Abstract
Background and Objective
The interwoven immunological, biological, and genetic complexity of thyroid diseases makes suitable targeted therapies particularly challenging to develop. Stemming from ancient practices, al-hijamah, or wet cupping, has achieved notable popularity in recent years, leading to unique applications in modern medicine. By grappling with the current literature that links the effects of wet cupping with the immune system in patients with Hashimoto’s thyroiditis (HT), this narrative review aims to compose a comprehensive assessment of this adjunctive treatment based on evidence of its integration into practice.
Methods
Between upregulating critical players of the innate immune system, such as immunostimulatory cytokines, white blood cells (WBCs) and natural killer (NK) cells, and downregulating essential thyroid antibodies (anti-thyroid peroxidase and anti-thyroglobulin) and inflammatory markers (C-reactive protein and erythrocyte sedimentation rate), wet cupping practices provide promising complementary therapy for hypothyroidism.
Key Content and Findings
Wet cupping manipulates in vivo molecular mechanisms, as outlined in hemodynamic and microparticle clearance theories, to slow disease progression and even development in disease-free populations. Given the established utilization of wet cupping in the context of autoimmune diseases and inflammatory conditions, the emerging utility of wet cupping continues to gain credibility.
Conclusions
This literature review illuminates the documented improvements in immune and biological function due to cupping therapeutic practices and sheds light on its appropriate application in the clinical setting for patients with HT. Furthermore, this review proposes a clear need for implementing future clinical trials, which may effectively bridge pathophysiological causes of hypothyroidism with underrated techniques for enhanced thyroid health.
Keywords: Hashimoto, thyroiditis, autoimmune, cupping, hypothyroidism
Introduction
Overview of Hashimoto’s thyroiditis (HT)
HT involves the interplay of environmental and genetic factors and remains a highly problematic autoimmune disease that leads to hypothyroidism (1,2). This condition, commonly observed as the primary cause of chronic thyroid inflammation (2,3), is widely recognized as the leading contributor to thyroid disorders among adolescents (4,5). HT boasts an incidence of 0.3–3.7% in the general United States population (6), though rising rates of certain autoimmune diseases, including HT, have been observed globally in recent years (7,8). Furthermore, HT tends to be underdiagnosed due to symptomatic dormancy (1). Hallmark characteristics of the disease emphasize heightened anti-thyroid peroxidase (anti-TPO) levels and anti-thyroglobulin (anti-Tg) levels combined with homeostatic imbalances in hypothyroidism (1,8). Thyroxine (T4) hormone replacement monotherapy or T4 and triiodothyronine (T3) combination therapy is the gold standard of treatment for HT (9,10). This treatment acts similarly to what would typically be endogenously produced by the thyroid gland under healthy physiological functioning (6,10). These hormone replacement options aid in restoring normal thyroid hormone levels in vivo so long as dosage is administered based on case-specific hormone levels and associated symptoms (11-13). Nonetheless, applying such therapies fails to fully demonstrate symptom-alleviation, as patients continue to present post-T4 treatment with persistent fatigue and lethargy and low overall treatment satisfaction (6). Lifestyle changes involving diet and exercise have also emerged to lower hormone levels (13,14). Overarchingly, extensive evidence points to a thyroid-gut axis that mediates autoimmune function (14,15).
A brief history of wet cupping
Al-hijamah, called “wet cupping”, describes a therapeutic technique practiced across diverse cultures for centuries (16,17). It involves applying suction cups to the skin’s surface, generating a vacuum effect such that small amounts of blood are extracted from the body (16). The collected blood has been thought to carry toxins, and thus, removing such blood has been believed to restore balance and promote a state of well-being (11). Since the dawn of health practices in Asia, the Middle East, and other ancient societies, wet cupping has long-standing historical ties. Having been practiced since its inception by Egyptians in 1550 BC (17,18), wet cupping has been utilized in many cultures. The origin of such techniques has consistently emphasized the concept of ridding toxins from the body via the removal of blood, believed to harbor a variety of impurities that lead to infection and disease, overall having the potential to mediate recovery from illness (19,20).
The practice and protocol of wet cupping
The procedure commences with creating small incisions on the skin within the confines outlined by the borders of cup placement. After making the micro-incisions, the cup is placed in contact with the skin to form a seal, thus producing a partial vacuum effect that draws the blood from the skin into the cup (16). The distribution of cups and total surface area over which the procedure is conducted varies widely depending on the intended function or individual nature of the ailment (21). These cups are typically left on the skin surface for a few minutes, facilitating controlled extraction of a small quantity of blood into each respective cup.
Wet cupping techniques have appeared in a range of settings for a broad spectrum of conditions, primarily due to the proven benefits of the procedure in providing pain relief. The practice has also been utilized for resolving respiratory issues and other non-specific symptoms of illness and disease (22). Wet cupping has even seen successful application in patients with migraines (23). Indeed, it has gained significant credibility in modern medicine.
Despite the growing awareness of its recognized therapeutic potential, the mechanisms of these healing techniques still need to be explored within the infrastructure of traditional research. Because wet cupping is derived from theories centered around purging and cleansing the blood of toxins and waste products, it has shown remarkable utility in promoting improved physiological functioning, resulting in significant health benefits. Nonetheless, a critical eye on literature in this domain of study remains necessary given the limited and anecdotal supply of evidence that advocates for this procedure’s efficacy in treating thyroid diseases.
Examining effects of wet cupping on HT
With an understanding of the above-stated limitations regarding available empirical evidence, wet cupping intrigues researchers and clinicians alike. The current literature explores the practice, therapeutic mechanisms, and potential benefits of wet cupping related to enhanced human biological and immunological function in the context of HT. By delving into documented literature on wet cupping’s effects on the immune system, the current review seeks to balance both the cultural significance with the scientific foundations of the practice, ultimately contributing to a more comprehensive assessment of benefits and limitations as applied to the resolution of disease and ailments through clinical and surgical settings. We present this article in accordance with the Narrative Review reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-24-173/rc).
Methods
This narrative review entailed initial searches of PubMed (NLM), Embase (Elsevier), Web of Science Core Collection, and PsycInfo (EBSCOhost) for articles pertaining to wet cupping practices and clinical utility as related to HT. The criteria of this search were expanded to include literature in which this treatment was applied in the context of autoimmune conditions for the purpose of examining unique biological and immunological effects of wet cupping therapy. Referenced articles within articles found in this search were also reviewed for applicability. Searches were completed on November 12, 2023. Three authors (M.B.L., A.A., J.A.J.) independently conducted searches on the limited literature pertaining to this topic, all of which were compiled for incorporation into the final review. Articles that were not peer-reviewed, lacked text in English, or did not contribute evidence-based insight on mechanistic or clinical knowledge were excluded (Table 1).
