Abstract
Purpose
To evaluate retinal thickness changes of individual retinal layers using spectral-domain optical coherence tomography (SD-OCT) after uneventful cataract surgery over a 3-months period.
Design
Prospective cohort study.
Methods
41 patients who underwent uneventful cataract surgery were included. Retinal SD-OCT images of both eyes were acquired preoperatively, 1 day after surgery as well as 1 month and 3 months postoperatively. Changes of retinal layer thickness were estimated after semi-automated segmentation for the following individual layers in the central subfield (CS, 1 mm) and inner ring (IR, 1–3 mm) of the early treatment diabetic retinopathy study (ETDRS) grid: retinal nerve fiber layer (RNFL), ganglion cell layer (GCL), inner plexiform layer (IPL), RNFL-GCL-IPL complex, inner nuclear layer (INL), outer plexiform layer (OPL), INL-OPL complex, outer nuclear layer (ONL), inner retina layer (IRL) and the total retina (TR). Furthermore, a sub-analysis with exclusion of patients devoid CME and an analysis in regard of patient age, lens status of the fellow eye, best corrected visual acuity and duration of surgery was conducted.
Results
This study found significant alterations in all analysed retinal layers except for the RNFL (p = 0.33) and the GCL (p = 0.06) in the central subfield and the INL-OPL complex (p = 0.07) in the inner ring over the 3-months period (all p < 0.05). Retinal thickness decreases on the first postoperative day, followed by a significant increase 1 month after surgery and subsequent reduction at 3 months following uneventful cataract surgery could be observed.
Conclusion
These results assume the hypothesis that the apex of inflammatory response, characterized by an augmentation in the thickness of individual retinal layers, occurs around 1 month after uneventful cataract surgery, and subsequently experience a reduction in activity. Therefore, we suggest that additional therapy for cystoid macular edema does not have to be initiated as early as the first month in mild cases.
1. Introduction
Cystoid macular edema (CME) is one of the most prevalent postoperative complications after otherwise uncomplicated cataract surgery. It usually develops within 3 months postoperatively, with a peak incidence at 4–6 weeks after surgery [[1], [2], [3]].
It is considered the most important cause of suboptimal visual acuity within the first weeks postoperatively. Most cases are self-limiting and patients experience no or only minimal reduction in visual acuity [4]. Up to 6 % of nondiabetic subjects develop visual complaints and suffer from CME [5,6].
Etiologically, CME occurs due to the breakdown of the inner blood-retina barrier secondary to an inflammatory process mediated by prostaglandins and other inflammatory mediators. These inflammatory mediators result from the surgical trauma to lens epithelial cells, the iris and ciliary body and then diffuse into the vitreous cavity and retina leading to the breakdown of the blood-retinal barrier (BRB) and subsequent leakage of fluids across the retinal vessel wall and into the perifoveal retinal tissues [[7], [8], [9]].
The development of spectral-domain optical coherence tomography (SD-OCT) was a milestone in ophthalmological clinical practice and retinal research. Using OCT disclosed that pseudophakic CME occurs in a significantly higher extent than previously thought, ranging from 4 to 41 % [[10], [11], [12]].
The investigations conducted so far [[11], [12], [13], [14], [15], [16], [17]] have yielded incongruous findings regarding alterations in retinal layer thickness. These findings have encompassed variations within the inner retinal layers, changes in the outer retinal layers, as well as modifications across all retinal layers.
The objective of our investigation is to assess the average alterations in retinal layer thickness following uncomplicated phacoemulsification surgery in healthy eyes, employing SD-OCT scans up to 3-months postoperatively considering patient age, lens status of the fellow eye (phakic and pseudophakic), duration of surgery and best corrected visual acuity preoperative.
2. Methods
We recruited 41 patients who were scheduled for cataract surgery between February 2016 and October 2017 at the Department of Ophthalmology, Medical University Graz, Austria.
The exclusion criteria comprised the following conditions: macular pathologies, retinal vascular occlusion, a prior history of ocular disorders (inclusive of uveitis, eye trauma, glaucoma), previous ocular surgeries, systemic disorders such as diabetes and systemic inflammation as well as ongoing usage of topical anti-inflammatory medications or systemic anti-inflammatory agents as well as topical glaucoma agents, and intraoperative complications such as posterior capsular rupture, vitreous loss, iris prolapse, and impossibility of SD-OCT examination due to dense cataracts at baseline examination.
Cataract surgery was performed by phacoemulsification and intraocular lens (IOL) insertion within the capsular bag. At the end of the surgery, 1 mg cefuroxime was injected into the anterior chamber in all patients (Curocef®, Glaxo Wellcome Pharma, Vienna, Austria). All operations were performed by 13 experienced cataract surgeons with at least 323 operations before the respective cataract procedure.
Postoperatively the patients were locally treated with neomycin and betamethasone drops (Betnesol N®, TubiluxPharma, Rome, Italy) 5 times per day in a tapered frequency for 5 weeks and with a dexamethasone and gentamycin ointment (Dexagenta®, Croma Pharma GmbH, Leobendorf, Austria) once a day in the evening for 1 week.
2.1. SD-OCT and grading modalities
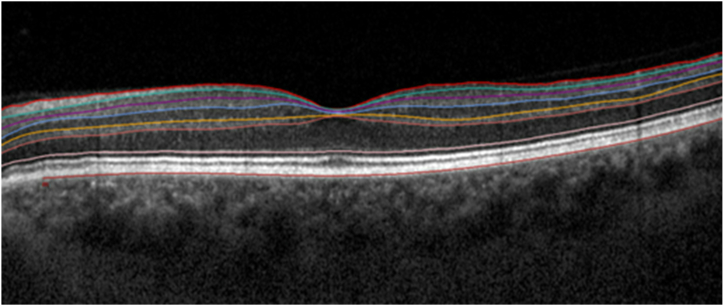
Retinal layer thickness analysis was conducted using SD-OCT scans using volume scanning with 31 B-scans and a wavelength of 825 nm. Images have a resolution of 7 μm axially x 14 μm laterally and a distance of 240 μm between sections and are centered on the anatomical fovea perioperative, one day, one month and three months after cataract surgery. These scans were processed and examined using a Heidelberg SpectralisTM device (HEYEX version 2.5.6, Heidelberg Engineering, Heidelberg, Germany). The baseline assessment was carried out before cataract surgery, while follow-up assessments occurred at one day, one month, and three months post-surgery, utilizing the device's built-in follow-up mode. Image acquisition was followed by an automated intraretinal layer segmentation process carried out by the integrated Spectralis software, with manual adjustments made as necessary by individuals JG and DD. Retinal thickness measurements were derived from the "early treatment diabetic retinopathy study" (ETDRS) grid, specifically focusing on the 1 mm central subfield and the 1–3 mm inner ring. The retinal layer thickness of the inner ring was evaluated in the 4 quadrants (inferior, superior, nasal and temporal). These 4 quadrants were combined, and the mean thickness of individual retinal layers was calculated to ensure better readability of the study. Analysis of the SD-OCT scans involved evaluating changes in the mean thickness of the retinal layers, as described below (Fig. 1).
