Abstract
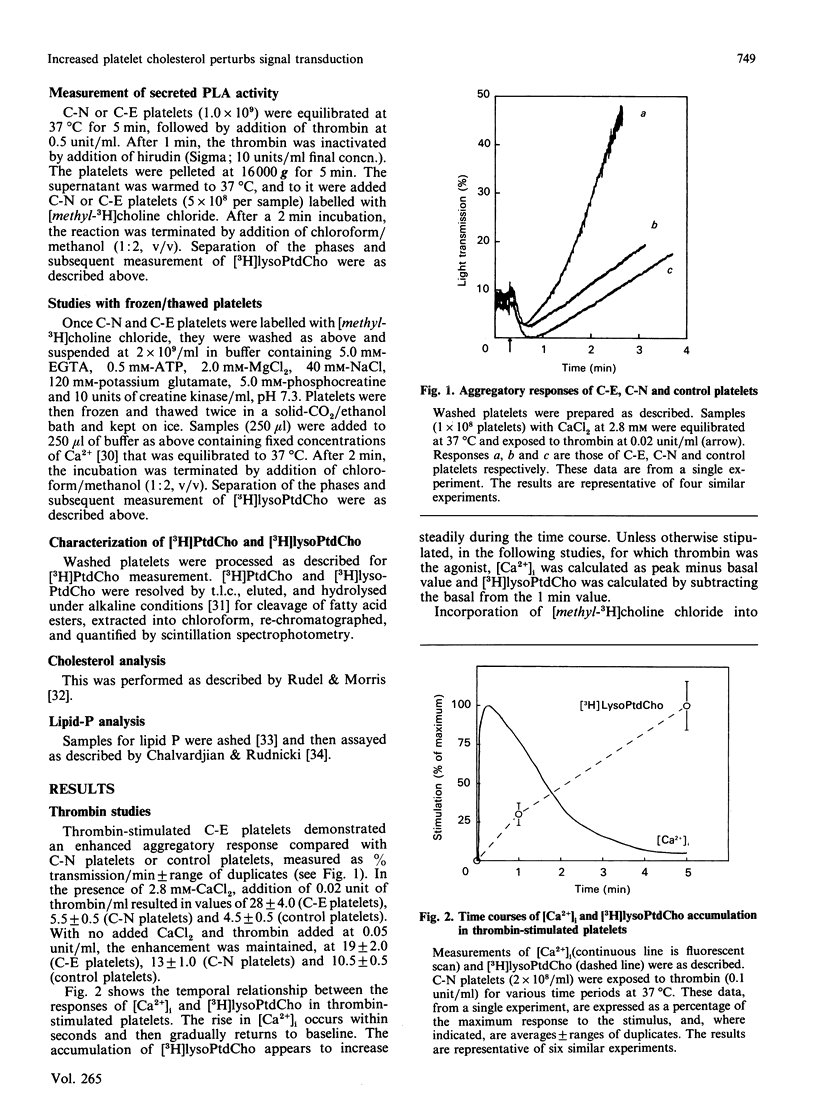
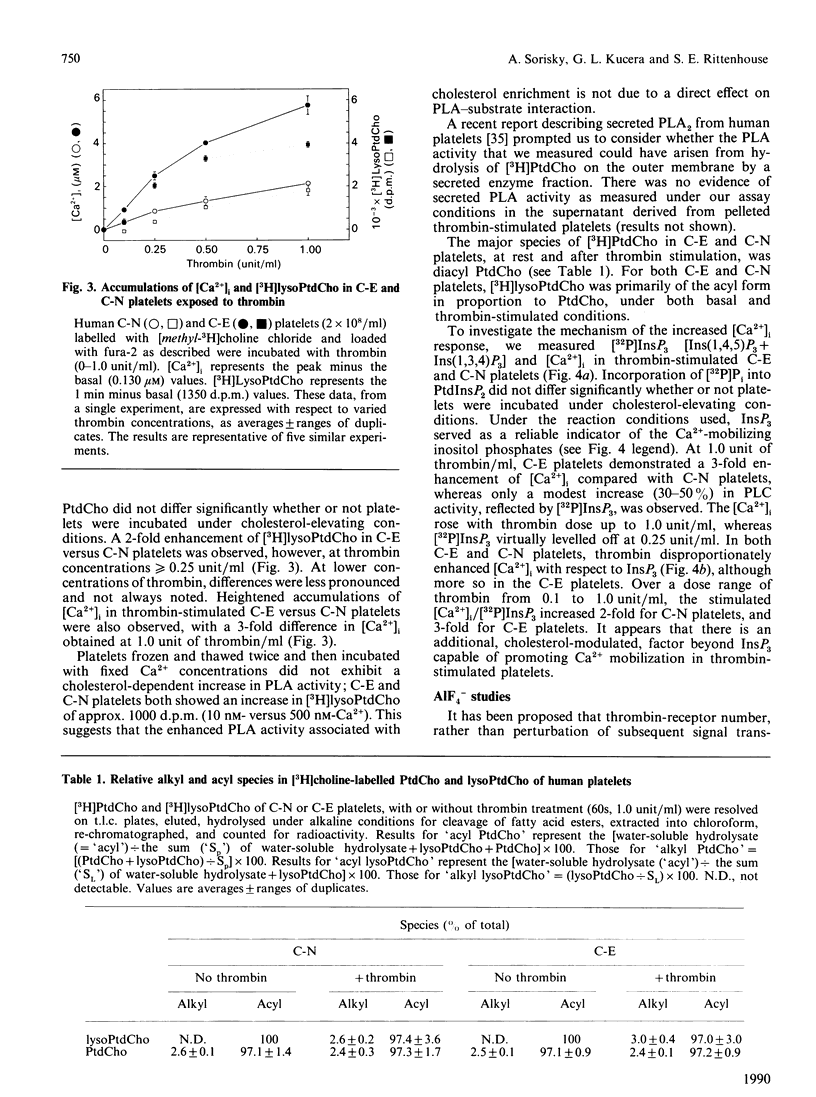
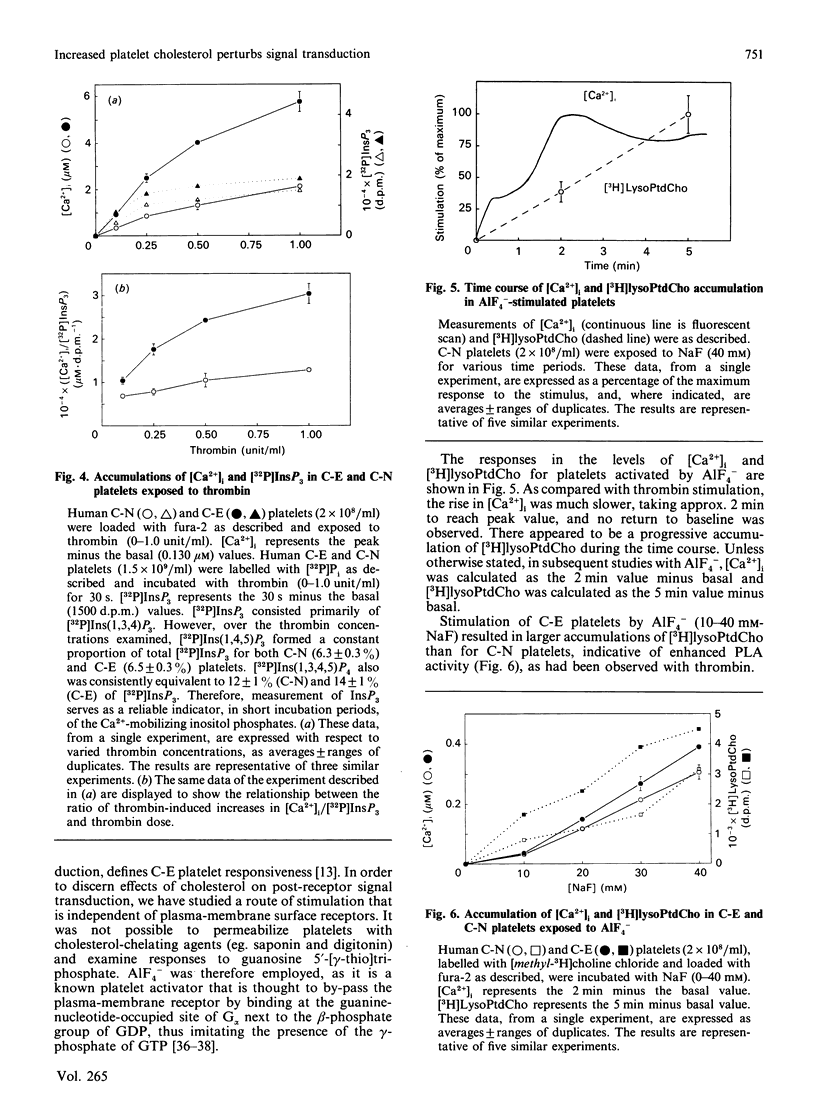
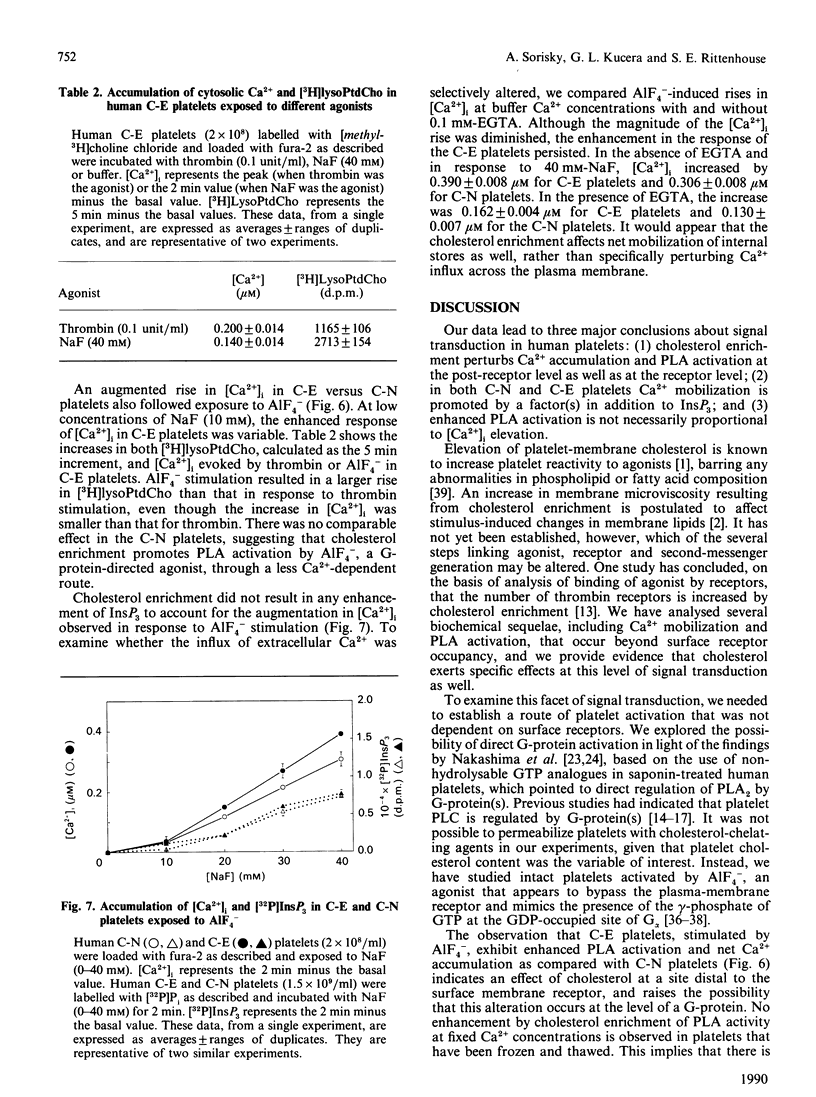
To investigate the mechanism of enhanced responsiveness of cholesterol-enriched human platelets, we compared stimulation by surface-membrane-receptor (thrombin) and post-receptor (AlF4-) G-protein-directed pathways. Platelets were labelled with [32P]Pi and [methyl-3H] choline chloride, incubated with sonicated lipid dispersions of various ratios of cholesterol and phospholipid, and loaded with the fluorescent Ca2+ indicator fura-2. We report the following. (1) Cholesterol enrichment enhances cytosolic Ca2+ accumulation and phospholipase A activation in response to both receptor-directed and post-receptor-directed agonists. No enhancement by cholesterol of phospholipase A activity at fixed Ca2+ concentrations is observed in lysed platelets, implying that no perturbation by cholesterol of phospholipase A/substrate interaction occurs in our preparations. (2) In both normal and cholesterol-enriched platelets, Ca2+ mobilization is promoted by a factor(s) apart from InsP3 that appear(s) to be modulated by cholesterol. A disproportionate increase in cytosolic Ca2+ relative to [32P]InsP3 is observed with increasing doses of thrombin in normal, and to a larger extent in cholesterol-enriched, platelets. When AlF4- is the agonist, there is no cholesterol-associated enhancement in [32P]InsP3 to account for the heightened Ca2+ rise seen with cholesterol enrichment. (3) Enhanced phospholipase A activation is not necessarily proportional to cytosolic Ca2+ increase. The magnitude of the increase in phospholipase A activity for a given rise in cytosolic Ca2+ is greater in cholesterol-enriched platelets that are stimulated by AlF4- than in those stimulated by thrombin. We conclude that increased membrane microviscosity associated with cholesterol enrichment may promote G-protein/phospholipase A interaction as well as the Ca2(+)-release mechanism, without significantly altering G-protein/phospholipase C interaction.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Ballou L. R., DeWitt L. M., Cheung W. Y. Substrate-specific forms of human platelet phospholipase A2. J Biol Chem. 1986 Mar 5;261(7):3107–3111. [PubMed] [Google Scholar]
