Abstract
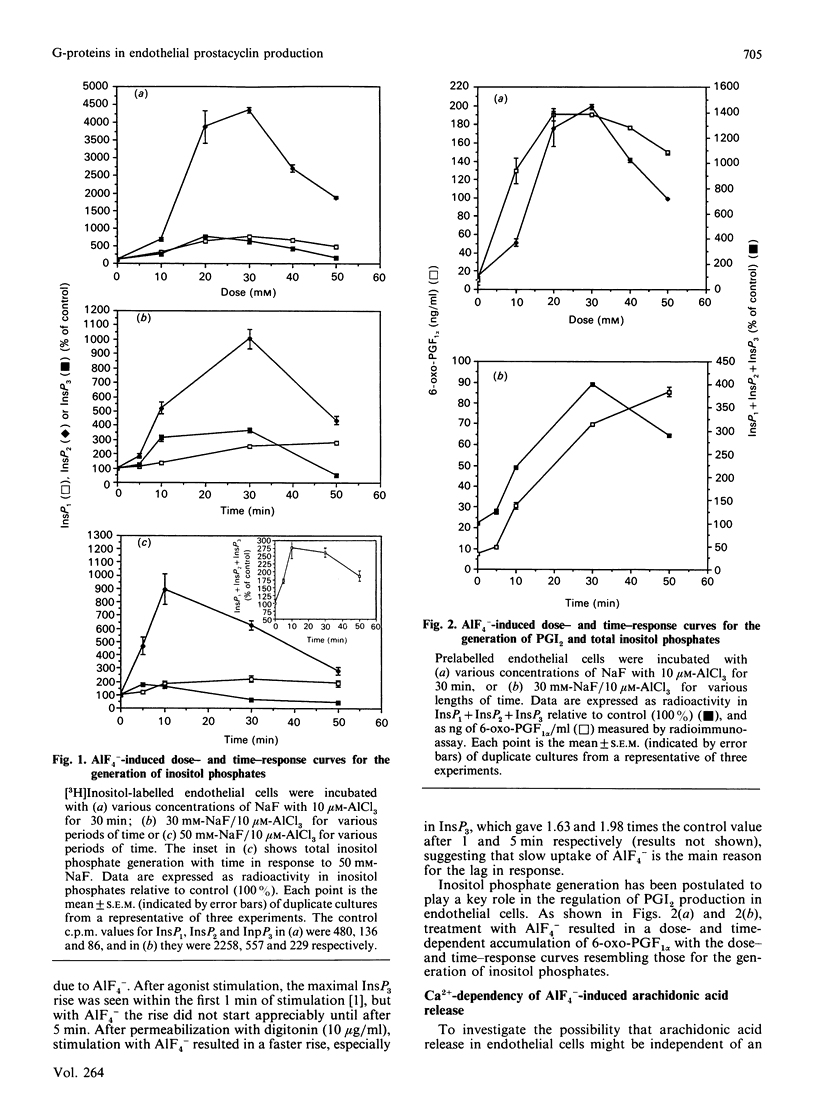
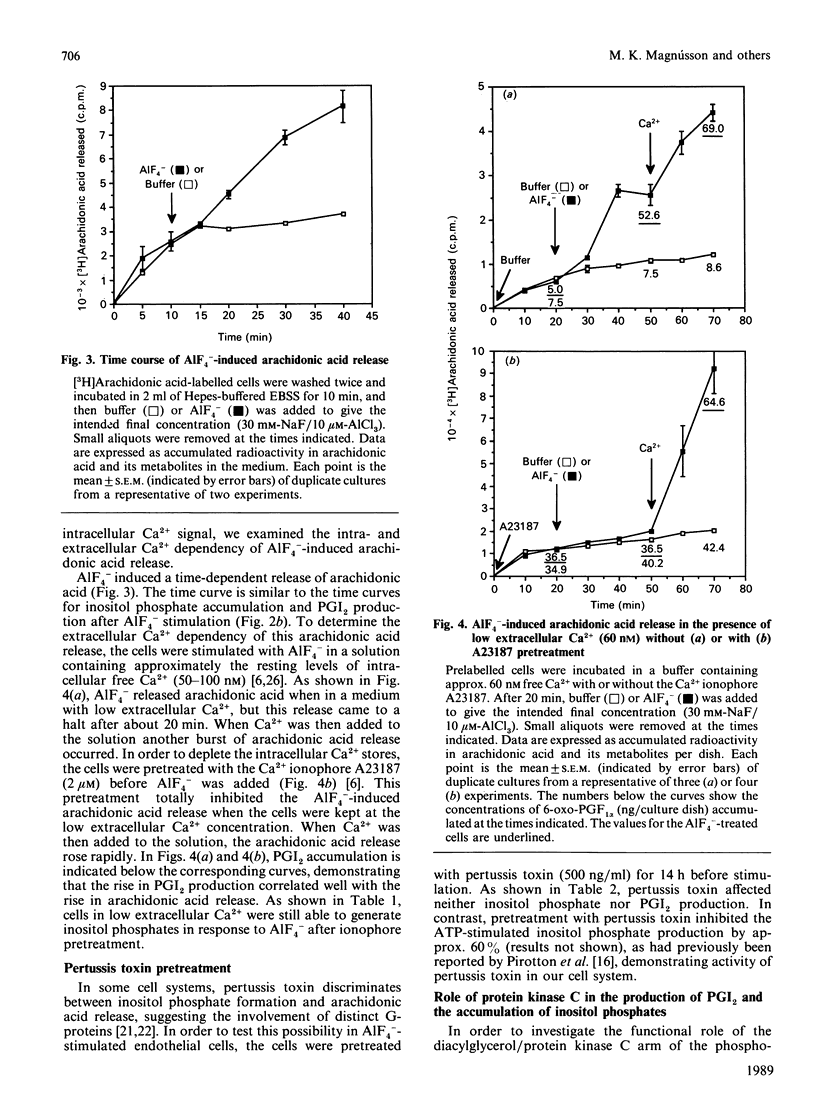
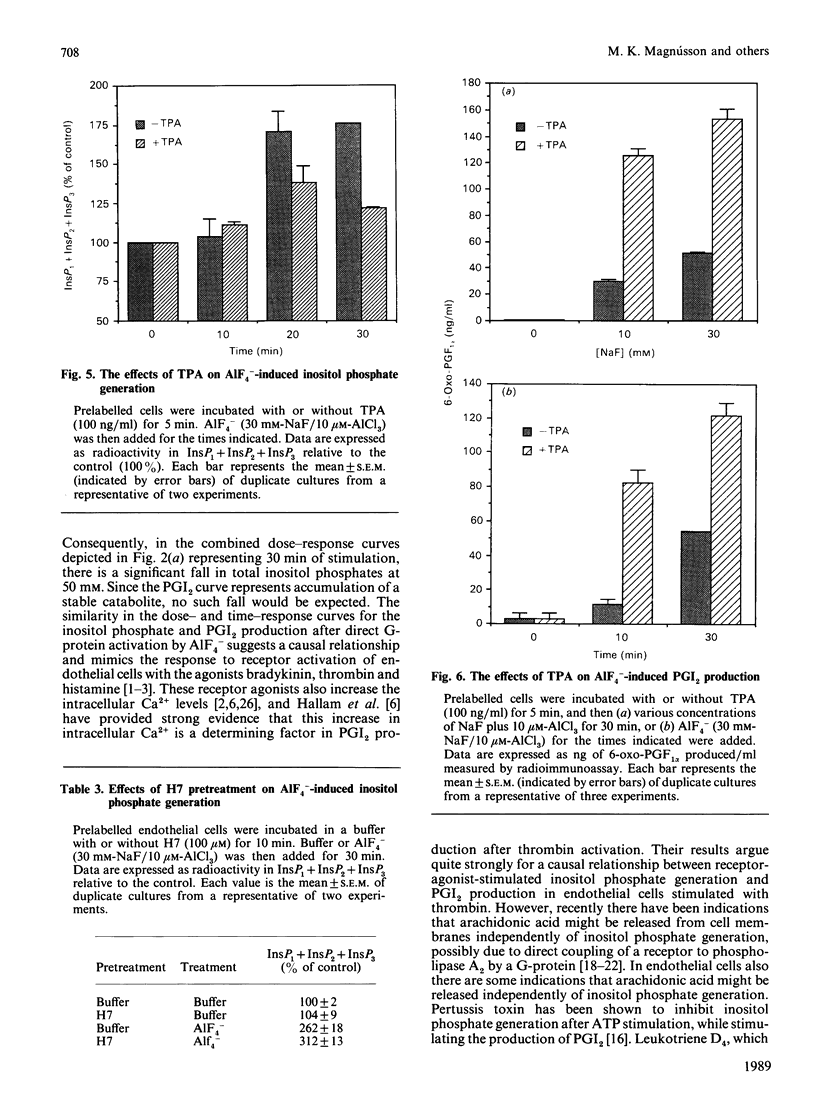
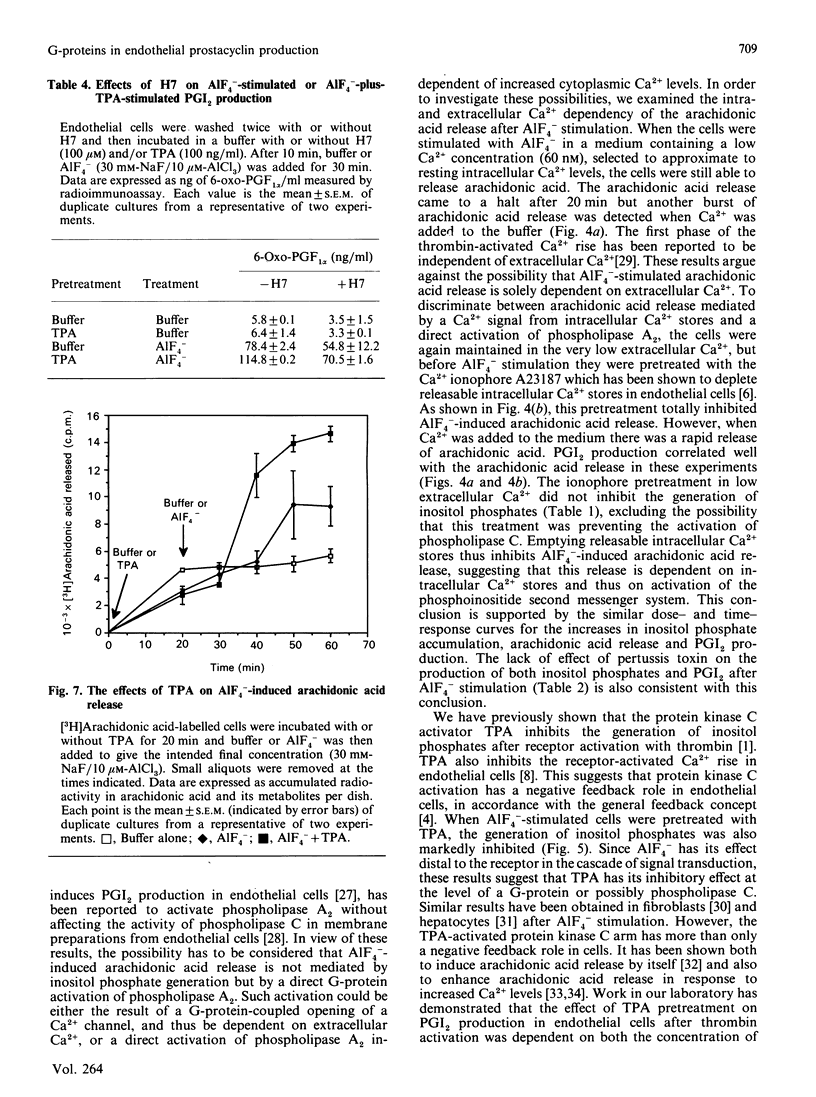
In order to elucidate the role of guanine-nucleotide-binding proteins (G-proteins) in endothelial prostacyclin (PGI2) production, human umbilical vein endothelial cells, prelabelled with either [3H]inositol or [3H]arachidonic acid, were stimulated with the non-specific G-protein activator aluminium fluoride (AlF4-). AlF4- caused a dose- and time-dependent generation of inositol phosphates, release of arachidonic acid and production of PGI2. The curves for the three events were similar. When the cells were stimulated in low extracellular calcium (60 nM), they released [3H]arachidonic acid and produced PGI2, but depleting the intracellular Ca2+ stores by pretreatment with the Ca2+ ionophore A23187 totally inhibited both events, although the cells still responded when extracellular Ca2+ was added. The Ca2+ ionophore did not inhibit the generation of inositol phosphates in cells maintained at low extracellular Ca2+. Pertussis toxin pretreatment (14 h) altered neither inositol phosphate nor PGI2 production in response to AlF4-. To investigate the functional role of the diacylglycerol/protein kinase C arm of the phosphoinositide system, the cells were pretreated with the protein kinase C activator 12-O-tetradecanoylphorbol 13-acetate (TPA) or the protein kinase C inhibitor 1-(5-isoquinolinylsulphonyl)-2-methylpiperazine (H7). TPA inhibited the AlF4(-)-induced inositol phosphate generation but stimulated both the release of arachidonic acid and the production of PGI2. H7 had opposite effects both on inositol phosphate generation and on PGI2 production. These results suggest that AlF4(-)-induced PGI2 production is mediated by a pertussis-toxin-insensitive G-protein which activates the phosphoinositide second messenger system. This production of PGI2 can be modulated by protein kinase C activation, both at the level of inositol phosphate generation and at the level of arachidonic acid release.