derived from the Calotropis procera plant's leaves has significant promise as an antimicrobial agent for the treatment of infectious diseases. The inhibitory concentration of a plant extract against test microorganisms indicates its substantial antibacterial properties. This validates previous studies in the literature that demonstrated the potent antibacterial activity of crude extracts. Additionally, it has been noted that phenolic compounds, among other diverse chemical components, are present in crude extracts of spices.
Table 4.
Inhibition zone measurement for Calotrpis procera extract.
| Name of plant extract | (50 μL) crude extracts concentration | Name of bacteria | Inhibition zone diameter (mm) |
|---|---|---|---|
| Mean ± standard deviation | |||
| Calotropis procera extracts | 1 mg/ml | Staphylococcus aureus | 6.33 ± 0.57 |
| Escherichia coli | 11.33 ± 1.15 | ||
| +ve Control | (5 μg Ciprofloxacin) | Staphylococcus aureus | 28.67 ± 0.57 |
| Escherichia coli | 38 ± 1.00 | ||
| -ve Control | (50 μL Dimethyl sulphoxide) | 0 | 0 |
The crude extract from Calotropis procera leaves exhibits antibacterial activity against both gram-negative and gram-positive bacteria through multiple mechanisms. These mechanisms likely involve disruption of the bacterial cell membrane by phytochemicals like saponins and alkaloids, leading to leakage of cellular contents and cell death. The extract may also inhibit protein synthesis, essential for bacterial growth and replication, by targeting bacterial ribosomes. Furthermore, the extract can interfere with cell wall synthesis, a mechanism particularly effective against gram-positive bacteria due to their thicker cell walls. Additionally, the extract might inhibit bacterial nucleic acid synthesis, hindering replication, and induce oxidative stress through its antioxidant properties, leading to the production of damaging reactive oxygen species within the bacteria. These combined effects contribute to the observed antibacterial activity of the Calotropis procera leaf extract. The potent antibacterial effect of Calotropis procera leaf extract is orchestrated by a synergistic interplay of diverse natural chemicals within its composition. Some, like alkaloids, saponins, and triterpenoids, puncture bacterial cell walls, causing leakage and cell death. Others, like flavonoids and phenolics, throw a wrench in the works of essential bacterial enzymes and disrupt their membranes. Interestingly, even cardiac glycosides, at lower doses, can join the fight by interfering with bacterial growth through ion transport. This impressive teamwork by these diverse chemicals makes the extract's antimicrobial activity even stronger than any single one could achieve alone.
4. Conclusion
In this present study, it can be concluded that the Calotropis procera plant's leave has a medicinal property; scientists can utilize Calotropis procera plant part for ant-microbial (anti-viral, anti-fungal and anti-bacterial) and ant-inflammatory treatment in a scientific way rather than a traditional practitioner. The Calotropis procera leaves had saponin, flavonoids, alkaloids, tan,nins, and phenols which have medicinal properties. Calotropis procera exthas ha ave Hydroxyl group, alkane, Carbonyl, aldehyde, ketone, phenols, ester, alcohol, and Methylene. GC-MS identifies the active compounds of Calotropis procera have Neophytadiene, Hexahydrofarnesyl, lanosterol, 2, 4-dimethylbenzo [H]quinoand lone, Squalene. Those active ingredients have anti-bacterial, anti-inflammation, ant-oxidant, ant-microbial, and anti-cancer activities. The collective reason is that Calotropis procerleafnt's leaf extracts can have substantial uses for wart medicine. Specifically, for the substantial uses for the treatment of warts, that can cause HPV and inflammation of the human body.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. Material/referenced in the article.
CRediT authorship contribution statement
Yassin Adem Endris: writing –original research, conceptualization, writing –review & editing, Methodology, investigation, conceptualization.Kedir Yesuf Abdu: Writing – original draft, Resources, Conceptualization, Writing – review & editing, Methodology, Investigation, Formal analysis. Solomon Getachew Abate: Methodology, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e34687.
Contributor Information
Yassin Adem Endris, Email: yassinadem67@gmail.com, yassinadem67@kiot.edu.et.
