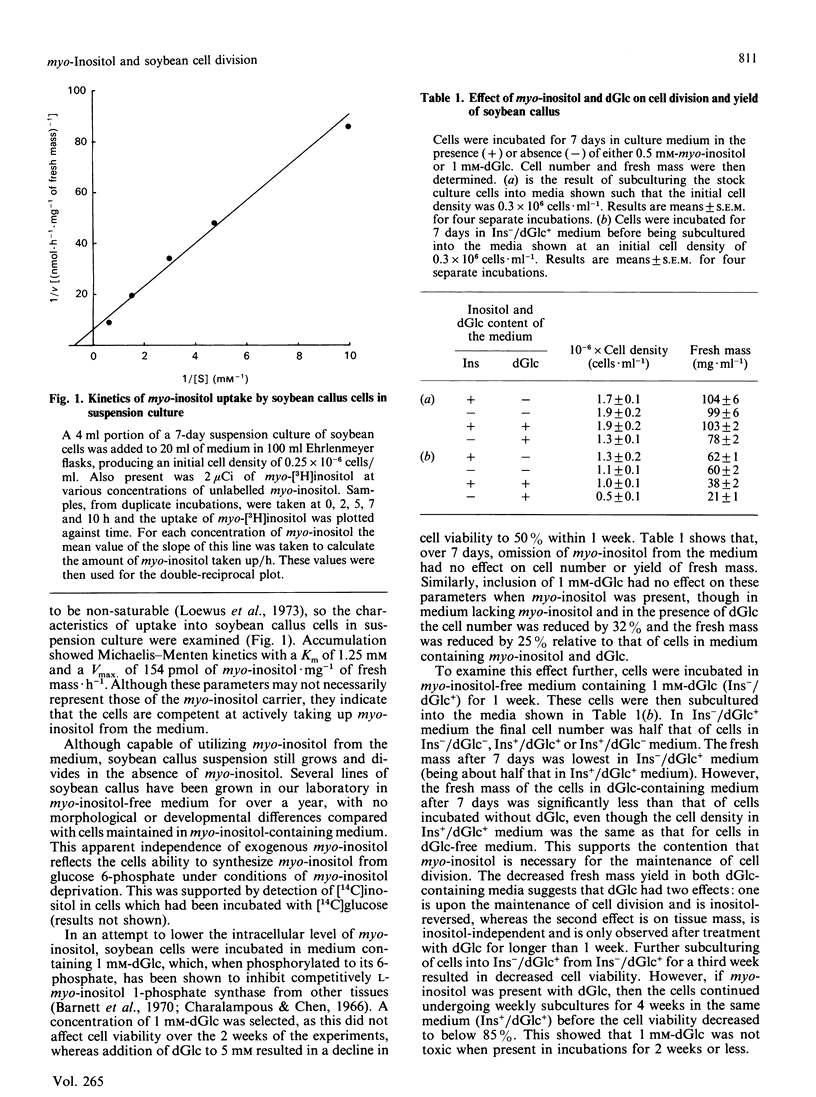
Abstract
Although myo-inositol is included in media for the successful growth of plant tissues, the actual requirement of most tissues, including soybean (Glycine max) callus in suspension culture, for myo-inositol has not been demonstrated. We have made use of deoxyglucose to reduce intracellular levels of myo-inositol. Deoxyglucose is phosphorylated to deoxyglucose 6-phosphate, which inhibits L-myo-inositol 1-phosphate synthase, an important enzyme in the synthesis of myo-inositol. Addition of deoxyglucose to the medium resulted in a decrease in the intracellular level of myo-inositol that corresponded with a decrease in cell division. Cell viability was not affected. When myo-inositol was added to cells along with deoxyglucose, cell division was restored, as were intracellular levels of myo-inositol. Addition of myo-inositol had no affect on the uptake or metabolism of deoxyglucose. From these results we propose that myo-inositol has a role in maintaining cell division in soybean callus tissue in suspension culture.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett J. E., Brice R. E., Corina D. L. A colorimetric determination of inositol monophosphates as an assay for D-glucose 6-phosphate-1L-myoinositol 1-phosphate cyclase. Biochem J. 1970 Sep;119(2):183–186. doi: 10.1042/bj1190183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker G. W., Lester R. L. Changes in phospholipids of Saccharomyces cerevisiae associated with inositol-less death. J Biol Chem. 1977 Dec 10;252(23):8684–8691. [PubMed] [Google Scholar]
- Berridge M. J. Ernst Klenk Lecture, November 1985. Intracellular signalling through inositol trisphosphate and diacylglycerol. Biol Chem Hoppe Seyler. 1986 Jun;367(6):447–456. [PubMed] [Google Scholar]
- Burton L. E., Wells W. W. Studies on the effect of 5-thio-D-glucose and 2-deoxy-D-glucose on myo-inositol metabolism. Arch Biochem Biophys. 1977 Jun;181(2):384–392. doi: 10.1016/0003-9861(77)90243-0. [DOI] [PubMed] [Google Scholar]
- Drøbak B. K., Ferguson I. B., Dawson A. P., Irvine R. F. Inositol-containing lipids in suspension-cultured plant cells: an isotopic study. Plant Physiol. 1988 May;87(1):217–222. doi: 10.1104/pp.87.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettlinger C., Lehle L. Auxin induces rapid changes in phosphatidylinositol metabolites. Nature. 1988 Jan 14;331(6152):176–178. doi: 10.1038/331176a0. [DOI] [PubMed] [Google Scholar]
- Farkas V., Svoboda A., Bauer S. Secretion of cell-wall glycoproteins by yeast protoplasts. Effect of 2-deoxy-D-glucose and cycloheximide. Biochem J. 1970 Aug;118(5):755–758. doi: 10.1042/bj1180755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funkhouser E. A., Loewus F. A. Purification of myo-Inositol 1-Phosphate Synthase from Rice Cell Culture by Affinity Chromatography. Plant Physiol. 1975 Dec;56(6):786–790. doi: 10.1104/pp.56.6.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEREDIA C. F., DELAFUENTE G., SOLS A. METABOLIC STUDIES WITH 2-DEOXYHEXOSES. I. MECHANISMS OF INHIBITION OF GROWTH AND FERMENTATION IN BAKER'S YEAST. Biochim Biophys Acta. 1964 May 11;86:216–223. doi: 10.1016/0304-4165(64)90045-5. [DOI] [PubMed] [Google Scholar]
