Abstract
This study investigates the application of crude glycerol to the production of 1,3-propanediol by immobilized cells of Bacillus pumilus. This is a novel application of a naturally occurring producer obtained from a wastewater storage pond in Thailand. Crude glycerol was obtained through the methanolysis of palm oil, which was catalyzed using rice bran lipase. Ten components of the fermentation medium were screened using a Plackett-Burman design. The statistical significance of the results was determined using multiple linear regression with a backward elimination approach. The significance level was set to 5 % (p < 0.05). Only crude glycerol, (NH4)2SO4, MgSO4, and CaCl2 significantly affected 1,3-propanediol production by immobilized B. pumilus. Furthermore, preliminary screenings of environmental conditions used for 1,3-propanediol production were conducted using a Plackett-Burman design. The results showed that the temperature, time, and quantity of immobilized cells were factors that significantly affected 1,3-propanediol yield. Therefore, the quantities of crude glycerol, (NH4)2SO4, MgSO4, and CaCl2 and the temperature, time, and quantity of immobilized cells were optimized using response surface methodology based on a Box-Behnken design. The model predicted a maximum 1,3-propanediol yield of 45.68 g/L with the following conditions: 60 g/L crude glycerol, 5 g/L (NH4)2SO4, 0.55 g/L MgSO4, 0.05 g/L CaCl2, a fermentation duration of 101 h, and a temperature of 25 °C, with 250 g of immobilized cells. The validation trials confirmed a production level of 44.12 ± 1.81 g/L, indicating a 2.86-fold production increase relative to the control group. Overall, this study demonstrates the potential of using crude glycerol as a substrate to improve the yields of 1,3-propanediol produced by B. pumilus.
Keywords: Optimization; Production; 1,3-Propanediol; Crude glycerol; Immobilized Bacillus pumilus
1. Introduction
Crude glycerol is a by-product of the manufacturing of biodiesel through transesterification of oil catalyzed by an acid, base, or enzyme. Around 1 kg of glycerol is produced from 10 kg of biodiesel production [1], and it consists of glycerol (75 %) and various impurities such as water (10 %), ash (10 %), and non-glycerol organic matter (5 %) [2]. In the future, the availability of crude glycerol will increase due to increased biodiesel production. As a result, there will be a substantial accumulation of surplus crude glycerol, which has the potential to cause environmental issues [3]. It is noteworthy that crude glycerol may be used as a substrate for technologies utilizing microorganisms [4,5]. Therefore, converting crude glycerol to value-added products (e.g., 1,3-propanediol (1,3-PDO), acrolein, and hydrogen) is an alternative method for crude glycerol disposal [6].
1,3-PDO is a precursor in the production of partially biobased biopolymers such as polytrimethylene terephthalate (PTT) and polytrimethylene 2,5-furandicarboxylate (PTF), cosmetics, foods, lubricants, medicine, adhesives, laminates, detergent, coatings, antifreeze additives, and freshness-preserving agents [7,8]. The 1,3-PDO polymerization process yields products with improved specificity, increased industrial safety, and enhanced biodegradability [4,5]. 1,3-PDO can be manufactured from glycerol by either a chemical process (dihydroxylation and hydrogenolysis) or a fermentation process. The chemical process generates hazardous intermediate compounds, uses expensive catalysts, and requires high pressures and temperatures [9]. In contrast, the advantages of the fermentation process include its mild condition and environmentally friendly nature. The fermentation process occurs in a two-step reaction sequence: 1) the conversion of glycerol to 3-hydroxypropionaldehyde (3-HPA) and water, which is catalyzed by the glycerol dehydratase enzyme; and 2) the subsequent reduction of 3-HPA with a reduced form of nicotinamide adenine dinucleotide (NADH) to 1,3-PDO, which is catalyzed by the 1,3-PDO dehydrogenase enzyme [10]. NADH is generated during the oxidation reaction of glycerol to pyruvate which also yields several other organic products such as organic acid (acetic acid, butyric acid, lactic acid, and succinic acid) and alcohol (ethanol and butanol), depending on the type of microorganism [11].
Many types of bacteria, such as Klebsiella pneumoniae [12,13], Clostridium butyricum [14,15], Clostridium beijerinckii [16], Clostridium pasteurianum [17], Escherichia coli [18], Citrobacter freundii and Hafnia alvei [12], Lactobacillus brevis [11], Lactobacillus diolivorans [19], and Lactobacillus reuteri [20], have been reported as capable of producing 1,3-PDO from the fermentation of glycerol. Problems encountered in 1,3-PDO production by fermentation of glycerol include low yields, inhibition by substrates and products, formation of by-products, and high costs of both substrate and fermentation medium components. One way to solve this problem is to use immobilized microbial cells instead of free cells in the fermentation process; this allows reusage of microbial cells, high cell density, protection from inhibition by substrates and products, and easy product harvesting [21]. However, the production of 1,3-PDO from glycerol fermentation with the use of immobilized cells is still limited. Previous research has reported that Bacillus pumilus, which was obtained from the wastewater of a Nham factory, has the distinctive characteristic of exhibiting resistance to inorganic salts and the ability to produce polyhydroxyalkanoate via oxidation of crude glycerol [22]. Therefore, the production of 1,3-PDO from crude glycerol using immobilized B. pumilus cells is a potentially promising approach that merits further investigation. In this study, the Plackett-Burman design (PBD) was employed to decrease the number of trials required as it selectively determines only the factors that affect the synthesis of 1,3-PDO [23]. In addition, the most favorable concentrations of culture medium components and appropriate environmental conditions to produce 1,3-PDO are investigated based on the response surface methodology (RSM). Overall, this study's findings can be used as guidelines to reduce the cost of 1,3-PDO production by glycerol fermentation and can be implemented in industrial settings.
2. Materials and methods
2.1. Microorganism and pre-culturing
The wastewater of a Nham factory in Uttaradit Province, Thailand, was utilized to isolate the bacterial strain B. pumilus [22]. Microbial cells (one loop) were inoculated in a 200-mL pre-culture medium containing 10 g/L peptone, 10 g/L yeast extract, 5 g/L beef extract, 5 g/L ammonium sulfate, and 2 g/L pure glycerol. This inoculation was performed at a temperature of 30 °C in a shaking incubator operating at 200 rpm for 30 h. The cells were isolated from the pre-culture medium using centrifugation (10 min at 5000 g and 4 °C). The cell precipitate was rinsed with sterile distilled water (5 mL) three times and then dissolved in 70 mL of sterile distilled water and retained for use in the next experiment.
2.2. Cell immobilization
The sodium alginate solution was prepared by dissolving 2.1 g of sodium alginate (Sigma-Aldrich, Saint Louis, USA) in 70 mL of 0.85 % w/v NaCl. The mixture was stirred until homogeneous (about 1 h). Subsequently, 70 mL of B. pumilus suspension from Section 2.1 was added to the above sodium alginate solution and gently stirred. The solution of the cells and the sodium alginate were dropped into 105 mL of 2 % w/v CaCl2 to form beads using a 10-mL graduated syringe and shaken gently for 1 h in a separate beaker. The calcium alginate beads were stored in the CaCl2 solution at 4 °C and washed with 0.85 % w/v NaCl before further use [24].