Fig. 1.
Boundaries of segmented retinal layers on spectral domain optical coherence. The segmented boundaries in order from top to bottom are: Internal Limiting Membrane (ILM); Retinal Nerve Fiber Layer (RNFL) outer border; Ganglion Cell Layer (GCL) outer border; Inner Plexiform Layer (IPL) outer border; Inner Nuclear Layer (INL) outer border; Outer Plexiform Layer (OPL) outer border; External Limiting Membrane (ELM) and Bruch's membrane.
2.2. Boundaries and layers
-
•
Retinal Nerve Fiber Layer (RNFL) – region between the inner limiting membrane (ILM) and the outer boundary of the RNFL.
-
•
Ganglion Cell Layer (GCL) – region between the outer boundary of the RNFL and the outer boundary of the GCL.
-
•
Inner Plexiform Layer (IPL) – region between the outer boundary of the GCL and the outer boundary of the IPL.
-
•
Superficial capillary plexus – region between the ILM and the outer boundary of the IPL.
-
•
Inner Nuclear Layer (INL) – region between the outer boundary of the IPL and the outer boundary of the INL.
-
•
Outer Plexiform Layer (OPL) – the region between the outer boundary of the INL and the outer boundary of the OPL.
-
•
Deep capillary plexus – region between the outer boundary of the IPL and the outer boundary of the OPL.
-
•
Outer Nuclear Layer (ONL) – region between the outer boundary of the OPL and external limiting membrane (ELM).
-
•
Inner Retinal Layer (IRL) – region between the Inner ILM and the ELM.
-
•
Total Retina (TR) – region between the ILM and Bruch's membrane.
CME was identified as a distinct region of any reduced reflectivity within the neurosensory retina using SD-OCT. Patients presenting with CME (5 patients) were excluded in a subsequent subgroup-analysis to more comprehensively evaluate both the subclinical and clinical progression (Table 1, Table 2).
Table 1.
Estimated changes in the individual retinal layer thickness [μm] in the central subfield and the inner ring analysed accross the 4 visits with exclusion of patients with occurrence of cystoid macular edema adjusted for patient age, best corrected visual acuity, lens status of the fellow eye (phakic and pseudophakic) and duration of surgery.
| retinal layers | ETDRS grid | Baseline values of the mean thickness [μm] ± standard deviation |
p-value | |||
|---|---|---|---|---|---|---|
| Preoperative n = 35 | day 1 postoperative n = 25 |
month 1 postoperative n = 36 |
month 3 postoperative n = 30 |
|||
| RNFL | central subfield | 13.86± 2.92 |
13.42± 2.87 |
13.44± 2.80 |
13.17± 2.95 |
0.5930 |
| inner ring | 21.60± 2.29 |
21.32± 2.08 |
23.28± 3.41 |
22.54± 3.03 |
<0.0001 | |
| GCL | central subfield | 15.44± 3.94 |
14.38± 3.25 |
15.94± 4.55 |
15.73± 4.51 |
0.1243 |
| inner ring | 46.80± 5.61 |
46.36± 6.39 |
50.37± 6.48 |
49.70± 6.39 |
<0.0001 | |
| IPL | central subfield | 20.32± 3.92 |
20.08± 4.86 |
22.00± 4.50 |
21.83± 4.27 |
0.0009 |
| inner ring | 38.83± 4.41 |
38.50± 4.48 |
41.58± 4.70 |
41.03± 4.33 |
<0.0001 | |
| RNFL-GCL-IPL Complex | central subfield | 49.53± 9.68 |
47.88± 9.62 |
51.39± 11.20 |
50.73± 10.88 |
0.1578 |
| inner ring | 107.18 ± 10.90 | 106.17 ± 11.65 | 115.24 ± 13.18 | 113.27 ± 12.66 | <0.0001 | |
| INL | central subfield | 21.91± 6.69 |
20.92± 6.47 |
23.36± 7.68 |
22.90± 7.31 |
0.1558 |
| inner ring | 40.08± 4.63 |
39.38± 4.98 |
41.81± 5.20 |
40.69± 4.39 |
0.0009 | |
| OPL | central subfield | 27.21± 7.91 |
26.19± 7.79 |
26.44± 7.84 |
25.10± 6.54 |
0.1159 |
| inner ring | 34.11± 5.00 |
33.53± 5.20 |
32.59± 4.85 |
32.93± 5.19 |
0.0803 | |
| INL-OPL Complex | central subfield | 49.12 ± 11.67 | 47.12± 12.50 |
49.81± 12.90 |
48.00± 11.73 |
0.1242 |
| inner ring | 74.19± 7.60 |
72.91± 7.87 |
74.40± 7.86 |
73.63± 7.14 |
0.4100 | |
| ONL | central subfield | 93.15 ± 11.15 | 90.81± 11.71 |
100.64 ± 17.43 | 98.60± 12.93 |
<0.0001 |
| inner ring | 68.71± 6.97 |
69.15± 7.91 |
76.22± 11.25 |
74.09± 10.82 |
<0.0001 | |
| IRL | central subfield | 189.56 ± 19.70 | 182.77 ± 20.19 | 200.17 ± 29.99 | 195.37 ± 23.64 | <0.0001 |
| inner ring | 250.17 ± 17.59 | 248.34 ± 18.35 | 265.85 ± 23.20 | 260.83 ± 19.81 | <0.0001 | |
| TR | central subfield | 276.77 ± 19.12 | 269.15 ± 19.39 | 288.14 ± 31.65 | 282.57 ± 24.47 | <0.0001 |
| inner ring | 330.81 ± 18.34 | 327.72 ± 19.57 | 346.86± 24.01 |
341.33 ± 20.23 | <0.0001 | |
Legend: RNFL = Retinal Nerve Fiber Layer; GCL = Ganglion Cell Layer; IPL = Inner Plexiform Layer; RNFL-GCL-IPL Complex = RNFL + GCL + IPL combined; INL =Inner Nuclear Layer; OPL = Outer Plexiform Layer; INL-OPL Complex = INL + OPL combined; ONL =Outer Nuclear Layer; IRL = Inner Retinal Layer; TR = Total Retina; Bold: Disparity is statistically significant (p < 0.05).
Table 2.
