Abstract
The blood-brain interface poses formidable obstacles in addressing neurological conditions such as Alzheimer's, Multiple Sclerosis, brain cancers, and cerebrovascular accidents. Serving as a safeguard against potential threats in the blood, this barrier hinders direct drug delivery to affected cells, necessitating specialized transport mechanisms. Within the realm of nanotechnology, the creation of nanoscale carriers, including macromolecules such as polymers, lipids, and metallic nanoparticles, is gaining prominence. These carriers, tailored in diverse forms and sizes and enriched with specific functional groups for enhanced penetration and targeting, are capturing growing interest. This revised abstract explores the macromolecular dimension in understanding how nanoparticles interact with the blood-brain barrier. It re-evaluates the structure and function of the blood-brain barrier, highlighting macromolecular nanocarriers utilized in drug delivery to the brain. The discussion delves into the intricate pathways through which drugs navigate the blood-brain barrier, emphasizing the distinctive attributes of macromolecular nanocarriers. Additionally, it explores recent innovations in nanotechnology and unconventional approaches to drug delivery. Ultimately, the paper addresses the intricacies and considerations in developing macromolecular-based nanomedicines for the brain, aiming to advance the creation and evolution of nanomedicines for neurological ailments.
Keywords: Drug delivery, Blood-brain barrier, Lipid nanoparticles
1. Introduction
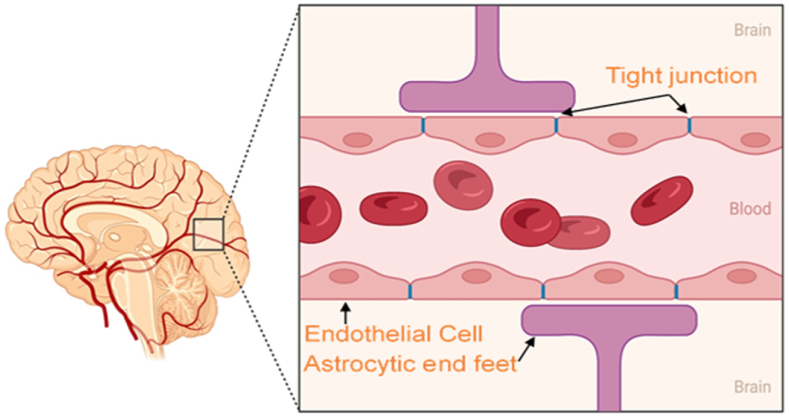
Physiological barriers such as the blood-brain barrier (BBB), intestinal barrier, and cerebrospinal fluid barrier prevent the entry of external substances into the brain [1]. Among these, the BBB operates as a sophisticated, dynamic, and adaptable interface that exchanges materials between the central nervous system (CNS) and the circulatory system, safeguarding the CNS as its most impenetrable barrier. This barrier is defined by a periphery of astrocytic endfeet, lumen, and pericyte cells, and is fundamentally made up of a web of tightly bound brain endothelial cells [2,3].
Within the BBB, endothelial cells are linked by tight junctions that create a network that restricts intercellular communication. Pericytes, or microvascular mural cells, play a significant role in managing the BBB's regulatory functions and lend structural support to the brain's capillary endothelium. These cells, along with the endothelial cells, are structurally integrated into the BBB via the basal lamina and astrocytic perivascular endfeet [4]. Due to the chemical equilibrium maintained by astrocytes, neurons in the vicinity of the BBB can effectively communicate. These interacting cells at the barrier manage the operations of tight junctions, transportation systems, and enzymatic frameworks. The processes of astrocytes encircling the brain capillaries are instrumental in the early establishment of endothelial tight junctions [5].
They are also responsible for secreting factors that augment cellular proliferation and differentiation [6]. In the context of inflammation, activated perivascular macrophages and microglia can act as a component of the brain's immune defense. The BBB's robust architecture selectively filters out foreign entities and toxic agents, enabling only certain molecules, such as oxygen, carbon dioxide, glucose, and ethanol, to permeate through specialized transport mechanisms [7]. This selective permeability of the BBB presents a significant hurdle in developing efficacious treatments for neurological disorders like Alzheimer's, stroke, glioma, and Parkinson's disease, as it impedes the delivery of therapeutic agents [8]. Solutions to overcome these challenges are actively being researched. One promising approach involves the use of nanotechnology. Nanomaterials crafted from polymers, lipids, and inorganic substances are proving to be particularly efficacious in medication delivery across the BBB. These nanocarriers can be engineered to exploit various transport mechanisms, such as passive diffusion, carrier-mediated transport, transport through aqueous channels, and active transport involving protein carriers with specific binding sites. By designing nanoparticles that can mimic or hijack these transport pathways, researchers can enhance the delivery of drugs to the CNS. The selectively semipermeable nature of the BBB typically hinders the entry of molecules needed to manage various ailments, including small-molecule pharmaceuticals and protein-based treatments. Modes of transport through the BBB encompass passive diffusion, carrier-mediated transport, transport through aqueous channels, and active transport involving protein carriers with specific binding sites [9]. The trafficking of substances across the BBB is contingent upon both transcellular and paracellular diffusion mechanisms. Transcellular diffusion permits the movement of lipophilic molecules across the endothelial cell membranes, while paracellular diffusion is tightly regulated by junctional complexes that limit the passage of small polar molecules [10].
The regulation of the BBB is pivotal for drug distribution [11]. Tight junctions have been observed to loosen, thereby increasing permeability under certain pathological states. Receptors capable of modulating the BBB have been thoroughly examined and cataloged [12]. Adenosine triphosphate, for instance, has been identified to influence the barrier through ligand-receptor interactions and endocytosis processes [13]. Alterations in specific chemical concentrations can also affect signaling pathways, thereby modifying physiological reactions. The practicability and potency of nano-based delivery systems are under active scrutiny.
Comprehending the interplay between endothelial cells and astrocytes within the BBB could enhance treatments for conditions that affect BBB integrity and permeability [14]. Utilizing steroids and other therapeutic compounds can facilitate the repair and increased permeability of the BBB, thus allowing medications to pass into the brain from the bloodstream [15]. Although the modulation of the BBB is strategic, additional research is necessary to refine drug delivery systems that enable significant therapeutic agents to penetrate the brain's cortex [16,17]. Conversely, functional nanomaterials are poised to transform the therapeutic landscape for a plethora of conditions, such as cancer and infectious diseases [18]. The Food and Drug Administration (FDA) has approved several lipid and polymer-based nanomedical formulations for clinical use, which have shown promise in treating breast and lung cancers [19], largely due to their proficiency in facilitating drug transit across the BBB, which is crucial for treating brain metastases that can arise from primary cancers such as breast cancer. For instance, nanoparticles can be functionalized with ligands that target specific receptors on the BBB, thereby enhancing drug delivery to the brain [20]. These targeted nanocarriers can improve the specificity and efficiency of drug delivery, reducing side effects and increasing therapeutic efficacy. In addition to nanotechnology, other innovative strategies are being explored. The modulation of the BBB using pharmacological agents can temporarily increase its permeability, allowing therapeutic compounds to enter the brain. Steroids and other compounds have been shown to facilitate the repair and increased permeability of the BBB [21]. Moreover, understanding the interplay between endothelial cells and astrocytes within the BBB can lead to the development of treatments that maintain or restore BBB integrity and functionality. This research aims to provide a synopsis of the most advanced nanotechnological innovations devised for the treatment of central nervous system (CNS) ailments. It discusses the roles of polymer, lipid, and inorganic nanocarriers within these drug delivery systems and highlights the potential of these approaches to overcome the challenges posed by the BBB. By leveraging these innovative solutions, significant progress can be made in the treatment of neurological disorders, ultimately improving patient outcomes. (Fig. 1).