Table 1. Search summary strategy.
| Items | Specification |
|---|---|
| Date of search | November 12, 2023 |
| Databases and other sources searched | PubMed (NLM), Embase (Elsevier), Web of Science Core Collection, and PsycInfo (EBSCOhost) |
| Search terms used | “Hashimoto’s Thyroiditis and Wet Cupping”, “Autoimmune Diseases and Wet Cupping”, “Benefits of Al-Hijamah”, “Cupping and Hypothyroidism” |
| Timeframe | No specific filters for timeframe were utilized in this search |
| Inclusion and exclusion criteria | Non-peer-reviewed articles, studies without English text available, and papers that contained no evidence-based insight on biological/immunological mechanisms or clinical relevance were excluded. All other literature was included so long as it pertained to the practices of wet cupping as a potential adjunctive treatment for inflammatory, autoimmune, and endocrine conditions |
| Selection process | 3 authors (M.B.L., A.A., J.A.J.) conducted searches continuously to net all available literature related to the core topic of this review. Duplicates were removed |
| Additional considerations | This methodology was selected via mutual consensus among the authors due to the limited number of studies initially found through pilot searches and the goal of generating a comprehensive a pool of literature for utilization |
Key content and findings
Proposed immunological mechanisms induced by wet cupping
Negative pressure suction and promotion of blood flow theories
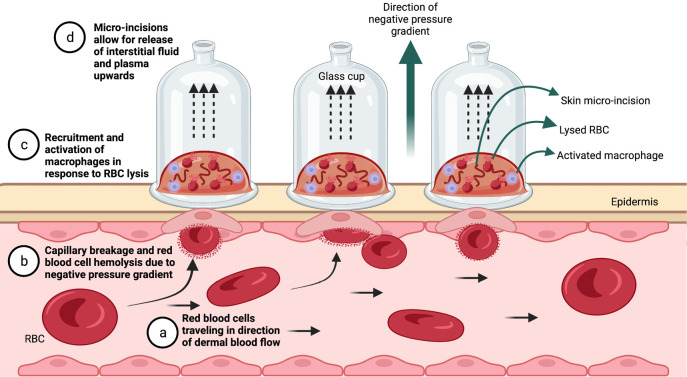
Several physiological models have been anticipated to explain the effects of wet cupping, which invokes a negative pressure suction effect, increases dermal blood flow, and activates the immune system (Figure 1). However, prior characterization of data remains inadequate to draw significant conclusions. A comprehensive understanding of the cupping procedure can be achieved by linking cupping therapy effects with its mechanisms based on viable theories, despite the exact mechanism of action for improving autoimmune thyroid health needing to be better understood.
Figure 1.
Visual of wet cupping on the human epidermis. a: RBCs travel in the direction of dermal flow along the capillary. b: capillary rupture due to the presence of the cup suction induces hemolysis and pulls the RBCs out of circulation via a negative pressure gradient (arrows). c: lysis of the RBCs and potential recruitment of activated macrophages (purple) for RBC phagocytosis occurs just beneath epidermal surface. d: interstitial fluid and plasma escape micro-incisions and collect in the upper portion of the cup. The image was created with BioRender.com. RBC, red blood cell.
Mechanical effect and hemodynamic theory
The mechanical effect and hemodynamic theory propose that wet cupping therapy is an artificial surgical excretory procedure that clears blood and interstitial fluids containing causative pathological substances (CPS) in a two-stage process. It is suggested in the first step that the negative pressure gradient inside the confines of the cup compiles a mixture of interstitial fluid and plasma. Then, rupturing the skin with micro-incisions overcomes barriers to exit for toxins, enhances natural excretory functions of the skin, augments innate immunity, and increases filtration at capillary ends to clear the blood of CPS to restore physiology and homeostasis (24). Sucking pressure inside the cups applied to the skin disseminates around skin capillaries, adding to capillary hydrostatic pressure, which counteracts capillary osmotic forces (24). This creates a pressure gradient and traction force across skin/capillaries and increases filtration at arterial and venous ends of capillaries, resulting in clearance of CPS (iron, ferritin, and hemolyzed blood cells) (24).
Negative pressure to the skin stimulates blood flow when the cup is applied, as a differential pressure is created between the epidermis and the underlying blood vessels, resulting in a five-fold increase in vascular perfusion within the area (25). In a 2017 study, the relationship between negative pressure applied to the skin and heme oxygenase-1 (HO-1) was explored further, leading to the discovery that negative pressure caused the rupture of superficial capillaries, leaking red blood cells (RBCs) into the interstitial space. Macrophages then phagocytosed the leaked RBCs, triggering the release of HO-1, responsible for metabolizing the heme and ultimately leading to the production of carbon monoxide (CO), biliverdin (BV), and bilirubin (BR) (26). These substances have been shown to (I) possess antioxidant and anti-inflammatory effects in both animal and human systems and (II) stimulate a shift of macrophages to the anti-inflammatory M2 phenotype (26). Activation of the HO-1 enzyme system has been shown to have potent anti-inflammatory effects. For instance, HO-1 has demonstrated reduced pro-inflammatory cytokines via regulation of the inflammasome system. Inflammasomes are cytosolic protein complexes that promote the cleavage of caspase 1, which leads to the maturation and secretion of pro-inflammatory cytokines, including IL-1b and IL-18 (27). Increased production of IL-10, an anti-inflammatory cytokine that promotes inflammation resolution, has also been observed (28). IL-10 inhibition downregulates other inflammatory mediators, such as tumor necrosis factor-alpha (TNF-α) and IL-6, in addition to inhibiting the effects of IL-1b (28-30).
Microparticle clearance theory
Hassan et al. proposed a novel mechanism for the action of wet cupping therapy: Microparticle Clearance Theory. Microparticles, called extracellular vesicles (EVs), are released by aging erythrocytes, platelets, endothelial cells, or leukocytes (31). Erythrocyte microparticles (EMPs) have been associated with systemic inflammatory conditions (32-34), and are involved in pro-inflammatory immune system priming following transfusion (35). Microparticles have been shown to stimulate immune cells by activating the nuclear factor-kappa B (NF-kB) and inducing endothelial dysfunction via the downregulation of nitric oxide synthase (36), promoting in situ thrombosis (37). Cupping-mediated clearance of plasma microparticles may afford a protective effect via the downregulation of downstream inflammatory signals and endothelial protection (38).
Hypothyroidism: biological signaling and autoimmune implications
Immunomodulation of the innate immune system
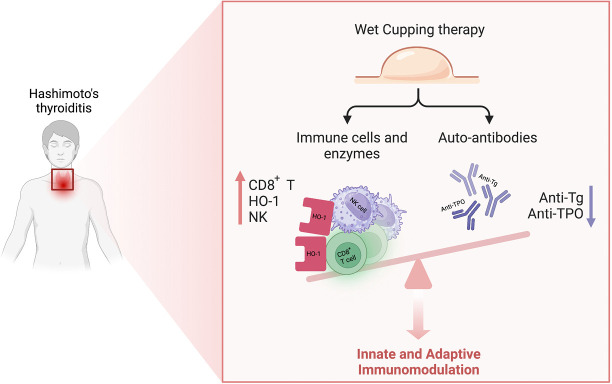
The immunological mechanisms underlying the practice of wet cupping provide ample protection for normal thyroid function. Due to the intimate relationship between natural immunity and metabolic homeostasis, cupping has been shown to influence immunomodulation and mitigate pathophysiological ramifications indirectly. As such, though few studies detail the implementation of wet cupping practices to reduce inflammation directly, various papers include anti-inflammatory markers as measurable outcomes of patient treatment. Anti-TPO and anti-thyroid-stimulating hormone (TSH) have been reduced after treatment, for instance, in rheumatoid arthritis (RA) and assorted dermatological disorders following cupping procedures (39). Such outcomes confer the potential advantages of wet cupping as it relates to reductions in specific inflammatory markers, thus combatting major or ancillary inflammatory events (Figure 2).
