Abstract
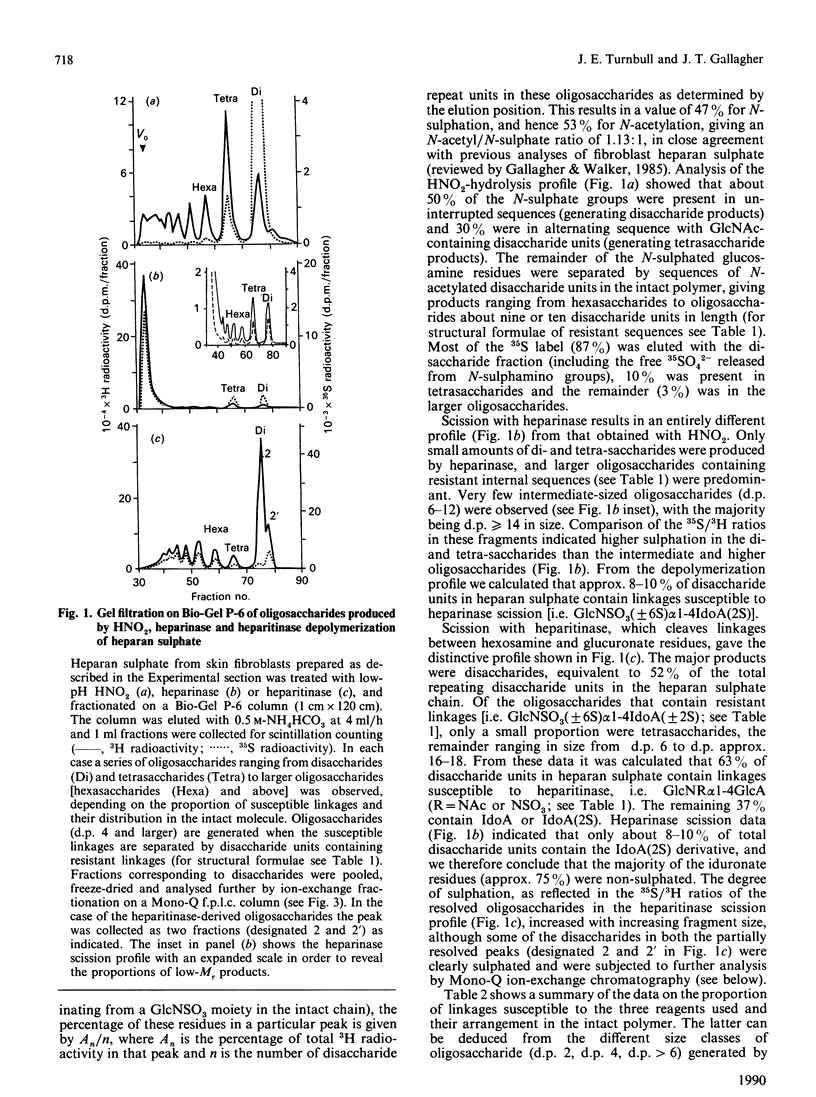
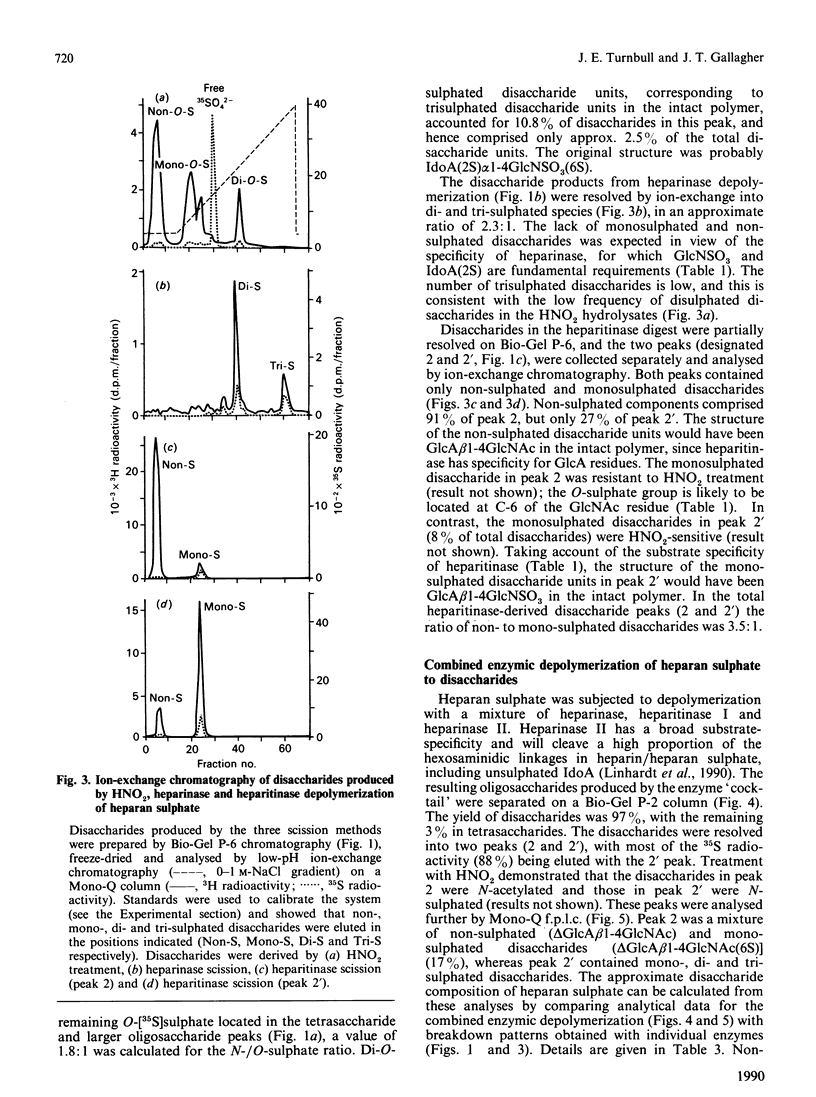
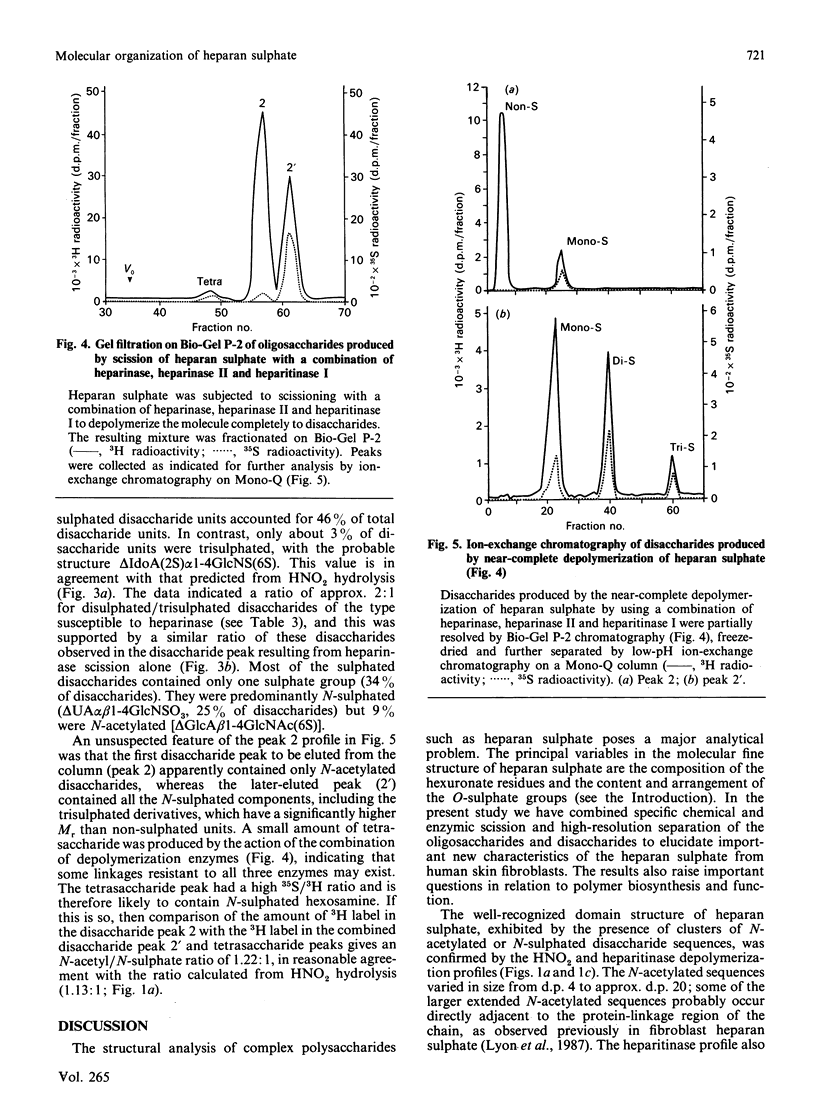
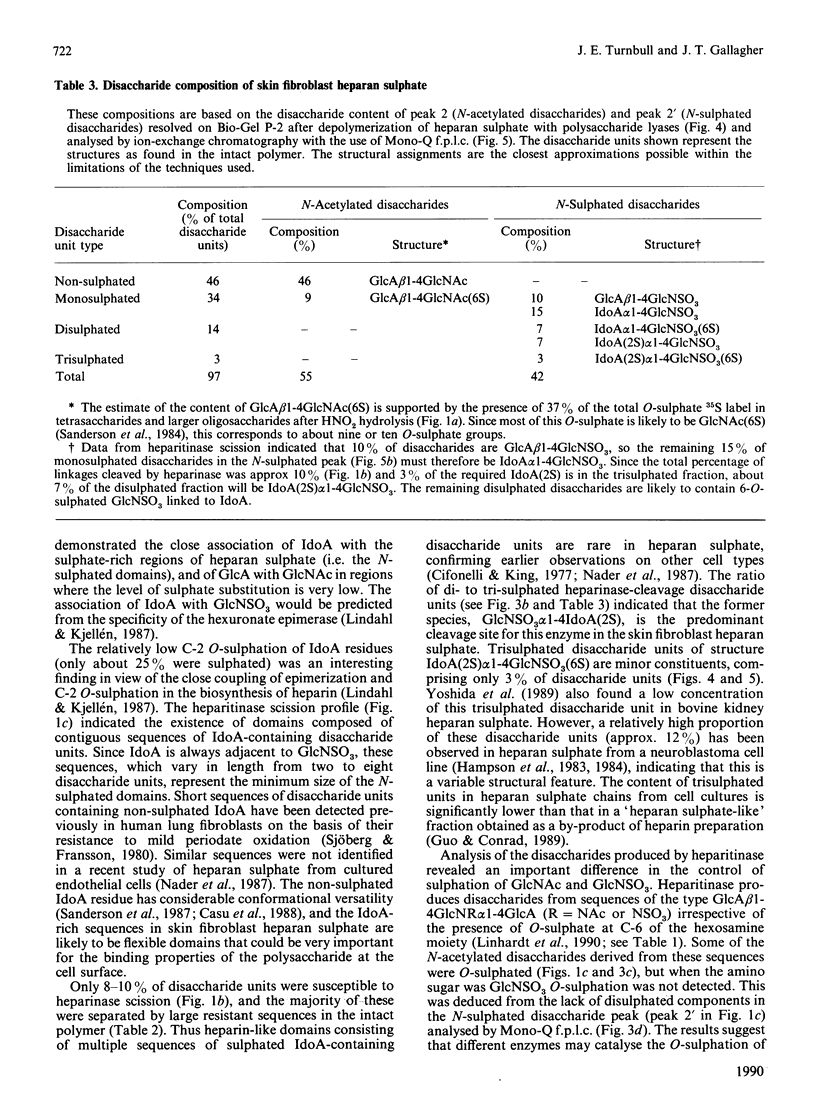
The molecular structure of human skin fibroblast heparan sulphate was examined by specific chemical or enzymic depolymerization and high-resolution separation of the resulting oligosaccharides and disaccharides. Important features of the molecular organization, disaccharide composition and O-sulphate disposition of this heparan sulphate were identified. Analysis of the products of HNO2 hydrolysis revealed a polymer in which 53% of disaccharide units were N-acetylated and 47% N-sulphated, with an N-/O-sulphate ratio of 1.8:1. These two types of disaccharide unit were mainly located in separate domains. Heparitinase and heparinase scission indicated that the iduronate residues (37% of total hexuronate) were largely present in contiguous disaccharide sequences of variable size that also contained the majority of the N-sulphate groups. Most of the iduronate residues (approx. 70%) were non-sulphated. About 8-10% of disaccharide units were cleaved by heparinase, but only a minority of these originated from contiguous sequences in the intact polymer. Trisulphated disaccharide units [alpha-N-sulpho-6-sulphoglucosaminyl-(1----4)-iduronate 2-sulphate], which are the major structural units in heparin, made up only 3% of the disaccharide units in heparan sulphate. O-Sulphate groups (approx. 26 per 100 disaccharide units) were distributed almost evenly among C-6 of N-acetylglucosamine, C-2 of iduronate and C-6 of N-sulphated glucosamine residues. The results indicate that the sulphated regions of heparan sulphate have distinctive and potentially variable structural characteristics. The high content of non-sulphated iduronate in this heparan sulphate species suggests a conformational versatility that could have important implications for the biological properties of the polymer.