Abstract
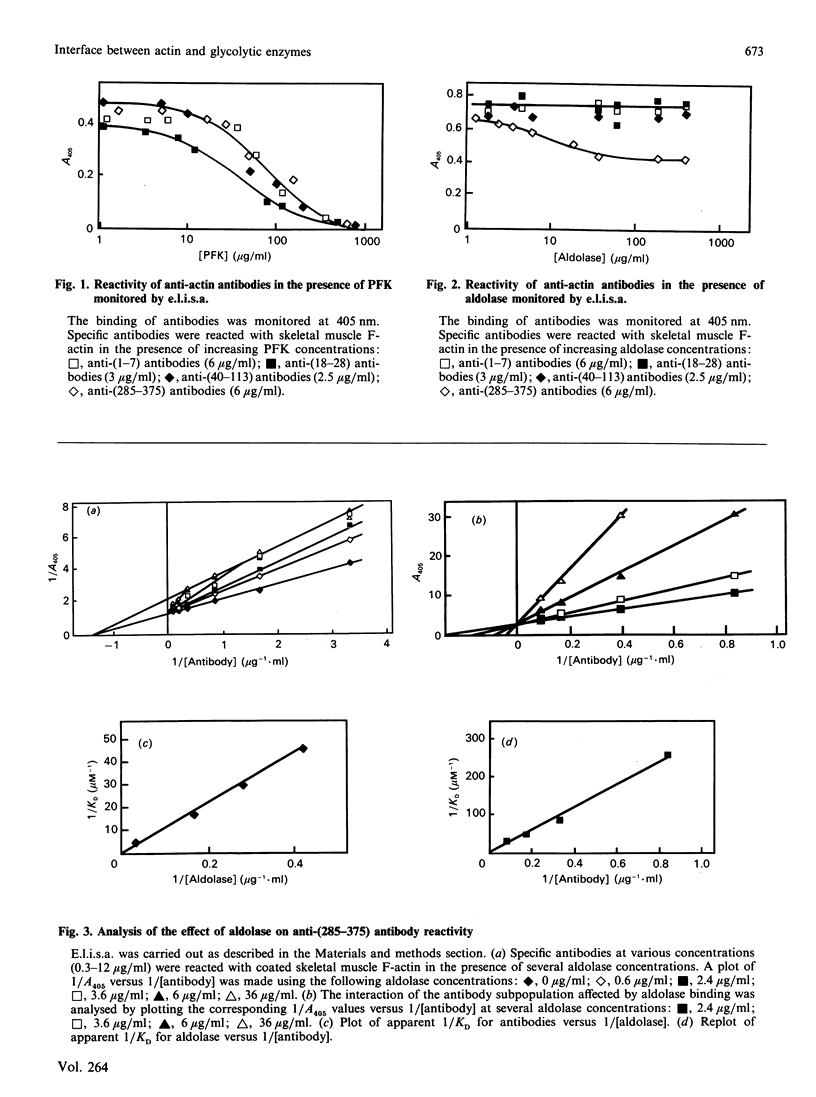
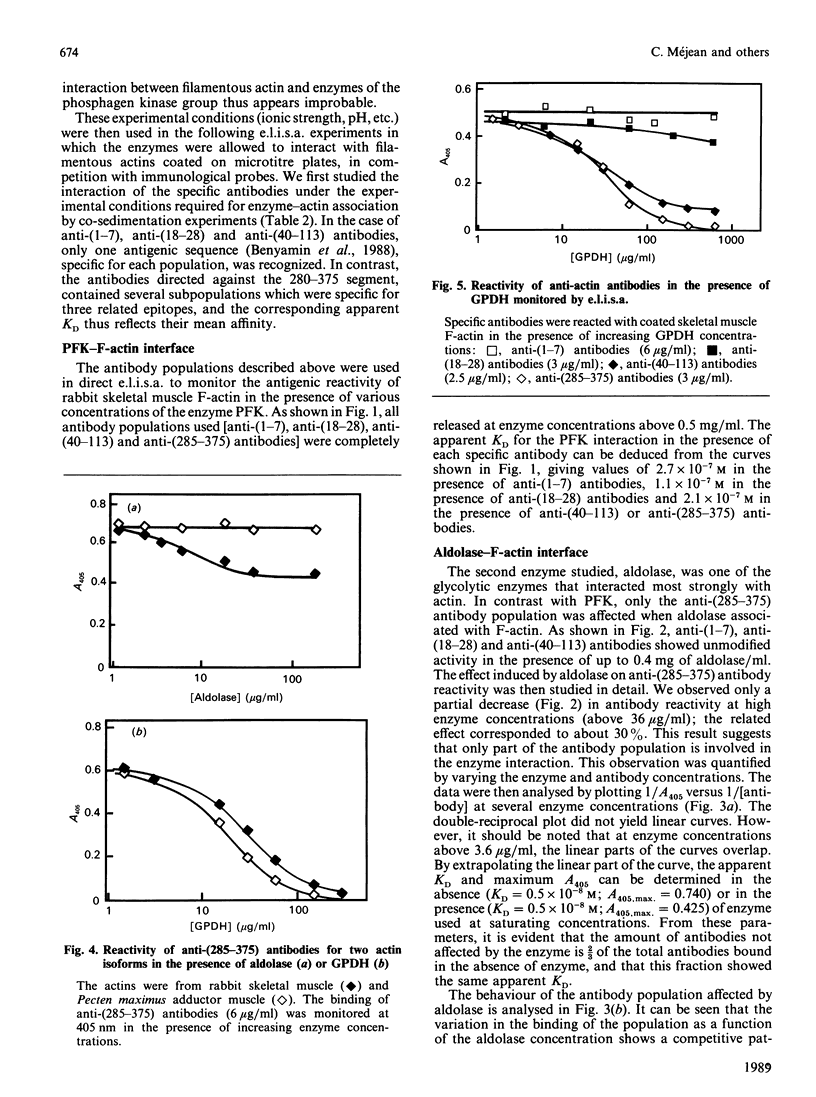
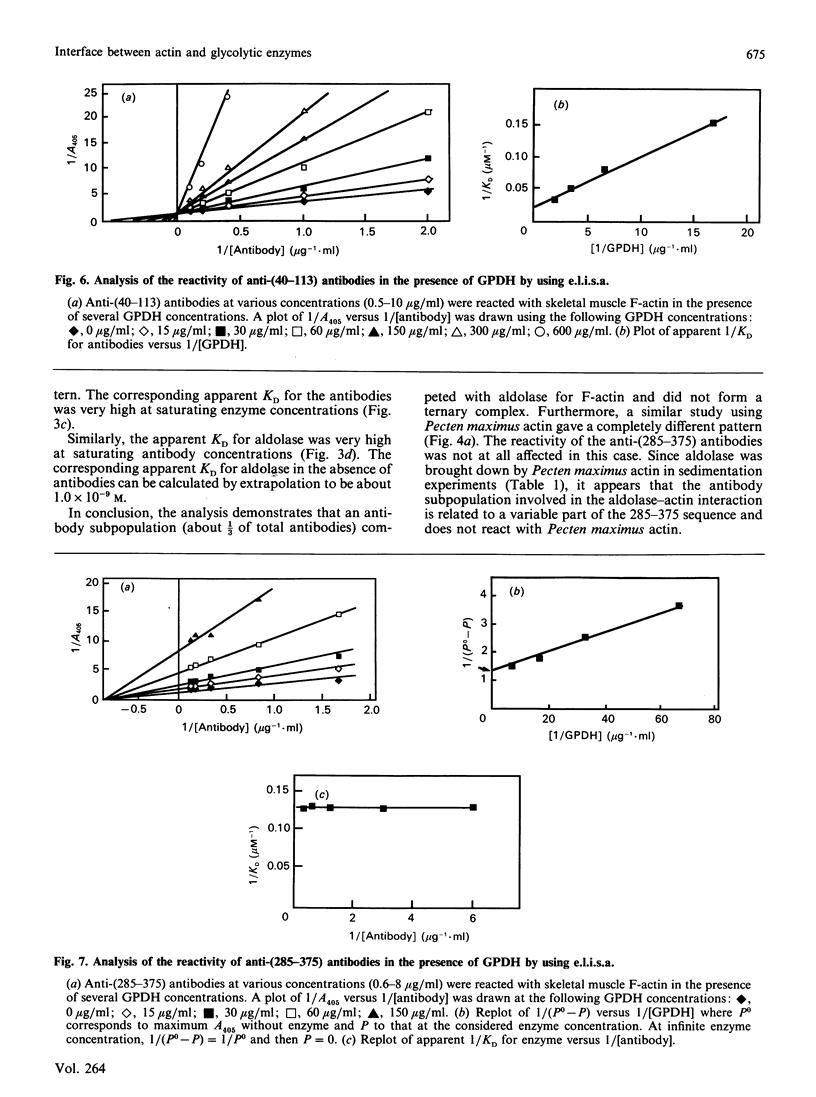
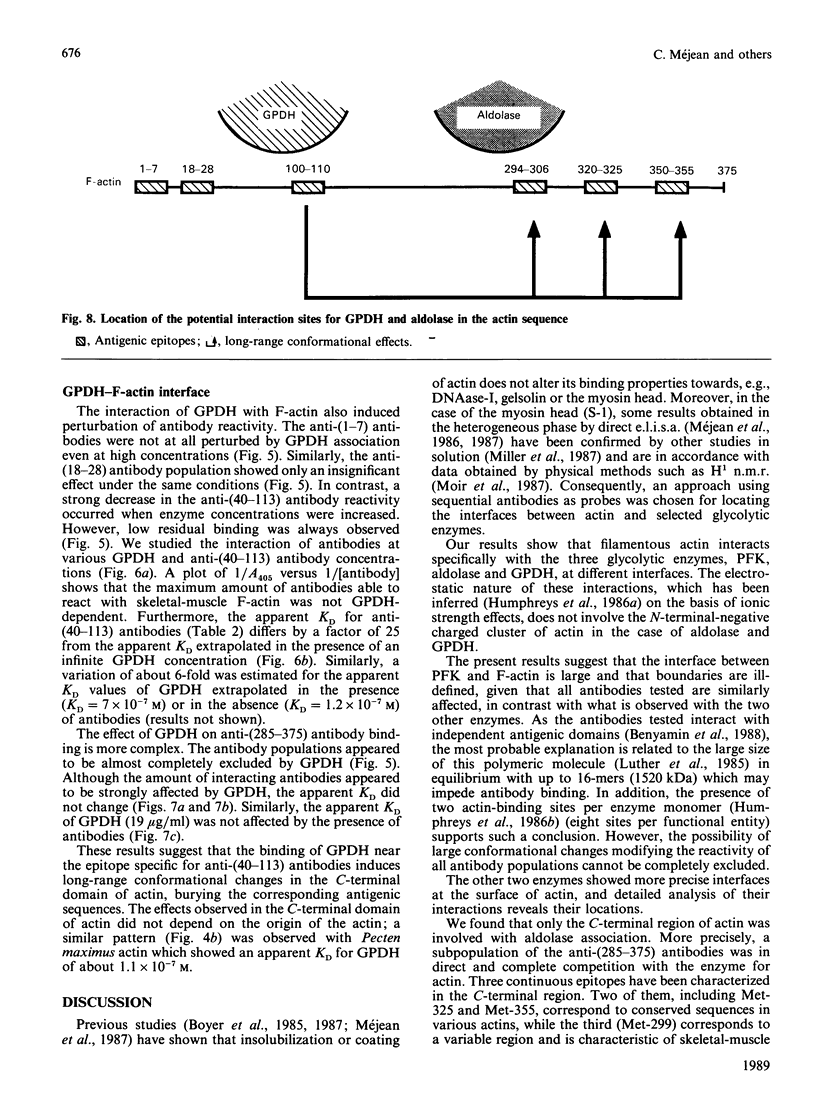
The topology of the interfaces between actin monomers in microfilaments and three glycolytic enzymes (glyceraldehyde-3-phosphate dehydrogenase, aldolase and phosphofructokinase) was investigated using several specific antibodies directed against precisely located sequences in actin. A major contact area for glyceraldehyde-3-phosphate dehydrogenase was characterized in a region near residue 103. This interaction altered, by long-range conformational changes, the reactivity of antigenic epitopes in the C-terminal part of actin. The interface between actin and aldolase appeared to involve a sequence around residue 299 in the C-terminal region of actin. The interaction of phosphofructokinase, in contrast, modified the reactivity of all antibodies tested. Finally, the phosphagen kinases arginine kinase and creatine kinase showed no interaction with the microfilament.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold H., Henning R., Pette D. Quantitative comparison of the binding of various glycolytic enzymes to F-actin and the interaction of aldolase with G-actin. Eur J Biochem. 1971 Sep 13;22(1):121–126. doi: 10.1111/j.1432-1033.1971.tb01522.x. [DOI] [PubMed] [Google Scholar]
