Abstract
The solar pyrolysis of materials has emerged as a promising technology for their efficient conversion into solid char, syngas and oil. The technology has its challenges, however, as constraints such as solar intermittence and scalability must be overcame for solar pyrolysis to thrive. The present work presents a review of the developments in solar pyrolysis considering a such as development by country, solar technology employed, etcetera. Moreover, details on the challenges and potential future developments are presented. It was found that most of the development in solar pyrolysis has been focused on waste-handling, and that a particular challenge exists in an adequate control system to achieve the desired end products.
Keywords: Solar pyrolysis, Solar biofuels, Pyrolysis, Concentrated solar energy, Solar reactor, Biochar, Bio-oil, Syngas, Biomass
Graphical abstract
Highlights
-
•
Solar pyrolysis has been used mostly for waste handling.
-
•
Solar pyrolysis-produced biochar can be useful for carbon capture.
-
•
Challenges like solar intermittence and optical efficiency must be tackled.
-
•
Some countries have potential for solar pyrolysis and must invest in R + D.
1. Introduction
The climate change has resulted in raised environmental concerns and awareness worldwide. In order to tackle climate change, several lines of action are being followed, for example the adoption of a circular economic model, the establishment of a carbon economy, and the transition to renewable and clean sources of energy such as wind, geothermal, solar and biomass energy [[1], [2], [3]]. Besides reducing the dependence in the limited availability of fossil fuels, a goal of renewable energy is to decrease the greenhouse gas emissions; in this regard, biomass has received attention since it can be used to produce solid, liquid or gaseous fuels for combustion engines without a significant production of greenhouse gases or contribution to the greenhouse effect [4]. Evidence exists that indicates that under adequate process designs, biomass energy can be carbon neutral [5,6]. Biomass, which is organic material composed of mostly carbon, hydrogen and oxygen, contains chemical energy that can be directly released by combustion [3]. Alternatively, biomass can receive different biological or thermochemical treatments and become energy-dense liquids, solids or gases; an overview of the different processes can be found in Fig. 1.
Fig. 1.
Overview of the different biomass treatments and their products.
Biological treatments include digestion and fermentation, while thermochemical treatments include torrefaction, gasification and pyrolysis (and the corresponding advanced processes, such as catalytic pyrolysis, or supercritical gasification) [3]. Although biological treatments tend to be less expensive than thermochemical treatments, these can be constrained by exceedingly long reaction times even in small reactor scales [7]. On the other hand, thermochemical technologies have a much shorter processing time and can be commercially attractive, even if the technologies have their respective challenges and limitations (such as the production of tars in the case of gasification) [2]. Gasification consists of heating a feedstock under a controlled oxidant atmosphere for the production of a combination of H2, CO, CO2 and other gases called syngas, a solid called biochar and a mixture of condensable hydrocarbons deemed tar; typical gasification agents are air, oxygen and steam [8]. Contrary to gasification, pyrolysis and torrefaction occur in an inert atmosphere, hence the products stem from the decomposition of the feedstock with no primary gas-phase reactions occurring. Pyrolysis leads to the formation of a liquid product (often called bio-oil), a combination of permanent gases (H2, CO, CO2, CH4 among others) and biochar [3]. Finally, torrefaction is a process very similar to pyrolysis, where the main differences lie in the pressure and heating rates, where the pressure is usually much higher than in pyrolysis [3,5]. Fig. 2 portrays the reaction steps depending on the thermochemical process. In all the thermochemical technologies the product composition depends on a number of factors such as the reaction temperature, reaction time, feedstock composition, and reactor geometry [[9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20]]. From the diverse product composition and process operation variables it can be inferred that thermochemical processes, although promising, are complex in nature and present a challenge to achieve the desired end-products [[9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20]]. Some researchers have dedicated their efforts to the optimization of the operation conditions [3] or the development and implementation of different advanced technologies such as plasma, microwave and solar pyrolysis and gasification [20,21].
Fig. 2.
Reaction steps during thermochemical processing of biomass.
On the other hand, solar energy, which is the electromagnetic radiation from the sun that strikes the Earth, has enormous potential for employment in energy systems. It has been shown that the solar energy that strikes the Earth in a single day is more than that consumed by the whole population in an entire year [2]. Alas, not all the solar energy can be harnessed and turned into useful work or electricity; plenty of room for research, development and improvement exists in the field of solar energy since the efficiency of the system is constrained by the available technology and the laws of thermodynamics [22]. Different technologies have been developed to improve the process efficiency and can be categorized in three main groups: photochemical, photovoltaic and photothermal (or thermal) technologies. Photochemical technologies make use of sunlight as a source of energy for chemical reactions (such as degradation and synthesis reactions) [22,23]. Photovoltaic technologies (such as solar panels) are based on the photovoltaic effect to convert solar irradiation into electricity [24]. Finally, thermal technologies convert solar radiation into heat which can be employed in a particular process such as electricity generation [25].
Solar energy is promising and can potentially be the primary source of power in the decades to come, nonetheless, it is not a “condensed” form of energy and cannot be used directly in devices such as combustion engines. Fortunately, since solar thermal technologies can heat samples at different rates and to temperatures over 1000 °C, solar energy can be coupled with thermochemical biomass conversion technologies for the production of chemicals of interest. With adequate development it has been projected that together, solar and biomass energy should be able to satisfy society's energy requirements by 2050 [25,26]. To this end, solar pyrolysis has been studied worldwide and has resulted in different scale experimental facilities [12,[27], [28], [29], [30], [31], [32], [33]]. With the aim of helping the development of further studies, several reviews on the combination of solar thermal technologies and biomass energy have been published. Zhang et al. [34] conducted a literature review on the use of thermochemical technologies (including novel methodologies such as microwave-assisted and solar-assisted technologies) for the production of aviation fuels from waste biomass, with special emphasis in the product properties as a function of the solar pyrolysis conditions. Hamilton et al. [35] developed a review describing the implementation of solar-assisted pyrolysis based on the needs and resources available in Victoria, Australia, outlining the advantages of incorporating concentrated solar technology into fuel production. Zeng et al. [36] reviewed published works related to the conversion of solar energy into fuels by integration of pyrolysis, with special emphasis on the parameters that have an impact on the solar pyrolysis products (for example, temperature and employed solar technology). Abanades et al. [37] developed and published a manuscript describing the theoretical background on solar gasification and pyrolysis, together with a collection of recent works. Hamed et al. [38] conducted a literature review to assess the feasibility of using solar pyrolysis and the different available technologies to produce compounds of interest from oil palm biomass, focusing on the Malaysian climate. Parthasarathy [39] et al. published a review where the potential of different animal and municipal waste for biochar generation using conventional and solar pyrolysis is analyzed; from the review, the authors determined that solar pyrolysis is a promising option to produce biochars for different uses, where the potential use is mainly driven by the feedstock type. Mondal et al. [40] conducted a review with the goal of assessing the use of solar energy for biofuel production; in the work, the authors reported that solar-thermal technologies are less costly than photovoltaic technologies for biofuel production, and with further technological advance, the gap in costs will widen.
There are literature reviews that analyze published works related to solar pyrolysis focusing on different aspects. However, none of the available works discuss the amount projects and the relationship between that number and the solar potential per region.
The structure of the work is as follows: firstly, the pyrolysis process is explained, where details on conventional and solar pyrolysis are given and the parameters of importance during the process are explained. Afterwards, an insight on the reaction mechanism is given, where details regarding the decomposition of biomass as a whole, as well as the constituent components (lignin, cellulose and hemicellulose) and subsequent products and their distribution, are conferred. The next section consists of a review of advances on solar pyrolysis, where a bibliometric analysis is also provided. Additionally, the advances and projects related to solar pyrolysis around the world are discussed, and potential details that could be useful to improve the development of the technology are given. Further details on solar pyrolysis and potential classifications are presented at the end of the section. Subsequently, challenges found from the literature review are conferred in the second to last section, and the manuscript closes with a series of concluding remarks.
2. Description of the technologies
2.1. Pyrolysis
Pyrolysis is the thermal decomposition of a feedstock in an inert atmosphere which results in the production of gases, solids, and liquids. The formal study of pyrolysis has increased in recent decades, with interest arising from the need of solid and liquid fuels, as well as the potential “chemical recycling” of waste [7,20]. Studies have focused on understanding the feedstock parameters (for example biomass composition, feedstock particle size, presence of moisture, co-feeding) and reaction parameters (temperature, heating rate and reaction time) in the process. The reaction parameters have resulted in a classification of pyrolysis under different categories: based on the operation temperature and the heating rate, pyrolysis can be classified as slow, fast and flash pyrolysis [9,41]. Slow pyrolysis occurs at heating rates of around 0.005 °C/s and temperatures between 200 and 600 °C, fast pyrolysis occurs at heating rates of between 5 and 100 °C/s and temperatures between 350 and 650 °C, and flash pyrolysis occurs at heating rates above 100 °C/s and temperatures above 400 °C [9,41]. Regarding residence time, slow pyrolysis can last for days, while fast and flash pyrolysis usually last seconds or fractions of seconds.
The variability of pyrolysis make it a versatile process, therefore, researchers with different interests have worked in its development: the development of catalysts or catalyst supports [8,[42], [43], [44]], biochar for carbon capture [[45], [46], [47], [48], [49]], bio-oil production [34,[50], [51], [52], [53]], hydrogen production [[54], [55], [56], [57]], syngas production [31,44,58], amongst other uses. Particularly, authors have discovered that the incorporation of catalysts during pyrolysis improve the product quality and favor the formation of a particular type of compound [11,12,50]. Besides the gas, liquid and solid yields, several criteria are used to assess the process performance. In terms of the gas, it is desirable for the gas to have a high calorific value, a H2/CO ratio larger than 1 and as low as possible CO2 contents [31,[59], [60], [61]]. On the other hand, for pyrolysis oil, it is desirable to have high H/C ratios, low O/C ratios, high calorific values and densities similar to that of conventional fuels [[62], [63], [64]]. Finally, the desirable properties in biochar are high porosity, lack of pollutants, functional groups, high surface area and a structured carbonaceous matrix [[46], [47], [48],[65], [66], [67]].
2.2. Solar pyrolysis
Solar pyrolysis uses solar energy as a source of heat for pyrolysis. Solar pyrolysis has several advantages over other technologies: from an energy balance perspective, the process can be considered as an “upgrade” to conventional biomass energy, or as solar energy storage [62,68]. With respect to emissions, solar pyrolysis is technically emission-free since any produced gases are inside the carbon cycle and solar energy does not produce greenhouse gases [3]. Still, as promising as the technology may sound, it is not without is challenges since for example, it is highly weather dependent, hence, it can be difficult to achieve constant results.
3. Description of the pyrolysis mechanism
3.1. Biomass decomposition
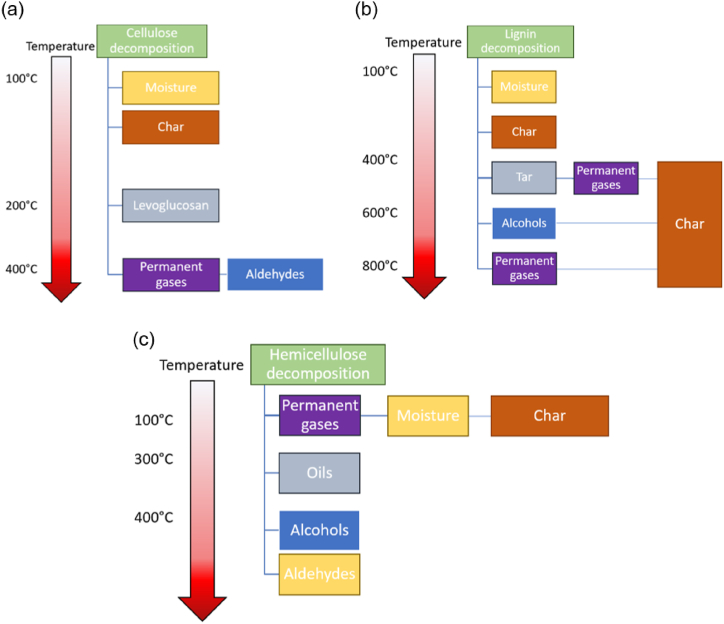
Biomass is primarily composed of cellulose (CL), hemicellulose (HM) and lignin (LI), all organic polymers intertwined together that interact during changes occurring to the biomass. During pyrolysis, the three components decompose at different temperatures and rates, and the resulting products interact with the remaining components [69,70]. Due to their polymeric and variable nature, the thermal decomposition of the biomass components is complicated and has been studied thoroughly for decades with varying degrees of detail [20,41]. The three polymers interact during pyrolysis and result in the formation of a number of products depending on operation conditions such as temperature, reaction time and heating rate; Fig. 3 shows an overview of the pyrolysis process of CL, HM and LI. As can be seen in Fig. 3, CL, HM and LI decompose in “stages”, where the first stage consists on the activation of the polymer, followed by devolatilization, carbonization and decomposition reactions.
Fig. 3.
Representation of the products of the decomposition of (a) cellulose, (b) lignin and (c) hemicellulose across different temperature ranges.
CL is a linear polymer consisting of linked d-glucose units bonded via ether glycosidic bonds [71,72]. The main difference between the mentioned models lies in the number of reactions considered and the incorporation of different CL/HM/LI monomers [[73], [74], [75]].
Firstly, (circa 240 °C), cellulose becomes “activated”, the degree of cellulose polymerization decreases, and water and some permanent gases are released, and as temperature increases, some carboxyl and carbonyl groups are formed and dehydrated sugars are produced [70,76,77]. At temperatures between 250 and 400 °C the chemical structure is rearranged, and volatiles are released; at this stage levoglucosan (a typical cellulose model compound) is formed [78]. Finally, at temperatures >400 °C the levoglucosan decomposes, secondary cellulose pyrolysis occurs and gases such as H2, CO, CO2, along with simple hydrocarbons such as hydroxyacetaldehyde are formed [70].
HM degrades at temperatures between 200 and 300 °C. Volatiles are released at temperatures around 250 °C, followed by the production of oils at temperatures between 350 and 450 °C; at this temperature, compounds such as hydroxypyrene and 2-furaldehyde are produced. As the temperature increases past 450 °C, the liquid yield is replaced by lighter compounds such as formic acid, furfural and acetaldehyde [71,79].