- Banga H. S., Walker R. K., Winberry L. K., Rittenhouse S. E. Platelet adenylate cyclase and phospholipase C are affected differentially by ADP-ribosylation. Effects on thrombin-mediated responses. Biochem J. 1988 May 15;252(1):297–300. doi: 10.1042/bj2520297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bigay J., Deterre P., Pfister C., Chabre M. Fluoroaluminates activate transducin-GDP by mimicking the gamma-phosphate of GTP in its binding site. FEBS Lett. 1985 Oct 28;191(2):181–185. doi: 10.1016/0014-5793(85)80004-1. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G., Cuatrecasas P. Phospholipase A2 activity specific for phosphatidic acid. A possible mechanism for the production of arachidonic acid in platelets. J Biol Chem. 1981 Jun 10;256(11):5399–5403. [PubMed] [Google Scholar]
- Brass L. F., Joseph S. K. A role for inositol triphosphate in intracellular Ca2+ mobilization and granule secretion in platelets. J Biol Chem. 1985 Dec 5;260(28):15172–15179. [PubMed] [Google Scholar]
- Brass L. F., Laposata M., Banga H. S., Rittenhouse S. E. Regulation of the phosphoinositide hydrolysis pathway in thrombin-stimulated platelets by a pertussis toxin-sensitive guanine nucleotide-binding protein. Evaluation of its contribution to platelet activation and comparisons with the adenylate cyclase inhibitory protein, Gi. J Biol Chem. 1986 Dec 25;261(36):16838–16847. [PubMed] [Google Scholar]
- Broekman M. J., Ward J. W., Marcus A. J. Phospholipid metabolism in stimulated human platelets. Changes in phosphatidylinositol, phosphatidic acid, and lysophospholipids. J Clin Invest. 1980 Aug;66(2):275–283. doi: 10.1172/JCI109854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A. C., Colman R. W., Lees R. S. Platelet function in hyperlipoproteinemia. N Engl J Med. 1974 Feb 21;290(8):434–438. doi: 10.1056/NEJM197402212900805. [DOI] [PubMed] [Google Scholar]
