Abstract
In a survey of the arthropod fauna of 33 Urban Green Spaces (UGS) in Bogotá, Colombia, between 2017 and 2019, 21 species (3,825 specimens) of Psylloidea were collected. These represent all seven recognised families of jumping plant-lice and include seven species identified only to genus. The specimens, all adults, were collected on 30 plant species used for arborization in the UGS. Two species are described as new (Mastigimaslongicaudatus Rendón-Mera, Burckhardt & Vargas-Fonseca, sp. nov. and Leuronotaalbilinea Rendón-Mera, Burckhardt & Vargas-Fonseca, sp. nov.), one species is redescribed (Mastigimascolombianus Burckhardt, Queiroz & Drohojowska) and one species is recorded for the first time from Colombia (Calindatrinervis Olivares & Burckhardt). Among the seven species identified only to genus is an undescribed species of Melanastera, representing a genus not previously known from Colombia. Fourteen species found during the survey are probably native (66%) and seven (33%) adventive. Our findings highlight the significance of UGS for preservation of biological diversity and stress the importance of using native plants in urban landscape planning for the conservation of the native entomofauna.
Key words: Biodiversity, city parks, insect–plant interactions, Neotropical region, psyllids, Sternorrhyncha, taxonomy, urbanisation
Introduction
Urbanisation, the most irreversible form of land-use by the ever increasing human population, is one of the main drivers of the current extinction crisis (McKinney 2002; Seto et al. 2012; Díaz et al. 2019; Kong et al. 2021; Jaureguiberry et al. 2022). Accompanied by the degradation, fragmentation and loss of natural habitats (Foley et al. 2005; Elmqvist et al. 2013; Kong et al. 2021), urbanisation usually favours the presence of exotic species, leads to biotic homogeneity, and ultimately results in the loss of native species (McKinney 2002, 2008; Elmqvist et al. 2013; McDonald et al. 2018). Cities have dramatically expanded during the last decades and, as of today, more than half of the world’s population resides in urban areas with an expected increase to 70% by 2050 (Elmqvist et al. 2013; United Nations 2019).
As cities grow, Urban Green Spaces (UGS) become increasingly critical for supporting native organisms (Goddard et al. 2010; Aronson et al. 2014; Ives et al. 2016). These spaces comprise natural, semi-natural and artificial habitats, including remnants of native vegetation, parks, gardens, urban wastelands and green infrastructure (Tzoulas et al. 2007; Aronson et al. 2017; Lepczyk et al. 2017). However, not all UGS have equal conservation value, as the degree to which they can support biodiversity depends on several factors such as quality, size, connectivity, biotic interactions, land-use history and human population density (Aronson et al. 2017). Consequently, it is necessary to integrate ecological and biodiversity aspects into urban planning, to develop strategies for the design and management of these spaces to serve biodiversity conservation (McKinney 2002; Elmqvist et al. 2013; Aronson et al. 2017; McDonald et al. 2018).
Colombia is located in the north-west of South America and is one of the world’s megadiverse countries, home to approximately 10% of the world’s species and two of the world’s biodiversity hotspots: Tropical Andes and Tumbes–Chocó–Magdalena (Myers et al. 2000; Baptiste et al. 2017). At the same time, it is a highly urbanised country, with ~ 80% of its 50 million human inhabitants residing in urban areas (OECD 2022). This contrast is particularly evident in the Andean region, which exhibits both the highest levels of biological diversity and endemism, and of urbanisation and population density (Anselm et al. 2018; Carvajal-Castro et al. 2019). The Colombian capital Bogotá, the largest city in the country, is located in the middle of the Andes mountains, in the Eastern Ranges. Like other Latin American cities, much of Bogotá’s urban growth during the last two centuries has been unplanned and informal (Andrade et al. 2013), driven by an accelerated increase of rural-to-urban migration (Dufour and Piperata 2004). As a result, UGS only began to appear by the end of the 19th century and, as late as the end of the 20th century, became relevant under the concept of “Ecological Main Structure” (Andrade et al. 2013, 2014). Today, the concept has been decreed as one of the environmental determinants of land use-planning (Andrade et al. 2013, 2014). Bogotá has around 7,000 UGS of different scale and function, and ~ 1.4 million urban trees (Alcaldía Mayor de Bogotá 2009; Jardín Botánico de Bogotá 2023). However, despite the need for information on ecology and biodiversity to develop these strategies (McKinney 2002; Elmqvist et al. 2013; Aronson et al. 2017; McDonald et al. 2018), there are only a few studies that explore urban biodiversity in Colombia (e.g. Marín-Gómez et al. 2016; Ocampo Flórez et al. 2018; Durán-Prieto and Ocampo 2019; Durán-Prieto et al. 2020, 2023; Martínez and Morales 2020; Garizábal-Carmona and Mancera-Rodríguez 2021; Olaya-Arenas et al. 2022; Roncallo et al. 2022).
Psylloidea (jumping plant-lice or psyllids) constitute one of the superfamilies of Sternorrhyncha with more than 4,000 described and probably just as many undescribed species (Burckhardt et al. 2021; Ouvrard 2023). Psyllids are generally monophagous or narrowly oligophagous on one or a few closely related host plant species (Hodkinson 1974; Burckhardt et al. 2014; Ouvrard et al. 2015). A host plant is defined as that plant “on which a psyllid species completes its immature-to-adult life cycle” (Burckhardt et al. 2014). In practice, a host plant can be recognised by the presence of fifth instar immatures. Unlike the relatively immobile immatures, the winged adults disperse through flight or by air currents and are often found also on non-host plants (Burckhardt et al. 2014).
Psyllids are found in all biogeographic realms but are probably most species-rich in the tropics and the south temperate regions though these faunas are only poorly known, particularly those of the Afrotropical and Neotropical realms (Hollis 2004; Hodkinson 2009; Burckhardt and Queiroz 2020; Mauck et al. 2024). Little is known about the psyllid fauna of Colombia. Rendón-Mera et al. (2017) published a generic overview on the Colombian psyllids with a list of species known at the time. Additional information on psyllids from Bogotá is provided by Pinzón et al. (2002).
Here, the psyllids collected during a survey of the arthropod fauna of 33 UGS in Bogotá by the Botanical Garden “José Celestino Mutis” of Bogotá are discussed. The survey was conducted between 2017 and 2019, focussing on 30 species of native and exotic plants.
Material and methods
Material
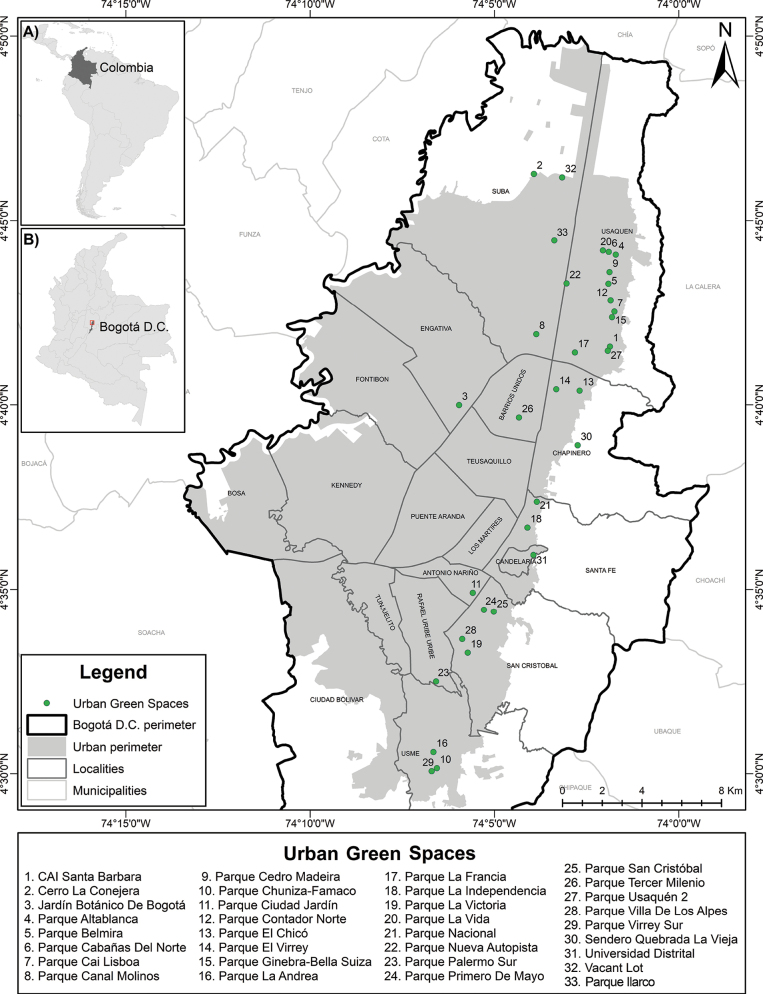
Collections were conducted between 2017 and 2019 in 33 Urban Green Spaces (UGS) of nine of the 19 urban districts (“localidades”) of Bogotá (Figs 1, 2, Table 1, Appendix 1). Specimens were collected using sweep nets and entomological aspirators on the tree/shrub canopy cover of 30 plant species used for arborization in the city (Table 2). Unless stated otherwise, material is preserved pinned.
Figure 1.
Some urban green spaces of Bogotá A Parque Altablanca B Parque Ginebra-Bella Suiza C Sendero Quebrada la Vieja D Parque La Francia E Parque San Cristóbal F Parque La Independencia.
Figure 2.
Map of Bogotá indicating localities and sampled urban green spaces.
Table 1.
Urban Green Spaces (UGS) with examined plants and psyllid species with number of collected adults. Plants confirmed in the literature as hosts or likely hosts are marked with § (see also text).
| UGS | Plant species | Psyllid species | Number of adults |
|---|---|---|---|
| CAI Santa Barbara | Quercushumboldtii (Fagaceae) | Calophyaschini | 1 |
| Cerro La Conejera | § Acaciadealbata (Fabaceae) | Acizziaacaciaebaileyanae | 4 |
| Cerro La Conejera | § Acaciamelanoxylon (Fabaceae) | Acizziauncatoides | 2 |
| Cerro La Conejera | § Baccharis sp. (Asteraceae) | Calindagibbosa | 1 |
| Cerro La Conejera | Myrcianthesleucoxyla (Myrtaceae) | Tuthillialatipennis | 1 |
| Jardín Botánico de Bogotá | Myrcianthes sp. (Myrtaceae) | Trioza sp. 1 | 1 |
| Jardín Botánico de Bogotá | Myrcianthes sp. (Myrtaceae) | Tuthillialatipennis | 1 |
| Parque Altablanca | Lafoensiaacuminata (Lythraceae) | Synozacornutiventris | 2 |
| Parque Belmira | Schinusareira (Anacardiaceae) | Acizziaacaciaebaileyanae | 1 |
| Parque Belmira | § Schinusareira (Anacardiaceae) | Calophyaschini | 8 |
| Parque Cabañas del Norte | Lafoensiaacuminata (Lythraceae) | Acizziaacaciaebaileyanae | 5 |
| Parque Cabañas del Norte | Lafoensiaacuminata (Lythraceae) | Calophyaschini | 1 |
| Parque Cabañas del Norte | Lafoensiaacuminata (Lythraceae) | Ctenarytainaspatulata | 1 |
| Parque Cabañas del Norte | Lafoensiaacuminata (Lythraceae) | Mastigimascolombianus | 1 |
| Parque Cabañas del Norte | Pittosporumundulatum (Pittosporaceae) | Mastigimaslongicaudatus Rendón-Mera, Burckhardt & Vargas-Fonseca, sp. nov. | 1 |
| Parque Cabañas del Norte | § Schinusareira (Anacardiaceae) | Calophyaschini | 419 |
| Parque CAI Lisboa | Pittosporumundulatum (Pittosporaceae) | Acizziauncatoides | 2 |
| Parque CAI Lisboa | Pittosporumundulatum (Pittosporaceae) | Synozacornutiventris | 6 |
| Parque Canal Molinos | Bocconiafrutescens (Papaveraceae) | Acizziaacaciaebaileyanae | 2 |
| Parque Canal Molinos | § Cedrelamontana (Meliaceae) | Mastigimascolombianus | 17 |
| Parque Canal Molinos | § Cedrelamontana (Meliaceae) | Mastigimaslongicaudatus Rendón-Mera, Burckhardt & Vargas-Fonseca, sp. nov. | 38 |
| Parque Canal Molinos | § Ficusamericanasubsp.andicola (Moraceae) | Synozacornutiventris | 9 |
| Parque Cedro Madeira | § Ficus sp. (Moraceae) | Synozacornutiventris | 17 |
| Parque Chuniza-Famaco | Quercushumboldtii (Fagaceae) | Calophyaschini | 2 |
| Parque Chuniza-Famaco | § Schinusareira (Anacardiaceae) | Calophyaschini | 558 |
| Parque Ciudad Jardín | § Ficus sp. (Moraceae) | Synozacornutiventris | 9 |
| Parque Ciudad Jardín | Lafoensiaacuminata (Lythraceae) | Calophyaschini | 2 |
| Parque Ciudad Jardín | Lafoensiaacuminata (Lythraceae) | Syncoptozusmexicanus | 1 |
| Parque Ciudad Jardín | § Schinusareira (Anacardiaceae) | Calophyaschini | 726 |
| Parque Contador Norte | § Ficus sp. (Moraceae) | Synozacornutiventris | 117 |
| Parque Contador Norte | Lafoensiaacuminata (Lythraceae) | Acizziaacaciaebaileyanae | 2 |
| Parque Contador Norte | Liquidambarstyraciflua (Altingiaceae) | Acizziaacaciaebaileyanae | 2 |
| Parque Contador Norte | Liquidambarstyraciflua (Altingiaceae) | Calophyaschini | 1 |
| Parque Contador Norte | Liquidambarstyraciflua (Altingiaceae) | Ctenarytainaspatulata | 1 |
| Parque Contador Norte | Liquidambarstyraciflua (Altingiaceae) | Synozacornutiventris | 2 |
| Parque Contador Norte | Pittosporumundulatum (Pittosporaceae) | Synozacornutiventris | 1 |
| Parque El Chicó | § Ficus sp. (Moraceae) | Synozacornutiventris | 10 |
| Parque El Chicó | § Schinusareira (Anacardiaceae) | Calophyaschini | 7 |
| Parque El Virrey | § Acaciamelanoxylon (Fabaceae) | Acizziauncatoides | 19 |
| Parque El Virrey | § Cedrelamontana (Meliaceae) | Mastigimascolombianus | 38 |
| Parque El Virrey | § Cedrelamontana (Meliaceae) | Mastigimaslongicaudatus Rendón-Mera, Burckhardt & Vargas-Fonseca, sp. nov. | 13 |
| Parque El Virrey | Crotoncoriaceus (Euphorbiaceae) | Calophyaschini | 14 |
| Parque El Virrey | Delostomaintegrifolium (Bignoniaceae) | Synozacornutiventris | 1 |
| Parque El Virrey | Feijoasellowiana (Myrtaceae) | Glycaspisbrimblecombei | 1 |
| Parque El Virrey | Fraxinuschinensis (Oleaceae) | Syncoptozusmexicanus | 1 |
| Parque El Virrey | Ligustrum sp. (Oleaceae) | Syncoptozusmexicanus | 2 |
| Parque El Virrey | Magnoliagrandiflora (Magnoliaceae) | Acizziauncatoides | 28 |
| Parque El Virrey | § Magnoliagrandiflora (Magnoliaceae) | Syncoptozusmexicanus | 37 |