Fig. 1.
Schematic representation of different types of nanoparticles (NPs) divided into polymeric, inorganic, and lipid-based nanocarriers.
2. The use of nanocarriers for drug delivery
Nanotechnology stands as a formidable tool with applications in the treatment of diseases like cancer, heart disease, and infectious diseases such as bacterial infections, viral infections (including influenza and HIV), fungal infections (such as candidiasis), and parasitic infections (like malaria). For example, nanocarriers, which are nanomaterials designed for drug transport, enhance the solubility and bioavailability of drugs that are typically poorly soluble, thereby improving their efficacy while reducing side effects [22,23]. The principal strategies for ferrying nanocarriers across the BBB for cerebral drug delivery (Table 1, Fig. 2) include (i) the disruption of tight junctions between endothelial cells or inducing localized toxic effects to facilitate drug/nanoparticle (NP) penetration; (ii) transcytosis or a combination of transcytosis and endocytosis through endothelial cells; (iii) employing endocytic endocytosis to transport substances through endothelial cells, wherein the cargo is discharged into the cytoplasm and subsequently exocytosed on the abluminal side of the endothelial cells [24,25].
Table 1.
Some of the recent nanomedicine for BBB penetration and their exclusive properties.
| Platform | Type | Size (nm) | Therapeutic agent | Application | Ref. |
|---|---|---|---|---|---|
| Liposomes | Lipid-Based Nanoparticles | 100to 125 | Doxorubicin | Chemotherapeutics for glioma therapy | [26] |
| zein nanoparticles | Nanocarriers | ∼150 | curcumin | Glioma therapy | [27] |
| Polymersome | Lipid-Based Nanoparticles | ∼76 | Saporin | Protein toxin delivery | [28] |
| PLGA |
Polymer-Based Nanocarriers | 28–98 | 3,30-Diindolylmethane | Preventing glioma progression | [29] |
| ApoE-ARTPC@TMZ liposomes | Lipid-Based Nanoparticles | Temozolomide and artesunate-phosphatidylcholine | therapy for resistant glioblastoma |
[30] | |
| PEG-PLA NPs (tLyp-1 peptide) | Polymer-Based Nanocarriers | 111.30 ±15.64 |
Paclitaxel | Glioma therapy | [31] |
| Solid lipid NPs | Lipid-Based Nanoparticles | 100–120 | – | A feasible strategy for improving brain delivery of therapeutics |
[32] |
| CMC-coated Fe3O4 NPs |
Inorganic Nanoparticles | 14.05 ± 1.70 | Dopamine hydrochloride | An agent for MRI and targeted drug delivery | [33] |
| PLA coated MSNs |
Polymer-Based Nanocarriers | 200 | Resveratrol | Oxidative stress therapy in CNS | [34] |
| poly (amidoamine) (PAMAM) | Dendrimers | siRNA | Targeted Intracellular Delivery in Glioblastoma | [35] | |
| Amphiphilic polymer lipid NPs |
Polymer-Based Nanocarriers | 100.1 ± 2.6 | Docetaxel | Treatment of brain metastasis of triple-negative breast cancer |
[36] |
| Au NPs |
Inorganic Nanoparticles | 3.5 ± 0.8 | – | Evolution of protein corona NPs across the BBB |
[37] |
| Si NPs | Inorganic Nanoparticles | 25, 50, 100 | Ruby dye | Evolution of Si NPs across the BBB | [38] |
| Den-RGD | Dendrimers | ∼10 | Cy5.5 | Photoacoustic shockwave therapy for glioblastoma | [39] |
Fig. 2.
The schematic shows the structure of the BBB.
The aspiration is for targeted nanocarrier drug delivery systems to supersede existing methods. The binding of specific surface ligands to nanocarriers, such as targeting antibodies, can enhance the selectivity and permeability of these systems through molecular recognition. NP-based systems allow for the controlled release of the drug payload or for release that is responsive to external stimuli, such as changes in pH or temperature. Dodecamer peptide (G23)-functionalized polydopamine-coated curcumin-loaded zein nanoparticles (CUR-ZpD-G23 NPs) efficiently traversed the BBB, delivering curcumin to glioblastoma cells and enhancing cellular uptake. These NPs inhibited proliferation, and migration, induced apoptosis in C6 glioma cells, and demonstrated circulation post-intravenous injection in zebrafish, showcasing potential as a targeted GBM therapy. Bradykinin aggregation-induced-emission nanoparticles (BK@AIE NPs) are designed for enhanced penetration through the blood–tumor barrier and efficient absorption in the near-infrared region. Under a 980 nm NIR laser, BK@AIE NPs demonstrate high photothermal conversion, effectively treating deep-seated glioblastomas and activating local brain immune responses [[27], [40], [41], [42]].
2.1. Polymer-based nanocarriers
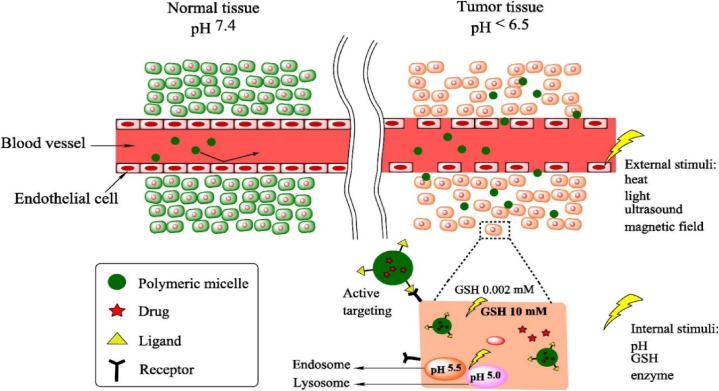
Polymeric substances, both synthetic and natural, are increasingly recognized as viable mediums for drug delivery. Their biocompatibility and degradability make them suitable for various pharmaceutical applications [43]. The specificity, bioavailability, adverse effects, and pharmacokinetic profiles of polymer-based nanomaterials, including polymeric nanoparticles (PNPs) and polymer-drug conjugates, have been extensively studied. Polymer-based carriers can shield therapeutic agents from enzymatic degradation, enabling their passive or active transport across cellular barriers (Fig. 3) [44]. To enhance Glioblastoma therapy, 3,3′-diindolylmethane was encapsulated in somatostatin receptor 2 SSTR2-targeted poly (lactic-co-glycolic acid) nanoparticles, showcasing efficient BBB penetration and glioma cell apoptosis. These nanoparticles were tagged with a novel peptide against somatostatin receptor 2 (SSTR2), a potential target in glioma. SSTR2 is a G-protein-coupled receptor that is overexpressed in various tumor cells, including gliomas. It plays a critical role in inhibiting cell proliferation and inducing apoptosis when activated. This nanoformulation demonstrates promising therapeutic potential, offering targeted delivery and inhibiting the epidermal growth factor receptor pathway in glioma [29]. Researchers introduce multifunctional polymeric nanoparticles (ASPTT NPs) designed for targeted stroke treatment and drug delivery to ischemic brain tissue. Utilizing ROS-reactive PTT polymer and AMD3100-mediated brain penetration, ASPTT NPs efficiently target ischemic regions after intravenous administration. By encapsulating glyburide, these NPs offer enhanced anti-edema and antioxidant therapy, demonstrating the potential for effective stroke treatment with superior therapeutic outcomes [45]. Drug-loaded polymeric nanocarriers have shown enhanced brain penetration, resulting in increased drug concentration and therapeutic efficacy.