Investigation of alterations in the mean retinal thickness across distinct retinal layers throughout the visits with exclusion of patients with occurrence of cystoid macular edema adjusted for patient age, best corrected visual acuity, lens status of the fellow eye (phakic and pseudophakic) and duration of surgery (n = 36).
| Retinal layer | ETDRS | Presented are the least square mean differences and 95 % confidence intervals (Bonferroni corrected) in μm |
|||||
|---|---|---|---|---|---|---|---|
| Pre OP - Day 1 | Pre OP - Month 1 | Pre OP - Month 3 | Day 1 - Month 1 | Day 1 - Month 3 | Month 1 - Month 3 | ||
| RNFL | CS | 0.03 (−0.93, 0.99) | 0.34 (−0.78, 1.45) | 0.57 (−0.6, 1.74) | 0.31 (−1, 1.61) | 0.54 (−0.98, 2.06) | 0.23 (−0.91, 1.37) |
| IR | 0.15 (−0.3, 0.6) | −1.6 (-2.55, -0.64) | −1.29 (-2.01, -0.57) | −1.75 (-2.72, -0.78) | −1.44 (-2.2, -0.69) | 0.3 (−0.23, 0.84) | |
| GCL | CS | 0.66 (−0.71, 2.02) | −0.58 (−1.7, 0.53) | −0.33 (−1.25, 0.6) | −1.24 (−2.66, 0.19) | −0.98 (−2.48, 0.52) | 0.25 (−0.74, 1.25) |
| IR | −0.52 (−1.68, 0.64) | −3.43 (-4.65, -2.2) | −2.96 (-3.97, -1.96) | −2.91 (-4.34, -1.47) | −2.45 (-3.64, -1.25) | 0.46 (−0.2, 1.13) | |
| IPL | CS | −0.48 (−1.91, 0.94) | −1.71 (-2.89, -0.53) | −1.43 (-2.34, -0.52) | −1.23 (−2.78, 0.32) | −0.95 (−2.48, 0.59) | 0.28 (−0.54, 1.1) |
| IR | −0.26 (−1.16, 0.64) | −2.64 (-3.55, -1.73) | −2.23 (-3.18, -1.29) | −2.39 (-3.45, -1.32) | −1.98 (-2.7, -1.25) | 0.41 (−0.48, 1.29) | |
| RNFL + GCL + IPL | CS | 0 (−2.1, 2.11) | −2.02 (−4.52, 0.48) | −1.24 (−3.09, 0.61) | −2.02 (−5.04, 0.99) | −1.25 (−3.72, 1.23) | 0.78 (−1.04, 2.6) |
| IR | −0.59 (−2.12, 0.94) | −7.64 (-9.96, -5.32) | −6.37 (-8.15, -4.6) | −7.05 (-9.91, -4.19) | −5.78 (-8.09, -3.48) | 1.26 (−0.16, 2.69) | |
| INL | CS | 0.07 (−2.36, 2.5) | −1.7 (−3.98, 0.58) | −1.52 (−3.58, 0.54) | −1.77 (−4.56, 1.02) | −1.59 (−4.16, 0.98) | 0.18 (−1.15, 1.51) |
| IR | 0.43 (−0.9, 1.76) | −1.81 (-3.08, -0.54) | −0.77 (−1.84, 0.3) | −2.24 (-3.77, -0.71) | −1.2 (-2.39, -0.01) | 1.04 (0.02, 2.05) | |
| OPL | CS | 0.82 (−1.87, 3.52) | 0.31 (−2.69, 3.32) | 1.91 (−1.49, 5.3) | −0.51 (−3.8, 2.78) | 1.08 (−1.51, 3.67) | 1.59 (−0.43, 3.62) |
| IR | 0.48 (−1.7, 2.66) | 1.42 (−0.59, 3.44) | 1.35 (−0.7, 3.4) | 0.94 (−0.6, 2.48) | 0.87 (−0.34, 2.07) | −0.08 (−1.26, 1.11) | |
| INL + OPL | CS | 0.57 (−3.47, 4.6) | −1.31 (−5.39, 2.77) | 0.64 (−3.57, 4.85) | −1.88 (−6.34, 2.59) | 0.08 (−3.42, 3.57) | 1.95 (−0.38, 4.29) |
| IR | 0.87 (−1.57, 3.3) | −0.36 (−2.67, 1.95) | 0.6 (−1.66, 2.86) | −1.23 (−3.65, 1.2) | −0.27 (−2.08, 1.54) | 0.96 (−0.71, 2.62) | |
| ONL | CS | 2.92 (−1.89, 7.73) | −7.26 (-13.05, -1.48) | −6.12 (-10.67, -1.56) | −10.18 (-16.25, -4.12) | −9.04 (-12.85, -5.23) | 1.14 (−3.93, 6.21) |
| IR | −0.6 (−3.15, 1.94) | −7.43 (-10.37, -4.5) | −6.23 (-8.93, -3.53) | −6.83 (-9.61, -4.05) | −5.63 (-7.74, -3.52) | 1.2 (−0.4, 2.8) | |
| IRL | CS | 4.51 (1.32, 7.71) | −11.13 (-20.36, -1.9) | −7.51 (-11.79, -3.24) | −15.64 (-25.33, -5.96) | −12.02 (-17.24, -6.81) | 3.62 (−3.34, 10.58) |
| IR | 0.28 (−2.71, 3.27) | −15.31 (-20.37, -10.25) | −11.62 (-15.28, -7.96) | −15.59 (-21.65, -9.52) | −11.9 (-16.47, -7.33) | 3.69 (0.38, 7) | |
| TR | CS | 4.45 (0.7, 8.19) | −11.88 (-22.34, -1.43) | −7.22 (-12.3, -2.14) | −16.33 (-27.59, -5.07) | −11.67 (-17.67, -5.67) | 4.66 (−3.34, 12.66) |
| IR | 1.76 (−3.12, 6.65) | −15.91 (-21.64, -10.18) | −11.71 (-16.69, -6.74) | −17.68 (-25.68, -9.68) | −13.48 (-20.36, -6.59) | 4.2 (−0.53, 8.94) | |
Legend: ETDRS = Early Treatment Diabetic Retinopathy Study; CS= Central Subfield; IR= Inner Ring; RNFL = Retinal Nerve Fiber Layer; GCL = Ganglion Cell Layer; IPL = Inner Plexiform Layer; RNFL-GCL-IPL = RNFL + GCL + IPL combined; INL =Inner Nuclear Layer; OPL = Outer Plexiform Layer; INL-OPL =INL + OPL combined; ONL =Outer Nuclear Layer; IRL = Inner Retinal Layer; TR = Total Retina; Bold: Disparity is statistically significant (p < 0.05).
No postoperative complications (e.g., increased intraocular pressure, excessive fibrin reaction or endophthalmitis) were observed.
2.3. Statistical analysis
Descriptive results are presented as mean ± standard deviation for continuous variables and as n and percent for categorical variables. To investigate changes in the retinal layer thickness over time linear models accounting for the repeated measures by using an unstructured covariance structure were used for the investigated layers separately. Visit was included as categorical parameter and overall significance was tested by type 3 tests. The least square means (LSMs) at the single visits (preoperative, postoperative: day 1, month 1 and month 3) were compared with each other and presented with LSM-differences (LSMDs) and their corresponding 95 % confidence intervals (CIs). All models were adjusted for patient age, lens status of the fellow eye (phakic and pseudophakic), duration of surgery and best corrected visual acuity preoperative. For the comparisons of the changes between the visits, Bonferroni correction was applied. No imputation for missing data was applied. All analyses were performed in the whole cohort (n = 41 patients), and in the sub-cohort without CME cases (n = 36 patients). For statistical analysis, SAS 9.4 (SAS Institute, Cary NC) was used.