2.3. Crude glycerol preparation
Crude glycerol was collected from the chemistry laboratory of Uttaradit Rajabhat University. This was produced from the transesterification of palm oil and methanol at a mole ratio of 1:6 oil to methanol in chloroform, which was catalyzed by rice bran lipase. The reaction was performed at 40 °C for 36 h. The crude glycerol was light brown, had a pH of 6.71 ± 0.15, and contained 80.04 ± 4.00 % glycerol, 5 ± 0.25 % NaCl, and 3 ± 0.15 % water.
2.4. Optimization experiments design
2.4.1. Optimization of culture medium components
A PBD was employed to assess the impact of different nutrients in the culture medium on the synthesis of 1,3-propanediol (1,3-PDO). The independent variables in the liquid medium consisted of 10 nutrients: crude glycerol, K2HPO4, KH2PO4, (NH4)2SO4, MgSO4.7H2O, CaCl2.2H2O, FeCl2.7H2O, FeSO4.7H2O, yeast extract, and mineral solution. Two levels, low (−) and high (+), were used to evaluate each nutrient. The low level was taken from a literature review [25], and this was increased 1.25-fold to determine the high level. The experimental concentrations of each nutrient are presented in Supplement 1. The experimental design consisted of 16 runs (each row), in which 10 factors were adjusted at two different levels, as shown in Table 1. Each test was conducted in a 250-mL Erlenmeyer flask, with 100 mL of culture media containing the 10 nutrients (Table 1) and 200 g of immobilized B. pumilus. The flask was placed in a shaking incubator at a temperature of 30 °C and shaken at a speed of 200 rpm for 72 h. The experiments were performed in triplicate. The culture broth (20 mL) from each experimental run was centrifuged at 8000 g for 10 min. Subsequently, the residual glycerol, 1,3-PDO, and organic acids were collected for further examination. The statistical significance of the results was determined using multiple linear regression with a backward elimination approach. The significance level was set to 5 % (p < 0.05).
Table 1.
Plackett–Burman design for culture medium components to metabolite production.
| Run | A | B | C | D | E | F | G | H | I | J | 1,3-PDO (g/L) | Acetic acid (g/L) | Residual glycerol (g/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | −1 | 1 | −1 | 1 | 1 | −1 | 13.55 | 2.85 | 8.60 |
| 2 | 1 | 1 | 1 | −1 | 1 | −1 | 1 | 1 | −1 | −1 | 13.76 | 2.89 | 8.35 |
| 3 | 1 | 1 | −1 | 1 | −1 | 1 | 1 | −1 | −1 | 1 | 13.52 | 2.84 | 8.64 |
| 4 | 1 | −1 | 1 | −1 | 1 | 1 | −1 | −1 | 1 | −1 | 13.53 | 2.84 | 8.63 |
| 5 | −1 | 1 | −1 | 1 | 1 | −1 | −1 | 1 | −1 | −1 | 13.29 | 2.80 | 3.91 |
| 6 | 1 | −1 | 1 | 1 | −1 | −1 | 1 | −1 | −1 | −1 | 13.33 | 2.80 | 8.87 |
| 7 | −1 | 1 | 1 | −1 | −1 | 1 | −1 | −1 | −1 | 1 | 13.23 | 2.78 | 3.99 |
| 8 | 1 | 1 | −1 | −1 | 1 | −1 | −1 | −1 | 1 | 1 | 13.11 | 2.76 | 9.13 |
| 9 | 1 | −1 | −1 | 1 | −1 | −1 | −1 | 1 | 1 | 1 | 12.82 | 2.70 | 9.48 |
| 10 | −1 | −1 | 1 | −1 | −1 | −1 | 1 | 1 | 1 | 1 | 11.96 | 2.52 | 5.52 |
| 11 | −1 | 1 | −1 | −1 | −1 | 1 | 1 | 1 | 1 | −1 | 11.90 | 2.50 | 5.60 |
| 12 | 1 | −1 | −1 | −1 | 1 | 1 | 1 | 1 | −1 | 1 | 13.69 | 2.88 | 8.43 |
| 13 | −1 | −1 | −1 | 1 | 1 | 1 | 1 | −1 | 1 | −1 | 13.72 | 2.88 | 3.40 |
| 14 | −1 | −1 | 1 | 1 | 1 | 1 | −1 | 1 | −1 | 1 | 13.54 | 2.84 | 3.62 |
| 15 | −1 | 1 | 1 | 1 | 1 | −1 | 1 | −1 | 1 | 1 | 13.43 | 2.82 | 3.75 |
| 16 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | 10.86 | 2.28 | 6.86 |
A, crude glycerol; B, K2HPO4; C, KH2PO4; D, (NH4)2SO4; E, MgSO47H2O; F, CaCl2.2H2O; G, FeCl2.7H2O; H, FeSO4.7H2O; I, yeast extract; J, mineral solution.
Based on the Plackett-Berman experiment, the independent variables selected in relation to 1,3-PDO production by immobilized B. pumilus (crude glycerol, (NH4)2SO4, MgSO4.7H2O, and CaCl2.2H2O) were to be further assessed using a Box-Behnken design to identify the optimal quantity for the culture medium. The experimental design consisted of 29 runs, as shown in Table 2. Similar to the PBD experiments, the tests were conducted using a 250-mL Erlenmeyer flask with 100 mL of growth medium consisting of 3.4 g/L K2HPO4, 1.3 g/L KH2PO4, and 0.01 g/L FeCl2.7H2O, 0.005 g/L FeSO4.7H2O, 2.0 g yeast extract, and 2 mL mineral solution, which consisted of 70 mg/L ZnCl2 and 100 mg/L MnCl2.4H2O, 60 mg/L H3BO3, 200 mg/L CoCl2.4H2O, 20 mg/L CaCl2.2H2O, 25 mg/L NiCl2.6H2O, 35 mg/L Na2MoO4.2H2O, and 4 mL/L HCl. The concentrations of crude glycerol, (NH4)2SO4, CaCl2.2H2O, and MgSO4.7H2O used will be coded into three distinct levels, namely low, middle, and high, which are denoted as −1, 0, and 1, respectively, as presented in Supplement 2. Fermentation was initiated by adding 200 g of immobilized B. pumilus. The flask was placed in a shaking incubator at a temperature of 30 °C and incubated at a speed of 200 rpm for 72 h. All of the experiments were conducted in triplicate. The 1,3-PDO content was chosen as the response variable, Y. The mathematical relationship between the response and independent variables, X (X1: crude glycerol; X2: ammonium sulfate; X3: magnesium sulfate; and ×4: calcium chloride), was calculated based on the response surface regression Equation [1], as follows:
| Equation [1] |
Table 2.