| Parque El Virrey | Pittosporumundulatum (Pittosporaceae) | Acizziauncatoides | 1 |
| Parque El Virrey | Salixhumboldtiana (Salicaceae) | Calophyaschini | 4 |
| Parque Ginebra-Bella Suiza | Ficus sp. (Moraceae) | Triozidae gen. sp. 3 | 1 |
| Parque Ginebra-Bella Suiza | Liquidambarstyraciflua (Altingiaceae) | Mastigimaslongicaudatus Rendón-Mera, Burckhardt & Vargas-Fonseca, sp. nov. | 1 |
| Parque Ginebra-Bella Suiza | § Schinusareira (Anacardiaceae) | Calophyaschini | 148 |
| Parque Ginebra-Bella Suiza | Schinusareira (Anacardiaceae) | Mastigimaslongicaudatus Rendón-Mera, Burckhardt & Vargas-Fonseca, sp. nov. | 2 |
| Parque Ginebra-Bella Suiza | Schinusareira (Anacardiaceae) | Synozacornutiventris | 1 |
| Parque Tercer Ilarco | Bocconiafrutescens (Papaveraceae) | Acizziaacaciaebaileyanae | 2 |
| Parque Tercer Ilarco | Clusia sp. (Clusiaceae) | Glycaspisbrimblecombei | 1 |
| Parque Tercer Ilarco | Crotoncoriaceus (Euphorbiaceae) | Calinda sp. | 1 |
| Parque Tercer Ilarco | Crotoncoriaceus (Euphorbiaceae) | Mastigimascolombianus | 1 |
| Parque Tercer Ilarco | Crotoncoriaceus (Euphorbiaceae) | Platycorypha sp. | 1 |
| Parque Tercer Ilarco | Crotoncoriaceus (Euphorbiaceae) | Synozacornutiventris | 1 |
| Parque Tercer Ilarco | Prunusserotina (Rosaceae) | Leuronotaalbilinea Rendón-Mera, Burckhardt & Vargas-Fonseca, sp. nov. | 1 |
| Parque Tercer Ilarco | § Schinusareira (Anacardiaceae) | Calophyaschini | 8 |
| Parque La Andrea | § Ficus sp. (Moraceae) | Synozacornutiventris | 99 |
| Parque La Francia | Lafoensiaacuminata (Lythraceae) | Synozacornutiventris | 2 |
| Parque La Francia | § Schinusareira (Anacardiaceae) | Calophyaschini | 7 |
| Parque La Independencia | § Ficus sp. (Moraceae) | Synozacornutiventris | 30 |
| Parque La Independencia | Liquidambarstyraciflua (Altingiaceae) | Synozacornutiventris | 1 |
| Parque La Independencia | Quercushumboldtii (Fagaceae) | Synozacornutiventris | 1 |
| Parque La Victoria | Pittosporumundulatum (Pittosporaceae) | Calophyaschini | 1 |
| Parque La Victoria | Quercushumboldtii (Fagaceae) | Calophyaschini | 5 |
| Parque La Victoria | § Schinusareira (Anacardiaceae) | Calophyaschini | 250 |
| Parque La Vida | § Ficus sp. (Moraceae) | Synozacornutiventris | 244 |
| Parque La Vida | Ficus sp. (Moraceae) | Triozidae gen. sp. 3 | 1 |
| Parque La Vida | Pittosporumundulatum (Pittosporaceae) | Synozacornutiventris | 6 |
| Parque Nacional | § Ficus sp. (Moraceae) | Synozacornutiventris | 4 |
| Parque Nueva Autopista | Ficus sp. (Moraceae) | Calophyaschini | 1 |
| Parque Nueva Autopista | § Ficus sp. (Moraceae) | Synozacornutiventris | 3 |
| Parque Palermo Sur | § Ficus sp. (Moraceae) | Synozacornutiventris | 2 |
| Parque Palermo Sur | Pittosporumundulatum (Pittosporaceae) | Acizziauncatoides | 1 |
| Parque Palermo Sur | Pittosporumundulatum (Pittosporaceae) | Calophyaschini | 1 |
| Parque Palermo Sur | Pittosporumundulatum (Pittosporaceae) | Triozidae gen. sp. 2 | 1 |
| Parque Palermo Sur | § Schinusareira (Anacardiaceae) | Calophyaschini | 26 |
| Parque Primero de Mayo | § Ficus sp. (Moraceae) | Synozacornutiventris | 106 |
| Parque Primero de Mayo | Quercushumboldtii (Fagaceae) | Synozacornutiventris | 1 |
| Parque San Cristóbal | Lafoensiaacuminata (Lythraceae) | Synozacornutiventris | 1 |
| Parque San Cristóbal | Pittosporumundulatum (Pittosporaceae) | Synozacornutiventris | 1 |
| Parque San Cristóbal | § Schinusareira (Anacardiaceae) | Calophyaschini | 89 |
| Parque Tercer Milenio | § Clusia sp. (Clusiaceae) | Leuronotaalbilinea Rendón-Mera, Burckhardt & Vargas-Fonseca, sp. nov. | 85 |
| Parque Tercer Milenio | Magnoliagrandiflora (Magnoliaceae) | Calophyaschini | 6 |
| Parque Tercer Milenio | § Magnoliagrandiflora (Magnoliaceae) | Syncoptozusmexicanus | 33 |
| Parque Tercer Milenio | Sambucusnigra (Viburnaceae) | Mastigimaslongicaudatus Rendón-Mera, Burckhardt & Vargas-Fonseca, sp. nov. | 2 |
| Parque Usaquén 2 | Ficus sp. (Moraceae) | Calophyaschini | 1 |
| Parque Usaquén 2 | § Ficus sp. (Moraceae) | Synozacornutiventris | 4 |
| Parque Usaquén 2 | Liquidambarstyraciflua (Altingiaceae) | Calophyaschini | 1 |
| Parque Usaquén 2 | Pittosporumundulatum (Pittosporaceae) | Calophyaschini | 1 |
| Parque Usaquén 2 | Pittosporumundulatum (Pittosporaceae) | Synozacornutiventris | 1 |
| Parque Villa de los Alpes | § Ficus sp. (Moraceae) | Synozacornutiventris | 120 |
| Parque Villa de los Alpes | § Schinusareira (Anacardiaceae) | Calophyaschini | 81 |
| Parque Virrey Sur | § Ficus sp. (Moraceae) | Synozacornutiventris | 29 |
| Parque Virrey Sur | § Schinusareira (Anacardiaceae) | Calophyaschini | 51 |
| Sendero Quebrada La Vieja | Miconiaelaeoides (Melastomataceae) | Ctenarytainaspatulata | 1 |
| Sendero Quebrada La Vieja | Miconiaelaeoides (Melastomataceae) | Melanastera sp. | 1 |
| Sendero Quebrada La Vieja | Piperbogotense (Piperaceae) | Acizziauncatoides | 1 |
| Sendero Quebrada La Vieja | Piperbogotense (Piperaceae) | Ctenarytainaeucalypti | 5 |
| Universidad Distrital | Acaciadecurrens (Fabaceae) | Acizziauncatoides | 15 |
| Universidad Distrital | § Acaciamelanoxylon (Fabaceae) | Acizziauncatoides | 125 |
| Universidad Distrital | § Baccharislatifolia (Asteraceae) | Calindagibbosa | 3 |
| Universidad Distrital | Baccharislatifolia (Asteraceae) | Calindatrinervis | 1 |
| Universidad Distrital | Crotoncoriaceus (Euphorbiaceae) | Acizziauncatoides | 2 |
| Universidad Distrital | Lyciantheslycioides (Solanaceae) | Acizziauncatoides | 24 |
| Universidad Distrital | Oreopanaxincisus (Araliaceae) | Acizziauncatoides | 1 |
| Universidad Distrital | Oreopanaxincisus (Araliaceae) | Ctenarytainaspatulata | 7 |
| Universidad Distrital | Quercushumboldtii (Fagaceae) | Acizziauncatoides | 1 |
| Universidad Distrital | Quercushumboldtii (Fagaceae) | Calindagibbosa | 1 |
| Universidad Distrital | Quercushumboldtii (Fagaceae) | Ctenarytainaspatulata | 1 |
| Universidad Distrital | Quercushumboldtii (Fagaceae) | Triozidae gen. sp. 3 | 1 |
| vacant lot | Quercushumboldtii (Fagaceae) | Acizziauncatoides | 1 |
| vacant lot | Quercushumboldtii (Fagaceae) | Triozidae gen. sp. 1 | 1 |
Table 2.
Psyllid species, hosts (cf. text) and numbers of adult psyllid specimens collected on hosts and non-hosts.
| Psyllid species | Host taxon | Adults on host | Adults on non-host |
|---|---|---|---|
| Calophyaschini | Schinusareira | 2378 | 42 (= 1.8%) |
| Synozacornutiventris | Ficus spp. | 803 | 28 (= 3.5%) |
| Acizziauncatoides | mimosoid Fabaceae | 161 | 62 (= 38.5%) |
| Leuronotaalbilinea Rendón-Mera, Burckhardt & Vargas-Fonseca, sp. nov. | Clusia sp. | 85 | 1 (= 1.2%) |
| Mastigimascolombianus | Cedrelamontana | 55 | 2 (= 3.6%) |
| Mastigimaslongicaudatus Rendón-Mera, Burckhardt & Vargas-Fonseca, sp. nov. | Cedrelamontana | 51 | 6 (= 11.8%) |
| Syncoptozusmexicanus | Magnoliagrandiflora | 30 | 4 (= 13.3%) |
| Acizziaacaciaebaileyanae | mimosoid Fabaceae | 4 | 12 |
| Calindagibbosa | Baccharis spp. | 4 | 1 |
| Ctenarytainaspatulata | Eucalyptus spp. | 11 | |
| Ctenarytainaeucalypti | Eucalyptus spp. | 5 | |
| Triozidae gen. sp. 3 | unknown | 3 | |
| Glycaspisbrimblecombei | Eucalyptus spp. | 2 | |
| Tuthillialatipennis | Myrcianthes spp. | 2 | |
| Calindatrinervis | unknown | 1 | |
| Calinda sp. | unknown | 1 | |
| Melanastera sp. | unknown | 1 | |
| Platycorypha sp. | unknown | 1 | |
| Trioza sp. 1 | unknown | 1 | |
| Triozidae gen. sp. 1 | unknown | 1 | |
| Triozidae gen. sp. 2 | unknown | 1 |
Holotypes are deposited in the entomological collection of the Museo Javeriano de Historia Natural of the Pontificia Universidad Javeriana, Bogotá, Colombia (MPUJ_ENT). Paratypes and non-type material are deposited in MPUJ_ENT and the Naturhistorisches Museum, Basel, Switzerland (NHMB).
Species description
Morphological terminology follows Bastin et al. (2023). Body length was taken from ethanol-preserved specimens in lateral view, measuring the distance from the tip of genal process to the tip of wings when folded over the body. All other measurements were taken from slide mounted specimens as indicated in Bastin et al. (2023). In Leuronota, vein length is measured as a linear distance. Measurements are given in mm and expressed as range (mean ± standard deviation). Slide preparation protocol follows Queiroz et al. (2017).
Conventions
Taxa are arranged alphabetically (families and genera) following the classification of Burckhardt et al. (2021). Plant names and information of their origin correspond to POWO (2023). The following markings are used: (*) for new species records for Colombia and (‡) for adventive species. Material examined is presented per urban district, written in bold and arranged alphabetically. Plants mentioned in this section are those from which specimens were collected and not necessarily host plants as defined by Burckhardt et al. (2014). Distribution in Colombia is presented by department.
Host plants
No immature psyllids were collected during the survey and none of the sampled plant species could, therefore, be confirmed as host in the sense of Burckhardt et al. (2014). Under “Host plant” we cite reliable literature records with the respective reference, or we discuss reasons for assuming that a particular plant constitutes a host. In Table 1 we use this information to classify plants into hosts (marked with §) and non-hosts.
Abbreviations
AL—Antenna length; AP—Apical portion of female proctiger length; BL—Body length; CRL—Circumanal ring length; DL—Distal segment of aedeagus length; FL—Forewing length; FP—Female proctiger length; FW—Forewing width; GL—Genal processes length; HW—Head width; MP—Male proctiger length; PL—Paramere length; SP—Female subgenital plate length; TL—Metatibia length; UGS—Urban Green Space; VL—Vertex length.
Taxonomy
Psylloidea Latreille, 1807
Aphalaridae Löw, 1879
‡. Ctenarytaina eucalypti
(Maskell, 1890)
D8EBD340-06E2-52B1-B3B4-A172D589C5CA
Material examined.
Chapinero: • 1 ♂, 4 ♀; Quebrada La Vieja; 4.6495, –74.0466; 2764 m; 06.iv.2017; J. Duran leg.; Piperbogotense (Piperaceae); MPUJ_ENT.
Distribution.
Colombia: Boyacá and Bogotá (Pinzón et al. 2002; Rendón-Mera et al. 2017).—Native to Australia, introduced into Africa, the Americas, Asia, Europe, and New Zealand (Makunde et al. 2020).
Host plant.
Eucalyptus L’Hér. spp. (Myrtaceae) (Makunde et al. 2020).
‡. Ctenarytaina spatulata
Taylor, 1997
3DDFA55D-86A8-5871-8ED0-BBF7F591D80E
Material examined.
Chapinero: • 1 ♀; Quebrada La Vieja; 4.6474, –74.0447; 2785 m; 20.vi.2017; J. Duran leg.; Miconiaelaeoides (Melastomataceae); MPUJ_ENT. Santa Fe: • 4 ♂, 3 ♀; Universidad Distrital; 4.5989, –74.0656; 2701 m; 05.v.2017; J. Duran leg.; Oreopanaxincisus (Araliaceae); MPUJ_ENT• 1 ♀; same but 4.5987, –74.0653; 2713 m; Quercushumboldtii (Fagaceae); MPUJ_ENT. Usaquén: • 1 ♀; Parque Cabañas del Norte; 4.7359, –74.0318; 2575 m; 16.iii.2018; V. Ocampo leg.; Lafoensiaacuminata (Lythraceae); MPUJ_ENT • 1 ♀; Parque Contador Norte; 4.715, –74.0302; 2595 m; 02.iv.2018; V. Ocampo leg.; Liquidambarstyraciflua (Altingiaceae); MPUJ_ENT.
Distribution.
Colombia: Bogotá (Rendón-Mera et al. 2017).—Native to Australia, introduced into the Americas, Europe, and New Zealand (Makunde et al. 2020).
Host plant.
Eucalyptus L’Hér. spp. (Myrtaceae) (Makunde et al. 2020).
‡. Glycaspis brimblecombei
Moore, 1964
89043217-29F8-557B-93CD-1FB5B4DFD00D
Material examined.
Chapinero: • 1 ♀; Parque El Virrey; 4.6736, –74.0548; 2590 m; 28.iii.2017; J. Duran leg.; Feijoasellowiana (Myrtaceae); MPUJ_ENT. Santa Fe: • 1 ♀; Parque Ilarco; 4.7003, –74.0655; 2569 m; 19.ix.2017; J. Duran leg.; Clusia sp. (Clusiaceae); MPUJ_ENT.
Distribution.
Colombia: Antioquia, Bogotá, Casanare, Risaralda, and Valle del Cauca (Rodas et al. 2014; Rendón-Mera et al. 2017).—Native to Australia, introduced into Africa, the Americas, Asia, Europe, and New Zealand (Pugh et al. 2017; Makunde et al. 2020).
Host plant.
Corymbia K.D.Hill and L.A.S.Johnson, and Eucalyptus L’Hér. spp. (Myrtaceae) (Makunde et al. 2020).
‡. Syncoptozus mexicanus
Hodkinson, 1990
7B8EB967-0875-582D-8AFD-FBBB0055C3DC
Material examined.
