Abstract
Noninvasive stimulation of the nervous system is of growing interest in Parkinson's disease (PD) to slow-down motor decline and decrease medication and its side-effects. Repetitive transcranial magnetic stimulation (rTMS) used in PD to modulate the excitability of the primary motor cortex (M1) provided controversial results, in part because of interactions with medication. This warrants to administer rTMS in drug-free patients. Repetitive peripheral magnetic stimulation (rPMS of muscles) has not yet been tested in PD. Its influence on M1 plasticity (as tested by TMS, transcranial magnetic stimulation) and sensorimotor disorders in other health conditions makes it worth be explored in PD. Thus, rTMS and rPMS were tested in a drug-free woman (52 years old, PD-diagnosed 10 years ago) in four different rTMS + rPMS combinations (one week apart): sham-sham, real-real, real-sham, sham-real. rTMS was applied over M1 contralateral to the most impaired bodyside, and rPMS on muscles of the legs, trunk, and arms, bilaterally. M1 plasticity (TMS measures) and motor symptoms and function (clinical outcomes) were measured at different timepoints. The real-real session induced the largest motor improvements, with possible summation of effects between sessions, and maintenance at follow-up (80 days later). This was paralleled by changes of M1 facilitation and inhibition. This sheds a new light on the link between TMS measures of M1 plasticity and motor changes in PD and informs on the remaining potential for neuroplasticity and functional improvement after 10 years of PD with no antiparkinsonian drug. De novo patients with PD (drug-free) should be motivated to participate in future randomized clinical trials to further test the slow-down or delay of motor decline under noninvasive neurostimulation regimens, whatever the stage of the disease.
Keywords: Repetitive transcranial magnetic stimulation, Repetitive peripheral magnetic stimulation, Parkinson's disease, Drug-free, Motor symptoms, TMS measures, Case report
Graphical abstract
Highlights
-
•
Muscle + brain stimulation improved motricity in a drug-free Parkinsonian case.
-
•
Changes of motricity and function was maintained at 80-day follow-up.
-
•
Improvement was paralleled by plastic changes in the primary motor cortex.
1. Introduction
Parkinson's disease (PD) is the second progressive neurodegenerative disorder worldwide after Alzheimer's disease and the 1.3 % PD-diagnosed people present with motor and nonmotor symptoms [1]. PD results from the progressive loss of nigrostriatal connections from the dopaminergic (DA) neurons of the substantia nigra pars compacta (SNc) to their target neurons in the striatum [2]. The lack of incoming nigrostriatal DA imbalances the activity between direct and indirect striatal GABAergic outputs to the internal globus pallidus (GPi) and the substantia nigra pars reticulata (SNr), which results in an increase of thalamus inhibition by GPi and SNr and an hyperpolarization of the thalamo-cortical glutamatergic connections [[3], [4], [5]]. The by-product of the loss of nigrostriatal connections is thus the hypoactivation of the primary motor cortex (M1) and the impaired activation of the cortico-striato-thalamo-cortical loops; this impacts the control of movement, from sensorimotor integration to motor sequence planning, programming of actions and task execution [3] leading to tremor, rigidity, akinesia, postural instability [6] and slow acquisition of new motor skills with impaired consolidation and retention of these skills [7,8]. From this etiology, it is logically assumed that any intervention that increases M1 excitability (such as drugs or noninvasive neurostimulation) could contribute to slow-down the progressive decline of motor control.
The gold-standard drug treatment in PD is the L-3,4-dihydroxy-phenylalanine (L-Dopa) whose antiparkinsonian effects are better than with other drugs [9]. L-Dopa in-take aims at compensating for the missing striatal DA and reactivating M1. It can be complemented by alternative therapies such as physical exercises and diet adaptation. Physical exercises such as yoga, Tai Chi, etc. have become of great clinical interest due to associated neuroprotective effects and improvement of motor and cognitive function [10]. Change of diet responds to the concept of the Brain Gut Microbiome system [11]: it was shown in 706 elders that the Mediterranean Intervention for Neurodegenerative Delay (MIND, Horn et al., 2022), i.e. the consumption of berries and green leafy vegetables in higher quantity than in the traditional Mediterranean diet, could reduce the risk of developing Parkinsonism and slow-down the progression of PD symptoms [12].
Given the large body of epidemiological evidence supporting the beneficial effects of these alternative therapies (physical exercises, diet adaptation), some patients can choose to adopt them without any L-Dopa treatment because they are reluctant to the drug in-take and its debilitating side-effects, such as nausea, visual hallucinations or the risk of developing long-term motor complications, i.e., L-Dopa-induced dyskinesias [13]. These drug-free people who have lived with PD for many years are of main interest to explore the influence of non-pharmacological and noninvasive interventions, such as stimulation of the nervous system, on M1 plasticity and motor symptoms: indeed, the common target between the antiparkinsonian drugs and other interventions to slow-down the functional decline is to up-regulate M1 excitability, via an action for instance on the glutamatergic NMDA-receptors. It is thus possible to test in drug-free people with PD the actual influence of other interventions (than drugs) on the disease itself, i.e., without any bias or contamination of results by drug interactions.
Noninvasive magnetic stimulation of nervous system consists of a transient electromagnetic induction administered via a coil positioned on the scalp to activate the electrical tissues beneath: this influences the excitability of the stimulated area and its networks [14]. Magnetic stimulation is painless (no recruitment of nociceptors), and it can be used as an exploration tool (non-repetitive mode) or an intervention (repetitive mode). Exploration of M1 excitability and its plastic changes after an intervention uses transcranial magnetic stimulation (TMS testing) to depolarize corticospinal cells and study the motor evoked potentials (MEP) elicited in a contralateral muscle recorded by surface electromyography [[15], [16], [17], [18], [19], [20]]. More precisely, single-pulse TMS (to collect test MEP) and paired-pulse TMS (to collect conditioned MEP) enable to test the excitability of different components of motor control, namely the corticospinal tract from M1 and the intrinsic inhibition and facilitation circuits surrounding the corticospinal cells in M1, respectively [16,[20], [21], [22], [23]]. These TMS measures are useful biomarkers of practice-dependent plasticity in M1 circuits [24] and it was shown for example that M1 facilitation was abnormally increased in patients with PD, likely due to abnormal glutamatergic neurotransmission [25]. Repetitive magnetic stimulation is an intervention to influence neuroplasticity and it can be administered over the central nervous system, e.g., repetitive TMS or rTMS of brain [26,27] or over the peripheral nervous system, e.g., repetitive peripheral magnetic stimulation or rPMS of nerve, muscles or spinal roots [14,[28], [29], [30]]. rTMS of M1 is a top-down approach to influence directly brain plasticity (in occurrence, to up-regulate M1 hypoactivation in the drug-free participant) and improve the symptoms, as already shown in other motor disorders, in cognitive disorders or chronic pain [26,27,[31], [32], [33], [34], [35], [36], [37], [38], [39], [40]]. rPMS of muscles is a bottom-up approach to generate specific movement-like activation of sensorimotor areas (influence of the proprioceptive systems, thalamo-cortico-thalamic loops, fronto-parietal networks) and improve motor disorders and pain [[28], [29], [30],[41], [42], [43], [44], [45], [46], [47], [48], [49]]. It has never been tested in PD but worth be for its genuine influence on M1 and thalamo-cortical circuits (lemniscal flows of proprioceptive information) which are overinhibited by GPi and SNr. It is proposed that top-down rTMS of M1 and bottom-up rPMS of muscles, alone or in combination, could influence M1 excitability and the reciprocal cortico-striato-cortical DA loops involved in motor planning, execution, and learning. Such influence could improve the motor function in PD [50,51], especially in a person whose central nervous system has not been altered by drugs. Of note, rTMS and rPMS methods are safe and rigorous following guidelines published [26,29].
Therefore, this open-label case study aimed at exploring the acute effects of rTMS and rPMS and their combination, on the motor symptoms, function and M1 plasticity in a drug-free participant whose 10-year PD progression and associated symptoms had been treated only by alternative therapies (physical exercises, diet adaptation), and no drug. Four stimulation sessions (rTMS + rPMS combination) were conducted one week apart to test separately rTMSsham + rPMSsham (placebo effect), rTMSreal + rPMSreal (M1 and muscles stimulated), rTMSreal + rPMSsham (M1 only stimulated), rTMSsham + rPMSreal (muscles only stimulated). M1 plasticity was probed per session by single- and paired-pulse TMS of M1 at pre- and post-stimulation. Motor symptoms, function and quality of movement were examined at baseline and one week after each session by the Unified Parkinson Disease Rating Scale part III (UPDRS-III) [[52], [53], [54]] and the Patient Specific Functional Scale (PSFS) [[55], [56], [57]]. PSFS is clinically useful in PD to self-report the evolution of chosen activities that patients personally find difficult and would like to improve [58]. We hypothesized and showed that the rTMSreal + rPMSreal session would have the largest influence on M1 and the symptoms. The results are discussed owing to the placebo after-effects (sham-sham), the progressive clinical changes until potential ceiling effects, M1 plasticity likely underlying the clinical changes, and the specific after-effects of the original combination of rPMS with rTMS. It is noteworthy to the results were obtained in a drug-free patient where no antiparkinsonian drug could have interacted with the after-effects of each stimulation suit.
2. Materials and methods
2.1. Case description and study design
A 52-years-old woman who had been diagnosed with Parkinson's disease 10 years ago (stage 3 on the Hoehn & Yahr scale) [59] was recruited in the present study under the written informed consent approved by the research ethics boards of the CHU de Québec - Université Laval. Tremor and bilateral rigidity in the limbs (scores of 12/28 and 7/20, see Table 1, clinical characteristics at baseline) and movement slowness (score of 19/68 for bradykinesia) impaired postural stability and confidence (score of 7/40). After having received the PD diagnosis, she declined medication in-take and rather chose an alternative natural approach to counter the effects of the disease, such as and adapted Mediterranean-like diet, naturopathy, meditation, and daily physical exercises (e.g., muscles stretching, squat and small distance walking 2–3 times per day). She worked from home (due to PD) to promote health-enhancing natural products and tried to maintain most of her daily activities in her new routine established after the onset of the disease.
Table 1.
Clinical outcomes.
| Effect of → | Baseline | rTMSSHAM |
rTMSREAL |
rTMSREAL |
rTMSSHAM |
|---|---|---|---|---|---|
| rPMSSHAM | rPMSREAL | rPMSSHAM | rPMSREAL | ||
| UPDRS III (improve = decrease) | |||||
| Global/156 | 45 | 42 | 33 | 30 | 29 |
| CID (with previous) | Moderate | Moderate | ns | ||
| CID (with baseline) | Small | Large | Large | Large | |
| UPDRS III Symptoms category (improve = decrease) | |||||
| Bradykinesia/68 | 19 | 17 | 16 | 14 | 14 |
| Rigidity/20 | 7 | 6 | 2 | 2 | 3 |
| Tremor/28 | 12 | 12 | 9 | 8 | 8 |
| Body & postural instability/40 | 7 | 7 | 6 | 6 | 4 |
| ANOVA (Dunnett's) with baseline | p = 0.61 | p=0.002 | p=0.0003 | p=0.0002 | |
| PSFS (improve = increase)/10 per item | |||||
| Arm swing in gait | 2 | 2 | 2 | 4 | 4 |
| CID (with baseline) | ns | ns | Small | Small | |
| Balance control | 2 | 2 | 2 | 3 | 5 |
| CID (with baseline) | ns | ns | ns | Large | |
| Fine motor control | 2 | 2 | 2 | 2 | 3 |
| CID (with baseline) | ns | ns | ns | ns | |
| Cut with a knife | 3 | 3 | 3 | 3 | 3 |
| CID (with baseline) | ns | ns | ns | ns | |
| TOTAL/40 | 9 | 9 | 9 | 12 | 15 |
| CID (with baseline) | ns | ns | Large | Large | |
| ANOVA (Dunnett's) with baseline | p > 0.99 | p > 0.99 | p = 0.35 | p=0.02 | |
Protocols of stimulation were conducted at one week apart. The baseline measure outcomes were collected immediately before the first stimulation session. Per protocol, the post-stimulation measure outcomes were collected one week after the stimulation, just before the next stimulation session, except for the sham-real combo (80 days later). The CID for UPDRS-III (clinical important difference) was small for a score reduction of [2.5; 5.2[, moderate at [5.2; 10.8] and large for a reduction greater than 10.8 points (Shulman et al., 2010). The CID for PSFS was small for a point increase of [1.3; 2.3[, medium at [2.3; 2.7[ and large for an increase from 2.7 points (Abbott and Schmitt 2014). UPDRS III: Unified Parkinson Disease Rating Scale (motor examination); ns: not significant; CID: clinically important difference.