- Chabre M. Aluminofluoride action on G-proteins of the adenylate cyclase system is not different from that on transducin. Biochem J. 1989 Mar 15;258(3):931–932. doi: 10.1042/bj2580931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalvardjian A., Rudnicki E. Determination of lipid phosphorus in the nanomolar range. Anal Biochem. 1970 Jul;36(1):225–226. doi: 10.1016/0003-2697(70)90352-0. [DOI] [PubMed] [Google Scholar]
- Colard O., Breton M., Pepin D., Chevy F., Bereziat G., Polonovski J. Arachidonate cannot be released directly from diacyl-sn-glycero-3-phosphocholine in thrombin-stimulated platelets. Biochem J. 1989 Apr 15;259(2):333–339. doi: 10.1042/bj2590333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. A., Leslie M. H., Fischkoff S., Shinitzky M., Shattil S. J. Factors influencing the lipid composition and fluidity of red cell membranes in vitro: production of red cells possessing more than two cholesterols per phospholipid. Biochemistry. 1978 Jan 24;17(2):327–331. doi: 10.1021/bi00595a021. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Fisher G. J., Bakshian S., Baldassare J. J. Activation of human platelets by ADP causes a rapid rise in cytosolic free calcium without hydrolysis of phosphatidylinositol-4,5-bisphosphate. Biochem Biophys Res Commun. 1985 Jun 28;129(3):958–964. doi: 10.1016/0006-291x(85)91984-9. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Hallam T. J., Rink T. J. Responses to adenosine diphosphate in human platelets loaded with the fluorescent calcium indicator quin2. J Physiol. 1985 Nov;368:131–146. doi: 10.1113/jphysiol.1985.sp015850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon J. T., Tandon N. N., Hoeg J. M., Jamieson G. A. Thrombin binding and response in platelets from patients with dyslipoproteinemias: increased stimulus-response coupling in type II hyperlipoproteinemia. Blood. 1986 Aug;68(2):498–505. [PubMed] [Google Scholar]