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Blackmore P. F., Bocckino S. B., Waynick L. E., Exton J. H. Role of a guanine nucleotide-binding regulatory protein in the hydrolysis of hepatocyte phosphatidylinositol 4,5-bisphosphate by calcium-mobilizing hormones and the control of cell calcium. Studies utilizing aluminum fluoride. J Biol Chem. 1985 Nov 25;260(27):14477–14483. [PubMed] [Google Scholar]
- Blackmore P. F., Exton J. H. Studies on the hepatic calcium-mobilizing activity of aluminum fluoride and glucagon. Modulation by cAMP and phorbol myristate acetate. J Biol Chem. 1986 Aug 25;261(24):11056–11063. [PubMed] [Google Scholar]
- Bonventre J. V., Swidler M. Calcium dependency of prostaglandin E2 production in rat glomerular mesangial cells. Evidence that protein kinase C modulates the Ca2+-dependent activation of phospholipase A2. J Clin Invest. 1988 Jul;82(1):168–176. doi: 10.1172/JCI113566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T. A., Capasso E. A. Thrombin and histamine activate phospholipase C in human endothelial cells via a phorbol ester-sensitive pathway. J Cell Physiol. 1988 Jul;136(1):54–62. doi: 10.1002/jcp.1041360107. [DOI] [PubMed] [Google Scholar]
- Burch R. M., Axelrod J. Dissociation of bradykinin-induced prostaglandin formation from phosphatidylinositol turnover in Swiss 3T3 fibroblasts: evidence for G protein regulation of phospholipase A2. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6374–6378. doi: 10.1073/pnas.84.18.