The inclusion criteria for this study were to be 18 years of age or older, capable of an informed decision making, and to have a PD diagnosis stage 1–4 on the Hoehn & Yahr scale [59]. The exclusion criteria included dementia diagnosed by the attending physician, cervical facet denervation, thermocoagulation, spinal surgery or rhizotomy, nonconsolidated fracture, neurological or psychiatric disorders (other than PD), Parkinson's syndrome due to chronic use of neuroleptics or other degenerative disorders, delirium within the last 6 months, alcohol, or drug abuse, and significant uncorrected visual and/or hearing impairment. Additional exclusion criteria were related to TMS and included metal plate at skull or jaw, history or presence of brain tumor or infection, cerebrospinal fluid bypass system, visceral or cardiac pump, implanted pacemaker, pregnancy, epilepsy not controlled by medication, one or more episodes of convulsions in the last 6 months, recurrent syncope, intracranial surgery or presence of intracranial clips, or presence of head implants including cochlear implants [60,61]. Exclusion criteria related to rPMS were metal, wound or history of cancer at the stimulation zones [29].
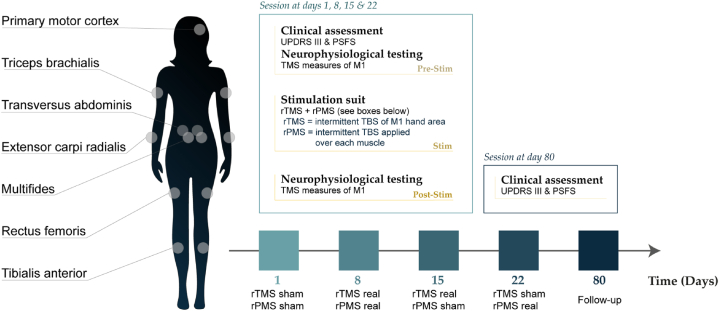
Fig. 1 presents the study design in five sessions, i.e., four stimulation sessions at one week apart and a follow-up 80 days later. The rTMS + rPMS suit sessions were in the following order week after week: sham-sham (placebo testing), real-real (combined effect), real-sham (rTMS effects), sham-real (rPMS effects). The clinical outcomes (symptoms and function) were collected at the onset of each session, immediately before the stimulation suit (at pre-stimulation per session) and the TMS measures (M1 excitability) were collected at pre- and post-stimulation per session. At pre-intervention, the TMS measures were always collected after the clinical outcomes (to avoid fatigue or lassitude during clinical testing). This study was thus designed to test the after-effects of each session one week after the session (at pre-stimulation of the next session). Of note, for the sham-sham session (first session), the pre-stimulation measurements tested the baseline clinical scores and baseline M1 excitability. TMS measures replicated at pre- and post-stimulation per session allowed to probe the acute after-effects per session (i.e., per stimulation suit). Due to COVID-19 issues, the participant could not come to the laboratory one week after the sham-real session (last session), but 80 days later: no TMS data were collected at that time and this was rather considered as a clinical follow-up. The participant was blind to the type of stimulation she received per session over brain and over muscles (sham or real). The stimulation suits were administered by an experimenter not involved in data collection and analyses. The latter were conducted by the first author (EG) who was out of the room during the intervention and remained blind to stimulation and timepoint until completion of analyses (data codified for stimulation and timepoint).
Fig. 1.
Design of the study. The protocol was conducted over four sessions of rTMS + rPMS suits (sham-sham, real-real, real-sham, sham-real) and a follow-up (80 days after the last session). Clinical assessment (UPDRS-III and the PSFS scales) was conducted one week after each session (i.e. at the onset of next session, at pre-stimulation), except for the follow-up. M1 excitability was tested by TMS at pre- and post-stimulation per session. M1: primary motor cortex (hand area); PSFS: patient specific functional scale; rPMS: repetitive peripheral magnetic stimulation; rTMS: repetitive transcranial magnetic stimulation; Stim: stimulation; TBS: theta burst stimulation; TMS: transcranial magnetic stimulation; UPDRS-III: Unified Parkinson's Disease Rating Scale (motor examination).
2.2. Clinical testing
The scores of the UPDRS-III and PSFS scales were used to characterize the participant at baseline and to delineate motor and functional changes over the experiment.
The UPDRS-III is mostly used to assess people with PD [52,54]. Four main categories of symptoms were assessed by a trained experimenter: bradykinesia (68 points/156 points of UPDRS-III score), rigidity (20/156), tremor (28/156), and body affectation and postural instability (40/156). The clinical important difference (CID) for UPDRS-III was originally described as “the smallest difference in score in the area of interest, which patients perceive as beneficial and which would lead, in the absence of annoying side effects and excessive costs, to a change in patient management”: CID is considered “small” when the reduction of UPDRS-III score is greater than or equal to 2.5 points, “moderate” when the reduction is greater than 5.2 points, and “large” when the reduction is greater than 10.8 points [62].
The PSFS is self-administered and it results in scores on one's ability to perform chosen tasks of daily life, i.e. functional limitations that one would like to improve [63]. The participant was required to choose four daily life activities whose performance challenged her and that she wanted to improve. The functional limitation, session per session, was scored by a 0–10 numerical scale [64], 0 corresponding to “unable to perform the activity” and 10 to “No problem” or “it's like before Parkinson's disease”. PSFS is acknowledged as feasible, valid, and reliable in participants with Parkinson's disease [58]. The CID for PSFS, also referred to as “minimal important difference or change”, is used to better understand the magnitude of motor improvements in clinical practice and in research: for the average score or each task score, a CID is small for an increase of 1.3 points, medium for an increase of 2.3 points, and large for an increase of 2.7 points [57].
2.3. TMS testing of M1 hand area
The TMS procedures were strictly replicated at all timepoints (pre- and post-intervention per session). The participant was comfortably seated in a reclining-adjustable chair with limb supports, forearms in pronation and the tested hand resting on the arm support. The instructions were to remain as relaxed as possible. Surface EMG electrodes (1-cm diameter) with adhesive skin interfaces (16-channel Bagnoli EMG System, Delsys Inc., Boston, MA) were installed on the first dorsal interosseus (FDI) muscle (index finger abductor) of the side the most affected by the disease (right, dominant side, in occurrence) after skin resistance reduction by slight friction with alcohol. A common ground electrode was positioned on the ulnar head of the hand tested. EMG signals were bandpass-filtered (10–450 Hz), amplified (x1500) before digitization (2 kHz), and stored for offline analysis (PowerLab acquisition system; LabChart-ADInstruments, Colorado Springs, CO). Magnetic stimuli were applied over FDI M1 area (left hemisphere) with a figure-of-eight coil (7-cm outer diameter each wing; Magstim Company Limited; Whitland, UK) which was held tangentially to the scalp and its midline being 45-deg switched from the nasion-inion line so that M1 cells were recruited by TMS in a postero-anterior direction current within brain [65]. The “hotspot” was first determined as the coil's location eliciting the largest amplitude of FDI MEP at the lowest TMS intensity [19]. The interactive frameless stereotaxic neuronavigation system (Brainsight, Rogue Industries, Montreal, Canada) was then used in real-time (TMS coil guidance and recordings of scalp coordinates) for reliability of the TMS coil position between timepoints. The resting motor threshold (RMT, informing on M1 basic excitability) was defined as the TMS intensity eliciting at least five MEP >50 μV out of 10 trials in the relaxed contralateral FDI [19,65]. The corticospinal excitability was assessed by the mean peak-to-peak amplitude of 15 test MEP generated in FDI by single-pulse TMS at an intensity of 120 % RMT [19,65]. The mean test MEP amplitude informs on the volume of M1 cells activated by TMS and the synchronicity of the corticospinal volleys to depolarize the spinal alpha-motoneurons innervating the FDI muscle [65,66]. The paired-pulse TMS paradigm (coil connected to two Magstim 2002 stimulators synchronized with a BiStim2 module; Magstim Company Limited; Whitland, UK) was used to test the excitability of the inhibition and facilitation circuits surrounding M1 cells. Precisely, the short-interval intracortical inhibition (SICI) informs on the excitability of the GABAA circuits of M1 [19,22]. SICI reduction favors the induction of mechanisms of plasticity in M1 circuits [24]. SICI was tested by the pairing of a subthreshold conditioning TMS (intensity of 80 % RMT) and a test TMS (120 % RMT) at an inter-stimulus interval (ISI) of 3 ms [67]. The intracortical facilitation (ICF) informs on the combined facilitation and inhibition of glutamate and GABAA circuits of M1 [68]. For ICF, the same conditioning and test TMS were paired (than for SICI) but at an ISI of 15 ms [67]. The short-interval intracortical facilitation (SICF) informs on the excitability of facilitation NMDA circuits of M1 acting at the corticospinal axon hillock [69] and may interact with SICI circuits [70]. SICF was tested by the pairing of a test TMS at 100 % RMT and a conditioning TMS at 90 % RMT at an ISI of 1 ms [25,70,71]. The conditioned MEP (mean of 15 trials for each of the three paired-pulse testing above) are usually of smaller amplitude than the test MEP in SICI, higher in ICF at rest, and higher in SICF; they are expressed in percentage of the mean test MEP amplitude. Any trial with FDI EMG background activity over 10 μV (electrical noise) was discarded and recollected.
2.4. rTMS & rPMS intervention and sham stimulation
The rTMS + rPMS suit procedures (either sham or real stimulation) were strictly replicated in the four sessions (sham-sham, real-real, real-sham, sham-real). The participant was comfortably seated on the reclining-adjustable chair for rTMS of M1 and she was supine on a therapeutic table for rPMS. Instructions were to remain as relaxed as possible, on the chair or on the table, and to avoid talking during the intervention. The rTMS (of M1) and rPMS (of muscles) were successively applied by means of the same air film cooled figure-of-eight coil (7-cm outer diameter each wing) connected to two rapid-rate magnetic stimulators (Rapid2; The Magstim Company Limited; Whitland, UK). The stimulation paradigm was excitatory for both rTMS and rPMS and consisted of the patterned theta-burst stimulation (TBS), i.e. 5-Hz trains of three 50-Hz TMS pulses [72] applied at an intermittent mode (iTBS), i.e. 2sec ON, 8sec OFF for 100 to 200 sec, corresponding to a total of 300 to 600 pulses, respectively [73] (200 sec for rTMS; 100sec for rPMS per muscle). Precisely, iTBS was shown to induce larger and longer increase of corticospinal excitability (outlasting the period of stimulation) than standard excitatory rTMS (>1Hz), for a shorter period of application and at a lower intensity [72,73]. Hence, iTBS appears as a relevant option to slow-down the functional decline in PD [38,72]: iTBS of M1 could normalize M1 hypoactivation (a by-product of nigrostriatal DA loss) [39] via its influence on NMDA-dependent plasticity [74]; iTBS of muscles could indirectly reactivate M1 by mimicking muscle contraction/relaxation and eliciting massive flows of proprioceptive information coherent with motor control and learning, as already shown in other conditions [28,30,42,43,45,48,49,[75], [76], [77], [78], [79]].
rTMS was applied over the left FDI M1 area (contralateral to the more affected side). In line, M1 had already been identified as an optimal site of stimulation to induce motor improvements in PD [39] and this stimulation could result in variations of extracellular DA concentration in the striatum, meaning a possible striatal release of DA following frontal stimulation [80,81]. The reliability of the rTMS coil positioning was insured by real-time neuronavigation (Brainsight, Rogue Industries, Montreal, Canada) using FDI M1 area coordinates of TMS testing. The 200-sec rTMS intensity was set at 90 % RMT. In rPMS, the intensity was set to produce a muscle contraction which had to be comfortable for the participant, but sufficient to generate massive flows of proprioceptive inputs able to influence M1 [28,29,43]. This intensity was set at 35–42 % of the maximal output of the stimulator, depending on the muscle. Precisely, rPMS was applied bilaterally during 100 sec per muscle, first on the most affected side, then on the other side, in the following order: tibialis anterior (ankle dorsiflexors), rectus femoris (hip flexors), transversus abdominis (deep abdominal muscles, spine stabilisators), multifidi (paravertebral muscles, spine stabilisators), triceps brachialis (elbow flexors) and extensor carpi radialis (wrist extensors), i.e. six couples of muscles (see Fig. 1) and a total rPMS intervention time of 20 minutes. The rTMS + rPMS intervention thus lasted 23min20sec per session. The sham stimulation (placebo) of rTMS and rPMS was administrated with the coil upside down, i.e., with the same parameters as for real stimulation (iTBS, same intensity, same duration, same M1 area, same muscles) but the stimulation was in the air and inactive for M1 (rTMSsham) or the muscles (rPMSsham). Maintenance of the participant's blinding (sham/real) throughout the different sessions was easy for rTMS (coil positioned on the head) and body parts stimulated were hidden during rPMS. The patient was naïve to neurostimulation and could not a priori discriminate between real or sham stimulation, but a questionnaire was administrated at the end of her participation to ask if she had guessed what she was receiving per session.
2.5. Data reduction and statistical analysis
Two clinical outcomes were collected: the UPDRS-III score (motor examination of different symptoms) and the PSFS score (different activities of daily living, self-administrated). Four TMS variables were analyzed: the peak-to-peak amplitude of the test MEP (in mV or in % pre-stimulation) and the peak-to-peak amplitudes of the conditioned MEP (in % test MEP or in % pre-stimulation) related to SICI, ICF and SICF. Statistical analyses were performed using Prism 10.1.0 software (GraphPad, CA, USA) with significance level set at p ≤ 0.05.