Box–Behnken experiments design matrix with experimental and value of 1,3-propanediol yield for culture medium component.
| Run | X1 | X2 | X3 | X4 | Crude Glycerol (g/L) |
(NH4)2SO4 (g/L) |
MgSO4.7H2O (g/L) | CaCl22H2O (g/L) | 1,3-PDO (g/L) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | −1 | −1 | 0 | 0 | 25 | 2.5 | 0.5 | 0.05 | 15.00 |
| 2 | 1 | −1 | 0 | 0 | 75 | 2.5 | 0.5 | 0.05 | 24.98 |
| 3 | 0 | 0 | −1 | 1 | 50 | 5 | 0.25 | 0.075 | 25.91 |
| 4 | −1 | 1 | 0 | 0 | 25 | 7.5 | 0.5 | 0.05 | 20.24 |
| 5 | 0 | 0 | 1 | −1 | 50 | 5 | 0.75 | 0.025 | 23.84 |
| 6 | 1 | 1 | 0 | 0 | 75 | 7.5 | 0.5 | 0.05 | 38.54 |
| 7 | 0 | 0 | 1 | 1 | 50 | 5 | 0.75 | 0.075 | 27.67 |
| 8 | −1 | 0 | 0 | −1 | 25 | 5 | 0.5 | 0.025 | 18.84 |
| 9 | −1 | 0 | 0 | 1 | 25 | 5 | 0.5 | 0.075 | 20.32 |
| 10 | 0 | −1 | 0 | 1 | 50 | 2.5 | 0.5 | 0.075 | 28.24 |
| 11 | 1 | 0 | 0 | −1 | 75 | 5 | 0.5 | 0.025 | 31.24 |
| 12 | 1 | 0 | 0 | 1 | 75 | 5 | 0.5 | 0.075 | 34.24 |
| 13 | 0 | −1 | −1 | 0 | 50 | 2.5 | 0.25 | 0.05 | 24.00 |
| 14 | −1 | 0 | −1 | 0 | 25 | 5 | 0.25 | 0.05 | 20.84 |
| 15 | 0 | 0 | −1 | −1 | 50 | 5 | 0.25 | 0.025 | 19.24 |
| 16 | 0 | −1 | 1 | 0 | 50 | 2.5 | 0.75 | 0.05 | 17.00 |
| 17 | 0 | 0 | 0 | 0 | 50 | 5 | 0.5 | 0.05 | 25.30 |
| 18 | 0 | 0 | 0 | 0 | 50 | 5 | 0.5 | 0.05 | 26.24 |
| 19 | 0 | 0 | 0 | 0 | 50 | 5 | 0.5 | 0.05 | 25.14 |
| 20 | 0 | 0 | 0 | 0 | 50 | 5 | 0.5 | 0.05 | 24.74 |
| 21 | 0 | 0 | 0 | 0 | 50 | 5 | 0.5 | 0.05 | 24.27 |
| 22 | 0 | 1 | −1 | 0 | 50 | 7.5 | 0.25 | 0.05 | 29.24 |
| 23 | 0 | 1 | 1 | 0 | 50 | 7.5 | 0.75 | 0.05 | 29.94 |
| 24 | −1 | 0 | 1 | 0 | 25 | 5 | 0.75 | 0.05 | 15.84 |
| 25 | 1 | 0 | −1 | 0 | 75 | 5 | 0.25 | 0.05 | 32.94 |
| 26 | 1 | 0 | 1 | 0 | 75 | 5 | 0.75 | 0.05 | 35.00 |
| 27 | 0 | −1 | 0 | −1 | 50 | 2.5 | 0.5 | 0.025 | 14.24 |
| 28 | 0 | 1 | 0 | −1 | 50 | 7.5 | 0.5 | 0.025 | 27.24 |
| 29 | 0 | 1 | 0 | 1 | 50 | 7.5 | 0.5 | 0.075 | 23.00 |
where Y represents the anticipated outcome for the parameter. β0 represents a constant, while βi (i = 1, 2, 3, 4) and βij (i = 1, 2, 3, 4; j = 1, 2, 3, 4; i ≠ j) represent the model's coefficients. The independent variables are denoted as Xi and Xj.
2.4.2. Optimization of fermentation conditions
Once the composition of the culture medium was optimized, the PBD was employed to identify the key environmental factors that influence 1,3-PDO production. This experiment included five distinct independent variables: shaking speed of the incubator (rpm), incubation temperature (οC), initial pH of the culture medium, incubation time (hours), and weight of immobilized B. pumilus (g), which were denoted as A, B, C, D, and E, respectively. Additionally, two factors (F and G) were included as dummy variables. Each independent variable was assigned two levels (low and high, denoted as −1 and +1). The low and high values of each variable were as follows: A {100, 200}, B {30, 40}, C {7, 9}, D {24, 72}, and E {100, 200}. The studies were performed using a 250-mL Erlenmeyer flask containing 100 mL of the growth medium, which consists of all the above compositions except crude glycerol, (NH4)2SO4, CaCl2.2H2O, and MgSO4. 7H2O will be used at the optimal quantities derived from Equation [2]. Eight experiments were conducted in triplicate, as listed in Supplement 3. The culture broth (20 mL) from each experimental run was subjected to centrifugation at 8000 g for 10 min to remove cell pellets. The supernatant was subsequently analyzed for residual glycerol, 1,3-PDO, and organic acids. The statistical significance of the results was determined using multiple linear regression with a backward elimination approach in conjunction with factors, with a significance level of 5 % (p < 0.05).
2.4.3. Experimental evaluation
The production efficiency of 1,3-PDO was enhanced by tests that identified the optimal growth medium components and experimental conditions, as explained in Sections 2.4.1, 2.4.2. The 100 mL of liquid growth medium containing the optimal nutrient composition was prepared and adjusted to pH 7. The optimum quantity of immobilized cells was added. The fermentation was carried out at the optimum temperature and time derived from Equation (3) while maintaining a shaking speed of 100 rpm. The experiment was repeated three times. A volume of 20 mL of culture broth was centrifuged at a force of 8000 g for 10 min to allow subsequent analysis of residual glycerol, 1,3-PDO, and organic acids. Subsequently, the mean amount of synthesized 1,3-PDO was compared to the values predicted by the regression model. The experimental data should exhibit a maximum deviation of 5 % from the predictions [26].
2.5. Analytical method
The glycerol, 1,3-PDO, and organic acid contents of the samples were analyzed using high-performance liquid chromatography (HPLC). The injection sample (10 μL) was acquired by passing it through a 0.22 μm membrane (Millex-GS, Millipore, USA). The mobile phase (0.004 M H2SO4) was passed through an Aminex HPX-87H 300 mm × 7.8 mm column (Bio-Rad) at a flow rate of 0.6 mL/min. A consistent temperature of 65 οC was maintained during this experiment. Peak areas were identified and quantified using external standards, namely 1,3-PDO, glycerol, butyric acid, and acetic acid. The retention periods of 1,3-PDO, glycerol, butyric acid, and acetic acid were measured as 17.17, 13.03, 20.57, and 14.4 min, respectively [4].