Kedir Yesuf Abdu, Email: kedir34@gmail.com.
Solomon Getachew Abate, Email: solgech62@gmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Melese A.T., Ayele D.T., Aljerf L., Al-Fekaiki D.F., Akele M.L. Investigating the phytoavailability of metals in roots of Croton macrostachyus and Phytolacca dodecandra: induced rhizosphere processes. Biometals. 2023;36:1347–1359. doi: 10.1007/s10534-023-00522-9. [DOI] [PubMed] [Google Scholar]
- 2.Awulachew M.T. Hand book of common Ethiopian traditional medicinal plants : their parts and uses for human and animal treatments hand book of common Ethiopian traditional medicinal plants : their parts and uses for human and animal treatments. 2021. [DOI]
- 3.Amsalu N., Bezie Y., Fentahun M., Alemayehu A., Amsalu G. Use and conservation of medicinal plants by indigenous people of gozamin wereda , east gojjam zone of Amhara region , Ethiopia : an ethnobotanical approach, 2018. 2018. [DOI] [PMC free article] [PubMed]
- 4.Kundu S. A mini review on Calotropis procera and tapping its phytochemical and pharmacological potential. J. Phytopharm. 2021;10:277–280. doi: 10.31254/phyto.2021.10411. [DOI] [Google Scholar]
- 5.Shahid S.K. Recent patents in anti-wart treatment. Pharm. Pat. Anal. 2020;9:53–62. doi: 10.4155/ppa-2019-0028. [DOI] [PubMed] [Google Scholar]
- 6.Mousavi Z.B., Mehrabian A., Golfakhrabadi F., Namjoyan F. A clinical study of efficacy of garlic extract versus cryotherapy in the treatment of male genital wart. Dermatol. Sin. 2018;36:196–199. doi: 10.1016/j.dsi.2018.06.007. [DOI] [Google Scholar]
- 7.Kaur A., Batish D.R., Kaur S., Chauhan B.S. An overview of the characteristics and potential of calotropis procera from botanical, ecological, and economic perspectives. Front. Plant Sci. 2021;12 doi: 10.3389/fpls.2021.690806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Endris Y.A., Mekonnen K.D. Formulation of neem leaf and Croton seed essential oils as a natural insecticide tested on mosquitoes and cockroaches. ACS Omega. 2023;8:15052–15061. doi: 10.1021/acsomega.2c08026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alqethami A., Aldhebiani A.Y. Medicinal plants used in jeddah, Saudi arabia: phytochemical screening. Saudi J. Biol. Sci. 2021;28:805–812. doi: 10.1016/j.sjbs.2020.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agidew M.G. Phytochemical analysis of some selected traditional medicinal plants in Ethiopia. Bull. Natl. Res. Cent. 2022;46:87. doi: 10.1186/s42269-022-00770-8. [DOI] [Google Scholar]
- 11.Weil A. Nutrition and health. Explore. 2005;1:65–66. doi: 10.1016/j.explore.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Adem Y., Yesuf kedir, Getachew S., Derbie kedir. Phytochemical property and antimicrobial activity of Ficifolius A. Rich root extract: advancing Ethiopian indigenous wart curing medicinal plant. Heliyon. 2024;10 doi: 10.1016/j.heliyon.2024.e31921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sim S.F., Lee T.Z.E., Mohd Irwan Lu N.A.L., Samling B. Synchronized analysis of FTIR spectra and GCMS chromatograms for evaluation of the thermally degraded vegetable oils. J. Anal. Methods Chem. 2014;2014:1–9. doi: 10.1155/2014/271970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adem Y., Yesuf kedir, Getachew S., Derbie kedir. Phytochemical property and antimicrobial activity of Ficifolius A. Rich root extract: advancing Ethiopian indigenous wart curing medicinal plant. Heliyon. 2024;10 doi: 10.1016/j.heliyon.2024.e31921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abew B., Sahile S., Moges F. In vitro antibacterial activity of leaf extracts of Zehneria scabra and Ricinus communis against Escherichia coli and methicillin resistance Staphylococcus aureus. Asian Pac. J. Trop. Biomed. 2014;4:816–820. doi: 10.12980/APJTB.4.201414B16. [DOI] [Google Scholar]