Figure 2.
Overview of main immunological benefits, including upregulation of key innate immune system cellular components, NK cells and CD8+ T-cells, the enzyme HO-1, and downregulation of adaptive immune system elements, anti-Tg and anti-TPO. The image was created with BioRender.com. HO-1, heme oxygenase-1; NK, natural killer; Tg, thyroglobulin; TPO, thyroid peroxidase.
Regarding the theory concerning wet cupping’s immune system activation, Ahmed et al. conducted a randomized clinical trial in 2005 to assess the efficacy of bloodletting cupping (BLC) as adjunctive therapy in RA (40). There was an observed early marked reduction in laboratory values of inflammatory markers for C-reactive protein (CRP) and for erythrocyte sedimentation rate (ESR). At the same time, the effects of conventional therapy appeared later, after 3 months of treatment. Consequently, in RA patients, it was found that CRP significantly decreased in the experimental group compared to the control group. Another study led by Abdulaziz et al. discovered that in female patients with chronic pelvic pain, wet cupping therapy was associated with a decrease in high-sensitivity CRP (hs-CRP) (41).
The effects on natural killer (NK) cells vary according to the disease state. In healthy populations, wet cupping usage showed decreased NK cell activity and cytotoxicity and increased NK cell levels in RA patients (40,42). Ahmed et al. claimed that the treatment significantly reduces the laboratory markers of RA pathology and modulates cellular conditions, particularly of the innate immune response (NK cells) and adaptive cellular immune response [soluble interleukin-2 receptor (sIL-2R)]. Conventional therapy, according to the original study by Ahmed et al., induced significant depression in white blood cell levels. In contrast, combination T3 + T4 therapy induced marked elevation since the first month. There was a significant decrease in NK cells with conventional therapy, while combination therapy increased significantly (42). Another study by Obeid et al. in Saudi Arabia demonstrated that HT patients had decreased ESR at 1 month and 3 months following wet cupping therapy (43). Ahmed et al.’s study results coincide logically with findings on ESR in RA patients in that those who underwent cupping therapy showed a significant decrease in ESR (40). At the same time, no change in ESR was noted in healthy populations, according to Obohat et al. (44).
As previously mentioned, HO-1 activation is associated with a shift in macrophage phenotypes from a pro-inflammatory M1 subtype to an M2 subtype (26). The M2 macrophage phenotype results from stimulation with IL-4 or IL-13 and is associated with a Th2 environment (45-48). M2 macrophages have been implicated in fighting parasitic infections and attenuating excessive inflammation (48-50). IL-4 and IL-13 modulate the shifting towards the macrophage M2 subtype, which emphasizes the downregulation of inflammasome activity (51).
Immunomodulation of the adaptive immune system
Wet cupping has demonstrated notable results in terms of T lymphocyte changes (Table 2). T-helper cell subsets have been studied in the context of HT patients and have been shown to play a role in the pathogenesis of the condition. Among HT patients, expression of T-bet and GATA-3 was significantly elevated, while FOXP3 expression was diminished considerably (59). Expression of T-bet/FOXP3, GATA-3/FOXP3, and RORα/FOXP3 ratios were increased among HT patients in comparison with the controls. At the level of the transcription factors, there was an evident imbalance between Th1/Treg, Th2/Treg, and Th17/Treg lymphocytes deviating towards Th1, Th2, and Th17 cells in HT patients, which, if corrected, could be of therapeutic value (59). Wet cupping therapy in healthy populations displays a decreased expression of T-bet/GATA-3 (Th1/Th2) (52), possibly correcting the T lymphocyte imbalance in HT and, consequently, serving as a potential therapeutic asset.
Table 2. Studies of healthy and diseased populations denote relevant immunological mechanisms associated with wet cupping.
| Category | Author | Year | Cohorts | Immunomodulation |
|---|---|---|---|---|
| Normal | Dons'koi (42) | 2016 | Health population | Decreased NK cell activity |
| No change in CD4+ T cells and CD8+ T cells | ||||
| Obohat (44) | 2020 | Health population | Decreased fasting blood glucose and uric acid | |
| No change in WBC, ESR, or ferritin | ||||
| Soleimani (52) | 2020 | Health population | Increased expression of transcription factors: GATA-3, RORγt, FOXP3 | |
| Decreased T-bet/GATA-3 (Th1/Th2) | ||||
| Disease | Ahmed (40) | 2005 | Rheumatoid arthritis | Increased NK cells and WBC |
| Decreased RF, ESR, CRP, and sIL-2R | ||||
| Decreased CRP (hs-CRP) | ||||
| Abdulaziz (41) | 2021 | Female chronic pelvic pain | Decreased CRP (hs-CRP) | |
| Obeid (43) | 2022 | Hashimoto’s thyroiditis | Decreased anti-TPO and anti-Tg | |
| Decreased ESR, prolactin, and TSH. No change in T4 | ||||
| El-Shanshory (53) | 2022 | Thalassemia | Increased CD4/CD8 ratio and TAC/MDA ratio | |
| Yin (54) | 2022 | Functional diarrhea | Increased CD4+ T cells, CD8+ T cells, and T17 helper lymphocytes | |
| Zhang (55) | 2006 | Bronchial asthma | Increased C3, C4, IgG, IgM, IgA, CD4+ T cells, CD4+/CD8+ ratio, IL-2, and IFN-γ | |
| Decreased IgE, IL-4, IL-10, and CD8+ T cells | ||||
| Sun (56) | 2016 | Acute scapulohumeral peri-arthritis | Decreased 5-HT and PGE2 | |
| El-Domyati (57) | 2013 | Dermatologic diseases | Increased C3 | |
| Decreased IgE and IL-2 | ||||
| Tian (58) | 2013 | Postherpetic neuralgia | Decreased P substance |
WBC, white blood cell; ESR, erythrocyte sedimentation rate; NK, natural killer; RF, rheumatoid factor; CRP, C-reactive protein; sIL-2R, soluble interleukin-2 receptor; hs-CRP, high-sensitivity CRP; TPO, thyroid peroxidase; Tg, thyroglobulin; TSH, thyroid-stimulating hormone; TAC/MDA, total antioxidant capacity/malondialdehyde; IFN, interferon; 5-HT, 5-hydroxy-tryptamine; PGE2, prostaglandin E2.