Antonio Nariño: • 1 ♂; Parque Ciudad Jardín; 4.5819, –74.0937; 2601 m; 13.iv.2018; V. Ocampo leg.; Lafoensiaacuminata (Lythraceae); MPUJ_ENT. Chapinero: • 1 ♂; Parque El Virrey; 4.6744, –74.0571; 2580 m; 20.vi.2017; J. Duran leg.; Fraxinuschinensis (Oleaceae); MPUJ_ENT • 1 ♂, 1 ♀; same but 4.674, –74.0565; 2581 m; Ligustrum sp. (Oleaceae); MPUJ_ENT • 3 ♂, 6 ♀; same but 4.6712, –74.0497; 2583 m; Magnoliagrandiflora (Magnoliaceae); MPUJ_ENT • 12 ♂, 16 ♀; same but 4.6753, –74.0581; 2579 m; 28.iii.2017; MPUJ_ENT. Santa Fe: • 14 ♂, 17 ♀; Parque Tercer Milenio; 4.5971, –74.0830; 2607 m; 23.iii.2017; J. Duran leg.; Magnoliagrandiflora (Magnoliaceae); MPUJ_ENT • 1 ♂, 1 ♀; same but 4.5971, –74.0829; 2606 m; 19.ix.2017; MPUJ_ENT.
Distribution.
Colombia: Bogotá (Rendón-Mera et al. 2017), Mexico (Hodkinson 1990).
Host plant.
Magnoliagrandiflora L. (Magnoliaceae) (unpublished NHMB data from Mexico).
Calophyidae Vondráček, 1957
‡. Calophya schini
Tuthill, 1959
09CC5398-48B7-5A93-B504-247EED7BB84D
Material examined.
Antonio Nariño: • 1 ♀; Parque Ciudad Jardín; 4.5818, –74.0933; 2601 m; 13.iv.2018; V. Ocampo leg.; Lafoensiaacuminata (Lythraceae); MPUJ_ENT • 1 ♀; same but 4.5819, –74.0937; 2601 m; MPUJ_ENT • 58 ♂, 73 ♀; same but 4.5814, –74.0932; 2601 m; Schinusareira (Anacardiaceae); MPUJ_ENT • 116 ♂, 104 ♀; same but 4.5816, –74.0931; 2600 m; MPUJ_ENT • 114 ♂, 159 ♀; same but 4.5817, –74.0931; 2602 m; MPUJ_ENT • 1 ♀; same but 4.5821, –74.0914; 2599 m; MPUJ_ENT • 45 ♂, 56 ♀; same but 4.5822, –74.0932; 2597 m; MPUJ_ENT. Chapinero: • 1 ♂, 6 ♀; Parque El Chicó; 4.673, –74.0452; 2599 m; 27.iv.2018; V. Ocampo leg.; Schinusareira (Anacardiaceae); MPUJ_ENT • 1 ♂, 2 ♀; Parque El Virrey; 4.6754, –74.0581; 2579 m; 25.ix.2017; J. Duran leg.; Crotoncoriaceus (Euphorbiaceae); MPUJ_ENT • 6 ♂, 5 ♀; same but 28.iii.2017; MPUJ_ENT • 2 ♂, 2 ♀; same but 4.6739, –74.0557; 2580 m; Salixhumboldtiana (Salicaceae); MPUJ_ENT. Rafael Uribe Uribe: • 1 ♀; Parque Palermo Sur; 4.5412, –74.1100; 2698 m; 09.iv.2018; V. Ocampo leg.; Pittosporumundulatum (Pittosporaceae); MPUJ_ENT • 10 ♂, 16 ♀; same but 4.5417, –74.1097; 2689 m; Schinusareira (Anacardiaceae); MPUJ_ENT. San Cristóbal: • 1 ♀; Parque La Victoria; 4.5546, –74.0954; 2757 m; 09.iv.2018; V. Ocampo leg.; Pittosporumundulatum (Pittosporaceae); MPUJ_ENT • 4 ♂, 1 ♀; same but 4.5548, –74.0955; 2764 m; Quercushumboldtii (Fagaceae); MPUJ_ENT • 79 ♂, 92 ♀; same but 4.5546, –74.0953; 2759 m; Schinusareira (Anacardiaceae); MPUJ_ENT • 31 ♂, 48 ♀; same but 4.5547, –74.0953; 2760 m; MPUJ_ENT • 3 ♂, 4 ♀; Parque San Cristóbal: 4.5735, –74.0832; 2639 m; 13.iv.2018; V. Ocampo leg.; Schinusareira (Anacardiaceae); MPUJ_ENT • 2 ♂, 3 ♀; same but 4.5736, –74.0834; 2638 m; MPUJ_ENT • 60 ♂, 17 ♀; same but 4.5736, –74.0827; 2642 m; MPUJ_ENT • 44 ♂, 37 ♀; Parque Villa de los Alpes; 4.5593, –74.0977; 2692 m; 13.iv.2018; V. Ocampo leg.; Schinusareira (Anacardiaceae); MPUJ_ENT. Santa Fe: • 4 ♂, 2 ♀; Parque Tercer Milenio; 4.5971, –74.0829; 2606 m; 19.ix.2017; J. Duran leg.; Magnoliagrandiflora (Magnoliaceae); MPUJ_ENT • 1 ♂, 7 ♀; same but 4.7011, –74.0655; 2570 m; 23.iii.2017; Schinusareira (Anacardiaceae); MPUJ_ENT. Usaquén: • 1 ♀; CAI Santa Barbara; 4.693, –74.0311; 2601 m; 06.iv.2018; V. Ocampo leg.; Quercushumboldtii (Fagaceae); MPUJ_ENT • 4 ♂, 4 ♀; Parque Belmira; 4.7215, –74.0318; 2576 m; 02.iv.2018; V. Ocampo leg.; Schinusareira (Anacardiaceae); MPUJ_ENT • 1 ♂; Parque Cabañas del Norte; 4.7359, –74.0318; 2575 m; 16.iii.2018; V. Ocampo leg.; Lafoensiaacuminata (Lythraceae); MPUJ_ENT • 33 ♂, 27 ♀; same but 4.7359, –74.0317; 2574 m; Schinusareira (Anacardiaceae); MPUJ_ENT • 102 ♂, 116 ♀; same but 4.7359, –74.0315; 2576 m; MPUJ_ENT • 19 ♂, 18 ♀; same but 4.7359, –74.0316; 2575 m; MPUJ_ENT • 48 ♂, 56 ♀; same but 4.736, –74.0317; 2571 m; MPUJ_ENT • 1 ♀; Parque Contador Norte; 4.7152, –74.0297; 2599 m; 02.iv.2018; V. Ocampo leg.; Liquidambarstyraciflua (Altingiaceae); MPUJ_ENT • 19 ♂, 21 ♀; Parque Ginebra-Bella Suiza; 4.7061, –74.0300; 2595 m; 06.iv.2018; V. Ocampo leg.; Schinusareira (Anacardiaceae); MPUJ_ENT • 13 ♂, 6 ♀; same but 4.7062, –74.0305; 2596 m; MPUJ_ENT • 55 ♂, 34 ♀; same but 4.7067, –74.0299; 2601 m; MPUJ_ENT • 2 ♂, 1 ♀; Parque La Francia; 4.6896, –74.0470; 2577 m; 05.iii.2018; V. Ocampo leg.; Schinusareira (Anacardiaceae); MPUJ_ENT • 1 ♀; same but 4.6902, –74.0464; 2577 m; MPUJ_ENT • 1 ♀; same but 4.6906, –74.0464; 2575 m; MPUJ_ENT • 1 ♂, 1 ♀; same but 4.6908, –74.0466; 2577 m; MPUJ_ENT • 1 ♀; Parque Nueva Autopista; 4.7217, –74.0507; 2579 m; 05.iii.2018; V. Ocampo leg.; Ficus sp. (Moraceae); MPUJ_ENT • 1 ♀; Parque Usaquén 2; 4.691, –74.0323; 2586 m; 05.iii.2018; V. Ocampo leg.; Ficus sp. (Moraceae); MPUJ_ENT • 1 ♂; same but 4.691, –74.0320; 2591 m; 27.iv.2018; Liquidambarstyraciflua (Altingiaceae); MPUJ_ENT • 1 ♀; same but 4.6912, –74.0317; 2571 m; 05.iii.2018; Pittosporumundulatum (Pittosporaceae); MPUJ_ENT. Usme: • 1 ♂, 1 ♀; Parque Chuniza-Famaco; 4.5018, –74.1086; 2775 m; 09.iv.2018; V. Ocampo leg.; Quercushumboldtii (Fagaceae); MPUJ_ENT • 158 ♂, 173 ♀; same but 4.5015, –74.1088; 2686 m; Schinusareira (Anacardiaceae); MPUJ_ENT • 28 ♂, 31 ♀; same but 4.5018, –74.1087; 2774 m; MPUJ_ENT • 55 ♂, 44 ♀; same but 4.5031, –74.1100; 2759 m; MPUJ_ENT • 16 ♂, 15 ♀; same but 4.5032, –74.1097; 2761 m; MPUJ_ENT • 20 ♂, 18 ♀; same but 4.5036, –74.1101; 2754 m; MPUJ_ENT • 22 ♂, 29 ♀; Parque Virrey Sur; 4.5009, –74.1125; 2768 m; 23.iv.2018; V. Ocampo leg.; Schinusareira (Anacardiaceae); MPUJ_ENT.
Distribution.
Colombia: Bogotá (Pinzón and González 2002).—Probably originating from Bolivia or Peru, adventive elsewhere in the Americas, Africa, Europe, and New Zealand (Burckhardt et al. 2018).
Host plant.
Schinusareira L. (Anacardiaceae) (Burckhardt et al. 2018).
Carsidaridae Crawford, 1911
. Synoza cornutiventris
Enderlein, 1918
D25F3784-7CFD-5299-AD85-8462FC81DFAD
Material examined.
Antonio Nariño: • 4 ♂, 5 ♀; Parque Ciudad Jardín; 4.5818, –74.0932; 2601 m; 23.iv.2018; V. Ocampo leg.; Ficus sp. (Moraceae); MPUJ_ENT. Chapinero: • 2 ♀; Parque El Chicó; 4.6731, –74.0447; 2605 m; 27.iv.2018; V. Ocampo leg.; Ficus sp. (Moraceae); MPUJ_ENT • 6 ♂, 2 ♀; same but 4.6732, –74.0445; MPUJ_ENT • 1 ♂; Parque El Virrey; 4.6733, –74.0554; 2591 m; 28.iii.2017; J. Duran leg.; Delostomaintegrifolium (Bignoniaceae); MPUJ_ENT. Rafael Uribe Uribe: • 2 ♂; Parque Palermo Sur; 4.5423, –74.1102; 2676 m; 09.iv.2018; V. Ocampo leg.; Ficus sp. (Moraceae); MPUJ_ENT. San Cristóbal: • 8 ♂, 7 ♀; Parque Primero de Mayo; 4.5734, –74.0882; 2625 m; 23.iv.2018; V. Ocampo leg.; Ficus sp. (Moraceae); MPUJ_ENT • 28 ♂, 24 ♀; same but 4.5738, –74.0879; 2622 m; 13.iv.2018; MPUJ_ENT • 25 ♂, 14 ♀; same but 4.5745, –74.0881; 2621 m; MPUJ_ENT • 1 ♀; same but 4.5746, –74.0880; 2621 m; Quercushumboldtii (Fagaceae); MPUJ_ENT • 1 ♂; Parque San Cristóbal: 4.5728, –74.0848; 2638 m; 13.iv.2018; V. Ocampo leg.; Lafoensiaacuminata (Lythraceae); MPUJ_ENT • 1 ♂; same but 4.5728, –74.0838; Pittosporumundulatum (Pittosporaceae); MPUJ_ENT • 36 ♂, 24 ♀; Parque Villa de los Alpes; 4.5591, –74.0974; 2698 m; 23.iv.2018; V. Ocampo leg.; Ficus sp. (Moraceae); MPUJ_ENT • 11 ♂, 4 ♀; same but 4.5593, –74.0972; 2695 m; MPUJ_ENT • 11 ♂, 16 ♀; same but 4.5595, –74.0978; 2686 m; MPUJ_ENT • 1 ♀; same but 4.5621, –74.0982; 2676 m; 13.iv.2018; MPUJ_ENT • 4 ♂, 6 ♀; same but 4.5624, –74.0983; 2667 m; MPUJ_ENT • 4 ♂, 1 ♀; same but 4.5625, –74.0983; 2665 m; 13.iv.2018; MPUJ_ENT • 2 ♀; same but 4.5628, –74.0982; 2658 m; 23.iv.2018; MPUJ_ENT. Santa Fe: • 5 ♂, 1 ♀; Parque La Independencia; 4.6108, –74.0678; 2645 m; 27.iv.2018; V. Ocampo leg.; Ficus sp. (Moraceae); MPUJ_ENT • 12 ♂, 11 ♀; same but 4.6114, –74.0682; 2639 m; MPUJ_ENT • 1 ♂; same but 4.6116, –74.0687; 2631 m; MPUJ_ENT • 1 ♂; same but 4.6108, –74.0678; 2644 m; Liquidambarstyraciflua (Altingiaceae); MPUJ_ENT • 1 ♂; same but 4.6119, –74.0694; 2583 m; Quercushumboldtii (Fagaceae); MPUJ_ENT • 1 ♂; Parque Nacional; 4.6217, –74.0643; 2576 m; 27.iv.2018; V. Ocampo leg.; Ficus sp. (Moraceae); MPUJ_ENT • 3 ♀; same but 4.6242, –74.0640; 2624 m; MPUJ_ENT • 1 ♂; Parque Ilarco; 4.7008, –74.0657; 2569 m; 23.iii.2017; J. Duran leg.; Crotoncoriaceus (Euphorbiaceae); MPUJ_ENT. Suba: • 4 ♂, 5 ♀; Parque Canal Molinos; 4.6981, –74.0634; 2575 m; 23.iii.2017; J. Duran leg.; Ficusamericanasubsp.andicola (Moraceae); MPUJ_ENT. Usaquén: • 1 ♂, 1 ♀; Parque Altablanca; 4.7347, –74.0285; 2581 m; 16.iii.2018; V. Ocampo leg.; Lafoensiaacuminata (Lythraceae); MPUJ_ENT • 3 ♂, 2 ♀; Parque CAI Lisboa; 4.7085, –74.0290; 2604 m; 06.iv.2018; V. Ocampo leg.; Pittosporumundulatum (Pittosporaceae); MPUJ_ENT • 1 ♀; same but 4.7088, –74.0292; 2599 m; MPUJ_ENT • 12 ♂, 5 ♀; Parque Cedro Madeira; 4.7268, –74.0313; 2574 m; 23.iii.2018; V. Ocampo leg.; Ficus sp. (Moraceae); MPUJ_ENT • 40 ♂, 24 ♀; Parque Contador Norte; 4.7127, –74.0312; 2594 m; 06.iv.2018; V. Ocampo leg.; Ficus sp. (Moraceae); MPUJ_ENT • 13 ♂, 13 ♀; same but 4.7129, –74.0312; 2601 m; MPUJ_ENT • 14 ♂, 13 ♀; same but 4.713, –74.0312; 2598 m; MPUJ_ENT • 1 ♂, 1 ♀; same but 4.7132, –74.0314; 2593 m; Liquidambarstyraciflua (Altingiaceae); MPUJ_ENT • 1 ♂; same but 4.7128, –74.0311; 2603 m; Pittosporumundulatum (Pittosporaceae); MPUJ_ENT • 1 ♀; Parque Ginebra-Bella Suiza; 4.7067, –74.0298; 2601 m; 06.iv.2018; V. Ocampo leg.; Schinusareira (Anacardiaceae); MPUJ_ENT • 2 ♂, 2 ♀; Parque La Francia; 4.6899, –74.0466; 2579 m; 05.iii.2018; V. Ocampo leg.; Ficus sp. (Moraceae); MPUJ_ENT • 2 ♀; same but 4.6908, –74.0480; 2580 m; MPUJ_ENT • 2 ♂; same but 4.6905, –74.0475; 2581 m; Lafoensiaacuminata (Lythraceae); MPUJ_ENT • 21 ♂, 13 ♀; Parque La Vida; 4.7361, –74.0339; 2585 m; 16.iii.2018; V. Ocampo leg.; Ficus sp. (Moraceae); MPUJ_ENT • 39 ♂, 22 ♀; same but 4.7362, –74.0339; 2586 m; MPUJ_ENT • 32 ♂, 32 ♀; same but 4.7365, –74.0341; 2577 m; MPUJ_ENT • 12 ♂, 6 ♀; same but 4.7367, –74.0342; 2579 m; MPUJ_ENT • 17 ♂, 17 ♀; same but 4.7369, –74.0350; 2576 m; MPUJ_ENT • 23 ♂, 10 ♀; same but 4.737, –74.0344; 2573 m; MPUJ_ENT • 1 ♂, 5 ♀; same but 4.7371, –74.0352; 2572 m; Pittosporumundulatum (Pittosporaceae); MPUJ_ENT • 1 ♂, 1 ♀; Parque Nueva Autopista; 4.7216, –74.0507; 2571 m; 05.iii.2018; V. Ocampo leg.; Ficus sp. (Moraceae); MPUJ_ENT • 1 ♂; same but 4.7217, –74.0507; 2579 m; MPUJ_ENT • 2 ♂, 2 ♀; Parque Usaquén 2; 4.691, –74.0323; 2586 m; 05.iii.2018; V. Ocampo leg.; Ficus sp. (Moraceae); MPUJ_ENT • 1 ♂; same but 4.6912, –74.0323; 2587 m; Pittosporumundulatum (Pittosporaceae); MPUJ_ENT. Usme: • 7 ♂, 12 ♀; Parque Diana Turbay; 4.5478, –74.1015; 2672 m; 23.iv.2018; V. Ocampo leg.; Ficus sp. (Moraceae); MPUJ_ENT • 5 ♂, 10 ♀; same but 4.5483, –74.1013; MPUJ_ENT • 14 ♂, 8 ♀; Parque La Andrea; 4.5098, –74.1109; 2741 m; 09.iv.2018; V. Ocampo leg.; Ficus sp. (Moraceae); MPUJ_ENT • 39 ♂, 32 ♀; same but 4.5098, –74.1109; 2701 m; MPUJ_ENT • 6 ♂, 7 ♀; Parque Virrey Sur; 4.5009, –74.1115; 2779 m; 23.iv.2018; V. Ocampo leg.; Ficus sp. (Moraceae); MPUJ_ENT • 4 ♀; same but 4.5012, –74.1113; 2780 m; MPUJ_ENT • 4 ♂, 1 ♀; same but 4.5013, –74.1114; 2781 m; MPUJ_ENT • 4 ♂, 3 ♀; same but 4.5014, –74.1116; 2778 m; MPUJ_ENT.