3. Results and discussions
3.1. The effect of selecting culture medium components on 1,3-PDO production
A statistical analysis was conducted to assess the impact of culture medium composition variables on the synthesis of 1,3-PDO by immobilized B. pumilus cells. A PBD was employed to screen the independent variables that significantly influence 1,3-PDO production. Table 1 presents the outcomes of each series of studies. The 1,3-PDO yield data were analyzed using multiple linear regression with a backward elimination approach, which is an effective way to remove the independent variables with the highest p-values (the least important variables) one by one until the most important variables remain. The findings revealed that the independent variables that were found to significantly positively affect the production of 1,3-PDO included crude glycerol (A), KH2PO4 (C), (NH4)2SO4 (D), MgSO4.7H2O (E), and CaCl2 (F), which are presented in Supplement 5. The excluded independent variables were K2HPO4 (B), FeCl2.7H2O (G), FeSO4.7H2O (H), yeast extract (I), and mineral solution (J) because they had high p-values (p > 0.05). This result is opposite to the 1,3-PDO production from crude glycerol by mixed culture obtained from a poultry slaughterhouse [27] which showed that KH2PO4 and vitamin B12 were significant for 1,3-PDO yield. The most important medium component is carbon, which provides microorganisms with energy and helps them grow and produce primary and secondary metabolites (e.g., 1,3-PDO) [23]. In this study, crude glycerol is the sole carbon source that is also significant, as it directly controls the energy supply to B. pumilus (via oxidation), which is essential for their growth and the reduction of glycerol for 1,3-PDO production. (NH4)2SO4, which is an inorganic nitrogen source, had an influence on the production of 1,3-PDO by immobilized B. pumilus because it was used as a precursor for vitamins, amino acids, and growth factors. This result is in contrast with Moon et al.'s study, which reported that (NH4)2SO4 had a negative effect on 1,3-PDO production while yeast extract had a positive effect on 1,3-PDO production by Clostridium pasteurianum [17].
Although KH2PO4 and K2HPO4 function as phosphate sources, this is crucial for preserving the buffer capacity of the culture medium, thereby facilitating the growth and energy generation of bacterial cells. Furthermore, it functions as a phosphorus source, which is essential for producing deoxyribonucleic acid (DNA), ribonucleic acid (RNA), phospholipids (a constituent of the plasma membrane), and adenosine triphosphate (ATP) from adenosine diphosphate (ADP) via the oxidative phosphorylation process [27]. The result of the present study did not show a significant effect on 1,3-PDO production from crude glycerol by immobilized B. pumilus (p values of KH2PO4 and K2HPO4 equal 0.067 and 0.171, respectively, p > 0.05). This is contrary to the study of Paranhos and Silva (2018) [27] which reported that the variables KH2PO4 and vitamin B12 were significant for 1,3-PDO yield (p < 0.05) produced from crude glycerol using mixed cultures in batch and continuous reactors. The maximum 1,3-PDO yields occurred when using high concentrations of vitamin B12 and low levels of KH2PO4.
According to Supplement 5, the variable MgSO4 was the most significant for 1,3-PDO production (p = 0.002). This finding is consistent with a study conducted by Li-Xia et al. (2020) [28], which found that the addition of MgSO4 significantly affected fermentation by Bacillus subtilis B201. If the amount of MgSO4 in the culture medium exceeds 0.87 %, it affects the pH, creating a weakly alkaline environment that inhibits microorganism growth. The variable CaCl2 is also significant for 1,3-PDO yield (p = 0.032). This substance liberates calcium ions, which are required for the respiration and fundamental cellular pathways of bacteria [29]. Therefore, in the present study, crude glycerol, (NH4)2SO4, MgSO4.7H2O, and CaCl2.2H2O (variables A, D, E, and F, respectively) were selected for the Box-Behnken design experiment to optimize 1,3-PDO yields. The results of the experiment are presented in Table 2. These results were statistically analyzed using multiple linear regression, and the findings, as presented in Table 3, were then utilized to determine correlations between different variables and the yield of 1,3-PDO. These are represented by a quadratic equation, as shown in Equation [2].
| Equation [2] |
Table 3.
The least-square fit and parameters (significant of regression coefficient) of culture medium component.
| Model | Unstandardized Coefficients |
Standardized Coefficients |
t |
Sig |
95.0 % Confidence Interval for B |
||
|---|---|---|---|---|---|---|---|
| B | Std. Error | Beta | Lower Bound | Upper Bound | |||
| Constant | 35.274 | 1.012 | 34.858 | <0.001 | 33.104 | 37.444 | |
| X1 (Crude glycerol) | 9.318 | 0.653 | 0.663 | 14.266 | <0.001 | 7.917 | 10.719 |
| X2 ((NH4)2SO4) | 0.818 | 0.653 | 0.058 | 1.253 | 0.231 | −0.583 | 2.219 |
| X3 (MgSO4) | 1.939 | 0.653 | 0.138 | 2.969 | 0.010 | 0.538 | 3.340 |
| X4 (CaCl2) | 1.912 | 0.653 | 0.136 | 2.928 | 0.011 | 0.512 | 3.313 |
| X12 | −12.260 | 0.888 | −0.667 | −13.799 | <0.001 | −14.165 | −10.354 |
| X22 | −5.740 | 0.888 | −0.312 | −6.460 | <0.001 | −7.645 | −3.834 |
| X32 | −3.826 | 0.888 | −0.208 | −4.306 | 0.001 | −5.731 | −1.920 |
| X42 | −0.803 | 0.888 | −0.044 | −0.904 | 0.381 | −2.709 | 1.102 |
| X1X2 | 0.542 | 1.131 | 0.022 | 0.480 | 0.639 | −1.884 | 2.969 |
| X1X3 | 1.715 | 1.131 | 0.070 | 1.516 | 0.152 | −0.712 | 4.142 |
| X1X4 | 1.313 | 1.131 | 0.054 | 1.160 | 0.265 | −1.114 | 3.739 |
| X2X3 | −3.310 | 1.131 | −0.136 | −2.926 | 0.011 | −5.737 | −0.883 |
| X2X4 | −0.848 | 1.131 | −0.035 | −0.749 | 0.466 | −3.274 | 1.579 |
| X3X4 | 0.802 | 1.131 | 0.033 | 0.709 | 0.490 | −1.624 | 3.229 |
Dependent Variables: 1,3-PDO.