3. Results
41 eyes of 41 patients were included, consisting of 24 right eyes (58.5 %) and 17 left eyes (41.5 %). Of the 41 fellow eyes, 27 (65.85 %%) were phakic, and 14 (34.15 %) were pseudophakic. In one patient, baseline data for retinal layer thickness was missing due to insufficient image quality (individual retinal layers could not be distinguished). Thus 40 SD-OCT images fulfilled these requirements preoperative. Owing to the non-appearance at postoperative assessments, we were able to analyse a total of 30 SD-OCT scans on day 1, 41 SD-OCT scans at the end of the first month, and 35 SD-OCT scans at the end of the third month. The 41 (100 %) fellow surgery native eyes showed no significant alterations in any of the measured parameters over the course of the observation period. The age of enrolled participants averaged was 71.5 ± 8.3 years; 20 participants (48.8 %) were male and 21 (51.2 %) were female. 5 eyes (12.9 %) showed CME 1 month after cataract surgery. The mean best corrected visual acuity (BCVA) measured 0.4 ± 0.1 logMAR prior to the surgery, 0.1 ± 0.1 logMAR on the first day following the surgery, 0.0 ± 0.1 logMAR one month after the surgery, and 0.0 ± 0.1 logMAR three months after the surgery.
3.1. Central subfield
In the central subfield, significant (all p < 0.05) changes were observed across all patients in each of the four assessments for every retinal layer, except for the RNFL (p = 0.35) the GCL (p = 0.06), while considering patient age, lens status of the fellow eye (phakic or pseudophakic) and best corrected visual acuity as presented in Table 3)
Table 3.
Estimated changes in the individual retinal layer thickness [μm] in the central subfield and the inner ring analysed over 3 months adjusted for patient age, best corrected visual acuity, lens status of the fellow eye (phakic and pseudophakic) and duration of surgery.
| retinal layers | ETDRS grid | Baseline values of the mean thickness [μm] ± standard deviation |
p-value | |||
|---|---|---|---|---|---|---|
| Preoperative n = 40 | day 1 postoperative n = 30 | month 1 postoperative n = 41 |
month 3 postoperative n = 35 |
|||
| RNFL | central subfield | 13.90± 2.79 |
13.53± 2.78 |
13.80± 2.96 |
13.17± 2.92 |
0.3463 |
| inner ring | 21.46± 2.23 |
21.28± 2.03 |
23.22± 3.22 |
22.51± 2.90 |
<0.0001 | |
| GCL | central subfield | 15.33± 3.81 |
14.53± 3.09 |
16.27± 4.77 |
15.66± 4.24 |
0.0635 |
| inner ring | 46.76± 5.34 |
46.28± 6.17 |
50.45± 6.13 |
49.56± 6.01 |
<0.0001 | |
| IPL | central subfield | 20.41± 3.75 |
19.97± 4.80 |
22.56± 4.62 |
21.80± 4.11 |
<0.0001 |
| inner ring | 38.74± 4.14 |
38.53± 4.21 |
41.73± 4.45 |
41.02± 4.03 |
<0.0001 | |
| RNFL-GCL-IPL Complex | central subfield | 49.56± 9.20 |
48.03± 9.38 |
52.63± 11.46 |
50.63± 10.42 |
0.0188 |
| inner ring | 106.92 ± 10.32 | 106.08 ± 11.13 | 115.40 ± 12.45 | 113.09 ± 11.87 | <0.0001 | |
| INL | central subfield | 21.90± 6.37 |
21.23± 6.37 |
24.22± 7.84 |
23.14± 7.16 |
0.0294 |
| inner ring | 40.06± 4.39 |
39.43± 4.71 |
42.11± 5.01 |
40.67± 4.08 |
<0.0001 | |
| OPL | central subfield | 27.44± 7.46 |
26.57± 7.62 |
26.61± 7.56 |
24.86± 6.22 |
0.0341 |
| inner ring | 34.44± 5.10 |
33.43± 5.07 |
32.89± 5.02 |
32.68± 4.90 |
0.0268 | |
| INL-OPL Complex | central subfield | 49.33 ± 11.07 | 47.80± 12.35 |
50.83± 12.84 |
48.00± 11.05 |
0.0144 |
| inner ring | 74.51± 7.37 |
72.86± 7.43 |
75.00± 7.74 |
73.35± 6.68 |
0.0676 | |
| ONL | central subfield | 94.23 ± 11.19 | 91.63± 12.34 |
102.76 ± 18.11 | 101.03 ± 14.21 | <0.0001 |
| inner ring | 69.26± 6.92 |
70.04± 7.79 |
77.51± 11.38 |
75.44± 10.59 |
<0.0001 | |
| IRL | central subfield | 191.05 ± 19.06 | 184.53 ± 19.54 | 204.56 ± 30.74 | 197.71 ± 23.03 | <0.0001 |
| inner ring | 250.79± 16.57 |
249.03± 17.29 |
267.91 ± 22.49 | 261.76 ± 18.56 | <0.0001 | |
| TR | central subfield | 278.18 ± 18.84 | 270.57 ± 18.62 | 292.51 ± 32.08 | 284.71 ± 23.78 | <0.0001 |
| inner ring | 331.53± 17.33 |
328.4± 18.47 |
348.76± 23.11 |
342.49 ± 19.08 | <0.0001 | |
Legend: RNFL = Retinal Nerve Fiber Layer; GCL = Ganglion Cell Layer; IPL = Inner Plexiform Layer; RNFL-GCL-IPL Complex = RNFL + GCL + IPL combined; INL =Inner Nuclear Layer; OPL = Outer Plexiform Layer; INL-OPL Complex = INL + OPL combined; ONL =Outer Nuclear Layer; IRL = Inner Retinal Layer; TR = Total Retina; Bold: Disparity is statistically significant (p < 0.05).
In comparing the preoperative assessment with the evaluation at 1 day post-operation, retinal layers exhibited a reduction in thickness, with a LSMD of the TR measuring 5.39 (95 % CI 1.8, 8.98) μm.
Furthermore, from 1 day postoperatively to 1 month after surgery, retinal layers demonstrated a subsequent increase in thickness. The RNFL-GCL-IPL complex, exhibited an LSMD of −3.43 (95 % CI -6.7, −0.16) μm, the INL-OPL -2.49 (95 % CI -6.78, 1.8) μm, and the ONL -11.62 (95 % CI -17.38, −5.87).
Subsequently, from one month postoperatively to 3 months after surgery, a decrease in mean thickness was evident in all retinal layers. The RNFL-GCL-IPL complex showed a LSMD of 2.11 (95 % CI -0.4, 4.62) μm, the INL-OPL complex 3.04 (95 % CI 0.52, 5.55) μm, and the ONL 1.16 (95 % CI -3.55, 5.87) μm, as visualized in Table 4.