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch R. M., Luini A., Axelrod J. Phospholipase A2 and phospholipase C are activated by distinct GTP-binding proteins in response to alpha 1-adrenergic stimulation in FRTL5 thyroid cells. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7201–7205. doi: 10.1073/pnas.83.19.7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter T. D., Hallam T. J., Cusack N. J., Pearson J. D. Regulation of P2y-purinoceptor-mediated prostacyclin release from human endothelial cells by cytoplasmic calcium concentration. Br J Pharmacol. 1988 Dec;95(4):1181–1190. doi: 10.1111/j.1476-5381.1988.tb11754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M. A., Littlejohn D., Conway T. M., Mong S., Steiner S., Crooke S. T. Leukotriene D4 treatment of bovine aortic endothelial cells and murine smooth muscle cells in culture results in an increase in phospholipase A2 activity. J Biol Chem. 1986 Aug 15;261(23):10713–10718. [PubMed] [Google Scholar]
- Clark M. A., Littlejohn D., Mong S., Crooke S. T. Effect of leukotrienes, bradykinin and calcium ionophore (A 23187) on bovine endothelial cells: release of prostacyclin. Prostaglandins. 1986 Jan;31(1):157–166. doi: 10.1016/0090-6980(86)90233-9. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Role of guanine nucleotide binding protein in the activation of polyphosphoinositide phosphodiesterase. Nature. 1985 Apr 11;314(6011):534–536. doi: 10.1038/314534a0. [DOI] [PubMed] [Google Scholar]
- Demolle D., Lecomte M., Boeynaems J. M. Pattern of protein phosphorylation in aortic endothelial cells. Modulation by adenine nucleotides and bradykinin. J Biol Chem. 1988 Dec 5;263(34):18459–18465. [PubMed] [Google Scholar]
- Derian C. K., Moskowitz M. A. Polyphosphoinositide hydrolysis in endothelial cells and carotid artery segments. Bradykinin-2 receptor stimulation is calcium-independent. J Biol Chem. 1986 Mar 15;261(8):3831–3837. [PubMed] [Google Scholar]
