Abstract
Knowledge regarding the influence of individual traits on interaction patterns in nature can help understand the topological role of individuals within a network of intrapopulation interactions. We tested hypotheses on the relationships between individuals’ positions within networks (specialization and centrality) of 4 populations of the mouse opossum Gracilinanus agilis and their traits (i.e., body length, body condition, tail length relative to body length, sex, reproductive condition, and botfly parasitism) and also seasonal effects in the Brazilian savanna. Individuals with lower body length, better body condition, and relatively shorter tail were more specialized (i.e., less connected within the network). Individuals were also more specialized and less connected during the warm-wet season. The relationship between individuals’ position in the network and body traits, however, was independent of season. We propose that specialization may arise not only as a result of preferred feeding strategies by more capable individuals (i.e., those with better body condition and potentially prone to defend and access high-quality food resources) but also because of morphological constraints. Smaller/younger individuals (consequently with less experience in foraging) and short-tailed individuals (less skilled to explore the vertical strata of the vegetation) would feed only on a subset of the available food resources and consequently become more specialized. Moreover, individuals are more specialized during the warm-wet season because of high competition (population-dense period) and higher ecological opportunities (resource-rich period). Therefore, our study reveals the relevance of individual traits in shaping interaction patterns and specialization in populations.
Keywords: body traits, centrality, Cerrado, Gracilinanus agilis, seasonality, specialization
Individuals in natural animal populations may vary behaviorally and morphologically. This variation may affect how they explore, utilize, and ultimately compete for food resources (e.g., Dingemanse and Réale 2005; Svanbäck and Bolnick 2007; Oliveira et al. 2020). Individual feeding specialization has been widely acknowledged in natural populations of several taxa (Bolnick et al. 2003). Knowledge regarding the influence of individual traits on interaction patterns in nature, however, is incipient for most species, and can be highly context-dependent (Svanbäck and Bolnick 2007; Miguel et al. 2018; Campião and Dáttilo 2020). These ecological differences between individuals play critical roles in various ecological aspects that can affect community responses and ecosystem functions (Guscelli et al. 2019).
Individual feeding specialization may vary mainly according to predation risk, level of competition, and ecological opportunity (e.g., diversity and abundance of food resources availability) (Araújo et al. 2011). Both level of competition and ecological opportunity are factors highly relevant to inter-individual variation in resource use in seasonal environments for different vertebrates, including fish (Costa‐Pereira et al. 2017), frogs (e.g., fishes and frogs; Araújo et al. 2007; Costa‐Pereira et al. 2017), and small mammals (Araújo et al. 2010; Camargo et al. 2019, 2021). For example, in the highly seasonal Brazilian savanna (locally known as the Cerrado), the gracile mouse opossum Gracilinanus agilis tends to present higher individual specialization during the resource-rich and population-dense period (i.e., warm-wet season), through a reduction in trophic niche width (Camargo et al. 2014b). In fact, population-based networks indicate that groups of G. agilis individuals tend to specialize in subsets of available food resources (forming food groups or modules), which may dampen intra and interspecific competition (Camargo et al. 2019).
Competition and other complex ecological relationships can be represented as a network comprised of nodes (e.g., individuals or species in ecological networks), and links between nodes represent interactions (van Veen et al. 2008). Depending on how the interactions between species are organized, the distribution patterns of links lead to different network structures (Guimarães 2020). These structures can be measured with specific network metrics (Costa 2007; Guimarães 2020). Modularity, for instance, measures whether networks are organized in such a way that groups of nodes present high overlap in their interactions forming modules, but sparse connections with nodes of other modules (Olesen et al. 2007). Moreover, there are metrics that indicate the relative importance of nodes (or node position within the network) in interaction patterns (Strona and Veech 2015). In ecological networks at the community level, for instance, the central species (potentially indicated by distinct centrality metrics; Oldham et al. 2019) are more abundant or generalists, and are generally highly connected with several partners in the network (González et al. 2010; Sazima et al. 2010). A similar inference can be extended to individual-based networks (e.g., Gómez and Perfectti 2012). Network centrality metrics (i.e., closeness, betweenness, and degree centrality—Kolaczyk 2009) are a proxy to infer levels of specialization, since in general they identify the most (generalists) and less (specialists) connected nodes (Gómez and Perfectti 2012) in the network. There are other metrics, however, that have also been specifically developed to measure node specialization (e.g., nested rank and resource range—Alarcón et al. 2008; Poisot et al. 2012).
The identification of individual node positions in the network contributes to the understanding of the potential interdependence of different organizational levels of interaction (e.g. individual–individual, individual–species, and species–species) (Tur et al. 2014; Melián et al. 2018; Guimarães 2020). For instance, specialized conspecifics interacting with different subsets of the total resources available, would lead to a pattern that the species is considered generalist in a species-based network (Guimarães 2020). Therefore, downscaling networks from species to individuals is a valuable way to explore mechanisms acting at the individual level, which may affect species network structure (Tur et al. 2014), and to understand the niche dynamics of natural populations according to food resources and competition variation (Araújo et al. 2008; Tur et al. 2014; Camargo et al. 2019). Moreover, identifying node positions within networks has been useful to better comprehend the topological role of individuals with different traits as well as the benefits of occupying a certain position in the network (e.g., individual fitness; Gómez and Perfectti 2012).
Despite the knowledge advances on the patterns that affect individual resource specialization, the assessment of which traits relate to the individual specialization of small mammals, or in an individual-based network context, their network position, remains little explored. Individuals may present age-dependent foraging skills, which has been reported in several animal taxa (Werner and Gilliam 1984). Younger individuals may present a more constrained diet or dietary shifts because of their reduced ability to capture and handle certain food resources (Werner and Gilliam 1984; Rutz et al. 2006; Camargo et al. 2014b). Behavior and dietary shifts have also been reported in parasitized individuals for both vertebrates and invertebrates (Moore 1995), leading to the addition of new food resource types (e.g., medicinal foods—Huffman 1997; Karban and English-Loeb 1997) or avoiding certain types (e.g., larger preys—Moore and Gotelli 1990). For some species, parasites may also affect the competitive ability of individual hosts (Schall 1992; Hudson and Greenman 1998). Moreover, differences in resource use between subgroups of natural populations (e.g., sex, age, and reproductive conditions) have been reported in several studies. These resource-use differences are related to variations in reproductive strategies, body size, home range area, and energetic and nutritional demands (e.g., Cruz‐Neto and Bozinovic 2004; Beck et al. 2007; Camargo et al. 2014a). Therefore, all the above-mentioned factors potentially affect individual positions within an ecological network of interactions.
In this study, we investigated the relationship between individual traits and the position of individuals within networks in 4 distinct populations of the marsupial G. agilis in the Brazilian savanna. We specifically tested whether there is a relationship between individual specialization (based on network metrics of node centrality and specialization—hereafter referred as “node metrics”) and traits related to body measurements (body length, body condition, and tail length), sex, female reproduction, and infestation by the botfly Cuterebra apicalis. In addition, we verified whether these potential relationships vary temporally according to the season. In the present study we considered specialization or generalization under the network perspective, which is commonly evaluated considering the number of realized links by a node (in our case, a marsupial individual) (Blüthgen et al. 2006, 2007).
We expected that small-bodied, heavier, and short-tailed individuals would occupy less central and more specialized positions in the network. Our expectation was based on the low ability of smaller (and younger) individuals to forage (Camargo et al. 2014a); the high ability of individuals with better body condition to defend high-quality resources (Schoener 1983; Persson 1985); and the low ability of short-tailed individual to explore the vertical space to find food resources (Smartt and Lemen 1980). We also expected that females in general, nonreproductive females, and botfly-infected individuals would be more specialists. Females tend to present small home range sizes in comparison to males (Shibuya et al. 2018), accessing only a subset of the available food resources; nonreproductive females are less dependent of energy intake and nutritional requirements in comparison to reproductive ones (Julien-Laferrière 1995; Gentile et al. 1997; Ribeiro 2011); and botfly-infected individuals present low foraging capacity due to their weakened condition to anemia (Zangrandi et al. 2019) and negatively affected movements (Wecker 1962; Smith 1978). Finally, we expected that less connected individuals and feeding specialization occurs during the warm-wet season, since this season represents the resource-rich (high ecological opportunity) and population-dense (increased competition) period (Camargo et al. 2019).
