Abstract
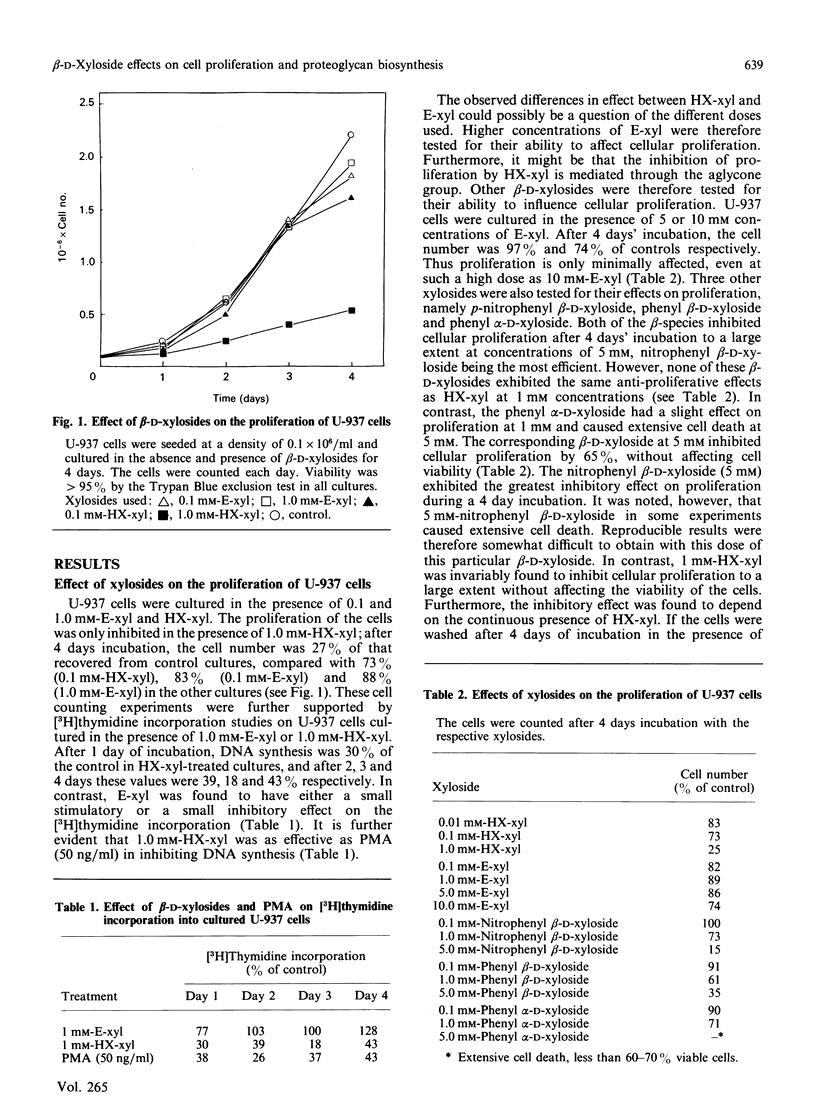
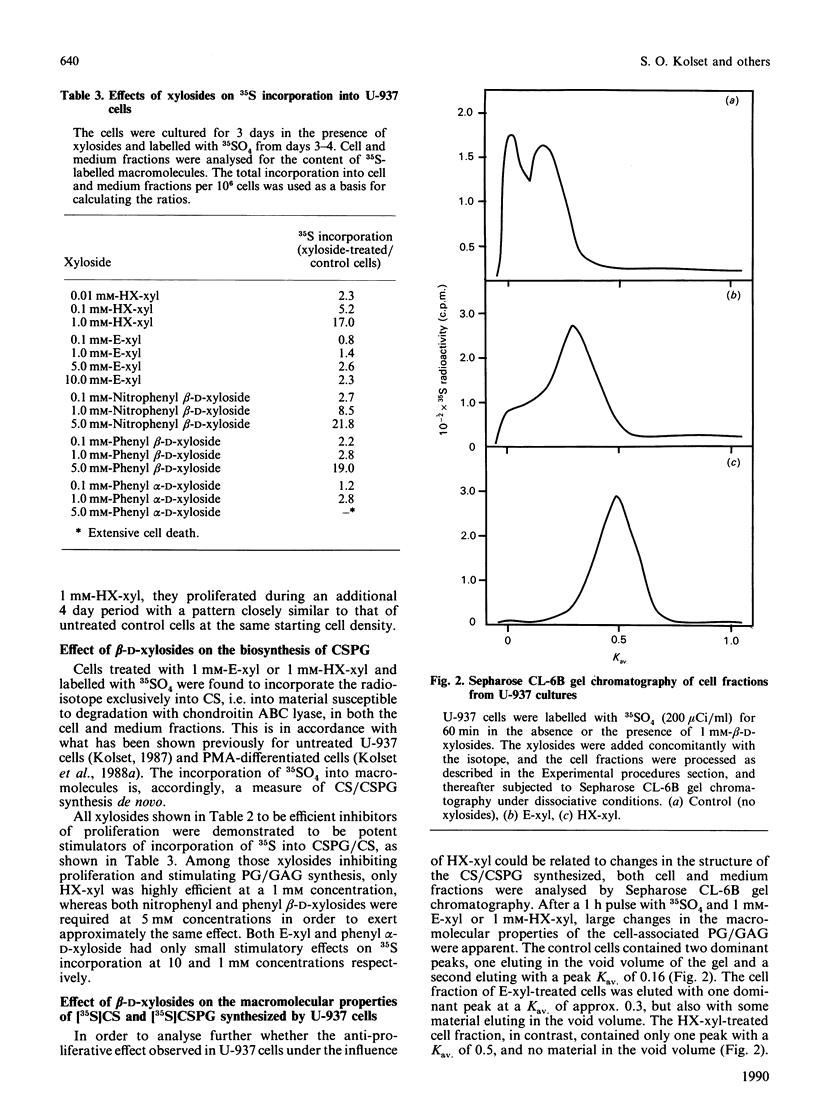
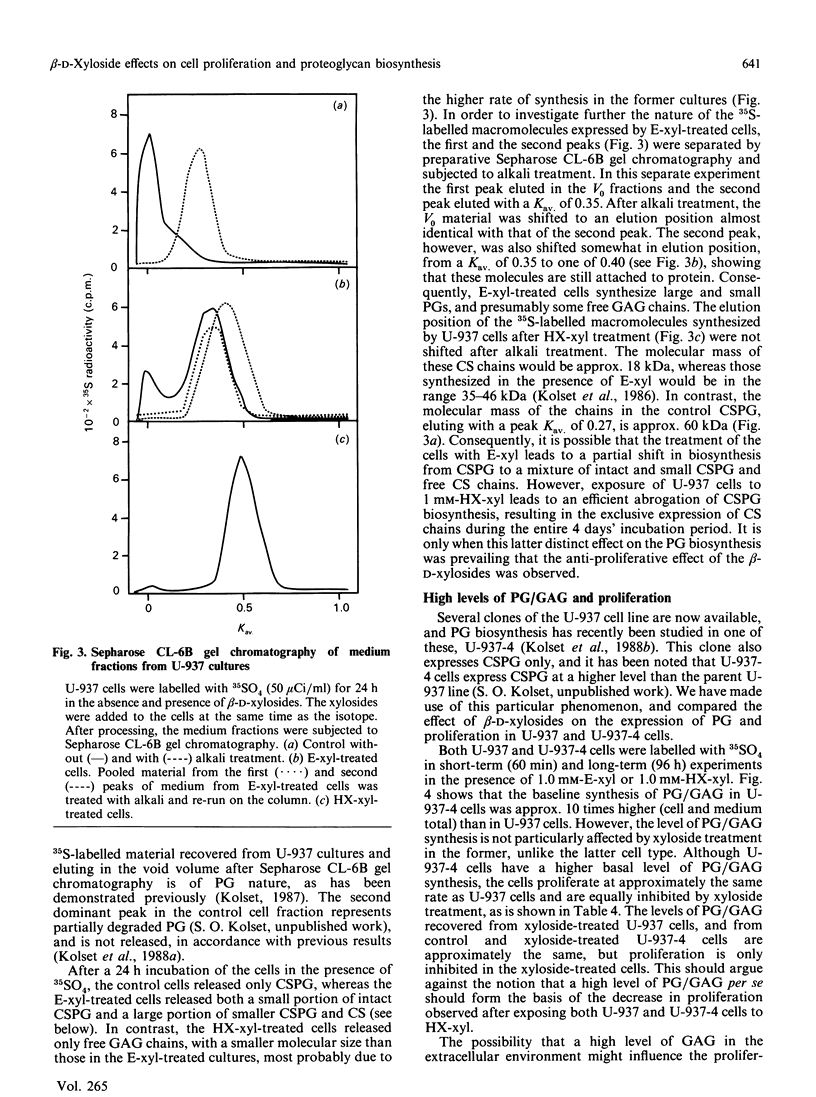
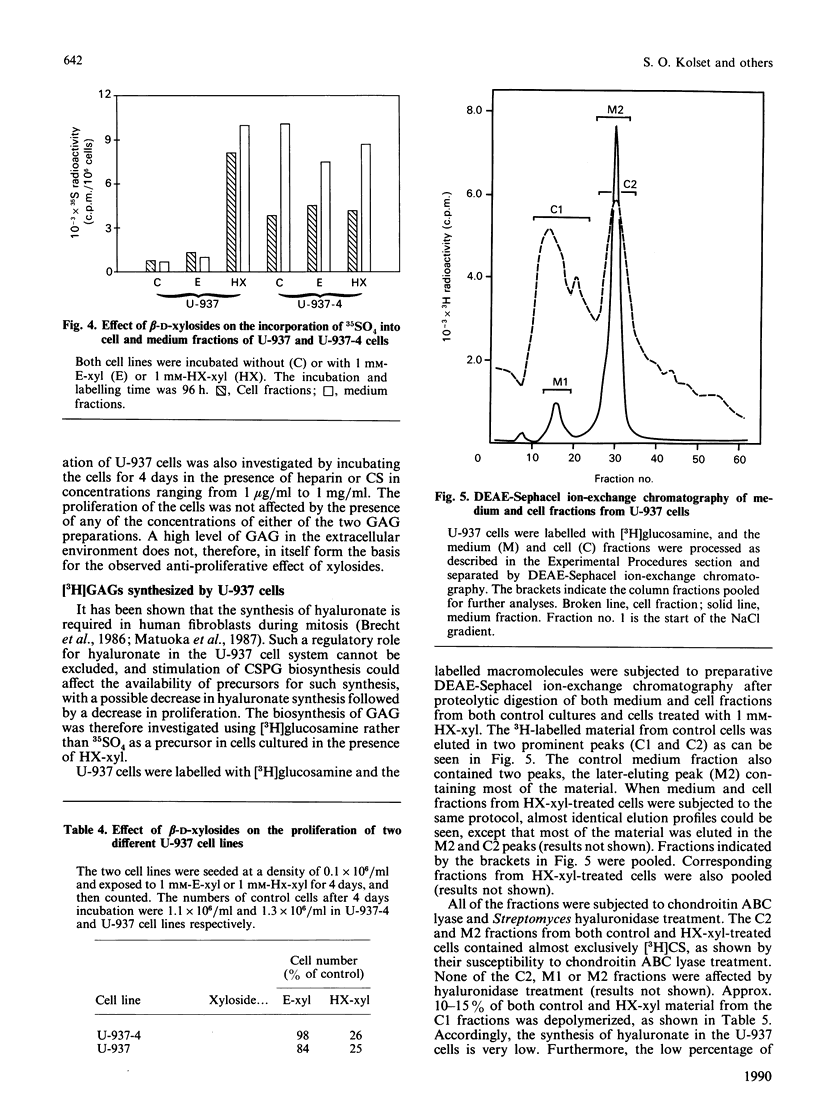
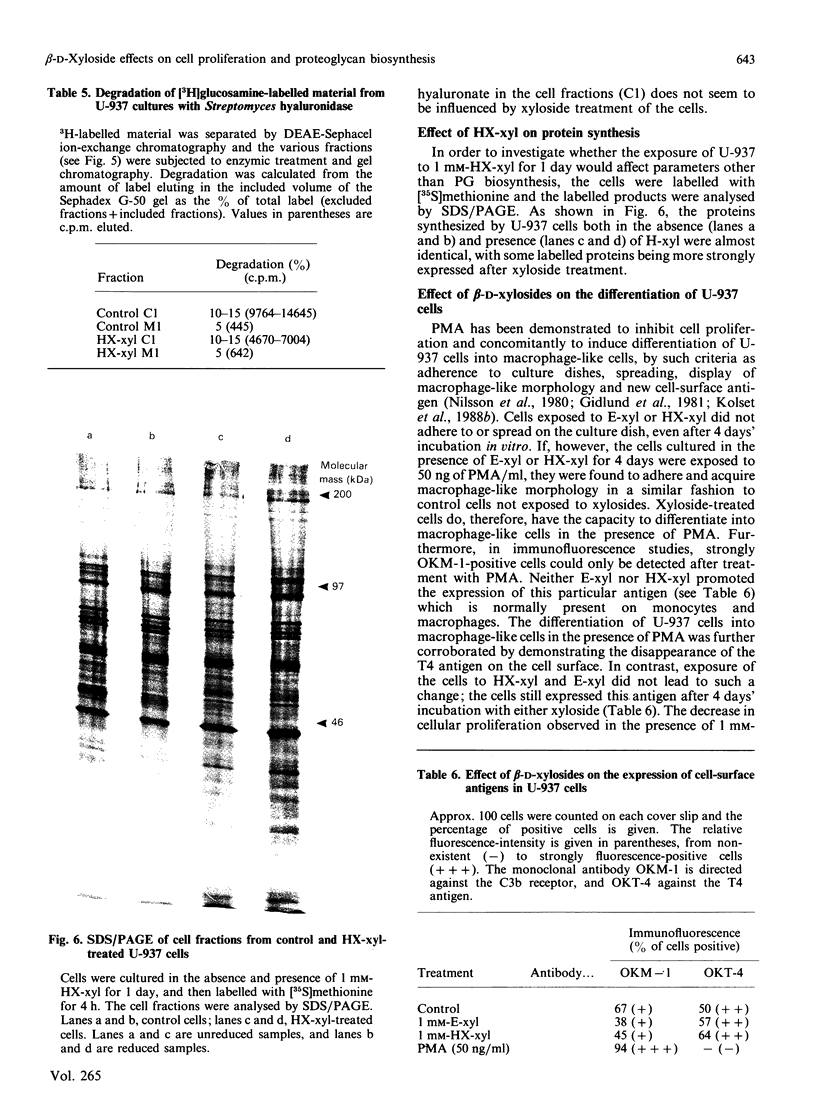
The monoblastic cell line U-937 was cultured in the presence of C-ethyl beta-D-xyloside (E-xyl), hexyl beta-D-thioxyloside (HX-xyl), p-nitrophenyl beta-D-xyloside, phenyl beta-D-xyloside or phenyl alpha-D-xyloside. All of the beta-D-xylosides inhibited proliferation, but HX-xyl was by far the most efficient, and had a maximum effect at 1 mM concentration. The inhibitory effect of HX-xyl could be reversed; after washing, the HX-xyl-treated cells proliferated with a pattern similar to that of control cells. For more detailed analysis of the effects of beta-D-xylosides on cell proliferation and chondroitin sulphate (CS)/chondroitin sulphate proteoglycan (CSPG) structure, a comparison between the effects of E-xyl and HX-xyl was made. Treating the cells with 1 mM-HX-xyl resulted in a large increase in CS synthesis, whereas 1 mM-E-xyl had only minor effects on the rate of PG/glycosaminoglycan synthesis. Sepharose CL-6B gel chromatography of medium and cell fractions from 35S-labelled cells revealed that HX-xyl treatment resulted in the expression of only free CS chains, whereas E-xyl exposure leads to the synthesis of both large and