Abstract
Anterior spinal artery occlusion resulting in bilateral medial medullary infarction (bMMI) and anterior spinal artery syndrome (ASAS) simultaneously has been rarely described. To the best of our knowledge, this is the first report of such occurrence during pregnancy. A 23-year-old preeclamptic parturient at 374/7 weeks underwent an emergent cesarean section after developing gradual neurological deficits. Her symptoms started with a severe occipital headache and progressed to right-hand tingling, left-hand weakness, dyspnea, and elevated blood pressure (165/117 mmHg). Spinal anesthesia was performed by injection of bupivacaine 0.5% with no complications. Twenty minutes into the surgery, after the patient's systolic pressure fell below 85 mmHg, a bolus dose of ephedrine was administered. After a while, the patient presented with sudden respiratory distress and declining consciousness, prompting her immediate intubation. In the intensive care unit, she initially exhibited flaccid quadriplegia, sensory loss, areflexia, upward vertical nystagmus, and some cranial nerve (CN) palsy, including CN 9, 10, and 12, indicative of a medullary-level infarction extending downward. The magnetic resonance imaging (MRI) of the brain revealed a heart-shaped sign in the medulla, suggesting bMMI as a result of anterior spinal artery (ASA) occlusion. During the course of hospitalization, the patient regained the senses of vibration, touch, and proprioception; however, she has remained quadriplegic up to now.
Keywords: Anterior spinal artery, Medullary infarction, Quadriplegia, Pregnancy, Hypertension, Spinal anesthesia, Cesarean section, Case report
1. Introduction
The cervical anterior spinal artery (ASA) arises from the vertebral, anterior, and/or deep cervical arteries and provides blood supply to the anterior two-thirds of the spinal cord [1]. In addition, the paramedian branches of ASA supply the medial part of the medulla [2]. There have been a few documented instances of medullary-level occlusion of the ASA leading to medial medullary infarction, also known as Dejerine syndrome [[3], [4], [5]]. This study's primary objective is to report a case of a 23-year-old pregnant woman with preeclampsia who developed anterior spinal artery syndrome (ASAS) and bilateral medial medullary infarction (bMMI) due to ASA occlusion. To the best of our knowledge, this is the first reported case of such occurrence in a pregnant woman.
2. Case presentation
A 23-year-old nulliparous woman at 374/7 weeks of gestational age was admitted after exhibiting high blood pressure (BP) (130/90 mmHg) in a routine obstetrics visit. The obstetric sonography evaluations of the first, second, and third trimesters showed normal findings. Furthermore, no abnormalities were reported during prenatal checkups, and her pregnancy period was generally uneventful. Also, she had an unremarkable drug and family history. However, the patient had a history of transient left-sided Bell's palsy approximately one year before becoming pregnant, which was treated with an intravenous (IV) corticosteroid.
Due to documenting an asymptomatic increased BP during an outpatient appointment, the patient was referred to an active labor unit for close observation and further investigations. Upon hospitalization, laboratory tests and ultrasound scans were performed, which showed no pathologies. Within 10 hours of admission, she presented with a severe new-onset occipital headache accompanied by a sudden increase in BP (158/117 mmHg) (T0), meeting the criteria for preeclampsia. Simultaneously, she developed a worsening dyspnea (T0) with a respiratory rate of 25 per minute and O2 saturation (SpO2) of 88%. For primary management of dyspnea, oxygen with a mask was applied. Soon after, the patient also complained of noticeable right-hand tingling (T0 + 0:07), followed by a progressive left upper extremity weakness (T0 + 0:10). Her BP promptly increased to 165/117 mmHg (T0 + 0:12). Subsequently, two doses of labetalol (20 mg) were administered with a 10-min interval, along with one dose of magnesium sulfate (4 gr). The motor power of the right and left upper extremities decreased to grades two and four, respectively (based on the Medical Research Council scale), while the lower extremities remained unaffected (Table 1). Eventually, the persistence of her clinical manifestations led to an immediate decision to terminate the pregnancy by an emergent cesarean section (CS) (T0 + 0:22).
Table 1.