In patients with HT, there is lower mRNA expression of Th1 cell-related T-bet and interferon-γ (IFN-γ) in peripheral blood mononuclear cells (60). At the same time, there was a sizable increase in the expression level in Th17 coherent retinoic acid-related orphan nuclear receptor gamma t (RORγt) and IL-17 mRNAs. In HT, a negative correlation between T-bet and RORγt mRNA expression was found, and a similar phenomenon was noted on the levels of mRNA and plasma concentration between IFN-γ and IL-17. This suggests that Th17 cells might be involved in the pathogenesis of HT (60). In fact, other studies have found a positive relationship between TSH levels and anti-CD3/anti-CD28-induced Th17 cells (61). Imbalances of T17/Treg in HT also serve to inform prognosis of gland damage in HT; therefore, the utilization of gene panels and advances in immunotherapeutic treatments may help to enhance treatment strategies for affected individuals (62).
Tg antibodies
Tg stands as the core substance within the thyroid gland’s follicular colloid (63). It is essential in storing iodine and the biosynthesis of thyroid hormones: T4 and T3. Antibodies against Tg predominantly belong to the IgG category and span across all four subclasses of IgG. In the case of HT, IgG2 is the more prevalent subclass, whereas IgG4 is commonly found in greater concentrations in individuals afflicted with Graves’ disease, non-toxic goiter, and differentiated thyroid carcinoma (64,65). Tg antibodies are considered non-pathogenic because serum does not usually transfer thyroiditis. Yet, they are frequently present in the sera of patients with autoimmune thyroid disease (AITD). However, thyroid autoantibody measurements for monitoring the treatment for AITD are generally not recommended. Treatment for AITD focuses on managing the thyroid dysfunction that results from the disorder rather than targeting the underlying autoimmunity that causes the disease. Nonetheless, changes in autoantibody concentrations often reflect a change in disease activity. For instance, thyroid cancer is related to prolonged exposure to high thyroid autoantibody levels and an associated pro-inflammatory environment (66-68).
Tg antibodies are also found in non-thyroid autoimmune diseases, such as Sjögren’s syndrome, myasthenia gravis, celiac disease, and type 1 diabetes (69). The thyroid autoantibodies prevalence is higher among patients with autoimmune disorders not related to the thyroid, such as type 1 diabetes and pernicious anemia (70). Measuring Tg antibodies is also used to assess the risk of developing autoimmune thyroiditis. A 20-year community-based study in England has shown that detecting thyroid-specific autoantibodies is a critical determinant for identifying individuals at elevated risk for thyroiditis. The presence of antibodies against Tg is beneficial for evaluating the potential onset of postpartum thyroiditis (71).
TPO antibodies
TPO is a membrane-associated glycoprotein expressed only in thyrocytes, where it is present primarily on the apical surface (72). Several mechanisms have been proposed by which TPO antibodies can damage thyroid follicular cells (71). Deposition of complement components has been proved in the thyroid gland of patients with AITD, and TPO antibodies are known to activate the complement cascade. Most TPO antibodies are of the IgG1 subclass (a suitable complement activator), strengthening the possibility that antibodies could directly induce cytotoxicity via the activation of the complement system. Anti-TPO plays a role in hypothyroidism development and progression. The National Health and Nutrition Examination Survey (NHANES) found that positive anti-TPO tests were significantly associated with hypothyroidism in disease-free populations (73). The presence of elevated anti-TPO titers in patients with subclinical hypothyroidism helps to predict progression to overt hypothyroidism—4.3% per year with anti-TPO vs. 2.6% per year without elevated anti-TPO titers (74,75).
The diagnosis of HT is based on the clinical manifestation of symptoms in conjunction with laboratory evidence indicating raised levels of TSH and accompanying T4 concentrations that range from normal to decreased levels. One of the keys to diagnosing any AITD is determining the presence of a high anti-thyroid antibody titer. Several patients with chronic autoimmune thyroiditis present with biochemical profiles indicative of euthyroidism. Nevertheless, about three-quarters of these patients exhibit increased titers of anti-thyroid antibodies. Once present, these antibodies generally persist (76).
To date, human investigations have not established a definitive link between the severity of thyroid disorders and the concentration of anti-TPO antibodies in serum. Nonetheless, it is recognized that a positive serum anti-TPO antibody is associated with the active phase of thyroid pathology (77). In disease-free populations, anti-TPO detectability was associated with a higher risk of overall, cancer-related, and cardiovascular mortality, and this finding was more prominent in men than women (78).
Women with positive anti-TPO may have an increased risk for first-trimester miscarriage (79), preterm delivery (80), and offspring with impaired cognitive development (81). This risk may be due to reduced thyroid functional reserve from chronic autoimmune thyroiditis leading to subtle hypothyroidism (82).
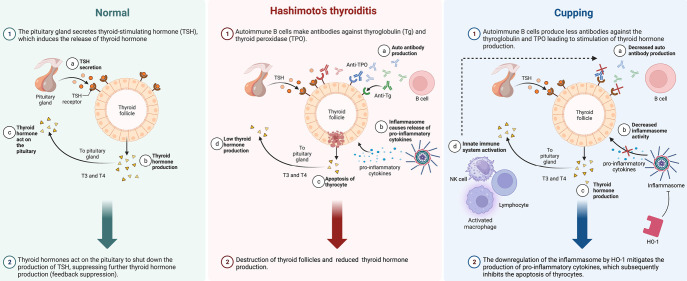
According to the results of Obeid et al.’s 2022 controlled trial among HT patients, there was a significant decrease in anti-TPO and anti-Tg in the wet cupping group vs. an increase in the control group, and the intervention group comparison was statistically significant. On a fixed T4 supplement for each patient, a significant decrease in levels of anti-TPO and anti-Tg occurred, supporting the immuno-modulatory role of wet cupping (43) (Figure 3).
Figure 3.
Immunomodulation overview consisting of a side-by-side comparison between mechanisms in the healthy state, Hashimoto’s thyroiditis, and Hashimoto’s thyroiditis following wet cupping. Normal thyroid functioning consists of uninterrupted signaling from the pituitary gland to thyrocytes via TSH, producing thyroid hormone production within the thyroid follicle, which partakes in a negative feedback loop to maintain appropriate hormone production levels (left column). The altered immunomodulation of thyroid hormone production in patients with Hashimoto’s thyroiditis entails elevated autoantibody levels (anti-TPO and anti-Tg) and upregulation of inflammasome activity, leading to pathologic production of pro-inflammatory cytokines and subsequent cell death of thyrocytes, resulting in under-production of thyroid hormone (center column). This pathologic immunomodulation is manipulated via cupping by way of downregulation of anti-Tg and anti-TPO, with concurrent decreased activity of inflammasomes due to inhibition by HO-1, resulting in lowered levels of pro-inflammatory cytokines; thyrocyte activity incurs partial restoration with adequate thyroid hormone production, and reinforcement of depressed autoantibody levels, maintained by recruitment of NK cells, lymphocytes, and macrophages, diminishing apoptosis of thyrocytes (right column). TSH, thyroid-stimulating hormone; Tg, thyroglobulin; TPO, thyroid peroxidase; HO-1, heme oxygenase-1; NK, natural killer.