- Arrio-Dupont M. An example of substrate channeling between co-immobilized enzymes. Coupled activity of myosin ATPase and creatine kinase bound to frog heart myofilaments. FEBS Lett. 1988 Nov 21;240(1-2):181–185. doi: 10.1016/0014-5793(88)80364-8. [DOI] [PubMed] [Google Scholar]
- Arrio-Dupont M., Coulet P. R., Gautheron D. C. Coupled reaction of immobilized aspartate aminotransferase and malate dehydrogenase. A plausible model for the cellular behaviour of these enzymes. Biochim Biophys Acta. 1985 May 20;829(1):58–68. doi: 10.1016/0167-4838(85)90068-8. [DOI] [PubMed] [Google Scholar]
- Benyamin Y., Roustan C., Boyer M. Anti-actin antibodies. Chemical modification allows the selective production of antibodies to the N-terminal region. J Immunol Methods. 1986 Jan 22;86(1):21–29. doi: 10.1016/0022-1759(86)90260-7. [DOI] [PubMed] [Google Scholar]
- Boyer M., Feinberg J., Hue H. K., Capony J. P., Benyamin Y., Roustan C. Antigenic probes locate a serum-gelsolin-interaction site on the C-terminal part of actin. Biochem J. 1987 Dec 1;248(2):359–364. doi: 10.1042/bj2480359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer M., Roustan C., Benyamin Y. DNAse I-actin complex: an immunological study. Biosci Rep. 1985 Jan;5(1):39–46. doi: 10.1007/BF01117439. [DOI] [PubMed] [Google Scholar]
- Clarke F. M., Masters C. J. On the association of glycolytic enzymes with structural proteins of skeletal muscle. Biochim Biophys Acta. 1975 Jan 13;381(1):37–46. doi: 10.1016/0304-4165(75)90187-7. [DOI] [PubMed] [Google Scholar]