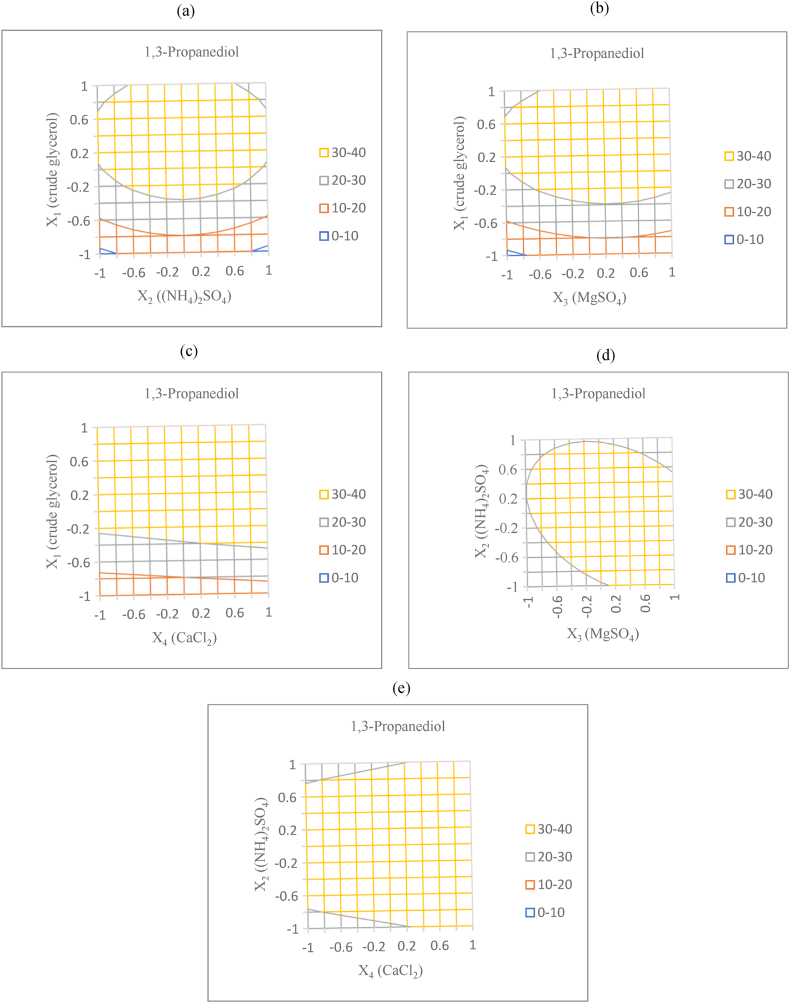
The regression equation of the relationship between variables was used to predict the appropriate number of components in the culture medium that were significantly related to the amount of 1,3-PDO. When calculating the 1,3-PDO values from Equation [2], the relationships between pairs of variables were considered to create wireframe contour plots. Therefore, the other variables that were not being considered were set to a constant value of 0, and the studied pair of variables was set to a range of −1 to 1, as shown in Fig. 1a–e. The ellipses in Fig. 1a and b shows significant interactions between crude glycerol and (NH4)2SO4 and between crude glycerol and MgSO4, respectively. In contrast, Fig. 1c–e shows circular shapes, indicating a lack of interactions between the remaining variables [30]. The relationship between crude glycerol and (NH4)2SO4 (Fig. 1a) revealed that the maximum 1,3-PDO yield (37.04 g/L) occurs when using an X1 (crude glycerol) level of 0.4 (equivalent to 60 g/L) and an X2 ((NH4)2SO4) level of 0 (equivalent to 5 g/L). The relationship between crude glycerol and MgSO4 (Fig. 1b) revealed that the maximum 1,3-PDO yield (37.27 g/L) occurs when using an X1 (crude glycerol) level of 0.4 (equivalent to 60 g/L) and an X3 (MgSO4) level of 0.2 (equivalent to 0.55 g/L). The relationship between crude glycerol and CaCl2 (Fig. 1c) revealed the highest 1,3-PDO production (37.04 g/L) corresponded to an X1 (crude glycerol) level of 0.4 (equivalent to 60 g/L) and an X4 (CaCl2) level of 0 (equivalent to 0.05 g/L). The correlation between (NH4)2SO4 and MgSO4 (Fig. 1d) indicated that an amount of 1,3-PDO (35.51 g/L) was produced when using an X2 (NH4)2SO4 level of 0 (equivalent to 5 g/L) and an X3 (MgSO4) level of 0.2 (equivalent to 0.55 g/L), while the relationship between (NH4)2SO4 and CaCl2 (Fig. 1e) indicated a maximum 1,3-PDO yield (37.19 g/L) when using an X2 (NH4)2SO4 level of 0 (equivalent to 5 g/L) and an X4 (CaCl2) level of 1 (equivalent to 0.075 g/L). Overall, the values for the components of the culture medium in Equation [2] indicate that using raw glycerol at 60 g/L, (NH4)2 SO4 at 5 g/L, MgSO4 at 0.55 g/L, and CaCl2 at 0.05 g/L in the culture medium will result in the maximum 1,3-PDO yield (37.27 g/L). However, the output of the predicted 1,3-PDO amount was less than Zhang's study, which found that the prediction of 1,3-PDO production of E. coli JM109 (pHsh-dhaB-yqhD) gave the highest yield of 43.86 g/L when 61.8 g/L glycerol, 6.2 g/L yeast extract, and 49 mg/L vitamin B12 were used [26].
Fig. 1.
Contour plot for 1,3-PDO yield as a function of crude glycerol and (NH4)2SO4 (a); crude glycerol and MgSO4 (b); crude glycerol and CaCl2 (c); (NH4)2SO4 and MgSO4 (d); and (NH4)2SO4 and CaCl2 (e).
3.2. Suitable conditions for production 1,3-PDO
The fermentation efficiency of 1,3-PDO generation from crude glycerol by immobilized B. pumilus cells is influenced by various factors, aside from the components of the culture medium. These factors include the shaking speed, temperature, pH, reaction time, and quantity of immobilized cells. This study employed the PBD approach to determine the primary physical parameters that control 1,3-PDO production. The experimental findings are presented in Supplement 3, illustrating the spectrum of 1,3-PDO production yields, which varied between 29.33 and 42.89 g/L. The statistical analysis employed a multiple linear regression technique to conduct a backward elimination analysis. The primary determinants influencing 1,3-PDO production were the quantity of immobilized cells (p = 0.014), followed by time (p = 0.025) and temperature (p = 0.028). A positive correlation affecting 1,3-PDO production is between the mass of immobilized cells and the duration of the experiment. But the temperature showed a negative correlation with 1,3-PDO generation (Supplement 6). This study found the same thing as another one that looked at how the fermentation environment affected the production of 1,3-PDO by E. coli JM109 (pHsh-dhaB-yqhD) using a one-factor-at-a-time method [26]. That study found that the time of fermentation was a key factor that affected the yield of 1,3-propanediol. Hence, a Box-Behnken experimental design was employed to determine the optimal conditions to enhance 1,3-PDO production efficiency, which involved selecting the appropriate values of time, temperature, and immobilized cell mass (Supplement 4). A prediction equation was constructed using multiple linear regression, and the findings are listed in Table 4. Linear regression coefficients and quadratic coefficients (p-value <0.05) were used to establish correlations between the three physical characteristics (temperature, time, and mass of immobilized cells) and 1,3-PDO production, as shown in Equation [3]:
| Equation [3] |
Table 4.