Preoperative and postoperative motor examination of four limbs based on the Medical Research Council Scale.
|
Immediately prior to the operation |
Postoperative day 1 |
Postoperative month 12 |
Postoperative month 18 |
|||||
|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | Left | Right | |
| Upper extremities | ||||||||
| Hand palmar flexion | 5 | 4 | 0 | 0 | 1 | 1 | 3 | 3 |
| Wrist dorsiflexion | 5 | 4 | 0 | 0 | 1 | 1 | 3 | 3 |
| Elbow flexion | 5 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| Elbow extension | 5 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| Shoulder flexion | 4 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Shoulder extension | 4 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lower extremities | ||||||||
| Foot Plantar flexion | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Foot dorsiflexion | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 1 |
| Knee flexors | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Knee extensors | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hip flexors | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hip extensors | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
Upon arrival at the operating room, the patient's hemodynamics were adequately under control. Spinal anesthesia was applied by injecting 10 mg of bupivacaine 0.5% at the L4-L5 level. Following that, surgery commenced at T0 + 0:45. Approximately 20 minutes into the surgery, her systolic BP fell below 85 mmHg, and in response, a bolus of 5 mg of IV ephedrine was administered. A live baby weighing 2400 g was born and exhibited Apgar scores of 8 and 10 at 1 and 5 minutes, respectively. Near the end of surgery (T0 + 1:15), the patient manifested respiratory distress (SpO2, 85%), followed by declining consciousness and reduced responsiveness. Correspondingly, she was immediately intubated, and general anesthesia was induced. Subsequently, the patient was placed under mechanical ventilation (MV) with anesthetic maintenance. The estimated blood loss was 750 mL.
After the surgery ended (T0 + 1:45), the attempts to reverse general anesthesia were unsuccessful. Therefore, the patient was transferred to the intensive care unit (ICU) under MV in a stable condition (BP, 110/60 mmHg; heart rate, 78 beats/min; SpO2, 99%). The attending anesthesiologist suspected a compressive spinal lesion, such as a hematoma, as a possible explanation for the patient's condition.
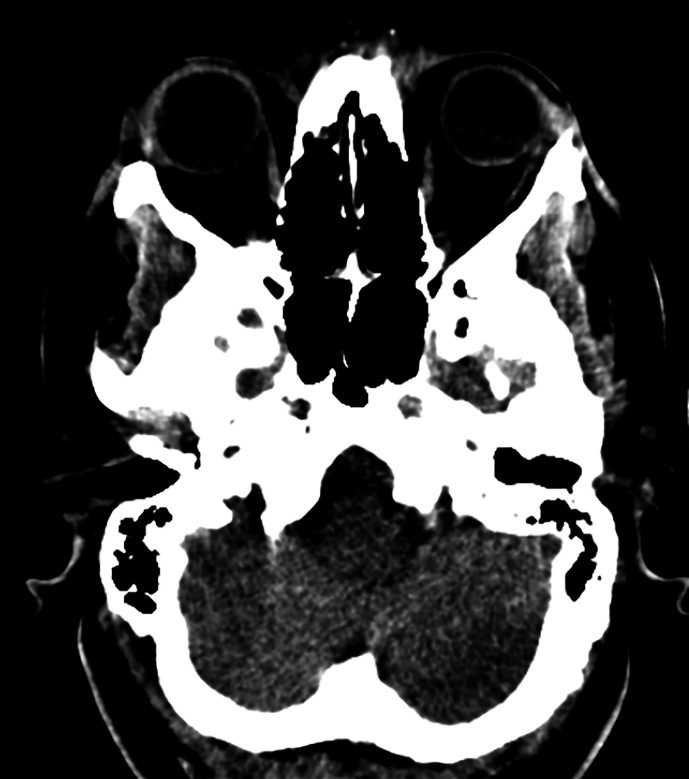
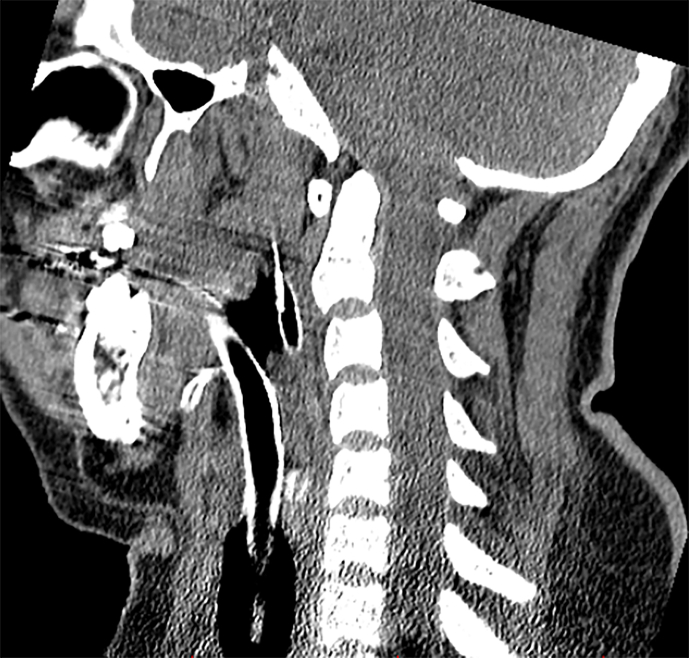
In the ICU, the patient exhibited flaccid quadriplegia as well as fecal and urinary incontinence (T0 + 2:20). She was able to open her eyes spontaneously and follow commands; however, upward vertical nystagmus throughout all directions was detected. The abnormal cranial nerve findings, which were examinable, included the absence of gag reflex, right-sided tongue deviation, and impaired corneal reflexes bilaterally. Moreover, the neurological examination demonstrated bilateral down-going plantar reflexes and absent deep tendon reflexes. The patient suffered from a complete sensory loss and motor block below the region of C2 innervation (Table 1). She was promptly started on supportive treatments to limit spinal cord inflammation (methylprednisolone, 250 mg twice daily), prevent coagulation (heparin, 5000 IU twice daily), and expand blood volume (500 mL of crystalloid). Her spinal and brain computed tomography (CT) were normal, showing no signs of hematoma, infarct lesions, or mass effect (T0 + 3:10) (Supplementary Fig. 1). The evolution of the patient's signs and symptoms up to the first hour of ICU admission is illustrated in Fig. 1.