LI is an amorphous polymer consisting of phenyl units linked by C–C and C–O that degrades at relatively high temperatures (>400 °C). Reports have indicated that even if CL and HM contribute, LI is largely responsible for the formation of the mixture of aromatic hydrocarbons deemed tar [[80], [81], [82]]. Volatiles form from LI pyrolysis at temperatures between around 400 and 700 °C, aromatic hydrocarbons form at temperatures around 500–700 °C and light pyrolysis oil is mostly prevalent between 700 and 900 °C [70]. Importantly, the pyrolytic behavior of LI is influenced by the interaction with HM and CL, as well as the biomass type and structure [69].
3.2. Kinetic models
Experimental data provides valuable information for the design and understanding of pyrolysis systems, however, in order to study the decomposition mechanism kinetic analysis is useful and often the most economically and timely viable option. For example, to design an adequate solar pyrolysis system it is important to understand what kind of products can be formed in the available conditions. For kinetic analysis models consider extended, lumped or model compound mechanisms; the mechanisms can be employed to understand the relationship between different operation parameters and the products. Extended mechanisms consider the biomass as the main reactant which decomposes into intermediate species and end-products; extended mechanisms often yield the most significant results, but can be complicated to use due to computational demand and lack of kinetic information, which is often acquired using computational methods such as Density Functional Theory simulations [69,83,84]; some of the most widely used decomposition models were developed by Shafizadeh [72] and Ranzi [76]. Lumped mechanisms, which are less computationally demanding and still provide significant information make use of “lumps”, which are a group of compounds with a limited number of primary and secondary reactions [33,73]. Finally, model compound models only consider certain compounds representative of the products or intermediates, based on their physicochemical properties, and while useful, give the least information on the pyrolysis kinetics [80,85].
Diverse mathematical abstractions are used to determine pyrolysis kinetics; amongst the most commonly used methods are the Kissinger-Akahira-Sunose, the Distributed Activation Energy model and the model-free method [[86], [87], [88]]. Differently from conventional graphical methods based on the Arrhenius equation slope, pyrolysis kinetic methodologies incorporate parameters such as the effect of the heating rate in the thermal degradation, hence, offer more robust estimations.
3.3. Product distribution
The products from pyrolysis have a high degree of variability. Fig. 4 portrays the effect of pyrolysis temperature in (a) slow, (b) fast and (c) flash pyrolysis in the product distribution between liquids, solids and gases [9]. As can be seen in the figures, the liquid (bio-oil) yield increases with temperature, reaches a peak at around 600 °C and then starts decreasing. On the other hand, the gas yield increases with temperature; the constant increase of gas yield is related to the decrease in liquid due to secondary oil/tar decomposition reactions [33]. Finally, with regards to the solid (biochar) yield, it is favored at low temperatures and decreases with increasing temperature; at sufficiently high temperatures, the solid interacts with the liquid and gas and reforms to enhance the production of gases [8]. Slow pyrolysis favors the formation of solids while fast pyrolysis exhibits the best behavior for the production of gases. Finally, flash pyrolysis favors the formation of liquids, especially at low and intermediate temperatures (400–600 °C).
Fig. 4.
Products from the pyrolysis of biomass at different temperatures [[89], [90], [91], [92], [93], [94], [95], [96], [97]] considering (a) slow, (b) fast and (c) flash pyrolysis.
4. Literature review
Solar pyrolysis has been studied for biofuel production since it requires less energy when compared to the conventional pyrolysis process [98,99], where the main sought after product is bio-oil [100]. Studies have focused on understanding different involved aspects, such as the feedstock parameters (for example biomass composition, feedstock particle size, presence of moisture, co-feeding) and reaction parameters (temperature, heating rate and reaction time) in the process.
Solar pyrolysis has been of interest for decades, stemming in a range of research works being published and developed with the goal of understanding the effect of different parameters in the operation and products. From the review conducted in this work, the solar pyrolysis efforts can be classified according to the type of solar concentration into two main categories: linear concentration technology, including Parabolic trough and Linear Fresnel, and point concentration technology such as Fresnel lens, Parabolic dish, solar simulators, solar furnaces, and solar tower. From this analysis, it was identified that 82 % of the studies utilize point concentration technologies, which are particularly suitable for Fast and Flash pyrolysis processes due to the high temperatures required. In contrast, only 18 % of the solar pyrolysis works rely on linear concentration systems. The preference for point concentration systems is driven by their ability to achieve the necessary high temperatures for efficient pyrolysis. Further detail on the information recovered from the revised articles can be found in the Supplementary Material. A summary on the findings from different articles is presented in Table 1.
Table 1.
Summary of solar pyrolysis experiments reported in literature.
| Ref. | Feedstock | Solar technology | Varied parameter | Summary of findings |
|---|---|---|---|---|
| [29,101,102] | Beech wood sawdust | Solar furnace | Temperature (900, 1200, 1600 & 2000 °C) | The structure of the produced biochar becomes more ordered with increasing temperature and decreased heating rate. |
| Heating rate (10 & 150 °C/s) | Biochar pore volume and surface area increases with temperature then decreases due to sintering. | |||
| [103] | Heavy metal-contaminated willow | Solar furnace | Temperature (600–1600 °C) | The presence of heavy metals can enhance the gas yield. |
| Heating rate (10 & 50 °C/s) | ||||
| [104] | Pine sawdust | Solar furnace | Temperature (800–2000 °C) | Gas yield increases with temperature and heating rate. |
| Peach pit | Heating rate (10–150 °C/s) | Bio-oil yield decreases with temperature and heating rate. | ||
| Grape stem | ||||
| Grape stalk | ||||
| [105] | Orange peel | Parabolic trough | Heating rate (1, 5, 10, 20 & 40 °C/s) | The feedstock properties affect the heat losses in a parabolic trough receiver. |
| [106,107] | Waste tires | Fresnel lens | Presence of a catalyst (H-beta, H-USY & TiO2) | TiO2 has a negligible effect in the pyrolysis. |
| H-beta enhances the pyrolysis products. | ||||
| [108] | Algae | Solar simulator | Carrier gas composition (Argon, Argon/steam) | Gasification can be conducted with a solar simulator. |
| Wheat Straw | Irradiation time (10 & 20 min) | Wheat straw can be an adequate feedstock for solar gasification. | ||
| Sewage sludges | ||||
| [62] | Jatropha seeds | Parabolic dish | Heat flux (0.03–0.07 MW/m2) | Bio-oil yield decreases with temperature. |
| Jatropha seeds can be used to produce liquid fuel precursors. | ||||
| [53] | Peanut shell | Parabolic dish | Aqueous-phase bio-oil wash | Washing increases the bio-oil yield. |
| Soybean straw | ||||
| Pinewood | ||||
| [109] | Cotton stalks | Parabolic dish | Presence of a catalyst (HZSM-5 zeolite) | Catalysts enhance the biochar yield and reduce the formation of coke. |
| Pretreatment (torrefaction) | ||||
| [110] | Rice husk | Parabolic dish | Temperature (500–800 °C) | Bio-oil and biochar yield decreases with temperature and heating rate. |
| [111] | Chicken litter | Parabolic dish | Presence of a catalyst (CaO, char) | The particle size changes the product yield. |
| Particle sizes | ||||
| [112] | Rice husk | Parabolic dish | Temperature (800–1600 °C) | Bio-oil and biochar yield decreases with temperature and heating rate. |
| Chicken litter | Heating rate (10–500 °C/s) | |||
| [113] | Willow pellets | Solar simulator | Pellet diameter (0.5, 1 & 3 mm) | The reflectivity of biochar increases with reaction time. |
| Heat flux (0.5 & 1.1 MW/m2) | The biochar surface functional groups change with the heat flux. | |||
| Reaction time (5, 10 & 21 s) | ||||
| [114] | Waste wood | Solar simulator | Heating rate (∼5–5.5 °C/min) | The biochar surface area decreases with heating rate. |
| Waste straw | Gas yield increases with temperature. | |||
| Sewage sludge | ||||
| [31] | Sawdust pellets | Parabolic dish | Pellet size (5, 10 & 15 mm) | Particle size changed the product yield. |
| Temperature (800, 1200 & 1600 °C) | ||||
| Heating rate (10 & 50 °C/s) | ||||
| [115] | Pine sawdust | Solar simulator | Heat flux (0.274–0.959 MW/m2) | Product yield depended on maximum achieved temperature and not on heating rate. |
| [116] | Microalgae | Fresnel lens | Presence of a catalyst (Calcined & non-calcined hydrocalumite) | The reaction time and catalysts had a negligible impact in the species present in the bio-oil. |
| Reaction time (2.27, 6, 15, 24 & 27.73 min) | The catalysts prevent the formation of coke. | |||
| The presence of long-chain and aromatic hydrocarbons is enhanced with increasing reaction time and catalyst loading. | ||||
| [117] | Microalgae | Parabolic dish | Presence of a downstream catalytic reactor (hydrocalcite) | The catalyst in the downstream reactor. |
| Biomass loading (1.98, 2.50, 3.75, 5.0 & 5.52 g) | The catalyst losses effectivity but can be regenerated. | |||
| [118] | Microalgae | Parabolic dish | Presence of a downstream catalytic reactor (hydrocalcite) | The hydrogen atmosphere decreases the formation of aliphatic compounds in the pyrolysis bio-oil. |
| Atmosphere (Vacuum & hydrogen) | The bio-oil yield increases with reaction time and the presence of a catalyst. | |||
| Biomass loading (1.98, 2.50, 3.75, 5.0 & 5.52 g) | ||||
| Reaction time (2, 6, 10, 13, 20, 23, 30 & 34 min) | ||||
| [119] | Walnut shell | Solar simulator | Heat flux (234, 482 & 725 W) | Heating of biochar in a secondary reactor above the pyrolysis temperature leads to the formation of tar due to secondary reactions. |
| Vocalic stone | ||||
| [120] | Agave waste | Solar simulator | Temperature (500, 700 & 900 °C) | The produced biochar surface area and electrokinetic parameters decrease with temperature. |
| [121] | Agave waste | Solar furnace | Temperature (450, 600, 800, 935, 1100, 1430 & 1564 °C) | Biochars produced from solar pyrolysis can be employed in supercapacitors, where the temperature must be controlled to prevent biochar sintering. |
| Tomato plant waste | ||||
| [122] | Coconut shell | Solar simulator | Temperature (400, 600, 800 °C) | The pyrolysis temperature has an important effect in the biochar microporosity, while the heating rate has an important effect in the pore volume. |
| Heating rate (50, 200 & 500 °C/min) |
4.1. Bibliometric analysis of keywords in solar pyrolysis projects
Fig. 5 is a keyword density visualization map generated with VOSviewer based on the literature review presented in this study, using databases focused on Scopus and supplemented with Google Scholar and Mendeley. This map shows the most common keywords in studies related to the reviewed articles on solar pyrolysis.
Fig. 5.
Visualization Map of Keyword Density, generated by VOSviewer v 1.6.19.
This analysis offers several important observations about the trends and focus areas in this field. Solar pyrolysis and biomass occupy a central position in the density map, with terms like "solar pyrolysis" and "biomass" standing out. This indicates that much of the research focuses on biomass pyrolysis using solar energy, emphasizing its importance for converting biomass into valuable products such as biochar, bio-oil, and syngas. The prominence of "solar pyrolysis" highlights the relevance of this process in current research, while "biomass" underscores the importance of biomass as a raw material in these studies. Another key aspect is the focus on solar energy and its optimization. Terms like "solar energy" and "concentrated solar energy" show high density, indicating significant interest in how to harness and optimize solar energy in pyrolysis processes. This includes research into solar concentration technologies that can maximize the capture and utilization of solar radiation, which is crucial for improving the efficiency of solar pyrolysis processes. The diversification of feedstocks used in solar pyrolysis is another highlighted area. The appearance of terms like "algae," "beech wood" and “sewage sludge” indicates that researchers are exploring a variety of feedstocks for solar pyrolysis, since these feedstocks have significantly different physicochemical characteristics. This diversification is essential for finding sustainable and efficient biomass sources that can be used in energy and chemical production. Based on the analysis, several research areas can be highlighted for further exploration to advance the development of solar pyrolysis, as shown in Table 2.
Table 2.
Potential research areas found in a bibliometric analysis.
| Research Areas | Description |
|---|---|
| Improvements in Solar Concentration Technology | Future research could focus on developing more advanced concentration and reactors technologies that maximize the capture and utilization of solar radiation. It is also crucial to create scaling strategies that facilitate practical large-scale application. Additionally, exploring the integration of molten salt thermal energy storage systems can provide a way to store thermal energy efficiently, ensuring continuous operation even when solar radiation is not available. |
| Exploration of New Feedstocks | The solar pyrolysis of different types of biomass, including industrial and municipal waste, as well as the implementation of co-pyrolysis, should be extensively researched. |
| Development of New Catalysts and Materials | Studies could focus on identifying and synthesizing new catalytic materials that are more active, selective, and durable under solar pyrolysis conditions. |
| Life Cycle and Economic Feasibility Studies | These studies will help to better understand the environmental and economic impacts of these technologies and identify areas for improvement. |
| Innovative Applications of Biochar and Other Products | Investigating new applications of biochar, such as soil remediation, contaminant adsorption, and energy storage technologies, could open new opportunities for its use. Innovative applications for other products derived from solar pyrolysis should also be explored. |
| Integration of Hybrid Technologies | Combining solar pyrolysis with other renewable technologies, such as wind, geothermal or photovoltaic energy, could result in more robust and efficient hybrid energy systems. |
4.2. Literature reviews on solar pyrolysis
Pyrolysis is a relatively well studied (alas, not completely understood) technology, hence, a number of studies have been reported, including reviews. Reviews have focused on studies to upgrade syngas [99,123,124] and to produce liquid fuels from coals [36]. The review by Zeng et al. [36] is dedicated to discuss articles that explore the solar coal pyrolysis process efficiency with different solar furnaces. Nzihou et al. [125] discussed the advances in the production of liquid fuels from with concentrated solar power, while Chintala discusses solar pyrolysis as an opportunity for upgradation [126]. Solar gasification has seen some effort can be a promising process for autothermal gas production. Piatkowski et al. [127] discussed the kinetics and thermodynamics involved in solar gasification, as well as the science that must be developed to improve the technology. Parthasarathy et al. [128] assessed works focused on the production of biochar from animal and municipal waste via solar pyrolysis, mainly discussing the effect of physicochemical properties of the biomass in the process optimization. Hamed et al. [124] also discussed the effect of physicochemical parameters, but their review involves the use of mainly palm tree waste. Reviews have also been conducted to determine how different arrangements of the solar power technologies can be used for solar pyrolysis [129,130].