Distribution.
Colombia: Bogotá, Cundinamarca, Meta (Brown and Hodkinson 1988; Rendón-Mera et al. 2017), Costa Rica, Panama, and Peru (Brown and Hodkinson 1988; Hollis 2000).
Host plant.
Ficushartwegii Miq. (Moraceae) (Hollis 2000). Several adults were collected in the present study on Ficusamericanasubsp.andicola (Standl.) C.C.Berg. This species has to be confirmed as host. Many adults were collected on unidentified Ficus trees. It is possible that these also constitute hosts, but they should be identified to species and examined for psyllid immatures for further conclusions.
Liviidae Löw, 1879
*. Melanastera
sp.
EE9DD33F-D092-5658-A72B-08263410008C
Material examined.
Chapinero: • 1 ♀; Quebrada La Vieja; 4.6474, –74.0447; 2785 m; 20.vi.2017; J. Duran leg.; Miconiaelaeoides (Melastomataceae); MPUJ_ENT.
Distribution.
Colombia: Bogotá.
Host plant.
Unknown.
Comments.
The single female appears to belong to an undescribed species of Melanastera, a predominantly Neotropical genus associated with Melastomataceae, Annonaceae, and other plant families (Burckhardt et al. 2024). This is the first record of the genus from Colombia.
Mastigimatidae Bekker-Migdisova, 1973
. Mastigimas colombianus
Burckhardt, Queiroz & Drohojowska, 2013
DF505AB8-18C9-5BEE-8706-CE5579EC6068
Figure 3.
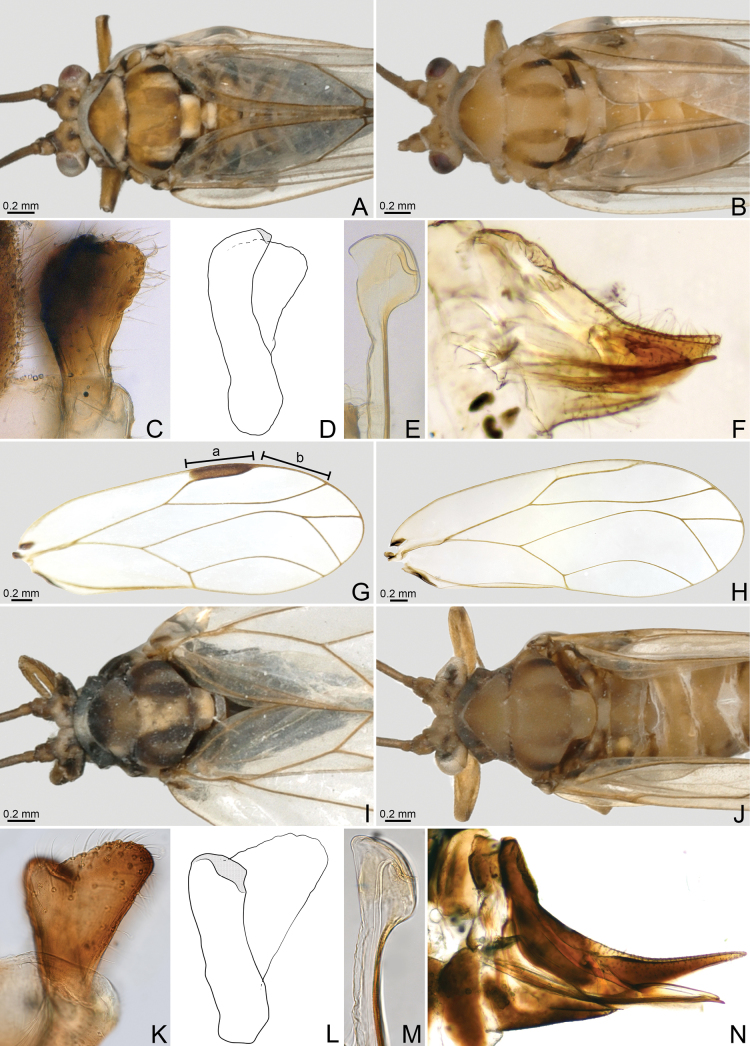
A–GMastigimascolombianus Burckhardt, Queiroz & Drohojowska, 2013 H–NMastigimaslongicaudatus Rendón-Mera, Burckhardt & Vargas-Fonseca, sp. nov. A, I male, dorsal view B, J female, dorsal view C, K paramere, outer surface, lateral view D, L paramere, inner surface, lateral view E, M distal segment of aedeagus, lateral view F, N female terminalia, lateral view G, H forewing.
Material examined.
Chapinero: • 17 ♂, 17 ♀; Parque El Virrey; 4.6728, –74.0533; 2581 m; 28.iii.2017; J. Duran leg.; Cedrelamontana (Meliaceae); MPUJ_ENT • 2 ♂, 2 ♀; same but NMHB • 2 ♂, 2 ♀; same but slide mounted; NMHB. Santa Fe: • 1 ♂; Parque Ilarco; 4.7008, –74.0657; 2569 m; 23.iii.2017; J. Duran leg.; Crotoncoriaceus (Euphorbiaceae); MPUJ_ENT. Suba: • 9 ♂, 6 ♀; Parque Canal Molinos; 4.6976, –74.0637; 2575 m; 10.vii.2017; J. Duran leg.; Cedrelamontana (Meliaceae); MPUJ_ENT • 1 ♂, 1 ♀; same but 03.x.2017; MPUJ_ENT. Usaquén: • 1 ♀; Parque Cabañas del Norte; 4.7358, –74.0315; 2578 m; 16.iii.2018; V. Ocampo leg.; Lafoensiaacuminata (Lythraceae); MPUJ_ENT.
Redescription.
Colouration. Male (Fig. 3A) dark yellow with dark brown markings. Vertex with pale brown longitudinal stripe along lateral and anterior margins on either side; discal foveae with dark brown spot; margin of toruli brown. Genal processes and clypeus whitish. Antennal segments 1 and 2 yellow, segment 3 yellow basally, gradually darkening to dark brown apex, segments 4–10 dark brown. Pronotum whitish with lateral sutures brown. Mesopraescutum pale yellow along posterior margins. Mesoscutum with two dark yellow longitudinal stripes on either side, the outer one black posteriorly. Mesoscutellum and metascutellum whitish. Metapostnotum with dark brown spots medially and laterally. Pleura whitish, propleurites black dorsally. Mesosternum brown. Forewing colourless, with black spot at base of C+Sc and basally on anal cell; veins and pterostigma brown. Fore and mid legs with femur dark yellow, tibia and tarsi brown; hind leg with femur dark brown, tibia and tarsi pale yellow. Abdomen brown with yellow spot medially, narrowing to apex; intersegmental membrane straw-coloured. Terminalia dark brown, parameres black, subgenital plate pale yellow dorsally.—Female (Fig. 3B) yellow with only a few black markings. Discal foveae dark yellow. Pronotum with lateral indentations dark yellow. Meso- and metanotum as in male but markings dark yellow, with outermost stripes on mesoscutum black posteriorly. Forewing as in male but pterostigma colourless. Pleura as in male. Fore and mid legs with femora pale yellow, tibiae dark yellow, and tarsi brown, hind leg pale yellow. Terminalia yellow, apex of proctiger black.
Structure. Antenna 4.0–4.1× as long as head width; segment 3 1.3–1.4× as long as segment 4. Forewing (Fig. 3G) 4.5–5.3× as long as head width, and 2.6× as long as wide, pterostigma long and narrow, ratio a/b 0.9–1.0, cell cu1 long and flat, length/height ratio 3.3.
Terminalia. Male. Paramere (Fig. 3C, D) bifid, clavate, outer lobe rounded anteriorly and angular posteriorly. Apical dilatation of aedeagus with small blunt apico-ventral hook (Fig. 3E), 1.2× as long as paramere.—Female (Fig. 3F). Terminalia short and cuneate, dorsal outline of proctiger slightly concave; proctiger as long as head width, and 2.4× as long as subgenital plate.
Measurements (in mm). BL 2 ♂ 3.6–5.0 (4.36±0.74), 2 ♀ 5.3–5.4 (5.31±0.08); HW ♂ 0.85, ♀ 0.82; AL ♂ 3.44, ♀ 3.39; FL ♂ 3.8, ♀ 4.36; FW ♂ 1.47, ♀ 1.67; PL ♂ 0.24; DL ♂ 0.28; FP ♀ 0.8; FS ♀ 0.34.
Distribution.
Colombia: Bogotá (Burckhardt et al. 2013).
Host plant.
Most adults (types and material at hand) were collected on Cedrelamontana Turcz. (Meliaceae). Mastigimas species develop, as far as known, on Cedrela, suggesting that C.montana is a host.
Comments.
Mastigimascolombianus was described from two males and two females collected in Bogotá (Burckhardt et al. 2013). As more material is available from this study, a redescription of the species is provided here. The females in the material at hand fit the original description perfectly but the male paramere is slightly variable with respect to the shape of the outer lobe. As in the two type specimens, the paramere in the material at hand is strongly sclerotised which seems characteristic for the species.
*. Mastigimas longicaudatus
Rendón-Mera, Burckhardt & Vargas-Fonseca sp. nov.
28962111-4B8A-5089-94C4-BD2D3474B4B6
https://zoobank.org/7AED528B-ED19-4544-96D1-15488A4D5A2F
Type locality.
Colombia, Bogotá: Suba, Parque Canal Molinos, 4.6976389, –74.063694, 2575 m.
Type material.
Holotype: Colombia • ♂, pinned; Bogotá, Suba, Parque Canal Molinos; 4.6976389, –74.063694; 2575 m; 03.x.2017; J. Duran leg; on Cedrelamontana (Meliaceae); MPUJ_ENT0074272. Paratypes: Chapinero: • 6 ♂, 7 ♀; Parque El Virrey; 4.6728, –74.0533; 2581 m; 28.iii.2017; J. Duran leg.; Cedrelamontana (Meliaceae); MPUJ_ENT. Santa Fe: • 2 ♀; Parque Tercer Milenio; 4.5974, –74.0835; 2605 m; 23.iii.2017; J. Duran leg.; Sambucusnigra (Viburnaceae); MPUJ_ENT. Suba: • 8 ♂, 3 ♀; Parque Canal Molinos; 4.6976, –74.0637; 2575 m; 10.vii.2017; J. Duran leg.; Cedrelamontana (Meliaceae); MPUJ_ENT • 11 ♂, 13 ♀; same data as for holotype • 1 ♂, 1 ♀; same data as for holotype but NHMB • 1 ♂, 1 ♀; same data as for holotype but slide mounted; NHMB. Usaquén: • 1 ♀; Parque Cabañas del Norte; 4.7363, –74.0317; 2575 m; 16.iii.2018; V. Ocampo leg.; Pittosporumundulatum (Pittosporaceae); MPUJ_ENT • 1 ♂; Parque Ginebra-Bella Suiza; 4.7061, –74.0304; 2596 m; 06.iv.2018; V. Ocampo leg.; Liquidambarstyraciflua (Altingiaceae); MPUJ_ENT • 2 ♂; same but 4.7062, –74.0305; Schinusareira (Anacardiaceae); MPUJ_ENT.
Diagnosis.
Forewing (Fig. 3H) with pterostigma long and narrow, ratio a/b 1.2 Antennal segment 3 approx. as long as segment 4. Paramere (Fig. 3K, L) bifid, irregularly triangular, strongly widening to apex. Aedeagal head lacking apico-ventral hook. Female terminalia (Fig. 3N) elongate, falcate; proctiger 1.0–1.2× as long as head width.
Description.
Colouration. Male (Fig. 3I) dark brown. Head pale yellow; vertex with pale brown longitudinal stripe along lateral and anterior margins on either side; discal foveae with dark brown spot, sometimes much expanded; margin of toruli brown. Genal processes and clypeus whitish. Antennae yellowish brown. Pronotum whitish with lateral quarter dark brown. Mesopraescutum with dark brown polygon-shaped spot anteriorly. Mesoscutum with two dark brown longitudinal stripes on either side. Mesoscutellum and metascutellum whitish. Metapostnotum dark brown. Pleura whitish, with dark brown markings dorsally. Mesosternum dark brown. Forewing colourless, with brown spot at base of C+Sc and base of anal cell; veins yellow; pterostigma dark brown or yellowish brown. Fore and mid legs brown and yellowish brown, metafemur and base of metatibia dark brown, rest of hind leg pale yellow. Abdomen dark brown; intersegmental membrane straw-coloured. Terminalia dark brown, parameres sometimes dark yellow.—Female (Fig. 3J) yellow. Discal foveae dark brown; margin of toruli brown. Pronotum with lateral indentations brown. Tergum as in male but markings dark yellow on mesopraescutum and brownish on mesoscutum, with outermost stripe dark brown posteriorly. Forewing as in male but pterostigma colourless. Pleura and legs as in male. Abdominal sclerites usually brown laterally. Terminalia yellow, brown apically, proctiger sometimes completely brown.