2.5.1. Clinical outcomes: changes over the sessions
Scores (UPDRS-III, PSFS) were used. The CID was used to detect clinically significant changes of UPDRS-III and PSFS scores, i.e. the absolute change at each timepoint compared with baseline. In addition, for UPDRS-III, the CID was used to detect the relative additional change (from one timepoint to the next). One-way repeated-measure analyses of variance (ANOVARM) using the factor Time (n = 5: at baseline and after each of the four stimulation suits, the last being the follow-up) were applied on the datasets of the UPDRS-III symptom categories and of the PSFS tasks with the Dunnett's multiple comparison test (adjusted p-values). The Shapiro-Wilk normality test on residuals verified the Gaussian distribution of the two datasets.
2.5.2. TMS measures: acute changes per session
Descriptive statistics were used (mean, SD) to present each TMS variable at pre- and post-stimulation in each of the four stimulation suits. The 5 % ROUT methods (Prism software) was used to detect outliers (further than ± 2 SD from the mean), i.e. values different than the 95 % following the Gaussian distribution [82]. Each TMS variable at post-stimulation was expressed in % pre-stimulation per suit and one-way ANOVA using the factor Suit (n = 4) was applied to detect if the immediate after-effects on M1 excitability were different between stimulation suits. The Tukey's multiple comparisons tests determined where differences lay between suits. Log-transformation was applied on a variable when one or more pre- or post-stimulation data did not respect the distribution normality (Shapiro-Wilk test on residuals). If this reestablished normality, then ANOVA was applied on the log-transformed data (other statistical rules for non-reestablishment of normality by log-transformation were not required). A two-way ANOVA with factors Suit (n = 4) and Time (pre-stimulation vs. post-stimulation) was then applied on the test and conditioned MEP with Sídák's multiple comparisons test, to detect if post-stimulation changes were significant per suit.
2.5.3. Correlations
Pearson's correlation matrices were produced to examine the relationships between changes of different clinical outcomes (global scores or components) between stimulation suits and test the the link between clinical and TMS changes. This was done for the longer-term changes of TMS data, i.e. data at pre-stimulation of the next session (thus at timepoints of clinical outcomes) and expressed in % of their values at pre-stimulation of the previous session. Long-term TMS changes of sham-real suit were not measured due to COVID-19 and the last session (80 days after sham-real) was considered as a follow-up.
3. Results
All procedures were well tolerated by the participant and no adverse effect was reported. She did not guess which type of stimulation (real/sham) she received per session on brain and muscles.
3.1. Clinical outcomes
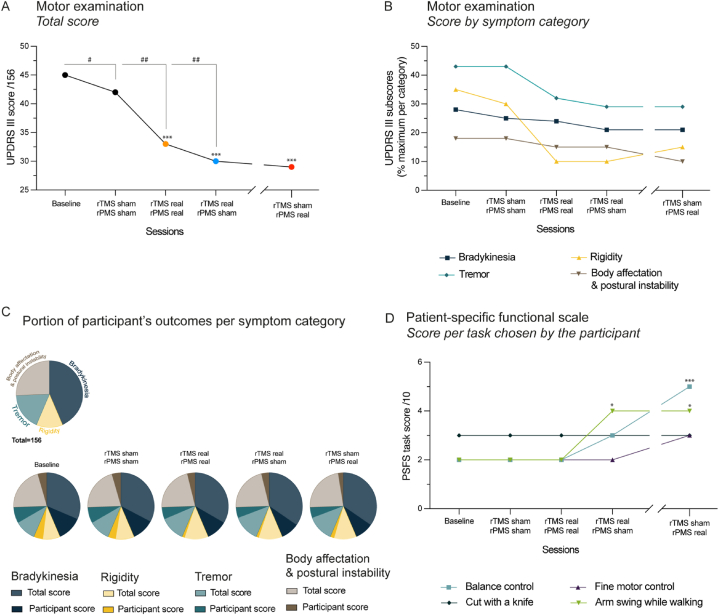
Table 1 and Fig. 2 present the clinical data collected per timepoint one week after each rTMS + rPMS suit. In Fig. 2, stars (*) and pads (#) were used to indicate a clinically significant effect owing to the scale CID used.
Fig. 2.
Clinical outcomes. Motor function was assessed by UPDRS-III and PSFS scales one week after each stimulation suit (except for the last session, followed-up at 80 days later). A. Improvement (decrease) of the total UPDRS-III score per timepoint. The asterisks (*) inform on the clinically important difference (CID) between the timepoint and the baseline (i.e., *small, **moderate or ***large CID) and the sharps (#) on the CID between two successive stimulation suits (i.e., #small, ##moderate or ###large CID; see Clinical testing or Table 1 for CID values). B. Changes of UPDRS-III subscores, i.e. results per symptom category per timepoint. C. Pie visualization of the proportion of the participant's outcomes per symptom category per timepoint, i.e., score obtained by the participant for each UPDRS-III symptom superimposed on the maximal score per category (decrease of proportion was an improvement of the participant's function per category). D. PSFS scores per timepoint for each task chosen by the participant. The asterisks (*) inform on the CID between the timepoint and the baseline (i.e., *small, **moderate or ***large CID, see Clinical testing or Table 1). UPDRS-III: Unified Parkinson's Disease Rating Scale (motor examination); PSFS: patient-specific functional scale; rPMS: repetitive peripheral magnetic stimulation; rTMS: repetitive transcranial magnetic stimulation; wk: week.
3.1.1. UPDRS-III scores
Table 1 and Fig. 2A present that the UPDRS-III score was 3-point lower than baseline (45 points) after sham-sham suit (42 points, small effect with respect to CID), 12-point lower after real-real (33 points, large effect), 15-point lower after real-sham (30 points, large effect) and still 16-point lower at follow-up (29 points, large effect). This corresponded to a progressive decrease of the score after sham-sham until follow-up, with additional moderate effects after real-real (9 points less) and real-sham (3 points less) and changes maintained at follow-up (1 point less).
Table 1, Fig. 2B (symptom categories) and Fig. 2C (proportion per maximum score of each category) present that the UPDRS-III global score reduction was explained by a decrease of rigidity and tremor after real-real and by a decrease of body affectation and postural instability at follow-up. Data distribution for symptoms passed the normality test. The ANOVA applied on the symptoms detected a main effect of Time (F4,12 = 15.65, p = 0.0001) with a statistically significant reduction as compared to baseline after real-real (p = 0.002), real-sham (p = 0.0003) and at follow-up (p = 0.0002, see Table 1). Precisely (not reported on the figures), these symptoms were improved on both sides for movement and resting posture, with more velocity of the left hand, faster alternate movements and more postural stability after real-real, then lesser resting tremor of the right hand and a better hand motricity on both sides after real-sham and finally, further reduction of the left-hand tremor (movement, resting posture) at follow-up. Overall, Table 1 shows that, at follow-up compared to baseline, bradykinesia was 5-point decreased (14/68 vs. 19/68 at baseline), rigidity was 4-point decreased (3/20 vs. 7/20), so was tremor (8/28 vs. 12/28), and body affectation and postural instability was 3-point decreased (4/40 vs. 7/40).
3.1.2. PSFS scores
Table 1 and Fig. 2D detail the evolution of the four tasks chosen by the participant for the PSFS self-administration (total score/40, 0–10 score per task). No change was noted as compared to baseline until the large effect perceived two weeks after real-real (i.e., one week after real-sham) with a 9-pt to 12-pt increase of the total score (see Table 1). This was due to improvement of arm swing during gait (+2 points, small effect with respect to CID) and balance control (+1 point, nonsignificant). This large effect was maintained at follow-up (up to 15 points total) with the same improvement of arm swing during gait, improvement of balance control (+3 points, large effect) and increase of fine motor control (+1 point, nonsignificant). Data distribution for tasks passed the normality test. The ANOVA applied on the tasks scores detected a main effect of Time (F4,12 = 4.15, p = 0.024) with a statistically significant increase at follow-up as compared to baseline (p = 0.02, see Table 1).
3.2. TMS measures
Table 2 presents the TMS data collected at pre- and post-stimulation per suit, the expression of post-stimulation in % pre-stimulation per suit (acute changes per suit) and the expression of pre-stimulation at the next suit in % pre-stimulation of previous suit (i.e., changes after a week, see section 3.3). Fig. 3 presents the box-and-whiskers graphs of the acute changes per suit per TMS variable (% pre-stimulation).
Table 2.
TMS measures.
| Effect of | rTMSSHAM |
rTMSREAL |
rTMSREAL |
rTMSSHAM |
||||
|---|---|---|---|---|---|---|---|---|
| rPMSSHAM |
rPMSREAL |
rPMSSHAM |
rPMSREAL |
|||||
|
Pre |
Post |
Pre |
Post |
Pre |
Post |
Pre |
Post |
|
| Baseline | sham-sham | real-real | real-sham | |||||
| Test MEP | ||||||||
|
mV Mean |
2.48 | 1.83 | 4.84 | 5.15 | 2.87 | 2.31 | 3.91 | 5.32 |
| SD | 1.78 | 0.83 | 2.91 | 3.05 | 2.33 | 1.76 | 2.76 | 3.02 |
| Post in % pre-stimulation (same session) | ||||||||
| Mean | – | 73.8 | – | 106.4 | – | 80.7 | – | 136.1 |
| SD | – | 33.5 | – | 62.9 | – | 61.5 | – | 77.3 |
| Pre in % pre-stimulation of previous session | ||||||||
| Mean | – | – | 195.1 | – | 59.3 | – | 136.2 | – |
| SD | – | – | 117.3 | 48.1 | 96.2 | – | ||
| SICI-conditioned MEP and log-transformed data | ||||||||
|
% test MEP Mean |
26.6 | 80.3 | 60.4 | 56.4 | 78.4 | 61.8 | 6.4 | 74.8 |
| SD | 28.4 | 32.7 | 46.5 | 51.9 | 71 | 58.8 | 3.9 | 57.6 |
|
Log-data Mean |
1.23 | 1.87 | 1.62 | 1.50 | 1.69 | 1.56 | 0.73 | 1.73 |
| SD | 0.41 | 0.17 | 0.42 | 0.57 | 0.49 | 0.50 | 0.26 | 0.38 |
| Post in % pre-stimulation (same session, log-data) | ||||||||
| Mean | – | 152.3 | – | 92.4 | – | 92.5 | – | 237.6 |
| SD | – | 13.9 | – | 35.1 | – | 29.4 | – | 52.5 |
| Pre in % pre-stimulation of previous session | ||||||||
| Mean | – | – | 131.7 | – | 104.3 | – | 43.2 | – |
| SD | – | – | 34.1 | 30 | 15.4 | – | ||
| ICF-conditioned MEP % test MEP | ||||||||
| Mean | 141 | 110.6 | 92.2 | 115.3 | 161.2 | 121 | 78.1 | 126 |
| SD | 122.4 | 45.2 | 53.8 | 48.1 | 86.9 | 73.3 | 66.7 | 49.6 |
| Post in % pre-stimulation (same session) | ||||||||
| Mean | – | 78.5 | – | 125.1 | 75 | 161.1 | ||
| SD | – | 32 | – | 52.2 | 45.5 | 63.5 | ||
| Pre in % pre-stimulation of previous session | ||||||||
| Mean | – | – | 65.4 | – | 174.8 | – | 48.5 | – |
| SD | – | – | 38.2 | 94.3 | 41.2 | – | ||
| SICF conditioned MEP % test MEP | ||||||||
| Mean | 1531 | 472 | 4980 | 8211 | 4480 | 1316 | 2122 | 3337 |
| SD | 1561 | 202.1 | 4623 | 7482 | 4155 | 1042 | 1494 | 1822 |
| Post in % pre-stimulation (same session) | ||||||||
| Mean | – | 30.8 | – | 164.9 | – | 29.4 | – | 157.3 |
| SD | – | 13.2 | – | 150 | – | 23.3 | – | 85.8 |
| Pre in % pre-stimulation of previous session | ||||||||
| Mean | – | – | 325.3 | – | 89.96 | – | 47.37 | – |
| SD | – | – | 302 | 83.4 | 33.3 | – | ||
Test MEP was elicited at 120 % RMT for SICI and ICF (paired-pulse TMS at 80/120 % RMT, ISI at 3 and 15 ms, respectively) and at 100 % RMT for SICF (paired-pulse TMS at 100/90 % RMT, ISI at 1 ms). SICI data were log-transformed (non-respect of Gaussian distribution) and % pre-stimulation was calculated on log-data. N: number of measures per timepoint; ISI: inter-stimulus interval; MEP: Motor evoked potential; pre: before stimulation at each session; post: after stimulation at each session; RMT: resting motor threshold; SD: Standard deviation; ICF: intracortical facilitation; SICF: short-interval intracortical facilitation; SICI: short-interval intracortical inhibition. Significant differences for pre/post-stimulation changes (% pre-stimulation) are denoted in Fig. 3.
