Abstract
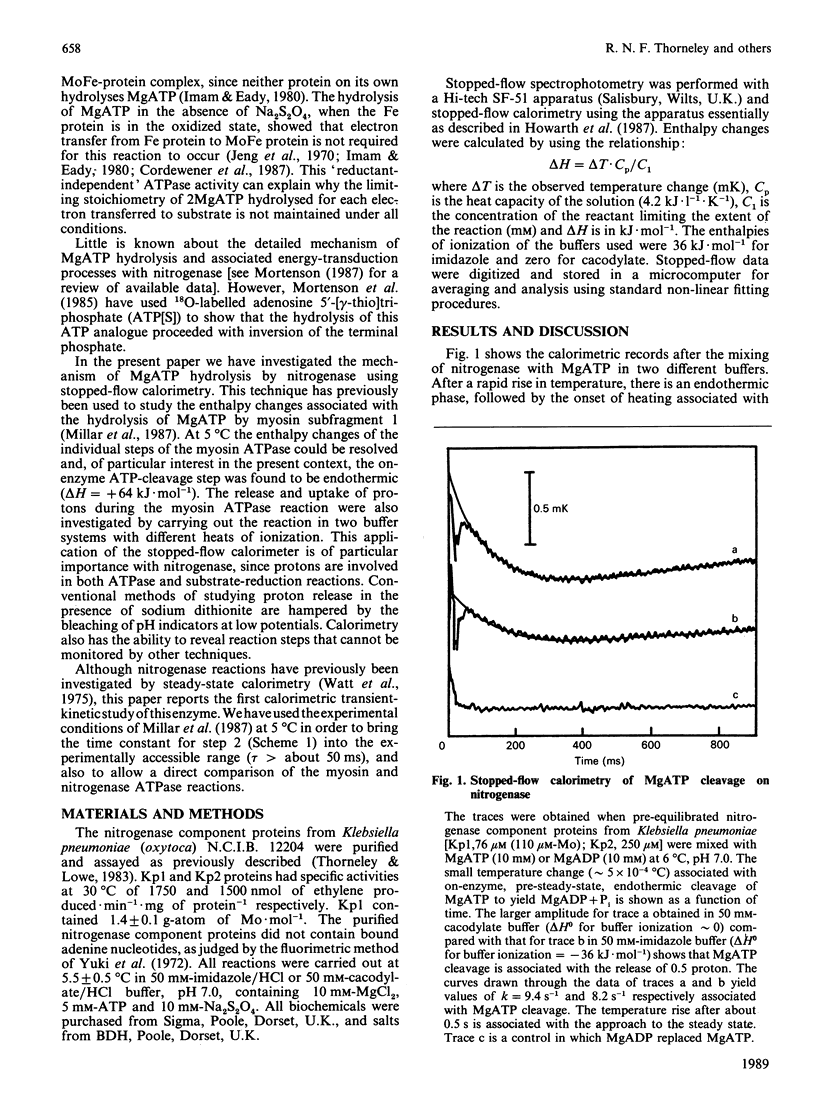
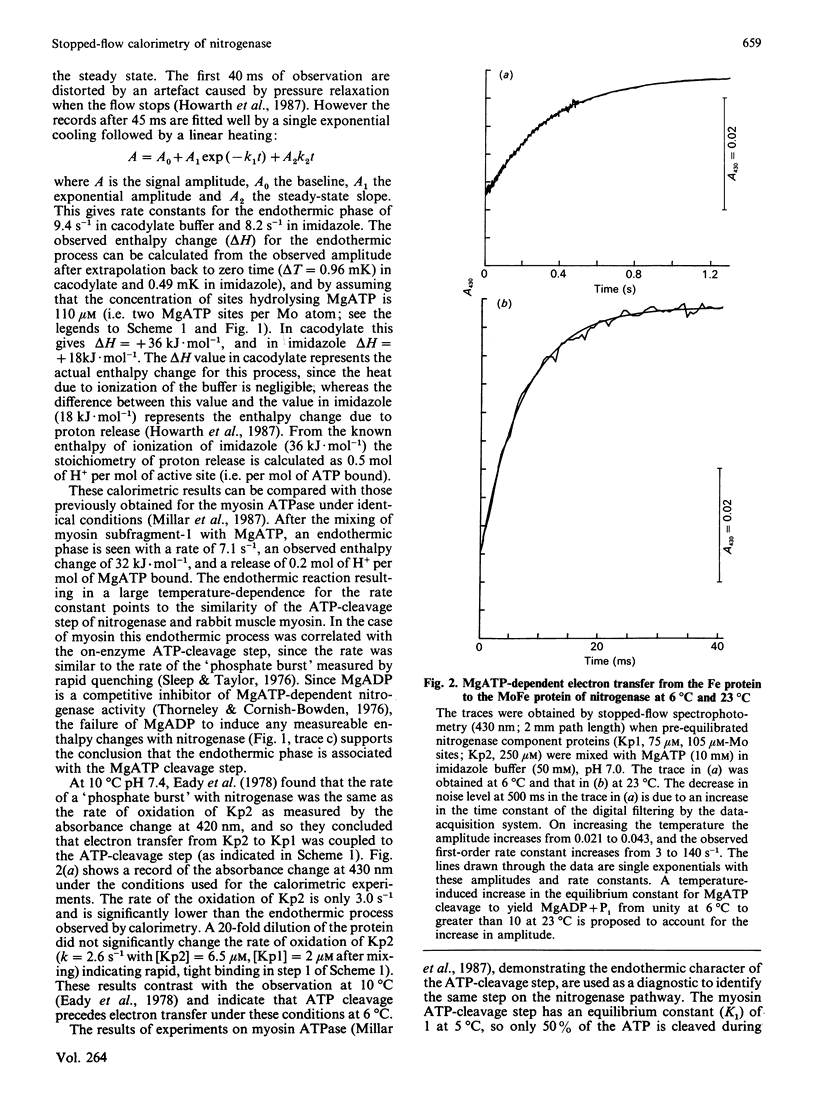
The pre-steady-state kinetics of MgATP hydrolysis by nitrogenase from Klebsiella pneumoniae were studied by stopped-flow calorimetry at 6 degrees C and at pH 7.0. An endothermic reaction (delta Hobs. = +36 kJ.mol of ATP-1; kobs. = 9.4 s-1) in which 0.5 proton.mol of ATP-1 was released, has been assigned to the on-enzyme cleavage of MgATP to yield bound MgADP + Pi. The assignment is based on the similarity of these parameters to those of the corresponding reaction that occurs with rabbit muscle myosin subfragment-1 (delta Hobs. = +32 kJ.mol of ATP-1; kobs. = 7.1 s-1; 0.2 proton released.mol of ATP-1) [Millar, Howarth & Gutfreund (1987) Biochem. J. 248, 683-690]. MgATP-dependent electron transfer from the nitrogenase Fe-protein to the MoFe-protein was monitored by stopped-flow spectrophotometry at 430 nm and occurred with kobs. value of 3.0 s-1 at 6 degrees C. Thus, under these conditions, hydrolysis of MgATP precedes electron transfer within the protein complex. Evidence is presented that suggests that MgATP cleavage and subsequent electron transfer are reversible at 6 degrees C with an overall equilibrium constant close to unity, but that, at 23 degrees C, the reactions are essentially irreversible, with an overall equilibrium constant greater than or equal to 10.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashby G. A., Thorneley R. N. Nitrogenase of Klebsiella pneumoniae. Kinetic studies on the Fe protein involving reduction by sodium dithionite, the binding of MgADP and a conformation change that alters the reactivity of the 4Fe-4S centre. Biochem J. 1987 Sep 1;246(2):455–465. doi: 10.1042/bj2460455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagshaw C. R., Eccleston J. F., Eckstein F., Goody R. S., Gutfreund H., Trentham D. R. The magnesium ion-dependent adenosine triphosphatase of myosin. Two-step processes of adenosine triphosphate association and adenosine diphosphate dissociation. Biochem J. 1974 Aug;141(2):351–364. doi: 10.1042/bj1410351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagshaw C. R., Trentham D. R. The reversibility of adenosine triphosphate cleavage by myosin. Biochem J. 1973 Jun;133(2):323–328. doi: 10.1042/bj1330323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordewener J., ten Asbroek A., Wassink H., Eady R., Haaker H., Veeger C. Binding of ADP and orthophosphate during the ATPase reaction of nitrogenase. Eur J Biochem. 1987 Jan 15;162(2):265–270. doi: 10.1111/j.1432-1033.1987.tb10594.x. [DOI] [PubMed] [Google Scholar]