Fig. 3.
Transport of stimuli-responsive polymeric nanocarriers through normal (left) and tumor (right) tissues via several stimuli-responsive delivery strategies. Reproduced with permission of ref [44].
2.1.1. Polymeric nanoparticles (PNPs)
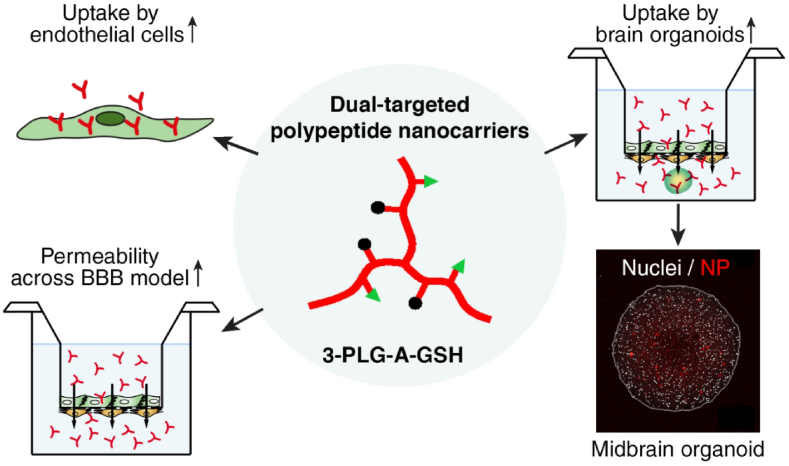
PNPs are the subject of substantial research within the realm of polymeric drug delivery. Polymers such as poly (d,l-lactide-co-glycolide) (PLGA), poly (lactic acid) (PLA), polyethylene glycol (PEG), poly (caprolactone), poly (glutamic acid), N-(2-hydroxypropyl)-methacrylamide copolymers (HPMA), and others have been widely used to fabricate PNPs. Dual-targeted polypeptide nanoparticles (NPs) were developed using alanine and glutathione ligands, enhancing permeability across a human BBB co-culture model. The NPs exhibited elevated cellular uptake, improved BBB permeability, and successfully entered midbrain-like organoids derived from both healthy and Parkinson's disease-specific stem cells. These findings underscore the potential of poly (l-glutamic acid) NPs for targeted drug delivery to the brain [46,47] (Fig. 4). Recently research presents hyaluronic acid (HA) and polycaprolactone (PCL)-based amphiphilic block copolymers assembled into targeted polymersomes using a solvent substitution method. Coated with polysorbate-80 (P80) for blood-brain barrier receptor targeting, these nanopolymersomes efficiently encapsulated celecoxib (COXI) with enhanced release properties. The P80-coated, COXI-loaded nanopolymersomes demonstrated sustained drug release for up to 72 h, showcasing potential as a passive targeting system for the blood-brain barrier [48].
Fig. 4.
Figure displaying the potential of poly (l-glutamic acid) NPs for targeted drug delivery to Human Brain Organoids. Reproduced with permission Ref [46].
Through ester bond hydrolysis in the polymer matrix, these nanoparticles can tailor the drug release profile and mitigate toxicity. PLGA nanoparticles, for instance, with an average size of 70.19 nm, can penetrate substantial brain volumes in animal models, showcasing the potential for deep brain delivery [49]. The controlled release from these NPs has led to increased survival rates in mice with brain cancer stem cell-derived xenografts. PEGylation of PNPs has been shown to enhance penetration into brain tissue, reduce systemic clearance, and improve BBB crossing capabilities, thereby extending the drug's presence in circulation and enhancing brain accumulation [50]. Amphiphilic block copolymers can self-assemble into micelles with a hydrophobic core and a hydrophilic shell. Due to their uniform size and distribution, micellar nanocarriers are favored for drug delivery applications [51]. Both polymeric and phospholipid micelles can overcome issues related to NP toxicity and rapid clearance by the body's immune system and can be modified to increase drug encapsulation and penetration efficacy. Such micelle delivery systems have demonstrated the capability to traverse the BBB [52]. Researchers devised an amphiphilic polymer-lipid nanoparticle system to deliver docetaxel (DTX) across the BBB for treating brain metastasis of triple-negative breast cancer (TNBC). This DTX-NP formulation exhibited enhanced brain accumulation, and prolonged circulation, and effectively inhibited tumor growth, surpassing the efficacy of the clinically used Taxotere®. Ex vivo fluorescence imaging further validated the nanoparticle's potential for assessing drug distribution in the brain, highlighting its promise as a therapeutic strategy for TNBC brain metastases [36]. The conjugation of targeting moieties, such as proteins or peptides, to PEG-PLGA and PEG-PLA-based nanoparticles, can further enhance their circulation time and BBB permeability. For example, cRGD-functionalized PEG micelles have shown enhanced targeting and penetration abilities against glioblastoma tumors [53,54].In other research utilizing the overexpression of neuropilin (NRP) on glioma and endothelial cells, tLyp-1 peptide-functionalized PEG-ePLA nanoparticles demonstrated enhanced glioma targeting, cellular uptake, and deep penetration, enhancing paclitaxel (PTX) efficacy. This dual-targeting nanoparticle DDS, confirmed by in vivo imaging, significantly improved survival in mice with intracranial C6 glioma, suggesting its potential as an effective anti-glioma drug carrier [31]. Similar brain-targeting effects were observed with PEGylated poly (2-dimethylamino) ethyl methacrylate (PEG-PDMAEMA) micelles conjugated with a 12-amino acid peptide [55]. ANG-PS efficiently delivers saponin (SAP) to human glioblastoma in mice, showcasing potent tumor inhibition and BBB penetration. Mimicking viruses in function and size, ANG-PS offers targeted payload delivery, demonstrating promising therapeutic potential for glioblastoma treatment [28]. Despite their potential, the biological stability of micelles, the rate of drug release, and retention time in the biological system require further investigation.
2.1.2. Dendrimers
Dendrimers, a class of synthetic macromolecules with a distinct three-dimensional, highly branched structure, stand out for their symmetrical architecture and core-centric configuration [10]. Unlike conventional polymers, dendrimers offer a high degree of control over their surface functional groups, which can engage in both covalent and noncovalent interactions with therapeutic agents [56]. These interactions are critical as they can enhance the water solubility of drugs through encapsulation and facilitate targeted delivery via surface electrostatic interactions. A notable application of dendrimers was their use in delivering siRNA to HIV-infected human astrocytes in a mouse model, where a carbosilane dendrimer effectively targeted and silenced a specific gene. A specialized nanoparticle, Den-RGD/CGS/Cy5.5, has been developed for targeted glioblastoma treatment. Utilizing pulsed laser energy, this nanoparticle induces an ultrasonic shockwave that causes localized damage to tumor cells while preserving adjacent tissue. This approach leverages temporary modulation of the BBB, ensuring specificity for glioblastoma and minimizing harm to surrounding brain tissue. It offers a promising precision therapy for glioblastoma [39].