Fig. 1.
The evolution of the patient's clinical manifestations and the key events until the patient's arrival at the ICU
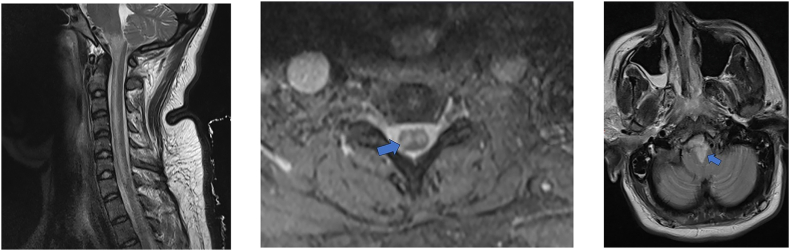
On the first postoperative day (POD 1), a neurological reassessment revealed no improvement in motor deficits; nevertheless, the vertical nystagmus had disappeared. In lab data, the patient's hemoglobin level was 13.3 g/dL, and blood test results for coagulation and inflammatory markers were reassuring. Furthermore, the autoimmune antibodies associated with prothrombotic diseases such as systemic lupus erythematosus, vasculitis, and antiphospholipid syndrome were notably absent. Shortly afterward, she underwent both brain and cervical spine magnetic resonance imaging (MRI) examinations. The T2-weighted MRI images of the cervical spine showed increased signal intensity extending from the medulla to the C7 level (Fig. 2A). From the axial view of the bilateral hyperintensities of the anterior horns, an “owl's eyes” sign was noticeable (Fig. 2B). In addition, the brain MRI confirmed bilateral medial medullary infarction that extended to the pyramidal tracts bilaterally, manifesting a “heart-shaped” sign (Fig. 2C).
Fig. 2.
Postoperative MRI images of the patient. (A) Sagittal T2-weighed MRI image of the cervical spine shows hyperintensity in the areas supplied by the ASA. The cervical vertebrae and the intervertebral disc spaces appeared normal, with no indications of discopathy or spinal cord compression. (B) The cervical spine MRI in the axial plane demonstrates T2 hyperintensity, which is indicative of the “owl's eye” sign (arrow). (C) Axial view of the brain T2-weighted image reveals a “heart-shaped” hyperintensity (arrow), characteristic of bMMI.