small CSPGs, as well as some free CS chains. The expression of elevated levels of free CS chains was clearly correlated to the inhibition of proliferation. The proliferation of U-937-4, a clone of U-937 synthesizing ten times more CSPG/CS than the parent line, was equally inhibited by HX-xyl treatment. With this clone, however, there was no stimulation of CS synthesis after xyloside exposure, indicating that the elevated level of CS evident after xyloside treatment of the parent cell line is not causing the inhibition of proliferation. Furthermore, the biosynthesis of hyaluronate was shown not to be implicated in the xyloside-induced decrease in proliferation. The inhibition of proliferation observed in the presence of 1 mM-HX-xyl did not lead to differentiation of the cells into macrophage-like cells, as is observed when the cells are cultured in the presence of phorbol esters, agents also known to inhibit proliferation of U-937 cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brecht M., Mayer U., Schlosser E., Prehm P. Increased hyaluronate synthesis is required for fibroblast detachment and mitosis. Biochem J. 1986 Oct 15;239(2):445–450. doi: 10.1042/bj2390445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christmas S. E., Steward W. P., Lyon M., Gallagher J. T., Moore M. Chondroitin sulphate proteoglycan production by NK cells and T cells: effects of xylosides on proliferation and cytotoxic function. Immunology. 1988 Feb;63(2):225–231. [PMC free article] [PubMed] [Google Scholar]