The size-dependent efficacy of dendrimers has been explored through the development of PAMAM-based dendrimers of different diameters, demonstrating that a 6.7 nm dendrimer had a more pronounced effect on accumulation within the damaged brain tissue than a smaller 4.3 nm counterpart. Despite these advancements, the application of dendrimers as carriers for brain delivery remains limited, primarily due to challenges in targeting and penetrating the BBB [57]. In another study, poly (2-methacryloyloxyethyl phosphorylcholine) (PMPC) is utilized to modify PAMAM dendrimers, aiming to enhance targeted drug delivery for glioma while reducing toxicity. PMPC effectively diminishes PAMAM's cytotoxicity and improves brain tumor targeting. In vivo tests demonstrate that DOX-loaded PAMAM-PMPC minimizes side effects, enhances therapeutic efficacy against glioma by 80.76 %, and reduces cardiotoxicity, suggesting PMPC as a promising nanocarrier strategy for glioma treatment [58]. Development of covalently conjugated PAMAM dendrimer–siRNA conjugates with glutathione-sensitive linkers enhances siRNA stability, achieves significant gene knockdown, and facilitates targeted delivery to tumor-associated macrophages in glioblastoma, presenting a promising strategy for clinical translation of RNAi therapies [35] (Fig. 5). Innovations such as DAB-based dendrimers conjugated with transferrin have shown promise, significantly enhancing brain gene expression compared to non-targeted counterparts [59,60].
Fig. 5.
Dendrimer−siRNA Nanoconstructs Target Tumor-Associated Macrophages (TAMs) in a GL261 Mouse Model. Reproduced with permission Ref [35].
Nevertheless, concerns regarding the unpredictability of drug release kinetics and long-term safety profiles temper the widespread adoption of dendrimers for BBB delivery, in contrast to more established carriers like PLGA and PLA [61]. PEGOL-60, a neuroinflammation-targeting dendrimer synthesized via click chemistry, effectively crosses impaired CNS barriers, targeting activated microglia/macrophages in conditions like cerebral palsy and glioblastoma. This innovative approach demonstrates promise for targeted therapy in neuroinflammatory diseases [62]. The continual chemical refinement of dendrimers aims to improve their brain delivery efficacy. In the broader context of polymer-drug conjugates, substantial research and clinical trials are ongoing, such as the Phase II studies for ProLindac (HPMA copolymer–diaminocyclohexane platinum complex) in recurrent ovarian cancer. Although polymer-drug conjugates have not been extensively tested in CNS disorders, their targeted delivery and controlled release characteristics position them as potentially powerful therapeutic tools. The future of drug delivery for CNS conditions relies on the further development of polymeric nanocarriers designed for precise and regulated transport through the BBB.
2.1.3. Lipid-based nanoparticles (NPs)
Liposomes, which are vesicles composed of bilayer phospholipid membranes encasing aqueous compartments, can be synthesized from various components such as fatty acids, cholesterol, or phospholipids [63]. Their biocompatibility and biodegradability confer a natural affinity for the BBB, extending their circulation time within the bloodstream. The bioavailability of drugs encapsulated in liposomes can be significantly improved due to the liposome's ability to incorporate both hydrophilic and hydrophobic substances [64]. Modifying the size, charge, and composition of liposomes simplifies the transport of nucleic acids and enzymes. Conjugation of liposomes with ligands or monoclonal antibodies that target BBB-specific receptors can further facilitate drug transport across the BBB [65]. Liposomes' capacity to sequester both lipophilic and hydrophilic molecules also protects them from degradation, enabling targeted and controlled release at specific sites [66]. This makes liposomes highly effective nanocarriers for transporting therapeutic agents across the BBB for the treatment of various brain diseases. A study developed a dual-modified liposomal system (c (RGDfK)/Pep-22-DOX-LP) enhancing BBB/BBTB crossing and glioma targeting, significantly improving therapeutic efficacy in glioma-bearing mice [26]. In other research, Solid lipid nanoparticles (SLN) with surface-modified Apolipoprotein E-derived peptide (SLN-mApoE) exhibit enhanced BBB penetration, especially when administered via the pulmonary route, offering a promising strategy for neurotherapeutics. The warm microemulsion technique facilitates easy SLN preparation, with pulmonary SLN-mApoE administration showing superior brain bioavailability over intravenous and intraperitoneal routes without inducing lung inflammation. This approach underscores the potential of alternative administration methods and SLN design for effective brain-targeted drug delivery, benefiting patients on chronic treatments [32].In research, p-aminophenyl-d-mannopyranoside-based liposomes (MAN-LIP) were investigated for their role in the distribution of glucose and liposome transporters within cellular systems, with findings indicating a significant accumulation of MAN-LIP in the brain tissue of mice. These MAN-based liposomes demonstrated an enhanced ability to traverse LV-GLUT1/GLUT3/bEND.3 cell monolayers, suggesting their potential efficacy in brain drug delivery [67,68]. Cationic liposomes, composed of Dipalmitoyl phosphatidylcholin (DPPC), cholesterol, and diverse gemini amphiphiles, were investigated for BBB penetration. The study also explored the impact of incorporating a mannosylated lipid targeting the GLUT1 transporter on liposomal properties. Utilizing hiPSC-derived BMECs and a co-culture transwell BBB model, the findings underscored the potential of these formulations for enhanced CNS drug delivery [69]. The phosphatidylcholine (ARTPC) encapsulated with temozolomide (ApoE-ARTPC@TMZ) nanoplatform effectively co-delivered ART and TMZ to TMZ-resistant U251-TR GBM, enhancing DNA damage and apoptosis via Wnt/β-catenin signaling. Utilizing LDLRs-mediated transcytosis, the targeted liposomes penetrated the BBB and significantly reduced glioma burden in vivo, improving mouse survival. This approach minimizes TMZ-induced systemic toxicity, highlighting its clinical potential for enhancing CNS tumor chemotherapy outcomes [30] (Fig. 6).
Fig. 6.
Schematic illustration displaying the preparation of ApoE-peptide decorated combination liposomes for GBM targeting and therapeutics. Reproduced with permission Ref [30].
Liposomes engineered for dual-targeting are considered superior for drug delivery due to their improved permeability and targeting capabilities, proving more effective in treating brain gliomas and reducing side effects in control groups (DOX solution only). Furthermore, the innovation of magnetic fluid-based liposomal nanocarriers has been explored [70], where liposomes loaded with iron oxide nanoparticles exhibited selective targeting of glioblastoma cells and enhanced the permeability and retention effect, indicating promising applications in targeted cancer therapy.