- Haslam R. J., Davidson M. M. Receptor-induced diacylglycerol formation in permeabilized platelets; possible role for a GTP-binding protein. J Recept Res. 1984;4(1-6):605–629. doi: 10.3109/10799898409042576. [DOI] [PubMed] [Google Scholar]
- Hawkins P. T., Stephens L., Downes C. P. Rapid formation of inositol 1,3,4,5-tetrakisphosphate and inositol 1,3,4-trisphosphate in rat parotid glands may both result indirectly from receptor-stimulated release of inositol 1,4,5-trisphosphate from phosphatidylinositol 4,5-bisphosphate. Biochem J. 1986 Sep 1;238(2):507–516. doi: 10.1042/bj2380507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Lewis D. L., Graziadei L., Neer E. J., Bar-Sagi D., Clapham D. E. G-protein beta gamma-subunits activate the cardiac muscarinic K+-channel via phospholipase A2. Nature. 1989 Feb 9;337(6207):557–560. doi: 10.1038/337557a0. [DOI] [PubMed] [Google Scholar]
- Kramer R. M., Hession C., Johansen B., Hayes G., McGray P., Chow E. P., Tizard R., Pepinsky R. B. Structure and properties of a human non-pancreatic phospholipase A2. J Biol Chem. 1989 Apr 5;264(10):5768–5775. [PubMed] [Google Scholar]
- Kramer R. M., Jakubowski J. A., Deykin D. Hydrolysis of 1-alkyl-2-arachidonoyl-sn-glycero-3-phosphocholine, a common precursor of platelet-activating factor and eicosanoids, by human platelet phospholipase A2. Biochim Biophys Acta. 1988 Apr 15;959(3):269–279. doi: 10.1016/0005-2760(88)90200-7. [DOI] [PubMed] [Google Scholar]
- Kramer R. M., Jakubowski J. A., Vaillancourt R., Deykin D. Effect of membrane cholesterol on phospholipid metabolism in thrombin-stimulated platelets. Enhanced activation of platelet phospholipase(s) for liberation of arachidonic acid. J Biol Chem. 1982 Jun 25;257(12):6844–6849. [PubMed] [Google Scholar]
- Kucera G. L., Rittenhouse S. E. Inhibition of GDP beta S of agonist-activated phospholipase C in human platelets requires cell permeabilization. Biochem Biophys Res Commun. 1988 May 31;153(1):417–421. doi: 10.1016/s0006-291x(88)81240-3. [DOI] [PubMed] [Google Scholar]