- Fuse I., Tai H. H. Stimulations of arachidonate release and inositol-1,4,5-triphosphate formation are mediated by distinct G-proteins in human platelets. Biochem Biophys Res Commun. 1987 Jul 31;146(2):659–665. doi: 10.1016/0006-291x(87)90579-1. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Halenda S. P., Rehm A. G. Thrombin and C-kinase activators potentiate calcium-stimulated arachidonic acid release in human platelets. Biochem J. 1987 Dec 1;248(2):471–475. doi: 10.1042/bj2480471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam T. J., Jacob R., Merritt J. E. Evidence that agonists stimulate bivalent-cation influx into human endothelial cells. Biochem J. 1988 Oct 1;255(1):179–184. doi: 10.1042/bj2550179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam T. J., Pearson J. D., Needham L. A. Thrombin-stimulated elevation of human endothelial-cell cytoplasmic free calcium concentration causes prostacyclin production. Biochem J. 1988 Apr 1;251(1):243–249. doi: 10.1042/bj2510243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halldórsson H., Kjeld M., Thorgeirsson G. Role of phosphoinositides in the regulation of endothelial prostacyclin production. Arteriosclerosis. 1988 Mar-Apr;8(2):147–154. doi: 10.1161/01.atv.8.2.147. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Grulich J., Weksler B. B., Hampel G., Watanabe K. Correlation between thrombin-induced prostacyclin production and inositol trisphosphate and cytosolic free calcium levels in cultured human endothelial cells. J Biol Chem. 1987 Jun 25;262(18):8557–8565. [PubMed] [Google Scholar]
- Lambert T. L., Kent R. S., Whorton A. R. Bradykinin stimulation of inositol polyphosphate production in porcine aortic endothelial cells. J Biol Chem. 1986 Nov 15;261(32):15288–15293. [PubMed] [Google Scholar]