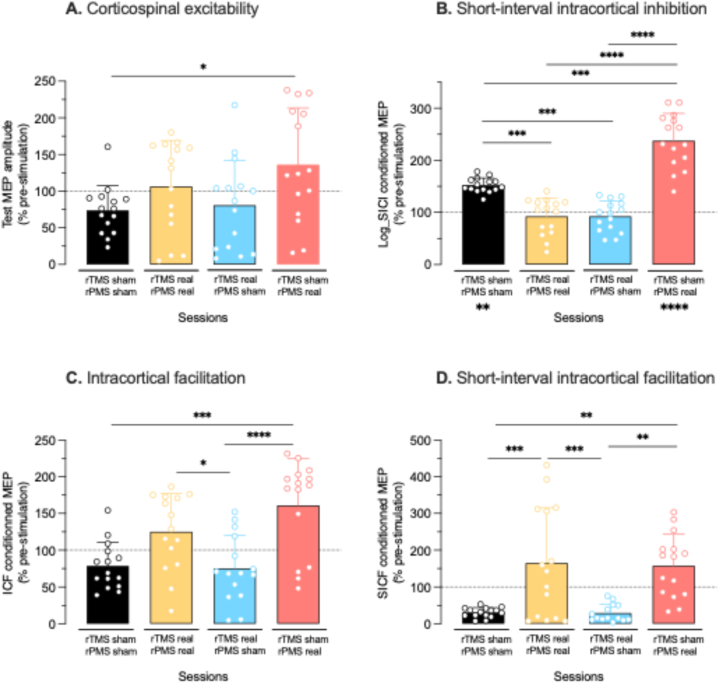
Fig. 3.
Acute changes of TMS measures per stimulation suit (% pre-stimulation per session). In the box-and-whisker representation, the line at the center of the box gives the median value, the lower and upper limits of the box give the 25 % and 75 % percentiles and the whiskers delineate the extreme values. The individual trials are superimposed (empty circles). A. Corticospinal excitability assessed by the peak-to-peak amplitude of the test MEP amplitude elicited by single-pulse TMS at 120 % RMT. B. Short-interval intracortical inhibition (SICI) assessed by the peak-to-peak amplitude of the conditioned MEP (log-transformed) elicited by paired-pulse TMS at 80/120 % RMT (ISI of 3 msec). The asterisks under the sham-sham and sham-real histograms denote a significant increase of the conditioned MEP at post-stimulation as compared to pre-stimulation. C. Intracortical facilitation (ICF) assessed by the peak-to-peak amplitude of the conditioned MEP elicited by paired-pulse TMS at 80/120 % RMT (ISI of 15 msec). D. Short-interval intracortical facilitation assessed by the amplitude of the peak-to-peak conditioned MEP elicited by paired-pulse TMS at 100/90 % RMT (ISI of 1 msec). Note the overall tendency to get larger changes at post-stimulation when the suit included real rPMS. ISI: inter-stimulus interval; MEP: motor evoked potential; rPMS: repetitive peripheral magnetic stimulation; rTMS: repetitive transcranial magnetic stimulation. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Test MEP. Data passed normality test and no outlier was detected. The ANOVA detected a Suit effect (F3,56 = 3.24, p = 0.029; Fig. 3A) with the increase of test MEP amplitude higher after sham-real (136.1 ± 77.3 % pre-stimulation) than after sham-sham (73.74 ± 33.5 %, p = 0.034) and close to be significantly higher than after real-sham (80.7 ± 61.5 %, p = 0.073). The two-way ANOVA applied on the pre- and post-stimulation data confirmed a Suit effect (F3,112 = 10.38, p < 0.0001) but the Sídák's multiple comparisons test did not detect any significant change at post-stimulation as compared to pre-stimulation in any session (see data in Table 2). However, the medians (central lines of the boxes) show that the test MEP amplitude tended to be similarly increased after real-real and sham-real sessions, and not increased in sham-sham and real-sham (Fig. 3A).
SICI. Two outliers were removed at pre-stimulation in sham-sham and sham-real sessions. Data did not pass normality test thus were first log-transformed at pre and post-stimulation (see Table 2) before expressing the post-stimulation data in % pre-stimulation. These data (% pre-stimulation from SICI log-transformed data) passed normality test. The ANOVA applied on these data detected a Suit effect (F3,56 = 29.9, p < 0.0001) with the increase of log-transformed conditioned MEP (i.e., decrease of SICI) the greatest after sham-real (237.6 ± 52.5 % pre-stimulation, Fig. 3B) than after all other suits, i.e., than after sham-sham (152.3 ± 13.9 %, p < 0.0001), real-real (92.4 ± 29.4 %, p < 0.0001), and real-sham (92.5 ± 29.4 %, p < 0.0001). SICI reduction was also greater after sham-sham than after real-real and real-sham (p = 0.0001 each). The two-way ANOVA applied on the pre- and post-stimulation log-transformed data detected the Suit × Time interaction (F3,108 = 12.98, p < 0.0001) with log-conditioned MEP significantly higher (SICI reduced) at post-stimulation than at pre-stimulation in sham-sham suit (p = 0.0004) and in sham-real (p < 0.0001) and not in real-real (p = 0.90) or real-sham (p = 0.87, see data in Table 2). The medians illustrate the SICI decrease (conditioned MEP increase) only after sham-real and sham-sham, and to a larger extent after sham-real.
ICF. Data passed normality test and no outlier was detected. The ANOVA detected a Suit effect (F3,56 = 10.27, p < 0.0001) with the conditioned MEP amplitudes higher after sham-real (161.1 ± 63.5 % pre-stimulation, Fig. 3C) than after sham-sham (78.5 ± 32 %, p = 0.0002) and real-sham (75 ± 45.5 %, p < 0.0001), and higher after real-real (125.1 ± 52.2 %) than after real-sham (p = 0.04) and almost than sham-sham (p = 0.06). The two-way ANOVA applied on the pre- and post-stimulation data was close to detect a Suit × Time interaction (F3,112 = 2.55, p = 0.06, see data in Table 2). The medians confirm the ICF increase only after sham-real and real-real, with a tendency to a larger extent after sham-real, and no increase after sham-sham and real-sham.
SICF. Data passed normality test. Four outliers were removed at pre-stimulation in sham-sham, real-real and sham-real sessions and two in real-sham session; one outlier was removed at post-stimulation in sham-real session. The ANOVA detected a Suit effect (F3,55 = 10.83, p < 0.0001) with the conditioned MEP amplitudes higher after sham-real (157.3 ± 85.8 % pre-stimulation, Fig. 3D) than after sham-sham (30.8 ± 13.2 %, p = 0.001) and real-sham (29.4 ± 23.3 %, p = 0.002), and higher after real-real (164.9 ± 150 %) than after sham-sham (p = 0.0006) and real-sham (p = 0.0007). The two-way ANOVA applied on the pre- and post-stimulation data confirmed a Suit × Time interaction (F3,97 = 3.7, p = 0.014) but the Sídák's multiple comparisons test did not detect any significant change at post-stimulation as compared to pre-stimulation in any session (see data in Table 2). The medians show the SICF increase only after sham-real and real-real, and not after sham-sham and real-sham.
No other effect was detected for the variables of interest.
3.3. Correlations
Fig. 4A shows the links between the global score of UPDRS-III and each of its symptom categories, i.e., stronger relationships with tremor (r = 0.99, p = 0.002), bradykinesia (r = 0.95, p = 0.01) and rigidity (r = 0.94, p = 0.02) than with body affectation and postural stability (r = 0.82, p = 0.09). Fig. 4B shows the relationships between the changes of scores of UPDRS-III and of the auto-administered PSFS. This graph denotes that an improvement in the activities chosen by the participant (score increase) was perceived only when the UPDRS-III score was below 33 points/156 which was obtained after real-real stimulation suit: the PSFS score was 3-point increased after the real-sham suit (third session: improvement of arm swing during gait and of balance control) when the UPDRS-III score was 3-point decreased to 30 points (see the greyed area, Fig. 4B). Also, at follow-up (F–U), the PSFS score was further 3-point increased (improvement of arm swing during gait, balance control and fine motor control) together with 1-point decrease of UPDRS-III score. No relationship was detected between the acute TMS changes (measured immediately after each stimulation suit) and these clinical changes (tested one week after each stimulation suit).
Fig. 4.
Correlations of clinical outcomes and TMS measures. A. Link between the global score of UPDRS-III and each of its symptom categories after baseline and after each stimulation suit. The timeline is from right (Baseline) to left (last session: sham-real). B. Relationship between the changes of UPDRS-III and of PSFS scores (four activities chosen by the participant, PSFS auto-administrated for perception of change). Note that the PSFS was improved only for an UPDRS-III score below 33 points (within the greyed area). C–F. Links between the changes of clinical outcomes between sessions and the long-term changes of SICF, SICI and ICF. These long-term changes of TMS measures were the data collected at pre-stimulation of a session (at same timepoint of clinical testing) and expressed in % of their values at pre-stimulation of the previous session. The long-term changes of sham-real could not be measured given that the sham-real suit was the last session of TMS measurements. C–D: Decrease of the UDPRS-III and the tremor scores against the long-term decrease of SICF. E: Decrease of the bradykinesia score against the long-term increase of SICI. F: Relative decrease of rigidity from one session to the other against the long-term increase of ICF. Sham-sham, real-real, real-sham: brain-muscle stimulation suit; UPDRS-III: Unified Parkinson's Disease Rating Scale (motor examination); BPI: body affectation and postural instability; PSFS: patient-specific functional scale; F–U: follow-up (80 days after the sham-real suit); SICF, SICI: short-interval intracortical facilitation, inhibition; ICF: intracortical facilitation (different circuits than the SICF ones); MEP: motor evoked potential (corticospinal excitability).
Fig. 4C–F present however the links detected between the clinical changes and the long-term changes of SICF, SICI, ICF after sham-sham, real-real, and real-sham stimulation suits. These long-term changes were the data measured just before the next stimulation session, i.e. concurrently to clinical assessment, and expressed in % of their values at pre-stimulation of the previous session. Of note, the long-term changes of sham-real suit session could not be measured given that this session was the last of TMS measurements. Fig. 4C–D show that the more SICF was decreased from one session to the other, the better (decrease) were the UDPRS-III and the tremor scores (r = 0.99, p = 0.05). Fig. 4E shows that the more SICI was increased from one session to the other, the better (decrease) was the bradykinesia score (r = −0.99, p = 0.017). In these Fig. 4C–E, the relationships detected followed the order of the sessions (1. sham-sham, 2. real-real, 3. real-sham), sham-sham being with the lesser score and real-sham with the better. Fig. 4F presents that the more the ICF was increased from one session to the other, the better was the decrease of rigidity from one session to the other (r = −0.99, p = 0.049). In this case, real-real stimulation suit induced the highest relative improvement.
4. Discussion
This case study tested whether four different rTMS + rPMS suits could each improve the condition of a drug-free woman (no antiparkinsonian medication) who had been living with PD's diagnosis for 10 years: sham + sham, then real + real, real + sham, and sham + real. UPDRS-III score improved more after real-real and this was maintained at follow-up. PSFS score improved once the UPDRS-III score was below 33 points. These effects were concomitant to changes of M1 excitability, thus denoting a link with M1 plasticity induced by rTMS + rPMS. Results are discussed owing to the existence of small placebo after-effects (sham-sham session), the clinical changes over the four suits likely due to summative effects and potential ceiling mechanisms, the underlying M1 plasticity concomitant to the clinical improvements and the specific rPMS after-effects. Of note, our drug-free participant could be considered as a de novo patient because no antiparkinsonian drug could have interacted with the stimulation after-effects. That said, studies in drug-free patients are scarce and our findings could be compared with results from studies in OFF-medication patients, i.e. during wash-out of L-Dopa action. However, caution must be taken because OFF-periods cannot completely free patients from residual effects of medication and some chronic benefits of L-Dopa treatment have been reported during OFF-periods [83].
4.1. Placebo effects
A 3-point improvement of the UPDRS-III motor examination score was obtained one week after sham-sham stimulation session (placebo). One question could be whether this was related to intra-rater variability, but the UPDRS-III intra-rater reliability was reported as good to very good [84]. However, a 3-point improvement is a small clinical effect owing to CID of the UPDRS-III score, i.e. in the [2.5; 5.2] points range [62]. And such small effects (but true effects) after placebo are expected in PD clinical trials because patients expect a clinical benefit, especially if they are optimistic persons, with a somatic focus or empathic resonance [85]. These placebo effects due to expectation could be due to the increase of striatal and mesoaccumbens dopamine release, triggered by the perception on skull of the magnetic brain stimulation coil, even during placebo stimulation [86] and by the informed possibility to receive a real treatment, as compared to uninformed [87]. Of note, dopamine is mainly contained in three main pathways [88] and the nigrostriatal and mesolimbic pathways involved in placebo effects may be different than the corticostriatal networks mediating local dopamine release in response to actual magnetic stimulation [86].