Materials and Methods
Study species
Gracilinanus agilis is a small (mean body mass [g] ± standard deviation [SD] = 23.5 ± 9.2; mean head-body length [mm] ± SD = 85.7 ± 9.5; number of individuals = 345), solitary, nocturnal, and scansorial marsupial (Emmons and Feer 1997; Vieira et al. 2017; Camargo et al. 2018), that is abundant in forest formations present in the Brazilian Cerrado (Nitikman and Mares 1987). Its reproduction is highly seasonal, occurring from the end of the cool-dry season (i.e., September) to the middle/end of the warm-wet season (i.e., between December and March; Martins et al. 2006b). The diet of G. agilis is comprised mainly by pioneer fruits and invertebrates, but it also occasionally feeds on birds (Camargo et al. 2014a).
Study area
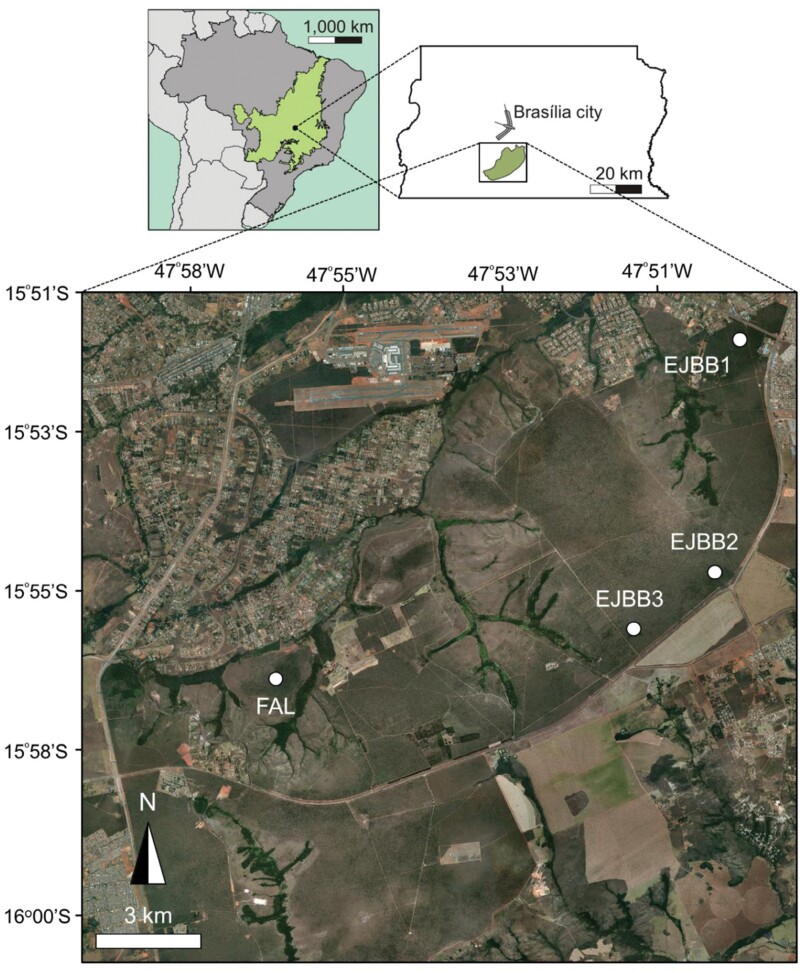
This study was conducted in the core area of the Cerrado, which contains vegetation types ranging from grasslands to typical savanna habitats and forests (Ribeiro and Walter 1998). In the Cerrado, the warm-wet season occurs from October to April; this period receives 90% of the annual precipitation of 1100–1600 mm (Miranda et al. 1993). Field data were collected between 2009 and 2010 at 4 sites in savanna woodlands (locally known as cerrradão) near the city of Brasília in the Federal District of Brazil, Figure 1). The vegetation is a xeromorphic forest formation with trees ranging from 8 to 15 m, and a tree layer cover between 50% and 90% (Ribeiro and Walter 1998). The 4 forest sites were located at the Ecological Station of the Botanic Garden of Brasília (EEJBB in Portuguese; 15° 52ʹ S, 47° 50ʹ W) and Fazenda Água Limpa, the ecological and agricultural field station of the University of Brasília (FAL in Portuguese; 15° 58ʹ S, 47° 59ʹ W). These 2 locations are part of the area of environmental protection (APA, from the original in Portuguese), Gama and Cabeça-de-Veado, which covers approximately 15,000 hectares of continuous Cerrado vegetation. Three sites were located in EEJBB (coded as EEJBB1, EEJBB2, and EEJBB3), and 1 in FAL (coded as FAL).
Figure 1.
Location of the study sites in Cerrado, showed as a green area in the Brazil’s map (dark gray area) on the top left. These sites were located near Brasília city in the Federal District of Brazil (top right inset). The bottom map indicates the detailed location of the 4 sampled sites of savanna woodland (cerradão), 3 at the Botanical Garden of Brasília (EEJBB1, EEJBB2, and EEJBB3) and 1 site at the ecological and agricultural field station of the University of Brasília (FAL).
Gracilinanus agilis capture and identification of food items
We captured G. agilis individuals in 4 grids (1 in each forest site). Each grid was composed of 144 (12 × 12) capture stations spaced at 15-m intervals, where we randomly placed 160 Sherman® live traps (80 on the ground and 80 in the understory, between 1.5 and 2.0 m high). Trap locations were randomly selected for each capture session, which lasted 6 consecutive days. For each grid, we conducted 3 capture sessions (interval of 3–4 weeks between sessions) in each of the 2 seasons (dry and wet), totaling 23,040 trapping nights. We used a mixture of peanut butter, corn flour, mashed bananas, cod liver oil, and vanilla essence in the taps as bait. We ear-tagged each captured individual for further identification and registered the sex, reproductive condition, and botfly presence. The reproductive condition of the females was inferred from swelling of the nipples (Pinheiro et al. 2002). However, data on male reproductive conditions based on external characteristics are inaccurate (Quental et al. 2001). Therefore, we did not evaluate this condition in the males. Individuals were considered as parasitized when they presented with botfly larvae or had scar tissue indicating recent larval emergence (as in Zangrandi et al. 2019). For each individual, we also recorded the head-body and tail lengths (to the nearest mm) with a ruler and body weight (to the nearest 0.1 g) with a Pesola® dynamometer. If present, parasitized individuals had their botflies removed before the body weight was obtained.
To evaluate the diet, we collected scats from traps or during the handling of trapped animals. In the laboratory, we washed the fecal samples in 2 superimposed sieve meshes (0.1 mm and 0.7 mm) to obtain the remains of the food items for further identification under a stereoscopic microscope. The food categories were identified as the narrowest possible taxonomic category by comparison with a reference collection of invertebrates and fruits from the study area. Details regarding the methodology can be found in other studies based on the same database used in this study (Camargo et al. 2014a, 2014b). This study was approved by the Institutional Animal Care and Use Committee of the University of Brasília (no. 44743/2019).
Data analysis
Network analysis
We evaluated the relationship between the animals and food resources for each of the 4 G. agilis populations. For most of food categories we were able to identify food categories in a coarse level of taxonomic identification (arthropods = order; vertebrate = class; fruits = family, genus, and species) due to the highly fragmented remains found in fecal samples. Therefore, we opted to construct unweighted networks compiling observed interactions into presence (assigned as 1) and absence (assigned as 0) matrices, with each cell value representing the interaction between a marsupial individual (columns) and food resources (rows). We calculated node metrics of centrality (degree centrality [DC], closeness centrality [CC], and betweenness centrality [BC]) and specialization (nested rank [NR] and resource range [RR]) for each individual using the function specieslevel in the bipartite package (Dormann et al. 2008) in the software R version 4.0.5 (R Development Core Team 2021). Centrality and specialization metrics measure how well an individual is connected within the network (Gómez and Perfectti 2012; Poisot et al. 2012).
The 3 calculated centrality metrics represent distinct perspectives to evaluate node position in the network (Oldham et al. 2019). DC measures how well connected a node is according to the fraction of links connected to a node in relation to the maximum possible connections (Nieminen 1974). The closeness of a node in relation to the other nodes in the network is provided by the CC. This centrality metric describes the number of intermediaries that exist in a node that interacts with another node, considering the shortest available path (González et al. 2010). The third centrality metric, BC, measures the number of shortest paths between 2 nodes passing through a node of interest. This metric indicates how relevant a node is, acting as a bridge connecting different portions of the network (González et al. 2010). Since network sizes may vary between each other, to minimize possible size effects on centralities, we used normalized versions of each node metric (ranging from 0 to 1) (Dormann et al. 2008; Abbasi et al. 2011).