4.3. Solar pyrolysis around the world
Researchers from all over the world have conducted experiments under different conditions using various setups with varied results; an overview of the number of research projects conducted by region between 2014 and 2024 is depicted in Fig. 6a, while Fig. 6b displays the number of projects per country along with their minimum and maximum Direct Normal Irradiance (DNI), which is the solar radiation received by an area always normal to the solar rays, in kWh/m2 per day, taken from the Global Solar Atlas platform [131]. One would expect a correlation between the DNI and the number of projects per country, however, from Fig. 6a, the clear leader is Asia, even if the DNI in the lead Asiatic country (China) is not the highest. This might suggest strong investments in research and technology favor the development of the technology, or a strong group of researchers focusing in publications or grants exist in the countries. On the other hand, countries like Chile, Mexico, South Africa, Australia and Saudi Arabia have a high DNI but not as many reported projects and could benefit from research incentives.
Fig. 6.
Global distribution of Solar pyrolysis projects: (a) Regional publication count and (b) country-wise project distribution with Max and Min DNI.
Policies and incentives are necessary for different countries to foster solar pyrolysis as a waste management and energy production technology [132]. For example, in some developing countries, an important bottleneck for waste management is the collection and separation of waste, as well as the lack of a clear Extended Producer Responsibility (EPR). EPR could be developed by incorporating and incentivizing the handling of waste and regulating the productions of CO2. For example, organizations that produce a large amount of waste could generate electricity from the pyrolysis of plastics such as tires and be further aided with the incorporation of political instruments such as feed in tariffs.
On the other hand, investment in research and development, and aligning the needs of society with for example, the Sustainable Development Goals, could benefit the economy and welfare of countries. Information from the World Bank indicates a relationship with countries having “underutilized” solar energy and low investment in research and development [133]. Countries like Mexico and Chile could use solar energy and energy storage to satisfy its needs [134,135]. Moreover, waste produced in large quantities could be valorized via solar pyrolysis or gasification, and the information should be diffused as consumers “will not invest in what they do not know”. Wastes such as tire waste, agave waste, avocado waste and palm tree waste are produced in considerable amounts in Latin America and might not be disposed of correctly [120,[136], [137], [138], [139]].
4.4. Classification of solar pyrolysis
In general, the experiments can be categorized based on (1) whether the irradiation comes from the sun or from a solar simulator, (2) whether the solar thermal energy is focused to the feedstock (direct heating) or not (indirect heating), (3) the solar thermal technology and (4) the type of reactor [38,62,108,115,140,141]. Additional important factors to consider include the pyrolysis atmosphere, the type of feedstock, the biomass feeding method to the reactor (continuous or batch), and whether the reactor is equipped with a temperature control system; a summary of the reported studies based on these classifications is presented in Fig. 7. Table 3 and Fig. 7a portray details a classification, based on the solar technology, while Table 4 showcases some characteristics of the continuously fed (flow) and batch reactors. It can be seen that most efforts involve the use of solar furnace, parabolic dish and solar simulators, while Fresnel lenses and solar photovoltaic modules are the least popular technologies.
Fig. 7.
A classification of solar pyrolysis studies categorized as follows: (a) solar technology, (b) solar thermal energy, (c) conditions of inert atmosphere, (d) method of biomass feeding to the reactor.
Table 3.
Summary of the different technologies found in the reviewed Works.
| Solar technology | % of total works | References |
|---|---|---|
| Solar furnace | 26 | [[142], [143], [144], [145], [146], [147], [148], [149], [150], [151], [152], [153], [154], [155], [156], [157], [158], [159], [160], [161], [162], [163]] |
| Parabolic dish | 20 | [[164], [165], [166], [167], [168], [169], [170], [171], [172], [173], [174], [175], [176], [177], [178], [179], [180], [181]] |
| Solar simulator (furnace) | 15 | [[182], [183], [184], [185], [186], [187], [188], [189], [190], [191], [192], [193], [194], [195]] |
| Parabolic-trough | 15 | [178,[196], [197], [198], [199], [200], [201], [202], [203], [204], [205], [206], [207]] |
| Solar Tower | 14 | [203,[208], [209], [210], [211], [212], [213], [214], [215], [216], [217], [218]] |
| Others (Fresnel lens, linear Fresnel reflector, solar photovoltaic, etc) | 10 | [173,173,[219], [220], [221], [222], [223], [224]] |
Table 4.
Overview of the characteristics of batch and flow reactors used in solar pyrolysis.
| Description | Flow reactor | Batch reactor | |
|---|---|---|---|
| Cost | Intermediate to high | Low to intermediate | [225] |
| Time to develop | High | Low | [225] |
| Operation mode | Uninterrupted or constant operation | Operates by batches and might require some time for start-up/cooling down between them. | [166] |
| Capacity | Industrial scale | Small scale and specific products | [225] |
| Other details | Usually safe to operate. | Higher flexibility and less cost in initial research stages. | [225] |
| Can be initially hard to control. | It might be easier to reproduce previously reported work. | ||
| Promotes high heat and mass transfer and leads to optimal reaction yields. | Feeding the reactor is usually simple. |
For solar pyrolysis, the employed reactors are either fixed or fluidized bed. The difference between fixed and fluidized bed reactors lies in the hydrodynamic behavior of the feedstock and/or catalyst particles inside the reactor: in fixed bed reactors, the particles do not move significantly; the carrier gas enters on one side (usually the top) and exits through the bottom [140,226]. On the other hand, in fluidized bed reactors, a gas is injected in the reactor to mobilize the particles; the extent and topology of the fluidization can vary, leading to further conceptualization such as bubbling bed, spouted bed and circulating bed, among others [140,226]. Fixed bed reactors are the most common due to their relative simplicity and ease of operation but can be constrained by non-uniform heat transfer and pressure drop [140]. Fluidized bed reactors do not suffer of the formation of hot spots but can be complicated to design and operate due to problems such as pellet agglomeration, complicated control systems and complicated scalation [227].
According to Rahman et al. [140], solar pyrolysis can be divided into three types based on how heat is transferred to the biomass: direct heated reactor, indirect heated reactor, and separated reactor system, the latter using a heat transfer fluid (HTF). Based on this classification, it was found that 58 % of the reviewed studies operate with a direct heated reactor, 24 % with an indirect heated reactor, and 18 % with a separated reactor system using some HTF (Fig. 7b). Upon investigating HTFs, it was discovered that 80 % of them are molten salts, primarily associated with solar tower systems. The remaining 20 % of the implemented HTFs include nitrogen (N2), steam, and hot air. Pyrolysis is typically carried out in the absence of or with a minimal amount of O2 in the reaction chamber, making it crucial to maintain a controlled atmosphere. Fig. 7c shows a comparison of the different inert atmospheres studied in solar pyrolysis. From the studies reviewed, it was found that 47 % used Ar as a carrier gas for the gases generated during pyrolysis, while 46 % opted for N2. Other studies implemented atmospheres of hydrogen (in what is called hydropyrolysis), vacuum, and helium (He). Fig. 4d compares the types of reactors used in solar pyrolysis systems, classifying them into two groups based on the reactor type as continuous flow or batch reactor. Fifteen percent of the reviewed projects study continuous reactors, while the majority focus on batch-type studies. Additionally, it was found that out of the total reported projects, 74 % correspond to experimental studies, 16 % to theoretical research, and 9 % to numerical analyses.
Regarding the heating technology, direct heating technologies are the most common; in these, the radiation is concentrated into a vessel (usually a quartz tube) where the feedstock lies, and the decomposition occurs. On the other hand, in the case of indirect heating technologies the sunlight is concentrated into an opaque surface and then, using a heat exchanger, the energy is passed to the feedstock for decomposition [140].
Finally, Fig. 8 provides an overview of the feedstocks used in solar pyrolysis projects. The classification of these feedstocks into four main categories reveals a clear trend in current research. Agricultural, plant, and organic waste (APOW) is the most commonly used category, with 52 studies, highlighting the abundance and availability of these residues for solar pyrolysis. The use of APOW also reflects the interest in utilizing agricultural and organic waste for energy and chemical production, promoting more sustainable practices and reducing environmental impact. The second most popular type of feedstock are wood and forestry products, with 34 studies, underscoring the importance of forestry and wood industry residues as viable sources for solar pyrolysis, leveraging their high energy content and availability. 9 studies were found that focus on the pyrolysis of plastic and synthetic material waste. Finally, 2 studies that use coal were documented; this reduced number can be associated to research focusing on decarbonization.
Fig. 8.
Main types of feedstocks used in solar pyrolysis studies.
It is crucial to consider that the feedstocks used in solar pyrolysis must be readily available in the regions where the pyrolysis plants will be established for it to be feasible. The presented results likely reflect the abundance of these feedstocks in the various regions where these studies are conducted, emphasizing the importance of local availability in the selection of feedstocks. This highlights the need for regional assessments of biomass resources to ensure sustainable and efficient implementation of solar pyrolysis technologies.
4.5. Description of solar thermal technologies
Solar simulators are devices that use an arc lamp (usually based of xenon, argon or halides) to exert a concentrated light flux similar to solar radiation in controlled conditions in a zone of interest [26,114]. Natural sunlight can be unpredictable and has a direct effect on the system performance since; in this case, the efficiency of solar thermal system depends on the solar intensity, presence of clouds, geographical location of the system, among other variables, making solar simulators attractive for preliminary design. Contrary to the sun, solar simulators offer the capability of climate and time of day-independent radiation; however, solar simulator-based systems offer an ideal scenario, and the obtained results can be opposite from those obtained with the sun [228].
With respect to actual solar thermal technologies, while there is a wide range available, four stand out for their frequent use in pyrolysis: (a) Solar furnace (Heliostat + parabolic dish), (b) Parabolic dish, (c) Parabolic trough, and (d) solar tower. Among these, three are of point concentration and one of linear concentration, as indicated in Table 5, Table 6, respectively. Fig. 9a provides a physical representation of these solar thermal technologies, while Fig. 9b illustrates the temperatures that can be reached based on the concentration ratio.
Table 5.
| Solar Technology | Achievable |
Solar Flux |
Optical efficiency | Axis tracking | Receiver type | Technology suitability |
|---|---|---|---|---|---|---|
| Temperature (°C) | Concentration ratio (Suns)a | |||||
| Parabolic trough | 350–550 | 70–80 | Medium | Single | Moving | Slow pyrolysis/Torrefaction |
| Linear Fresnel | 390 | >60 | Low | Single | Fixed | Slow pyrolysis/Torrefaction |
1 Sun = 1000 W/m.2.
Table 6.
| Solar Technology | Achievable |
Solar Flux |
Optical efficiency | Axis tracking | Receiver type | Technology suitability |
|---|---|---|---|---|---|---|
| Temperature (°C) | Concentration ratio | |||||
| Fresnel lens | 350 | <50 | High | Dual | Moving | Slow pyrolysis |
| Parabolic dish | 1200 | 100–1000 | High | Dual | Moving | Fast pyrolysis |
| Solar Tower (Heliostats) | 150–1000 | 100–1500 | Medium | Dual | Fixed | Fast pyrolysis |
| Solar furnace | >2000 | >10000 | High | Dual | Fixed | Fast/Flash pyrolysis |
Fig. 9.
Schematic Diagram of Various Solar Thermal Technologies. (a) Physical Representation and (b) Temperature vs. Concentration Ratio for Different Solar Pyrolysis Technologies.
Heliostats are devices that consists of mirrors with a solar-tracking system which direct sunlight towards a receiver, which acts as a furnace. Heliostats are usually arranged in groups and can heat the receiver to more than 1000 °C, depending on the solar irradiance and the number of heliostats [229].
On the other hand, the solar furnaces reviewed in this study are smaller-scale technologies that involve heliostats directing solar radiation towards a secondary parabolic optic. This optic then reconcentrates the radiation at a focal point, where the pyrolytic reactors are situated. These technologies, along with solar simulators—which act as a type of solar furnace—have been more extensively studied [230].
Fig. 10 showcases a schematic distribution of the regions explored for solar pyrolysis in a map generated based on the information gathered in this study. The figure illustrates the types of technologies implemented in the various countries shown in Fig. 6, overlaid on a map indicating the distribution of direct solar radiation, sourced from Ref. [131]. However, countries and regions with limited solar resources can still explore solar pyrolysis through the use of solar simulators.
Fig. 10.
Global Distribution of Solar Technologies Studied for Solar Pyrolysis. Map obtained from the “Global Solar Atlas 2.0, a free, web-based application is developed and operated by the company Solargis s.r.o. on behalf of the World Bank Group, utilizing Solargis data, with funding provided by the Energy Sector Management Assistance Program (ESMAP). For additional information: https://globalsolaratlas.info.
5. Challenges of solar pyrolysis
Although promising, solar pyrolysis faces significant challenges before the technology is mature enough to be commercially viable, according to the technology readiness level scale [1]. A key finding from the review is that there is a predominant focus on solar pyrolysis for solid waste management, utilizing a variety of raw materials, including agricultural, industrial, and forest waste, sewage sludge from wastewater treatment plants, and tire waste (Fig. 4d). This underscores the emerging potential of solar pyrolysis as an ally in converting these wastes into valuable fuels and chemicals, with the additional benefit of reducing greenhouse gas emissions.
Additionally, it is observed that the majority of the reviewed studies are conducted in Ar and N2 atmospheres, emphasizing the need to explore experimental continuous systems at the laboratory scale to address emerging technical challenges, such as the management of pyrolytic oils and the separation of inert gases from synthesis gas. This is crucial for advancing towards industrial-scale implementation.