4.2. Neurostimulation influence: cumulative then ceiling effects?
iTBS of motor areas has already been tested in PD but results remain controversial between improvement [38,89,90] and no change of symptoms [[91], [92], [93], [94], [95]]. These discrepancies could be partly related to the interplay of anti-glutamatergic drugs (e.g., L-Dopa) with M1 responsiveness to iTBS acting via glutamatergic plasticity [74] but also to the variety of stimulation parameters and protocols inducing different metaplasticity phenomena between studies [96]. This motivated the present testing of responsiveness to neurostimulation in a drug-free people living with PD, which had never been tested before. Our results can only be compared to studies in patients with OFF medication status, which is not quite the same status as in our participant considered as de novo with no influence ever of L-Dopa in her metabolism. These studies in OFF-medication patients used rTMS and applied iTBS (as in our study) or its counterpart, the inhibitory continuous TBS (40-sec cTBS, not used here). One study applied cTBS over the left supplementary motor area (SMA) and reported UPDRS-III score improvement [90]. Other studies reported no change of UPDRS-III score after one session of iTBS over M1 [97] but rather improvement of dexterity [89], or gait improvement with iTBS of SMA [98] or no change with cTBS of the cerebellum [99]. Thus, our study is the first to have combined iTBS of M1 with rPMS of muscles and shown improvement of UPDRS-III score. These changes were significant statistically and clinically (CID over a 10.8-point change) [62]. This large effect was mainly due to real-real stimulation suit (9-point decrease in addition to the 3-point of placebo effect thus 12 points total) and the maintenance at follow-up of a 16-point decrease. One could question whether the effects cumulated between sessions (with larger effects induced by real-real), i.e. the benefits of a previous session being added to the benefits of the next session, as already reported in other studies involving rPMS of muscles [28,48]. The existence of ceiling effects throughout different sessions is also questioned given the stabilization of UPDRS III score decrease (see the time-course on Fig. 2A). Indeed, based on PD's stage equivalences with MDS-UPDRS scores [100], the reduction of UPDRS-III scores obtained in the present study changed the participant's PD stage from moderate to mild: this is a significant improvement that could have reached a plateau, thus limiting additional changes. Cumulative effects and ceiling limitations could be explained by the different time-course of symptoms improvement. Indeed, the symptom categories more affected at baseline, i.e. with a higher impact on the function (rigidity and tremor: 35 % and 43 % of maximal impact, see Fig. 2C) were more improved by real-real suit than the less affected (bradykinesia and postural instability: 28 % and 18 % of maximal impact). The latter, and especially the postural instability, were however improved at follow-up. It is questioned whether several sessions of the real-real intervention could have led to more improvement of these less affected symptom categories (bradykinesia, postural instability). That said, the persistence of the after-effects 80 days later at least is promising. Future studies should test whether a treatment regimen with the repetition of the real-real stimulation suit once per week during four weeks for instance enhances the clinical benefits obtained here and with longer-term persistence.
The PSFS scored how the participant perceived her performance at daily functional tasks that were meaningful in her life. This score was also improved and denoted a clinically large effect owing to CID (>2.7 points) [57]. This large effect was maintained at follow-up where the perceived changes were statistically different as compared to baseline. Of note, the main changes concerned the balance control and the arm swing during gait (see Fig. 2D), which can be related to the changes of tremor, bradykinesia and rigidity measured with the UPDRS-III (see Fig. 4A). However, the time-course of improvement was different between the two clinical outcomes. Indeed, PSFS score began to increase once UPDRS-III score was decreased to 33 points at least (see Fig. 4B). This reflects that moving from moderate to mild stage of PD could have been a prerequisite to favor the practice of the more ecological daily activities chosen by the participant. Also, changes in daily activities can be time-lagged, as compared to lab outcome measures (such as UPDRS III), owing to the time it can take for the participant to be aware of changes or for actual changes denoted in lab to be transferred in daily life. This is obvious in PD where abnormalities of the nigrostriatal system in PD can slow-down motor learning and alter consolidation and retention [8]. One recent neural marker of motor symptoms identified in PD, and especially in relation to the pervasive deficits of cognitive-motor control of obstacles avoidance while walking, was the reduced modulation of the theta frequency band [101]. It is proposed that iTBS used for rTMS of M1 and for rPMS of muscles could have positively influenced this impaired brain resonance in PD.
4.3. Potential M1 plastic changes underlying clinical changes
Our original data showed that the clinical improvements were related to the long-term changes of TMS measures (from one session to the other). Precisely, the improvement of UPDRS-III and tremor scores were related to SICF decrease, the improvement of the bradykinesia score to SICI increase (conditioned MEP decrease) and the improvement of rigidity to ICF increase. Given TMS measures are markers of M1 plasticity [24,96], these correlations highlight for the first time the potential mechanisms of M1 plasticity at the origin of the clinical improvements following noninvasive neurostimulation in PD. No link was however detected with test MEP amplitude and this may be related to its high variability that could have limited acute after-effects (see Fig. 3A). MEP variability is related to the random fluctuations of corticomotoneuronal synaptic transmission and motoneuron membrane potentials [102] and in PD, this phenomenon could be larger and could mask any influence of rTMS, as already observed in most PD studies [83,90,103]. The subsections that follow discuss the potential of SICF, SICI and ICF as biomarkers of clinical improvement in PD.
The SICF decrease in the left M1 was related to UPDRS-III and tremor improvements (Fig. 4C–D). This result is supported at least by three previous studies, the first with the use of cTBS (inhibition of left SMA) [90], the second with iTBS of the right M1 (interhemispheric inhibition of the left) [38] and the third with short-term and long-term treatments with safinamide, a monoamine oxidase type-B inhibitor with anti-glutamatergic properties [25]. These studies indirectly or directly targeted the decrease of the NMDA receptor-dependent SICF in the left M1 and reported improvement of UPDRS-III [90], of metaphor comprehension [38], and of M1 responsiveness to neurostimulation (iTBS-induced plasticity) [25]. In the latter, it was shown that SICF was too much enhanced in PD and hindered iTBS-induced plasticity, likely in relation to the abnormal glutamatergic neurotransmission in M1; the safinamide treatment over 14 days reduced SICF and improved iTBS-induced plasticity of M1 [25]. Changes of SICF may have partly influenced SICI which acts via GABAA receptors [69]. Indeed, SICF up-regulation in patients with PD or with neurological or psychiatric disorders has been associated with the reduction of normal levels of SICI [70,104]. A corollary proposal is that the SICF decrease over time in our study should have been concomitant to SICI increase. In line, we obtained a correlation between the long-term SICI increase and bradykinesia improvement (see Fig. 4E). This result is further supported by the fact that SICI increase is involved in motor planning, learning and motor gains [23,24] and that higher levels of SICI in OFF-medication patients could be more likely related to better motor improvement under dopaminergic medication [105]. The long-term changes of ICF were related to the decrease of rigidity between sessions (Fig. 4F). ICF reflects the combined activity of NMDA and GABAA circuits within M1, thus it is enhanced by glutamatergic facilitation and GABAergic inhibition [69]. ICF was reported as normal or abnormally reduced in PD but patients with higher ICF were more likely to get better therapeutic effects with lower doses of dopaminergic medication [105]. In line, our results showed that the higher the ICF from one session to the next, the greater the rigidity decrease (Fig. 4F). Thus, in our study, SICF decrease, SICI increase and ICF increase from one session to another may have promoted M1 responsiveness to neurostimulation, as already shown for SICF [25] and maintained the improvement of motor symptoms, tremor, bradykinesia and rigidity, respectively, via the regulation of NMDA receptors [74,106,107] and GABAergic inhibition [69]. Of note, the influence of rPMS and rTMS + rPMS on these markers of M1 plasticity and in relation to motor improvement in PD are reported here for the first time whereas the non-effect of rTMS on ICF (real-sham session, Fig. 4F) had already been reported [97].
4.4. After-effects of rPMS conditioned by rTMS
Conversely to the top-down rTMS, rPMS of muscles is a bottom-up approach that generates specific movement-like activation of sensorimotor areas: it mimics the mechanisms of muscle contraction-relaxation with sensorimotor coherence in brain, i.e. it generates massive flows of proprioceptive information that influence the lemniscal and spinocerebellar pathways, the thalamo-cortico-thalamic and cerebellar loops, and fronto-parietal areas, all involved in motor planning and execution, thus improving sensorimotor disorders in various conditions [[28], [29], [30],[41], [42], [43],45,[47], [48], [49],[75], [76], [77],[108], [109], [110], [111], [112], [113], [114], [115], [116], [117], [118]]. rPMS was used here for the first time in PD and the bilateral motor symptoms required to stimulate the legs, trunk and arms muscles bilaterally. This may have induced proprioceptive information meaningful for the sensorimotor control of the whole body, as supported by the self-perception of a better balance control (PSFS item) and the improvement of tremor and rigidity (UPDRS-III item).
rTMS was applied only over the FDI M1 area contralateral to the most affected side. Thus, an acceptable assumption is that, in our case study, the whole-body sensorimotor improvements was induced more by bilateral rPMS than rTMS of M1. rPMS may have increased the excitability of the thalamo-cortical connections (overinhibited by GPi and SNr) and its combination with rTMS of M1 influenced the cortico-striato-cortical DA loops, the basal ganglia plasticity and the endogenous DA release [80,81]. DA release could have been at the origin of the acute increase of facilitation (SICF, ICF), as already suggested in PD [97]. In accordance, an immediate increase of ICF and SICF was obtained in the two sessions with real rPMS of muscles, i.e. real-real and sham-real, and not with rTMS alone (real-sham) (see Fig. 3C–D). Also, an immediate decrease of SICI was obtained after rPMS alone session (sham-real, Fig. 3B), as in chronic stroke [75], and it is acknowledged that M1 disinhibition favors task-dependent plastic changes at the origin of functional improvement [24]. There was thus an indirect link, that future studies will have to elucidate, between the acute changes of M1 facilitation following rPMS of muscles and the large motor and functional improvement. Furthermore, it was suggested that in PD the functional segregation of M1 information to the striatum was lost and that rTMS of M1 alone induced the release of DA (of smaller quantity than in normal conditions) in an enlarged aspecific area [80]. The addition of muscle-specific activation of sensorimotor areas by rPMS could have improved the corticostriatal segregation, thus enhancing plausible DA release and the motor and functional after-effects.
The SICI variable was not influenced after real-real session like the ICF and SICF were. One explanation is that iTBS of M1 might not influence SICI circuits [97,119]. This is supported by the absence of SICI after real-sham too (rTMS alone, Fig. 3B). However, as previously explained for the long-term changes, the SICF increase after real-real (Fig. 3D) should have been associated with SICI reduction after real-real (Fig. 3B) [70,104]. Thus, another explanation could be related to the stimulation suit itself: the combination of real rTMS and real rPMS, two interventions that likely influenced M1 plasticity, could have cancelled the neuroplastic influence that rPMS could have had on SICI circuits [75]; this could be related to the mechanisms of immediate homeostatic metaplasticity when the increase of M1 excitability by iTBS acting on NMDA glutamatergic receptors could have reversed or cancelled the influence of rPMS on the same NMDA receptors [120,121]. Nevertheless, SICI longer term changes could have been related to bradykinesia (Fig. 4E) and future studies should test the long-term changes of TMS variables after sham-real session (rPMS alone). In this session, the concomitance of SICI decrease and SICF increase (Fig. 3B and D) suggests that our drug-free participant, still 10 years after PD's diagnosis, presented with SICF–SICI interactions similar to those reported in healthy subjects [70]. SICI acute decrease after sham-sham suit was not accompanied by SICF changes and was likely related to placebo-induced neurotransmitters release [86].
Our original and promising results must be replicated in larger samples of people with PD, drug-free or not, to test for example the effects between ON- and OFF-medication periods, gender and different stages of the disease.
4.5. Methodological considerations and limitations of the study
This study tends to support more effects of rPMS (bottom-up intervention) than rTMS (top-down) in a drug-free person with effects lasting 2 months and half at least. However, this should be discussed with caution given that only one session was administered per protocol in one person. Such a case study raised new hypotheses and its promising results, rather than be generalizable, warrant the conduction of prospective larger-sampled experimental studies with drug-free patients, tested in more than one session per stimulation suit and with sufficient washout periods between conditions to avoid cumulative changes. Results on the rPMS after-effects have been mitigated by the fact that it was not possible to test the rTMSsham + rPMSreal suit at one week later, but rather 80 days later, due to COVID-19 issues. The new version of UPDRS-III, the MDS-UPRDS-III (MDS for movement disorder revised) should be used in future studies to assess motor symptoms: the guidelines are more detailed for the experimenter and the patients, and the version appears to be more suitable for clinical research [122,123]. Testing a drug-free patient living for 10 years with PD was a rare opportunity to better understand M1 plasticity and motor and functional changes associated with the disease itself and not influenced by exogenous dopamine intake, chronic dopamine receptor stimulation or other complications related to PD pharmacological treatment. However, comparison with other neurostimulation PD studies remains challenging, even during OFF periods which could not completely free patients from years of chronic medication and its interplay with M1 plastic phenomena [83].