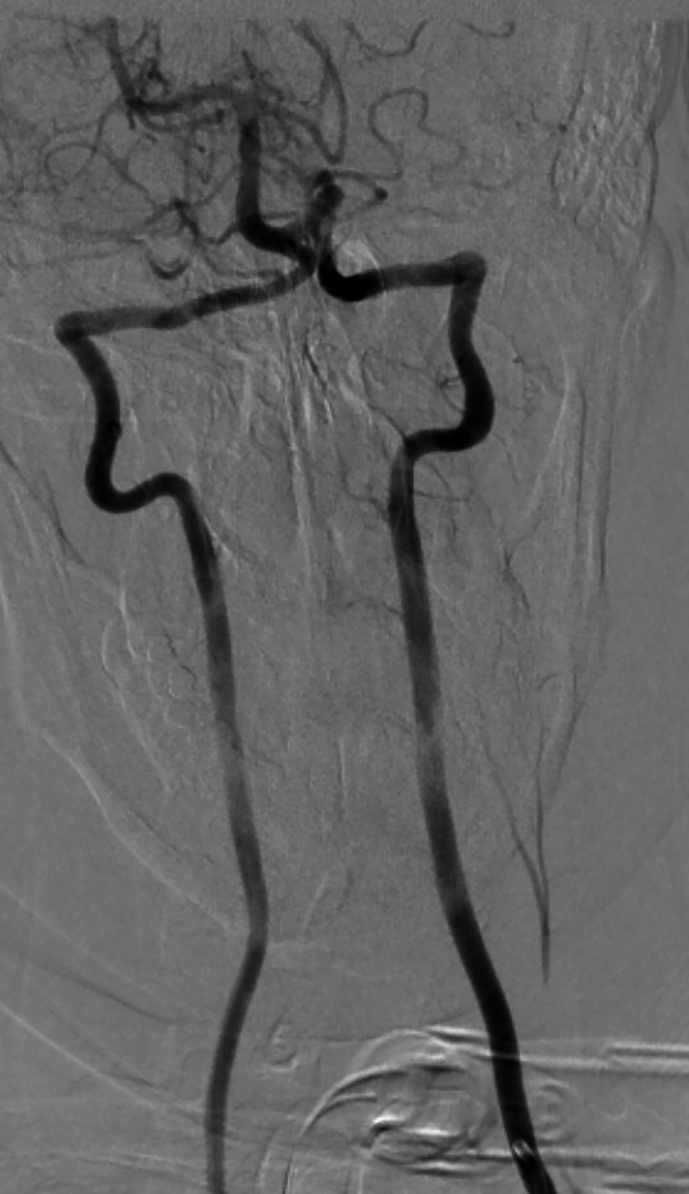
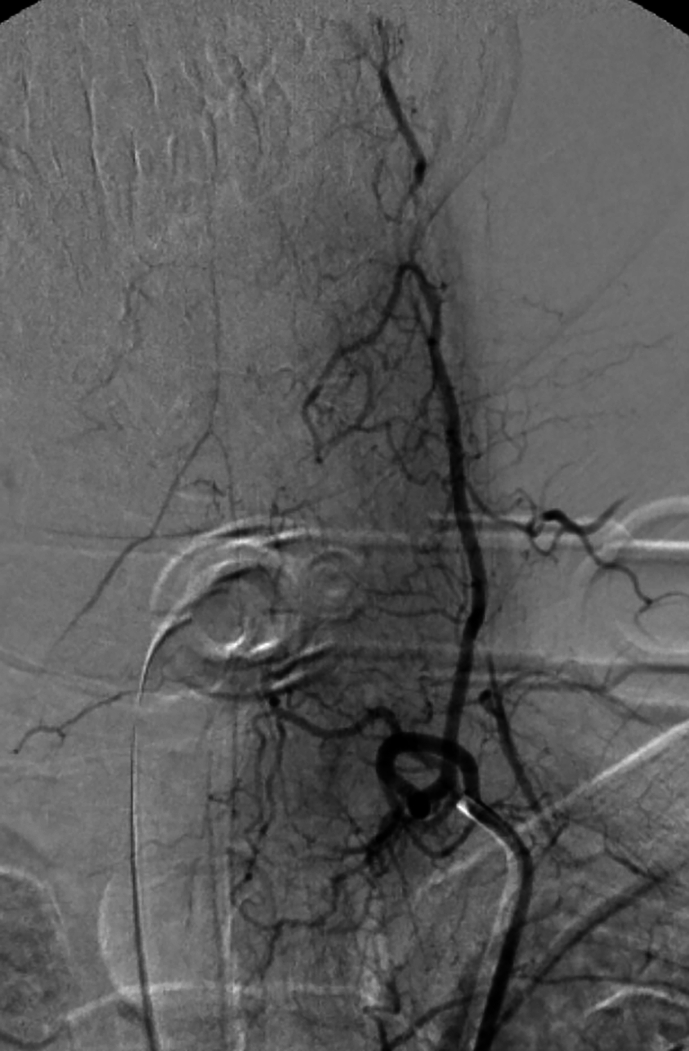
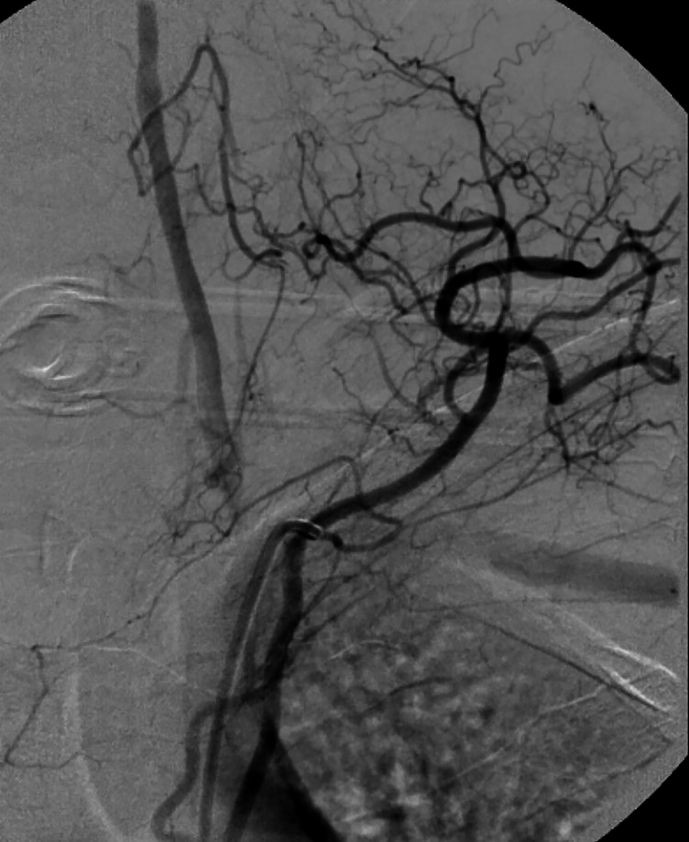
On POD 2, aorta CT angiography was performed and showed no abnormal findings. Lumbar puncture (LP) was not conducted, because the patient's guardian did not give consent. During the first week, the patient showed signs of gradual improvement in vibration, touch, and proprioception sensation. Notably, bilateral Babinski's sign, symmetrical corneal reflexes, and gag reflex were first observed on POD 7. After three days (POD 10), she underwent selective cerebrospinal angiography (Supplementary Fig. 2). The upper part of the ASA was not visualized after both vertebral arteries were injected; however, the ASA was visible in the lower cervical region following the injection of the ascending cervical artery. During the first month of hospitalization, her muscle tone continued to improve gradually, leading to a transition into a state of spastic quadriplegia by POD 30. The patient was transferred to the ward on POD 90.
At ward admission, the patient remained reliant on the ventilator, received nutrition through a percutaneous endoscopic gastrostomy (PEG) tube, and continuously benefited from physiotherapy. A cervical cord MRI 12 months after surgery showed a high signal in the T2-sequence and atrophy of the cervical spinal cord. At the 12-month reevaluation, she tolerated respiration without requiring MV. Her muscle strength also continued to improve during her convalescence at the hospital (Table 1). Eighteen months post-injury, the patient fully regained the ability to swallow; accordingly, the PEG tube was decannulated. Since our patient maintains critical medical conditions, she has not been discharged home yet. Ultimately, her final diagnosis was ASAS and bMMI following ASA occlusion.