2.2. Inorganic nanoparticles (NPs)
Inorganic nanoparticles, including those made of gold (Au), iron oxide, silica, and silver, possess unique attributes that render them suitable for brain drug delivery systems [71]. These nanoparticles can be chemically tailored for conjugation with ligands or polymers to enhance their biocompatibility and targeted delivery capacity [72]. In research innovatively, a PLA-coated MSNP system functionalized with LDLR ligand peptides facilitates BBB penetration and targeted RSV delivery for oxidative stress mitigation in CNS disorders. Utilizing an in vitro BBB/inflammation model, the system demonstrated ROS-responsive RSV release upon encountering activated microglia, highlighting its therapeutic promise [34]. In other research, silica nanoparticles (Si NPs) were functionalized with lactoferrin (Lf) and polyethylene glycol for improved brain drug delivery. Utilizing an in vitro BBB model, the study found that Lf-modified Si NPs exhibited enhanced transport efficiency across the BBB, particularly with 25 nm diameter particles, suggesting potential applications for targeted brain drug and imaging probe delivery [38]. Other research delves into the dynamics of the ‘protein corona' formed on nanoparticles as they interact with the BBB. Using a cellular BBB model with gold nanoparticles, findings reveal that the corona composition undergoes significant shifts during BBB traversal, stabilizing post-crossing. These insights highlight the pivotal role of the corona in nanoparticle-cell interactions and underscore the need for refined biological models in nanoparticle biomedical evaluations [37]. In vitro brain spheroids composed of six cell types demonstrate ultrasmall gold nanoparticles (2 nm core diameter) crossing the BBB, offering the potential for targeted brain therapies. These nanoparticles permeated the barrier more efficiently after hypoxia exposure, allowing enhanced drug delivery capabilities. However, further research in animal models is essential to understand particle clearance kinetics and potential human applications [60]. The fine-tuning of their size, shape, and porosity allows for their specialized application in both therapeutic and diagnostic processes for brain disorders. The use of magnetic fields and near-infrared (NIR) radiation with these nanoparticles facilitates improved imaging techniques and the controlled release of drugs across the BBB on demand (Fig. 7(a–c)) [73]. In research -coated magnetic nanoparticles (Fe3O4@CMC) demonstrate promise for BBB-crossing drug delivery, with proven biocompatibility and controlled release capabilities. In this research, magnetic nanoparticles (Fe3O4) are coated with carboxymethyl cellulose (CMC). The CMC coating provides biocompatibility, stability, and functionalization sites for drug loading. This combination aims to enhance the nanoparticles' ability to cross the blood-brain barrier for targeted drug delivery. Their hydrophilic CMC coating prevents agglomeration, enhances dispersion, and facilitates functionalization for targeted therapy, potentially minimizing neurological treatment side effects. These cost-effective, biodegradable nanoparticles offer a targeted approach for efficient brain drug delivery, highlighting their significance in neurotherapeutics [33]. Gold nanoparticles (AuNPs), particularly when surface-modified (i.e., PEG), offer a promising platform for drug delivery [60,74].
Fig. 7.
The cell associations at the BBB (a). Structure of junctions at the BBB (b). Transport routes across the BBB (c). Reproduced with permission Ref [74].
A diagnostic probe utilizing DNA-modified AuNPs has been developed to detect amyloid-derived diffusible ligands, highlighting their potential in diagnostics [73]. Additionally, AuNPs engineered for theranostic applications have demonstrated the ability to penetrate the BBB and tumors, showing promise for targeted brain drug delivery due to their selectivity and low toxicity [75].
Functionalized AuNPs with transferrin (Tf) have shown remarkable penetration into mice, employing the receptor transcytosis pathway [76,77]. AuNPs with a cleavable acid linkage and Tf coating were more effective in brain accumulation than their non-cleavable counterparts due to the cleavable process that allowed for enhanced drug release into the brain.
The integration of iron oxide nanoparticles (IONPs) and superparamagnetic iron oxide nanoparticles (SPIONs) with magnetic resonance imaging (MRI) has seen an uptick in brain drug delivery research. A traceable SPION system, coupled with a cell-penetrating peptide (CPP) targeting ligand, improved MRI contrast due to increased proton relaxation in the brain tissue [78]. Studies have shown that magnetic nanoparticles (MNPs) can cross the BBB without the need for an external magnetic field and without compromising the barrier's integrity [79]. By magnetically disrupting the BBB, MNPs offer a non-invasive method for drug delivery.
While inorganic nanoparticles (NPs) offer benefits such as an enhanced permeability and retention (EPR) effect, antibacterial properties, unique sizes, low cost, and ease of modification, they face challenges such as low biodegradability and potential toxicity, which hinder their clinical advancement [80]. In contrast, polymeric and lipid-based NPs, despite their biodegradability, biocompatibility, and high drug loading capabilities, are limited by short circulation times, large size, suboptimal targeting, and production challenges [74]. The development and clinical application of inorganic NPs for brain diseases continues to be a complex field with significant hurdles to overcome.
3. Conclusion, challenges, and feature prospects
The field of NP-based drug delivery has witnessed remarkable strides, especially concerning treatments targeting the brain. However, despite the progress fueled by nanotechnology, the realm of nanomedicine for brain delivery is still fraught with uncertainties regarding the creation of optimal nanomedicine formulations. Successes in animal models are merely indicative, not definitive, and the journey from preclinical models to a marketable delivery system that can traverse the BBB effectively remains arduous.
Before reaching the brain, NP-based formulations often accumulate in major organs like the liver, spleen, lungs, and kidneys, posing significant challenges for regulatory approval and commercialization. The potential risks of nanomaterials to human health stand as formidable barriers. The intricate interactions between therapeutic nanomaterials, the immune system, and biological barriers are not fully understood, especially regarding their overall kinetic and spatial dynamics.
Apart from experimental challenges in evaluating brain-targeting NP formulations, issues with scaling, regulation, and clinical validation pose additional hurdles. These multifaceted challenges offer rich research opportunities for the next wave of brain drug delivery systems and their clinical assessment.
Given the intricate nature of brain disorders, a multidisciplinary approach is likely necessary for effective treatment, requiring a deep clinical and scientific understanding of both disease mechanisms and drug delivery barriers. Advances in our comprehension of nanomaterials' interactions with biological systems could lead to the rational design of nanomedicines with optimal biodistribution.
Material scientists are encouraged to innovate novel nanomaterials and refine their design, synthesis, and characterization processes, particularly for BBB penetration, focusing on biodegradability, biocompatibility, precise targeting, and protection against degradation. The particle size sweet spot for delivering therapeutics to the brain is believed to be between 10 and 150 nm, balancing efficacy and clearance considerations. The surface charge of nanoparticles is another critical factor influencing their toxicity, circulation half-life, biodistribution, cellular uptake, and interaction with biological barriers.
Furthermore, enhancing the bioavailability of nanoformulations at the intended site is critical. The use of surface ligands that target brain endothelial cell receptors may improve BBB penetration. In summary, nanomaterials offer vast potential in the realm of brain-targeted therapies, from early detection to effective treatment. The journey to develop nanomaterial-based medical products is laden with scientific and regulatory challenges, yet the prospects for treating complex diseases with brain-targeting nanomedicine are promising. A thorough evaluation of clinical needs and a nuanced understanding of disease biology will support the transition of nanomedicine from bench to bedside.
Transparency document
The Transparency document associated with this article can be found in the online version.
CRediT authorship contribution statement
Elham Zeynalzadeh: Writing – review & editing, Investigation, Conceptualization. Ehsan Khodadadi: Writing – review & editing, Validation, Investigation. Ehsaneh Khodadadi: Writing – review & editing, Validation, Investigation. Zainab Ahmadian: Writing – review & editing, Validation, Investigation. Fahimeh Kazeminava: Writing – review & editing, Visualization, Validation, Supervision, Resources, Project administration, Investigation. Monireh Rasoulzadehzali: Writing – review & editing, Validation, Investigation. Hossein Samadi Kafil: Writing – review & editing, Supervision, Resources, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Fahimeh Kazeminava, Email: fahimehnava@yahoo.com.
Hossein Samadi Kafil, Email: kafilhs@tbzmed.ac.ir.
