Abstract
Picrasma quassioides (D.Don) Benn is a member of the Simaroubaceae family, which has a long history of medicinal use in China, the composition of compounds is complex, mainly including alkaloids, lignin, triterpenoids, and other compounds. As a traditional Chinese medicine, P. quassioides has pharmacological effects such as anti-inflammatory, antipyretic, antiviral, blood pressure lowering and anticancer. Scholars at home and abroad have been studying P. quassioides for about 50 years. In the present review, the research status of the chemical composition, pharmacological activity and pharmacokinetics of P. quassioides was provided, as a reference for further developing the value of P. quassioides.
Keywords: Picrasma quassioides, Chemical composition, Biological activity, Metabolism
Picrasma quassioides (D.Don) Benn is a member of the Simaroubaceae family, is a traditional Chinese herb with a long history of medicinal value, which is bitter, cold in nature, and belongs to the lung and large intestine meridians. According to the 2020 edition of the Chinese Pharmacopoeia, P. quassioides has the effect of clearing heat and removing dampness which is used for wind-heat colds, sore throats, damp-heat diarrhea and dysentery, eczema, sores, and snake and insect bites [1]. The chemical composition of P. quassioides is complex and rich, containing triterpenoids, volatile oils, flavonoids, sterols, saponins, coumarins, and phenolic glycosides in addition to the characteristic P. quassioides alkaloids and bittersweet components [2]. Modern pharmacological studies have shown that P. quassioides has anticancer, anti-inflammatory, antibacterial, antihypertensive, antipyretic, antivenom, antimalarial, and stomachic effects [3]. Clinically, a variety of active ingredients in P. quassioides have been used in herbal preparations, such as P. quassioides injection, P. quassioides combination, compound P. quassioides anti-inflammatory capsule, lotus gall bladder anti-inflammatory tablets, and anti-inflammatory and cholagogue tablets, and have achieved good efficacy in the treatment of various inflammatory diseases such as upper respiratory tract infections, cholecystitis, and enteritis [[4], [5], [6]]. In addition to its significant anti-inflammatory effects, P. quassioides contains components such as P. quassioides alkaloids and bittersweet, which have various pharmacological activities such as anti-tumor, blood pressure lowering, and anti-pathogenic microorganisms [7,8]. This study reviews the chemical composition, pharmacological activity, and pharmacokinetic studies of P. quassioides in the past 50 years to provide a basis for better development and utilization of the Chinese medicine P. quassioides.
1. Chemical composition of P. quassioides
The chemical composition of P. quassioides is relatively complex. A β-carboline alkaloid was first isolated and identified from P. quassioides by Japanese scholars Kondo and Takemoto [9] in 1973, and subsequently, its chemical composition has been extensively studied by domestic and foreign scholars. It was found that the main chemical composition of P. quassioides are alkaloids and bittersweet substances, and the P. quassioides alkaloids have received wide attention due to their significant pharmacological activity and have been used as marker compounds for quality control of P. quassioides and its related preparations.
1.1. Alkaloids
P. quassioides alkaloids are classified into β-carboline alkaloids, canthin-6-one alkaloids, and alkaloid dimers. According to the literature, 86 alkaloids have been identified in the P. quassioides, including 60 β-carbolines, 15 canthin-6-ones, and 11 alkaloid dimers.
1.1.1. β-carboline alkaloids
The most abundant alkaloids in P. quassioides plants are the β-carboline alkaloids, of which there are 60 species [[10], [11], [12], [13], [14], [15], [16], [9], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29]]. Among them, there are 58 compounds with the parent nucleus structure in Fig. 1 A and two compounds with the parent nucleus structure in Fig. 1 B.
Fig. 1.
Parent nucleus structure of β-carboline alkaloids in P. quassioides. (A) The parent nucleus structure of 58 β-carboline alkaloid compounds. (B) The parent nucleus structure of two other β-carboline alkaloid compounds.
1.1.2. Canthin-6-one alkaloids
There are 15 canthin-6-one alkaloids [16,9,18,[21], [22], [23],26,27,30,31] in the P. quassioides plant, as shown in Fig. 2. There are 11 compounds with the parent nucleus structure in Fig. 2 A, one with the parent nucleus structure in Fig. 2 B, and three with the parent nucleus structure in Fig. 2 C.
Fig. 2.
Parent nucleus structure of canthin-6-one alkaloids in P. quassioides. (A) The parent nucleus structure of 11 canthin-6-one alkaloid compounds. (B) The parent nucleus structure of one canthin-6-one alkaloid compound. (C) The parent nucleus structure of three other canthin-6-one alkaloid compounds.