- Eady R. R., Lowe D. J., Thorneley R. N. Nitrogenase of Klebsiella pneumoniae: a pre-steady state burst of ATP hydrolysis is coupled to electron transfer between the component proteins. FEBS Lett. 1978 Nov 15;95(2):211–213. doi: 10.1016/0014-5793(78)80995-8. [DOI] [PubMed] [Google Scholar]
- Hageman R. V., Burris R. H. Kinetic studies on electron transfer and interaction between nitrogenase components from Azotobacter vinelandii. Biochemistry. 1978 Oct 3;17(20):4117–4124. doi: 10.1021/bi00613a002. [DOI] [PubMed] [Google Scholar]
- Hageman R. V., Orme-Johnson W. H., Burris R. H. Role of magnesium adenosine 5'-triphosphate in the hydrogen evolution reaction catalyzed by nitrogenase from Azotobacter vinelandii. Biochemistry. 1980 May 27;19(11):2333–2342. doi: 10.1021/bi00552a009. [DOI] [PubMed] [Google Scholar]
- Hibberd M. G., Trentham D. R. Relationships between chemical and mechanical events during muscular contraction. Annu Rev Biophys Biophys Chem. 1986;15:119–161. doi: 10.1146/annurev.bb.15.060186.001003. [DOI] [PubMed] [Google Scholar]
- Howarth J. V., Millar N. C., Gutfreund H. A stopped-flow calorimeter for biochemical applications. Biochem J. 1987 Dec 15;248(3):677–682. doi: 10.1042/bj2480677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imam S., Eady R. R. Nitrogenase of Klebsiella pneumoniae: reductant-independent ATP hydrolysis and the effect of pH on the efficiency of coupling of ATP hydrolysis to substrate reduction. FEBS Lett. 1980 Jan 28;110(1):35–38. doi: 10.1016/0014-5793(80)80016-0. [DOI] [PubMed] [Google Scholar]
- Jeng D. Y., Morris J. A., Mortenson L. E. The effect of reductant in inorganic phosphate release from adenosine 5'-triphosphate by purified nitrogenase of Clostridium pasteurianum. J Biol Chem. 1970 Jun 10;245(11):2809–2813. [PubMed] [Google Scholar]
- Lowe D. J., Thorneley R. N. The mechanism of Klebsiella pneumoniae nitrogenase action. Pre-steady-state kinetics of H2 formation. Biochem J. 1984 Dec 15;224(3):877–886. doi: 10.1042/bj2240877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D. J., Thorneley R. N. The mechanism of Klebsiella pneumoniae nitrogenase action. The determination of rate constants required for the simulation of the kinetics of N2 reduction and H2 evolution. Biochem J. 1984 Dec 15;224(3):895–901. doi: 10.1042/bj2240895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymn R. W., Taylor E. W. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971 Dec 7;10(25):4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- Millar N. C., Howarth J. V., Gutfreund H. A transient kinetic study of enthalpy changes during the reaction of myosin subfragment 1 with ATP. Biochem J. 1987 Dec 15;248(3):683–690. doi: 10.1042/bj2480683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme-Johnson W. H. Molecular basis of biological nitrogen fixation. Annu Rev Biophys Biophys Chem. 1985;14:419–459. doi: 10.1146/annurev.bb.14.060185.002223. [DOI] [PubMed] [Google Scholar]