The least–square fit and parameters (significant of regression coefficient) (Fermentation condition).
| Model | Unstandardized Coefficients |
Standardized Coefficients |
t | Sig. | 95.0 % Confidence Interval for B |
||
|---|---|---|---|---|---|---|---|
| B | Std. Error | Beta | Lower Bound | Upper Bound | |||
| 1 (Constant) | 45.460 | 0.439 | 103.475 | <0.001 | 44.421 | 46.499 | |
| X1 (Temp) | −1.465 | 0.347 | −0.126 | −4.218 | 0.004 | −2.286 | −0.644 |
| X2 (Time) | 2.453 | 0.347 | 0.210 | 7.061 | <0.001 | 1.631 | 3.274 |
| X3 (immobilized cell) | 0.663 | 0.347 | 0.057 | 1.907 | 0.098 | −0.159 | 1.484 |
| X12 | −13.049 | 0.479 | −0.813 | −27.256 | <0.001 | −14.181 | −11.917 |
| X22 | −6.809 | 0.479 | −0.424 | −14.222 | <0.001 | −7.941 | −5.677 |
| X32 | −2.569 | 0.479 | −0.160 | −5.366 | 0.001 | −3.701 | −1.437 |
| X1X2 | 0.532 | 0.491 | 0.032 | 1.084 | 0.314 | −0.629 | 1.694 |
| X1X3 | 0.392 | 0.491 | 0.024 | 0.799 | 0.451 | −0.769 | 1.554 |
| X2X3 | 0.627 | 0.491 | 0.038 | 1.278 | 0.242 | −0.534 | 1.789 |
Dependent Variables: 1,3-PDO.
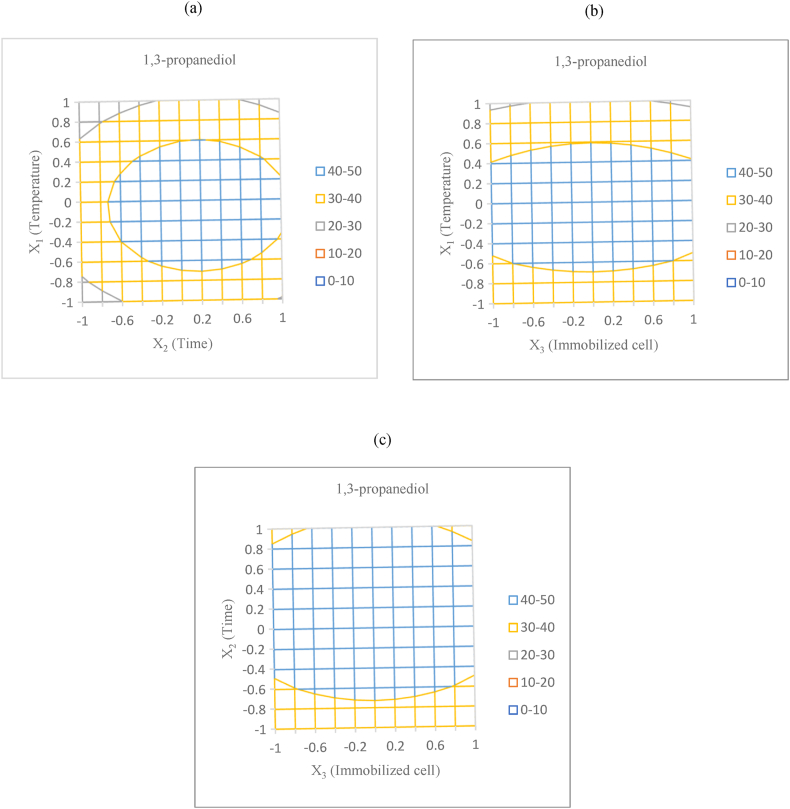
The relationships to predict the levels of these physical factors, including temperature, time, and the mass of immobilized cells, with respect to 1,3-PDO production are shown in Fig. 2(a–c). Fig. 2a shows the contour plots of the relationship between the two independent variables, such as temperature (X1) and time (X2), and 1,3-PDO yield. It was displayed that the 1,3-PDO yield will increase with an increase in temperature and time. A maximum yield of 1,3-PDO was predicted at 45.68 g/L under a fermentation time of level 0.2 (equivalent to 101 h) and a temperature of level 0 (equivalent to 25 °C). In terms of the relationship between temperature and the mass of immobilized cells (Fig. 2b), the yield of 1,3-PDO increases when increasing the immobilized cell from level −1 to level 0. If the mass of immobilized cells increases above level 0, it will reduce the amount of 1,3-PDO produced. The relationship between time and the mass of immobilized cells (Fig. 2c) showed the result in the same way. Therefore, the maximum 1,3-PDO yield of 45.68 g/L will be produced under optimum conditions: a temperature of level 0 (equivalent to 25 °C), a mass of immobilized cells at level 0 (equivalent to 250 g), and a fermentation time of level 0.2 (equivalent to 101 h). The predicted maximum yield of 1,3-PDO was obtained by adjusting the components of the culture medium and the fermentation conditions according to Equations Equation [2], Equation [3]), respectively. The predicted optimum temperature of immobilized B. pumilus for 1,3-PDO production (25 °C) was not consistent with the optimum temperature of polyhydroxyalkanoate production (40 °C) by B. pumilus [22]. This difference can be explained by previous studies reporting that B. pumilus can grow at temperatures of approximately 5–50 °C [31].
Fig. 2.
Contour plot for 1,3-PDO yield as a function of: Temperature and time (a); Temperature and immobilized cell (b); and Time and immobilized cell (c).