- 16.Aljerf L., Aljerf N. Food products quality and nutrition in relation to public. Balancing health and disease. Prog. Nutr. 2023;25 doi: 10.23751/pn.v25i1.13928. [DOI] [Google Scholar]
- 17.Swamy M.K., Arumugam G., Kaur R., Ghasemzadeh A., Yusoff M.M., Sinniah U.R. GC-MS based metabolite profiling, antioxidant and antimicrobial properties of different solvent extracts of Malaysian plectranthus amboinicus leaves, evidence-based complement. Altern. Med. 2017;2017 doi: 10.1155/2017/1517683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avoseh O.N., Mtunzi F.M., Ogunwande I.A., Ascrizzi R., Guido F. Albizia lebbeck and Albizia zygia volatile oils exhibit anti-nociceptive and anti-inflammatory properties in pain models. J. Ethnopharmacol. 2021;268 doi: 10.1016/j.jep.2020.113676. [DOI] [PubMed] [Google Scholar]
- 19.Kim S., Chen J., Cheng T., Gindulyte A., He J., He S., Li Q., Shoemaker B.A., Thiessen P.A., Yu B., Zaslavsky L., Zhang J., Bolton E.E. PubChem 2023 update. Nucleic Acids Res. 2023;51:D1373–D1380. doi: 10.1093/nar/gkac956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makhwitine J.P., Kumalo H.M., Ndlovu S.I., Mkhwanazi N.P. Epigenetic induction of secondary metabolites production in endophytic fungi Penicillium chrysogenum and GC-MS analysis of crude metabolites with anti-HIV-1 activity. Microorganisms. 2023;11:1–18. doi: 10.3390/microorganisms11061404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gnes F. Medical use of squalene as a natural antioxidant. J. Marmara Univ. Inst. Heal. Sci. 2013;3:220–228. doi: 10.5455/musbed.20131213100404. [DOI] [Google Scholar]
- 22.Gonzalez-Rivera M.L., Barragan-Galvez J.C., Gasca-Martínez D., Hidalgo-Figueroa S., Isiordia-Espinoza M., Alonso-Castro A.J. In vivo neuropharmacological effects of neophytadiene. Molecules. 2023;28:1–12. doi: 10.3390/molecules28083457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bondzie-Quaye P., Swallah M.S., Acheampong A., Elsherbiny S.M., Acheampong E.O., Huang Q. Advances in the biosynthesis, diversification, and hyperproduction of ganoderic acids in Ganoderma lucidum, Mycol. Prog. 2023;22:31. doi: 10.1007/s11557-023-01881-w. [DOI] [Google Scholar]
- 24.Huang Z.-R., Lin Y.-K., Fang J.-Y. Biological and pharmacological activities of squalene and related compounds: potential uses in cosmetic dermatology. Molecules. 2009;14:540–554. doi: 10.3390/molecules14010540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar L.R.G., Tejpal C.S., Anas K.K., Chatterjee N.S., Anandan R., Mathew S., Ravishankar C.N. Mar. Antioxidants Prep. Synth. Appl. Elsevier; 2022. Squalene: bioactivity, extraction, encapsulation, and future perspectives; pp. 409–419. [DOI] [Google Scholar]
- 26.Mekonnen K.D. Fourier transform infrared spectroscopy as a tool for identifying the unique characteristic bands of lipid in oilseed components: confirmed via Ethiopian indigenous desert date fruit. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e14699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nandiyanto A.B.D., Oktiani R., Ragadhita R. How to read and interpret ftir spectroscope of organic material. Indones. J. Sci. Technol. 2019;4:97–118. doi: 10.17509/ijost.v4i1.15806. [DOI] [Google Scholar]
- 28.Ahmad Nejhad A., Alizadeh Behbahani B., Hojjati M., Vasiee A., Mehrnia M.A. Identification of phytochemical, antioxidant, anticancer and antimicrobial potential of Calotropis procera leaf aqueous extract. Sci. Rep. 2023;13 doi: 10.1038/s41598-023-42086-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. Material/referenced in the article.