Structure. Conforms to the generic description of Brown and Hodkinson (1988). Antenna 4.3–4.5× as long as head width; segment 3 1.1–1.3× as long as segment 4. Forewing (Fig. 3H) 4.8–5.2× as long as head width, and 2.6–2.7× as long as wide, pterostigma long and narrow, ratio a/b 1.1–1.2, cell cu1 long and flat, length/height ratio 3.5–3.8.
Terminalia. Paramere (Fig. 3K, L), bifid, in lateral view irregularly triangular, strongly widening to apex. Apical dilatation of aedeagus lenticular (Fig. 3M), 1.5× as long as paramere.—Female terminalia (Fig. 3N) elongate, falcate; proctiger 1.5× as long as head width, and 1.9× as long as subgenital plate.
Measurements (in mm). BL ♂ 4.8, 2 ♀ 5.8–5.9 (5.85±0.1); HW ♂ 0.88, ♀ 0.92; AL ♂ 3.95, ♀ 3.93; FL ♂ 4.23, ♀ 4.78; FW ♂ 1.57, ♀ 1.85; PL ♂ 0.24; DL ♂ 0.36; FP ♀ 1.26; FS ♀ 0.65.
Etymology.
From Latin longus = long, and caudatus = bearing a tail, referring to the long female terminalia. Adjective.
Distribution.
Colombia: Bogotá.
Host plant.
Most of the examined adults were collected on Cedrelamontana Turcz. (Meliaceae). Mastigimas species develop, as far as known, on Cedrela, suggesting that C.montana is a host.
Comments.
Mastigimaslongicaudatus Rendón-Mera, Burckhardt & Vargas-Fonseca, sp. nov. resembles M.anjosiBurckhardt et al., 2011 (known from Brazil, Trinidad, and Venezuela) in the irregularly triangular paramere and the elongate, falcate female terminalia; it differs in the antennal segment 3 approx. as long as segment 4 (instead of twice as long), and the aedeagal head lacking an apico-ventral hook (Burckhardt et al. 2011, 2013). The falcate female terminalia are shared also with M.drepanodis Burckhardt, Queiroz & Drohojowska, 2013 (Brazil) which differs in the slenderer paramere (Burckhardt et al. 2013). In the key by Burckhardt et al. (2013), the new species keys out with M.colombianus from which it differs in details of the male and female terminalia.
Psyllidae Latreille, 1807
‡. Acizzia acaciaebaileyanae
(Froggatt, 1901)
6688CCC2-7EDF-5FF0-A0E2-151AA34573F5
Material examined.
Suba: • 1 ♂, 3 ♀; Cerro La Conejera; 4.7705, –74.0656; 2620 m; 28.iii.2017; J. Duran leg.; Acaciadealbata (Fabaceae); MPUJ_ENT • 1 ♀; Parque Ilarco; 4.701, –74.0663; 2567 m; 23.iii.2017; J. Duran leg.; Bocconiafrutescens (Papaveraceae); MPUJ_ENT • 1 ♂; same but 10.vii.2017; MPUJ_ENT. Usaquén: • 1 ♂; Parque Belmira; 4.7215, –74.0318; 2576 m; 02.iv.2018; V. Ocampo leg.; Schinusareira (Anacardiaceae); MPUJ_ENT • 5 ♂; Parque Cabañas del Norte; 4.7358, –74.0315; 2578 m; 16.iii.2018; V. Ocampo leg.; Lafoensiaacuminata (Lythraceae); MPUJ_ENT • 1 ♂, 1 ♀; Parque Contador Norte; 4.7158, –74.0322; 2581 m; 06.iv.2018; V. Ocampo leg.; Lafoensiaacuminata (Lythraceae); MPUJ_ENT • 1 ♂; same but 4.715, –74.0301; 2597 m; 02.iv.2018; Liquidambarstyraciflua (Altingiaceae); MPUJ_ENT • 1 ♀; same but 4.7151, –74.0299; 2600 m; MPUJ_ENT.
Distribution.
Colombia: Bogotá (Rendón-Mera et al. 2017).—Native to Australia, adventive in Africa, North America, Asia, Europe, and New Zealand (Ouvrard 2023).
Host plant.
Acacia Mill. and Samanea (Benth.) Merr. spp. (Fabaceae) (Ouvrard 2023).
‡. Acizzia uncatoides
(Ferris & Klyver, 1932)
94BC505B-D1A6-5B09-9E3C-AE8531FE53AA
Material examined.
Chapinero: • 9 ♂, 10 ♀; Parque El Virrey; 4.6713, –74.0504; 2591 m; 28.iii.2017; J. Duran leg.; Acaciamelanoxylon (Fabaceae); MPUJ_ENT • 18 ♂, 10 ♀; same but 4.6753, –74.0581; 2579 m; Magnoliagrandiflora (Magnoliaceae); MPUJ_ENT • 1 ♀; same but 4.6738, –74.0563; 2581 m; Pittosporumundulatum (Pittosporaceae); MPUJ_ENT • 1 ♂; Quebrada La Vieja; 4.6495, –74.0466; 2764 m; 06.iv.2017; J. Duran leg.; Piperbogotense (Piperaceae); MPUJ_ENT. Rafael Uribe Uribe: • 1 ♂; Parque Palermo Sur; 4.542, –74.1089; 2692 m; 09.iv.2018; V. Ocampo leg.; Pittosporumundulatum (Pittosporaceae); MPUJ_ENT. Santa Fe: • 9 ♂, 6 ♀; Universidad Distrital; 4.5991, –74.0656; 2695 m; 19.ix.2017; J. Duran leg.; Acaciadecurrens (Fabaceae); MPUJ_ENT • 1 ♂, 1 ♀; same but 4.5986, –74.0656; 2702 m; 05.v.2017; Acaciamelanoxylon (Fabaceae); MPUJ_ENT • 71 ♂, 52 ♀; same but 4.5987, –74.0667; 2667 m; 19.ix.2017; MPUJ_ENT • 1 ♂, 1 ♀; same but 4.5983, –74.0654; 2712 m; 05.v.2017; Crotoncoriaceus (Euphorbiaceae); MPUJ_ENT • 10 ♂, 14 ♀; same but 4.5985, –74.0655; 2704 m; Lyciantheslycioides (Solanaceae); MPUJ_ENT • 1 ♂; same but 4.5989, –74.0656; 2701 m; Oreopanaxincisus (Araliaceae); MPUJ_ENT • 1 ♂; same but 4.5987, –74.0653; 2713 m; Quercushumboldtii (Fagaceae); MPUJ_ENT. Suba: • 2 ♀; Cerro La Conejera; 4.7718, –74.0648; 2622 m; 10.vii.2017; J. Duran leg.; Acaciamelanoxylon (Fabaceae); MPUJ_ENT • 1 ♂; same but 4.7695, –74.0527; 2674 m; 03.x.2017; Quercushumboldtii (Fagaceae); MPUJ_ENT. Usaquén: • 1 ♂; Parque CAI Lisboa; 4.7088, –74.0292; 2599 m; 06.iv.2018; V. Ocampo leg.; Pittosporumundulatum (Pittosporaceae); MPUJ_ENT • 1 ♂; same but 4.7094, –74.0291; 2590 m; MPUJ_ENT.
Distribution.
Colombia: Bogotá, Cundinamarca, Huila (Rendón-Mera et al. 2017).—Native to Australia, adventive in Africa, the Americas, Asia, Europe, North Africa, and New Zealand (Ouvrard 2023).
Host plant.
Acacia Mill. and Albizia A. ex Benth. (Fabaceae) (Halbert and Burckhardt 2020); in this survey several adults were collected on Acaciadecurrens (J.C.Wendl.) Willd. and A.melanoxylon R.Br. While the latter is confirmed in the literature as host, the former is not. Further studies will be necessary to find out whether A.decurrens serves as host to A.uncatoides.
. Platycorypha
sp.
9A01B3A7-D758-537B-89A0-05202973E3D3
Material examined.
Santa Fe: • 1 ♀; Parque Ilarco; 4.7008, –74.0657; 2569 m; 23.iii.2017; J. Duran leg.; Crotoncoriaceus (Euphorbiaceae); MPUJ_ENT.
Distribution.
Colombia: Bogotá, Magdalena (Rendón-Mera et al. 2017).
Host plant.
Unknown. The single female at hand was collected on Croton, an unlikely host as all Platycorypha species, for which hosts are known, develop on Fabaceae (Burckhardt and Queiroz 2020).
Comments.
The single female at hand resembles specimens reported as Platycoryphaerythrinae (Lizer) from Panama (Brown and Hodkinson 1988) and Peru (Burckhardt 1987). These specimens are probably not conspecific with P.erythrinae from Argentina, Brazil, Paraguay, and Uruguay, but represent an undescribed species. The specimens from Colombia, Panama and Peru differ from the latter in the presence of distinct brown dots on the radular areas of the forewing and the small hook on the apex of the female proctiger. More material is required for solving this issue.
. Tuthillia latipennis
Hodkinson, Brown & Burckhardt, 1986
3B5C6D03-47BF-5181-B8D7-E8600F7370CD
Material examined.
Suba: • 1 ♀; Cerro La Conejera; 4.7718, –74.0651; 2631 m; 03.x.2017; J. Duran leg.; Myrcianthesleucoxyla (Myrtaceae); MPUJ_ENT. Engativá: • 1 ♂; Jardín Botánico de Bogotá; 4.6666, –74.0993; 2553 m; 16.x.2019; S. Vargas leg.; Myrcianthes sp. (Myrtaceae); MPUJ_ENT.
Distribution.
Colombia: Bogotá (Rendón-Mera et al. 2017), Costa Rica, Panama (Brown and Hodkinson 1988; Hollis 2000).
Host plant.
Myrcianthesfragrans (Sw.) McVaugh (Myrtaceae) (Hollis 2000). If Myrcianthesleucoxyla (Ortega) McVaugh, on which one female was collected, also constitutes a host, needs further observations.
Triozidae Löw, 1879
. Calinda gibbosa
(Tuthill, 1959)
60355E31-B6A1-5065-952B-DAB7F87E5466
Material examined.
Santa Fe: • 1 ♂, 2 ♀; Universidad Distrital; 4.5995, –74.0664; 2673 m; 19.ix.2017; J. Duran leg.; Baccharislatifolia (Asteraceae); MPUJ_ENT • 1 ♀; same but 4.5997, –74.0653; 2692 m; Quercushumboldtii (Fagaceae); MPUJ_ENT. Suba: • 1 ♀; Cerro La Conejera; 4.7702, –74.0664; 2634 m; 10.vii.2017; J. Duran leg.; Baccharis sp. (Asteraceae); MPUJ_ENT.
Distribution.
Colombia: Antioquia, Bogotá, Boyacá, Cundinamarca, Nariño (Olivares and Burckhardt 1997; Rendón-Mera et al. 2017), Cuba, Ecuador, Peru, Venezuela (Olivares and Burckhardt 1997).
Host plant.
Baccharislatifolia Pers. (Asteraceae) (Olivares and Burckhardt 1997).
*. Calinda trinervis
Olivares & Burckhardt, 1997
E91217EC-CE71-51F6-B30E-BCE42D784B62
Material examined.
Santa Fe: • 1 ♀; Universidad Distrital; 4.5995, –74.0664; 2673 m; 19.ix.2017; J. Duran leg.; Baccharislatifolia (Asteraceae); MPUJ_ENT.
Distribution.
Colombia: Bogotá, Costa Rica, Panama (Olivares and Burckhardt 1997).
Host plant.
Unknown. Adults from Colombia were collected on Baccharislatifolia Pers. and adults from Costa Rica on B.trinervis Pers. (Asteraceae). Both should be checked to determine whether they are hosts.
Comments.
Calindatrinervis is reported here for the first time from Colombia.
. Calinda
sp.
A2EDB7F9-024E-5C33-8AF1-BE3451909F4B
Material examined.
Santa Fe: • 1 ♀; Parque Ilarco; 4.7008, –74.0657; 2569 m; 23.iii.2017; J. Duran leg.; Crotoncoriaceus (Euphorbiaceae); MPUJ_ENT.
Distribution.
Colombia: Bogotá.
Host plant.
Unknown.
Comments.
The single female at hand represents probably an undescribed species. It shares the following characters with Calindaalbonigra Olivares & Burckhardt, 1997 and C.gladiformis Olivares & Burckhardt, 1997: antenna shorter than 1.2 mm; forewing lacking surface spinules in distal 1/2; apical projection of proctiger well delimited from base, not inflated, straight, pointed apically, with well-defined teeth along dorsal margin; subgenital plate long; valvula dorsalis long; ventral saw of valvula ventralis not well delimited at base. From the former it differs in the relatively longer processes on the proctiger and subgenital plate. From the latter it differs in the relatively shorter apical process of the proctiger and the presence of a small ventral hump in the basal 1/3 of the subgenital plate.
*. Leuronota albilinea
Rendón-Mera, Burckhardt & Vargas-Fonseca sp. nov.
58A1AB79-83A8-5420-B7BE-2EF628E2CCC5
https://zoobank.org/B778B877-DF95-4CAD-9A27-D325DE459AF0
Figure 4.
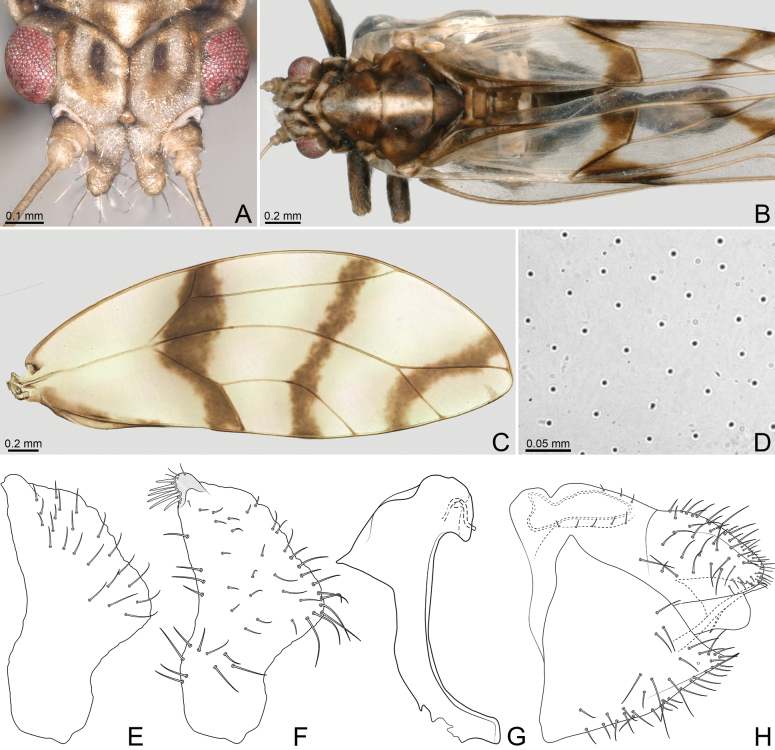
Leuronotaalbilinea Rendón-Mera, Burckhardt & Vargas-Fonseca, sp. nov. A head, dorsal view B habitus, dorsal view C forewing D surface spinules E–G male terminalia, lateral view E paramere, outer surface F paramere, inner surface G distal segment of aedeagus H female terminalia, lateral view.