With regard to the evaluation of individual specialization within a network, NR measures the specialization of a node in relation to the rearranged network to its maximal possible nestedness (Guimarães and Guimarães 2006; Alarcón et al. 2008). A network can be considered as perfectly nested when all resources consumed by specialist individuals are a subset of the resources consumed by the generalist ones. Then, the rank position of an individual within the rearranged matrix provides its level of specialization. Individuals with high values (within the range of 0 to 1) are more specialized. The other specialization metric used (RR), measures the interaction specificity of each node (in our case, a marsupial individual) by normalizing the number of links connected to a node by the possible links in the network (Poisot et al. 2012). Individuals with high values of RR (within the range of 0 to 1) are more specialized. The formulas to calculate each node metric of centrality and specialization can be verified in the Supporting Information.
To construct the networks and obtain the node metrics, we included only samples from sub-adult and adult individuals (i.e., excluding data from infants and juveniles), which were identified on the eruption of the last superior molars (M4). Both age classes are sexually mature but only adults present fully fourth-erupted molars (Macedo et al. 2006). We analyzed 347 fecal samples from 301 individuals. In each forest site, the number of fecal samples obtained during the cool-dry and warm-wet season were as follow: FAL: 42, 70; EEJBB1: 33, 47; EEJBB2: 44, 48; EEJBB3: 36, 27. In total, we included in the analysis 143 samples of males, 204 of females, 47 of individuals parasitized by botflies, 300 of individuals nonparasitized by botflies, and 44 of reproductive females and 93 of nonreproductive females from the warm-wet season.
Statistical analysis
To verify whether there is a relationship between both the position of individuals within the network and their traits, we first performed principal component analysis (PCA) to obtain 2 new variables (PC1 and PC2) containing most of the variation (>75%; see results for more details) of the 5 node metrics. This approach helped summarize individual-based networks, considering all metrics together. We then used generalized linear mixed-effects models (GLMMs) considering forest site as a random effect, using the individual scores of PC1 and PC2 as response variables and individual traits as predictor factors.
To construct the linear models, we used the tail length relative to the body length (hereafter relative tail length) of the individual (i.e., in body length units). We used the relative tail length as a proxy for arboreal activity in mammals because this variable is commonly used in ecological studies and is considered to be functionally informative (Hayssen 2008; Mincer and Russo 2020). Moreover, we included in the linear models a scaled mass index (SMI) instead of body weight of each individual. The SMI standardizes body mass for a fixed linear measurement (in our case, body length) based on the scaling relationship between mass and length obtained using a standardized major axis regression (Peig and Green 2009). Since males (88.6 cm ± 11.5 cm [SD]) and females (83.3 cm ± 11.1 cm) presented sexual dimorphism in relation to body length (ANOVA: F1, 345 = 19.066, P = 0.001), we calculated SMI for both sexes separately. We log-transformed all morphological traits to improve data normality. We considered season (cool-dry or warm-wet), botfly presence, sex, and reproductive conditions as categorical variables in the linear model. For the entire population in each forest site, we ran a model considering the factors botfly occurrence, season, relative tail length, body length, SMI, and sex as predictor variables in the initial model (i.e., considering all interactions). However, since we were able to accurately determine reproductive conditions in the field only for females, we also ran a separate model containing only the data for females from the warm-wet season (i.e., when reproduction occurs). Thus, in this initial model, we considered reproductive condition, botfly presence, and all morphological traits.
We conducted a GLMM analysis using the function lmer in the lme4 package (Bates et al. 2015). Considering that the response variables (PC1 and PC2 scores of the network node metrics) presented negative and positive continuous values, we used a Gaussian family with identity link function and confirmed adequacy with residual plots. We first tested a model containing all interactions between the predictor factors against another model containing only the main effects (i.e., without interactions between variables) using the function Anova in the R stats library (R Development Core Team 2021). We found no statistically significant differences between these models for the model with all individuals (PC1: χ²48 = 55.877, P = 0.203; PC2: χ²48 = 53.770, P = 0.263) and for the model considering only the nonreproductive and reproductive females from the warm-wet season (PC1:χ²24 = 29.914, P = 0.188; PC2:χ²24 = 55.877, P = 0.203). Therefore, we used the most parsimonious models as global models (i.e., main-effects models). Because we found no relevant interactions between reproductive condition and the other predictor factors (botfly infection and morphological traits) in the female-only model from the warm-wet season, we conducted the analysis considering only the reproductive condition factor, since the other factors were already evaluated in the whole-population model.
After choosing the fittest global models, we performed a model selection using the Akaike Information Criterion corrected for small sample sizes (AICc) with the dredge function in the package MuMIn (Bartoń 2020) to fit all possible models. To delineate the “best” model set, we considered ∆AICc ≤ 2 as a threshold (Grueber et al. 2011). We used t-tests to identify predictor variables that had statistically significant effects on the best model sets (as in López‐Uribe et al. 2019; Laurindo et al. 2020). The relative importance of each predictor variable was calculated as the sum of the Akaike weights, considering all models in which the parameter of interest appeared. We checked for multicollinearity among factors by evaluating the variance inflation factor (VIF) using the vif function with the package car (Fox and Weisberg 2019). VIF values above 5 indicate high multicolinearity (Akinwande et al. 2015). However, our analyses showed no such effect (VIF range = 1.08–2.57).
Results
Network analysis
We found 20 distinct food items in the scats of G. agilis. These items comprised 10 arthropod orders (9 of insects and 1 of arachnid), pulp, fiber, and seeds of 5 plant species, 3 morphotypes of unidentified fruit fibers, and young bird remains (see Camargo et al. 2014a and Data accessibility section for more details). All the populations from the studied sites presented very similar diet compositions. Only 3 food items (the insect orders Diptera and Odonata, and the fruit Miconia pepericarpo [Melastomataceae]) were exclusively consumed in 1 out of the 4 forest sites. Network sizes (total number of opossum individuals and food resource types) had in average 53 ± 15(SD) nodes (range: 39–84), and 125 ± 26 interactions (range: 72–162).
The mouse opossum individuals presented, on average, a moderate DC value and a wide range of values in the network (average ± SD, 0.277 ± 0.146; range, 0.070–0.714). This range indicates that individuals can be both well and poorly connected with the use of distinct food resources relative to the most connected individual in the network. However, both CC (0.023 ± 0.006; range = 0.001–0.039) and BC (0.017 ± 0.022; range = 0.000–0.180) values were generally low, indicating that individuals tended not to occupy central positions in the networks or play major roles as connectors. The feeding specialization of G. agilis was relatively high, with a wide range of NR (0.500 ± 0.296; range = 0.000–1.000) and RR (0.795 ± 0.148; range = 0.333–1.000) values among individuals.
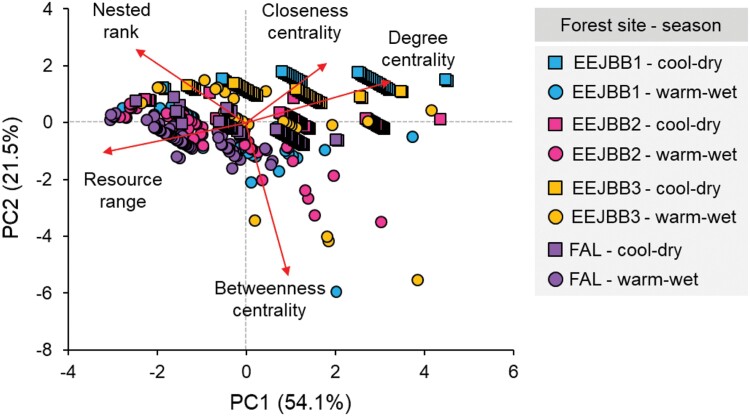
In the PCA used to summarize the individual´s position in the networks, PC1 explained 54.1% of the variance and PC2 explained 21.5% (Figure 2 and Supplementary Table S1). PC1 was associated (absolute factor loading ≥ 0.5; Supplementary Table S1) with almost all network metrics, except for BC (Supplementary Table S1). Specialization metrics (both NR and RR) were negatively associated with the 1st PCA axis, whereas centrality metrics (DC and CC) were positively associated with this axis. Therefore, the 1st axis (PC1) represents a gradient of individuals from more specialized and less connected (low scores) to individuals less specialized and more connected in the network (high scores). For the 2nd axis (PC2), the negative relationship with BC indicated a gradient of more connected (low scores) to less connected individuals (high scores). We found no clear patterns of differences among sites except for a trend for low scores along the 1st axis of individuals from the FAL forest site (Figure 2).
Figure 2.