References
- 1.Daneman R., Prat A. The blood–brain barrier. Cold Spring Harbor Perspect. Biol. 2015;7:a020412. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbott N.J., Patabendige A.A., Dolman D.E., Yusof S.R., Begley D.J. Structure and function of the blood–brain barrier. Neurobiol. Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 3.Hourfar H., Aliakbari F., Aqdam S.R., Nayeri Z., Bardania H., Otzen D.E., et al. The impact of α-synuclein aggregates on blood-brain barrier integrity in the presence of neurovascular unit cells. Int. J. Biol. Macromol. 2023;229:305–320. doi: 10.1016/j.ijbiomac.2022.12.134. [DOI] [PubMed] [Google Scholar]
- 4.Saunders A., Macosko E.Z., Wysoker A., Goldman M., Krienen F.M., de Rivera H., et al. Molecular diversity and specializations among the cells of the adult mouse brain. Cell. 2018;174:1015–1030. e16. doi: 10.1016/j.cell.2018.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hashimoto Y., Campbell M. Tight junction modulation at the blood-brain barrier: current and future perspectives. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2020;1862 doi: 10.1016/j.bbamem.2020.183298. [DOI] [PubMed] [Google Scholar]
- 6.Kadry H., Noorani B., Cucullo L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS. 2020;17:1–24. doi: 10.1186/s12987-020-00230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Segarra M., Aburto M.R., Acker-Palmer A. Blood–brain barrier dynamics to maintain brain homeostasis. Trends Neurosci. 2021;44:393–405. doi: 10.1016/j.tins.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Ma M., Liu Z., Gao N., Pi Z., Du X., Ren J., et al. Self-protecting biomimetic nanozyme for selective and synergistic clearance of peripheral amyloid-β in an Alzheimer's disease model. J. Am. Chem. Soc. 2020;142:21702–21711. doi: 10.1021/jacs.0c08395. [DOI] [PubMed] [Google Scholar]
- 9.Rhea E.M., Banks W.A. Role of the blood-brain barrier in central nervous system insulin resistance. Front. Neurosci. 2019;13:521. doi: 10.3389/fnins.2019.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu Y., Liu C., Pang Z. Dendrimer-based drug delivery systems for brain targeting. Biomolecules. 2019;9:790. doi: 10.3390/biom9120790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stone N.L., England T.J., O'Sullivan S.E. A novel transwell blood brain barrier model using primary human cells. Front. Cell. Neurosci. 2019;13:230. doi: 10.3389/fncel.2019.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lochhead J.J., Yang J., Ronaldson P.T., Davis T.P. Structure, function, and regulation of the blood-brain barrier tight junction in central nervous system disorders. Front. Physiol. 2020;11 doi: 10.3389/fphys.2020.00914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergmann S., Lawler S.E., Qu Y., Fadzen C.M., Wolfe J.M., Regan M.S., et al. Blood–brain-barrier organoids for investigating the permeability of CNS therapeutics. Nat. Protoc. 2018;13:2827–2843. doi: 10.1038/s41596-018-0066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao Y., Chen X., Liu H. A facile approach for synthesis of nano-CeO2 particles loaded co-polymer matrix and their colossal role for blood-brain barrier permeability in Cerebral Ischemia. J. Photochem. Photobiol. B Biol. 2018;187:184–189. doi: 10.1016/j.jphotobiol.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Qian C., Yuan C., Li C., Liu H., Wang X. Multifunctional nano-enabled delivery systems in Alzheimer's disease management. Biomater. Sci. 2020;8:5538–5554. doi: 10.1039/d0bm00756k. [DOI] [PubMed] [Google Scholar]
- 16.Baniamerian H., Isfahani P.G., Tsapekos P., Alvarado-Morales M., Shahrokhi M., Vossoughi M., et al. Application of nano-structured materials in anaerobic digestion: current status and perspectives. Chemosphere. 2019;229:188–199. doi: 10.1016/j.chemosphere.2019.04.193. [DOI] [PubMed] [Google Scholar]
- 17.Bayir E., Celtikoglu M.M., Sendemir A. The use of bacterial cellulose as a basement membrane improves the plausibility of the static in vitro blood-brain barrier model. Int. J. Biol. Macromol. 2019;126:1002–1013. doi: 10.1016/j.ijbiomac.2018.12.257. [DOI] [PubMed] [Google Scholar]
- 18.Mahmoudi M., Pakpour S., Perry G. Drug-abuse nanotechnology: opportunities and challenges. ACS Chem. Neurosci. 2018;9:2288–2298. doi: 10.1021/acschemneuro.8b00127. [DOI] [PubMed] [Google Scholar]
- 19.Singh M., Thakur V., Deshmukh R., Mishra N. Role of nanocarriers for drug delivery to brain. J. Chem. Pharmaceut. Res. 2018;10:155–168. [Google Scholar]
- 20.Hersh A.M., Alomari S., Tyler B.M. Crossing the blood-brain barrier: advances in nanoparticle technology for drug delivery in neuro-oncology. Int. J. Mol. Sci. 2022;23:4153. doi: 10.3390/ijms23084153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vitku J., Hill M., Kolatorova L., Kubala Havrdova E., Kancheva R. Steroid sulfation in neurodegenerative diseases. Front. Mol. Biosci. 2022;9 doi: 10.3389/fmolb.2022.839887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joseph A., Simo G.M., Gao T., Alhindi N., Xu N., Graham D.J., et al. Surfactants influence polymer nanoparticle fate within the brain. Biomaterials. 2021;277 doi: 10.1016/j.biomaterials.2021.121086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salehi R., Alizadeh E., Kafil H.S., Hassanzadeh A.M., Mahkam M. pH-Controlled multiple-drug delivery by a novel antibacterial nanocomposite for combination therapy. RSC Adv. 2015;5:105678–105691. [Google Scholar]
- 24.Gosselet F., Loiola R.A., Roig A., Rosell A., Culot M. Central nervous system delivery of molecules across the blood-brain barrier. Neurochem. Int. 2021;144 doi: 10.1016/j.neuint.2020.104952. [DOI] [PubMed] [Google Scholar]
- 25.Aparicio-Blanco J., Pucci C., De Pasquale D., Marino A., Debellis D., Ciofani G. Development and characterization of lipid nanocapsules loaded with iron oxide nanoparticles for magnetic targeting to the blood–brain barrier. Drug Delivery and Translational Research. 2024:1–18. doi: 10.1007/s13346-024-01587-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen C., Duan Z., Yuan Y., Li R., Pang L., Liang J., et al. Peptide-22 and cyclic RGD functionalized liposomes for glioma targeting drug delivery overcoming BBB and BBTB. ACS Appl. Mater. Interfaces. 2017;9:5864–5873. doi: 10.1021/acsami.6b15831. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H., van Os W.L., Tian X., Zu G., Ribovski L., Bron R., et al. Development of curcumin-loaded zein nanoparticles for transport across the blood–brain barrier and inhibition of glioblastoma cell growth. Biomater. Sci. 2021;9:7092–7103. doi: 10.1039/d0bm01536a. [DOI] [PubMed] [Google Scholar]
- 28.Jiang Y., Yang W., Zhang J., Meng F., Zhong Z. Protein toxin chaperoned by LRP‐1‐targeted virus‐mimicking vesicles induces high‐efficiency glioblastoma therapy in vivo. Adv. Mater. 2018;30 doi: 10.1002/adma.201800316. [DOI] [PubMed] [Google Scholar]