- Clarke F. M., Stephan P., Huxham G., Hamilton D., Morton D. J. Metabolic dependence of glycolytic enzyme binding in rat and sheep heart. Eur J Biochem. 1984 Feb 1;138(3):643–649. doi: 10.1111/j.1432-1033.1984.tb07963.x. [DOI] [PubMed] [Google Scholar]
- Humphreys L., Reid S., Masters C. Evidence for the spatial separation of the binding sites for substrate and for cytoskeletal proteins on the enzyme aldolase. Int J Biochem. 1986;18(1):7–13. doi: 10.1016/0020-711x(86)90003-0. [DOI] [PubMed] [Google Scholar]
- Humphreys L., Reid S., Masters C. Studies on the topographical localization of the binding sites for substrate and for actin on the enzymes, glyceraldehydephosphate dehydrogenase and phosphofructokinase. Int J Biochem. 1986;18(5):445–451. doi: 10.1016/0020-711x(86)90187-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lang A. B., Wyss C., Eppenberger H. M. Localization of arginine kinase in muscles fibres of Drosophila melanogaster. J Muscle Res Cell Motil. 1980 Jun;1(2):147–161. doi: 10.1007/BF00711796. [DOI] [PubMed] [Google Scholar]
- Luther M. A., Hesterberg L. K., Lee J. C. Subunit interaction of rabbit muscle phosphofructokinase: effects of purification procedures. Biochemistry. 1985 May 7;24(10):2463–2470. doi: 10.1021/bi00331a011. [DOI] [PubMed] [Google Scholar]