- Gibson K. D., Doller H. J., Hoar R. M. beta-D-xylosides cause abnormalities of growth and development in chick embryos. Nature. 1978 May 11;273(5658):151–154. doi: 10.1038/273151a0. [DOI] [PubMed] [Google Scholar]
- Gidlund M., Orn A., Pattengale P. K., Jansson M., Wigzell H., Nilsson K. Natural killer cells kill tumour cells at a given stage of differentiation. Nature. 1981 Aug 27;292(5826):848–850. doi: 10.1038/292848a0. [DOI] [PubMed] [Google Scholar]
- Kinoshita S., Saiga H. The role of proteoglycan in the development of sea urchins. I. Abnormal development of sea urchin embryos caused by the disturbance of proteoglycan synthesis. Exp Cell Res. 1979 Oct 15;123(2):229–236. doi: 10.1016/0014-4827(79)90463-4. [DOI] [PubMed] [Google Scholar]
- Kolset S. O., Ehlorsson J., Kjellén L., Lindahl U. Effect of benzyl beta-D-xyloside on the biosynthesis of chondroitin sulphate proteoglycan in cultured human monocytes. Biochem J. 1986 Aug 15;238(1):209–216. doi: 10.1042/bj2380209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolset S. O., Ivhed I., Overvatn A., Nilsson K. Differentiation-associated changes in the expression of chondroitin sulfate proteoglycan in induced U-937 cells. Cancer Res. 1988 Nov 1;48(21):6103–6108. [PubMed] [Google Scholar]