There is also a notable interest in solar pyrolysis in regions with high DNI, such as South Africa, northern Mexico, Australia, and Saudi Arabia, as well as in countries with lower DNI like France and China (Fig. 3b). This indicates that the intensity of the solar resource does not necessarily limit the study of solar pyrolysis, opening the door to the use of Xenon lamp solar simulators in places with lower solar radiation (Fig. 6). Moreover, solar furnaces stand out as the most studied solar technology (Fig. 4a), capable of reaching temperatures up to 2000 °C and offering precise control over the reaction temperature through the use of attenuators. This precision is essential for flash pyrolysis, especially when small amounts of biomass (∼0.3 g) are used and direct irradiation is required, commonly employing Pyrex reactors supported on stainless steel bases.
However, a question of scalability arises. Although some of the mentioned solar furnaces are among the largest in the world, the technology has been primarily explored at the laboratory scale, with studies focusing on biomass amounts in the order of grams. This raises questions about the applicability of this technology at an industrial level, especially considering the operational cost of using inert gases like argon and nitrogen on a large scale (Fig. 4c).
The challenges are composed of different design, physical, or technical aspects.
-
-
Solar intermittence
-
-
Controlling reactor temperature and heating rates
-
-
Optical efficiency
-
-
Reactor thermal efficiency
-
-
Heat distribution in the pyrolysis reactor
-
-
High cost due to the operation atmosphere
Table 7 shows a summary of some of the challenges and associated research gaps that must be addressed.
Table 7.
| Challenge | Research gap that must be addressed |
|---|---|
| Variability in solar irradiation |
|
| Improving the bio-oil quality |
|
| Solar reactor configuration |
|
| Mixed waste pyrolysis |
|
| Hybrid technologies |
|
To address the variability in solar irradiation different methodologies called Thermal Energy Storage (TES) have been proposed [130,232]. Broadly speaking, the TES systems depend on three technologies: sensible heat storage, latent heat storage and thermochemical storage. Sensible heat storage consists in heating materials such as oils and molten salts as heat reservoirs [233]. Latent heat storage consists in the use of phase change materials, that is, materials with a large latent heat, to store energy [234]. Finally, thermochemical storage involves materials that can undergo exothermic reversible reactions [235].
6. Concluding remarks
Pyrolysis is a promising technology with an important limitation stemming from the energy demand, therefore, the use of solar energy as an energy source can potentially catapult pyrolysis to become the de facto choice for biofuel production and chemical recycling. The present work conferred an overview of the advances in solar pyrolysis worldwide and provided a series of prospectives for development. Solar technologies have several variables that affect their performance and can be a bottleneck for commercialization. At the same time, pyrolysis has the recurrent challenge of non-uniformity in the products; therefore, coupling pyrolysis with solar technologies can be challenging and requires extensive use of mathematical models or methodologies for adequate design and optimization. Experiments under similar conditions have resulted in different results. Fortunately, AI has become sufficiently advanced as to provide opportunities to contribute in the development of solar pyrolysis. AI has attracted interest due to the capabilities to predict phenomena based on historic data, where Machine Learning has seen popularity in scientific and engineering bio-energy related applications. However, due to the nature of the “algorithm” behind AI functioning as a black box, uncertainty exists on the actual accuracy of a generalized AI ML model. Moreover, given that the product yield distribution follows different trends with different biomasses and reactors, the actual accuracy of AI ML model is compromised until the technology becomes more developed, or enough training data becomes available. Therefore, with enough training data become available, a groundbreaking advance in solar pyrolysis could stem from the incorporation of AI.
On the other hand, co-pyrolysis of different feedstocks has become the de facto alternative to handle waste and biomass; evidence has shown that co-processing can be used to improve the quality of the products and is a feasible alternative for chemical recycling. Naturally, post-combustion CO2 capture technologies are under constant development and might be one of the driving forces behind solar pyrolysis, given that solar-produced-biochar for carbon capture will have less carbon footprint than conventionally produced biochar. As the regulation behind biochar for carbon sequestration becomes mature, the market will start favoring the use of environmentally friendly carbon, which can more-often-than-not complicated to produce. When the solar pyrolysis reaches enough maturity, by using energy from the sun as a heat source, solar pyrolysis and co-pyrolysis can pave the way for carbon capture and will provide an alternative to upgrade fuels with chemical recycling and a lesser carbon footprint.
From the reviewed, the following concluding remarks are provided.
-
-
The effect of the process parameters in the product composition must be understood in detail to maximize the utility of solar pyrolysis. This represents a challenge, since solar intermittence results in a difficult temperature control, hence, difficult process controls. However, the uprising of Artificial Intelligence can potentially ease the mentioned problems.
-
-
Solar pyrolysis focused on biochar production is a promising carbon capture alternative. We expect the solar pyrolysis technology to see investment and development in the future.
-
-
While current studies have provided a solid knowledge base, there is a clear need to advance towards the study and development of continuous systems that can address the technical and economic challenges for the large-scale implementation of solar pyrolysis.
-
-
Exploring theoretical and numerical models and the detailed design of reactors and entire plants in continuous mode are essential steps towards the industrial viability of this promising technology.
CRediT authorship contribution statement
V.M. Maytorena: Writing – review & editing, Writing – original draft, Investigation. D.A. Buentello-Montoya: Writing – review & editing, Conceptualization.
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work the author(s) used DALL-E 2 in order to aid in the development of some figures. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e35464.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.International Energy Agency . IEA: World Energy Outlook; 2017. WEO 2017 Chapter 1: Introduction and Scope; pp. 33–61. [DOI] [Google Scholar]
- 2.Capuano L. Dr. July; 2018. International Energy Outlook 2018 (IEO2018) p. 21.www.eia.gov [Online]. Available: [Google Scholar]
- 3.Brown R.C. 2011. Thermochemical Processing of Biomass: Conversion into Fuels, Chemicals and Power. [DOI] [Google Scholar]
- 4.Sulaiman C., Abdul-Rahim A.S., Ofozor C.A. Does wood biomass energy use reduce CO2 emissions in European Union member countries? Evidence from 27 members. J. Clean. Prod. Apr. 2020;253 doi: 10.1016/j.jclepro.2020.119996. [DOI] [Google Scholar]
- 5.Basu P. 2008. Biomass Gasification, Pyrolisis and Torrefaction. [Google Scholar]
- 6.Krivov A. 2018. Anju; Dahiya, Bioenergy : Biomass to Biofuels: an Overview. [Google Scholar]
- 7.Wang G., et al. A review of recent advances in biomass pyrolysis. Am. Chem. Soc. Dec. 17, 2020 doi: 10.1021/acs.energyfuels.0c03107. [DOI] [Google Scholar]
- 8.Buentello-Montoya D.A., Zhang X., Li J. Elsevier Ltd; Jun. 01, 2019. The Use of Gasification Solid Products as Catalysts for Tar Reforming. [DOI] [Google Scholar]
- 9.Zhang X. Essential scientific mapping of the value chain of thermochemically converted second-generation bio-fuels. Green Chem. 2016;18(19):5086–5117. doi: 10.1039/C6GC02335E. [DOI] [Google Scholar]
- 10.Wang Y.G., et al. In situ catalyzing gas conversion using char as a catalyst/support during brown coal gasification. Energy Fuel. 2015;29(3):1590–1596. doi: 10.1021/ef502761d. [DOI] [Google Scholar]
- 11.Shen Y., Zhao P., Shao Q., Ma D., Takahashi F., Yoshikawa K. In-situ catalytic conversion of tar using rice husk char-supported nickel-iron catalysts for biomass pyrolysis/gasification. Appl. Catal., B. 2014;152–153(1):140–151. doi: 10.1016/j.apcatb.2014.01.032. [DOI] [Google Scholar]
- 12.Andrade L.A., Barrozo M.A.S., Vieira L.G.M. Catalytic solar pyrolysis of microalgae Chlamydomonas reinhardtii. Sol. Energy. Oct. 2018;173:928–938. doi: 10.1016/j.solener.2018.08.035. [DOI] [Google Scholar]
- 13.Shahbaz M., Yusup S., Inayat A., Patrick D.O., Ammar M. The influence of catalysts in biomass steam gasification and catalytic potential of coal bottom ash in biomass steam gasification: a review. Renew. Sustain. Energy Rev. 2017;73(January):468–476. doi: 10.1016/j.rser.2017.01.153. [DOI] [Google Scholar]
- 14.Min Z., Lin J.Y., Yimsiri P., Asadullah M., Li C.Z. Catalytic reforming of tar during gasification. Part V. Decomposition of NOx precursors on the char-supported iron catalyst. Fuel. 2014;116(x):19–24. doi: 10.1016/j.fuel.2013.07.080. [DOI] [Google Scholar]
- 15.Buentello-Montoya D., Zhang X., Li J., Ranade V., Marques S., Geron M. Performance of biochar as a catalyst for tar steam reforming: effect of the porous structure. Appl. Energy. 2020;259(Feb) doi: 10.1016/j.apenergy.2019.114176. [DOI] [Google Scholar]
- 16.Nilsson S., Gómez-Barea A., Fuentes-Cano D., Ollero P. Gasification of biomass and waste in a staged fluidized bed gasifier: modeling and comparison with one-stage units. Fuel. 2012;97:730–740. doi: 10.1016/j.fuel.2012.02.044. [DOI] [Google Scholar]
- 17.Wang Y., Yoshikawa K., Namioka T., Hashimoto Y. Performance optimization of two-staged gasification system for woody biomass. Fuel Process. Technol. 2007;88(3):243–250. doi: 10.1016/j.fuproc.2006.10.002. [DOI] [Google Scholar]
- 18.Leijenhorst E.J., Wolters W., Van De Beld B., Prins W. Staged biomass gasification by autothermal catalytic reforming of fast pyrolysis vapors. Energy Fuel. 2015;29(11):7395–7407. doi: 10.1021/acs.energyfuels.5b01912. [DOI] [Google Scholar]
- 19.Alvarez J., et al. Hydrogen production from biomass and plastic mixtures by pyrolysis-gasification. Int. J. Hydrogen Energy. 2014;39(21):10883–10891. doi: 10.1016/j.ijhydene.2014.04.189. [DOI] [Google Scholar]
- 20.Fahmy T.Y.A., Fahmy Y., Mobarak F., El-Sakhawy M., Abou-Zeid R.E. Springer; Jan. 01, 2020. Biomass Pyrolysis: Past, Present, and Future. [DOI] [Google Scholar]
- 21.Sikarwar V.S., et al. An overview of advances in biomass gasification. Energy Environ. Sci. 2016;9(10):2939–2977. doi: 10.1039/C6EE00935B. [DOI] [Google Scholar]
- 22.Spasiano D., Marotta R., Malato S., Fernandez-Ibañez P., Di Somma I. A Comprehensive Approach. Elsevier; Jul. 01, 2015. “Solar photocatalysis: materials, reactors, some commercial, and pre-industrialized applications. [DOI] [Google Scholar]
- 23.Cambié D., et al. Energy‐efficient solar photochemistry with luminescent solar concentrator based photomicroreactors. Angew. Chem. Oct. 2019;131(40):14512–14516. doi: 10.1002/ange.201908553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sobri S., Koohi-Kamali S., Rahim N.A. Elsevier Ltd; Jan. 15, 2018. Solar Photovoltaic Generation Forecasting Methods: A Review. [DOI] [Google Scholar]
- 25.Bashir M., Yu X., Hassan M., Makkawi Y. Modeling and performance analysis of biomass fast pyrolysis in a solar-thermal reactor. ACS Sustain. Chem. Eng. May 2017;5(5):3795–3807. doi: 10.1021/acssuschemeng.6b02806. [DOI] [Google Scholar]
- 26.Ndukwu M.C., Horsfall I.T., Ubouh E.A., Orji F.N., Ekop I.E., Ezejiofor N.R. King Saud University; Sep. 01, 2021. Review of Solar-Biomass Pyrolysis Systems: Focus on the Configuration of Thermal-Solar Systems and Reactor Orientation. [DOI] [Google Scholar]
- 27.Zeng K., Gauthier D., Li R., Flamant G. Solar pyrolysis of beech wood: effects of pyrolysis parameters on the product distribution and gas product composition. Energy. 2015;93:1648–1657. doi: 10.1016/j.energy.2015.10.008. [DOI] [Google Scholar]
- 28.Zeng K., Gauthier D., Lu J., Flamant G. Parametric study and process optimization for solar pyrolysis of beech wood. Energy Convers. Manag. Dec. 2015;106:987–998. doi: 10.1016/j.enconman.2015.10.039. [DOI] [Google Scholar]
- 29.Zeng K., Gauthier D., Li R., Flamant G. Combined effects of initial water content and heating parameters on solar pyrolysis of beech wood. Energy. 2017;125:552–561. doi: 10.1016/j.energy.2017.02.173. [DOI] [Google Scholar]
- 30.Melchior T., Perkins C., Lichty P., Weimer A.W., Steinfeld A. Solar-driven biochar gasification in a particle-flow reactor. Chem. Eng. Process: Process Intensif. Aug. 2009;48(8):1279–1287. doi: 10.1016/j.cep.2009.05.006. [DOI] [Google Scholar]
- 31.Soria J., Li R., Flamant G., Mazza G.D. Influence of pellet size on product yields and syngas composition during solar-driven high temperature fast pyrolysis of biomass. J. Anal. Appl. Pyrolysis. Jun. 2019;140:299–311. doi: 10.1016/j.jaap.2019.04.007. [DOI] [Google Scholar]