3. Discussion
The first confirmed case of ASAS due to ASA occlusion during pregnancy was reported by Barre and D'Andrade in 1938 [6]. Patients with ASAS typically experience paraplegia or quadriplegia, incontinence, and loss of pain and temperature sensation [7]. More seldom, ASA occlusion could lead to bMMI, a rare type of stroke [8]. A heart-shaped sign in the medulla appears as the most distinctive feature on the brain MRIs of such patients [9]. Common symptoms among bMMI patients include spastic or flaccid quadriplegia, dysarthria, and hypoglossal nerve palsy [9]. Several studies have suggested various contributing factors for ASA occlusion such as the hypercoagulable state of pregnancy [10,11], arteriosclerosis [10], the vasoconstrictive effect of drugs [11], varicella zoster virus (VZV) vasculopathy [12], and fibrous cartilage emboli (FCE) [3,4,13].
An increase in the coagulation factors during pregnancy can enhance the chances of thrombotic infarction [14]. Dunn et al. [10] presented the case of a previously healthy 26-year-old woman who developed sudden flaccid paraplegia 20 days post-delivery. Eventually, she was diagnosed clinically with ASAS, and the authors considered the hypercoagulable state of the pregnancy and arteriosclerosis as the main underlying factors. However, in our case, the patient's young age and the progressive deterioration of the neurological symptoms make the aforementioned factors improbable [15].