- Kolset S. O., Kjellén L., Seljelid R., Lindahl U. Changes in glycosaminoglycan biosynthesis during differentiation in vitro of human monocytes. Biochem J. 1983 Mar 15;210(3):661–667. doi: 10.1042/bj2100661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolset S. O. Proteoglycans in normal and neoplastic monocytes. Exp Cell Res. 1987 Feb;168(2):318–324. doi: 10.1016/0014-4827(87)90004-8. [DOI] [PubMed] [Google Scholar]
- Levitt D., Olmstead L. Stimulation of mouse B cells by a factor that coisolates with T-cell proteoglycan. Cell Immunol. 1986 Mar;98(1):78–92. doi: 10.1016/0008-8749(86)90269-8. [DOI] [PubMed] [Google Scholar]
- Lindahl U., Hök M. Glycosaminoglycans and their binding to biological macromolecules. Annu Rev Biochem. 1978;47:385–417. doi: 10.1146/annurev.bi.47.070178.002125. [DOI] [PubMed] [Google Scholar]
- Matuoka K., Namba M., Mitsui Y. Hyaluronate synthetase inhibition by normal and transformed human fibroblasts during growth reduction. J Cell Biol. 1987 Apr;104(4):1105–1115. doi: 10.1083/jcb.104.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscicki R. A., Amento E. P., Krane S. M., Kurnick J. T., Colvin R. B. Modulation of surface antigens of a human monocyte cell line, U937, during incubation with T lymphocyte-conditioned medium: detection of T4 antigen and its presence on normal blood monocytes. J Immunol. 1983 Aug;131(2):743–748. [PubMed] [Google Scholar]