Immunology-driven advancements to combat autoimmunity
Relating immunological therapies to thyroid function
The delicate interplay between the innate immune system, the adaptive immune system, and the molecular and cellular foundation of inflammatory effects confer the potential to inflict serious bodily harm when dysregulation ensues. It has been well-established that thyroid-specific genes and downstream signaling pathways influence autoimmune-related diseases of the thyroid (83). Both cytokines and antibodies in HT create a self-perpetuating autoimmune response, where the activation of immune cells and pro-inflammatory cytokines promotes the production of antibodies, which, in turn, contribute to heightened inflammation and tissue damage within the thyroid gland. Understanding the nature of these mechanisms in the healthy and diseased states remains vital for synthesizing effective treatments for HT.
As previously alluded, therapeutic approaches attempt to suppress over-active autoimmune responses, restoring the gentle balance of immunological function at the heart of glandular activity. Decreased levels of thyroid autoantibodies can potentially ameliorate the symptoms of HT, consequently improving thyroid hormone synthesis and mending malfunctioning immune responsiveness. Though anti-Tg and anti-TPO antibodies are non-pathologic, their presence is a signature of AITD. The medication levothyroxine has been specifically designed to replace thyroid hormones and immunosuppressive medications on the market (84). However, these pharmacokinetic remedies can be costly for patients (85-87). More novel treatments have enabled targeted therapies emphasizing specific cytokines and immune cells (88,89), made possible by the growing pool of scientific evidence pointing to particular molecular mechanisms at play in HT. As such, the evolution of our biomechanical knowledge concerning thyroid diseases heavily contributes to the direction of translational research concerning targeted therapies for autoimmune dysregulation involving the thyroid; innovative efforts in basic and clinical research continue to inform and direct the usage of newfound insights to probe the interrelationship between all aspects of immune function in HT.
Potential uses of wet cupping in patients with HT
Of significance, existing modalities for treating HT aim to address thyroid dysfunction instead of targeting the underlying autoimmune process. Wet cupping techniques prevail by delving into the fundamentals of innate and adaptive immune functioning, manipulating the raw interactions at play, and targeting feasible steps in molecular pathways for autoimmune therapeutic intervention.
While the efficacy and application of wet cupping harbor scarce prior research directly studying its effects on the immune system and improved physiological function in HT patients, the potential for its utility remains viably beneficial. Both extremely valuable in enhancing health outcomes and widely underutilized, wet cupping offers the possibility of diminishing classic HT symptoms. It even prompts a first line of defense in the form of preventative care for the disease-free population. HT is a common autoimmune disorder associated with systematic chronic inflammation and thyroid malfunction. By mediating adaptive and innate cell signaling, these control centers within the thyroid effectively modulate diminished immune responses via wet cupping. Although complete functional restoration of thyroid activity is beyond the current realm of existent treatment options, wet cupping manipulates the core components of immunity to combat autoimmunity itself, consequently dampening the immunosuppressive ramifications of autoimmune disease. The elective practice of wet cupping for those with genetic or environmental susceptibility to HT may also mitigate the risk of compromised autoimmune function. Additionally, for already immunosuppressed individuals with thyroid cancer, evidence points to the positive facets of patient outcomes that may accompany the prospect of routinely administering such treatment among these patients.
This review is meant to highlight key biological and immunological mechanisms influenced by the practice of wet cupping and shed light on the impact of its implementation in the clinical setting in hopes to illuminate the benefit of its role in management of HT. The comprehensive approach to methodology grants thorough review of the limited established research pertaining to the topic, grappling with this viable treatment modality on a molecular and scientific level, appreciating the longstanding significance of this therapy throughout time. Nevertheless, the limited quantity of evidence-based studies directly examining the impact of this treatment in those with HT make direct quantitative analysis difficult to accomplish.
Conclusions
Few studies exist examining the therapeutic effects of wet cupping on autoimmune diseases. Nevertheless, no study undergone in the United States focuses the therapy solely on HT, and such therapy among this population has yet to be practiced domestically in modern medicine for this reason. This review elucidates the physiological implications of the practice and purports to motivate healthcare providers to trial this therapy on HT patients.
Studies that have examined the effects of cupping and patient outcomes of wet cupping as an adjunctive autoimmune treatment to hormone replacement therapy require clear consensus. As such, we advocate for future clinical trials dedicated towards any of three main objectives: (I) consider the influence of genetic characteristics and epigenetic alterations on the immunological mechanisms of wet cupping, (II) identify the most suitable location for physical positioning of the cups for promoting beneficial immunostimulatory effects in HT patients, and (III) evaluate specific immunological markers via pre- and post-treatment blood sampling in an HT patient population undergoing wet cupping for therapeutic purposes.
Large-scale clinical trials targeting any one of these three future directions will significantly advance our understanding of wet cupping therapy’s immunological mechanisms. This knowledge can improve patient selection and treatment outcomes, help design more personalized modes of therapy for autoimmune diseases, and contribute to evidence-based integration of wet cupping into clinical practice.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-24-173/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-24-173/coif). E.K. serves as an Editor-in-Chief of Gland Surgery from May 2024 to April 2026. The other authors have no conflicts of interest to declare.