5. Conclusion
This study in a drug-free woman living with PD for 10 years showed that combining rPMS of muscles and rTMS of M1 significantly improved motor symptoms (UPDRS-III scores) and daily function (PSFS scores) and changed the disease stage from moderate to mild for at least 80 days. Concomitant changes of M1 plasticity shed a new light on the link between TMS measures and the changes of motor symptoms in PD. This informs on the potential for plastic and functional changes still 10 years after PD diagnosis and in the absence of antiparkinsonian drugs. De novo patients with PD (drug-free) should participate in future clinical trials on noninvasive neurostimulation aiming at slowing-down motor decline or delaying its progression whatever the stage of the disease.
Funding
This work was supported by the Canadian Foundation for Innovation (grant number 10071), the Chronic Pain Network (CPN) of the Canadian Institutes for Health Research – Strategy for Patient-Oriented Research (grant number 358108) and the Foundation of the CHU de Québec for clinical research (grant number 91067-CHU#3151). EG was supported by different PhD scholarships from foundations, MITACS, Université Laval and CPN.
Ethical approval Statement
This study was reviewed and approved by the research ethics boards of the CHU de Québec - Université Laval with the approval number 2022–6288, dated April 4, 2022, including an informed consent form for the patient's data publication.
Data Availability Statement
Our data will be available on request and, to date, have not been deposited into a publicly available site or platform.
CRediT authorship contribution statement
Estelle Gouriou: Writing – original draft, Visualization, Methodology, Investigation, Formal analysis. Cyril Schneider: Writing – review & editing, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are grateful to the participant who complied to all procedures of the study.
References
- 1.Ball N., Teo W.P., Chandra S., Chapman J. Parkinson's disease and the environment. Front. Neurol. 2019;10:218. doi: 10.3389/fneur.2019.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore R.Y., Bloom F.E. Central catecholamine neuron systems: anatomy and physiology of the dopamine systems. Annu. Rev. Neurosci. 1978;1:129–169. doi: 10.1146/annurev.ne.01.030178.001021. [DOI] [PubMed] [Google Scholar]
- 3.Calabresi P., Picconi B., Tozzi A., Ghiglieri V., Di Filippo M. Direct and indirect pathways of basal ganglia: a critical reappraisal. Nat. Neurosci. 2014;17:1022–1030. doi: 10.1038/nn.3743. [DOI] [PubMed] [Google Scholar]
- 4.McGregor M.M., Nelson A.B. Circuit mechanisms of Parkinson's disease. Neuron. 2019;101:1042–1056. doi: 10.1016/j.neuron.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Pandey S., Srivanitchapoom P. Levodopa-induced dyskinesia: clinical features, pathophysiology, and medical management. Ann. Indian Acad. Neurol. 2017;20:190–198. doi: 10.4103/aian.AIAN_239_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pirtosek Z., Bajenaru O., Kovacs N., Milanov I., Relja M., Skorvanek M. vol. 2020. Parkinsons Dis; 2020. (Update on the Management of Parkinson's Disease for General Neurologists). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marinelli L., Quartarone A., Hallett M., Frazzitta G., Ghilardi M.F. The many facets of motor learning and their relevance for Parkinson's disease. Clin. Neurophysiol. 2017;128:1127–1141. doi: 10.1016/j.clinph.2017.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Udupa K., Bhattacharya A., Bhardwaj S., Pal P.K., Chen R. Parkinson's disease: alterations of motor plasticity and motor learning. Handb. Clin. Neurol. 2022;184:135–151. doi: 10.1016/b978-0-12-819410-2.00007-2. [DOI] [PubMed] [Google Scholar]
- 9.LeWitt P.A. Levodopa therapy for Parkinson's disease: pharmacokinetics and pharmacodynamics. Mov. Disord. 2015;30:64–72. doi: 10.1002/mds.26082. [DOI] [PubMed] [Google Scholar]
- 10.Dong J., Cui Y., Li S., Le W. Current pharmaceutical treatments and alternative therapies of Parkinson's disease. Curr. Neuropharmacol. 2016;14:339–355. doi: 10.2174/1570159x14666151120123025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horn J., Mayer D.E., Chen S., Mayer E.A. Role of diet and its effects on the gut microbiome in the pathophysiology of mental disorders. Transl. Psychiatry. 2022;12:164. doi: 10.1038/s41398-022-01922-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agarwal P., Wang Y., Buchman A.S., Holland T.M., Bennett D.A., Morris M.C. MIND diet associated with reduced incidence and delayed progression of ParkinsonismA in old age. J. Nutr. Health Aging. 2018;22:1211–1215. doi: 10.1007/s12603-018-1094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armstrong M.J., Okun M.S. Diagnosis and treatment of Parkinson disease: a review. JAMA. 2020;323:548–560. doi: 10.1001/jama.2019.22360. [DOI] [PubMed] [Google Scholar]
- 14.Barker A.T. An introduction to the basic principles of magnetic nerve stimulation. J. Clin. Neurophysiol. 1991;8:26–37. doi: 10.1097/00004691-199101000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Beaulieu L.D., Flamand V.H., Masse-Alarie H., Schneider C. Reliability and minimal detectable change of transcranial magnetic stimulation outcomes in healthy adults: a systematic review. Brain Stimul. 2017;10:196–213. doi: 10.1016/j.brs.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Amassian V.E., Stewart M., Quirk G.J., Rosenthal J.L. Physiological basis of motor effects of a transient stimulus to cerebral cortex. Neurosurgery. 1987;20:74–93. [PubMed] [Google Scholar]
- 17.Barker A.T., Jalinous R., Freeston I.L. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 18.Chipchase L., Schabrun S., Cohen L., Hodges P., Ridding M., Rothwell J., Taylor J., Ziemann U. A checklist for assessing the methodological quality of studies using transcranial magnetic stimulation to study the motor system: an international consensus study. Clin. Neurophysiol. 2012;123:1698–1704. doi: 10.1016/j.clinph.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groppa S., Oliviero A., Eisen A., Quartarone A., Cohen L.G., Mall V., Kaelin-Lang A., Mima T., Rossi S., Thickbroom G.W., Rossini P.M., Ziemann U., Valls-Sole J., Siebner H.R. A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin. Neurophysiol. 2012;123:858–882. doi: 10.1016/j.clinph.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothwell J.C. Techniques and mechanisms of action of transcranial stimulation of the human motor cortex. J. Neurosci. Methods. 1997;74:113–122. doi: 10.1016/s0165-0270(97)02242-5. [DOI] [PubMed] [Google Scholar]
- 21.Capaday C. Neurophysiological methods for studies of the motor system in freely moving human subjects. J. Neurosci. Methods. 1997;74:201–218. doi: 10.1016/s0165-0270(97)02250-4. [DOI] [PubMed] [Google Scholar]
- 22.Di Lazzaro V., Restuccia D., Oliviero A., Profice P., Ferrara L., Insola A., Mazzone P., Tonali P., Rothwell J.C. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp. Brain Res. 1998;119:265–268. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- 23.Ziemann U., Reis J., Schwenkreis P., Rosanova M., Strafella A., Badawy R., Muller-Dahlhaus F. TMS and drugs revisited 2014. Clin. Neurophysiol. 2015;126:1847–1868. doi: 10.1016/j.clinph.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 24.Ziemann U., Muellbacher W., Hallett M., Cohen L.G. Modulation of practice-dependent plasticity in human motor cortex. Brain. 2001;124:1171–1181. doi: 10.1093/brain/124.6.1171. [DOI] [PubMed] [Google Scholar]
- 25.Guerra A., Asci F., Zampogna A., D'Onofrio V., Suppa A., Fabbrini G., Berardelli A. Long-term changes in short-interval intracortical facilitation modulate motor cortex plasticity and L-dopa-induced dyskinesia in Parkinson's disease. Brain Stimul. 2022;15:99–108. doi: 10.1016/j.brs.2021.11.016. [DOI] [PubMed] [Google Scholar]
- 26.Lefaucheur J.P., Aleman A., Baeken C., Benninger D.H., Brunelin J., Di Lazzaro V., Filipovic S.R., Grefkes C., Hasan A., Hummel F.C., Jaaskelainen S.K., Langguth B., Leocani L., Londero A., Nardone R., Nguyen J.P., Nyffeler T., Oliveira-Maia A.J., Oliviero A., Padberg F., Palm U., Paulus W., Poulet E., Quartarone A., Rachid F., Rektorova I., Rossi S., Sahlsten H., Schecklmann M., Szekely D., Ziemann U. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014-2018) Clin. Neurophysiol. 2020;131:474–528. doi: 10.1016/j.clinph.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Lefaucheur J.P., Aleman A., Baeken C., Benninger D.H., Brunelin J., Di Lazzaro V., Filipovic S.R., Grefkes C., Hasan A., Hummel F.C., Jaaskelainen S.K., Langguth B., Leocani L., Londero A., Nardone R., Nguyen J.P., Nyffeler T., Oliveira-Maia A.J., Oliviero A., Padberg F., Palm U., Paulus W., Poulet E., Quartarone A., Rachid F., Rektorova I., Rossi S., Sahlsten H., Schecklmann M., Szekely D., Ziemann U. Corrigendum to "Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014-2018)". Clin. Neurophysiol. 2020;131:1168–1169. doi: 10.1016/j.clinph.2020.02.003. 131 (2020) 474-528], Clin Neurophysiol. [DOI] [PubMed] [Google Scholar]
- 28.Beaulieu L.D., Schneider C. Effects of repetitive peripheral magnetic stimulation on normal or impaired motor control. A review. Neurophysiol. Clin. 2013;43:251–260. doi: 10.1016/j.neucli.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Schneider C., Zangrandi A., Sollmann N., Bonfert M.V., Beaulieu L.D., C.G. r P.M.S. Checklist on the quality of the repetitive peripheral magnetic stimulation (rPMS) methods in research: an international delphi study. Front. Neurol. 2022;13 doi: 10.3389/fneur.2022.852848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zschorlich V., Yamaguchi T., Schneider C. Editorial: the use of repetitive peripheral magnetic stimulation (rPMS) in neurological disorders and neurorehabilitation. Front. Neurol. 2023;14 doi: 10.3389/fneur.2023.1324882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ackerley S.J., Stinear C.M., Barber P.A., Byblow W.D. Combining theta burst stimulation with training after subcortical stroke. Stroke. 2010;41:1568–1572. doi: 10.1161/STROKEAHA.110.583278. [DOI] [PubMed] [Google Scholar]
- 32.Beaulieu L.D., Masse-Alarie H., Ribot-Ciscar E., Schneider C. Reliability of lower limb transcranial magnetic stimulation outcomes in the ipsi- and contralesional hemispheres of adults with chronic stroke. Clin. Neurophysiol. 2017;128:1290–1298. doi: 10.1016/j.clinph.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 33.Choo Y.J., Kwak S.G., Chang M.C. Effectiveness of repetitive transcranial magnetic stimulation on managing fibromyalgia: a systematic meta-analysis. Pain Med. 2022;23:1272–1282. doi: 10.1093/pm/pnab354. [DOI] [PubMed] [Google Scholar]
- 34.Comeau N., Monetta L., Schneider C. Noninvasive stimulation of the unlesioned hemisphere and phonological treatment in a case of chronic anomia post-stroke. Neurocase. 2022:1–12. doi: 10.1080/13554794.2022.2068374. [DOI] [PubMed] [Google Scholar]
- 35.Conchou F., Loubinoux I., Castel-Lacanal E., Le Tinnier A., Gerdelat-Mas A., Faure-Marie N., Gros H., Thalamas C., Calvas F., Berry I., Chollet F., Simonetta Moreau M. Neural substrates of low-frequency repetitive transcranial magnetic stimulation during movement in healthy subjects and acute stroke patients. A PET study. Hum. Brain Mapp. 2009;30:2542–2557. doi: 10.1002/hbm.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D'Amico J.M., Donges S.C., Taylor J.L. High-intensity, low-frequency repetitive transcranial magnetic stimulation enhances excitability of the human corticospinal pathway. J. Neurophysiol. 2020;123:1969–1978. doi: 10.1152/jn.00607.2019. [DOI] [PubMed] [Google Scholar]