Type locality.
Colombia, Bogotá: Santa Fe, Parque Tercer Milenio, 4.70025, –74.0654667, 2569 m.
Type material.
Holotype: Colombia • ♂, pinned; Bogotá, Santa Fe, Parque Tercer Milenio; 4.70025, –74.0654667; 2569 m; 19.ix.2017; J. Duran leg.; on Clusia sp. (Clusiaceae); MPUJ_ENT0074271. Paratypes: Santa Fe: • 28 ♂, 23 ♀; same data as for holotype but 4.7003, –74.0655; MPUJ_ENT • 1 ♂, 1 ♀; same data as for preceding but NHMB • 1 ♂, 1 ♀; same data as for preceding but slide mounted; NHMB • 4 ♂, 4 ♀; same data as for preceding but in ethanol 70%; NHMB • 19 ♂, 11 ♀; same data as for preceding but 27.vi.2017; MPUJ_ENT • 1 ♂; same data as for preceding but 4.5989, –74.0814; 2607 m; Prunusserotina (Rosaceae); MPUJ_ENT.
Diagnosis.
Mesonotum with white longitudinal stripe (Fig. 4B). Forewing (Fig. 4C) with three brown transverse bands as follows: one along vein R1, base of cells r2 and m2, vein Cu1 and apex of cell cu2 adjacent to vein Cu1b, one from subapex of cell r1, through approx. middle of r2 and m2, to radular spinules of cu1, and one from subapex of r2, through base of m1 to radular spinules of m2; clavus brown along A1 distal to apex of Cu2. Paramere (Fig. 4E, F) with apical process short and posterior margin with apical 1/2 sinuous. Female proctiger (Fig. 4H) with apical portion relatively slender.
Description.
Colouration. Head, pronotum and pleura white, rest of notum and abdomen dark brown (Fig. 4A, B). Vertex with dark brown longitudinal stripes adjacent to eyes, curving inwards distal to torulus; anterior margin usually brownish; discal foveae dark brown; margin of toruli brown. Genal process sometimes slightly darker apically. Antennal segments 1–8 pale-yellow, 9–10 black. Clypeus white, slightly brown posteriorly. Pronotum with two brown longitudinal stripes medially; sublateral and lateral indentations dark brown. Mesopraescutum and mesoscutum with white longitudinal stripe medially. Forewing membrane (Fig. 4C) colourless, with three brown transverse bands as follows: one along vein R1, base of cells r2 and m2, vein Cu1 and apex of cell cu2 adjacent to vein Cu1b, one from near apex of cell r1, through approx. middle of r2 and m2, to radular spinules of cu1, and one from near apex of r2, through base of m1 to radular spinules of m2; clavus brown along A1 distal to apex of Cu2; veins yellow, brown within the colour pattern; radular spinules brown. Fore, mid legs and metafemur brown, rest of hind leg yellow with apicotarsus brown. Abdominal basal sternites white or yellow medially; intersegmental membrane straw-coloured. Male terminalia dark brown. Female terminalia brown dorsally and ventrally, yellow apically.
Structure. Genal processes (Fig. 4A) 1.1–1.3× as long as vertex along midline, subcylindrical, slightly narrowing apically, sometimes slightly curved outwards, divergent; apex rounded. Antenna 3.1–3.6× as long as head width; longest terminal seta 3.5–4.0× as long as short seta, and 0.6–0.8× as long as segment 10. Labium with apical segment 0.3–0.4× as long as medial segment. Forewing (Fig. 4C) 5.2–5.6× as long as head width, and 2.5–2.6× as long as wide, obovate with angular apex; vein C+Sc evenly curved; vein Rs straight; vein M 2.1–2.3× as long as M1+2, bifurcating after imaginary line between apices of veins Rs and Cu1a; vein M1+2 reaching wing margin approximately at imaginary line through trifurcation of vein R+M+Cu and bifurcation of vein M; vein Cu 1.3–1.4× as long as R, and 1.6–1.8× as long as Cu1b; cell r1 approx. as wide as the narrowest section of r2. Surface spinules widely spaced (Fig. 4D), covering m1, cu1, and cu2, and colour pattern on r2 and m2. Radular spinules forming triangular fields. Metafemur with six or seven apical bristles; metatibia 1.2–1.4× as long as head width.
Terminalia. Male proctiger, in lateral view, subconical; apex constricted at anterior margin; anus large, occupying most part of apex, obliquely blunt. Paramere, in lateral view (Fig. 4E, F), 0.9× as long as proctiger; apical process short; bearing posterior lobe; anterior margin sinuous, concave submedially; posterior margin strongly irregular, concave in basal 1/3, strongly convex in median 1/3, sinuous in apical 1/3; outer surface (Fig. 4E) covered in medium long setae along posterior apical 1/2; inner surface (Fig. 4F) covered in short setae medially, long setae along anterior and posterior margins, and thick bristles anteriorly on apical tooth. Apical dilatation of aedeagus (Fig. 4G) with ventral extension beak-like, short; apically slightly convex, with small subapical hump; sclerotised end tube of ductus ejaculatorius short, weakly sinuate.—Female proctiger (Fig. 4H), in lateral view, 0.9× as long as head width; apical portion ~ 1/2 proctiger length; dorsal outline weakly incised at transverse groove, apical portion relatively slender, apex blunt; covered in long setae laterally, medium long setae dorsally, and short setae apically. Circumanal ring 0.4× as long as proctiger. Subgenital plate (Fig. 4H), in lateral view, 0.8× as long as proctiger; ventral outline straight in basal 1/2, weakly angular in the middle, straight in apical 1/2; sparsely covered in long setae, mostly ventrally and apically.
Measurements (in mm). BL 2 ♂ 3.7–4.1 (3.8±0.31), 2 ♀ 4.4–4.7 (4.79±0.37); HW ♂ 0.63, ♀ 0.67; VL ♂ 0.19, ♀ 0.18; GL ♂ 0.21, ♀ 0.23; AL ♂ 2.2, ♀ 1.78; LAB2 ♂ 0.22, ♀ 0.27; LAB3 ♂ 0.09, ♀ 0.09; FL ♂ 3.36, ♀ 3.46; TL ♂ 0.85, ♀ 0.78; MP 0.29; PL 0.26; FP 0.57; CRL 0.21; AP 0.25; SP 0.47.
Etymology.
From Latin albus = white, and linea = line, referring to the contrasting longitudinal white stripe on the mesonotum. Noun in the ablative case.
Distribution.
Colombia: Bogotá.
Host plant.
Unknown. Many adults were collected on Clusia sp. (Clusiaceae) in the same area suggesting it is a host rather than just a casual plant. Further studies are necessary to to check this assumption.
Comments.
Leuronotaalbilinea Rendón-Mera, Burckhardt & Vargas-Fonseca, sp. nov. resembles L.inusitata (Tuthill, 1944) (known from Costa Rica, Mexico, and Panama) in the brown body colour with white head and pleura, and white longitudinal stripe on mesonotum. It differs in the forewing pattern, the obovate forewing (vs ovate), the paramere with short (vs long) apical process and sinuous (vs concave) apical 1/2 of posterior margin, the aedeagal head with a weakly sinuous apical margin (vs evenly convex), and the female proctiger with a relatively slender apical portion (vs massive). In the key of Brown and Hodkinson (1988), L.albilinea Rendón-Mera, Burckhardt & Vargas-Fonseca, sp. nov. keys out with L.inusitata. In the key of Burckhardt (1988), the species keys out in couplet 4 with L.digitulata Burckhardt, 1988 (Paraguay) and L.fagarae Burckhardt, 1988 (Brazil, Ecuador, Mexico, Paraguay, USA), from which it differs in the forewing pattern with three brown transverse bands (vs restricted to anal margin or completely or almost completely covering the entire membrane).
*. Triozidae
gen. sp. 1
B6435409-A562-5887-B10B-BEFF81457DC4
Material examined.
Engativá: • 1 ♂; Jardín Botánico de Bogotá; 4.6666, –74.0993; 2553 m; 16.x.2019; S. Vargas leg.; Myrcianthes sp. (Myrtaceae); MPUJ_ENT. Suba: • 1 ♂; Cerro La Conejera; 4.7695, –74.0527; 2674 m; 03.x.2017; J. Duran leg.; Quercushumboldtii (Fagaceae); MPUJ_ENT.
Distribution.
Colombia: Bogotá.
Host plant.
Unknown.
Comments.
The two males at hand probably represent an undescribed species. More material is required for a proper identification.
*. Triozidae
gen. sp. 2
A27DB3F5-A342-5A9A-AF17-26588122FA5B
Material examined.
Rafael Uribe Uribe: • 1 ♀; Parque Palermo Sur; 4.5413, –74.1097; 2692 m; 09.iv.2018; V. Ocampo leg.; Pittosporumundulatum (Pittosporaceae); MPUJ_ENT.
Distribution.
Colombia: Bogotá.
Host plant.
Unknown.
Comments.
The single female at hand fits in the Triozapsyllihabitus species group of Brown and Hodkinson (1988). More material is required for a species identification.
*. Triozidae
gen. sp. 3
FC56B880-2559-587D-816A-8B5B6D08F249
Material examined.
Santa Fe: • 1 ♂; Universidad Distrital; 4.5987, –74.0653; 2713 m; 27.vi.2017; J. Duran leg.; Quercushumboldtii (Fagaceae); MPUJ_ENT. Usaquén: • 1 ♀; Parque Ginebra-Bella Suiza; 4.706, –74.0302; 2591 m; 06.iv.2018; V. Ocampo leg.; Ficus sp. (Moraceae); MPUJ_ENT • 1 ♀; Parque La Vida; 4.7362, –74.0339; 2586 m; 16.iii.2018; V. Ocampo leg.; Ficus sp. (Moraceae); MPUJ_ENT.
Distribution.
Colombia: Bogotá.
Host plant.
Unknown.
Comments.
The three specimens share the conspicuous dark longitudinal stripe on the forewing with species of Triozoida Crawford, 1911, a feature also found in other unrelated species of Triozidae (unpublished NHMB data). More material is required for a species identification.
Discussion and conclusions
During the survey of the arthropod fauna of 33 UGS in Bogotá between 2017 and 2019, 3,825 adult specimens of 21 psyllid species of seven families were found, seven species of which could be identified only to genus. Psyllids were found in all UGS ranging from 1–8 species per UGS. The UGS with the highest number (8 spp.) is Parque Ilarco, followed by Parque El Virrey (7 spp.), Parque Cabañas del Norte (5 spp.) and Universidad Distrital (Pueblo Viejo) (5 spp.) (Table 1). Parque El Virrey serves as a “contemplative” park while the other three UGS are designed for different purposes, primarily recreational use, and two of them, viz. Parque Ilarco and Parque Cabañas del Norte, are small parks with an area of less than 1 hectare each (Alcaldía Mayor de Bogotá 2021, 2022). At first sight this may be surprising, and one would expect that larger UGS specifically designed for conservation purposes would support the largest number of psyllid species. As psyllids are host specific, the presence of the host is the most important factor allowing the occurrence of psyllid species at a particular place. Local psyllid diversity usually reflects local host diversity.
The number of 21 species found during the survey is high in comparison to the number of taxa previously reported from Colombia: 34 identified species plus ten species identified only to genus (Pinzón et al. 2002; Rendón-Mera et al. 2017). This high percentage is, however, an artefact of the poor knowledge of the psyllid fauna of Colombia. From Brazil, whose psyllid diversity is slightly better known than that of Colombia, 163 species have been recorded (Burckhardt and Queiroz 2023). However, the actual number of species is likely to be in excess of 1000 (Burckhardt and Queiroz 2020). Comparing the number of plant species of the two countries with 44,000 species in Brazil and 37,000 species in Colombia (Flora e Funga do Brasil 2023; SiB Colombia 2023), it is reasonable to expect several hundreds of psyllid species in Colombia. The presence of previously undescribed species and the high percentage (38%) of species identified only to genus is a further indication of the hazy state of taxonomic knowledge.
Most specimens (3,800) were taken on plants which we consider hosts (vs 184 on non-hosts) (Table 2). Among the seven species with more than 20 collected specimens, less than 10% of the specimens were collected on non-hosts for four of them, while two species had between 10 and 15% of specimens on non-hosts. In only one species, Acizziauncatoides, almost 40% of specimens were collected on non-hosts, reflecting the high mobility of this invasive species. Of these seven species, two, viz. Calophyaschini and Syncoptozusmexicanus, are known to be monophagous, while the others are oligophagous. The suspected hosts of Leuronotaalbilinea Rendón-Mera, Burckhardt & Vargas-Fonseca, sp. nov. (Clusia sp.), Mastigimascolombianus (Cedrelamontana), M.longicaudatus Rendón-Mera, Burckhardt & Vargas-Fonseca, sp. nov. (Cedrelamontana) and Synozacornutiventris (Ficusamericanasubsp.andicola, Ficus sp.) are native, probably including those not identified to species.
A third of the psyllid species and more than 70% of the specimens found during the survey are exotic: the Australian Acizziaacaciaebaileyanae, A.uncatoides, Ctenarytainaeucalypti, C.spatulata and Glycaspisbrimblecombei, the North American Syncoptozusmexicanus, and the Peruvian Calophyaschini. The high abundance of these species is promoted by urban landscaping practices using exotic tree species (Molina-Prieto and Acosta-Hernández 2018; Bernal et al. 2022; Molina 2022), such as Acaciadecurrens, A.melanoxylon, Schinusareira, and Magnoliagrandiflora. Incidently, species like A.decurrens, A.melanoxylon, Eucalyptusglobulus, and S.areira were among the earliest species used for urban arborisation in Bogotá (Molina-Prieto and Acosta-Hernández 2018). Schinusareira, the host of C.schini, the most abundant psyllid species of the survey (63% of all specimens), constitutes one of the most characteristic trees of Bogotá (Jardín Botánico de Bogotá 2022). Native to Bolivia, northern Chile, and Peru (Bernal et al. 2016; POWO 2023), S.areira was introduced into Bogotá around 1850 (Molina-Prieto and Acosta-Hernández 2018) and now numbers approximately 24,000 trees (Jardín Botánico de Bogotá 2023). Immatures of C.schini induce pit-galls on the leaflets of their host (Pinzón and González 2002; Rendón-Mera et al. 2017), and it is not uncommon to find the heavily galled foliage of S.areira throughout the city (pers. observation of the authors).
There are twice as many native as exotic psyllid species (66%) but only four of these (Leuronotaalbilinea Rendón-Mera, Burckhardt & Vargas-Fonseca, sp. nov., Mastigimascolombianus, M.longicaudatus Rendón-Mera, Burckhardt & Vargas-Fonseca, sp. nov., and Synozacornutiventris) are represented by more than five individuals. Of the other ten species, three are identified to species and the other seven may be undescribed, but more material is needed to confirm this.
The psyllid data from our arthropod survey show that the UGS in Bogotá support a diverse psyllid fauna. The dominance of exotic tree species (Jardín Botánico de Bogotá 2023), however, promotes adventive, potentially invasive psyllids at the expense of the native fauna. For conservation of the native insect fauna, the use of native trees and shrubs should be considered a priority when new UGS are planned.
Supplementary Material
Acknowledgements
We are grateful to Giovanny Fagua, Dimitri Forero, Alejandra Rodríguez (MPUJ), and Francisco Serna (UNAB) for granting access to the collections; Kristian Rubiano for designing the map figure; Jowita Drohojowska, Diana Percy, Igor Malenovský, Alexandra Viertler, and Armando Rosario-Lebrón for useful comments on previous manuscript drafts. DIR-M and SAV-F would like to thank Sara for her assistance at the MPUJ. This study forms part of the work of DIR-M towards a Ph.D. degree at the University of Basel, funded by a Swiss Government Excellence Scholarship for Foreign Students from the Federal Commission (ESKAS), the Freiwillige Akademische Gesellschaft (FAG) and the Maria Parmigiani-Fonds, Basel.