- Hahn M. G., Darvill A. G., Albersheim P. Host-Pathogen Interactions : XIX. THE ENDOGENOUS ELICITOR, A FRAGMENT OF A PLANT CELL WALL POLYSACCHARIDE THAT ELICITS PHYTOALEXIN ACCUMULATION IN SOYBEANS. Plant Physiol. 1981 Nov;68(5):1161–1169. doi: 10.1104/pp.68.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim S., Wagner K. G. Evidence of phosphorylated phosphatidylinositols in the growth cycle of suspension cultured plant cells. Biochem Biophys Res Commun. 1986 Feb 13;134(3):1175–1181. doi: 10.1016/0006-291x(86)90374-8. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Lander D. J., Drøbak B. K., Dawson A. P., Musgrave A. Phosphatidylinositol(4,5)bisphosphate and Phosphatidylinositol(4)phosphate in Plant Tissues. Plant Physiol. 1989 Mar;89(3):888–892. doi: 10.1104/pp.89.3.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroh M., Loewus F. Biosynthesis of pectic substance in germinating pollen: labeling with myoinositol-2-14C. Science. 1968 Jun 21;160(3834):1352–1354. doi: 10.1126/science.160.3834.1352. [DOI] [PubMed] [Google Scholar]
- LOEWUS F. A., KELLY S. Conversion of glucose to inositol in parsley leaves. Biochem Biophys Res Commun. 1962 Apr 20;7:204–208. doi: 10.1016/0006-291x(62)90175-4. [DOI] [PubMed] [Google Scholar]
- Loewus M. W., Loewus F. d-Glucose 6-Phosphate Cycloaldolase: Inhibition Studies and Aldolase Function. Plant Physiol. 1973 Feb;51(2):263–266. doi: 10.1104/pp.51.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell R. H., Kirk C. J., Jones L. M., Downes C. P., Creba J. A. The stimulation of inositol lipid metabolism that accompanies calcium mobilization in stimulated cells: defined characteristics and unanswered questions. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):123–138. doi: 10.1098/rstb.1981.0177. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Loewus F. A. Metabolism of myo-[2-H]Inositol and scyllo-[R-H]Inositol in Ripening Wheat Kernels. Plant Physiol. 1980 Oct;66(4):740–745. doi: 10.1104/pp.66.4.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht J. Extraction and purification of polyphosphoinositides. Methods Enzymol. 1981;72:626–631. doi: 10.1016/s0076-6879(81)72054-8. [DOI] [PubMed] [Google Scholar]
- Steiner S., Lester R. L. Studies on the diversity of inositol-containing yeast phospholipids: incorporation of 2-deoxyglucose into lipid. J Bacteriol. 1972 Jan;109(1):81–88. doi: 10.1128/jb.109.1.81-88.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S. K., Mohindroo S. R., Kansal D. K. The maximal anaerobic power of different categories of players. J Sports Med Phys Fitness. 1979 Mar;19(1):55–62. [PubMed] [Google Scholar]
- Widholm J. M. The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol. 1972 Jul;47(4):189–194. doi: 10.3109/10520297209116483. [DOI] [PubMed] [Google Scholar]
- Yamazaki N., Fry S. C., Darvill A. G., Albersheim P. Host-Pathogen Interactions : XXIV. Fragments Isolated from Suspension-Cultured Sycamore Cell Walls Inhibit the Ability of the Cells to Incorporate [C]Leucine into Proteins. Plant Physiol. 1983 Jul;72(3):864–869. doi: 10.1104/pp.72.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]