Biplot showing the results of the PCA of individual-based network metrics (i.e. node metrics) with respect to specialization (nested rank and resource range) and centrality (degree, closeness, and betweenness) of the gracile mouse opossum Gracilinanus agilis in 4 dry woodland forest sites (EEJBB1-3 and FAL) during the cool-dry (squares) and warm-wet seasons (circles). Eigenvector coefficients are shown in Supplementary Table S1.
Position of individuals in the network
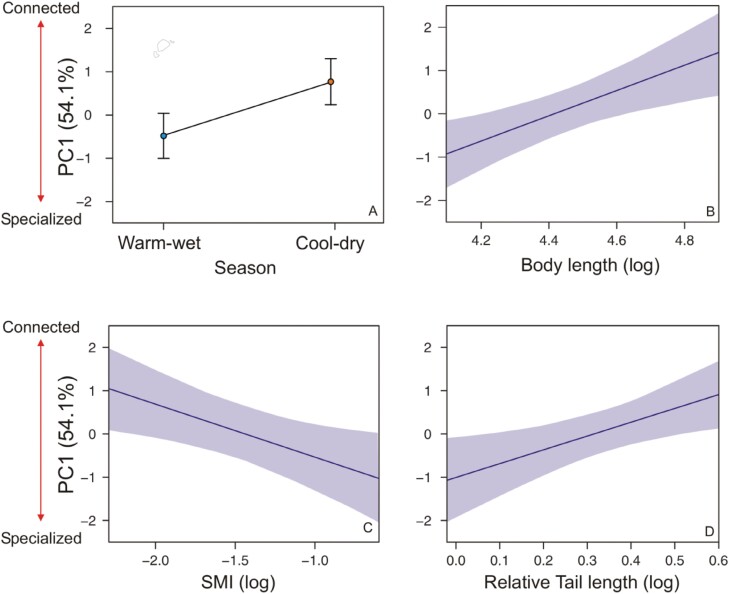
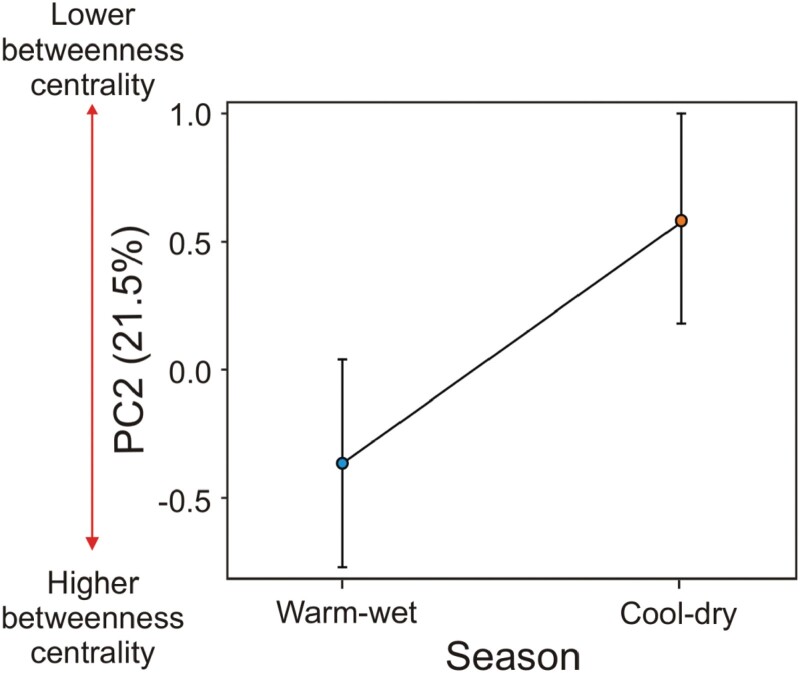
The model selection evaluating the relation between morphological traits and season of the year on the individuals’ position in the network (based on PC1 scores) indicated only 1 remaining “best” model (∆AICc ≤ 2). This model identified that body length (positive effect), body condition (negative effect), relative tail length (positive effect), and season had high relative importance (between 0.85 and 1.000) and statistically significant effects (Table 1 and Supplementary Table S2). Thus, individuals with small body length, high body condition (SMI), and short tail length relative to body length tended to be specialists and less connected in the network, and botfly presence and sex were factors with no relevance to predict individual’s position in the network. Individuals were also more specialized and less connected during the warm-wet season (Figure 3). For the 2nd axis of the PCA, the “best” set of models included the season, body length, and tail length (Table 1 and Supplementary Table S3). Thus, botfly presence, sex, and SMI were not selected as relevant factors to predict individual´s position in the network considering this PCA axis. However, among the selected factors, only season was present in all 4 models selected and had high relative importance (1.00) in comparison to the remaining factors (relative importance < 0.43 all; Table 1). These results indicate that during the warm-wet season, individuals had higher values of BC (Figure 4). Testing for the reproductive condition effect on the females’ position in the network, we found no statistical significance (GLMM) for both PC1 (F1,132.1 = 0.004; P = 0.952) and PC2 (F1,135.0 = 0.858; P = 0.356).
Table 1.
Results of the model selection assessing the individuals’ position (PC1 and PC2) in network of interactions of the gracile mouse opossum Gracilinanus agilis in 4 dry woodland forest (cerradão) sites in the Brazilian savanna (Cerrado), according to 6 predictor variables
| BF | SE | RTL | BL | SMI | SX | df | logLik | AICc | ∆AICc | Weight (w) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PC1 (54.1%) | |||||||||||
| SE (Cool-dry) + RTL + BL + SMI | 1.287 | 3.236 | 1.846 | −0.718 | 7 | −596.615 | 1207.561 | 0.000 | 0.449 | ||
| Relative importance | 0.254 | 1.000 | 0.918 | 0.860 | 0.865 | 0.167 | |||||
| PC2 (21.5%) | |||||||||||
| SE (Cool-dry) | 0.956 | 4 | −440.264 | 888.645 | 0.000 | 0.279 | |||||
| SE + RTL + BL | 1.018 | −0.879 | −0.772 | 6 | −438.679 | 889.605 | 0.960 | 0.172 | |||
| SE + BL | 0.979 | −0.297 | 5 | −440.033 | 890.243 | 1.598 | 0.125 | ||||
| SE + RTL | 0.957 | −0.118 | 5 | −440.056 | 890.288 | 1.643 | 0.123 | ||||
| Relative importance | 0.110 | 1.000 | 0.425 | 0.434 | 0.139 | 0.090 | |||||
PC1 and PC2 correspond to the PCA (see results for more details) of 5 individual-based network regarding specialization (NR and resource range) and centrality (degree, closeness, and betweenness). The percentage of the total variance explained by each PC is indicated between parentheses. The values given for each predictor variable are regression coefficients according to the GLMM using forest site as random effect. Numbers in bold indicate statistically significant effects (P ≤ 0.05) in the model. BF = botfly; SE = season; RTL = tail length relative to body size; BL = body length; SMI = scaled mass index (body condition); SX = sex.
Figure 3.
Relationship between the position of Gracilinanus agilis individuals in the network of interactions and independent variables evaluated (season [A] and morphological traits [B–D]). PC1 correspond to the 1st axis obtained in the PCA of 5 individual-based network metrics (see methods for more details). The predictor variables shown in the figure were among the fittest models according to the GLMM coupled with model AIC, and with statistically significant effects. The number in parenthesis represents the percentage of the total variance explained by the PCA axis. Vertical bars (A) and shaded areas (B–D) indicate 95% confidence intervals.
Figure 4.
Mean score values per season of the 2nd axis of the PCA, obtained with 5 individual-based network metrics (see methods for more details). The predictor variable (season) shown was among the fittest models according to the GLMM coupled with model AIC, with statistically significant effects. The number in parenthesis represents the percentage of the total variance explained in the PCA. The vertical bars present the 95% confidence intervals. The red arrow on the left side of y-axis indicates the direction of change of the individuals’ position in the network, according to most related network metric with PC2 (BC).
Discussion
Our results showed that the individual´s position in interaction networks in the dry woodland forests of the Brazilian savanna is related to both body traits of G. agilis and seasonality. Node metrics of specialization (nested rank and resource range) and centrality (degree, closeness, and betweenness) indicated that individuals with a small body length, better body condition (i.e., heavier relative to body length), and short tails relative to body length were more specialized (i.e., less connected in the network). Individuals were also more specialized during the warm-wet season. Currently, a major challenge in studying individual trait variation and trophic interactions is to identify which individual´s phenotypical traits mediate specialization patterns, as they can be diversified across taxa and context-dependent (e.g., Bolnick et al. 2003; Svanbäck and Bolnick 2007; Miguel et al. 2018; Campião and Dáttilo 2020). Few studies have identified traits that modulate individual specialization in vertebrates (but see Tinker et al. 2007; Exnerová et al. 2010; Montoya-Arango et al. 2019; Laurindo et al. 2020; Oliveira et al. 2020). Other studies have shown that G. agilis populations are composed of relatively specialized individuals (Araújo et al. 2010; Camargo et al. 2014, 2019). However, this is the first study to identify individual traits that are relevant in predicting specialization, helping us better understand the mechanisms of individual specialization in natural populations.