Appendix 1
Table A1.
Green Spaces (UGS) per district (“localidades”). In some instances, the park names as indicated on the collection labels differ from the official names, which are here provided in parentheses. See glossary of terms below, compilated from: Alcaldía Mayor de Bogotá 2021, 2022, IDRD 2023a, 2023b.
| Neighbourhood / UGS | Identifier | Park level and typology | Park classification |
|---|---|---|---|
| Antonio Nariño | |||
| Parque Ciudad Jardín | 15-027 | Structuring (sport) | Zonal |
| Chapinero | |||
| Parque El Chicó | 02-097 | Proximity | |
| Parque El Virrey | 02-014 | Structuring (contemplative) | Zonal |
| Sendero Quebrada La Vieja | – | Protected area | – |
| Engativá | |||
| Jardín Botánico de Bogotá | 10-291 | Structuring (contemplative) | Metropolitan |
| Rafael Uribe Uribe | |||
| Parque Palermo Sur | 18-035 | Proximity | Neighbourhood |
| San Cristóbal | |||
| Parque La Victoria | 04-122 | Structuring (sport) | Zonal |
| Parque Primero de Mayo (Deportivo Primero de Mayo) | 04-196 | Structuring (sport) | Metropolitan |
| Parque San Cristóbal | 04-127 | Structuring (cultural) | Metropolitan |
| Parque Villa de los Alpes | 04-075 | Structuring (sport) | Zonal |
| Santa Fe | |||
| Parque La Independencia (Independencia-Bicentenario) | 03-039 | Structuring (cultural) | Metropolitan |
| Parque Nacional | 03-035 | Structuring (cultural) | Metropolitan |
| Parque Ilarco | 11-007 | Proximity | Neighbourhood |
| Parque Tercer Milenio | 03-085 | Structuring (cultural) | Metropolitan |
| Universidad Distrital (Pueblo Viejo) | – | Structuring (NIA) | Zonal |
| Suba | |||
| Cerro La Conejera | – | Protected area | Ecological |
| Parque Canal Molinos (Parque La Alhambra Sector Sur) | 11-097 | Proximity | Neighbourhood |
| Vacant lot | – | ||
| Usaquén | |||
| CAI Santa Barbara (Urbanización Santa Bárbara Primer Sector) | 01-198 | Proximity | Neighbourhood |
| Parque Altablanca | 01-075 | Structuring (sport) | Zonal |
| Parque Belmira | 01-187 | Proximity | Neighbourhood |
| Parque Cabañas del Norte | 01-244 | Proximity | Neighbourhood |
| Parque CAI Lisboa (Urbanización Ginebra Norte) | 01-120 | Proximity | Neighbourhood |
| Parque Cedro Madeira (El Cedro Maderia) | 01-250 | Proximity | Neighbourhood |
| Parque Contador Norte | 01-083 | Proximity | Neighbourhood |
| Parque Ginebra-Bella Suiza | 01-106 | Proximity | Neighbourhood |
| Parque La Francia (Urbanización Los Molinos) | 01-118 | Proximity | Neighbourhood |
| Parque La Vida | 01-012 | Structuring (recreational) | Zonal |
| Parque Nueva Autopista | 01-064 | Structuring (contemplative) | Zonal |
| Parque Usaquén 2 (Santa Barbara Primer Sector) | 01-087 | Proximity | Neighbourhood |
| Usme | |||
| Parque Chuniza-Famaco (Famaco) | 05-086 | Structuring (cultural) | Zonal |
| Parque La Andrea | 05-004 | Structuring (sport) | Zonal |
| Parque Virrey Sur | 05-016 | Structuring (sport) | Zonal |
Glossary
Contemplative: Spaces designed to promote the richness and diversity of vegetation cover for environmental enjoyment and low-impact human activities. They focus on a contemplative and educational relationship achieved through both permanence and travel. Their main spatial design component is ecological.
Cultural: Spaces designed to serve as meeting places, promoting permanence for the development of civic or cultural activities and outdoor events that highlight cultural values, traditions, and collective memory. The design can incorporate various care and social services, with permanence as the main spatial design component.
Ecological park: Parks that due to their high scenic and/or biological value, as well as their location and accessibility, are intended for the preservation, restoration, and sustainable ecological use of their biophysical elements for environmental education and passive recreation.
Metropolitan park: Parks covering an area of more than 10 hectares, designated for the development of both active and passive recreational uses, aiming to generate landscape and environmental values. The influence of these spaces extends across the entire territory of the city.
Neighbourhood park: Parks with an area of less than one hectare, designed for the recreation, meeting, and integration of the community, addressing the specific needs of the neighbourhoods.
Pocket park: Neighbourhood-type parks but with an area of less than 0.1 hectares, intended primarily for the recreation of children and senior citizens.
Protected area: Spaces with unique value for the natural heritage of the Capital District, ecosystems, biodiversity conservation, and the evolution of culture in the area.
Proximity space: Spaces mostly smaller than one hectare that offer a diverse range of leisure activities at a local scale.
Recreational: Spaces designed to provide facilities for the development of recreational activities, promoting relationships between individuals, the development of skills, and engagement in both free and structured activities. Their main spatial design component is play.
Sport: Spaces designed to accommodate physical activities and sports practice at different levels, including recreational, training, and competitive levels. The activities focus on the physical conditioning of different age groups, either individually or collectively. The main spatial design component of these spaces is sports.
Structuring space: Spaces larger than one hectare that provide a diverse range of leisure activities, supporting both regional and district scales. These spaces contribute not only to human interactions but also to environmental and ecosystemic connectivity.
Zonal park: Parks ranging from 1 to 10 hectares designed to fulfil the active recreational needs of a group of neighbourhoods and can accommodate specialized sport facilities.
References
Alcaldía Mayor de Bogotá (2021) Anexo 03. Inventario de espacio público peatonal y para el encuentro. In: Plan de Ordenamiento Territorial: Bogotá Reverdece 2022–2035. Alcaldía Mayor de Bogotá, Secretaría Distrital de Planeación, Bogotá, 200. https://www.sdp.gov.co/sites/default/files/anexo_03_inventario_espacio_publico_0.pdf [Accessed 24 Nov 2023]
Alcaldía Mayor de Bogotá (2022) Manual de espacio público. Alcaldía Mayor de Bogotá, Secretaría Distrital de Planeación, Bogotá, 476 pp. https://www.sdp.gov.co/sites/default/files/generales/mep_p1-mon.pdf [Accessed 24 Nov 2023]
IDRD (2023a) Buscador de parques. https://portalciudadano.idrd.gov.co/parques/buscar [Accessed 24 Nov 2023]
IDRD (2023b) Parques. Instituto Distrital de Recreación y Deporte. https://sim1.idrd.gov.co/parques-0 [Accessed 24 Nov 2023]
Citation
Rendón-Mera DI, Burckhardt D, Durán J, Ocampo V, Vargas-Fonseca SA (2024) The jumping plant-lice (Hemiptera, Psylloidea) in Urban Green Spaces of Bogotá (Colombia), with descriptions of two new species and redescription of Mastigimas colombianus Burckhardt, Queiroz and Drohojowska. ZooKeys 1209: 199–230. https://doi.org/10.3897/zookeys.1209.117368
Additional information
Conflict of interest
The authors have declared that no competing interests exist.
Ethical statement
No ethical statement was reported.
Funding
DIR-M was funded by the Swiss Government Excellence Scholarship for Foreign Students from the Federal Commission (ESKAS), the Freiwillige Akademische Gesellschaft (FAG) and the Maria Parmigiani-Fonds, Basel.
Author contributions
Conceptualization: VO, DB, JD, SAVF, DIRM. Data curation: DIRM, SAVF, VO, JD. Formal analysis: DIRM, DB. Investigation: DB, DIRM. Methodology: VO, JD. Project administration: VO, JD, SAVF. Resources: SAVF, DB, DIRM, VO, JD. Supervision: SAVF, JD, DB, VO. Validation: DB, DIRM. Visualization: DIRM. Writing - original draft: DIRM. Writing - review and editing: DIRM, VO, SAVF, DB, JD.
Author ORCIDs
Diana Isabel Rendón-Mera https://orcid.org/0000-0003-2553-9486
Daniel Burckhardt https://orcid.org/0000-0001-8368-5268
Sergio Andrés Vargas-Fonseca https://orcid.org/0000-0002-6716-905X
Data availability
All of the data that support the findings of this study are available in the main text.
References
- Alcaldía Mayor de Bogotá (2009) Bogotá. Ciudad de estadísticas. No. 4. Ciudad Verde. Alcaldía Mayor de Bogotá, Secretaría Distrital de Planeación, Bogotá, 31 pp. https://observatorio.dadep.gov.co/documento/bogota-ciudad-de-estadisticas-no-4-ciudad-verde [accessed 24 Nov 2023] [Google Scholar]
- Alcaldía Mayor de Bogotá (2021) Anexo 03. Inventario de espacio público peatonal y para el encuentro. In: Plan de Ordenamiento Territorial: Bogotá Reverdece 2022–2035. Alcaldía Mayor de Bogotá, Secretaría Distrital de Planeación, Bogotá, 200. https://www.sdp.gov.co/sites/default/files/anexo_03_inventario_espacio_publico_0.pdf [accessed 24 Nov 2023]
- Alcaldía Mayor de Bogotá (2022) Manual de espacio público. Alcaldía Mayor de Bogotá, Secretaría Distrital de Planeación, Bogotá, 476 pp. https://www.sdp.gov.co/sites/default/files/generales/mep_p1-mon.pdf [accessed 24 Nov 2023] [Google Scholar]
- Andrade GI, Remolina F, Wiesner D. (2013) Assembling the pieces: A framework for the integration of multi-functional ecological main structure in the emerging urban region of Bogotá, Colombia. Urban Ecosystems 16(4): 723–739. 10.1007/s11252-013-0292-5 [DOI] [Google Scholar]
- Andrade GI, Montenegro F, Remolina F, Wiesner D. (2014) La Estructura Ecológica Principal en lo local. Propuesta de aplicación en la renovación urbana de Fenicia, Las Aguas, Bogotá. Revista Nodo 8: 42–54. https://revistas.uan.edu.co/index.php/nodo/article/view/100/81 [accessed 24 Nov 2023] [Google Scholar]
- Anselm N, Brokamp G, Schütt B. (2018) Assessment of land cover change in peri-urban high Andean environments south of Bogotá, Colombia. Land (Basel) 7(2): 1–28. 10.3390/land7020075 [DOI] [Google Scholar]
- Aronson MFJ, La Sorte FA, Nilon CH, Katti M, Goddard MA, Lepczyk CA, Warren PS, Williams NSG, Cilliers S, Clarkson B, Dobbs C, Dolan R, Hedblom M, Klotz S, Kooijmans JL, Kühn I, Macgregor-Fors I, Mcdonnell M, Mörtberg U, Pyšek P, Siebert S, Sushinsky J, Werner P, Winter M. (2014) A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proceedings of the Royal Society B: Biological Sciences 281. 10.1098/rspb.2013.3330 [DOI] [PMC free article] [PubMed]
- Aronson MFJ, Lepczyk CA, Evans KL, Goddard MA, Lerman SB, MacIvor JS, Nilon CH, Vargo T. (2017) Biodiversity in the city: Key challenges for urban green space management. Frontiers in Ecology and the Environment 15(4): 189–196. 10.1002/fee.1480 [DOI] [Google Scholar]
- Baptiste B, Pinedo-Vasquez M, Gutierrez-Velez VH, Andrade GI, Vieira P, Estupiñán-Suárez LM, Londoño MC, Laurance W, Lee TM. (2017) Greening peace in Colombia. Nature Ecology & Evolution 1(4): 0102. 10.1038/s41559-017-0102 [DOI] [PubMed] [Google Scholar]
- Bastin S, Burckhardt D, Reyes-Betancort JA, Hernández-Suárez E, Ouvrard D. (2023) A review of the jumping plant-lice (Hemiptera: Psylloidea) of the Canary Islands, with descriptions of two new genera and sixteen new species. Zootaxa 5313(1): 1–98. 10.11646/zootaxa.5313.1.1 [DOI] [PubMed] [Google Scholar]
- Bernal R, Gradstein SR, Celis M. (2016) Catálogo de plantas y liquenes de Colombia. Instituto de Ciencias Naturales, Universidad Nacional de Colombia, Bogotá. http://catalogoplantasdecolombia.unal.edu.co [accessed 24 Nov 2023]
- Bernal FA, Matulevich JA, Corredor JA, Coy-Barrera E. (2022) GC/MS-based fingerprinting reveals two chemotypes in the leaf essential oils from Magnoliagrandiflora trees within the urban forestry of a Colombian Andean plateau. Chemistry & Biodiversity 19(9): e202200448. 10.1002/cbdv.202200448 [DOI] [PubMed]
- Brown RG, Hodkinson ID. (1988) Taxonomy and ecology of the jumping plant-lice of Panama (Homoptera: Psylloidea). Lyneborg L (Ed.). E. J. Brill/Scandinavian Science Press Ltd., Leiden, 304 pp. 10.1163/9789004631304 [DOI] [Google Scholar]
- Burckhardt D. (1987) Jumping plant lice (Homoptera: Psylloidea) of the temperate neotropical region. Part 2: Psyllidae (subfamilies Diaphorininae, Acizziinae, Ciriacreminae and Psyllinae). Zoological Journal of the Linnean Society 90(2): 145–205. 10.1111/j.1096-3642.1987.tb01353.x [DOI] [Google Scholar]
- Burckhardt D. (1988) Jumping plant lice (Homoptera: Psylloidea) of the temperate neotropical region. Part 3: Calophyidae and Triozidae. Zoological Journal of the Linnean Society 92(2): 115–191. 10.1111/j.1096-3642.1988.tb00101.x [DOI] [Google Scholar]
- Burckhardt D, Queiroz DL. (2020) Neotropical jumping plant-lice (Hemiptera, Psylloidea) associated with plants of the tribe Detarieae (Leguminosae, Detarioideae). Zootaxa 4733(1): 1–73. 10.11646/zootaxa.4733.1.1 [DOI] [PubMed] [Google Scholar]
- Burckhardt D, Queiroz DL. (2023) Psylloidea. Catálogo Taxonômico da Fauna do Brasil. PNUD. http://fauna.jbrj.gov.br/fauna/faunadobrasil/97523 [accessed 24 Nov 2023]
- Burckhardt D, Queiroz DL, Queiroz EC, Andrade DP, Zanol K, Rezende MQ, Kotrba M. (2011) The jumping plant-louse Mastigimasanjosi spec. nov., a new pest of Toonaciliata (Meliaceae) in Brazil. Spixiana 34: 109–120. [Google Scholar]
- Burckhardt D, Queiroz DL, Drohojowska J. (2013) Revision of the neotropical jumping plant-louse genus Mastigimas (Hemiptera, Psylloidea) attacking Cedrela and Toona species (Meliaceae). Zootaxa 3745(1): 1–18. 10.11646/zootaxa.3745.1.1 [DOI] [PubMed] [Google Scholar]