VZV-induced vasculopathy has been associated with both the spinal cord [12,16,17] and medial medullary infarction [16]. The latent VZV in the cranial nerve ganglia can reactivate, then spread to the arterial adventitia, and finally induce pathological vascular remodeling [17]. McNamara et al. [12] reported on a 28-year-old pregnant woman who presented with a vesicular rash on her back and sudden-onset sharp, constant interscapular pain, followed by bilateral arm weakness gradually deteriorating to flaccid quadriplegia and sensory loss. Consistent with these clinical manifestations, MRI findings were indicative of ASAS. A positive polymerase chain reaction (PCR) result for VZV IgG made VZV vasculopathy the primary cause of ASAS in this case. Even though our patient neither reported a history of skin rash nor recalled being infected with varicella at a younger age, studies have shown that one-third of the patients with VZV vasculopathy experience no prior rash [17]. Altogether, VZV is a possible candidate given the patient's history of Bell's palsy symptoms; yet, as permission to perform LP was not obtained, no evidence can support this hypothesis.
Spinal infarction caused by FCE remains an understudied phenomenon with no definitive pre-mortem diagnosis or treatment up to now [18]. In contrast to thrombotic infarction, FCE predominantly affects young females with a median age of 22 [15,[18], [19], [20], [21]]. In the case published by Uppal et al. [13], a 41-year-old G5p3105 female presented with motor weakness in the lower extremities, sensory loss below the T10 level, and absence of rectal sphincter tone. The autopsy examinations revealed the occlusion of the spinal cord vessels by fibrocartilaginous material. The authors speculated that FCE correlated in some way with pregnancy but were not able to suggest a mechanism. During pregnancy, an increase in vertebral intraosseous pressure caused by hyperlordosis [15,22,23], and elevated intra-abdominal and intra-thoracic pressure [18,24] can trigger the retrograde transit of fibrocartilaginous material through the vessels to the ASA resulting in occlusion [18]. Taking into account our patient's gender, age, pregnancy, and the gradual deterioration of the neurological symptoms with trivial improvement, FCE appears to be a more probable cause than thrombotic infarction, despite the absence of supportive evidence [15,[18], [19], [20], [21]].
The definitive underlying factor causing the ASA occlusion in our patient has not been identified. Nevertheless, some other factors, such as excessive bleeding and certain medications, likely exacerbated the ischemia. Previous studies have demonstrated that elevated BP in acute stroke patients is associated with a poorer prognosis and an increased risk of in-hospital bleeding [25,26]. Our patient presented with hypertension before surgery, which increased intraoperative bleeding. Furthermore, her respiratory distress and subsequent induction of general anesthesia prolonged the surgery, resulting in considerable blood loss. It is noteworthy that excessive blood loss can lead to decreased perfusion, further expanding ischemia in the affected areas [27].
In addition to hypovolemia, the combination of sympathetic neural blockade of bupivacaine and the vasoconstriction effect of ephedrine administered during the surgery may have compromised perfusion in the previously ischemic areas of the spinal cord and medulla. Similar contributors were reported by Zaphiratos et al. in the case of a 32-year-old parturient with ASAS [11]. In essence, although the ischemia patently began prior to the CS, the aforementioned factors have possibly expedited the process.
4. Strengths and limitations
The strengths of this study included the long period of patient follow-up, the careful record of preoperative patient signs and symptoms in chronological order, the adequate access to her clinical documents, and the comprehensive reporting of all relevant findings in the case description. However, we encountered some limitations. For example, we were not allowed to perform an LP, which limited our ability to examine VZV IgG in the cerebrospinal fluid. In addition, conducting an MRI evaluation in the first hours of ICU admission was not possible due to her critical condition. Moreover, we were not able to perform some additional diagnostic tests such as genetic testing for vascular Ehlers-Danlos Syndrome, which increases the chances of aneurysm and dissection in patients.
5. Conclusion
In conclusion, the heart-shaped sign in the brain MRI was indicative of bMMI caused by occlusion of the ASA. The absence of concrete evidence makes it impossible to confirm the exact etiology, yet we believe that the devastating nature of the events is suggestive of the combined effect of multiple factors.
Data availability statement
Data will be made available on request.
Ethics declarations
This study was reviewed and approved by Fasa University of Medical Sciences, with the approval number: IR. FUMS.REC.1401.010. The patient provided informed consent to participate in the study. The patient provided informed consent for the publication of their anonymised case details and images.