Individuals with lower body lengths were more specialized than larger ones, as expected. Small-sized individuals normally represent younger individuals who may still be learning to forage and are less experienced and potentially less skillful in finding, capturing, and manipulating certain prey types (Werner and Gilliam 1984; Dickman 1988; Rutz et al. 2006). In fact, G. agilis presents a pattern of herbivory-to-carnivory switching related to ontogeny, where smaller/younger individuals mainly feed on fruits, whereas larger/older individuals tend to consume more animal prey (Camargo et al. 2014b). This pattern may reflect the lack of experience of younger individuals to capture mobile and agile prey. Dietary changes can be directly related to ontogenetic and allometric shifts in skull morphology (Thompson et al. 2003; Flores et al. 2010). Smaller individuals generally present lower bite force (Thompson et al. 2003) and smaller mouth size (e.g., Abdala et al. 2001; Sánchez-Hernández et al. 2019), hindering them while feeding on large prey, for example, on larger insects (with harder exoskeletons) and vertebrates. Therefore, older and large-bodied individuals are able to capture a wider range of prey body sizes and hardness, as seen for larger predators, including large mammals (Werner and Gilliam 1984; Owen‐Smith and Mills 2008). For our study species, we suggest that small-bodied individuals present individual specialization due to biomechanical and behavioral constraints.
We also found that individuals with better body condition (i.e., heavier in relation to their body size) were more specialized. We suggest that more profitable resources are more commonly accessed and dominated by individuals in better conditions, which would not need to widen their range of used resources. The relation between body size and weight (fat reserves and muscle mass) is directly related to the resource holding power (RHP) or fight ability of an individual (Nosil 2002; Hsu et al. 2006; Wright et al. 2019). RHP determines the success of an individual in a contest and may be decisive for its resource use and selection. A direct relationship between RHP and body weight has already been shown in several animals, including invertebrates and vertebrates, reflecting their ability to access different types of resources. For instance, heavier dung beetles (but not larger in body length; Salomão et al. 2019), gladiator frogs (Candaten et al. 2020), flying squirrels (Fokidis et al. 2007), and gorillas (Wright et al. 2019) are more prone to fight and defend resources than lighter individuals. Our results indicate a similar pattern, in which heavier individuals (relative to body length) possibly select a few preferable and energy-rewarding food items due to their higher RHP, thus being less connected in the network and more specialized. This specialization would arise as a selected feeding strategy and not imposed by size/age constraints, as in the case of more specialized smaller/younger individuals.
We predicted that individual specialization would be related to tail length relative to body size, as this trait is related to arboreality and, consequently, to the use of available resources. Our models confirm this prediction, indicating that individuals with relative short tails are more specialized and less connected in the network of interactions. Similar to body length, feeding specialization of short-tailed individuals is likely more related to certain constraints than to feeding choices and advantages related to energy intake, as proposed with respect to body condition. Relative tail length is positively related to arboreal activity and the ability to explore the vertical strata of different vertebrate taxa (Mincer and Russo 2020; Smith and Hilliard Young 2021). In fact, a better capacity of long-tailed animals for exploring vertical strata would also be expected at the individual level. For instance, the Cricetidae rodents Peromyscus truei and P. boylii showed a relationship between tail length and foraging habits, indicating that long-tailed individuals feed more on food resources available in the upper strata of the vegetation (Smartt and Lemen 1980). Therefore, we propose that G. agilis individuals with short tails (specialists and less connected ones) are prone to exploit resources mainly on the ground and in the lower vegetation strata. Long-tailed individuals, in turn, can better exploit all vertical strata (ground, understory, and canopy), being able to feed on a wider range of food resources. Thus, these individuals are potentially more likely to be generalists and connected than short-tailed individuals. This finding is in accordance with the pattern observed in previous studies where population-level networks of G. agilis are nested in more complex habitats (Camargo et al. 2019). In these habitats, the greater availability of vertical space potentially promotes segregation among individuals. This segregation results in more terrestrial individuals presenting a diet that is a subset of the food items consumed by the more arboreal individuals (Camargo et al. 2019).
Specialization was also affected by the time of year in the highly seasonal Cerrado. We verified that, on average, individuals are less connected and more specialized during the resource-rich period (i.e., warm-wet season). This result is in accordance with other studies showing that G. agilis (Camargo et al. 2014b, 2019) and other vertebrate populations (Araújo et al. 2007; Costa-Pereira et al. 2017; Camargo et al. 2021) tend to be more specialized during this season in the Cerrado. Population-level networks of G. agilis increase modularity during the warm-wet season, indicating that discrete groups of individuals tend to specialize in subsets of food resources available in the environment (Camargo et al. 2019). A similar pattern was also verified in climbing rats Rhipidomys macrurus based on stable isotope analysis, indicating a low individual isotopic niche overlap during the resource-rich season (Camargo et al. 2021). In contrast to G. agilis, however, this rodent does not exhibit between-season differences in individual niche widths. This specialization seems to be related to an increase in population densities of most small mammals (including G. agilis) due to recruitment by reproduction in the warm-wet season (Mares and Ernest 1995), resulting in increased competition during this period. However, at the same time, more food resources are available in the environment, increasing ecological opportunities (Camargo et al. 2019, 2021). Thus, in an environmental context where competition is high and different food resource types are more available, individuals would present feeding specialization leading to a reduced niche width (i.e., less connected in the network) and overlap to minimize the competition effects.
We also detected that BC was generally higher during the warm-wet season. Food web models indicate that, as modularity increases, the networks also present higher average BC, which indicates that the species have a more relevant role as connectors in more modular networks (Garay‐Narváez et al. 2014). In addition, there is a general pattern in which more central elements tend to be the more generalist ones in both individual- and species-level networks (González et al. 2010; Gómez and Perfectti 2012; Palacio et al. 2016). For instance, in plant-frugivore networks, it has been found that generalist birds act as local hubs in their own modules, and as connectors between modules (Palacio et al. 2016; Vidal et al. 2014). Therefore, it is likely that during the resource-rich and population-dense period, when population networks of G. agilis present higher modularity (Camargo et al. 2019), some relatively more-generalized individuals that feed on food resources from distinct modules may also play this role in the network, increasing the average BC during the warm-wet season. Although we did not find significant relationships between the measured individual traits and BC, 3 out of 4 best set of models according to the AIC included tail length and/or body length. This result could suggest a relative importance (but not clearly detected) of these traits in determining which individuals were acting as connectors in the networks. In these models, again, individuals with higher body and tail lengths were more generalists, potentially connecting different modules in the network.
The position of G. agilis within the network of interactions was not affected by botfly infestation, contrary to our expectations. Although parasitized individuals could have their movement negatively affected (Wecker 1962; Smith 1978) and present a weakened condition due to anemia (Zangrandi et al. 2019), these individuals were not specialized in their feeding habits due to these constraints. However, in the present study, we did not consider the number of botflies (Dunaway et al. 1967), the larval instar (Smith 1978), or the position of larvae in each individual (e.g., near hind limbs in the ingual region—Test and Test 1943), which could affect the degree of negative impacts of being parasitized. Nevertheless, botfly effects on mammalian hosts are highly variable and may have neutral (e.g., Bergallo et al. 2000; Spessot et al. 2013) or even positive (Jaffe et al. 2005; Cramer and Cameron 2006) effects.
The individual´s position in the network was not explained by the sex or reproductive condition of the females. Different intraspecific feeding patterns have been reported for G. agilis considering these 2 intrapopulational subgroups (e.g., Martins et al. 2006a; Camargo et al. 2011, 2014a). For example, G. agilis males and reproductive females feed more heavily on arthropods compared with females and nonreproductive females (Camargo et al. 2014a). Additionally, reproductive females also select ants and beetles, whereas nonreproductive females negatively select beetles (Camargo et al. 2014a). It is possible that the taxonomic resolution (Hemprich‐Bennett et al. 2021; Llopis‐Belenguer et al. 2022) of identification of certain food items (e.g., arthropods at the order level) that we used in the present study is not enough to detect such differences. Additionally, reproductive and nonreproductive females may present similar diet composition, but differ significantly in the intensity of food item consumption, which is not considered in unweighted networks. Therefore, these limitations could hinder the identification of any differences related to individuals’ positions in the network with respect to sex and reproductive conditions.