- Netzloff M. L., Streiff R. R., Frias J. L., Rennert O. M. Folate antagonism following teratogenic exposure to diphenylhydantoin. Teratology. 1979 Feb;19(1):45–49. doi: 10.1002/tera.1420190108. [DOI] [PubMed] [Google Scholar]
- Okayama M., Kimata K., Suzuki S. The influence of p-nitrophenyl beta-d-xyloside on the synthesis of proteochondroitin sulfate by slices of embryonic chick cartilage. J Biochem. 1973 Nov;74(5):1069–1073. [PubMed] [Google Scholar]
- Roberts R., Gallagher J., Spooncer E., Allen T. D., Bloomfield F., Dexter T. M. Heparan sulphate bound growth factors: a mechanism for stromal cell mediated haemopoiesis. Nature. 1988 Mar 24;332(6162):376–378. doi: 10.1038/332376a0. [DOI] [PubMed] [Google Scholar]
- Robinson H. C., Brett M. J., Tralaggan P. J., Lowther D. A., Okayama M. The effect of D-xylose, beta-D-xylosides and beta-D-galactosides on chondroitin sulphate biosynthesis in embryonic chicken cartilage. Biochem J. 1975 Apr;148(1):25–34. doi: 10.1042/bj1480025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. A., Robinson H. C. Control of chondroitin sulphate biosynthesis. beta-D-Xylopyranosides as substrates for UDP-galactose: D-xylose transferase from embryonic-chicken cartilage. Biochem J. 1981 Mar 15;194(3):839–846. doi: 