Table 4.
Investigation of alterations in the mean retinal thickness across distinct retinal layers throughout the visits adjusted for patient age, best corrected visual acuity, lens status of the fellow eye (phakic and pseudophakic) and duration of surgery (n = 41).
| Retinal layer | ETDRS | Presented are the least square mean differences and 95 % confidence interval (Bonferroni corrected) in μm |
|||||
|---|---|---|---|---|---|---|---|
| Pre OP - Day 1 | Pre OP - Month 1 | Pre OP - Month 3 | Day 1 - Month 1 | Day 1 - Month 3 | Month 1 - Month 3 | ||
| RNFL | CS | −0.03 (−0.88, 0.82) | 0.03 (−1.04, 1.09) | 0.63 (−0.42, 1.68) | 0.06 (−1.12, 1.24) | 0.66 (−0.65, 1.97) | 0.6 (−0.51, 1.72) |
| IR | 0.06 (−0.36, 0.48) | −1.68 (-2.52, -0.84) | −1.37 (-2.02, -0.73) | −1.74 (-2.58, -0.9) | −1.43 (-2.11, -0.75) | 0.31 (−0.19, 0.8) | |
| GCL | CS | 0.45 (−0.75, 1.66) | −1.01 (−2.27, 0.26) | −0.36 (−1.19, 0.48) | −1.46 (−2.96, 0.04) | −0.81 (−2.11, 0.49) | 0.65 (−0.5, 1.8) |
| IR | −0.44 (−1.47, 0.59) | −3.55 (-4.64, -2.47) | −2.84 (-3.73, -1.95) | −3.11 (-4.44, -1.78) | −2.4 (-3.49, -1.31) | 0.71 (0.07, 1.36) | |
| IPL | CS | −0.05 (−1.43, 1.34) | −2.17 (-3.4, -0.95) | −1.33 (-2.25, -0.41) | −2.13 (-3.89, -0.36) | −1.28 (−2.65, 0.09) | 0.84 (−0.4, 2.09) |
| IR | −0.27 (−1.1, 0.56) | −2.88 (-3.74, -2.02) | −2.26 (-3.08, -1.44) | −2.61 (-3.6, -1.62) | −1.99 (-2.71, -1.27) | 0.61 (−0.23, 1.46) | |
| RNFL + GCL + IPL | CS | 0.23 (−1.69, 2.15) | −3.2 (-5.93, -0.47) | −1.09 (−2.78, 0.6) | −3.43 (-6.7, -0.16) | −1.32 (−3.46, 0.81) | 2.11 (−0.4, 4.62) |
| IR | −0.57 (−2.02, 0.87) | −8.08 (-10.18, -5.98) | −6.37 (-7.92, -4.83) | −7.51 (-10.15, -4.87) | −5.8 (-8.03, -3.56) | 1.71 (0.33, 3.08) | |
| INL | CS | −0.1 (−2.32, 2.13) | −2.55 (-4.85, -0.24) | −1.67 (−3.59, 0.24) | −2.45 (−5.22, 0.32) | −1.58 (−4.22, 1.07) | 0.87 (−0.71, 2.46) |
| IR | 0.42 (−0.76, 1.59) | −2.11 (-3.28, -0.95) | −0.73 (−1.73, 0.27) | −2.53 (-3.91, -1.14) | −1.15 (-2.25, -0.04) | 1.38 (0.3, 2.47) | |
| OPL | CS | 0.67 (−1.8, 3.14) | 0.45 (−2.27, 3.18) | 2.4 (−0.65, 5.46) | −0.22 (−3.14, 2.7) | 1.73 (−0.81, 4.28) | 1.95 (−0.02, 3.93) |
| IR | 0.99 (−1.03, 3.01) | 1.48 (−0.32, 3.27) | 1.9 (−0.16, 3.95) | 0.48 (−1, 1.96) | 0.91 (−0.19, 2) | 0.42 (−1.03, 1.88) | |
| INL + OPL | CS | 0.46 (−3.22, 4.15) | −2.03 (−5.83, 1.77) | 1.01 (−2.7, 4.73) | −2.49 (−6.78, 1.8) | 0.55 (−2.87, 3.96) | 3.04 (0.52, 5.55) |
| IR | 1.26 (−0.94, 3.45) | −0.63 (−2.72, 1.46) | 1.2 (−0.98, 3.39) | −1.89 (−4.19, 0.41) | −0.05 (−1.63, 1.52) | 1.83 (−0.05, 3.72) | |
| ONL | CS | 3.25 (−1.19, 7.7) | −8.37 (-13.8, -2.95) | −7.21 (-11.44, -2.98) | −11.62 (-17.38, -5.87) | −10.46 (-14.38, -6.54) | 1.16 (−3.55, 5.87) |
| IR | −0.62 (−2.82, 1.57) | −8.22 (-11, -5.44) | −6.74 (-9.2, -4.29) | −7.6 (-10.15, -5.04) | −6.12 (-8.02, -4.21) | 1.48 (−0.33, 3.29) | |
| IRL | CS | 4.93 (1.95, 7.91) | −14.03 (-22.79, -5.26) | −7.8 (-11.59, -4.01) | −18.95 (-28.35, -9.56) | −12.73 (-17.52, -7.93) | 6.23 (−0.52, 12.98) |
| IR | 0.62 (−2.04, 3.27) | −16.81 (-21.6, -12.01) | −11.56 (-14.72, -8.4) | −17.42 (-23.15, -11.7) | −12.18 (-16.25, -8.1) | 5.25 (1.74, 8.75) | |
| TR | CS | 5.39 (1.8, 8.98) | −14.82 (-24.61, -5.04) | −7.42 (-11.88, -2.96) | −20.21 (-31.03, -9.38) | −12.81 (-18.1, -7.51) | 7.4 (−0.24, 15.05) |
| IR | 2.23 (−2.08, 6.53) | −17.12 (-22.35, -11.9) | −11.77 (-16.05, -7.48) | −19.35 (-26.66, -12.04) | −13.99 (-20.07, -7.92) | 5.36 (0.99, 9.72) | |
Legend: ETDRS = Early Treatment Diabetic Retinopathy Study; CS= Central Subfield; IR= Inner Ring; RNFL = Retinal Nerve Fiber Layer; GCL = Ganglion Cell Layer; IPL = Inner Plexiform Layer; RNFL-GCL-IPL = RNFL + GCL + IPL combined; INL =Inner Nuclear Layer; OPL = Outer Plexiform Layer; INL-OPL =INL + OPL combined; ONL =Outer Nuclear Layer; IRL = Inner Retinal Layer; TR = Total Retina; Bold: Disparity is statistically significant (p < 0.05).
3.2. Inner ring
The inner ring displayed statistically significant changes in mean retinal thickness across all analysed retinal layers in the whole cohort, with p-values less than 0.05 except for the INL-OPL complex (p = 0.07) at the four time points (Table 3).