- Logothetis D. E., Kim D. H., Northup J. K., Neer E. J., Clapham D. E. Specificity of action of guanine nucleotide-binding regulatory protein subunits on the cardiac muscarinic K+ channel. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5814–5818. doi: 10.1073/pnas.85.16.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis D. E., Kurachi Y., Galper J., Neer E. J., Clapham D. E. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987 Jan 22;325(6102):321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- McKean M. L., Smith J. B., Silver M. J. Formation of lysophosphatidylcholine by human platelets in response to thrombin. Support for the phospholipase A2 pathway for the liberation of arachidonic acid. J Biol Chem. 1981 Feb 25;256(4):1522–1524. [PubMed] [Google Scholar]
- Mendelsohn M. E., Loscalzo J. Role of platelets in cholesteryl ester formation by U-937 cells. J Clin Invest. 1988 Jan;81(1):62–68. doi: 10.1172/JCI113311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi C., Colli S., Tremoli E., Galli C. Phosphatidylinositol (PI) and PI-associated arachidonate are elevated in platelet total membranes of type IIa hypercholesterolemic subjects. Atherosclerosis. 1988 Aug;72(2-3):129–134. doi: 10.1016/0021-9150(88)90073-1. [DOI] [PubMed] [Google Scholar]
- Nakashima S., Suganuma A., Matsui A., Hattori H., Sato M., Takenaka A., Nozawa Y. Primary role of calcium ions in arachidonic acid release from rat platelet membranes. Comparison with human platelet membranes. Biochem J. 1989 Apr 1;259(1):139–144. doi: 10.1042/bj2590139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima S., Tohmatsu T., Hattori H., Suganuma A., Nozawa Y. Guanine nucleotides stimulate arachidonic acid release by phospholipase A2 in saponin-permeabilized human platelets. J Biochem. 1987 Apr;101(4):1055–1058. doi: 10.1093/oxfordjournals.jbchem.a121948. [DOI] [PubMed] [Google Scholar]
- Nakashima S., Tohmatsu T., Shirato L., Takenaka A., Nozawa Y. Neomycin is a potent agent for arachidonic acid release in human platelets. Biochem Biophys Res Commun. 1987 Jul 31;146(2):820–826. doi: 10.1016/0006-291x(87)90604-8. [DOI] [PubMed] [Google Scholar]