In conclusion, our study revealed the role of traits and season of the year on an individual’s position within the interaction networks of a neotropical marsupial. To the best of our knowledge, this is the first study of resource-consumer networks using this approach with a vertebrate as a model, increasing the knowledge regarding factors that underlie individual specialization in natural populations. Our results indicated that specialization (i.e., reduced connection within the ecological network) may arise not only as a result of preferred feeding strategies by more capable individuals (i.e., those with better body condition and potentially prone to defend and access high-quality food resources) but also because of morphological constraints. These constraints are related to body size and tail length (relative to body size). Smaller/younger individuals (consequently with less experience in foraging) and short-tailed individuals (less skilled to explore the vertical strata of the vegetation) would have access to a more limited range of resources and consequently become more specialized. These results indicate that feeding specialization is not only a product of individual choices and preferences regarding nutritional and energetic demands but also a result of behavioral and morphological constraints. Additionally, G. agilis presented feeding specialization during a resource-rich and population-dense period (warm-wet season). This seasonal pattern reinforces the role of competition and ecological opportunities in determining individual feeding patterns within natural populations in highly seasonal environments. The time of the year (dry or wet season), however, did not affect the relationship between individuals’ body traits and network position. This regular pattern indicates that G. agilis populations present both generalist and specialist individuals throughout the year, with body traits affecting the interactions within the ecological network.
Supplementary Material
Acknowledgments
We acknowledge and thank the permission granted by the EJBB and FAL to our fieldwork. We are also grateful to the staff of the Laboratory of Vertebrate Ecology of the Universidade de Brasilia (UnB) for fieldwork assistance, and the 3 anonymous reviewers for the valuable suggestions and improvement of the manuscript.
Contributor Information
Nícholas F de Camargo, Laboratório de Ecologia de Vertebrados, Departamento de Ecologia, Instituto de Ciências Biológicas, Universidade de Brasília, CP 04457, Brasília, DF, 70910-900, Brazil.
Hernani F M de Oliveira, Departamento de Zoologia, Setor de Ciências Biológicas, Universidade Federal do Paraná, Avenida Coronel Francisco Heráclito dos Santos100, Curitiba, PR, 81531980, Brazil.
Juliana F Ribeiro, Laboratório de Ecologia de Vertebrados, Departamento de Ecologia, Instituto de Ciências Biológicas, Universidade de Brasília, CP 04457, Brasília, DF, 70910-900, Brazil.
Amabílio J A de Camargo, Embrapa Cerrados, Rodovia BR 020, km 18, CP 08223, Planaltina, DF, 73310-970, Brazil.
Emerson M Vieira, Laboratório de Ecologia de Vertebrados, Departamento de Ecologia, Instituto de Ciências Biológicas, Universidade de Brasília, CP 04457, Brasília, DF, 70910-900, Brazil.
Funding
The present study was supported by graduated scholarships granted to NFC and JFR, by a Research Productivity Grant (No 308992/2013‐0) and a research funding (No 483117/2009‐9) granted to EMV, provided by the Brazilian National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico– CNPq).
Author Contributions
N.F.C., H.F.M.O. and E.M.V. conceived the idea of the study; N.F.C. and J.F.R. collected the data; N.F.C. and A.J.A.C. identified the arthropods and fruits found in the fecal samples; N.F.C. and H.F.M.O. conducted the network analysis; N.F.C. conducted the statistical analyses and wrote the manuscript. All authors revised the manuscript, and gave the final approval for publication.
Data Availability
Raw Data available in the Figshare Digital Repository http://doi.org/10.6084/m9.figshare.20736814
Conflict of Interest
The authors declare no conflict of interest.
References
- Abbasi A, Altmann J, Hossain L, 2011. Identifying the effects of co-authorship networks on the performance of scholars: A correlation and regression analysis of performance measures and social network analysis measures. J Informetr 5:594–607. [Google Scholar]
- Abdala F, Flores DA, Giannini NP, 2001. Postweaning ontogeny of the skull of Didelphis albiventris. J Mammal 82:190–200. [Google Scholar]
- Akinwande MO, Dikko HG, Samson A, 2015. Variance inflation factor: As a condition for the inclusion of suppressor variable(s) in regression analysis. Open J Stat 5:754–767. [Google Scholar]
- Alarcón R, Waser NM, Ollerton J, 2008. Year-to-year variation in the topology of a plant - pollinator interaction network. Oikos 117:1796–1807. [Google Scholar]
- Araújo MS, Bolnick DI, Layman CA, 2011. The ecological causes of individual specialisation. Ecol Lett 14:948–958. [DOI] [PubMed] [Google Scholar]
- Araújo MS, dos Reis SF, Giaretta AA, Machado G, Bolnick DI, 2007. Intrapopulation diet variation in four frogs (Leptodactylidae) of the Brazilian Savannah. Copeia 2007:855–865. [Google Scholar]
- Araújo MS, Guimarães PR, Svanbäck R, Pinheiro A, Guimarães P. et al. , 2008. Network analysis reveals contrasting effects of intraspecific competition on individual vs. population diets. Ecology 89:1981–1993. [DOI] [PubMed] [Google Scholar]
- Araújo MS, Martins EG, Cruz LD, Fernandes FR, Linhares AX. et al. , 2010. Nested diets: a novel pattern of individual-level resource use. Oikos 119:81–88. [Google Scholar]
- Bartoń K, 2020. MuMln: Multi-Model Inference. R package version 1.43.17. Vienna: The Comprehensive R Archive Network (CRAN). [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S, 2015. Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. [Google Scholar]
- Beck CA, Iverson SJ, Bowen WD, Blanchard W, 2007. Sex differences in grey seal diet reflect seasonal variation in foraging behaviour and reproductive expenditure: Evidence from quantitative fatty acid signature analysis. J Anim Ecol 76:490–502. [DOI] [PubMed] [Google Scholar]
- Bergallo H, Martins-Hatano F, Juca N, Gettinger D, 2000. The effect of botfly parasitism of Metacuterebra apicalis (Diptera) on reproduction, survival and general health of Oryzomys russatus (Rodentia), in southeastern Brazil. Mammalia 64:439–446. [Google Scholar]
- Blüthgen N, Menzel F, Blüthgen N, 2006. Measuring specialization in species interaction networks. BMC Ecol 6:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blüthgen N, Menzel F, Hovestadt T, Fiala B, Blüthgen N, 2007. Specialization, constraints, and conflicting interests in mutualistic networks. Curr Biol 17:341–346. [DOI] [PubMed] [Google Scholar]
- Bolnick D, Svanbäck R, Fordyce J, Yang L, Davis J. et al. , 2003. The ecology of individuals: incidence and implications of individual specialization. Am Nat 161:1–28. [DOI] [PubMed] [Google Scholar]
- Camargo NF, Cruz RMS, Ribeiro JF, Vieira EM, 2011. Frugivory and potential seed dispersal by the marsupial Gracilinanus agilis (Didelphidae: Didelphimorphia) in areas of Cerrado in central Brazil. Acta Bot Bras 25:646–656. [Google Scholar]
- Camargo NF, Oliveira HF, Ribeiro JF, Camargo AJ, Vieira EM, 2019. Availability of food resources and habitat structure shape the individual: Resource network of a Neotropical marsupial. Ecol Evol 9:3946–3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo NF, Reis GG, Camargo ACL, Nardoto GB, Kneitel JM. et al. , 2021. Seasonal isotopic niche of a rodent: High between-individual variation but no changes in individual niche width during the rich-resource period. Biotropica 53:966–975. [Google Scholar]
- Camargo NF, Ribeiro JF, Camargo AJ, Vieira EM, 2014a. Diet of the gracile mouse opossum Gracilinanus agilis (Didelphimorphia: Didelphidae) in a neotropical savanna: Intraspecific variation and resource selection. Acta Ther 59:183–191. [Google Scholar]