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bame K. J., Esko J. D. Undersulfated heparan sulfate in a Chinese hamster ovary cell mutant defective in heparan sulfate N-sulfotransferase. J Biol Chem. 1989 May 15;264(14):8059–8065. [PubMed] [Google Scholar]
- Bienkowski M. J., Conrad H. E. Structural characterization of the oligosaccharides formed by depolymerization of heparin with nitrous acid. J Biol Chem. 1985 Jan 10;260(1):356–365. [PubMed] [Google Scholar]
- Casu B., Petitou M., Provasoli M., Sinaÿ P. Conformational flexibility: a new concept for explaining binding and biological properties of iduronic acid-containing glycosaminoglycans. Trends Biochem Sci. 1988 Jun;13(6):221–225. doi: 10.1016/0968-0004(88)90088-6. [DOI] [PubMed] [Google Scholar]
- Cifonelli J. A., King J. A. Structural characteristics of heparan sulfates with varying sulfate contents. Biochemistry. 1977 May 17;16(10):2137–2141. doi: 10.1021/bi00629a014. [DOI] [PubMed] [Google Scholar]
- Fedarko N. S., Conrad H. E. A unique heparan sulfate in the nuclei of hepatocytes: structural changes with the growth state of the cells. J Cell Biol. 1986 Feb;102(2):587–599. doi: 10.1083/jcb.102.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson L. A., Sjöberg I., Havsmark B. Structural studies on heparan sulphates. Characterization of oligosaccharides; obtained by periodate oxidation and alkaline elimination. Eur J Biochem. 1980 May;106(1):59–69. [PubMed] [Google Scholar]