- 37.Elder G.J., Taylor J.P. Transcranial magnetic stimulation and transcranial direct current stimulation: treatments for cognitive and neuropsychiatric symptoms in the neurodegenerative dementias? Alzheimer's Res. Ther. 2014;6:74. doi: 10.1186/s13195-014-0074-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tremblay C., Monetta L., Langlois M., Schneider C. Intermittent theta-burst stimulation of the right dorsolateral prefrontal cortex to promote metaphor comprehension in Parkinson disease: a case study. Arch. Phys. Med. Rehabil. 2016;97:74–83. doi: 10.1016/j.apmr.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Yokoe M., Mano T., Maruo T., Hosomi K., Shimokawa T., Kishima H., Oshino S., Morris S., Kageyama Y., Goto Y., Shimizu T., Mochizuki H., Yoshimine T., Saitoh Y. The optimal stimulation site for high-frequency repetitive transcranial magnetic stimulation in Parkinson's disease: a double-blind crossover pilot study. J. Clin. Neurosci. 2018;47:72–78. doi: 10.1016/j.jocn.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 40.Yang Z., Yue T., Zschorlich V.R., Li D., Wang D., Qi F. Behavioral effects of repetitive transcranial magnetic stimulation in disorders of consciousness: a systematic review and meta-analysis. Brain Sci. 2023;13 doi: 10.3390/brainsci13101362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gallasch E., Christova M., Kunz A., Rafolt D., Golaszewski S. Modulation of sensorimotor cortex by repetitive peripheral magnetic stimulation. Front. Hum. Neurosci. 2015;9:407. doi: 10.3389/fnhum.2015.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Struppler A., Binkofski F., Angerer B., Bernhardt M., Spiegel S., Drzezga A., Bartenstein P. A fronto-parietal network is mediating improvement of motor function related to repetitive peripheral magnetic stimulation: a PET-H2O15 study. Neuroimage. 2007;36(Suppl 2):T174–T186. doi: 10.1016/j.neuroimage.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 43.Beaulieu L.D., Schneider C. Repetitive peripheral magnetic stimulation to reduce pain or improve sensorimotor impairments: a literature review on parameters of application and afferents recruitment. Neurophysiol. Clin. 2015;45:223–237. doi: 10.1016/j.neucli.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Borner C., Urban G., Beaulieu L.D., Sollmann N., Krieg S.M., Straube A., Renner T., Schandelmaier P., Lang M., Lechner M., Vill K., Gerstl L., Heinen F., Landgraf M.N., Bonfert M.V. The bottom-up approach: non-invasive peripheral neurostimulation methods to treat migraine: a scoping review from the child neurologist's perspective. Eur. J. Paediatr. Neurol. 2021;32:16–28. doi: 10.1016/j.ejpn.2021.02.008. [DOI] [PubMed] [Google Scholar]
- 45.Kunesch E., Knecht S., Classen J., Roick H., Tyercha C., Benecke R. Somatosensory evoked potentials (SEPs) elicited by magnetic nerve stimulation. Electroencephalogr. Clin. Neurophysiol. 1993;88:459–467. doi: 10.1016/0168-5597(93)90035-n. [DOI] [PubMed] [Google Scholar]
- 46.Sollmann N., Trepte-Freisleder F., Albers L., Jung N.H., Mall V., Meyer B., Heinen F., Krieg S.M., Landgraf M.N. Magnetic stimulation of the upper trapezius muscles in patients with migraine - a pilot study. Eur. J. Paediatr. Neurol. 2016;20:888–897. doi: 10.1016/j.ejpn.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 47.Zhu Y., Starr A. Magnetic stimulation of muscle evokes cerebral potentials. Muscle Nerve. 1991;14:721–732. doi: 10.1002/mus.880140806. [DOI] [PubMed] [Google Scholar]
- 48.Allen Demers F., Zangrandi A., Schneider C. Theta burst stimulation over forearm muscles in complex regional pain syndrome (CRPS): influence on brain and clinical outcomes. Frontiers in Pain Research - Pain Mechanisms. 2021 doi: 10.3389/fpain.2021.736806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Provencher J., Beaulieu-Guay E.M., Loranger S.D., Schneider C. Repetitive peripheral magnetic stimulation to improve ankle function and gait in cerebral palsy at adulthood: an open-label case study. Brain Res. 2022;1792 doi: 10.1016/j.brainres.2022.147999. [DOI] [PubMed] [Google Scholar]
- 50.Bhattacharjee S., Kashyap R., Abualait T., Annabel Chen S.H., Yoo W.K., Bashir S. The role of primary motor cortex: more than movement execution. J. Mot. Behav. 2021;53:258–274. doi: 10.1080/00222895.2020.1738992. [DOI] [PubMed] [Google Scholar]
- 51.Hosp J.A., Luft A.R. Dopaminergic meso-cortical projections to m1: role in motor learning and motor cortex plasticity. Front. Neurol. 2013;4:145. doi: 10.3389/fneur.2013.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramaker C., Marinus J., Stiggelbout A.M., Van Hilten B.J. Systematic evaluation of rating scales for impairment and disability in Parkinson's disease. Movement disorders: official journal of the Movement Disorder Society. 2002;17:867–876. doi: 10.1002/mds.10248. [DOI] [PubMed] [Google Scholar]
- 53.Fahn S. Unified Parkinson's disease rating scale. Recent developments in Parkinson's disease. 1987:153–163. [Google Scholar]
- 54.D. Movement Disorder Society Task Force on Rating Scales for Parkinson's The unified Parkinson's disease rating scale (UPDRS): status and recommendations. Mov. Disord. 2003;18:738–750. doi: 10.1002/mds.10473. [DOI] [PubMed] [Google Scholar]
- 55.Pathak A., Wilson R., Sharma S., Pryymachenko Y., Ribeiro D.C., Chua J., Abbott J.H. Measurement properties of the patient-specific functional scale and its current uses: an updated systematic review of 57 studies using COSMIN guidelines. J. Orthop. Sports Phys. Ther. 2022;52:262–275. doi: 10.2519/jospt.2022.10727. [DOI] [PubMed] [Google Scholar]
- 56.Stratford P.W., Binkley J. A review of the McMurray test: definition, interpretation, and clinical usefulness. J. Orthop. Sports Phys. Ther. 1995;22:116–120. doi: 10.2519/jospt.1995.22.3.116. [DOI] [PubMed] [Google Scholar]
- 57.Abbott J.H., Schmitt J. Minimum important differences for the patient-specific functional scale, 4 region-specific outcome measures, and the numeric pain rating scale. J. Orthop. Sports Phys. Ther. 2014;44:560–564. doi: 10.2519/jospt.2014.5248. [DOI] [PubMed] [Google Scholar]
- 58.Bohannon R.W., Nair P., Green M. Feasibility and informativeness of the patient-specific functional scale with patients with Parkinson's disease. Physiother. Theory Pract. 2020;36:1241–1244. doi: 10.1080/09593985.2019.1571134. [DOI] [PubMed] [Google Scholar]
- 59.Hoehn M.M., Yahr M.D. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 60.Rossi S., Antal A., Bestmann S., Bikson M., Brewer C., Brockmoller J., Carpenter L.L., Cincotta M., Chen R., Daskalakis J.D., Di Lazzaro V., Fox M.D., George M.S., Gilbert D., Kimiskidis V.K., Koch G., Ilmoniemi R.J., Pascal Lefaucheur J., Leocani L., Lisanby S.H., Miniussi C., Padberg F., Pascual-Leone A., Paulus W., Peterchev A.V., Quartarone A., Rotenberg A., Rothwell J., Rossini P.M., Santarnecchi E., Shafi M.M., Siebner H.R., Ugawa Y., Wassermann E.M., Zangen A., Ziemann U., Hallett M., F.o.T.M.S.S.E.G.S.O.u.t.A Basis of this article began with a Consensus Statement from the Ifcn Workshop on "Present, Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: expert Guidelines. Clin. Neurophysiol. 2021;132:269–306. doi: 10.1016/j.clinph.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rossi S., Hallett M., Rossini P.M., Pascual-Leone A., Safety of, Safety T.M.S.C.G. Ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shulman L.M., Gruber-Baldini A.L., Anderson K.E., Fishman P.S., Reich S.G., Weiner W.J. The clinically important difference on the unified Parkinson's disease rating scale. Arch. Neurol. 2010;67:64–70. doi: 10.1001/archneurol.2009.295. [DOI] [PubMed] [Google Scholar]
- 63.Stratford P., Gill C., Westaway M., Binkley J. Assessing disability and change on individual patients: a report of a patient specific measure. Physiotherapy canada. 1995;47:258–263. doi: 10.3138/ptc.47.4.258. [DOI] [Google Scholar]
- 64.Heldmann P., Hummel S., Bauknecht L., Bauer J.M., Werner C. Construct validity, test-retest reliability, sensitivity to change, and feasibility of the patient-specific functional scale in acutely hospitalized older patients with and without cognitive impairment. J. Geriatr. Phys. Ther. 2021 doi: 10.1519/jpt.0000000000000303. [DOI] [PubMed] [Google Scholar]
- 65.Rossini P.M., Burke D., Chen R., Cohen L.G., Daskalakis Z., Di Iorio R., Di Lazzaro V., Ferreri F., Fitzgerald P.B., George M.S., Hallett M., Lefaucheur J.P., Langguth B., Matsumoto H., Miniussi C., Nitsche M.A., Pascual-Leone A., Paulus W., Rossi S., Rothwell J.C., Siebner H.R., Ugawa Y., Walsh V., Ziemann U. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015;126:1071–1107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kobayashi M., Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2:145–156. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- 67.Kujirai T., Caramia M.D., Rothwell J.C., Day B.L., Thompson P.D., Ferbert A., Wroe S., Asselman P., Marsden C.D. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wiegel P., Niemann N., Rothwell J.C., Leukel C. Evidence for a subcortical contribution to intracortical facilitation. Eur. J. Neurosci. 2018;47:1311–1319. doi: 10.1111/ejn.13934. [DOI] [PubMed] [Google Scholar]
- 69.Reis J., Swayne O.B., Vandermeeren Y., Camus M., Dimyan M.A., Harris-Love M., Perez M.A., Ragert P., Rothwell J.C., Cohen L.G. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J Physiol. 2008;586:325–351. doi: 10.1113/jphysiol.2007.144824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peurala S.H., Muller-Dahlhaus J.F., Arai N., Ziemann U. Interference of short-interval intracortical inhibition (SICI) and short-interval intracortical facilitation (SICF) Clin. Neurophysiol. 2008;119:2291–2297. doi: 10.1016/j.clinph.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 71.Di Lazzaro V., Rothwell J., Capogna M. Noninvasive stimulation of the human brain: activation of multiple cortical circuits. Neuroscientist. 2018;24:246–260. doi: 10.1177/1073858417717660. [DOI] [PubMed] [Google Scholar]
- 72.Huang Y.Z., Edwards M.J., Rounis E., Bhatia K.P., Rothwell J.C. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 73.Huang Y.Z., Rothwell J.C. The effect of short-duration bursts of high-frequency, low-intensity transcranial magnetic stimulation on the human motor cortex. Clin. Neurophysiol. 2004;115:1069–1075. doi: 10.1016/j.clinph.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 74.Huang Y.Z., Rothwell J.C., Edwards M.J., Chen R.S. Effect of physiological activity on an NMDA-dependent form of cortical plasticity in human. Cereb Cortex. 2008;18:563–570. doi: 10.1093/cercor/bhm087. [DOI] [PubMed] [Google Scholar]
- 75.Beaulieu L.D., Masse-Alarie H., Camire-Bernier S., Ribot-Ciscar E., Schneider C. After-effects of peripheral neurostimulation on brain plasticity and ankle function in chronic stroke: the role of afferents recruited. Neurophysiol. Clin. 2017;47:275–291. doi: 10.1016/j.neucli.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 76.Struppler A., Angerer B., Havel P. Modulation of sensorimotor performances and cognition abilities induced by RPMS: clinical and experimental investigations. Suppl. Clin. neurophysiol. 2003;56:358–367. doi: 10.1016/s1567-424x(09)70239-9. [DOI] [PubMed] [Google Scholar]
- 77.Flamand V.H., Schneider C. Noninvasive and painless magnetic stimulation of nerves improved brain motor function and mobility in a cerebral palsy case. Arch. Phys. Med. Rehabil. 2014;95:1984–1990. doi: 10.1016/j.apmr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 78.Masse-Alarie H., Beaulieu L.D., Preuss R., Schneider C. Repetitive peripheral magnetic neurostimulation of multifidus muscles combined with motor training influences spine motor control and chronic low back pain. Clin. Neurophysiol. 2017;128:442–453. doi: 10.1016/j.clinph.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 79.Masse-Alarie H., Flamand V.H., Moffet H., Schneider C. Peripheral neurostimulation and specific motor training of deep abdominal muscles improve posturomotor control in chronic low back pain. Clin. J. Pain. 2013;29:814–823. doi: 10.1097/AJP.0b013e318276a058. [DOI] [PubMed] [Google Scholar]
- 80.Strafella A.P., Ko J.H., Grant J., Fraraccio M., Monchi O. Corticostriatal functional interactions in Parkinson's disease: a rTMS/[11C]raclopride PET study. Eur. J. Neurosci. 2005;22:2946–2952. doi: 10.1111/j.1460-9568.2005.04476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Strafella A.P., Paus T., Barrett J., Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J. Neurosci. 2001;21 doi: 10.1523/JNEUROSCI.21-15-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Motulsky H.J., Brown R.E. Detecting outliers when fitting data with nonlinear regression - a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinf. 2006;7:123. doi: 10.1186/1471-2105-7-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kishore A., Popa T., Velayudhan B., Joseph T., Balachandran A., Meunier S. Acute dopamine boost has a negative effect on plasticity of the primary motor cortex in advanced Parkinson's disease. Brain. 2012;135:2074–2088. doi: 10.1093/brain/aws124. [DOI] [PubMed] [Google Scholar]