- 32.Joardder M.U.H., Halder P.K., Rahim M.A., Masud M.H. Clean Energy for Sustainable Development: Comparisons and Contrasts of New Approaches. Elsevier Inc.; 2017. Solar pyrolysis: converting waste into asset using solar energy; pp. 213–235. [DOI] [Google Scholar]
- 33.Maytorena V.M., Hinojosa J.F., Iriarte-Cornejo C., Orozco D.A. Biomass solar fast pyrolysis with a biomimetic mini heliostat field and thermal receiver for nitrogen heating. Energy Convers. Manag. 2023;291(Sep) doi: 10.1016/j.enconman.2023.117307. [DOI] [Google Scholar]
- 34.Zhang Y., Fan S., Liu T., Xiong Q. A review of aviation oil production from organic wastes through thermochemical technologies. Applications in Energy and Combustion Science. 2022;9(Mar) doi: 10.1016/j.jaecs.2022.100058. [DOI] [Google Scholar]
- 35.Hamilton J., Seyedmahmoudian M., Jamei E., Horan B., Stojcevski A. Elsevier Ltd; Nov. 01, 2020. A Systematic Review of Solar Driven Waste to Fuel Pyrolysis Technology for the Australian State of Victoria. [DOI] [Google Scholar]
- 36.Zeng K., Gauthier D., Soria J., Mazza G., Flamant G. Solar pyrolysis of carbonaceous feedstocks: a review. Sol. Energy. Nov. 2017;156:73–92. doi: 10.1016/j.solener.2017.05.033. [DOI] [Google Scholar]
- 37.Abanades S., Rodat S., Boujjat H. Solar thermochemical green fuels production: a review of biomass pyro-gasification, solar reactor concepts and modelling methods. Energies. Mar. 2021;14(5) doi: 10.3390/en14051494. [DOI] [Google Scholar]
- 38.Hamed A.S.A., Yusof N.I.F.M., Yahya M.S., Cardozo E., Munajat N.F. Concentrated solar pyrolysis for oil palm biomass: an exploratory review within the Malaysian context. Renew. Sustain. Energy Rev. 2023;188(Dec) doi: 10.1016/j.rser.2023.113834. [DOI] [Google Scholar]
- 39.Parthasarathy P., Al-Ansari T., Mackey H.R., Sheeba Narayanan K., McKay G. A review on prominent animal and municipal wastes as potential feedstocks for solar pyrolysis for biochar production. Fuel. May 2022;316 doi: 10.1016/j.fuel.2022.123378. [DOI] [Google Scholar]
- 40.Mondal S., Mondal A.K., Chintala V., Tauseef S.M., Kumar S., Pandey J.K. Thermochemical pyrolysis of biomass using solar energy for efficient biofuel production: a review. Biofuels. 2021;12(2):125–134. doi: 10.1080/17597269.2018.1461512. [DOI] [Google Scholar]
- 41.Vuppaladadiyam A.K., et al. Elsevier Ltd; Nov. 01, 2022. Biomass Pyrolysis: A Review on Recent Advancements and Green Hydrogen Production. [DOI] [PubMed] [Google Scholar]
- 42.Wang D., Yuan W., Ji W. Char and char-supported nickel catalysts for secondary syngas cleanup and conditioning. Appl. Energy. 2011;88(5):1656–1663. doi: 10.1016/j.apenergy.2010.11.041. [DOI] [Google Scholar]
- 43.Wang Y., et al. Catalytic steam reforming of cellulose-derived compounds using a char-supported iron catalyst. Fuel Process. Technol. 2013;116:234–240. doi: 10.1016/j.fuproc.2013.07.014. [DOI] [Google Scholar]
- 44.Zhang S., Dong Q., Zhang L., Xiong Y. High quality syngas production from microwave pyrolysis of rice husk with char-supported metallic catalysts. Bioresour. Technol. 2015;191:17–23. doi: 10.1016/j.biortech.2015.04.114. [DOI] [PubMed] [Google Scholar]
- 45.EBC . European Biochar Foundation (EBC), Arbaz, Switzerland. 2012. European biochar certificate - guidelines for a sustainable production of biochar. Version 6.1 of 19th June, no. June, pp. 1–22, 2015. [DOI] [Google Scholar]
- 46.Masoumi S., Borugadda V.B., Nanda S., Dalai A.K. MDPI; Aug. 01, 2021. Hydrochar: A Review on its Production Technologies and Applications. [DOI] [Google Scholar]
- 47.Syuhada A.B., Shamshuddin J., Fauziah C.I., Rosenani A.B., Arifin A. Biochar as soil amendment: impact on chemical properties and corn nutrient uptake in a Podzol. Can. J. Soil Sci. Jun. 2016;96(4):400–412. doi: 10.1139/cjss-2015-0044. [DOI] [Google Scholar]
- 48.Yaashikaa P.R., Kumar P.S., Varjani S., Saravanan A. Elsevier B.V; Dec. 01, 2020. A Critical Review on the Biochar Production Techniques, Characterization, Stability and Applications for Circular Bioeconomy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malghani S., Gleixner G., Trumbore S.E. Chars produced by slow pyrolysis and hydrothermal carbonization vary in carbon sequestration potential and greenhouse gases emissions. Soil Biol. Biochem. Jul. 2013;62:137–146. doi: 10.1016/j.soilbio.2013.03.013. [DOI] [Google Scholar]
- 50.Abu Bakar M.S., Titiloye J.O. Catalytic pyrolysis of rice husk for bio-oil production. J. Anal. Appl. Pyrolysis. 2013;103:362–368. doi: 10.1016/j.jaap.2012.09.005. [DOI] [Google Scholar]
- 51.Lazzari E., et al. Production and chromatographic characterization of bio-oil from the pyrolysis of mango seed waste. Ind. Crops Prod. 2016;83:529–536. doi: 10.1016/j.indcrop.2015.12.073. [DOI] [Google Scholar]
- 52.Schnitzer M.I., Monreal C.M., Facey G.A., Fransham P.B. The conversion of chicken manure to biooil by fast pyrolysis I. Analyses of chicken manure, biooils and char by13C and1H NMR and FTIR spectrophotometry. J Environ Sci Health B. 2007;42(1):71–77. doi: 10.1080/03601230601020894. [DOI] [PubMed] [Google Scholar]
- 53.Chen D., Cen K., Cao X., Zhang J., Chen F., Zhou J. Upgrading of bio-oil via solar pyrolysis of the biomass pretreated with aqueous phase bio-oil washing, solar drying, and solar torrefaction. Bioresour. Technol. 2020;305(Jun) doi: 10.1016/j.biortech.2020.123130. [DOI] [PubMed] [Google Scholar]
- 54.Luo S., Guo J., Feng Y. Hydrogen-rich gas production from pyrolysis of wet sludge in situ steam agent. Int. J. Hydrogen Energy. 2017;42(29):18309–18314. doi: 10.1016/j.ijhydene.2017.04.165. [DOI] [Google Scholar]
- 55.Nandhini R., Berslin D., Sivaprakash B., Rajamohan N., Vo D.V.N. Thermochemical conversion of municipal solid waste into energy and hydrogen: a review. Environ. Chem. Lett. 2022;20(3):1645–1669. doi: 10.1007/s10311-022-01410-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ren S., Lei H., Wang L., Bu Q., Chen S., Wu J. Hydrocarbon and hydrogen-rich syngas production by biomass catalytic pyrolysis and bio-oil upgrading over biochar catalysts. RSC Adv. 2014;4(21) doi: 10.1039/c4ra00122b. [DOI] [Google Scholar]
- 57.Ma Z., Xiao R., Zhang H. Catalytic steam reforming of bio-oil model compounds for hydrogen-rich gas production using bio-char as catalyst. Int. J. Hydrogen Energy. 2017;42(6):3579–3585. doi: 10.1016/j.ijhydene.2016.11.107. [DOI] [Google Scholar]
- 58.Alipour Moghadam R., Yusup S., Azlina W., Nehzati S., Tavasoli A. Investigation on syngas production via biomass conversion through the integration of pyrolysis and air-steam gasification processes. Energy Convers. Manag. 2014;87:670–675. doi: 10.1016/j.enconman.2014.07.065. [DOI] [Google Scholar]
- 59.Liu Q., et al. High H2/CO ratio syngas production from chemical looping co-gasification of biomass and polyethylene with CaO/Fe2O3 oxygen carrier. Energy Convers. Manag. 2019;199(May) doi: 10.1016/j.enconman.2019.111951. [DOI] [Google Scholar]
- 60.Choi Y.K., Ko J.H., Kim J.S. A new type three-stage gasification of dried sewage sludge: effects of equivalence ratio, weight ratio of activated carbon to feed, and feed rate on gas composition and tar, NH3, and H2S removal and results of approximately 5 h gasification. Energy. 2017;118:139–146. doi: 10.1016/j.energy.2016.12.032. [DOI] [Google Scholar]
- 61.Song H., et al. Recent development of biomass gasification for H2 rich gas production. Applications in Energy and Combustion Science. 2022;10(March) doi: 10.1016/j.jaecs.2022.100059. [DOI] [Google Scholar]
- 62.Chintala V. Elsevier Ltd; Jul. 01, 2018. Production, Upgradation and Utilization of Solar Assisted Pyrolysis Fuels from Biomass – A Technical Review. [DOI] [Google Scholar]
- 63.Chintala V., Kumar S., Pandey J.K., Sharma A.K., Kumar S. Solar thermal pyrolysis of non-edible seeds to biofuels and their feasibility assessment. Energy Convers. Manag. Dec. 2017;153:482–492. doi: 10.1016/j.enconman.2017.10.029. [DOI] [Google Scholar]
- 64.Al-Salem S.M., Chandrasekaran S.R., Dutta A., Sharma B.K. Study of the fuel properties of extracted oils obtained from low and linear low density polyethylene pyrolysis. Fuel. 2021;304(Nov) doi: 10.1016/j.fuel.2021.121396. [DOI] [Google Scholar]
- 65.Cao X., Sun S., Sun R. Application of biochar-based catalysts in biomass upgrading: a review. RSC Adv. 2017;7(77):48793–48805. doi: 10.1039/C7RA09307A. [DOI] [Google Scholar]
- 66.Dudziak M., Werle S., Marszałek A., Sobek S., Magdziarz A. Comparative assessment of the biomass solar pyrolysis biochars combustion behavior and zinc Zn(II) adsorption. Energy. Dec. 2022;261 doi: 10.1016/j.energy.2022.125360. [DOI] [Google Scholar]
- 67.Ahmed M.J., Hameed B.H. Elsevier Ltd; Aug. 20, 2020. Insight into the Co-pyrolysis of Different Blended Feedstocks to Biochar for the Adsorption of Organic and Inorganic Pollutants: A Review. [DOI] [Google Scholar]
- 68.Mohan A., Dutta S., Madav V. Characterization and upgradation of crude tire pyrolysis oil (CTPO) obtained from a rotating autoclave reactor. Fuel. Aug. 2019;250:339–351. doi: 10.1016/j.fuel.2019.03.139. [DOI] [Google Scholar]
- 69.Wang G., Dai G., Ding S., Wu J., Wang S. A new insight into pyrolysis mechanism of three typical actual biomass: the influence of structural differences on pyrolysis process. J. Anal. Appl. Pyrolysis. 2021;156(Jun) doi: 10.1016/j.jaap.2021.105184. [DOI] [Google Scholar]
- 70.Liu J., et al. Biomass pyrolysis mechanism for carbon-based high-value products. Proc. Combust. Inst. Jan. 2023;39(3):3157–3181. doi: 10.1016/j.proci.2022.09.063. [DOI] [Google Scholar]
- 71.Yang H., Yan R., Chen H., Zheng C., Lee D.H., Liang D.T. In-depth investigation of biomass pyrolysis based on three major components: hemicellulose, cellulose and lignin. Energy & Fuels. 2006;20(17):388–393. doi: 10.1021/ie1025453. 2006. [DOI] [Google Scholar]
- 72.Lin Y.C., Cho J., Tompsett G.A., Westmoreland P.R., Huber G.W. Kinetics and mechanism of cellulose pyrolysis. J. Phys. Chem. C. 2009;113(46):20097–20107. doi: 10.1021/jp906702p. [DOI] [Google Scholar]
- 73.A. Cuoci et al., “A General Mathematical Model of Biomass Devolatilization Note 1. Lumped Kinetic Models of Cellulose, Hemicellulose and Lignin.”.
- 74.Cuoci A., et al. 2007. https://www.researchgate.net/publication/242165991 (A General Mathematical Model of Biomass Devolatilization Note 2. Detailed Kinetics of Volatile Species). [Online]. Available: [Google Scholar]
- 75.Peters J.F., Banks S.W., Bridgwater A.V., Dufour J. A kinetic reaction model for biomass pyrolysis processes in Aspen Plus. Appl. Energy. 2017;188:595–603. doi: 10.1016/j.apenergy.2016.12.030. [DOI] [Google Scholar]
- 76.Ranzi E., et al. Chemical kinetics of biomass pyrolysis. Energy Fuel. Nov. 2008;22(6):4292–4300. doi: 10.1021/ef800551t. [DOI] [Google Scholar]
- 77.Fu X., Wang X., Li Y., Xin Y., Li S. Enhancing and upgrading bio-oil during catalytic pyrolysis of cellulose: the synergistic effect of potassium cation and different anions impregnation. Fuel Process. Technol. Oct. 2019;193:338–347. doi: 10.1016/j.fuproc.2019.05.022. [DOI] [Google Scholar]
- 78.Fukutome A., Kawamoto H., Saka S. Processes forming gas, tar, and coke in cellulose gasification from gas-phase reactions of levoglucosan as intermediate. ChemSusChem. 2015;8(13):2240–2249. doi: 10.1002/cssc.201500275. [DOI] [PubMed] [Google Scholar]
- 79.Yu H., Zhang Z., Li Z., Chen D. Characteristics of tar formation during cellulose, hemicellulose and lignin gasification. Fuel. 2014;118:250–256. doi: 10.1016/j.fuel.2013.10.080. [DOI] [Google Scholar]
- 80.Font Palma C. Model for biomass gasification including tar formation and evolution. Energy & Fuels. 2013;27:2693–2702. doi: 10.1021/ef4004297. [DOI] [Google Scholar]
- 81.Dufour A., Masson E., Girods P., Rogaume Y., Zoulalian A. Evolution of aromatic tar composition in relation to methane and ethylene from biomass pyrolysis-gasification. Energy Fuel. 2011;25(9):4182–4189. doi: 10.1021/ef200846g. [DOI] [Google Scholar]
- 82.Zwart R.W.R., Vreugdenhil B.J. 2009. Tar Formation in Pyrolysis and Gasification; p. 37. June. [Google Scholar]
- 83.Zhang X., Yang W., Blasiak W. Kinetics study on thermal dissociation of levoglucosan during cellulose pyrolysis. Fuel. 2013;109:476–483. doi: 10.1016/j.fuel.2013.03.035. [DOI] [Google Scholar]
- 84.Leng E., Guo Y., Chen J., Liu S., E J., Xue Y. Elsevier Ltd; Feb. 01, 2022. A Comprehensive Review on Lignin Pyrolysis: Mechanism, Modeling and the Effects of Inherent Metals in Biomass. [DOI] [Google Scholar]
- 85.Wang R., Ben H. Accelerated aging process of bio-oil model compounds: a mechanism study. Front. Energy Res. May 2020;8 doi: 10.3389/fenrg.2020.00079. [DOI] [Google Scholar]
- 86.Cai J., Wu W., Liu R. Elsevier Ltd; 2014. An Overview of Distributed Activation Energy Model and its Application in the Pyrolysis of Lignocellulosic Biomass. [DOI] [Google Scholar]
- 87.Sobek S., Werle S. Kinetic modelling of waste wood devolatilization during pyrolysis based on thermogravimetric data and solar pyrolysis reactor performance. Fuel. 2020;261(Feb) doi: 10.1016/j.fuel.2019.116459. [DOI] [Google Scholar]
- 88.Lim A.C.R., Chin B.L.F., Jawad Z.A., Hii K.L. Procedia Engineering. Elsevier Ltd; 2016. Kinetic analysis of rice husk pyrolysis using kissinger-akahira-sunose (KAS) method; pp. 1247–1251. [DOI] [Google Scholar]
- 89.L. Fagbemi, L. Khezami, and R. Capart, “Pyrolysis products from different biomasses: application to the thermal cracking of tar.” [Online]. Available: www.elsevier.com/locate/apenergy.