From the preoperative examination to day 1 post-operation, retinal layers demonstrated an increase in mean thickness. The LSMD of the RNFL-GCL-IPL complex exhibited an increase of −0.57 (95 % CI -2.02, 0.87) μm, the INL-OPL 1.26 (95 % CI -0.94, 3.45) μm, and the ONL -0.62 (95 % CI -2.82, 1.57) μm.
Subsequently, retinal layers revealed an increase in thickness from day 1 to month 1 postoperatively, with the RNFL-GCL-IPL complex exhibiting an LSMD of −7.51 (95 % CI -10.15, −4.87) μm, the INL-OPL complex −1.89 (95 % CI -4.19, 0.41) μm, and the ONL -7.6 (95 % CI -10.15, −5.04) μm.
Moreover, between month 1 and month 3 after surgery, the retinal layers became thinner again without reaching the preoperative thickness. The LSMD of the RNFL-GCL-IPL complex showed a decrease of 1.71 (95 % CI 0.33, 3.08) μm, the INL-OPL complex 1.83 (95 % CI -0.05, 3.72) μm, and the ONL 1.48 (95 % CI -0.33, 3.29) μm (Results presented in Table 4).
Type 3 tests examining the possible influence of patient age, the duration of surgery, initial best corrected visual acuity, and the lens status of the other eye (phakic and pseudophakic) revealed no significant association with retinal layer thickness. In both the entire cohort and the sub-analysis excluding patients with CME, similar outcomes were observed in both groups in the central subfield and the inner ring (Table 1, Table 2, Table 3, Table 4).
4. Discussion
In this study, we performed a comprehensive evaluation of the thickness of individual retinal layers using SD-OCT in healthy eyes both pre- and post-uncomplicated cataract surgery, with a particular focus on the central subfield and the inner ring of the ETDRS grid considering patient age, lens status of the fellow eye (phakic and pseudophakic), duration of surgery and best visual acuity preoperative. Postoperative assessments were performed at 1 day, 1 month, and 3 months post-surgery. Subsequent analyses were conducted, excluding patients with CME, to enable investigation of retinal layers demonstrating initial thickening prior to the manifestation of clinically apparent edema. This approach facilitated a comprehensive assessment of both subclinical and clinical progression. Moreover, comparisons of the timepoints within each individual retinal layers were conducted. We observed significant changes (p < 0.05) in all retinal layers, except for the RNFL (p = 0.33) and the GCL (p = 0.06) in the central subfield. Additionally, significant alterations were noted in all retinal layers (p < 0.05), except for the INL-OPL complex (p = 0.07) within the inner ring of the ETDRS grid.
Our study demonstrates an increase in retinal layer thickness 1 month after uncomplicated catarct surgery, consistent with previous literature [[13], [14], [15],17]. Layers of both, the superficial (RNFL-GCL-IPL complex) and the deep capillary plexus (INL-OPL complex) as well as the ONL nourished by the choroidal vasculature revealed an increase in mean retinal thicknes from day 1 to month 1 post uncomplicated surgery. The RNFL-GCL-IPL complex showed an increase in LSMD of −3.43 (95 % CI -6.7, −0.16) μm, the INL-OPL -2.49 (95 % CI -6.78, 1.8) μm and the ONL of −11.62 (−17.38, −5.87) μm, within the central subfield and an increase in LSMD of the RNFL-GCL-IPL complex of −7.51 (95 % CI -10.15, −4.87) μm, the INL-OPL complex of −1.89 (95 % CI -4.19, 0.41) μm and the ONL of −7.6 (95 % CI -10.15, −5.04) μm in the inner ring from day 1 to month 1 postoperative in the whole group. Hence, we posit that the postoperative inflammatory cascade subsequent to uncomplicated cataract surgery exerts an influence on each retinal layer, resulting in heightened thickness of individual layers as well as cumulative retinal thickness peaking approximately one month post-surgery.
A retrospective study conducted by Sigler et al. [18] identified intraretinal cysts within the INL and the OPL preceding the onset of subretinal fluid in patients with CME. Comparing the subgroup excluding patients with CME regarding the increase in thickness of the INL -2.24 (95 % CI -3.77, −0.71) μm to the entire study population −2.53 (95 % CI -3.91, −1.14) μm, both groups demonstrated a significant increase in the thickness of the INL in the inner ring from the 1st day to the 1st month postoperative. The INL, comprising nuclei of bipolar, horizontal, amacrine, and Müller cells, has been observed to undergo thickening in other inflammatory conditions such as multiple sclerosis as well [19]. None of the patients in our study cohort displayed cystoid formation in the OPL or subretinal fluid, suggesting a lack of pronounced CME cases within our population.
Kurt et al. [16] conducted an investigation, revealing hardly any difference in the mean retinal thickness of both the central subfield and the inner ring in all retinal layers from postoperative month 1 to month 3. Moreover, they mentioned that there was no formation of intraretinal cysts during their observation. Conversely, our investigation unveiled a reduction in the LSMD in retinal thickness of retinal layers analysed in the whole group, reaching statistical significance in the INL-OPL complex with 3.04 (95 % CI 0.52, 5.55) μm within the central subfield and the GCL with 0.71 (95 % CI 0.07, 1.36) μm, the RNFL-GCL-IPL complex with 1.71 (95 % CI 0.33, 3.08) μm the INL with 1.38 (95 % CI 0.3, 2.47) μm, the IRL with 5.25 (95 % CI 1.74, 8.75) μm and the TR with 5.36 (95 % CI 0.99, 9.72) μm in the inner ring from postoperative month 1 to month 3. These alterations between the studies may arise due to the employment of non-steroidal anti-inflammatory drugs (NSAIDs) as a treatment regime in the study conducted by Kurt et al.
Xu et al. [20] noted that, in the aftermath of extracapsular cataract surgery, there is an elevation in the expression of acute pro-inflammatory genes and a consequent increase in protein response within the posterior segment of the eye in mice modelling. Moreover, they reckon that this phenomenon suggests the initiation of the inflammasome and concurrent activation of the complement system also in humans. Based on the changes examined in our study, we assume that these genes reach their peak expression level around month 1 post-surgery and subsequently undergo a downregulation afterwards in accordance with the retinal layers analysed in our study over 3 months.
Nakatani et al. [13] found a significant increase of the GCL after cataract surgery in patients with and without glaucoma. They assumed that their observation was attributable to the biased preoperative thickness measurement secondary to the low signal strength in eyes with cataract. The same observation as a decrease of retinal thickness on day 1 after surgery was already reported by Ching et al. [2] stating that this phenomenon is attributable to the increased signal strength after the replacement of the crystalline lens by an intraocular lens. We also observed a reduction in the mean thickness of the individual retinal layers examined one day after cataract operation compared to the preoperative control, with the exception of the ONL (increase of thickness from 68.71 ± 6.97 μm at the baseline visit to 69.17 ± 7.91 μm at day 1 postoperative) in the inner ring. In our investigation, the thickness of individual retinal layers increased until month 1, followed by a subsequent decrease from month 1 to month 3 in individual retinal layers analysed. We attribute this pattern to an inflammatory response, characterized by an initial increase in individual retinal thicknesses up to month 1, followed by a decline in inflammation between month 1 and month 3.