- Sleep J. A., Taylor E. W. Intermediate states of actomyosin adenosine triphosphatase. Biochemistry. 1976 Dec 28;15(26):5813–5817. doi: 10.1021/bi00671a019. [DOI] [PubMed] [Google Scholar]
- Thorneley R. N., Ashby G. A. Oxidation of nitrogenase iron protein by dioxygen without inactivation could contribute to high respiration rates of Azotobacter species and facilitate nitrogen fixation in other aerobic environments. Biochem J. 1989 Jul 1;261(1):181–187. doi: 10.1042/bj2610181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneley R. N., Cornish-Bowden A. Kinetics of nitrogenase of Klebsiella pneumoniae. Heterotropic interactions between magnesium-adenosine 5'-diphosphate and magnesium-adenosine 5'-triphosphate. Biochem J. 1977 Aug 1;165(2):255–262. doi: 10.1042/bj1650255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneley R. N., Lowe D. J. Nitrogenase of Klebsiella pneumoniae. Kinetics of the dissociation of oxidized iron protein from molybdenum-iron protein: identification of the rate-limiting step for substrate reduction. Biochem J. 1983 Nov 1;215(2):393–403. doi: 10.1042/bj2150393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneley R. N., Lowe D. J. The mechanism of Klebsiella pneumoniae nitrogenase action. Pre-steady-state kinetics of an enzyme-bound intermediate in N2 reduction and of NH3 formation. Biochem J. 1984 Dec 15;224(3):887–894. doi: 10.1042/bj2240887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneley R. N., Lowe D. J. The mechanism of Klebsiella pneumoniae nitrogenase action. Simulation of the dependences of H2-evolution rate on component-protein concentration and ratio and sodium dithionite concentration. Biochem J. 1984 Dec 15;224(3):903–909. doi: 10.1042/bj2240903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneley R. N. Nitrogenase of Klebsiella pneumoniae. A stopped-flow study of magnesium-adenosine triphosphate-induce electron transfer between the compeonent proteins. Biochem J. 1975 Feb;145(2):391–396. doi: 10.1042/bj1450391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt G. D., Bulen W. A., Burns A., Hadfield K. L. Stoichiometry, ATP/2e values, and energy requirements for reactions catalyzed by nitrogenase from Azotobacter vinelandii. Biochemistry. 1975 Sep 23;14(19):4266–4272. doi: 10.1021/bi00690a019. [DOI] [PubMed] [Google Scholar]
- Yuki H., Sempuku C., Park M., Takiura K. Fluorometric determination of adenine and its derivatives by reaction with glyoxal hydrate trimer. Anal Biochem. 1972 Mar;46(1):123–128. doi: 10.1016/0003-2697(72)90403-4. [DOI] [PubMed] [Google Scholar]