10.1042/bj1940839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz N. B., Galligani L., Ho P. L., Dorfman A. Stimulation of synthesis of free chondroitin sulfate chains by beta-D-xylosides in cultured cells. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4047–4051. doi: 10.1073/pnas.71.10.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobue M., Habuchi H., Ito K., Yonekura H., Oguri K., Sakurai K., Kamohara S., Ueno Y., Noyori R., Suzuki S. beta-D-xylosides and their analogues as artificial initiators of glycosaminoglycan chain synthesis. Aglycone-related variation in their effectiveness in vitro and in ovo. Biochem J. 1987 Jan 15;241(2):591–601. doi: 10.1042/bj2410591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooncer E., Gallagher J. T., Krizsa F., Dexter T. M. Regulation of haemopoiesis in long-term bone marrow cultures. IV. Glycosaminoglycan synthesis and the stimulation of haemopoiesis by beta-D-xylosides. J Cell Biol. 1983 Feb;96(2):510–514. doi: 10.1083/jcb.96.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Thompson H. A., Spooner B. S. Proteoglycan and glycosaminoglycan synthesis in embryonic mouse salivary glands: effects of beta-D-xyloside, an inhibitor of branching morphogenesis. J Cell Biol. 1983 May;96(5):1443–1450. doi: 10.1083/jcb.96.5.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]