Our study is subject to several limitations. Firstly, a larger sample size and a longer follow-up period would have enhanced the statistical robustness of our findings as the retinal thickness values showed a decrease from the 1 month postoperative to the 3-month postoperative without reaching baseline values. Therefore, the follow-up should be longer than 3 months. We did not include cataract grading, effective phacoemulsification time (EPT) and total energy (TE) in our analysis. EPT, total energy and dense cataract are known to be significantly correlated to visual impairment and development of CME [21]. Although we did not evaluate EPT and TE, longer duration of surgery possibly correlates to higher EPT and in accordance TE and increased manipulation during surgery, being a risk factor for CME itself and was therefore included in our model in addition to patient age, the lens status of the fellow eye (phakic and pseudophakic) and the best corrected visual acuity preoperative. However, we didn't assess alterations in individual retinal layers based on surgeons' training levels. Nonetheless, List et al. [22] showed that surgeons with a training level of ≤300 cataract operations were 1.58 times more prone to developing CME compared to those with ≥300 cataract operations. Given that all surgeons in our study had conducted a minimum of 323 operations before the respective cataract procedure, this influence should be considered negligible. Furthermore, we did not assess anterior chamber inflammation at various time points, despite its known impact on CME development [23]. Additionally, post-operative therapeutic regimen consisted of glucocorticoids and antibiotics what may have influenced the development of CME in our study cohort. However, it's important to note that the postoperative treatment regimen was identical for both groups, thus affecting them equally. A recent meta-analysis conducted by Li et al. [24] revealed that in postoperative scenarios, simultaneous administration of NSAIDs alongside corticosteroids resulted in a notable decrease in the risk of CME development compared to using either medication alone.
This study shows in a prospective and consecutive design changes in the retinal layers nourished by both the superficial and deep capillary plexus as well as the ONL nourished by the choroidal vasculature following uncomplicated cataract surgery. Furthermore, a reduction in the mean thickness of individual retinal layers 1-day post-surgery, followed by an increase in mean retinal layer thickness after 1 month postoperative and a decline of mean retinal thickness after 3 months postoperative were observed. Hence, it can be inferred that the apex of inflammatory response, characterized by an augmentation in the thickness of individual retinal layers, occurs around 1 month after uneventful surgery. These findings may be useful in further understanding the pathophysiological pathways of CME. During the period spanning from the first to the third month, the LSMD of the TR 7.4 (−0.24, 15.05) underwent a reduction within the central subfield, with a significant decrease of 5.36 μm (95 % CI 1.74, 8.75) observed in the inner ring. Thus, we assume that additional therapy for the treatment of CME in mild cases does not have to be initiated as early as the first month. Further studies with different therapeutic arms and a longer duration are necessary to see whether total retinal and segmental changes return to preoperative levels.
5. Ethics statement
The study was approved by the Ethics Committee of the Medical University of Graz (number 27–490 ex 14/15), Austria, and was conducted in accordance with the tenets of the Declaration of Helsinki [25]. All patients gave informed consent before enrolment.
Funding statement
This study was funded by means of the Medical University of Graz.
Data availability statement
Data cannot be shared publicly because data contain potentially identifying or sensitive patient information and restrictions are imposed by the institutional review board. Data are available from the Research Center of the Department of Ophthalmology, Medical University of Graz (Tel.: +4338512394, mail: augwww@medunigraz.at) for researchers who meet the criteria for access to confidential data.
CRediT authorship contribution statement
Manuel Großpötzl: Writing – review & editing, Writing – original draft, Supervision, Project administration, Methodology, Investigation, Data curation, Conceptualization. Eva Maria Malle: Writing – original draft, Data curation, Conceptualization. Regina Riedl: Formal analysis. Jakob Daniel Gran: Investigation. Daniel Djavid: Investigation. Laura Posch-Pertl: Investigation. Wilfried Maximilian Glatz: Investigation. Thomas Falb: Investigation. Ewald Lindner: Supervision. Andreas Wedrich: Supervision. Domagoj Ivastinovic: Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
References
- 1.Yonekawa Y., Kim I.K. Pseudophakic cystoid macular edema. Curr. Opin. Ophthalmol. 2012;23:26–32. doi: 10.1097/ICU.0b013e32834cd5f8. [DOI] [PubMed] [Google Scholar]
- 2.Ching H.-Y., Wong A.C., Wong C.-C., Woo D.C., Chan C.W. Cystoid macular oedema and changes in retinal thickness after phacoemulsification with optical coherence tomography. Eye. 2006;20:297–303. doi: 10.1038/sj.eye.6701864. [DOI] [PubMed] [Google Scholar]
- 3.Nicholas S., Riley A., Patel H., Neveldson B., Purdie G., Wells A.P. Correlations between optical coherence tomography measurement of macular thickness and visual acuity after cataract extraction. Clin. Exp. Ophthalmol. 2006;34:124–129. doi: 10.1111/j.1442-9071.2006.01169.x. [DOI] [PubMed] [Google Scholar]
- 4.Wielders L.H.P., Lambermont V.A., Schouten J.S.A.G., van den Biggelaar F.J.H.M., Worthy G., Simons R.W.P., Winkens B., Nuijts R.M.M.A. Prevention of cystoid macular edema after cataract surgery in nondiabetic and diabetic patients: a systematic review and meta-analysis. Am. J. Ophthalmol. 2015;160:968–981.e33. doi: 10.1016/j.ajo.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 5.Mentes J., Erakgun T., Afrashi F., Kerci G. Incidence of cystoid macular edema after uncomplicated phacoemulsification. Ophthalmologica. 2003;217:408–412. doi: 10.1159/000073070. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson U., Alm A., Bjärnhall G., Granstam E., Matsson A.W. Macular edema and visual outcome following cataract surgery in patients with diabetic retinopathy and controls. Graefes Arch. Clin. Exp. Ophthalmol. 2011;249:349–359. doi: 10.1007/s00417-010-1484-9. [DOI] [PubMed] [Google Scholar]