- Litosch I., Wallis C., Fain J. N. 5-Hydroxytryptamine stimulates inositol phosphate production in a cell-free system from blowfly salivary glands. Evidence for a role of GTP in coupling receptor activation to phosphoinositide breakdown. J Biol Chem. 1985 May 10;260(9):5464–5471. [PubMed] [Google Scholar]
- Masters S. B., Martin M. W., Harden T. K., Brown J. H. Pertussis toxin does not inhibit muscarinic-receptor-mediated phosphoinositide hydrolysis or calcium mobilization. Biochem J. 1985 May 1;227(3):933–937. doi: 10.1042/bj2270933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat J., Moreno F., García-Barreno P. Mitogenic activity and inositide metabolism in thrombin-stimulated pig aorta endothelial cells. Biochem Biophys Res Commun. 1987 Jun 30;145(3):1302–1309. doi: 10.1016/0006-291x(87)91579-8. [DOI] [PubMed] [Google Scholar]
- Muldoon L. L., Jamieson G. A., Jr, Villereal M. L. Calcium mobilization in permeabilized fibroblasts: effects of inositol trisphosphate, orthovanadate, mitogens, phorbol ester, and guanosine triphosphate. J Cell Physiol. 1987 Jan;130(1):29–36. doi: 10.1002/jcp.1041300106. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Ohta H., Okajima F., Ui M. Inhibition by islet-activating protein of a chemotactic peptide-induced early breakdown of inositol phospholipids and Ca2+ mobilization in guinea pig neutrophils. J Biol Chem. 1985 Dec 15;260(29):15771–15780. [PubMed] [Google Scholar]
- Paris S., Pouysségur J. Further evidence for a phospholipase C-coupled G protein in hamster fibroblasts. Induction of inositol phosphate formation by fluoroaluminate and vanadate and inhibition by pertussis toxin. J Biol Chem. 1987 Feb 15;262(5):1970–1976. [PubMed] [Google Scholar]