- O'Rourke F. A., Halenda S. P., Zavoico G. B., Feinstein M. B. Inositol 1,4,5-trisphosphate releases Ca2+ from a Ca2+-transporting membrane vesicle fraction derived from human platelets. J Biol Chem. 1985 Jan 25;260(2):956–962. [PubMed] [Google Scholar]
- Owen J. S., Hutton R. A., Day R. C., Bruckdorfer K. R., McIntyre N. Platelet lipid composition and platelet aggregation in human liver disease. J Lipid Res. 1981 Mar;22(3):423–430. [PubMed] [Google Scholar]
- Pollock W. K., Rink T. J., Irvine R. F. Liberation of [3H]arachidonic acid and changes in cytosolic free calcium in fura-2-loaded human platelets stimulated by ionomycin and collagen. Biochem J. 1986 May 1;235(3):869–877. doi: 10.1042/bj2350869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott S. M., Majerus P. W. Characterization of 1,2-diacylglycerol hydrolysis in human platelets. Demonstration of an arachidonoyl-monoacylglycerol intermediate. J Biol Chem. 1983 Jan 25;258(2):764–769. [PubMed] [Google Scholar]
- Rittenhouse-Simmons S. Production of diglyceride from phosphatidylinositol in activated human platelets. J Clin Invest. 1979 Apr;63(4):580–587. doi: 10.1172/JCI109339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse S. E. Activation of human platelet phospholipase C by ionophore A23187 is totally dependent upon cyclo-oxygenase products and ADP. Biochem J. 1984 Aug 15;222(1):103–110. doi: 10.1042/bj2220103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse S. E. Human platelets contain phospholipase C that hydrolyzes polyphosphoinositides. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5417–5420. doi: 10.1073/pnas.80.17.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Rudel L. L., Morris M. D. Determination of cholesterol using o-phthalaldehyde. J Lipid Res. 1973 May;14(3):364–366. [PubMed] [Google Scholar]
- Sage S. O., Rink T. J. Kinetic differences between thrombin-induced and ADP-induced calcium influx and release from internal stores in fura-2-loaded human platelets. Biochem Biophys Res Commun. 1986 May 14;136(3):1124–1129. doi: 10.1016/0006-291x(86)90450-x. [DOI] [PubMed] [Google Scholar]