References
- 1.Ma Y, Zeng J, Jiang Y, et al. Thyroid function and associated mood changes after COVID-19 vaccines in patients with Hashimoto thyroiditis. Front Immunol 2023;14:1129746. 10.3389/fimmu.2023.1129746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mincer DL, Jialal I. Hashimoto Thyroiditis. Treasure Island, FL, USA: StatPearls Publishing; 2023. [PubMed] [Google Scholar]
- 3.Almahari SA, Maki R, Al Teraifi N, et al. Hashimoto Thyroiditis beyond Cytology: A Correlation between Cytological, Hormonal, Serological, and Radiological Findings. J Thyroid Res 2023;2023:5707120. 10.1155/2023/5707120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lorini R, Gastaldi R, Traggiai C, et al. Hashimoto's Thyroiditis. Pediatr Endocrinol Rev 2003;1 Suppl 2:205-11; discussion 211. [PubMed] [Google Scholar]
- 5.Segni M. Disorders of the Thyroid Gland in Infancy, Childhood and Adolescence. In: Feingold KR, Anawalt B, Blackman MR, et al. editors. Endotext. South Dartmouth, MA, USA: MDText.com, Inc.; 2000. [PubMed] [Google Scholar]
- 6.Wiersinga WM. T4+T3 Combination Therapy: An Unsolved Problem of Increasing Magnitude and Complexity. Endocrinol Metab (Seoul) 2021;36:938-51. 10.3803/EnM.2021.501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conrad N, Misra S, Verbakel JY, et al. Incidence, prevalence, and co-occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: a population-based cohort study of 22 million individuals in the UK. Lancet 2023;401:1878-90. 10.1016/S0140-6736(23)00457-9 [DOI] [PubMed] [Google Scholar]
- 8.Akamizu T, Amino N. Hashimoto’s Thyroiditis. In: Feingold KR, Anawalt B, Blackman MR, et al. editors. Endotext. South Dartmouth, MA, USA: MDText.com, Inc.; 2000. [Google Scholar]
- 9.Ettleson MD, Bianco AC. Individualized Therapy for Hypothyroidism: Is T4 Enough for Everyone? J Clin Endocrinol Metab 2020;105:e3090-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonklaas J. Optimal Thyroid Hormone Replacement. Endocr Rev 2022;43:366-404. 10.1210/endrev/bnab031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid 2014;24:1670-751. 10.1089/thy.2014.0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor PN, Eligar V, Muller I, et al. Combination Thyroid Hormone Replacement; Knowns and Unknowns. Front Endocrinol (Lausanne) 2019;10:706. 10.3389/fendo.2019.00706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaker L, Razvi S, Bensenor IM, et al. Hypothyroidism. Nat Rev Dis Primers 2022;8:30. 10.1038/s41572-022-00357-7 [DOI] [PubMed] [Google Scholar]
- 14.Knezevic J, Starchl C, Tmava Berisha A, et al. Thyroid-Gut-Axis: How Does the Microbiota Influence Thyroid Function? Nutrients 2020;12:1769. 10.3390/nu12061769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danailova Y, Velikova T, Nikolaev G, et al. Nutritional Management of Thyroiditis of Hashimoto. Int J Mol Sci 2022;23:5144. 10.3390/ijms23095144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Bedah AMN, Elsubai IS, Qureshi NA, et al. The medical perspective of cupping therapy: Effects and mechanisms of action. J Tradit Complement Med 2019;9:90-7. 10.1016/j.jtcme.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qureshi NA, Ali GI, Abushanab TS, et al. History of cupping (Hijama): a narrative review of literature. J Integr Med 2017;15:172-81. 10.1016/S2095-4964(17)60339-X [DOI] [PubMed] [Google Scholar]
- 18.Aboushanab TS, AlSanad S. Cupping Therapy: An Overview from a Modern Medicine Perspective. J Acupunct Meridian Stud 2018;11:83-7. 10.1016/j.jams.2018.02.001 [DOI] [PubMed] [Google Scholar]
- 19.Danyali F, VaezMahvavi M, Ghazanfari T, et al. Comparison of the biochemical, hematological and immunological factors of "cupping" blood with normal venous blood. Journal of Physiology and Pharmacology 2009;13:78-87. [Google Scholar]
- 20.Gök S, Kazanci FH, Erdamar H, et al. Is it possible to remove heavy metals from the body by wet cupping therapy (Al-hijamah)? Indian Journal of Traditional Knowledge 2016;15:700-4. [Google Scholar]
- 21.Mehta P, Dhapte V. Cupping therapy: A prudent remedy for a plethora of medical ailments. J Tradit Complement Med 2015;5:127-34. 10.1016/j.jtcme.2014.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almaiman AA. Proteomic effects of wet cupping (Al-hijamah). Saudi Med J 2018;39:10-6. 10.15537/smj.2018.1.21212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ersoy S, Benli AR. Continue or stop applying wet cupping therapy (al-hijamah) in migraine headache:A randomized controlled trial. Complement Ther Clin Pract 2020;38:101065. 10.1016/j.ctcp.2019.101065 [DOI] [PubMed] [Google Scholar]
- 24.Sayed SME, Al-quliti A, Mahmoud HS, et al. Therapeutic Benefits of Al-hijamah: in Light of Modern Medicine and Prophetic Medicine. American Journal of Medical and Biological Research 2014;2:46-71. [Google Scholar]
- 25.Cunningham DD, Henning TP, Shain EB, et al. Blood extraction from lancet wounds using vacuum combined with skin stretching. J Appl Physiol (1985) 2002;92:1089-96. 10.1152/japplphysiol.00798.2001 [DOI] [PubMed] [Google Scholar]
- 26.Lowe DT. Cupping therapy: An analysis of the effects of suction on skin and the possible influence on human health. Complement Ther Clin Pract 2017;29:162-8. 10.1016/j.ctcp.2017.09.008 [DOI] [PubMed] [Google Scholar]
- 27.Ryter SW, Choi AM. Targeting heme oxygenase-1 and carbon monoxide for therapeutic modulation of inflammation. Transl Res 2016;167:7-34. 10.1016/j.trsl.2015.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jamal Uddin M, Joe Y, Kim SK, et al. IRG1 induced by heme oxygenase-1/carbon monoxide inhibits LPS-mediated sepsis and pro-inflammatory cytokine production. Cell Mol Immunol 2016;13:170-9. 10.1038/cmi.2015.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clérigues V, Guillén MI, Castejón MA, et al. Heme oxygenase-1 mediates protective effects on inflammatory, catabolic and senescence responses induced by interleukin-1β in osteoarthritic osteoblasts. Biochem Pharmacol 2012;83:395-405. 10.1016/j.bcp.2011.11.024 [DOI] [PubMed] [Google Scholar]
- 30.Clérigues V, Guillén MI, Gomar F, et al. Haem oxygenase-1 counteracts the effects of interleukin-1β on inflammatory and senescence markers in cartilage-subchondral bone explants from osteoarthritic patients. Clin Sci (Lond) 2012;122:239-50. 10.1042/CS20100519 [DOI] [PubMed] [Google Scholar]
- 31.Hassan N, Suleman R, Al-Azzani W, et al. Microparticle clearance theory: An update to the potential mechanisms of action of cupping therapy. Advances in Integrative Medicine 2021;8:68-72. [Google Scholar]