3.3. Confirmation of results
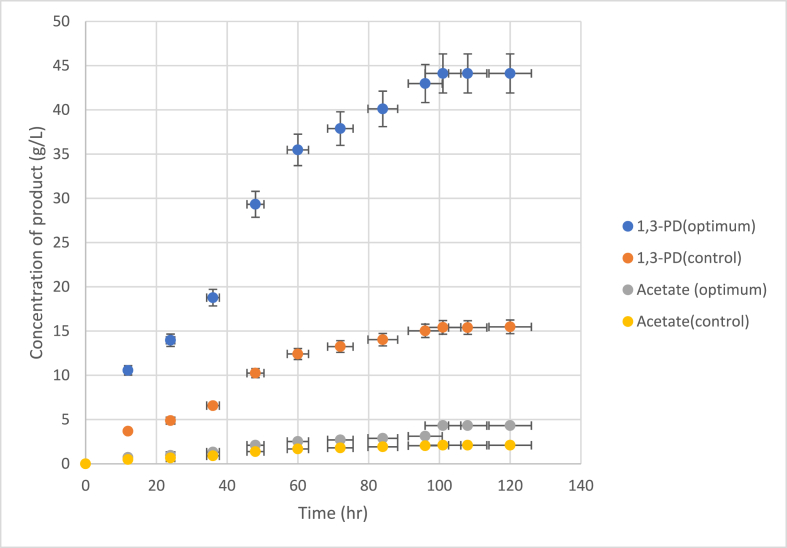
The experimentally determined maximum yield of 1,3-PDO under ideal circumstances was found to be 44.12 ± 1.81 g/L after 101 h (Fig. 3), which is 3.41 % less than the model prediction (45.68 g/L). The result showed that the mathematical model of Equations Equation [2], Equation [3]), which was used for the prediction of the optimum culture medium component and optimum condition for the production of 1,3-PDO by immobilized cells of B. pumilus, has a high level of reliability and suitability [26]. This finding is similar to that of a study on the optimum conditions for 1,3-PDO production by Klebsiella pneumoniae, which used RSM based on a 25-factorial central composite design (CCD) (Oh et al., 2008) [32]. They experimentally produced 1,3-PDO using 35 g/L of crude glycerol, 8 g/L of (NH4)2SO4, a pH of 7.37, and a temperature of 36.88 °C for 10.8 h, yielding approximately 13.88 g/L of 1,3-PDO, which was similar to that of the predicted model (13.74 g/L). Overall, the PBD and RSM approaches used in this experiment to identify the optimal reaction conditions and enhance 1,3-PDO production by B. pumilus resulted in a 2.86-fold rise in the yield of 1,3-PDO. The 1,3-PDO yield from the present study was better than Chatzifragkou's study (2011) [14], which found that naturally, Clostridium butyricum DSP1 and Lactococcus gasviea DSL1, which were isolated from the ruminal fluid of a cow, produced 29.1 and 19.8 g/L of 1,3-PDO, respectively. Moreover, it exceeded the efficiency of 1,3-PDO production by Lactobacillus diolivorans DSM 14421 with a mixed substrate of glucose and glycerol, which yielded 41.70 g/L after 139 h at 30 °C [33].
Fig. 3.
1,3-propanediol and acetate yield before and after the statistically optimized medium and physical conditions.
In addition to 1,3-PDO, acetic acid was produced as a major by-product during the fermentation of crude glycerol by B. pumilus (Fig. 3). These results differ from a study conducted by Pflugl et al. (2012) [33], which found that the co-fermentation of glucose and glycerol by Lactobacillus diolivorans produces 1,3-PDO, lactic acid, acetic acid, and ethanol. This phenomenon was found in the oxidation pathway of glycerol, which produces pyruvate and NADH. Then, the pyruvate further converts into acetyl phosphate and subsequently produces acetic acid and ATP. Microbial cells utilize ATP as a cellular energy source during the stationary phase. NADH is used in the reduction pathway of glycerol to 1,3-PDO, which is also used for NAD regeneration and the oxidation pathway of glycerol [16]. The advantage of the production of 1,3-propanediol from crude glycerol by immobilized cells of B. pumilus is that it is easy to purify 1,3-PDO from culture medium because only one by-product is obtained. Moreover, using immobilized cells will decrease the toxicity of the impurities contained in crude glycerol.
4. Conclusions
B. pumilus, isolated from Nham industrial effluent, can synthesize 1,3-PDO from crude glycerol. Plackett-Burman design revealed that variables including crude glycerol, (NH4)2SO4, MgSO4.7H2O, CaCl2.2H2O, time, temperature, and quantity of immobilized B. pumilus had a significant influence on 1,3-PDO yield. The predicted maximum yield of 1,3-PDO with response surface methodology was 45.68 g/L under 60 g/L crude glycerol, 5 g/L (NH4)2SO4, 0.55 g/L MgSO4.7H2O, 0.05 g/L CaCl2.2H2O, and 250 g of immobilized B. pumilus at a temperature of 25 °C for 101 h. The next step is the development of a pilot process for 1,3-PDO production by immobilized B. pumilus, including the study of batch and fed batch processes in a 5 L fermentor. The optimization of the feeding substrate, areation, temperature and pH control, and antifoaming will also be investigated to further scale up to industrial applications.
Data availability statement
Data will be made available on request.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
CRediT authorship contribution statement
Porntippa Pinyaphong: Writing – review & editing, Writing – original draft, Visualization, Resources, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Aroon La-up: Writing – review & editing, Validation, Methodology, Formal analysis, Data curation.
Declaration of AI and AI-assisted technologies in the writing process
During the preparation of this work the author(s) used ChatGPT 3.5 in order to preparation phase to detect and rectify grammatical problems that arose during the writing process. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are pleased to acknowledge financial support from the National Research Council of Thailand [Grant Number: 2560A16302031].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e35349.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Tan H.W., Abdul Aziz A.R., Aroua M.K. Glycerol production and its applications as a raw material: a review. Renew. Sustain. Energy Rev. 2013;27:118–127. [Google Scholar]
- 2.Thompson J., He B. Characterization of crude glycerol from biodiesel production from multiple feedstocks. Appl. Eng. Agric. 2006;22 [Google Scholar]
- 3.Johnson D.T., Taconi K.A. The glycerin glut: options for the value‐added conversion of crude glycerol resulting from biodiesel production. Environ. Prog. 2007;26(4):338–348. [Google Scholar]
- 4.Szymanowska-Powałowska D. 1,3-Propanediol production from crude glycerol by Clostridium butyricum DSP1 in repeated batch. Electron. J. Biotechnol. 2014;17(6):322–328. [Google Scholar]
- 5.Hu B., Das P., Lv X., Shi M., Aa J., Wang K., Duan L., Gilbert J., Nie Y., Wu x. Effects of ‘Healthy’ fecal microbiota transplantation against the deterioration of depression in fawn-hooded rats. mSystems. 2022;7(3) doi: 10.1128/msystems.00218-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ji L., Lei F., Zhang W., Song X., Jiang J., Wang K. Enhancement of bioethanol production from Moso bamboo pretreated with biodiesel crude glycerol: substrate digestibility, cellulase absorption and fermentability. Bioresour. Technol. 2019;276:300–309. doi: 10.1016/j.biortech.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 7.Fan X., Burton R., Zhou Y. Glycerol (byproduct of biodiesel production) as a source for fuels and chemicals mini review. Open Fuel Energy Sci. J. 2010;3(1) [Google Scholar]
- 8.Sun Y.-Q., Shen J.-T., Yan L., Zhou J.-J., Jiang L.-L., Chen Y., et al. Advances in bioconversion of glycerol to 1,3-propanediol: prospects and challenges. Process Biochem. 2018;71:134–146. [Google Scholar]