- 90.Horne P.A., Williams P.T. 1996. Influence of Temperature on the Products from the Flash Pyrolysis of Biomass. [Google Scholar]
- 91.Garcia-Perez M., et al. Fast pyrolysis of oil mallee woody biomass: effect of temperature on the yield and quality of pyrolysis products. Ind. Eng. Chem. Res. Mar. 2008;47(6):1846–1854. doi: 10.1021/ie071497p. [DOI] [Google Scholar]
- 92.Amini E., Safdari M.S., DeYoung J.T., Weise D.R., Fletcher T.H. Characterization of pyrolysis products from slow pyrolysis of live and dead vegetation native to the southern United States. Fuel. Jan. 2019;235:1475–1491. doi: 10.1016/j.fuel.2018.08.112. [DOI] [Google Scholar]
- 93.Kaur R., Tarun Kumar V., Krishna B.B., Bhaskar T. Characterization of slow pyrolysis products from three different cashew wastes. Bioresour. Technol. May 2023;376 doi: 10.1016/j.biortech.2023.128859. [DOI] [PubMed] [Google Scholar]
- 94.Onay O., Kockar O.M. Slow, fast and flash pyrolysis of rapeseed. Renew. Energy. 2003;28(15):2417–2433. doi: 10.1016/S0960-1481(03)00137-X. [DOI] [Google Scholar]
- 95.Demirbaş A. Analysis of liquid products from biomass via flash pyrolysis. Energy Sources. Apr. 2002;24(4):337–345. doi: 10.1080/00908310252888718. [DOI] [Google Scholar]
- 96.Pokorna E., Postelmans N., Jenicek P., Schreurs S., Carleer R., Yperman J. Study of bio-oils and solids from flash pyrolysis of sewage sludges. Fuel. Aug. 2009;88(8):1344–1350. doi: 10.1016/j.fuel.2009.02.020. [DOI] [Google Scholar]
- 97.Yorgun S., ¸ Ensö Z S.S., Koçkar M. 2001. www.elsevier.com/locate/jaap (Flash Pyrolysis of Sunflower Oil Cake for Production of Liquid Fuels). [Online]. Available: [Google Scholar]
- 98.Ndukwu M.C., Horsfall I.T., Ubouh E.A., Orji F.N., Ekop I.E., Ezejiofor N.R. Review of solar-biomass pyrolysis systems: focus on the configuration of thermal-solar systems and reactor orientation. Journal of King Saud University - Engineering Sciences. 2021;33(6):413–423. doi: 10.1016/j.jksues.2020.05.004. [DOI] [Google Scholar]
- 99.Abanades S., Rodat S., Boujjat H. Solar thermochemical green fuels production: a review of biomass pyro-gasification, solar reactor concepts and modelling methods. Energies. 2021;14(5) doi: 10.3390/en14051494. [DOI] [Google Scholar]
- 100.Bridgwater A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy. 2012;38:68–94. doi: 10.1016/j.biombioe.2011.01.048. [DOI] [Google Scholar]
- 101.Zeng K., Minh D.P., Gauthier D., Weiss-Hortala E., Nzihou A., Flamant G. The effect of temperature and heating rate on char properties obtained from solar pyrolysis of beech wood. Bioresour. Technol. Apr. 2015;182:114–119. doi: 10.1016/j.biortech.2015.01.112. [DOI] [PubMed] [Google Scholar]
- 102.Zeng K., Gauthier D., Minh D.P., Weiss-Hortala E., Nzihou A., Flamant G. Characterization of solar fuels obtained from beech wood solar pyrolysis. Fuel. Jan. 2017;188:285–293. doi: 10.1016/j.fuel.2016.10.036. [DOI] [Google Scholar]
- 103.Zeng K., et al. Characterization of char generated from solar pyrolysis of heavy metal contaminated biomass. Energy. 2020;206(Sep) doi: 10.1016/j.energy.2020.118128. [DOI] [Google Scholar]
- 104.Li R., et al. Product distribution from solar pyrolysis of agricultural and forestry biomass residues. Renew. Energy. Apr. 2016;89:27–35. doi: 10.1016/j.renene.2015.11.071. [DOI] [Google Scholar]
- 105.Morales S., Miranda R., Bustos D., Cazares T., Tran H. Solar biomass pyrolysis for the production of bio-fuels and chemical commodities. J. Anal. Appl. Pyrolysis. Sep. 2014;109:65–78. doi: 10.1016/j.jaap.2014.07.012. [DOI] [Google Scholar]
- 106.Zeaiter J., Ahmad M.N., Rooney D., Samneh B., Shammas E. Design of an automated solar concentrator for the pyrolysis of scrap rubber. Energy Convers. Manag. Jun. 2015;101:118–125. doi: 10.1016/j.enconman.2015.05.019. [DOI] [Google Scholar]
- 107.Hijazi A., Boyadjian C., Ahmad M.N., Zeaiter J. Solar pyrolysis of waste rubber tires using photoactive catalysts. Waste Management. Jul. 2018;77:10–21. doi: 10.1016/j.wasman.2018.04.044. [DOI] [PubMed] [Google Scholar]
- 108.Arribas L., et al. Solar-driven pyrolysis and gasification of low-grade carbonaceous materials. Int. J. Hydrogen Energy. May 2017;42(19):13598–13606. doi: 10.1016/j.ijhydene.2017.02.026. [DOI] [Google Scholar]
- 109.Chen D., Cen K., Chen F., Zhang Y. Solar pyrolysis of cotton stalks: combined effects of torrefaction pretreatment and HZSM-5 zeolite on the bio-fuels upgradation. Energy Convers. Manag. 2022;261(Jun) doi: 10.1016/j.enconman.2022.115640. [DOI] [Google Scholar]
- 110.Weldekidan H., Strezov V., Town G., Kan T. Production and analysis of fuels and chemicals obtained from rice husk pyrolysis with concentrated solar radiation. Fuel. Dec. 2018;233:396–403. doi: 10.1016/j.fuel.2018.06.061. [DOI] [Google Scholar]
- 111.Weldekidan H., Strezov V., Kan T., Kumar R., He J., Town G. Solar assisted catalytic pyrolysis of chicken-litter waste with in-situ and ex-situ loading of CaO and char. Fuel. Jun. 2019;246:408–416. doi: 10.1016/j.fuel.2019.02.135. [DOI] [Google Scholar]
- 112.Weldekidan H., et al. Distribution of solar pyrolysis products and product gas composition produced from agricultural residues and animal wastes at different operating parameters. Renew. Energy. May 2020;151:1102–1109. doi: 10.1016/j.renene.2019.11.107. [DOI] [Google Scholar]
- 113.Zhong D., et al. Solar pyrolysis of biomass - part II: the physicochemical structure evolution of char. Fuel. 2023;333(Feb) doi: 10.1016/j.fuel.2022.126474. [DOI] [Google Scholar]
- 114.Sobek S., Werle S. Solar pyrolysis of waste biomass: a comparative study of products distribution, in situ heating behavior, and application of model-free kinetic predictions. Fuel. May 2021;292 doi: 10.1016/j.fuel.2021.120365. [DOI] [Google Scholar]
- 115.Rony A.H., et al. A novel solar powered biomass pyrolysis reactor for producing fuels and chemicals. J. Anal. Appl. Pyrolysis. Jun. 2018;132:19–32. doi: 10.1016/j.jaap.2018.03.020. [DOI] [Google Scholar]
- 116.Martins M.P.B., Hori C.E., Barrozo M.A.S., Vieira L.G.M. Solar pyrolysis of spirulina platensis assisted by Fresnel lens using hydrocalumite-type precursors. Energies. Oct. 2022;15(20) doi: 10.3390/en15207590. [DOI] [Google Scholar]
- 117.Barbosa J.M., Rossi R.A.S., Andrade L.A., Barrozo M.A.S., Vieira L.G.M. A study of optimization of solar pyrolysis and catalyst recovery and reuse. Energy Convers. Manag. 2021;237(Jun) doi: 10.1016/j.enconman.2021.114094. [DOI] [Google Scholar]
- 118.Aparecida da Silveira Rossi R., Barbosa J.M., Antonio de Souza Barrozo M., Martins Vieira L.G. Solar assisted catalytic thermochemical processes: pyrolysis and hydropyrolysis of Chlamydomonas reinhardtii microalgae. Renew. Energy. Jun. 2021;170:669–682. doi: 10.1016/j.renene.2021.02.034. [DOI] [Google Scholar]
- 119.Aspiazu-Méndez A., Cisneros-Cárdenas N.A., Pérez-Rábago C., Pat-Espadas A.M., Manzini-Poli F., Estrada C.A. Analysis of the solar pyrolysis of a walnut shell: insights into the thermal behavior of biomaterials. Energies. Mar. 2024;17(6) doi: 10.3390/en17061435. [DOI] [Google Scholar]
- 120.Campos Roldán C.A., et al. Metal-free electrocatalysts obtained from agave waste by solar pyrolysis for oxygen reduction reaction. Int. J. Hydrogen Energy. Jul. 2021;46(51):26101–26109. doi: 10.1016/j.ijhydene.2020.12.095. [DOI] [Google Scholar]
- 121.Lobato-Peralta D.R., et al. Sustainable production of self-activated bio-derived carbons through solar pyrolysis for their use in supercapacitors. J. Anal. Appl. Pyrolysis. 2020;150(Sep) doi: 10.1016/j.jaap.2020.104901. [DOI] [Google Scholar]
- 122.Gongxiang S., et al. The heating rate and final temperature impacts on the coconut shell char structure characteristics during photo-thermal pyrolysis. J. Anal. Appl. Pyrolysis. 2022;167(Oct) doi: 10.1016/j.jaap.2022.105695. [DOI] [Google Scholar]
- 123.Weldekidan H., Strezov V., Town G. Review of solar energy for biofuel extraction. Renew. Sustain. Energy Rev. 2018;88:184–192. doi: 10.1016/j.rser.2018.02.027. [DOI] [Google Scholar]
- 124.Hamed A.S.A., Yusof N.I.F.M., Yahya M.S., Cardozo E., Munajat N.F. Concentrated solar pyrolysis for oil palm biomass: an exploratory review within the Malaysian context. Renew. Sustain. Energy Rev. 2023;188(September) doi: 10.1016/j.rser.2023.113834. [DOI] [Google Scholar]
- 125.Nzihou A., Flamant G., Stanmore B. Synthetic fuels from biomass using concentrated solar energy - a review. Energy. 2012;42(1):121–131. doi: 10.1016/j.energy.2012.03.077. [DOI] [Google Scholar]
- 126.Chintala V. Production, upgradation and utilization of solar assisted pyrolysis fuels from biomass – a technical review. Renew. Sustain. Energy Rev. 2018;90(x):120–130. doi: 10.1016/j.rser.2018.03.066. [DOI] [Google Scholar]
- 127.Piatkowski N., Wieckert C., Weimer A.W., Steinfeld A. Solar-driven gasification of carbonaceous feedstock - a review. Energy Environ. Sci. 2011;4(1):73–82. doi: 10.1039/c0ee00312c. [DOI] [Google Scholar]
- 128.Parthasarathy P., Al-Ansari T., Mackey H.R., Sheeba Narayanan K., McKay G. A review on prominent animal and municipal wastes as potential feedstocks for solar pyrolysis for biochar production. Fuel. 2022;316(December 2021) doi: 10.1016/j.fuel.2022.123378. [DOI] [Google Scholar]
- 129.Hamilton J., Seyedmahmoudian M., Jamei E., Horan B., Stojcevski A. A systematic review of solar driven waste to fuel pyrolysis technology for the Australian state of Victoria. Energy Rep. 2020;6:3212–3229. doi: 10.1016/j.egyr.2020.11.039. [DOI] [Google Scholar]
- 130.Rahman M.A., Parvej A.M., Aziz M.A. Concentrating technologies with reactor integration and effect of process variables on solar assisted pyrolysis: a critical review. Therm. Sci. Eng. Prog. 2021;25(May) doi: 10.1016/j.tsep.2021.100957. [DOI] [Google Scholar]
- 131.Global Solar Atlas.” [Online]. Available: https://globalsolaratlas.info/detail?c=28.971803,-110.99762,11&s=29.098177,-110.954361&m=site.
- 132.L. ; M. O. ; S. A. Ferrari, Transición energética justa y sustentable: Contexto y estrategias para México. Fondo de Cultura Económica y Consejo Nacional de Humanidades, Ciencias y Tecnologías (Conahcyt), 2023.
- 133.UNESCO Institute for Statistics, “Research and development expenditure (% GDP),” https://data.worldbank.org/indicator/GB.XPD.RSDV.GD.ZS?skipRedirection=true&view=map&year=2022.