- Schick B. P., Schick P. K. Cholesterol exchange in platelets, erythrocytes and megakaryocytes. Biochim Biophys Acta. 1985 Feb 8;833(2):281–290. doi: 10.1016/0005-2760(85)90200-0. [DOI] [PubMed] [Google Scholar]
- Shastri K. M., Carvalho A. C., Lees R. S. Platelet function and platelet lipid composition in the dyslipoproteinemias. J Lipid Res. 1980 May;21(4):467–472. [PubMed] [Google Scholar]
- Shattil S. J., Anaya-Galindo R., Bennett J., Colman R. W., Cooper R. A. Platelet hypersensitivity induced by cholesterol incorporation. J Clin Invest. 1975 Mar;55(3):636–643. doi: 10.1172/JCI107971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattil S. J., Bennett J. S., Colman R. W., Cooper R. A. Abnormalities of cholesterol-phospholipid composition in platelets and low-density lipoproteins of human hyperbetalipoproteinemia. J Lab Clin Med. 1977 Feb;89(2):341–353. [PubMed] [Google Scholar]
- Stephens L. R., Hawkins P. T., Barker C. J., Downes C. P. Synthesis of myo-inositol 1,3,4,5,6-pentakisphosphate from inositol phosphates generated by receptor activation. Biochem J. 1988 Aug 1;253(3):721–733. doi: 10.1042/bj2530721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi Y., Dyer C. A., Curtiss L. K. Platelet-enhanced apolipoprotein E production by human macrophages: a possible role in atherosclerosis. J Lipid Res. 1988 Jul;29(7):859–867. [PubMed] [Google Scholar]
- Tandon N., Harmon J. T., Rodbard D., Jamieson G. A. Thrombin receptors define responsiveness of cholesterol-modified platelets. J Biol Chem. 1983 Oct 10;258(19):11840–11845. [PubMed] [Google Scholar]
- Tarver A. P., King W. G., Rittenhouse S. E. Inositol 1,4,5-trisphosphate and inositol 1,2-cyclic 4,5-trisphosphate are minor components of total mass of inositol trisphosphate in thrombin-stimulated platelets. Rapid formation of inositol 1,3,4-trisphosphate. J Biol Chem. 1987 Dec 25;262(36):17268–17271. [PubMed] [Google Scholar]