- 29.Bhowmik A., Chakravarti S., Ghosh A., Shaw R., Bhandary S., Bhattacharyya S., et al. Anti-SSTR2 peptide based targeted delivery of potent PLGA encapsulated 3, 3’-diindolylmethane nanoparticles through blood brain barrier prevents glioma progression. Oncotarget. 2017;8 doi: 10.18632/oncotarget.18689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ismail M., Yang W., Li Y., Chai T., Zhang D., Du Q., et al. Targeted liposomes for combined delivery of artesunate and temozolomide to resistant glioblastoma. Biomaterials. 2022;287 doi: 10.1016/j.biomaterials.2022.121608. [DOI] [PubMed] [Google Scholar]
- 31.Hu Q., Gao X., Gu G., Kang T., Tu Y., Liu Z., et al. Glioma therapy using tumor homing and penetrating peptide-functionalized PEG–PLA nanoparticles loaded with paclitaxel. Biomaterials. 2013;34:5640–5650. doi: 10.1016/j.biomaterials.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 32.Dal Magro R., Ornaghi F., Cambianica I., Beretta S., Re F., Musicanti C., et al. ApoE-modified solid lipid nanoparticles: a feasible strategy to cross the blood-brain barrier. J. Contr. Release. 2017;249:103–110. doi: 10.1016/j.jconrel.2017.01.039. [DOI] [PubMed] [Google Scholar]
- 33.Aguilera G., Berry C.C., West R.M., Gonzalez-Monterrubio E., Angulo-Molina A., Arias-Carrión Ó., et al. Carboxymethyl cellulose coated magnetic nanoparticles transport across a human lung microvascular endothelial cell model of the blood–brain barrier. Nanoscale Adv. 2019;1:671–685. doi: 10.1039/c8na00010g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen Y., Cao B., Snyder N.R., Woeppel K.M., Eles J.R., Cui X.T. ROS responsive resveratrol delivery from LDLR peptide conjugated PLA-coated mesoporous silica nanoparticles across the blood–brain barrier. J. Nanobiotechnol. 2018;16:1–17. doi: 10.1186/s12951-018-0340-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liyanage W., Wu T., Kannan S., Kannan R.M. Dendrimer–siRNA conjugates for targeted intracellular delivery in glioblastoma animal models. ACS Appl. Mater. Interfaces. 2022;14:46290–46303. doi: 10.1021/acsami.2c13129. [DOI] [PubMed] [Google Scholar]
- 36.He C., Cai P., Li J., Zhang T., Lin L., Abbasi A.Z., et al. Blood-brain barrier-penetrating amphiphilic polymer nanoparticles deliver docetaxel for the treatment of brain metastases of triple negative breast cancer. J. Contr. Release. 2017;246:98–109. doi: 10.1016/j.jconrel.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 37.Cox A., Andreozzi P., Dal Magro R., Fiordaliso F., Corbelli A., Talamini L., et al. Evolution of nanoparticle protein corona across the blood–brain barrier. ACS Nano. 2018;12:7292–7300. doi: 10.1021/acsnano.8b03500. [DOI] [PubMed] [Google Scholar]
- 38.Song Y., Du D., Li L., Xu J., Dutta P., Lin Y. In vitro study of receptor-mediated silica nanoparticles delivery across blood–brain barrier. ACS Appl. Mater. Interfaces. 2017;9:20410–20416. doi: 10.1021/acsami.7b03504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu L., Chen Q., Wen L., Li C., Qin H., Xing D. Photoacoustic therapy for precise eradication of glioblastoma with a tumor site blood–brain barrier permeability upregulating nanoparticle. Adv. Funct. Mater. 2019;29 [Google Scholar]
- 40.Kim J., Ahn S.I., Kim Y. Nanotherapeutics engineered to cross the blood-brain barrier for advanced drug delivery to the central nervous system. J. Ind. Eng. Chem. 2019;73:8–18. doi: 10.1016/j.jiec.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poursadegh H., Amini-Fazl M.S., Javanbakht S., Kazeminava F. Magnetic nanocomposite through coating mannose-functionalized metal-organic framework with biopolymeric pectin hydrogel beads: a potential targeted anticancer oral delivery system. Int. J. Biol. Macromol. 2023 doi: 10.1016/j.ijbiomac.2023.127702. [DOI] [PubMed] [Google Scholar]
- 42.Zhang M., Wang W., Mohammadniaei M., Zheng T., Zhang Q., Ashley J., et al. Upregulating aggregation‐induced‐emission nanoparticles with blood–tumor‐barrier permeability for precise photothermal eradication of brain tumors and induction of local immune responses. Adv. Mater. 2021;33 doi: 10.1002/adma.202008802. [DOI] [PubMed] [Google Scholar]
- 43.Avramović N., Mandić B., Savić-Radojević A., Simić T. Polymeric nanocarriers of drug delivery systems in cancer therapy. Pharmaceutics. 2020;12:298. doi: 10.3390/pharmaceutics12040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alsehli M. Polymeric nanocarriers as stimuli-responsive systems for targeted tumor (cancer) therapy: recent advances in drug delivery. Saudi Pharmaceut. J. 2020;28:255–265. doi: 10.1016/j.jsps.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu H., Peng B., Mohammed F.S., Gao X., Qin Z., Sheth K.N., et al. Brain targeting, antioxidant polymeric nanoparticles for stroke drug delivery and therapy. Small. 2022;18 doi: 10.1002/smll.202107126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mészáros M., Phan T.H.M., Vigh J.P., Porkoláb G., Kocsis A., Páli E.K., et al. Targeting human endothelial cells with glutathione and alanine increases the crossing of a polypeptide nanocarrier through a blood–brain barrier model and entry to human brain organoids. Cells. 2023;12:503. doi: 10.3390/cells12030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Begines B., Ortiz T., Pérez-Aranda M., Martínez G., Merinero M., Argüelles-Arias F., et al. Polymeric nanoparticles for drug delivery: recent developments and future prospects. Nanomaterials. 2020;10:1403. doi: 10.3390/nano10071403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kiani-Dehkordi B., Vatanara A., Amini M., Hamidi M., Dibaei M., Norouzi P., et al. Preparation of polymersomes from synthesized hyaluronic acid-graft-poly (ε-caprolactone) copolymers for drug delivery to the brain. Mater. Today Chem. 2023;30 [Google Scholar]
- 49.Sur S., Rathore A., Dave V., Reddy K.R., Chouhan R.S., Sadhu V. Recent developments in functionalized polymer nanoparticles for efficient drug delivery system. Nano-Structures & Nano-Objects. 2019;20 [Google Scholar]
- 50.Leyva-Gómez G., Piñón-Segundo E., Mendoza-Muñoz N., Zambrano-Zaragoza M.L., Mendoza-Elvira S., Quintanar-Guerrero D. Approaches in polymeric nanoparticles for vaginal drug delivery: a review of the state of the art. Int. J. Mol. Sci. 2018;19:1549. doi: 10.3390/ijms19061549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang W.J., Hong C.Y., Pan C.Y. Polymerization‐induced self‐assembly of functionalized block copolymer nanoparticles and their application in drug delivery. Macromol. Rapid Commun. 2019;40 doi: 10.1002/marc.201800279. [DOI] [PubMed] [Google Scholar]