- 134.Saxe J.P., et al. Just or bust? Energy justice and the impacts of siting solar pyrolysis biochar production facilities. Energy Res Soc Sci. Dec. 2019;58 doi: 10.1016/j.erss.2019.101259. [DOI] [Google Scholar]
- 135.Martínez J.D. Proposing Pyrolysis for a Circular Economy. Elsevier Ltd; Jul. 01, 2021. “An overview of the end-of-life tires status in some Latin American countries. [DOI] [Google Scholar]
- 136.Díaz-Ferrán G., Chudinzow D., Kracht W., Eltrop L. AIP Conference Proceedings. American Institute of Physics Inc; Nov. 2018. Solar-powered pyrolysis of scrap rubber from mining truck end-of-life tires - a case study for the mining industry in the Atacama Desert, Chile. [DOI] [Google Scholar]
- 137.Amin M., Narayana M., Asadullah, Kamran sami S., Khan M.N., Sultan S.H. Bioenergy production from waste mango seed shell by thermo-chemical conversion and its importance for mango fruit processing industry. Journal.Buitms.Edu.Pk. 2019;1(9):48–52. http://journal.buitms.edu.pk/j/index.php/bj/article/view/272 [Online]. Available: [Google Scholar]
- 138.Perea-Moreno A.J., Perea-Moreno M.Á., Dorado M.P., Manzano-Agugliaro F. Mango stone properties as biofuel and its potential for reducing CO 2 emissions. J. Clean. Prod. 2018;190:53–62. doi: 10.1016/j.jclepro.2018.04.147. [DOI] [Google Scholar]
- 139.Perea-Moreno A.J., Aguilera-Ureña M.J., Manzano-Agugliaro F. Fuel properties of avocado stone. Fuel. Dec. 2016;186:358–364. doi: 10.1016/j.fuel.2016.08.101. [DOI] [Google Scholar]
- 140.Rahman M.A., Parvej A.M., Aziz M.A. Concentrating technologies with reactor integration and effect of process variables on solar assisted pyrolysis: a critical review. Therm. Sci. Eng. Prog. Oct. 2021;25 doi: 10.1016/j.tsep.2021.100957. [DOI] [Google Scholar]
- 141.Kodama T., Gokon N., Enomoto S.I., Itoh S., Hatamachi T. Coal coke gasification in a windowed solar chemical reactor for beam-down optics. Journal of Solar Energy Engineering, Transactions of the ASME. 2010;132(4) doi: 10.1115/1.4002081. [DOI] [Google Scholar]
- 142.Kenarsari S.D., Zheng Y. Fast pyrolysis of biomass pellets using concentrated solar radiation: a numerical study. Journal of Solar Energy Engineering, Transactions of the ASME. 2014;136(4):1–7. doi: 10.1115/1.4027266. [DOI] [Google Scholar]
- 143.Wu H., Gauthier D., Yu Y., Gao X., Flamant G. Solar-thermal pyrolysis of mallee wood at high temperatures. Energy Fuel. 2018;32(4):4350–4356. doi: 10.1021/acs.energyfuels.7b03091. [DOI] [Google Scholar]
- 144.Beattie W.H., Berjoan R., Coutures J.P. 1983. High-temperature Solar Pyrolysis of Coal. [DOI] [Google Scholar]
- 145.Lobato-Peralta D.R., et al. Sustainable production of self-activated bio-derived carbons through solar pyrolysis for their use in supercapacitors. J. Anal. Appl. Pyrolysis. 2020;150(June) doi: 10.1016/j.jaap.2020.104901. [DOI] [Google Scholar]
- 146.Lobato-Peralta D.R., et al. Activated carbons obtained by environmentally friendly activation using solar energy for their use in neutral electrolyte supercapacitors. J. Energy Storage. 2022;52(May) doi: 10.1016/j.est.2022.104888. [DOI] [Google Scholar]
- 147.Soria J., Li R., Flamant G., Mazza G.D. Influence of pellet size on product yields and syngas composition during solar-driven high temperature fast pyrolysis of biomass. J. Anal. Appl. Pyrolysis. 2019;140(January):299–311. doi: 10.1016/j.jaap.2019.04.007. [DOI] [Google Scholar]
- 148.Zeng K., Gauthier D., Minh D.P., Weiss-Hortala E., Nzihou A., Flamant G. Characterization of solar fuels obtained from beech wood solar pyrolysis. Fuel. 2017;188:285–293. doi: 10.1016/j.fuel.2016.10.036. [DOI] [Google Scholar]
- 149.Zeng K., Flamant G., Gauthier D., Guillot E. Solar pyrolysis of wood in a lab-scale solar reactor: influence of temperature and sweep gas flow rate on products distribution. Energy Proc. 2015;69:1849–1858. doi: 10.1016/j.egypro.2015.03.163. [DOI] [Google Scholar]
- 150.Soria J., Zeng K., Asensio D., Gauthier D., Flamant G., Mazza G. Comprehensive CFD modelling of solar fast pyrolysis of beech wood pellets. Fuel Process. Technol. 2017;158:226–237. doi: 10.1016/j.fuproc.2017.01.006. [DOI] [Google Scholar]
- 151.Ayala-Cortés A., et al. Exploring the influence of solar pyrolysis operation parameters on characteristics of carbon materials. J. Anal. Appl. Pyrolysis. 2019;140(February):290–298. doi: 10.1016/j.jaap.2019.04.006. [DOI] [Google Scholar]
- 152.Campos Roldán C.A., et al. Metal-free electrocatalysts obtained from agave waste by solar pyrolysis for oxygen reduction reaction. Int. J. Hydrogen Energy. 2021;46(51):26101–26109. doi: 10.1016/j.ijhydene.2020.12.095. [DOI] [Google Scholar]
- 153.Zeng K., Gauthier D., Lu J., Flamant G. Parametric study and process optimization for solar pyrolysis of beech wood. Energy Convers. Manag. 2015;106:987–998. doi: 10.1016/j.enconman.2015.10.039. [DOI] [Google Scholar]
- 154.Li R., et al. Product distribution from solar pyrolysis of agricultural and forestry biomass residues. Renew. Energy. 2016;89:27–35. doi: 10.1016/j.renene.2015.11.071. [DOI] [Google Scholar]
- 155.Rahman M.A. Valorizing of weeds algae through the solar assisted pyrolysis: effects of dependable parameters on yields and characterization of products. Renew. Energy. 2020;147:937–946. doi: 10.1016/j.renene.2019.09.046. [DOI] [Google Scholar]
- 156.Zeng K., Gauthier D., Li R., Flamant G. Solar pyrolysis of beech wood: effects of pyrolysis parameters on the product distribution and gas product composition. Energy. 2015;93:1648–1657. doi: 10.1016/j.energy.2015.10.008. [DOI] [Google Scholar]
- 157.Zeng K., Gauthier D., Li R., Flamant G. Combined effects of initial water content and heating parameters on solar pyrolysis of beech wood. Energy. 2017;125:552–561. doi: 10.1016/j.energy.2017.02.173. [DOI] [Google Scholar]
- 158.Zeng K., Soria J., Gauthier D., Mazza G., Flamant G. Modeling of beech wood pellet pyrolysis under concentrated solar radiation. Renew. Energy. 2016;99:721–729. doi: 10.1016/j.renene.2016.07.051. [DOI] [Google Scholar]
- 159.Zeng K., et al. Solar pyrolysis of heavy metal contaminated biomass for gas fuel production. Energy. 2019;187 doi: 10.1016/j.energy.2019.116016. [DOI] [Google Scholar]
- 160.Weldekidan H., et al. Distribution of solar pyrolysis products and product gas composition produced from agricultural residues and animal wastes at different operating parameters. Renew. Energy. 2020;151:1102–1109. doi: 10.1016/j.renene.2019.11.107. [DOI] [Google Scholar]
- 161.Zeng K., Minh D.P., Gauthier D., Weiss-Hortala E., Nzihou A., Flamant G. The effect of temperature and heating rate on char properties obtained from solar pyrolysis of beech wood. Bioresour. Technol. 2015;182:114–119. doi: 10.1016/j.biortech.2015.01.112. [DOI] [PubMed] [Google Scholar]
- 162.Rahman M.A., Aziz M.A. Solar pyrolysis of scrap tire: optimization of operating parameters. J. Mater. Cycles Waste Manag. 2018;20(2):1207–1215. doi: 10.1007/s10163-017-0686-1. [DOI] [Google Scholar]
- 163.Zeng K., et al. Characterization of char generated from solar pyrolysis of heavy metal contaminated biomass. Energy. 2020;206 doi: 10.1016/j.energy.2020.118128. [DOI] [Google Scholar]
- 164.Emran M., El-Gamal E.H., Mokhiamar O., Elsamni O., Rashad M. A novel solar disk chamber reactor for agricultural waste recycling and biochar production. Clean Technol. Environ. Policy. 2024;26(2):467–479. doi: 10.1007/s10098-023-02635-8. [DOI] [Google Scholar]
- 165.Weldekidan H., Strezov V., Town G., Kan T. Production and analysis of fuels and chemicals obtained from rice husk pyrolysis with concentrated solar radiation. Fuel. 2018;233(June):396–403. doi: 10.1016/j.fuel.2018.06.061. [DOI] [Google Scholar]
- 166.Singh Y., Singla A., Singh N.K., Sharma A. Production and feasibility characterization of bio-oil from jojoba seed–based biomass through solar thermal energy pyrolysis process. Biomass Convers Biorefin. 2024;14(4):5225–5237. doi: 10.1007/s13399-022-02686-9. [DOI] [Google Scholar]
- 167.Mondal S., Pandey J.K., Kumar S. Solar thermal pyrolysis of karanja seeds for a sustainable approach for liquid biofuel utilization. Nat. Environ. Pollut. Technol. 2019;18(3):941–948. [Google Scholar]
- 168.Weldekidan H., Strezov V., Kan T., Town G. Waste to energy conversion of chicken litter through a solar-driven pyrolysis process. Energy & Fuels. Apr. 2018;32(4):4341–4349. doi: 10.1021/acs.energyfuels.7b02977. [DOI] [Google Scholar]
- 169.de Souza P.H.C., Rocha S.D.F., de Rezende D.B. Luffa cylindrica slow pyrolysis and solar pyrolysis: impact of temperature and heating rate on biochar properties and iodine adsorption performance. Waste Biomass Valorization. 2023;14(5):1753–1768. doi: 10.1007/s12649-022-01954-z. [DOI] [Google Scholar]
- 170.Barbosa J.M., Rossi R.A.S., Andrade L.A., Barrozo M.A.S., Vieira L.G.M. A study of optimization of solar pyrolysis and catalyst recovery and reuse. Energy Convers. Manag. 2021;237(January) doi: 10.1016/j.enconman.2021.114094. [DOI] [Google Scholar]
- 171.Aparecida da Silveira Rossi R., Barbosa J.M., Antonio de Souza Barrozo M., Martins Vieira L.G. Solar assisted catalytic thermochemical processes: pyrolysis and hydropyrolysis of Chlamydomonas reinhardtii microalgae. Renew. Energy. 2021;170:669–682. doi: 10.1016/j.renene.2021.02.034. [DOI] [Google Scholar]
- 172.Rodríguez-Alejandro D.A., et al. Experimental and numerical investigation on a solar-driven torrefaction reactor using woody waste (Ashe Juniper) Energy Convers. Manag. 2023;288(April) doi: 10.1016/j.enconman.2023.117114. [DOI] [Google Scholar]
- 173.Chen D., Cen K., Chen F., Zhang Y. Solar pyrolysis of cotton stalks: combined effects of torrefaction pretreatment and HZSM-5 zeolite on the bio-fuels upgradation. Energy Convers. Manag. 2022;261 doi: 10.1016/j.enconman.2022.115640. [DOI] [Google Scholar]
- 174.Parvej A.M., Rahman M.A., Reza K.M.A. The combined effect of solar assisted torrefaction and pyrolysis on the production of valuable chemicals obtained from water hyacinth biomass. Cleaner Waste Systems. 2022;3(August) doi: 10.1016/j.clwas.2022.100027. [DOI] [Google Scholar]
- 175.Andrade L.A., Barrozo M.A.S., Vieira L.G.M. Catalytic solar pyrolysis of microalgae Chlamydomonas reinhardtii. Sol. Energy. 2018;173(August):928–938. doi: 10.1016/j.solener.2018.08.035. [DOI] [Google Scholar]
- 176.Chintala V., Kumar S., Pandey J.K., Sharma A.K., Kumar S. Solar thermal pyrolysis of non-edible seeds to biofuels and their feasibility assessment. Energy Convers. Manag. 2017;153(August):482–492. doi: 10.1016/j.enconman.2017.10.029. [DOI] [Google Scholar]
- 177.Barbosa J.M., Andrade L.A., Vieira L.G.M., Barrozo M.A.S. Multi-response optimization of bio-oil production from catalytic solar pyrolysis of Spirulina platensis. J. Energy Inst. 2020;93(4):1313–1323. doi: 10.1016/j.joei.2019.12.001. [DOI] [Google Scholar]
- 178.Chen D., Cen K., Cao X., Zhang J., Chen F., Zhou J. Upgrading of bio-oil via solar pyrolysis of the biomass pretreated with aqueous phase bio-oil washing, solar drying, and solar torrefaction. Bioresour. Technol. 2020;305(January) doi: 10.1016/j.biortech.2020.123130. [DOI] [PubMed] [Google Scholar]
- 179.Caputo C., Mašek O. Spear (Solar pyrolysis energy access reactor): theoretical design and evaluation of a small-scale low-cost pyrolysis unit for implementation in rural communities. Energies. 2021;14(8) doi: 10.3390/en14082189. [DOI] [Google Scholar]
- 180.Li R., et al. Comparative study of process simulation, energy and exergy analyses of solar enhanced char-cycling biomass pyrolysis process. Energy Convers. Manag. 2024;302(January) doi: 10.1016/j.enconman.2024.118082. [DOI] [Google Scholar]
- 181.Novita S.A., et al. Parabolic dish solar pyrolysis for bio-oil production: performance and energy analysis. Journal of Medicinal and Pharmaceutical Chemistry Research. 2024;6(1):89–110. doi: 10.48309/jmpcr.2024.423505.1030. [DOI] [Google Scholar]