- Mani R. S., Kay C. M. Physicochemical studies on the creatine kinase M-line protein and its interaction with myosin and myosin fragments. Biochim Biophys Acta. 1976 Dec 22;453(2):391–399. doi: 10.1016/0005-2795(76)90134-3. [DOI] [PubMed] [Google Scholar]
- Masters C. J. Interactions between soluble enzymes and subcellular structure. CRC Crit Rev Biochem. 1981;11(2):105–143. doi: 10.3109/10409238109108700. [DOI] [PubMed] [Google Scholar]
- Mejean C., Boyer M., Labbé J. P., Marlier L., Benyamin Y., Roustan C. Anti-actin antibodies. An immunological approach to the myosin-actin and the tropomyosin-actin interfaces. Biochem J. 1987 Jun 15;244(3):571–577. doi: 10.1042/bj2440571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L., Kalnoski M., Yunossi Z., Bulinski J. C., Reisler E. Antibodies directed against N-terminal residues on actin do not block acto-myosin binding. Biochemistry. 1987 Sep 22;26(19):6064–6070. doi: 10.1021/bi00393a018. [DOI] [PubMed] [Google Scholar]
- Méjean C., Boyer M., Labbé J. P., Derancourt J., Benyamin Y., Roustan C. Antigenic probes locate the myosin subfragment 1 interaction site on the N-terminal part of actin. Biosci Rep. 1986 May;6(5):493–499. doi: 10.1007/BF01116141. [DOI] [PubMed] [Google Scholar]
- Méjean C., Hué H. K., Pons F., Roustan C., Benyamin Y. Cation binding sites on actin: a structural relationship between antigenic epitopes and cation exchange. Biochem Biophys Res Commun. 1988 Apr 15;152(1):368–375. doi: 10.1016/s0006-291x(88)80723-x. [DOI] [PubMed] [Google Scholar]