- Camargo NF, Ribeiro JF, Camargo AJ, Vieira EM, 2014b. Intra-and inter-individual variation show distinct trends as drivers of seasonal changes in the resource use of a Neotropical marsupial. Biol J Linn Soc 111:737–747. [Google Scholar]
- Camargo NF, Sano N, Vieira E, 2018. Forest vertical complexity affects alpha and beta diversity of small mammals. J Mammal 99:1444–1454. [Google Scholar]
- Campião K, Dáttilo W, 2020. Biological drivers of individual-based anuran - parasite networks under contrasting environmental conditions. J Helminthol 94:e167. [DOI] [PubMed] [Google Scholar]
- Candaten A, Possenti AG, Mainardi AA, da Rocha MC, Palaoro AV, 2020. Fighting scars: Heavier gladiator frogs bear more injuries than lighter frogs. Acta Ethologica 23:39–44. [Google Scholar]
- Costa-Pereira R, Tavares LE, de Camargo PB, Araújo MS, 2017. Seasonal population and individual niche dynamics in a tetra fish in the Pantanal wetlands. Biotropica 49:531–538. [Google Scholar]
- Costa L, 2007. Characterization of complex networks: A survey of measurements. Adv Phys 56:167–242. [Google Scholar]
- Cramer MJ, Cameron GN, 2006. Effects of bot fly Cuterebra fontinella parasitism on a population of white-footed mice Peromyscus leucopus. J Mammal 87:1103–1111. [Google Scholar]
- Cruz-Neto AP, Bozinovic F, 2004. The Relationship between diet quality and basal metabolic rate in endotherms: insights from intraspecific analysis. Physiol Biochem Zool 77:877–889. [DOI] [PubMed] [Google Scholar]
- Dickman C, 1988. Age-related dietary change in the European hedgehog Erinaceus europaeus. J Zool 215:1–14. [Google Scholar]
- Dingemanse NJ, Réale D, 2005. Natural selection and animal personality. Behaviour 142:1159–1184. [Google Scholar]
- Dormann CF, Gruber B, Fründ J, 2008. Introducing the bipartite package: Analysing ecological networks. R News 8:8–11. [Google Scholar]
- Dunaway P, Payne J, Lewis L, Story J, 1967. Incidence and effects of Cuterebra in Peromyscus. J Mammal 48:38–51. [PubMed] [Google Scholar]
- Emmons LH, Feer F, 1997. Neotropical Rainforest Mammals: A Field Guide. Chicago: The University of Chicago Press. [Google Scholar]
- Exnerová A, Svádová KH, Fučíková E, Drent P, Štys P, 2010. Personality matters: Individual variation in reactions of naive bird predators to aposematic prey. Proc R Soc B 277:723–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores DA, Abdala F, Giannini N, 2010. Cranial ontogeny of Caluromys philander (Didelphidae: Caluromyinae): A qualitative and quantitative approach. J Mammal 91:539–550. [Google Scholar]
- Fokidis HB, Risch TS, Glenn TC, 2007. Reproductive and resource benefits to large female body size in a mammal with female-biased sexual size dimorphism. Anim Behav 73:479–488. [Google Scholar]
- Fox J, Weisberg S, 2019. An R Companion to Applied Regression. Thousand Oaks: Sage. [Google Scholar]
- Garay-Narváez L, Flores JD, Arim M, Ramos-Jiliberto R, 2014. Food web modularity and biodiversity promote species persistence in polluted environments. Oikos 123:583–588. [Google Scholar]
- Gentile R, D’Andrea PS, Cerqueira R, 1997. HRs of Philander frenata and Akodon cursor in a Brazilian restinga (Coastal shrubland). Mastozool Neotr 4:105–112. [Google Scholar]
- Gómez JM, Perfectti F, 2012. Fitness consequences of centrality in mutualistic individual-based networks. Proc R Soc B 279:1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González AMM, Dalsgaard B, Olesen JM, 2010. Centrality measures and the importance of generalist species in pollination networks. Ecol Complex 7:36–43. [Google Scholar]
- Grueber CE, Nakagawa S, Laws RJ, Jamieson IG, 2011. Multimodel inference in ecology and evolution: challenges and solutions. J Evol Biol 24:699–711. [DOI] [PubMed] [Google Scholar]
- Guimarães PR Jr, 2020. The structure of ecological networks across levels of organization. Annu Rev Ecol Evol Syst 51:433–460. [Google Scholar]
- Guimarães PR Jr, Guimarães P, 2006. Improving the analyses of nestedness for large sets of matrices. Environ Model Softw 21:1512–1513. [Google Scholar]
- Guscelli E, Spicer JI, Calosi P, 2019. The importance of inter-individual variation in predicting species’ responses to global change drivers. Ecol Evol 9:4327–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayssen V, 2008. Patterns of body and tail length and body mass in Sciuridae. J Mammal 89:852–873. [Google Scholar]
- Hemprich-Bennett DR, Oliveira HF, Le Comber SC, Rossiter SJ, Clare EL, 2021. Assessing the impact of taxon resolution on network structure. Ecology 102:e03256. [DOI] [PubMed] [Google Scholar]
- Hsu Y, Earley RL, Wolf LL, 2006. Modulation of aggressive behaviour by fighting experience: Mechanisms and contest outcomes. Biol Rev 81:33–74. [DOI] [PubMed] [Google Scholar]
- Hudson P, Greenman J, 1998. Competition mediated by parasites: biological and theoretical progress. Trends Ecol Evol 13:387–390. [DOI] [PubMed] [Google Scholar]
- Huffman MA, 1997. Current evidence for self-medication in primates: A multidisciplinary perspective. Am J Phys Anthropol 104:171–200. [Google Scholar]
- Jaffe G, Zegers DA, Steele MA, Merritt JF, 2005. Long-term patterns of botfly parasitism in Peromyscus maniculatus, P. leucopu, and Tamias striatus. J Mammal 86:39–45. [Google Scholar]
- Julien-Laferrière D, 1995. Use of space by the woolly opossum Caluromys philander (Marsupialia, Didelphidae) in French Guiana. Can J Zool 73:1280–1289. [Google Scholar]
- Karban R, English-Loeb G, 1997. Tachinid parasitoids affect host plant choice by caterpillars to increase caterpillar survival. Ecology 78:603–611. [Google Scholar]
- Kolaczyk E, 2009. Statistical Analysis of Network Data: Methods and Models. Germany: Springer. [Google Scholar]
- Laurindo RS, Vizentin-Bugoni J, Tavares DC, Mancini MCS, Mello RM. et al. , 2020. Drivers of bat roles in Neotropical seed dispersal networks: abundance is more important than functional traits. Oecologia 193:189–198. [DOI] [PubMed] [Google Scholar]
- Llopis-Belenguer C, Balbuena JA, Blasco-Costa I, Karvonen A, Sarabeev V. et al. , 2022. Sensitivity of bipartite network analyses to incomplete sampling and taxonomic uncertainty. Ecology 104:e3974. [DOI] [PubMed] [Google Scholar]
- López-Uribe MM, Jha S, Soro A, 2019. A trait-based approach to predict population genetic structure in bees. Mol Ecol 28:1919–1929. [DOI] [PubMed] [Google Scholar]
- Macedo J, Loretto D, Vieira MV, Cerqueira R, 2006. Classes de desenvolvimento em marsupiais: um método para animais vivos. Mastozool Neotr 13:133–136. [Google Scholar]
- Mares MA, Ernest KA, 1995. Population and community ecology of small mammals in a gallery forest of central Brazil. J Mammal 76:750–768. [Google Scholar]
- Martins E, Bonato V, Pinheiro H, Dos Reis S, 2006a. Diet of the gracile mouse opossum Gracilinanus microtarsus (Didelphimorphia: Didelphidae) in a Brazilian cerrado: patterns of food consumption and intrapopulation variation. J Zool 269:21–28. [Google Scholar]
- Martins EG, Bonato V, Da-Silva CQ, Dos Reis SF, 2006b. Seasonality in reproduction, age structure and density of the gracile mouse opossum Gracilinanus microtarsus (Marsupialia: Didelphidae) in a Brazilian cerrado. J Trop Ecol 22:461–468. [Google Scholar]
- Melián CJ, Matthews B, De Andreazzi CS, Rodríguez JP, Harmon LJ. et al. , 2018. Deciphering the interdependence between ecological and evolutionary networks. Trends Ecol Evol 33:504–512. [DOI] [PubMed] [Google Scholar]
- Miguel MF, Jordano P, Tabeni S, Campos CM, 2018. Context-dependency and anthropogenic effects on individual plant - frugivore networks. Oikos 127:1045–1059. [Google Scholar]
- Mincer ST, Russo GA, 2020. Substrate use drives the macroevolution of mammalian tail length diversity. Proc R Soc B 287:20192885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda A, Miranda H, Dias I, 1993. Soil and air temperatures during provocated cerrado fires in central Brazil. J Trop Ecol 9:313–320. [Google Scholar]