CRediT authorship contribution statement
Saman Rabiei: Writing – original draft, Validation, Conceptualization. Hosein Kouchaki: Writing – original draft, Visualization, Supervision, Project administration. Arad Iranmehr: Writing – review & editing. Alireza Ahmadkhani: Writing – original draft, Investigation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We gratefully acknowledge the patient for granting permission to publish this information. We also thank Dr. Abdollah Ghaferi (Department of Neurology) for providing postoperative information, Dr. Hashem Ranjbar (Department of Anesthesiology) for sharing data about the operation room events, Dr. Saeed Kafashan (Department of Radiology) for reviewing the CT scan and MRI images, Dr. Abdolhamid Shariat (Department of Clinical Neurology) for interpreting cerebrospinal angiography, Dr. Zahra Bagheri for gathering information from the patient's file, and Dr. Nastaran Samimi for reviewing our manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e35093.
Abbreviations
- bMMI
bilateral medial medullary infarction
- ASAS
anterior spinal artery syndrome
- CN
cranial nerve
- ASA
anterior spinal artery
- BP
blood pressure
- IV
intravenous
- SpO2
O2 saturation
- CS
cesarean section
- MV
mechanical ventilation
- ICU
intensive care unit
- CT
computed tomography
- MRI
magnetic resonance imaging
- POD
postoperative day
- LP
lumbar puncture
- PEG
percutaneous endoscopic gastrostomy
- VZV
varicella-zoster virus
- FCE
fibrous cartilage emboli
- PCR
polymerase chain reaction
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig. 1.
Postoperative CT scans of the patient. The four images demonstrate normal findings. (A, B) Axial planes of brain CT scan in medullary level. (C) Sagittal view of the cervical spine. (D) Axial view of the cervical spine.
Supplementary Fig. 2.
Postoperative selective cerebrospinal angiography of the patient. (A) The origin of left vertebral, thyrocervical, and costocervical vessels. (B) Anteroposterior injection of the bilateral vertebral arteries. (C) Lateral injection of the left vertebral artery. (D) Anteroposterior injection of the left ascending cervical artery. The anterior spinal artery can be seen in this projection. (E) Anteroposterior injection of the left costocervical artery.
figs3.
figs4.
figs5.
figs6.
figs7.
figs8.
figs9.
References
- 1.Sandoval J.I., De Jesus O. StatPearls [Internet] StatPearls Publishing; Treasure Island (FL): 2023. Anterior spinal artery syndrome.http://www.ncbi.nlm.nih.gov/books/NBK560731/ [cited 2023 Sep 11]. Available from: [Google Scholar]
- 2.Siddik A.B., Gupta V. StatPearls [Internet] StatPearls Publishing; Treasure Island (FL): 2023. Medial medullary syndrome.http://www.ncbi.nlm.nih.gov/books/NBK560590/ [cited 2023 Sep 11]. Available from: [Google Scholar]
- 3.Kase C.S., Varakis J.N., Stafford J.R., Mohr J.P. Medial medullary infarction from fibrocartilaginous embolism to the anterior spinal artery. Stroke. 1983;14(3):413–418. doi: 10.1161/01.str.14.3.413. [DOI] [PubMed] [Google Scholar]
- 4.Naiman J.L., Donohue W.L., Richard J.S. Fatal nucleus pulposus embolism of spinal cord after trauma. Neurology. 1961;11(1) doi: 10.1212/wnl.11.1.83. 83–83. [DOI] [PubMed] [Google Scholar]
- 5.Bodechtel G. On the differential diagnostic difficulties in the border fields between internal medicine and neurology. Munchener medizinische Wochenschrift (1950) 1968;110(16):969–980. [PubMed] [Google Scholar]
- 6.Barre J., d'Andrade C. Paraplegia par ramollissement aigu unisegmentaire de la moelle, survenue au cours de la grossesse. Rev Neurol. 1938;69:133–135. [Google Scholar]
- 7.Klakeel M., Thompson J., Srinivasan R., McDonald F. Baylor University Medical Center Proceedings. Taylor & Francis; 2015. Anterior spinal cord syndrome of unknown etiology; pp. 85–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pongmoragot J., Parthasarathy S., Selchen D., Saposnik G. Bilateral medial medullary infarction: a systematic review. J. Stroke Cerebrovasc. Dis. 2013;22(6):775–780. doi: 10.1016/j.jstrokecerebrovasdis.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Ali L., Amir N., Akhtar N., Elalamy O., Alhatou M. The heart shape-sign in bilateral medial medullary infarction; complicated with quadriparesis and respiratory failure. Clin Med Rev Case Rep. 2021;8:359. [Google Scholar]