- Roustan C., Benyamin Y., Boyer M., Cavadore J. C. Structural variations in actins. A study of the immunological reactivity of the N-terminal region. Biochem J. 1986 Jan 1;233(1):193–197. doi: 10.1042/bj2330193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roustan C., Pradel L. A., Kassab R., Van Thoai N. Studies on the partial exchange and overall reactions catalyzed by native and modified arginine kinase from Homarus vulgaris muscle. Biochim Biophys Acta. 1971 Oct;250(1):103–116. doi: 10.1016/0005-2744(71)90124-0. [DOI] [PubMed] [Google Scholar]
- Sigel P., Pette D. Intracellular localization of glycogenolytic and glycolytic enzymes in white and red rabbit skeletal muscle: a gel film method for coupled enzyme reactions in histochemistry. J Histochem Cytochem. 1969 Apr;17(4):225–237. doi: 10.1177/17.4.225. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Wallimann T., Turner D. C., Eppenberger H. M. Localization of creatine kinase isoenzymes in myofibrils. I. Chicken skeletal muscle. J Cell Biol. 1977 Nov;75(2 Pt 1):297–317. doi: 10.1083/jcb.75.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh T. P., Masters C. J., Morton D. J., Clarke F. M. The reversible binding of glycolytic enzymes in ovine skeletal muscle in response to tetanic stimulation. Biochim Biophys Acta. 1981 Jun 11;675(1):29–39. doi: 10.1016/0304-4165(81)90066-0. [DOI] [PubMed] [Google Scholar]