- 32.Dey-Hazra E, Hertel B, Kirsch T, et al. Detection of circulating microparticles by flow cytometry: influence of centrifugation, filtration of buffer, and freezing. Vasc Health Risk Manag 2010;6:1125-33. 10.2147/VHRM.S13236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diamant M, Tushuizen ME, Sturk A, et al. Cellular microparticles: new players in the field of vascular disease? Eur J Clin Invest 2004;34:392-401. 10.1111/j.1365-2362.2004.01355.x [DOI] [PubMed] [Google Scholar]
- 34.Nurden AT. Platelets, inflammation and tissue regeneration. Thromb Haemost 2011;105 Suppl 1:S13-33. 10.1160/THS10-11-0720 [DOI] [PubMed] [Google Scholar]
- 35.George FD. Microparticles in vascular diseases. Thromb Res 2008;122 Suppl 1:S55-9. 10.1016/S0049-3848(08)70020-3 [DOI] [PubMed] [Google Scholar]
- 36.Kent MW, Kelher MR, West FB, et al. The pro-inflammatory potential of microparticles in red blood cell units. Transfus Med 2014;24:176-81. 10.1111/tme.12123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Liu J, Chen X, et al. Dysfunctional endothelial-derived microparticles promote inflammatory macrophage formation via NF-кB and IL-1β signal pathways. J Cell Mol Med 2019;23:476-86. 10.1111/jcmm.13950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin ZB, Ci HB, Li Y, et al. Endothelial microparticles are increased in congenital heart diseases and contribute to endothelial dysfunction. J Transl Med 2017;15:4. 10.1186/s12967-016-1087-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fröhlich E, Wahl R. Thyroid Autoimmunity: Role of Anti-thyroid Antibodies in Thyroid and Extra-Thyroidal Diseases. Front Immunol 2017;8:521. 10.3389/fimmu.2017.00521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmed SM, Madbouly NH, Maklad SS, et al. Immunomodulatory effects of blood letting cupping therapy in patients with rheumatoid arthritis. Egypt J Immunol 2005;12:39-51. [PubMed] [Google Scholar]
- 41.Abdulaziz KS, Tareq Mohamad R, Saad El-Din Mahmoud L, et al. Effect of neurogenic acupoint cupping on high sensitive C-reactive protein and pain perception in female chronic pelvic pain: A randomized controlled trial. J Musculoskelet Neuronal Interact 2021;21:121-9. [PMC free article] [PubMed] [Google Scholar]
- 42.Dons'koi BV, Chernyshov VP, Osypchuk DV, et al. Repeated cupping manipulation temporary decreases natural killer lymphocyte frequency, activity and cytotoxicity. J Integr Med 2016;14:197-202. 10.1016/S2095-4964(16)60250-9 [DOI] [PubMed] [Google Scholar]
- 43.Obeid AM, Qari FA, Aljaouni SK, et al. The effect of wet-cupping therapy (hijama) in modulating autoimmune activity of Hashimoto's thyroiditis: A pilot controlled study. Saudi Med J 2022;43:45-52. 10.15537/smj.2022.43.1.20210755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Obohat R, Kazemeini S, Mansouri R, et al. Determination and comparison of laboratory parameters of venous blood before and after cupping therapy. PJMHS 2020;14:1405-9. Available online: https://pjmhsonline.com/2020/oct_dec/1405.pdf
- 45.Nair MG, Cochrane DW, Allen JE. Macrophages in chronic type 2 inflammation have a novel phenotype characterized by the abundant expression of Ym1 and Fizz1 that can be partly replicated in vitro. Immunol Lett 2003;85:173-80. 10.1016/s0165-2478(02)00225-0 [DOI] [PubMed] [Google Scholar]
- 46.Stein M, Keshav S, Harris N, et al. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med 1992;176:287-92. 10.1084/jem.176.1.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doyle AG, Herbein G, Montaner LJ, et al. Interleukin-13 alters the activation state of murine macrophages in vitro: comparison with interleukin-4 and interferon-gamma. Eur J Immunol 1994;24:1441-5. 10.1002/eji.1830240630 [DOI] [PubMed] [Google Scholar]
- 48.Fairweather D, Cihakova D. Alternatively activated macrophages in infection and autoimmunity. J Autoimmun 2009;33:222-30. 10.1016/j.jaut.2009.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity 2010;32:593-604. 10.1016/j.immuni.2010.05.007 [DOI] [PubMed] [Google Scholar]
- 50.Brombacher F, Arendse B, Peterson R, et al. Analyzing classical and alternative macrophage activation in macrophage/neutrophil-specific IL-4 receptor-alpha-deficient mice. Methods Mol Biol 2009;531:225-52. 10.1007/978-1-59745-396-7_15 [DOI] [PubMed] [Google Scholar]
- 51.Chen S, Saeed AFUH, Liu Q, et al. Macrophages in immunoregulation and therapeutics. Signal Transduct Target Ther 2023;8:207. 10.1038/s41392-023-01452-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soleimani R, Mohammadi M, Saghebi SA, et al. Comparison of Th1/Th2 and Treg/Th17 ratios between wet and dry cupping therapies in Persian medicine. Avicenna J Phytomed 2020;10:24-34. [PMC free article] [PubMed] [Google Scholar]
- 53.El-Shanshory M, Hablas NM, El-Tahlawi R, et al. Al-hijamah (the triple S treatment of prophetic medicine) significantly increases CD4/CD8 ratio in thalassemic patients via increasing TAC/MDA ratio: a clinical trial. Am J Blood Res 2022;12:125-35. [PMC free article] [PubMed] [Google Scholar]
- 54.Yin C, Fang Y, Yao D, et al. Influencing Mechanism of Cupping Moxibustion on Gastrointestinal Function and Immune Function in Patients with Functional Diarrhea. Cell Mol Biol (Noisy-le-grand) 2022;68:98-104. 10.14715/cmb/2022.68.6.16 [DOI] [PubMed] [Google Scholar]
- 55.Zhang CQ, Liang TJ, Zhang W. Effects of drug cupping therapy on immune function in chronic asthmatic bronchitis patients during protracted period. Zhongguo Zhong Xi Yi Jie He Za Zhi 2006;26:984-7. [PubMed] [Google Scholar]
- 56.Sun H, Wan H, Zhang L, et al. Clinical observation of blood-letting to reduce pressure plus electroacupuncture for acute scapulohumeral periarthritis. Zhongguo Zhen Jiu 2016;36:933-7. 10.13703/j.0255-2930.2016.09.010 [DOI] [PubMed] [Google Scholar]
- 57.El-Domyati M, Saleh F, Barakat M, et al. Evaluation of cupping therapy in some dermatoses. Egyptian Dermatology Online Journal 2013;9:2. [Google Scholar]
- 58.Tian H, Tian YJ, Wang B, et al. Impacts of bleeding and cupping therapy on serum P substance in patients of postherpetic neuralgia. Zhongguo Zhen Jiu 2013;33:678-81. [PubMed] [Google Scholar]
- 59.Safdari V, Alijani E, Nemati M, et al. Imbalances in T Cell-Related Transcription Factors Among Patients with Hashimoto's Thyroiditis. Sultan Qaboos Univ Med J 2017;17:e174-80. 10.18295/squmj.2016.17.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shi Y, Wang H, Su Z, et al. Differentiation imbalance of Th1/Th17 in peripheral blood mononuclear cells might contribute to pathogenesis of Hashimoto's thyroiditis. Scand J Immunol 2010;72:250-5. 10.1111/j.1365-3083.2010.02425.x [DOI] [PubMed] [Google Scholar]