- 182.Huang D., et al. Evolution mechanisms of bio-oil from conventional and nitrogen-rich biomass during photo-thermal pyrolysis. Energy. 2023;282(April) doi: 10.1016/j.energy.2023.128813. [DOI] [Google Scholar]
- 183.Zhu H., Yi B., Hu H., Fan Q., Wang H., Yao H. The effects of char and potassium on the fast pyrolysis behaviors of biomass in an infrared-heating condition. Energy. 2021;214 doi: 10.1016/j.energy.2020.119065. [DOI] [Google Scholar]
- 184.Luo H., et al. Solar pyrolysis of waste plastics with photothermal catalysts for high-value products. Fuel Process. Technol. 2022;230(January) doi: 10.1016/j.fuproc.2022.107205. [DOI] [Google Scholar]
- 185.Shagali A.A., et al. Pyrolysis characteristics and kinetic parameters assessment of typical agricultural residues using high heating photothermal TGA. J. Anal. Appl. Pyrolysis. 2023;174(April) doi: 10.1016/j.jaap.2023.106109. [DOI] [Google Scholar]
- 186.Werle S., Sobek S., Kaczor Z., Ziółkowski Ł., Buliński Z., Dudziak M. vol. 137. 2019. Experimental and numerical analysis of the biomass innovativ solar pyrolysis process; pp. 1–6. (E3S Web of Conferences). [DOI] [Google Scholar]
- 187.Gongxiang S., et al. The heating rate and final temperature impacts on the coconut shell char structure characteristics during photo-thermal pyrolysis. J. Anal. Appl. Pyrolysis. 2022;167(September) doi: 10.1016/j.jaap.2022.105695. [DOI] [Google Scholar]
- 188.Zhong D., et al. Solar pyrolysis of biomass - part I: volatile evolution mechanism. Energy Convers. Manag. 2022;267(July) doi: 10.1016/j.enconman.2022.115951. [DOI] [Google Scholar]
- 189.Sobek S., Werle S. Solar pyrolysis of waste biomass: a comparative study of products distribution, in situ heating behavior, and application of model-free kinetic predictions. Fuel. 2021;292(October 2020) doi: 10.1016/j.fuel.2021.120365. [DOI] [Google Scholar]
- 190.Arribas L., et al. Solar-driven pyrolysis and gasification of low-grade carbonaceous materials. Int. J. Hydrogen Energy. 2017;42(19):13598–13606. doi: 10.1016/j.ijhydene.2017.02.026. [DOI] [Google Scholar]
- 191.Zhong D., et al. Solar pyrolysis of biomass - part II: the physicochemical structure evolution of char. Fuel. 2023;333(P2) doi: 10.1016/j.fuel.2022.126474. [DOI] [Google Scholar]
- 192.Wang T., et al. Carbon nanofibers prepared from solar pyrolysis of pinewood as binder-free electrodes for flexible supercapacitors. Cell Rep Phys Sci. 2020;1(6) doi: 10.1016/j.xcrp.2020.100079. [DOI] [Google Scholar]
- 193.Xie Y., et al. Solar pyrolysis of cotton stalk in molten salt for bio-fuel production. Energy. 2019;179:1124–1132. doi: 10.1016/j.energy.2019.05.055. [DOI] [Google Scholar]
- 194.Dudziak M., Werle S., Marszałek A., Sobek S., Magdziarz A. Comparative assessment of the biomass solar pyrolysis biochars combustion behavior and zinc Zn(II) adsorption. Energy. 2022;261(July) doi: 10.1016/j.energy.2022.125360. [DOI] [Google Scholar]
- 195.Aspiazu-m A., Aracely N., Carlos P., Pat-espadas A.M., Manzini-poli F., Estrada C.A. 2024. Analysis of the Solar Pyrolysis of a Walnut Shell : Insights into the Thermal Behavior of Biomaterials. [Google Scholar]
- 196.Wu H., Liu Q., Bai Z., Xie G., Zheng J. A distributed cogeneration system with a two-stage solar-driven biomass gasifier for heating, power and hydrogen in Northern China. Energy Proc. 2018;152:1057–1062. doi: 10.1016/j.egypro.2018.09.258. [DOI] [Google Scholar]
- 197.Luo H., et al. Feasibility of biochar preparation with solar pyrolysis method. E3S Web of Conferences. 2021;299:1–9. doi: 10.1051/e3sconf/202129903003. [DOI] [Google Scholar]
- 198.Uzakov G.N., Almardanov X.A. Heliopyrolysis of sunflower waste using a parabolic solar concentrator. Appl. Sol. Energy. 2024;60(1):171–177. doi: 10.3103/S0003701X24600140. [DOI] [Google Scholar]
- 199.Uzakov G.N., Almardanov X.A., Kodirov I.N., Aliyarova L.A. Modeling the heat balance of a solar concentrator heliopyrolysis device reactor. BIO Web Conf. 2023;71(2023) doi: 10.1051/bioconf/20237101098. [DOI] [Google Scholar]
- 200.Uzakov G.N., Novik A.V., Davlonov X.A., Almardanov X.A., Chuliev S.E. Heat and material balance of heliopyrolysis device. Energetika. Proceedings of CIS Higher Education Institutions and Power Engineering Associations. 2023;66(1):57–65. doi: 10.21122/1029-7448-2023-66-1-57-65. [DOI] [Google Scholar]
- 201.Morales S., Miranda R., Bustos D., Cazares T., Tran H. Solar biomass pyrolysis for the production of bio-fuels and chemical commodities. J. Anal. Appl. Pyrolysis. 2014;109:65–78. doi: 10.1016/j.jaap.2014.07.012. [DOI] [Google Scholar]
- 202.Hou X., et al. Migration and transformation of heavy metals in Chinese medicine residues during the process of traditional pyrolysis and solar pyrolysis. Chemosphere. 2022;293(November 2021):1–9. doi: 10.1016/j.chemosphere.2022.133658. [DOI] [PubMed] [Google Scholar]
- 203.Hosseini T., Zhang L. Process modeling and techno-economic analysis of a solar thermal aided low-rank coal drying-pyrolysis process. Fuel Process. Technol. 2021;220(May) doi: 10.1016/j.fuproc.2021.106896. [DOI] [Google Scholar]
- 204.Bashir M., Yu X., Hassan M., Makkawi Y. Modeling and performance analysis of biomass fast pyrolysis in a solar-thermal reactor. ACS Sustain. Chem. Eng. 2017;5(5):3795–3807. doi: 10.1021/acssuschemeng.6b02806. [DOI] [Google Scholar]
- 205.Giwa A., Yusuf A., Ajumobi O., Dzidzienyo P. Pyrolysis of date palm waste to biochar using concentrated solar thermal energy: economic and sustainability implications. Waste Management. 2019;93:14–22. doi: 10.1016/j.wasman.2019.05.022. [DOI] [PubMed] [Google Scholar]
- 206.Eldredge T.V. The feasibility of solar assisted pyrolysis of sewer sludge and its potential for CO2 emissions reductions. Energy. 2021;226 doi: 10.1016/j.energy.2021.120296. [DOI] [Google Scholar]
- 207.Xin Y., Xing X., Li X., Hong H. A biomass–solar hybrid gasification system by solar pyrolysis and PV– Solid oxide electrolysis cell for sustainable fuel production. Appl. Energy. 2024;356(July 2023) doi: 10.1016/j.apenergy.2023.122419. [DOI] [Google Scholar]
- 208.Li J., et al. Algae pyrolysis in molten NaOH–Na2CO3 for hydrogen production. Environ. Sci. Technol. Apr. 2023;57(16):6485–6493. doi: 10.1021/acs.est.3c01325. [DOI] [PubMed] [Google Scholar]
- 209.Ratchahat S., Kodama S., Tanthapanichakoon W., Sekiguchi H. Combined molten salt-Ni/Al2O3 as synergistic medium for high-quality syngas production. Chem. Eng. J. 2015;278:224–233. doi: 10.1016/j.cej.2014.09.109. [DOI] [Google Scholar]
- 210.Zuo H., et al. Design of a self-management solar pyrolysis packed-bed reactor by coupling thermal energy storage. Energy & Fuels. Feb. 2023;37(3):2134–2148. doi: 10.1021/acs.energyfuels.2c03884. [DOI] [Google Scholar]
- 211.Azevedo P., Costa P. Electrical driven pyrolysis reactor retrofit for indirect concentrated solar heat. Therm. Sci. Eng. Prog. 2024;50(April 2022) doi: 10.1016/j.tsep.2024.102524. [DOI] [Google Scholar]
- 212.Khudayar D., Mehrpooya M., Moosavian S.M.A. Hybrid biomass fast pyrolysis process and solar thermochemical energy storage system, investigation and process development. Arab J Sci Eng. 2024;49(6):8341–8362. doi: 10.1007/s13369-024-08848-3. [DOI] [Google Scholar]
- 213.Zeng K., et al. Molten salt pyrolysis of biomass: the mechanism of volatile reforming and pyrolysis. Energy. 2020;213 doi: 10.1016/j.energy.2020.118801. [DOI] [Google Scholar]
- 214.He J., et al. Investigation of the reaction mechanism regarding thermal treatment of biomass using molten salts: a kinetic study of small molecule gases formation. J. Environ. Chem. Eng. 2023;11(1):1–10. doi: 10.1016/j.jece.2022.109094. [DOI] [Google Scholar]
- 215.Li J., et al. Solar pyrolysis of algae in molten salt for capacitive carbon preparation. J. Clean. Prod. 2023;406(November 2022) doi: 10.1016/j.jclepro.2023.136898. [DOI] [Google Scholar]
- 216.Chudinzow G.D.-F.D., Willy Kracht Ludger Eltrop . 2018. Solar-Powered Pyrolysis of Scrap Rubber from Mining. [Google Scholar]
- 217.Peng J., et al. Transformation of nitrogen during solar pyrolysis of algae in molten salt. Fuel Process. Technol. 2023;242(December 2022) doi: 10.1016/j.fuproc.2023.107664. [DOI] [Google Scholar]
- 218.Maytorena V.M., Hinojosa J.F., Iriarte-Cornejo C., Orozco D.A. Biomass solar fast pyrolysis with a biomimetic mini heliostat field and thermal receiver for nitrogen heating. Energy Convers. Manag. 2023;291(April) doi: 10.1016/j.enconman.2023.117307. [DOI] [Google Scholar]
- 219.Ramos G., Pérez-Márquez D. Design of semi-static solar concentrator for charcoal Production. Energy Proc. 2014;57:2167–2175. doi: 10.1016/j.egypro.2014.10.183. [DOI] [Google Scholar]
- 220.Martins M.P.B., Hori C.E., Barrozo M.A.S., Vieira L.G.M. Solar pyrolysis of spirulina platensis assisted by Fresnel lens using hydrocalumite-type precursors. Energies. 2022;15(20) doi: 10.3390/en15207590. [DOI] [Google Scholar]
- 221.Sánchez M., Clifford B., Nixon J.D. Modelling and evaluating a solar pyrolysis system. Renew. Energy. 2018;116:630–638. doi: 10.1016/j.renene.2017.10.023. [DOI] [Google Scholar]
- 222.Zeaiter J., Azizi F., Lameh M., Milani D., Ismail H.Y., Abbas A. Waste tire pyrolysis using thermal solar energy: an integrated approach. Renew. Energy. 2018;123:44–51. doi: 10.1016/j.renene.2018.02.030. [DOI] [Google Scholar]
- 223.Grassmann H., et al. Solar biomass pyrolysis with the linear mirror II. Smart Grid Renew. Energy. 2015;6(7):179–186. doi: 10.4236/sgre.2015.67016. [DOI] [Google Scholar]
- 224.Ghenai C., Rasheed M.A., Alshamsi M.J., Alkamali M.A., Ahmad F.F., Inayat A. Design of hybrid solar photovoltaics/shrouded wind turbine power system for thermal pyrolysis of plastic waste. Case Stud. Therm. Eng. 2020;22(October) doi: 10.1016/j.csite.2020.100773. [DOI] [Google Scholar]
- 225.Holtze C., Boehling R. Batch or flow chemistry? – a current industrial opinion on process selection. Curr Opin Chem Eng. 2022;36:1–7. doi: 10.1016/j.coche.2022.100798. [DOI] [Google Scholar]
- 226.Levenspiel O. Chemical reaction engineering. Ind. Eng. Chem. Res. 1999;38:4140–4143. doi: 10.1021/ie990488g. [DOI] [Google Scholar]
- 227.Warnecke R. Gasification of biomass: comparison of fixed bed and fluidized bed gasifier. Biomass Bioenergy. 2000;18(6):489–497. doi: 10.1016/S0961-9534(00)00009-X. [DOI] [Google Scholar]
- 228.Ascher S., Watson I., You S. 2021. Machine Learning Methods for Modelling the Gasification and Pyrolysis of Biomass and Waste. [Google Scholar]
- 229.Arancibia-Bulnes C. a, et al. Proceedings of the SolarPACES 2011 Congress. 2011. Heliostat testing at a new facility in Sonora, Mexico. April 2016. [Google Scholar]
- 230.Cisneros-Cárdenas N.A., et al. Thermal experimental study of a volumetric receiver-reactor using a Mini-Solar furnace. Appl. Therm. Eng. 2023;234(Nov) doi: 10.1016/j.applthermaleng.2023.121276. [DOI] [Google Scholar]
- 231.Zeng K., Gauthier D., Soria J., Mazza G., Flamant G. Solar pyrolysis of carbonaceous feedstocks: a review. Sol. Energy. 2017;156:73–92. doi: 10.1016/j.solener.2017.05.033. [DOI] [Google Scholar]
- 232.Yan Y., Wang K., Clough P.T., Anthony E.J. Developments in calcium/chemical looping and metal oxide redox cycles for high-temperature thermochemical energy storage: a review. Fuel Process. Technol. 2020;199 doi: 10.1016/j.fuproc.2019.106280. June 2019. [DOI] [Google Scholar]
- 233.Turchi C.S., Stekli J., Bueno P.C. Elsevier Ltd; 2017. Concentrating Solar Power. [DOI] [Google Scholar]
- 234.Jayathunga D.S., Karunathilake H.P., Narayana M., Witharana S. Phase change material (PCM) candidates for latent heat thermal energy storage (LHTES) in concentrated solar power (CSP) based thermal applications - a review. Renew. Sustain. Energy Rev. 2024;189(PB) doi: 10.1016/j.rser.2023.113904. [DOI] [Google Scholar]
- 235.Santamaría Padilla A., Romero-Paredes Rubio H. A thermochemical energy storage materials review based on solid-gas reactions for supercritical CO2 solar tower power plant with a Brayton cycle. J. Energy Storage. 2023;73(PB) doi: 10.1016/j.est.2023.108906. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.