- Montoya-Arango S, Acevedo-Quintero J, Parra J, 2019. Abundance and size of birds determine the position of the species in plant - frugivore interaction networks in fragmented forests. Commun Ecol 20:75–82. [Google Scholar]
- Moore J, 1995. The behavior of parasitized animals. Bioscience 45:89–96. [Google Scholar]
- Moore J, Gotelli NJ, 1990. A phylogenetic perspective on the evolution of altered host behaviours: a critical look at the manipulation hypothesis. In: Barnard C, Behnke J, editors. Parasitism and Host Behaviour. London: Taylor and Francis, 193-229. [Google Scholar]
- Nieminen J, 1974. On the centrality in a graph. Scand J Psychol 15:332–336. [DOI] [PubMed] [Google Scholar]
- Nitikman L, Mares M, 1987. Ecology of small mammals in a gallery forest of central Brazil. Ann Carneg Mus 56:75–95. [Google Scholar]
- Nosil P, 2002. Food fights in house crickets Acheta domesticus and the effects of body size and hunger level. Can J Zool 80:409–417. [Google Scholar]
- Oldham S, Fulcher B, Parkes L, Arnatkevic iūtė A, Suo C. et al. , 2019. Consistency and differences between centrality measures across distinct classes of networks. PLoS One 14:e0220061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen JM, Bascompte J, Dupont YL, Jordano P, 2007. The modularity of pollination networks. Proc Natl Acad Sci 104:19891–19896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira HFM, Camargo NF, Hemprich-Bennett DR, Rodríguez-Herrera B, Rossiter SJ. et al. , 2020. Wing morphology predicts individual niche specialization in Pteronotus mesoamericanus (Mammalia: Chiroptera). PLoS One 15:e0232601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen-Smith N, Mills MG, 2008. Predator - prey size relationships in an African large-mammal food web. J Anim Ecol 77:173–183. [DOI] [PubMed] [Google Scholar]
- Palacio RD, Valderrama-Ardila C, Kattan GH, 2016. Generalist species have a central role in a highly diverse plant - frugivore network. Biotropica 48:349–355. [Google Scholar]
- Peig J, Green AJ, 2009. New perspectives for estimating body condition from mass/length data: The scaled mass index as an alternative method. Oikos 118:1883–1891. [Google Scholar]
- Persson L, 1985. Asymmetrical competition: are larger animals competitively superior? Amer Natur 126:261–266. [Google Scholar]
- Pinheiro PS, Carvalho F, Fernandez FA, Nessimian JL, 2002. Diet of the marsupial Micoureus demerarae in small fragments of Atlantic forest in Southeastern Brazil. Stud Neotrop Fauna Environ 37:213–218. [Google Scholar]
- Poisot T, Canard E, Mouquet N, Hochberg ME, 2012. A comparative study of ecological specialization estimators. Meth Ecol Evol 3:537–544. [Google Scholar]
- Quental TB, Fernandez FADS, Dias ATC, Rocha FS, 2001. Population dynamics of the marsupial Micoureus demerarae in small fragments of Atlantic Coastal Forest in Brazil. J Trop Ecol 17:339–352. [Google Scholar]
- R Development Core Team, 2021. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Ribeiro J, 2011Avaliação do uso do espaço pelo marsupial Gracilinanus agilis em áreas de cerradão no Brasil Central. Master Dissertation, Universidade de Brasília, Brasília, DF. [Google Scholar]
- Ribeiro J, Walter B, 1998. Fitofisionomias do Cerrado. In: Sano S, Almeida S, editors. Cerrado: Ambiente e Flora. Plananaltina, DF: EMBRAPA-CPAC, 87-166. [Google Scholar]
- Rutz C, Whittingham MJ, Newton I, 2006. Age-dependent diet choice in an avian top predator. Proc R Soc B 273:579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomão RP, Favila ME, González-Tokman D, Chamorro-Florescano IA, 2019. Contest dynamics for food and reproductive resources are defined by health condition in a dung beetle. Ethology 125:343–350. [Google Scholar]
- Sánchez-Hernández J, Nunn AD, Adams CE, Amundsen PA, 2019. Causes and consequences of ontogenetic dietary shifts: A global synthesis using fish models. Biol Rev 94:539–554. [DOI] [PubMed] [Google Scholar]
- Sazima C, Guimarães PR Jr, Dos Reis SF, Sazima I, 2010. What makes a species central in a cleaning mutualism network? Oikos 119: 1319-1325. [Google Scholar]
- Schall JJ, 1992. Parasite-mediated competition in Anolis lizards. Oecologia 92:58–64. [DOI] [PubMed] [Google Scholar]
- Schoener TW, 1983. Field experiments on interspecific competition. Amer Natur 122:240–285. [Google Scholar]
- Shibuya PS, Melo GL, Cáceres NC, 2018. Determinants of home range size and spatial overlap of Gracilinanus agilis (Mammalia: Didelphidae) in central-western Brazil. Mammalia 82:328–337. [Google Scholar]
- Smartt RA, Lemen C, 1980. Intrapopulational morphological variation as a predictor of feeding behavior in deermice. Amer Natur 116:891–894. [Google Scholar]
- Smith D, 1978. Effects of bot fly (Cuterebra) parasitism on activity patterns of Peromyscus maniculatus in the laboratory. J Wildl Dis 14:28–39. [DOI] [PubMed] [Google Scholar]
- Smith SK, Hilliard Young VK, 2021. Balancing on a limb: effects of gravidity on locomotion in arboreal, limbed vertebrates. Integr Comp Biol 61:573–578. [DOI] [PubMed] [Google Scholar]
- Spessot MLG, Gomez MD, Priotto JW, 2013. Demographic responses of Akodon azarae (Rodentia: Cricetidae) enclosed populations to Rogenhofera bonaerensis bot fly parasitism. Mastozool Neotr 20:387–392. [Google Scholar]
- Strona G, Veech JA, 2015. A new measure of ecological network structure based on node overlap and segregation. Methods Ecol Evol 6:907–915. [Google Scholar]
- Svanbäck R, Bolnick DI, 2007. Intraspecific competition drives increased resource use diversity within a natural population. Proc R Soc B 274:839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Test FH, Test AR, 1943. Incidence of dipteran parasitosis in populations of small mammals. J Mammal 24:506–508. [Google Scholar]
- Thompson EN, Biknevicius AR, German RZ, 2003. Ontogeny of feeding function in the gray short-tailed opossum Monodelphis domestica: empirical support for the constrained model of jaw biomechanics. J Exp Biol 206:923–932. [DOI] [PubMed] [Google Scholar]
- Tinker M, Costa D, Estes J, Wieringa N, 2007. Individual dietary specialization and dive behaviour in the California sea otter: Using archival time - depth data to detect alternative foraging strategies. Deep Sea Res (II Top Stud Oceanogr) 54:330–342. [Google Scholar]
- Tur C, Vigalondo B, Trøjelsgaard K, Olesen JM, Traveset A, 2014. Downscaling pollen - transport networks to the level of individuals. J Anim Ecol 83:306–317. [DOI] [PubMed] [Google Scholar]
- van Veen F, Müller C, Pell J, Godfray H, 2008. Food web structure of three guilds of natural enemies: Predators, parasitoids and pathogens of aphids. J Anim Ecol 77:191–200. [DOI] [PubMed] [Google Scholar]
- Vidal MM, Hasui E, Pizo MA, Tamashiro JY, Silva WR. et al. , 2014. Frugivores at higher risk of extinction are the key elements of a mutualistic network. Ecology 95: 3440–3447. [Google Scholar]
- Vieira E, Camargo N, Colas P, Ribeiro J, Cruz-Neto A, 2017. Geographic variation in daily temporal activity patterns of a Neotropical marsupial Gracilinanus agilis. PLoS One 12:e0168495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wecker SC, 1962. The effects of bot fly parasitism on a local population of the white-footed mouse. Ecology 43:561–565. [Google Scholar]
- Werner EE, Gilliam JF, 1984. The ontogenetic niche and species interactions in size-structured populations. Annu Rev Ecol Syst 15:393–425. [Google Scholar]
- Wright E, Galbany J, McFarlin SC, Ndayishimiye E, Stoinski TS. et al. , 2019. Male body size, dominance rank and strategic use of aggression in a group-living mammal. Anim Behav 151:87–102. [Google Scholar]
- Zangrandi PL, Mendonça AF, Cruz-Neto AP, Boonstra R, Vieira EM, 2019. The impact of botfly parasitism on the health of the gracile mouse opossum Gracilinanus agilis. Parasitology 146:1013–1021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw Data available in the Figshare Digital Repository http://doi.org/10.6084/m9.figshare.20736814