- Parker J., Daniel L. W., Waite M. Evidence of protein kinase C involvement in phorbol diester-stimulated arachidonic acid release and prostaglandin synthesis. J Biol Chem. 1987 Apr 15;262(11):5385–5393. [PubMed] [Google Scholar]
- Pirotton S., Erneux C., Boeynaems J. M. Dual role of GTP-binding proteins in the control of endothelial prostacyclin. Biochem Biophys Res Commun. 1987 Sep 30;147(3):1113–1120. doi: 10.1016/s0006-291x(87)80185-7. [DOI] [PubMed] [Google Scholar]
- Rotrosen D., Gallin J. I. Histamine type I receptor occupancy increases endothelial cytosolic calcium, reduces F-actin, and promotes albumin diffusion across cultured endothelial monolayers. J Cell Biol. 1986 Dec;103(6 Pt 1):2379–2387. doi: 10.1083/jcb.103.6.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slivka S. R., Insel P. A. Alpha 1-adrenergic receptor-mediated phosphoinositide hydrolysis and prostaglandin E2 formation in Madin-Darby canine kidney cells. Possible parallel activation of phospholipase C and phospholipase A2. J Biol Chem. 1987 Mar 25;262(9):4200–4207. [PubMed] [Google Scholar]
- Slivka S. R., Insel P. A. Phorbol ester and neomycin dissociate bradykinin receptor-mediated arachidonic acid release and polyphosphoinositide hydrolysis in Madin-Darby canine kidney cells. Evidence that bradykinin mediates noninterdependent activation of phospholipases A2 and C. J Biol Chem. 1988 Oct 15;263(29):14640–14647. [PubMed] [Google Scholar]
- Thorgeirsson G., Robertson A. L., Jr, Cowan D. H. Migration of human vascular endothelial and smooth muscle cells. Lab Invest. 1979 Jul;41(1):51–62. [PubMed] [Google Scholar]
- Whatley R. E., Nelson P., Zimmerman G. A., Stevens D. L., Parker C. J., McIntyre T. M., Prescott S. M. The regulation of platelet-activating factor production in endothelial cells. The role of calcium and protein kinase C. J Biol Chem. 1989 Apr 15;264(11):6325–6333. [PubMed] [Google Scholar]