- Gallagher J. T., Lyon M., Steward W. P. Structure and function of heparan sulphate proteoglycans. Biochem J. 1986 Jun 1;236(2):313–325. doi: 10.1042/bj2360313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher J. T., Walker A. Molecular distinctions between heparan sulphate and heparin. Analysis of sulphation patterns indicates that heparan sulphate and heparin are separate families of N-sulphated polysaccharides. Biochem J. 1985 Sep 15;230(3):665–674. doi: 10.1042/bj2300665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y. C., Conrad H. E. The disaccharide composition of heparins and heparan sulfates. Anal Biochem. 1989 Jan;176(1):96–104. doi: 10.1016/0003-2697(89)90278-9. [DOI] [PubMed] [Google Scholar]
- Hampson I. N., Kumar S., Gallagher J. T. Differences in the distribution of O-sulphate groups of cell-surface and secreted heparan sulphate produced by human neuroblastoma cells in culture. Biochim Biophys Acta. 1983 Sep 22;763(2):183–190. doi: 10.1016/0167-4889(83)90043-5. [DOI] [PubMed] [Google Scholar]
- Hampson I. N., Kumar S., Gallagher J. T. Heterogeneity of cell-associated and secretory heparan sulphate proteoglycans produced by cultured human neuroblastoma cells. Biochim Biophys Acta. 1984 Sep 28;801(2):306–313. doi: 10.1016/0304-4165(84)90081-3. [DOI] [PubMed] [Google Scholar]
- Hök M., Kjellén L., Johansson S. Cell-surface glycosaminoglycans. Annu Rev Biochem. 1984;53:847–869. doi: 10.1146/annurev.bi.53.070184.004215. [DOI] [PubMed] [Google Scholar]
- Lindahl U., Thunberg L., Bäckström G., Riesenfeld J., Nordling K., Björk I. Extension and structural variability of the antithrombin-binding sequence in heparin. J Biol Chem. 1984 Oct 25;259(20):12368–12376. [PubMed] [Google Scholar]
- Linker A., Hovingh P. The uses of degradative enzymes as tools for identification and structural analysis of glycosaminoglycans. Fed Proc. 1977 Jan;36(1):43–46. [PubMed] [Google Scholar]