- 84.Post B., Merkus M.P., de Bie R.M., de Haan R.J., Speelman J.D. Unified Parkinson's disease rating scale motor examination: are ratings of nurses, residents in neurology, and movement disorders specialists interchangeable? Mov. Disord. 2005;20:1577–1584. doi: 10.1002/mds.20640. [DOI] [PubMed] [Google Scholar]
- 85.Lou J.S. Placebo responses in Parkinson's disease. Int. Rev. Neurobiol. 2020;153:187–211. doi: 10.1016/bs.irn.2020.03.031. [DOI] [PubMed] [Google Scholar]
- 86.Strafella A.P., Ko J.H., Monchi O. Therapeutic application of transcranial magnetic stimulation in Parkinson's disease: the contribution of expectation. Neuroimage. 2006;31:1666–1672. doi: 10.1016/j.neuroimage.2006.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lidstone S.C., Schulzer M., Dinelle K., Mak E., Sossi V., Ruth T.J., de la Fuente-Fernández R., Phillips A.G., Stoessl A.J. Effects of expectation on placebo-induced dopamine release in Parkinson disease. Arch Gen Psychiatry. 2010;67:857–865. doi: 10.1001/archgenpsychiatry.2010.88. [DOI] [PubMed] [Google Scholar]
- 88.de la Fuente-Fernández R., Stoessl A.J. The placebo effect in Parkinson's disease. Trends Neurosci. 2002;25:302–306. doi: 10.1016/s0166-2236(02)02181-1. [DOI] [PubMed] [Google Scholar]
- 89.Degardin A., Devos D., Defebvre L., Destee A., Plomhause L., Derambure P., Devanne H. Effect of intermittent theta-burst stimulation on akinesia and sensorimotor integration in patients with Parkinson's disease. Eur. J. Neurosci. 2012;36:2669–2678. doi: 10.1111/j.1460-9568.2012.08158.x. [DOI] [PubMed] [Google Scholar]
- 90.Eggers C., Gunther M., Rothwell J., Timmermann L., Ruge D. Theta burst stimulation over the supplementary motor area in Parkinson's disease. J. Neurol. 2015;262:357–364. doi: 10.1007/s00415-014-7572-8. [DOI] [PubMed] [Google Scholar]
- 91.Brugger F., Wegener R., Walch J., Galovic M., Hagele-Link S., Bohlhalter S., Kagi G. Altered activation and connectivity of the supplementary motor cortex at motor initiation in Parkinson's disease patients with freezing. Clin. Neurophysiol. 2020;131:2171–2180. doi: 10.1016/j.clinph.2020.05.023. [DOI] [PubMed] [Google Scholar]
- 92.Janssen A.M., Munneke M.A.M., Nonnekes J., van der Kraan T., Nieuwboer A., Toni I., Snijders A.H., Bloem B.R., Stegeman D.F. Cerebellar theta burst stimulation does not improve freezing of gait in patients with Parkinson's disease. J. Neurol. 2017;264:963–972. doi: 10.1007/s00415-017-8479-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tard C., Devanne H., Defebvre L., Delval A. Single session intermittent theta-burst stimulation on the left premotor cortex does not alleviate freezing of gait in Parkinson's disease. Neurosci. Lett. 2016;628:1–9. doi: 10.1016/j.neulet.2016.05.061. [DOI] [PubMed] [Google Scholar]
- 94.Vanbellingen T., Wapp M., Stegmayer K., Bertschi M., Abela E., Kubel S., Nyffeler T., Muri R., Walther S., Nef T., Hallett M., Bohlhalter S. Theta burst stimulation over premotor cortex in Parkinson's disease: an explorative study on manual dexterity. J. Neural. Transm. 2016;123:1387–1393. doi: 10.1007/s00702-016-1614-6. [DOI] [PubMed] [Google Scholar]
- 95.Benninger D.H., Berman B.D., Houdayer E., Pal N., Luckenbaugh D.A., Schneider L., Miranda S., Hallett M. Intermittent theta-burst transcranial magnetic stimulation for treatment of Parkinson disease. Neurology. 2011;76:601–609. doi: 10.1212/WNL.0b013e31820ce6bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abraham W.C. Metaplasticity: tuning synapses and networks for plasticity. Nat. Rev. Neurosci. 2008;9:387. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- 97.Zamir O., Gunraj C., Ni Z., Mazzella F., Chen R. Effects of theta burst stimulation on motor cortex excitability in Parkinson's disease. Clin. Neurophysiol. 2012;123:815–821. doi: 10.1016/j.clinph.2011.07.051. [DOI] [PubMed] [Google Scholar]
- 98.Brugger F., Wegener R., Baty F., Walch J., Krüger M.T., Hägele-Link S., Bohlhalter S., Kägi G. Facilitatory rTMS over the supplementary motor cortex impedes gait performance in Parkinson patients with freezing of gait. Brain Sci. 2021;11 doi: 10.3390/brainsci11030321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bologna M., Di Biasio F., Conte A., Iezzi E., Modugno N., Berardelli A. Effects of cerebellar continuous theta burst stimulation on resting tremor in Parkinson's disease. Parkinsonism Relat Disord. 2015;21:1061–1066. doi: 10.1016/j.parkreldis.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 100.Martínez-Martín P., Rodríguez-Blázquez C., Mario A., Arakaki T., Arillo V.C., Chaná P., Fernández W., Garretto N., Martínez-Castrillo J.C., Rodríguez-Violante M., Serrano-Dueñas M., Ballesteros D., Rojo-Abuin J.M., Chaudhuri K.R., Merello M. Parkinson's disease severity levels and MDS-Unified Parkinson's Disease Rating Scale. Parkinsonism Relat Disord. 2015;21:50–54. doi: 10.1016/j.parkreldis.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 101.Mustile M., Kourtis D., Edwards M.G., Ladouce S., Volpe D., Pilleri M., Pelosin E., Learmonth G., Donaldson D.I., Ietswaart M. Characterizing neurocognitive impairments in Parkinson's disease with mobile EEG when walking and stepping over obstacles. Brain Commun. 2023;5:fcad326. doi: 10.1093/braincomms/fcad326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Capaday C. On the variability of motor-evoked potentials: experimental results and mathematical model. Exp. Brain Res. 2021;239:2979–2995. doi: 10.1007/s00221-021-06169-7. [DOI] [PubMed] [Google Scholar]
- 103.Suppa A., Marsili L., Belvisi D., Conte A., Iezzi E., Modugno N., Fabbrini G., Berardelli A. Lack of LTP-like plasticity in primary motor cortex in Parkinson's disease. Exp. Neurol. 2011;227:296–301. doi: 10.1016/j.expneurol.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 104.Vucic S., Stanley Chen K.H., Kiernan M.C., Hallett M., Benninger D.H., Di Lazzaro V., Rossini P.M., Benussi A., Berardelli A., Curra A., Krieg S.M., Lefaucheur J.P., Long Lo Y., Macdonell R.A., Massimini M., Rosanova M., Picht T., Stinear C.M., Paulus W., Ugawa Y., Ziemann U., Chen R. Clinical diagnostic utility of transcranial magnetic stimulation in neurological disorders. Updated report of an IFCN committee. Clin. Neurophysiol. 2023;150:131–175. doi: 10.1016/j.clinph.2023.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Filipović S.R., Kačar A., Milanović S., Ljubisavljević M.R. Neurophysiological predictors of response to medication in Parkinson's disease. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.763911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huang Y.Z., Chen R.S., Rothwell J.C., Wen H.Y. The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin. Neurophysiol. 2007;118:1028–1032. doi: 10.1016/j.clinph.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 107.Huang Y.Z., Rothwell J.C., Lu C.S., Chuang W.L., Lin W.Y., Chen R.S. Reversal of plasticity-like effects in the human motor cortex. J Physiol. 2010;588:3683–3693. doi: 10.1113/jphysiol.2010.191361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Beaulieu L.D., Masse-Alarie H., Brouwer B., Schneider C. Noninvasive neurostimulation in chronic stroke: a double-blind randomized sham-controlled testing of clinical and corticomotor effects. Top. Stroke Rehabil. 2015;22:8–17. doi: 10.1179/1074935714Z.0000000032. [DOI] [PubMed] [Google Scholar]
- 109.Flamand V.H., Beaulieu L.D., Nadeau L., Schneider C. Peripheral magnetic stimulation to decrease spasticity in cerebral palsy. Pediatr. Neurol. 2012;47:345–348. doi: 10.1016/j.pediatrneurol.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 110.Krause P., Foerderreuther S., Straube A. Effects of conditioning peripheral repetitive magnetic stimulation in patients with complex regional pain syndrome. Neurol. Res. 2005;27:412–417. doi: 10.1179/016164105X17224. [DOI] [PubMed] [Google Scholar]
- 111.Krause P., Straube A. Reduction of spastic tone increase induced by peripheral repetitive magnetic stimulation is frequency-independent. NeuroRehabilitation. 2005;20:63–65. [PubMed] [Google Scholar]
- 112.Krewer C., Hartl S., Muller F., Koenig E. Effects of repetitive peripheral magnetic stimulation on upper-limb spasticity and impairment in patients with spastic hemiparesis: a randomized, double-blind, sham-controlled study. Arch. Phys. Med. Rehabil. 2014;95:1039–1047. doi: 10.1016/j.apmr.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 113.Masse-Alarie H., Schneider C. [Cerebral reorganization in chronic low back pain and neurostimulation to improve motor control] Neurophysiol. Clin. 2011;41:51–60. doi: 10.1016/j.neucli.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 114.Pan J.X., Diao Y.X., Peng H.Y., Wang X.Z., Liao L.R., Wang M.Y., Wen Y.L., Jia Y.B., Liu H. Effects of repetitive peripheral magnetic stimulation on spasticity evaluated with modified Ashworth scale/Ashworth scale in patients with spastic paralysis: a systematic review and meta-analysis. Front. Neurol. 2022;13 doi: 10.3389/fneur.2022.997913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Struppler A., Havel P., Muller-Barna P. Facilitation of skilled finger movements by repetitive peripheral magnetic stimulation (RPMS) - a new approach in central paresis. NeuroRehabilitation. 2003;18:69–82. [PubMed] [Google Scholar]
- 116.Tezer T., Yildiz N., Sarsan A., Alkan H. Short-term effect of magnetic stimulation added to bladder training in women with idiopathic overactive bladder: a prospective randomized controlled trial. Neurourol. Urodyn. 2022;41:1380–1389. doi: 10.1002/nau.24957. [DOI] [PubMed] [Google Scholar]
- 117.Wang F., Mi S.B., Guo H.P. Exploring the effect of bladder functional training combined with repetitive sacral root magnetic stimulation in the treatment of neurogenic bladder. World Neurosurg. 2023 doi: 10.1016/j.wneu.2023.06.072. [DOI] [PubMed] [Google Scholar]
- 118.Zschorlich V.R., Hillebrecht M., Tanjour T., Qi F., Behrendt F., Kirschstein T., Kohling R. Repetitive peripheral magnetic nerve stimulation (rPMS) as adjuvant therapy reduces skeletal muscle reflex activity. Front. Neurol. 2019;10:930. doi: 10.3389/fneur.2019.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Suppa A., Huang Y.Z., Funke K., Ridding M.C., Cheeran B., Di Lazzaro V., Ziemann U., Rothwell J.C. Ten years of theta burst stimulation in humans: established knowledge, unknowns and prospects. Brain Stimul. 2016;9:323–335. doi: 10.1016/j.brs.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 120.Gamboa O.L., Antal A., Laczo B., Moliadze V., Nitsche M.A., Paulus W. Impact of repetitive theta burst stimulation on motor cortex excitability. Brain Stimul. 2011;4:145–151. doi: 10.1016/j.brs.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 121.Schabrun S.M., Chipchase L.S., Zipf N., Thickbroom G.W., Hodges P.W. Interaction between simultaneously applied neuromodulatory interventions in humans. Brain Stimul. 2013;6:624–630. doi: 10.1016/j.brs.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 122.Goetz C.G., Nutt J.G., Stebbins G.T. The unified dyskinesia rating scale: presentation and clinimetric profile. Mov. Disord. 2008;23:2398–2403. doi: 10.1002/mds.22341. [DOI] [PubMed] [Google Scholar]
- 123.Goetz C.G., Tilley B.C., Shaftman S.R., Stebbins G.T., Fahn S., Martinez-Martin P., Poewe W., Sampaio C., Stern M.B., Dodel R., Dubois B., Holloway R., Jankovic J., Kulisevsky J., Lang A.E., Lees A., Leurgans S., LeWitt P.A., Nyenhuis D., Olanow C.W., Rascol O., Schrag A., Teresi J.A., van Hilten J.J., LaPelle N. Movement disorder society-sponsored revision of the unified Parkinson's disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Our data will be available on request and, to date, have not been deposited into a publicly available site or platform.