- 9.Oh B.-R., Lee S.-M., Heo S.-Y., Seo J.-W., Kim C.H. Efficient production of 1,3-propanediol from crude glycerol by repeated fed-batch fermentation strategy of a lactate and 2,3-butanediol deficient mutant of Klebsiella pneumoniae. Microb. Cell Factories. 2018;17(1):92. doi: 10.1186/s12934-018-0921-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olga Guerrero-Perez M., Rosas J., Bedia J., Rodríguez-Mirasol J., Cordero T. Recent inventions in glycerol transformations and processing. Recent Pat. Chem. Eng. 2009;2(11) [Google Scholar]
- 11.Vivek N., Pandey A., Binod P. Biological valorization of pure and crude glycerol into 1,3-propanediol using a novel isolate Lactobacillus brevis N1E9.3.3. Bioresour. Technol. 2016;213:222–230. doi: 10.1016/j.biortech.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 12.Drożdżyńska A., Pawlicka J., Kubiak P., Kośmider A., Pranke D., Olejnik-Schmidt A., Czaczyk K. Conversion of glycerol to 1,3-propanediol by Citrobacter freundii and Hafnia alvei - newly isolated strains from the Enterobacteriaceae. N. Biotech. 2014;31(5):402–410. doi: 10.1016/j.nbt.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Lee J.H., Jung M.-Y., Oh M.-K. High-yield production of 1,3-propanediol from glycerol by metabolically engineered Klebsiella pneumoniae. Biotechnol. Biofuels. 2018;11(1):104. doi: 10.1186/s13068-018-1100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chatzifragkou A., Papanikolaou S., Dietz D., Doulgeraki A.I., Nychas G.J., Zeng A.P. Production of 1,3-propanediol by Clostridium butyricum growing on biodiesel-derived crude glycerol through a non-sterilized fermentation process. Appl. Microbiol. Biotechnol. 2011;91(1):101–112. doi: 10.1007/s00253-011-3247-x. [DOI] [PubMed] [Google Scholar]
- 15.Yun J., Yang M., Magocha T.A., Zhang H., Xue Y., Zhang G., et al. Production of 1,3-propanediol using a novel 1,3-propanediol dehydrogenase from isolated Clostridium butyricum and co-biotransformation of whole cells. Bioresour. Technol. 2018;247:838–843. doi: 10.1016/j.biortech.2017.09.180. [DOI] [PubMed] [Google Scholar]
- 16.Wischral D., Zhang J., Cheng C., Lin M., De Souza L.M.G., Pessoa F.L.P., et al. Production of 1,3-propanediol by Clostridium beijerinckii DSM 791 from crude glycerol and corn steep liquor: process optimization and metabolic engineering. Bioresour. Technol. 2016;212:100–110. doi: 10.1016/j.biortech.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 17.Moon C., Lee C.H., Sang B.I., Um Y. Optimization of medium compositions favoring butanol and 1,3-propanediol production from glycerol by Clostridium pas teurianum. Bioresour. Technol. 2022;102:10561–10568. doi: 10.1016/j.biortech.2011.08.094. [DOI] [PubMed] [Google Scholar]
- 18.Przystałowska H., Zeyland J., Szymanowska-Powałowska D., Szalata M., Słomski R., Lipiński D. 1,3-Propanediol production by new recombinant Escherichia coli containing genes from pathogenic bacteria. Microbiol. Res. 2015;171:1–7. doi: 10.1016/j.micres.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Pflügl S., Marx H., Mattanovich D., Sauer M. Heading for an economic industrial upgrading of crude glycerol from biodiesel production to 1,3-propanediol by Lactobacillus diolivorans. Bioresour. Technol. 2014;152:499–504. doi: 10.1016/j.biortech.2013.11.041. [DOI] [PubMed] [Google Scholar]
- 20.Ricci M.A., Russo A., Pisano I., Palmieri L., de Angelis M., Agrimi G. Improved 1,3-propanediol synthesis from glycerol by the robust Lactobacillus reuteri strain DSM 20016. Journal of Microbiolofy and Biotechnology. 2015;25(6):893–902. doi: 10.4014/jmb.1411.11078. [DOI] [PubMed] [Google Scholar]
- 21.Kaur G., Srivastava A.K., Chand S. Advances in biotechnological production of 1,3-propanediol. Biochem. Eng. J. 2012;64:106–118. [Google Scholar]
- 22.Pinyaphong P., Sriburi P. Optimum condition for polyhydroxyalkanoate production from crude glycerol by Bacillus sp. isolated from lipid-containing wastewater. Trends in Sciences. 2022;19(23):2588. [Google Scholar]
- 23.Singh V., Haque S., Niwas R., Srivastava A., Pasupuleti M., Tripathi C.K.M. Strategies for fermentation medium optimization: an in-depth review. Front. Microbiol. 2017;7 doi: 10.3389/fmicb.2016.02087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duarte J.C., Rodrigues J.A., Moran P.J., Valença G.P., Nunhez J.R. Effect of immobilized cells in calcium alginate beads in alcoholic fermentation. Amb. Express. 2013;3(1):31. doi: 10.1186/2191-0855-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Y., Chen G., Yao S.J. Microbial production of 1,3-propanediol from glycerol by encapsulated Klebsiella pneumonia. Biochem. Eng. J. 2006;32:93–99. [Google Scholar]
- 26.Zhang X., Li Y., Zhuge B., Tang X., Shen W., Rao Z., et al. Construction of a novel recombinant Escherichia coli strain capable of producing 1,3–propanediol and optimization of fermentation parameters by statistical design. World J. Microbiol. Biotechnol. 2006;22(9):945–952. [Google Scholar]
- 27.Paranhos A.G.O., Silva E.L. Optimized 1,3-propanediol production from crude glycerol using mixed cultures in batch and continuous reactors. Bioproc. Biosyst. Eng. 2018;41(12):1807–1816. doi: 10.1007/s00449-018-2003-3. [DOI] [PubMed] [Google Scholar]
- 28.Li-xia Z., Fan Y., Kai L., Qi W. Effect of magnesium sulfate on fermentation of Bacillus subtilis. J. Microbiol. 2020;(6):53–58. [Google Scholar]
- 29.Shivaramu S., Tomasch J., Kopejtka K., Nupur Saini MK., Bokhari S.N.H., Kupper H., Koblizek M. The influence of calcium on the growth, morphology and gene regulation in Gemmatimonas phototrophica. Microorganisms. 2023;11(1):27. doi: 10.3390/microorganisms11010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elibol M. Optimization of medium composition for actinorhodin production by Streptomyces coelicolor A3(2) with response surface methodology. Process Biochem. 2004;39(9):1057–1062. [Google Scholar]
- 31.Parvathi A., Krishna K., Jose J., Joseph N., Nair S. Biochemical and molecular characterization of Bacillus pumilus isolated from coastal environment in cochin. Braz. J. Microbiol. 2009;40:269–275. doi: 10.1590/S1517-838220090002000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oh B.R., Seo J.W., Choi M.H., Kim C.H. Optimization of culture conditions for 1,3-propanediol production from crude glycerol by Klebsiella pneumoniae using response surface methodology. Biotechnol. Bioproc. Eng. 2008;13:666–670. [Google Scholar]
- 33.Pflügl S., Marx H., Mattanovich D., Sauer M. 1,3-Propanediol production from glycerol with Lactobacillus diolivorans. Bioresour. Technol. 2012;119:133–140. doi: 10.1016/j.biortech.2012.05.121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.