- 10.Dunn D.W., Ellison J. Anterior spinal artery syndrome during the postpartum period. Arch. Neurol. 1981;38(4) doi: 10.1001/archneur.1981.00510040089020. 263–263. [DOI] [PubMed] [Google Scholar]
- 11.Zaphiratos V., McKeen D.M., Macaulay B., George R.B. Persistent paralysis after spinal anesthesia for cesarean delivery. J. Clin. Anesth. 2015;27(1):68–72. doi: 10.1016/j.jclinane.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 12.McNamara J.F., Paterson D.L., Allworth A., Presneill J., O'Connell P., Henderson R.D. Re-activation of varicella zoster virus associated with anterior spinal cord stroke in pregnancy. Infectious Diseases. 2016;48(9):705–707. doi: 10.1080/23744235.2016.1185535. [DOI] [PubMed] [Google Scholar]
- 13.Uppal S., Dash S., Sharer L., Clark Lambert W., Heller D.S., Pullicino P. Spinal cord infarction secondary to nucleus pulposus embolization in pregnancy. Mod. Pathol. 2004;17(1):121–124. doi: 10.1038/sj.modpathol.3800037. [DOI] [PubMed] [Google Scholar]
- 14.Yoon H.J. Coagulation abnormalities and bleeding in pregnancy: an anesthesiologist's perspective. Anesthesia and pain medicine. 2019;14(4):371–379. doi: 10.17085/apm.2019.14.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tosi L., Rigoli G., Beltramello A. Fibrocartilaginous embolism of the spinal cord: a clinical and pathogenetic reconsideration. J. Neurol. Neurosurg. Psychiatr. 1996;60(1):55–60. doi: 10.1136/jnnp.60.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mizutani K., Oguri T., Sakurai K., Yuasa H., Takada K. Bilateral medial medullary infarction due to varicella‐zoster virus vasculopathy: a case report. Neurology and Clinical Neuroscience. 2023;11(4):245–247. [Google Scholar]
- 17.Nagel M.A. Varicella zoster virus vasculopathy: clinical features and pathogenesis. J. Neurovirol. 2014;20:157–163. doi: 10.1007/s13365-013-0183-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.AbdelRazek M.A., Mowla A., Farooq S., Silvestri N., Sawyer R., Wolfe G. Fibrocartilaginous embolism: a comprehensive review of an under-studied cause of spinal cord infarction and proposed diagnostic criteria. The Journal of Spinal Cord Medicine. 2016;39(2):146–154. doi: 10.1080/10790268.2015.1116726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sandson T.A., Friedman J.H. Spinal cord infarction: report of 8 cases and review of the literature. Medicine. 1989;68(5):282–292. [PubMed] [Google Scholar]
- 20.Monteiro L., Leite I., Pinto J.A., Stocker A. Spontaneous thoracolumbar spinal cord infarction: report of six cases. Acta Neurol. Scand. 1992;86(6):563–566. doi: 10.1111/j.1600-0404.1992.tb05487.x. [DOI] [PubMed] [Google Scholar]
- 21.Toro G., Roman G.C., Navarro-Roman L., Cantillo J., Serrano B., Vergara I. Natural history of spinal cord infarction caused by nucleus pulposus embolism. Spine. 1994;19(3):360–366. doi: 10.1097/00007632-199402000-00020. [DOI] [PubMed] [Google Scholar]
- 22.Quinn J.N., Breit H., Dafer R.M. Spinal cord infarction due to fibrocartilaginous embolism: a report of 3 cases. J. Stroke Cerebrovasc. Dis. 2019;28(6):e66–e67. doi: 10.1016/j.jstrokecerebrovasdis.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Ritchie J.R. Orthopedic considerations during pregnancy. Clin. Obstet. Gynecol. 2003;46(2):456–466. doi: 10.1097/00003081-200306000-00024. [DOI] [PubMed] [Google Scholar]
- 24.Al-Mujaini A.S., Montana C.C. Valsalva retinopathy in pregnancy: a case report. J. Med. Case Rep. 2008;2:1–3. doi: 10.1186/1752-1947-2-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson T., Waddington A., Ward-Close S., Taub N., Potter J. The predictive role of 24-hour compared to casual blood pressure levels on outcome following acute stroke. Cerebrovasc. Dis. 1997;7(5):264–272. [Google Scholar]
- 26.Ndrepepa G., Groha P., Lahmann A.L., Lohaus R., Cassese S., Schulz‐Schüpke S., et al. Increased bleeding risk during percutaneous coronary interventions by arterial hypertension. Cathet. Cardiovasc. Interv. 2016;88(2):184–190. doi: 10.1002/ccd.26272. [DOI] [PubMed] [Google Scholar]
- 27.Singh U., Silver J.R., Welply N.C. Hypotensive infarction of the spinal cord. Spinal Cord. 1994;32(5):314–322. doi: 10.1038/sc.1994.54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.