- 52.Zielińska A., Carreiró F., Oliveira A.M., Neves A., Pires B., Venkatesh D.N., et al. Polymeric nanoparticles: production, characterization, toxicology and ecotoxicology. Molecules. 2020;25:3731. doi: 10.3390/molecules25163731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karlsson J., Vaughan H.J., Green J.J. Biodegradable polymeric nanoparticles for therapeutic cancer treatments. Annu. Rev. Chem. Biomol. Eng. 2018;9:105–127. doi: 10.1146/annurev-chembioeng-060817-084055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhong Y., Wang C., Cheng R., Cheng L., Meng F., Liu Z., et al. cRGD-directed, NIR-responsive and robust AuNR/PEG–PCL hybrid nanoparticles for targeted chemotherapy of glioblastoma in vivo. J. Contr. Release. 2014;195:63–71. doi: 10.1016/j.jconrel.2014.07.054. [DOI] [PubMed] [Google Scholar]
- 55.Joseph A., Wood T., Chen C.-C., Corry K., Snyder J.M., Juul S.E., et al. Curcumin-loaded polymeric nanoparticles for neuroprotection in neonatal rats with hypoxic-ischemic encephalopathy. Nano Res. 2018;11:5670–5688. [Google Scholar]
- 56.Sherje A.P., Jadhav M., Dravyakar B.R., Kadam D. Dendrimers: a versatile nanocarrier for drug delivery and targeting. Int. J. Pharm. 2018;548:707–720. doi: 10.1016/j.ijpharm.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 57.Abedi-Gaballu F., Dehghan G., Ghaffari M., Yekta R., Abbaspour-Ravasjani S., Baradaran B., et al. PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl. Mater. Today. 2018;12:177–190. doi: 10.1016/j.apmt.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ban J., Li S., Zhan Q., Li X., Xing H., Chen N., et al. PMPC modified PAMAM dendrimer enhances brain tumor‐targeted drug delivery. Macromol. Biosci. 2021;21 doi: 10.1002/mabi.202000392. [DOI] [PubMed] [Google Scholar]
- 59.Tang L., Li J., Zhao Q., Pan T., Zhong H., Wang W. Advanced and innovative nano-systems for anticancer targeted drug delivery. Pharmaceutics. 2021;13:1151. doi: 10.3390/pharmaceutics13081151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sokolova V., Mekky G., van der Meer S.B., Seeds M.C., Atala A.J., Epple M. Transport of ultrasmall gold nanoparticles (2 nm) across the blood–brain barrier in a six-cell brain spheroid model. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-75125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pooresmaeil M., Namazi H. Advances in development of the dendrimers having natural saccharides in their structure for efficient and controlled drug delivery applications. Eur. Polym. J. 2021;148 [Google Scholar]
- 62.Sharma A., Sharma R., Zhang Z., Liaw K., Kambhampati S.P., Porterfield J.E., et al. Dense hydroxyl polyethylene glycol dendrimer targets activated glia in multiple CNS disorders. Sci. Adv. 2020;6 doi: 10.1126/sciadv.aay8514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nagaraju G.P., Srivani G., Dariya B., Chalikonda G., Farran B., Behera S.K., et al. Seminars in Cancer Biology. Elsevier; 2021. Nanoparticles guided drug delivery and imaging in gastric cancer; pp. 69–76. [DOI] [PubMed] [Google Scholar]
- 64.Raza A., Sime F.B., Cabot P.J., Maqbool F., Roberts J.A., Falconer J.R. Solid nanoparticles for oral antimicrobial drug delivery: a review. Drug Discov. Today. 2019;24:858–866. doi: 10.1016/j.drudis.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 65.Shah A., Aftab S., Nisar J., Ashiq M.N., Iftikhar F.J. Nanocarriers for targeted drug delivery. J. Drug Deliv. Sci. Technol. 2021;62 [Google Scholar]
- 66.Ertas Y.N., Abedi Dorcheh K., Akbari A., Jabbari E. Nanoparticles for targeted drug delivery to cancer stem cells: a review of recent advances. Nanomaterials. 2021;11:1755. doi: 10.3390/nano11071755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang J.-L., Chen H.-Z., Gao X.-L. Lipid-coated calcium phosphate nanoparticle and beyond: a versatile platform for drug delivery. J. Drug Target. 2018;26:398–406. doi: 10.1080/1061186X.2017.1419360. [DOI] [PubMed] [Google Scholar]
- 68.Du D., Chang N., Sun S., Li M., Yu H., Liu M., et al. The role of glucose transporters in the distribution of p-aminophenyl-α-d-mannopyranoside modified liposomes within mice brain. J. Contr. Release. 2014;182:99–110. doi: 10.1016/j.jconrel.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 69.Simonis B., Vignone D., Paz O.G., Donati E., Falchetti M.L., Bombelli C., et al. Transport of cationic liposomes in a human blood brain barrier model: role of the stereochemistry of the gemini amphiphile on liposome biological features. J. Colloid Interface Sci. 2022;627:283–298. doi: 10.1016/j.jcis.2022.07.025. [DOI] [PubMed] [Google Scholar]
- 70.Raj S., Khurana S., Choudhari R., Kesari K.K., Kamal M.A., Garg N., et al. Seminars in Cancer Biology. Elsevier; 2021. Specific targeting cancer cells with nanoparticles and drug delivery in cancer therapy; pp. 166–177. [DOI] [PubMed] [Google Scholar]
- 71.Carazo E., Borrego‐Sánchez A., García‐Villén F., Sánchez‐Espejo R., Cerezo P., Aguzzi C., et al. Advanced inorganic nanosystems for skin drug delivery. Chem. Rec. 2018;18:891–899. doi: 10.1002/tcr.201700061. [DOI] [PubMed] [Google Scholar]
- 72.Núñez C., Estévez S.V., del Pilar Chantada M. Inorganic nanoparticles in diagnosis and treatment of breast cancer. JBIC, J. Biol. Inorg. Chem. 2018;23:331–345. doi: 10.1007/s00775-018-1542-z. [DOI] [PubMed] [Google Scholar]
- 73.Choi G., Rejinold N.S., Piao H., Choy J.-H. Inorganic–inorganic nanohybrids for drug delivery, imaging and photo-therapy: recent developments and future scope. Chem. Sci. 2021;12:5044–5063. doi: 10.1039/d0sc06724e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ding S., Khan A.I., Cai X., Song Y., Lyu Z., Du D., et al. Overcoming blood–brain barrier transport: advances in nanoparticle-based drug delivery strategies. Mater. Today. 2020;37:112–125. doi: 10.1016/j.mattod.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mollazadeh S., Sahebkar A., Shahlaei M., Moradi S. Nano drug delivery systems: molecular dynamic simulation. J. Mol. Liq. 2021;332 [Google Scholar]
- 76.Zou Y., Huang B., Cao L., Deng Y., Su J. Tailored mesoporous inorganic biomaterials: assembly, functionalization, and drug delivery engineering. Adv. Mater. 2021;33 doi: 10.1002/adma.202005215. [DOI] [PubMed] [Google Scholar]
- 77.Fiedler A.M., Medeiros M., Fiedler H.D. Targeted glioblastoma treatment via synthesis and functionalization of gold nanoparticles with de novo–engineered transferrin-like peptides: protocol for a novel method. JMIR Research Protocols. 2023;12 doi: 10.2196/49417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nikezić A.V.V., Bondžić A.M., Vasić V.M. Drug delivery systems based on nanoparticles and related nanostructures. Eur. J. Pharmaceut. Sci. 2020;151 doi: 10.1016/j.ejps.2020.105412. [DOI] [PubMed] [Google Scholar]
- 79.Sharma S., Masud M.K., Kaneti Y.V., Rewatkar P., Koradia A., Hossain M.S.A., et al. Extracellular vesicle nanoarchitectonics for novel drug delivery applications. Small. 2021;17 doi: 10.1002/smll.202102220. [DOI] [PubMed] [Google Scholar]
- 80.Boztepe T., Castro G.R., Leon I.E. Lipid, polymeric, inorganic-based drug delivery applications for platinum-based anticancer drugs. Int. J. Pharm. 2021;605 doi: 10.1016/j.ijpharm.2021.120788. [DOI] [PubMed] [Google Scholar]