- Lyon M., Steward W. P., Hampson I. N., Gallagher J. T. Identification of an extended N-acetylated sequence adjacent to the protein-linkage region of fibroblast heparan sulphate. Biochem J. 1987 Mar 1;242(2):493–498. doi: 10.1042/bj2420493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader H. B., Dietrich C. P., Buonassisi V., Colburn P. Heparin sequences in the heparan sulfate chains of an endothelial cell proteoglycan. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3565–3569. doi: 10.1073/pnas.84.11.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pejler G., Bäckström G., Lindahl U., Paulsson M., Dziadek M., Fujiwara S., Timpl R. Structure and affinity for antithrombin of heparan sulfate chains derived from basement membrane proteoglycans. J Biol Chem. 1987 Apr 15;262(11):5036–5043. [PubMed] [Google Scholar]
- Pejler G., David G. Basement-membrane heparan sulphate with high affinity for antithrombin synthesized by normal and transformed mouse mammary epithelial cells. Biochem J. 1987 Nov 15;248(1):69–77. doi: 10.1042/bj2480069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice K. G., Linhardt R. J. Study of structurally defined oligosaccharide substrates of heparin and heparan monosulfate lyases. Carbohydr Res. 1989 Jul 15;190(2):219–233. doi: 10.1016/0008-6215(89)84127-8. [DOI] [PubMed] [Google Scholar]
- Riesenfeld J., Hözok M., Lindahl U. Biosynthesis of heparan sulfate in rat liver. Characterization of polysaccharides obtained with intact cells and with a cell-free system. J Biol Chem. 1982 Jun 25;257(12):7050–7055. [PubMed] [Google Scholar]
- Sanderson P. N., Huckerby T. N., Nieduszynski I. A. Conformational equilibria of alpha-L-iduronate residues in disaccharides derived from heparin. Biochem J. 1987 Apr 1;243(1):175–181. doi: 10.1042/bj2430175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson P. N., Huckerby T. N., Nieduszynski I. A. Very-high-field n.m.r. studies of bovine