- Burckhardt D, Ouvrard D, Queiroz D, Percy D. (2014) Psyllid Host-plants (Hemiptera: Psylloidea): resolving a semantic problem. The Florida Entomologist 97(1): 242–246. 10.1653/024.097.0132 [DOI] [Google Scholar]
- Burckhardt D, Cuda JP, Diaz R, Overholt W, Prade P, de Queiroz DL, Vitorino MD, Wheeler GS. (2018) Taxonomy of Calophya (Hemiptera: Calophyidae) Species Associated with Schinusterebinthifolia (Anacardiaceae). The Florida Entomologist 101(2): 178–188. 10.1653/024.101.0205 [DOI] [Google Scholar]
- Burckhardt D, Ouvrard D, Percy DM. (2021) An updated classification of the jumping plant-lice (Hemiptera: Psylloidea) integrating molecular and morphological evidence. European Journal of Taxonomy 736: 137–182. 10.5852/ejt.2021.736.1257 [DOI] [Google Scholar]
- Burckhardt D, Serbina LŠ, Malenovský I, Queiroz DL, Aléné DC, Cho G, Percy DM. (2024) Phylogeny and classification of jumping plant lice of the subfamily Liviinae (Hemiptera: Psylloidea: Liviidae) based on molecular and morphological data. Zoological Journal of the Linnean Society 201(2): 387–421. 10.1093/zoolinnean/zlad128 [DOI] [Google Scholar]
- Carvajal-Castro JD, Ana María Ospina L, Toro-López Y, Anny Pulido G, Cabrera-Casas LX, Guerrero-Peláez S, García-Merchán VH, Vargas-Salinas F. (2019) Birds vs bricks: Patterns of species diversity in response to urbanization in a Neotropical Andean city. PLoS ONE 14(6): 1–20. 10.1371/journal.pone.0218775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz S, Settele J, Brondízio ES, Ngo HT, Agard J, Arneth A, Balvanera P, Brauman KA, Butchart SHM, Chan KMA, Lucas AG, Ichii K, Liu J, Subramanian SM, Midgley GF, Miloslavich P, Molnár Z, Obura D, Pfaff A, Polasky S, Purvis A, Razzaque J, Reyers B, Chowdhury RR, Shin YJ, Visseren-Hamakers I, Willis KJ, Zayas CN. (2019) Pervasive human-driven decline of life on Earth points to the need for transformative change. Science 366(6471): eaax3100. 10.1126/science.aax3100 [DOI] [PubMed]
- Dufour DL, Piperata BA. (2004) Rural-to-urban migration in Latin America: An update and thoughts on the model. American Journal of Human Biology 16(4): 395–404. 10.1002/ajhb.20043 [DOI] [PubMed] [Google Scholar]
- Durán-Prieto J, Ocampo V. (2019) Registro de Diversinervuselegans Silvestri (Hymenoptera: Encyrtidae) para la ciudad de Bogotá, Colombia. Dugesiana 26(1): 51–52. 10.32870/dugesiana.v26i1.7057 [DOI] [Google Scholar]
- Durán-Prieto J, Tulande-Marín E, Ocampo-Flóres V. (2020) Avispas (Insecta: Hymenoptera) asociadas a árboles urbanos de la ciudad de Bogotá, Colombia. Revista Chilena de Entomologia 46(4): 681–698. 10.35249/rche.46.4.20.14 [DOI] [Google Scholar]
- Durán-Prieto J, Olmi M, Tulande-Marín E. (2023) New records of pincer wasps in urban parks of Bogotá city (Colombia) (Hymenoptera: Dryinidae). Osmia 11: 23–26. 10.47446/OSMIA11.5 [DOI] [Google Scholar]
- Elmqvist T, Fragkias M, Goodness J, Güneralp B, Marcotullio PJ, McDonald RI, Parnell S, Schewenius M, Sendstad M, Seto KC, Wilkinson C (Eds.) (2013) Urbanization, biodiversity and ecosystem services: challenges and opportunities. Springer Netherlands, Dordrecht, 453–459. 10.1007/978-94-007-7088-1 [DOI]
- Flora e Funga do Brasil (2023) Flora e Funga do Brasil. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br [accessed 24 Nov 2023]
- Foley JA, DeFries R, Asner GP, Barford C, Bonan G, Carpenter SR, Chapin FS, Coe MT, Daily GC, Gibbs HK, Helkowski JH, Holloway T, Howard EA, Kucharik CJ, Monfreda C, Patz JA, Prentice IC, Ramankutty N, Snyder PK. (2005) Global consequences of land use. Science 309(5734): 570–574. 10.1126/science.1111772 [DOI] [PubMed] [Google Scholar]
- Garizábal-Carmona JA, Mancera-Rodríguez NJ. (2021) Bird species richness across a Northern Andean city: Effects of size, shape, land cover, and vegetation of urban green spaces. Urban Forestry & Urban Greening 64: 127243. 10.1016/j.ufug.2021.127243 [DOI]
- Goddard MA, Dougill AJ, Benton TG. (2010) Scaling up from gardens: Biodiversity conservation in urban environments. Trends in Ecology & Evolution 25(2): 90–98. 10.1016/j.tree.2009.07.016 [DOI] [PubMed] [Google Scholar]
- Halbert SE, Burckhardt D. (2020) The psyllids (Hemiptera: Psylloidea) of Florida: newly established and rarely collected taxa and checklist. Insecta Mundi, 1–88. https://digitalcommons.unl.edu/insectamundi [accessed 24 Nov 2023]
- Hodkinson ID. (1974) The biology of the Psylloidea (Homoptera): A review. Bulletin of Entomological Research 64(2): 325–338. 10.1017/S0007485300031217 [DOI] [Google Scholar]
- Hodkinson ID. (1990) A new species of Syncoptozus Enderlein from Mexico with a redefinition of the subfamily Togepsyllinae Bekker-Migdisova (Insecta: Homoptera: Psylloidea). Journal of Natural History 24(3): 711–717. 10.1080/00222939000770491 [DOI] [Google Scholar]
- Hodkinson ID. (2009) Life cycle variation and adaptation in jumping plant lice (Insecta: Hemiptera: Psylloidea): a global synthesis. Journal of Natural History 43(1–2): 65–179. 10.1080/00222930802354167 [DOI] [Google Scholar]
- Hollis D. (2000) Preliminary list of Psylloidea known from Costa Rican cloud forests (1200–2000 m). In: Nadkarni NM, Wheelwright NT (Eds) Monteverde: ecology and conservation of a tropical cloud forest. Oxford University Press, New York, 573 pp. [Google Scholar]
- Hollis D. (2004) Australian Psylloidea: jumping plantlice and lerp insects. Australian Biological Resorces Study, Canberra, 216 pp. [Google Scholar]
- Ives CD, Lentini PE, Threlfall CG, Ikin K, Shanahan DF, Garrard GE, Bekessy SA, Fuller RA, Mumaw L, Rayner L, Rowe R, Valentine LE, Kendal D. (2016) Cities are hotspots for threatened species. Global Ecology and Biogeography 25(1): 117–126. 10.1111/geb.12404 [DOI] [Google Scholar]
- Jardín Botánico de Bogotá (2022) Crónica – Falso pimiento: un árbol apetecido por un insecto foráneo. https://jbb.gov.co/cronica-falso-pimiento-un-arbol-apetecido-por-un-insecto-foraneo/ [accessed 24 Nov 2023]
- Jardín Botánico de Bogotá (2023) Geoportal Datos Abiertos JBB. https://jardinbotanicobogota-jbb.hub.arcgis.com/ [accessed 24 Nov 2023]
- Jaureguiberry P, Titeux N, Wiemers M, Bowler DE, Coscieme L, Golden AS, Guerra CA, Jacob U, Takahashi Y, Settele J, Díaz S, Molnár Z, Purvis A. (2022) The direct drivers of recent global anthropogenic biodiversity loss. Science Advances 8(45): 1–12. 10.1126/sciadv.abm9982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X, Zhou Z, Jiao L. (2021) Hotspots of land-use change in global biodiversity hotspots. Resources, Conservation and Recycling 174: 105770. 10.1016/j.resconrec.2021.105770 [DOI]
- Lepczyk CA, Aronson MFJ, Evans KL, Goddard MA, Lerman SB, Macivor JS. (2017) Biodiversity in the city: Fundamental questions for understanding the ecology of urban green spaces for biodiversity conservation. Bioscience 67(9): 799–807. 10.1093/biosci/bix079 [DOI] [Google Scholar]
- Makunde PT, Slippers B, Burckhardt D, de Queiroz DL, Lawson SA, Hurley BP. (2020) Current and potential threat of psyllids (Hemiptera: Psylloidea) on eucalypts. Southern Forests 82(3): 233–242. 10.2989/20702620.2020.1813650 [DOI] [Google Scholar]
- Marín-Gómez OH, Garzón Zuluaga JI, Santa-Aristizabal DM, López JH, López-Garcia MM. (2016) Use of urban areas by two emblematic and threatened birds in the central Andes of Colombia. Revista Brasileira de Ornitologia 24(3): 260–266. 10.1007/BF03544353 [DOI] [Google Scholar]
- Martínez DC, Morales I. (2020) Annotated list of beetles (Insecta, Coleoptera) in an urban area of the Eastern Andes of Colombia. Check List 16(6): 1679–1693. 10.15560/16.6.1679 [DOI] [Google Scholar]
- Mauck KE, Gebiola M, Percy DM. (2024) The Hidden Secrets of Psylloidea: Biology, Behavior, Symbionts, and Ecology. Annual Review of Entomology 69(1): 277–302. 10.1146/annurev-ento-120120-114738 [DOI] [PubMed] [Google Scholar]
- McDonald RI, Colbert M, Hamann M, Simkin R, Walsh B. (2018) Nature in the Urban Century: A global assessment of where and how to conserve nature for biodiversity and human wellbeing. The Nature Conservancy, 78 pp. https://www.nature.org/en-us/what-we-do/our-insights/perspectives/nature-in-the-urban-century/
- McKinney ML. (2002) Urbanization, biodiversity, and conservation. Bioscience 52(10): 883–890. 10.1641/0006-3568(2002)052[0883:UBAC]2.0.CO;2 [DOI]
- McKinney ML. (2008) Effects of urbanization on species richness: A review of plants and animals. Urban Ecosystems 11(2): 161–176. 10.1007/s11252-007-0045-4 [DOI] [Google Scholar]
- Molina D. (2022) The forced retirement of a hard worker: The rise and fall of Eucalyptus in Bogotá. Environmental History 27(1): 58–85. 10.1086/717611 [DOI] [Google Scholar]
- Molina-Prieto LF, Acosta-Hernández CF. (2018) Orígenes y evolución de las arborizaciones urbanas en América Latina con énfasis en Bogotá y Medellín. Formas urbanas colonial, republicana y protomoderna. Gestion y Ambiente 21(2): 276–290. 10.15446/ga.v21n2.74906 [DOI] [Google Scholar]
- Myers N, Mittermeler RA, Mittermeler CG, Da Fonseca GAB, Kent J. (2000) Biodiversity hotspots for conservation priorities. Nature 2000 403: 853–858. 10.1038/35002501 [DOI] [PubMed]
- Ocampo Flórez V, Durán Prieto J, Albornoz M, Forero D. (2018) New plant associations for Monalonionvelezangeli (Hemiptera: Miridae) in green urban areas of Bogotá (Colombia). Acta Biologica Colombiana 23(2). 10.15446/abc.v23n2.69374 [DOI]
- OECD (2022) National urban policy review of Colombia. OECD Publishing, Paris, 316 pp. 10.1787/9ca1caae-en [DOI] [Google Scholar]
- Olaya-Arenas P, Durán-Prieto J, Pinzón-Florían OP, Becerra-Guerrero NS. (2022) Insectos del arbolado urbano de Bogotá (Colombia): explorando su diversidad y función. Jardín Botánico de Bogotá José Celestino Mutis, Instituto de Investigación de Recursos Biológicos Alexander von Humboldt., Bogotá, 99 pp. [Google Scholar]
- Olivares TS, Burckhardt D. (1997) Jumping plant-lice of the New World genus Calinda (Hemiptera: Psylloidea: Triozidae). Revue Suisse de Zoologie 104: 231–344. 10.5962/bhl.part.79999 [DOI] [Google Scholar]
- Ouvrard D. (2023) Planthoppers: Psylles Website. http://dbtnt.hemiptest.infosyslab.fr/psyllist/?db=psylles&page=explorer&card=genera&lang=en [accessed 24 Nov 2023]
- Ouvrard D, Chalise P, Percy DM. (2015) Host-plant leaps versus host-plant shuffle: A global survey reveals contrasting patterns in an oligophagous insect group (Hemiptera, Psylloidea). Systematics and Biodiversity 13(5): 434–454. 10.1080/14772000.2015.1046969 [DOI] [Google Scholar]
- Pinzón FOP, Guzmán CM, Navas NF. (2002) Contribución al conocimiento de la biología, enemigos naturales y daños del pulgón del eucalipto Ctenarytainaeucalypti (Homoptera: Psyllidae). Revista Colombiana de Entomologia 28(2): 123–128. 10.25100/socolen.v28i2.9636 [DOI] [Google Scholar]
- Pinzón OP, González RH. (2002) Caracterización biológica, hábitos, enemigos naturales y fluctuación poblacional de Calophyaschini Tuthill, en la especie forestal ornamental Schinus molle L. en Bogotá. Revista Científica, 2002, 137–154.
- POWO (2023) Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. https://powo.science.kew.org/ [accessed 24 Nov 2023]
- Pugh A, Withers T, Sopow S. (2017) New eucalypt feeding insect established in New Zealand. NZ Farm Forestry. https://www.nzffa.org.nz/farm-forestry-model/the-essentials/forest-health-pests-and-diseases/Pests/glycaspis-brimblecombei-red-gum-lerp-psyllid/new-eucalypt-feeding-insect-established-in-new-zealand/ [accessed 24 Nov 2023]
- Queiroz DL, Burckhardt D, Garrastazu MC. (2017) Protocolo de coleta e montagem de psilídeos. Comunicado Técnico Embrapa 393: 1–11. [Google Scholar]
- Rendón-Mera DI, Serna F, Burckhardt D. (2017) Generic synopsis of the jumping plant-lice (Hemiptera: Sternorrhyncha: Psylloidea) from Colombia. Zootaxa 4350(3): 436–468. 10.11646/zootaxa.4350.3.2 [DOI] [PubMed] [Google Scholar]
- Rodas CA, Serna R, Hurley BP, Bolaños MD, Granados GM, Wingfield MJ. (2014) Three new and important insect pests recorded for the first time in Colombian plantations. Southern Forests 76(4): 245–252. 10.2989/20702620.2014.965983 [DOI] [Google Scholar]
- Roncallo J, Ramos Ortega LM, Guerrero RJ, Sierra H. (2022) Las hormigas exóticas en ambientes urbanos de Santa Marta, Colombia. Intropica: 202–217. 10.21676/23897864.4758 [DOI]
- Seto KC, Güneralp B, Hutyra LR. (2012) Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proceedings of the National Academy of Sciences of the United States of America 109(40): 16083–16088. 10.1073/pnas.1211658109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SiB Colombia (2023) Biodiversidad en cifras. Sistema de Información sobre Biodiversidad de Colombia. https://cifras.biodiversidad.co/colombia (November 24, 2023).
- Tzoulas K, Korpela K, Venn S, Yli-Pelkonen V, Kaźmierczak A, Niemela J, James P. (2007) Promoting ecosystem and human health in urban areas using Green Infrastructure: A literature review. Landscape and Urban Planning 81(3): 167–178. 10.1016/j.landurbplan.2007.02.001 [DOI] [Google Scholar]
- United Nations (2019) World urbanization prospects 2018: highlights. United Nations, Department of Economic and Social Affairs, Population Division, New York, 30 pp. https://population.un.org/wup/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All of the data that support the findings of this study are available in the main text.