- 7.Miyake K., Ibaraki N. Prostaglandins and cystoid macular edema. Surv. Ophthalmol. 2002;47:S203–S218. doi: 10.1016/S0039-6257(02)00294-1. [DOI] [PubMed] [Google Scholar]
- 8.Ursell P.G., Spalton D.J., Whitcup S.M., Nussenblatt R.B. Cystoid macular edema after phacoemulsification: relationship to blood–aqueous barrier damage and visual acuity. J. Cataract Refract. Surg. 1999;25:1492–1497. doi: 10.1016/S0886-3350(99)00196-0. [DOI] [PubMed] [Google Scholar]
- 9.Lobo C. Pseudophakic cystoid macular edema. Ophthalmologica. 2012;227:61–67. doi: 10.1159/000331277. [DOI] [PubMed] [Google Scholar]
- 10.Bélair M.-L., Kim S.J., Thorne J.E., Dunn J.P., Kedhar S.R., Brown D.M., Jabs D.A. Incidence of cystoid macular edema after cataract surgery in patients with and without uveitis using optical coherence tomography. Am. J. Ophthalmol. 2009;148:128–135.e2. doi: 10.1016/j.ajo.2009.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perente I., Utine C.A., Ozturker C., Cakir M., Kaya V., Eren H., Kapran Z., Yilmaz O.F. Evaluation of macular changes after uncomplicated phacoemulsification surgery by optical coherence tomography. Curr. Eye Res. 2007;32:241–247. doi: 10.1080/02713680601160610. [DOI] [PubMed] [Google Scholar]
- 12.Lobo C.L., Faria P.M., Soares M.A., Bernardes R.C., Cunha-Vaz J.G. Macular alterations after small-incision cataract surgery, J. Cataract Refract. Surg. 2004;30:752–760. doi: 10.1016/S0886-3350(03)00582-0. [DOI] [PubMed] [Google Scholar]
- 13.Nakatani Y., Higashide T., Ohkubo S., Takeda H., Sugiyama K. Effect of cataract and its removal on ganglion cell complex thickness and peripapillary retinal nerve fiber layer thickness measurements by fourier-domain optical coherence tomography. J. Glaucoma. 2013;22:447–455. doi: 10.1097/IJG.0b013e3182894a16. [DOI] [PubMed] [Google Scholar]
- 14.Sari E.S., Ermis S.S., Yazici A., Koytak A., Sahin G., Kilic A. The effect of intracameral anesthesia on macular thickness and ganglion cell-inner plexiform layer thickness after uneventful phacoemulsification surgery: prospective and randomized controlled trial. Graefes Arch. Clin. Exp. Ophthalmol. 2014;252:433–439. doi: 10.1007/s00417-013-2557-3. [DOI] [PubMed] [Google Scholar]
- 15.Doncel-Fernández C.J., Alferez-Asenjo M.L., Quereda-Castañeda A., Castro-Luna G. Preoperative central macular thickness as a risk factor for pseudophakic macular edema. Graefes Arch. Clin. Exp. Ophthalmol. 2021;259:37–43. doi: 10.1007/s00417-020-04862-x. [DOI] [PubMed] [Google Scholar]
- 16.Kurt A., Kılıç R. The effects of uncomplicated cataract surgery on retinal layer thickness. J. Ophthalmol. 2018;2018:1–6. doi: 10.1155/2018/7218639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roh H.C., Park C.Y., Kim M. Changes of the macular ganglion cell-inner plexiform layer thickness after cataract surgery in glaucoma patients. J. Ophthalmol. 2016;2016:1–8. doi: 10.1155/2016/9785939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sigler E.J., Randolph J.C., Kiernan D.F. Longitudinal analysis of the structural pattern of pseudophakic cystoid macular edema using multimodal imaging. Graefes Arch. Clin. Exp. Ophthalmol. 2016;254:43–51. doi: 10.1007/s00417-015-3000-8. [DOI] [PubMed] [Google Scholar]
- 19.Petzold A., Balcer L.J., Calabresi P.A., Costello F., Frohman T.C., Frohman E.M., Martinez-Lapiscina E.H., Green A.J., Kardon R., Outteryck O., Paul F., Schippling S., Vermersch P., Villoslada P., Balk L.J., Aktas O., Albrecht P., Ashworth J., Asgari N., Balcer L., Balk L., Black G., Boehringer D., Behbehani R., Benson L., Bermel R., Bernard J., Brandt A., Burton J., Calabresi P., Calkwood J., Cordano C., Costello F., Courtney A., Cruz-Herranz A., Diem R., Daly A., Dollfus H., Fasser C., Finke C., Frederiksen J., Frohman E., Frohman T., Garcia-Martin E., Suárez I.G., Pihl-Jensen G., Graves J., Green A., Havla J., Hemmer B., Huang S.-C., Imitola J., Jiang H., Keegan D., Kildebeck E., Klistorner A., Knier B., Kolbe S., Korn T., LeRoy B., Leocani L., Leroux D., Levin N., Liskova P., Lorenz B., Preiningerova J.L., Martínez-Lapiscina E.H., Mikolajczak J., Montalban X., Morrow M., Nolan R., Oberwahrenbrock T., Oertel F.C., Oreja-Guevara C., Osborne B., Outteryck O., Papadopoulou A., Paul F., Petzold A., Ringelstein M., Saidha S., Sanchez-Dalmau B., Sastre-Garriga J., Schippling S., Shin R., Shuey N., Soelberg K., Toosy A., Torres R., Vidal-Jordana A., Villoslada P., Waldman A., White O., Yeh A., Wong S., Zimmermann H. Retinal layer segmentation in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol. 2017;16:797–812. doi: 10.1016/S1474-4422(17)30278-8. [DOI] [PubMed] [Google Scholar]
- 20.Xu H., Chen M., Forrester J.V., Lois N. Cataract surgery induces retinal pro-inflammatory gene expression and protein secretion. Investig. Opthalmology Vis. Sci. 2011;52:249. doi: 10.1167/iovs.10-6001. [DOI] [PubMed] [Google Scholar]
- 21.Ferrari T.M., Cavallo M., Durante G., Mininno L., Cardascia N. Macular edema induced by phacoemulsification. Doc. Ophthalmol. Adv. Ophthalmol. 1999;97:325–327. doi: 10.1023/a:1002142307952. [DOI] [PubMed] [Google Scholar]
- 22.List W., Steinwender G., Glatz W., Riedl R., Wedrich A., Ivastinovic D. The impact of surgeon's experience and sex on the incidence of cystoid macular edema after uneventful cataract surgery. PLoS One. 2022;17 doi: 10.1371/journal.pone.0279518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Maria M., Coassin M., Iannetta D., Fontana L. Laser flare and cell photometry to measure inflammation after cataract surgery: a tool to predict the risk of cystoid macular edema. Int. Ophthalmol. 2021;41:2293–2300. doi: 10.1007/s10792-021-01779-0. [DOI] [PubMed] [Google Scholar]
- 24.Li S.-S., Wang H.-H., Wang Y.-L., Zhang D.-W., Chen X. Comparison of the efficacy and safety of non-steroidal anti-inflammatory drugs and corticosteroid drugs for prevention of cystoid macular edema after cataract surgery. Int. Ophthalmol. 2022;43:271–284. doi: 10.1007/s10792-022-02426-y. [DOI] [PubMed] [Google Scholar]
- 25.World Medical Association Declaration of Helsinki Ethical principles for medical Research involving human subjects. JAMA. 2013;310:2191. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data cannot be shared publicly because data contain potentially identifying or sensitive patient information and restrictions are imposed by the institutional review board. Data are available from the Research Center of the Department of Ophthalmology, Medical University of Graz (Tel.: +4338512394, mail: augwww@medunigraz.at) for researchers who meet the criteria for access to confidential data.