- 61.Kristensen B, Hegedüs L, Madsen HO, et al. Altered balance between self-reactive T helper (Th)17 cells and Th10 cells and between full-length forkhead box protein 3 (FoxP3) and FoxP3 splice variants in Hashimoto's thyroiditis. Clin Exp Immunol 2015;180:58-69. 10.1111/cei.12557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mazzieri A, Montanucci P, Basta G, et al. The role behind the scenes of Tregs and Th17s in Hashimoto's thyroiditis: Toward a pivotal role of FOXP3 and BACH2. Front Immunol 2022;13:1098243. 10.3389/fimmu.2022.1098243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gentile F, Conte M, Formisano S. Thyroglobulin as an autoantigen: what can we learn about immunopathogenicity from the correlation of antigenic properties with protein structure? Immunology 2004;112:13-25. 10.1111/j.1365-2567.2004.01861.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Caturegli P, Kuppers RC, Mariotti S, et al. IgG subclass distribution of thyroglobulin antibodies in patients with thyroid disease. Clin Exp Immunol 1994;98:464-9. 10.1111/j.1365-2249.1994.tb05514.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Doullay F, Ruf J, Codaccioni JL, et al. Prevalence of autoantibodies to thyroperoxidase in patients with various thyroid and autoimmune diseases. Autoimmunity 1991;9:237-44. 10.3109/08916939109007649 [DOI] [PubMed] [Google Scholar]
- 66.Kim ES, Lim DJ, Baek KH, et al. Thyroglobulin antibody is associated with increased cancer risk in thyroid nodules. Thyroid 2010;20:885-91. 10.1089/thy.2009.0384 [DOI] [PubMed] [Google Scholar]
- 67.Muzza M, Degl'Innocenti D, Colombo C, et al. The tight relationship between papillary thyroid cancer, autoimmunity and inflammation: clinical and molecular studies. Clin Endocrinol (Oxf) 2010;72:702-8. 10.1111/j.1365-2265.2009.03699.x [DOI] [PubMed] [Google Scholar]
- 68.McLeod DS, Cooper DS, Ladenson PW, et al. Prognosis of differentiated thyroid cancer in relation to serum thyrotropin and thyroglobulin antibody status at time of diagnosis. Thyroid 2014;24:35-42. 10.1089/thy.2013.0062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Szyper-Kravitz M, Marai I, Shoenfeld Y. Coexistence of thyroid autoimmunity with other autoimmune diseases: friend or foe? Additional aspects on the mosaic of autoimmunity. Autoimmunity 2005;38:247-55. 10.1080/08916930500050194 [DOI] [PubMed] [Google Scholar]
- 70.Feldt-Rasmussen U, Høier-Madsen M, Bech K, et al. Anti-thyroid peroxidase antibodies in thyroid disorders and non-thyroid autoimmune diseases. Autoimmunity 1991;9:245-54. 10.3109/08916939109007650 [DOI] [PubMed] [Google Scholar]
- 71.Weetman AP. Autoimmune thyroid disease. Autoimmunity 2004;37:337-40. 10.1080/08916930410001705394 [DOI] [PubMed] [Google Scholar]
- 72.Rapoport B, McLachlan SM. Thyroid autoimmunity. J Clin Invest 2001;108:1253-9. 10.1172/JCI14321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baskin HJ, Cobin RH, Duick DS, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. Endocr Pract 2002;8:457-69. [PubMed] [Google Scholar]
- 74.Vanderpump MP, Tunbridge WM, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf) 1995;43:55-68. 10.1111/j.1365-2265.1995.tb01894.x [DOI] [PubMed] [Google Scholar]
- 75.Huber G, Staub JJ, Meier C, et al. Prospective study of the spontaneous course of subclinical hypothyroidism: prognostic value of thyrotropin, thyroid reserve, and thyroid antibodies. J Clin Endocrinol Metab 2002;87:3221-6. 10.1210/jcem.87.7.8678 [DOI] [PubMed] [Google Scholar]
- 76.Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract 2012;18:988-1028. 10.4158/EP12280.GL [DOI] [PubMed] [Google Scholar]
- 77.Williams DE, Le SN, Godlewska M, et al. Thyroid Peroxidase as an Autoantigen in Hashimoto's Disease: Structure, Function, and Antigenicity. Horm Metab Res 2018;50:908-21. 10.1055/a-0717-5514 [DOI] [PubMed] [Google Scholar]
- 78.Khan SR, Peeters RP, van Hagen PM, et al. Determinants and Clinical Implications of Thyroid Peroxidase Antibodies in Middle-Aged and Elderly Individuals: The Rotterdam Study. Thyroid 2022;32:78-89. 10.1089/thy.2021.0403 [DOI] [PubMed] [Google Scholar]
- 79.Stagnaro-Green A, Roman SH, Cobin RH, et al. Detection of at-risk pregnancy by means of highly sensitive assays for thyroid autoantibodies. JAMA 1990;264:1422-5. [PubMed] [Google Scholar]
- 80.Negro R, Schwartz A, Gismondi R, et al. Thyroid antibody positivity in the first trimester of pregnancy is associated with negative pregnancy outcomes. J Clin Endocrinol Metab 2011;96:E920-4. 10.1210/jc.2011-0026 [DOI] [PubMed] [Google Scholar]
- 81.Yu X, Shan Z, Teng W. A prospective study on impact of subclinical hypothyroidism during pregnancy receiving levothyroxine treatment or not on neuropsychological development of the offspring. In: International Thyroid Conference. Paris, France: 2010. [Google Scholar]
- 82.Glinoer D, Riahi M, Grün JP, et al. Risk of subclinical hypothyroidism in pregnant women with asymptomatic autoimmune thyroid disorders. J Clin Endocrinol Metab 1994;79:197-204. 10.1210/jcem.79.1.8027226 [DOI] [PubMed] [Google Scholar]
- 83.Weetman AP. The immunopathogenesis of chronic autoimmune thyroiditis one century after hashimoto. Eur Thyroid J 2013;1:243-50. 10.1159/000343834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Colucci P, Yue CS, Ducharme M, et al. A Review of the Pharmacokinetics of Levothyroxine for the Treatment of Hypothyroidism. Eur Endocrinol 2013;9:40-7. 10.17925/EE.2013.09.01.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hennessey JV, Espaillat R, Duan Y, et al. The Association Between Switching from Synthroid(®) and Clinical Outcomes: US Evidence from a Retrospective Database Analysis. Adv Ther 2021;38:337-49. 10.1007/s12325-020-01537-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Katz M, Scherger J, Conard S, et al. Healthcare costs associated with switching from brand to generic levothyroxine. Am Health Drug Benefits 2010;3:127-34. [PMC free article] [PubMed] [Google Scholar]
- 87.Khandelwal N, Johns B, Hepp Z, et al. The economic impact of switching from Synthroid for the treatment of hypothyroidism. J Med Econ 2018;21:518-24. 10.1080/13696998.2018.1443110 [DOI] [PubMed] [Google Scholar]
- 88.Ganesh BB, Bhattacharya P, Gopisetty A, et al. Role of cytokines in the pathogenesis and suppression of thyroid autoimmunity. J Interferon Cytokine Res 2011;31:721-31. 10.1089/jir.2011.0049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee HJ, Stefan-Lifshitz M, Li CW, et al. Genetics and epigenetics of autoimmune thyroid diseases: Translational implications. Best Pract Res Clin Endocrinol Metab 2023;37:101661. 10.1016/j.beem.2022.101661 [DOI] [PMC free article] [PubMed] [Google Scholar]