lung heparan sulphate tetrasaccharides produced by nitrous acid deaminative cleavage. Determination of saccharide sequence, uronate composition and degrees of sulphation. Biochem J. 1984 Oct 15;223(2):495–505. doi: 10.1042/bj2230495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Formation of anhydrosugars in the chemical depolymerization of heparin. Biochemistry. 1976 Sep 7;15(18):3932–3942. doi: 10.1021/bi00663a005. [DOI] [PubMed] [Google Scholar]
- Sjöberg I., Fransson L. A. Structural studies on heparan sulphate from human lung fibroblasts. Characterization of oligosaccharides obtained by selective periodate oxidation of D-glucuronic acid residues followed by scission in alkali. Biochem J. 1980 Oct 1;191(1):103–110. doi: 10.1042/bj1910103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull J. E., Gallagher J. T. Oligosaccharide mapping of heparan sulphate by polyacrylamide-gradient-gel electrophoresis and electrotransfer to nylon membrane. Biochem J. 1988 Apr 15;251(2):597–608. doi: 10.1042/bj2510597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasteson A. A method for the determination of the molecular weight and molecular-weight distribution of chondroitin sulphate. J Chromatogr. 1971 Jul 8;59(1):87–97. doi: 10.1016/s0021-9673(01)80009-1. [DOI] [PubMed] [Google Scholar]
- Winterbourne D. J., Mora P. T. Cells selected for high tumorigenicity or transformed by simian virus 40 synthesize heparan sulfate with reduced degree of sulfation. J Biol Chem. 1981 May 10;256(9):4310–4320. [PubMed] [Google Scholar]
- Yoshida K., Miyauchi S., Kikuchi H., Tawada A., Tokuyasu K. Analysis of unsaturated disaccharides from glycosaminoglycuronan by high-performance liquid chromatography. Anal Biochem. 1989 Mar;177(2):327–332. doi: 10.1016/0003-2697(89)90061-4. [DOI] [PubMed] [Google Scholar]