1.1.3. Alkaloid dimers
There are four parent nucleus structures of alkaloid dimer compounds in P. quassioides plants, totaling 11 compounds [24,26,[31], [32], [33], [34], [35], [36]]. Among them, there are two, two, three, and four compounds with the parent nucleus structures of Fig. 3 A, B, C, and D, respectively, and the structures are shown in Fig. 3.
Fig. 3.
Parent nucleus structure of alkaloid dimer in P. quassioides. (A) The parent nucleus structure of two alkaloid dimer compounds. (B) The parent nucleus structure of two alkaloid dimer compounds among them. (C) The parent nucleus structure of three alkaloid dimer compounds. (D) The parent nucleus structure of four other alkaloid dimer compounds.
1.2. Quassinoids
Quassinoids chemicals are characteristic components in the Chinese herbal medicine P. quassioides plant, and 64 quassinoids structures have been identified [[37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50]]. There are three types of their parent nucleus structures, hemiacetals and lactones. There are 24 compounds with the parent nucleus structure of Fig. 4 A, one compound with the parent nucleus structure of Fig. 4 B, and 39 compounds with the parent nucleus structure of Fig. 4 C. Their structures are shown in Fig. 4.
Fig. 4.
Parent nucleus structure of quassinoids in P. quassioides. (A) The parent nucleus structure of 24 quassinoid compounds. (B) The parent nucleus structure of one quassinoid compound. (C) The parent nucleus structure of 39 other quassinoid compounds.
1.3. Triterpenoids
A total of 42 triterpenoids were identified in P. quassioides [[51], [52], [53], [54], [55]], with two parent nucleus structures, as shown in Fig. 5. Among them, one compound with the structure of parent nucleus Fig. 5 A and 41 compounds with the structure of parent nucleus Fig. 5 B were identified.
Fig. 5.
Parent nucleus structure of triterpenoids in P. quassioides. (A) The parent nucleus structure of one triterpenoid compound. (B) The parent nucleus structure of 41 other triterpenoid compounds.
1.4. Other substances
So far, other compounds isolated from P. quassioides include volatile oils, phenylpropanoids, flavonoids, phenolic glycosides, phenolic acids, and violetone derivatives of cyclohexanone [[56], [57], [58]], including sesquiterpenes [12], cinnamamide derivative [15], neolignans [59,60], phenolic derivatives [61], and cyclization ionone derivative [62], which have been newly discovered in recent years.
1.5. Structural activity relationship (SAR) of P. quassioides
The structural activity relationship (SAR) of P. quassioides refers to the relationship between the structure of a specific chemical component in P. quassioides and its biological activity. The SAR of P. quassioides is mainly reflected in the close relationship between the structure of its chemical composition and its biological activity. The structural characteristics of these chemical components, such as containing nitrogen atoms and aromatic rings, give them unique pharmacological activities, such as antihypertensive, anti-cancer, anti-inflammatory and so on. Through the in-depth study of P. quassioides SAR, it can further reveal its pharmacological mechanism, and provide scientific basis for the quality control and clinical application of P. quassioides.
For example, alkaloids in P. quassioides, such as quassa, have an inhibitory effect on cAMP phosphodiesterase, which can affect intracellular signaling and thus affect blood pressure. In addition, the total alkaloids of P. quassioides promote the synthesis and release of vasodilator NO by endothelial cells through increasing the protein expression of eNOS, which makes blood vessels dilate and blood pressure decrease. In addition, the growth of lymphocytic leukemia P388 cell line was inhibited by picroside B in P. quassioides, which may be related to the specific structure of the compound, such as aromatic ring and nitrogen atom [37,63,64].
2. Biological activity of P. quassioides
2.1. Anti-cancer
According to recent literature, the anticancer effects and mechanisms of P. quassioides are as follows: treatment of lung cancer cells with P. quassioides extracts increases intracellular reactive oxygen species, decreases cell proliferation and migration capacity, and causes autophagy and apoptosis [65]. Compound d of the 4-methoxy-5-hydroxy-canthin-6-one (CAN1) backbone, an analog of canthin-6-one isolated from P. quassioides, exhibited stronger anti-tumor cell proliferative activity than 5-FU and induced apoptosis of human cancer cell lines, HepG2 and HCT116 more effectively [66]. P. quassioides extract can inhibit the growth of tumors in mice with gastric cancer by upregulating the p21 gene and improving the immune capacity of the body [67].
It was found that P. quassioides extract tonics have some efficacy in primary liver cancer, probably by modulating the levels of AFP, VEGF, nm23, CD44, and Bcl-2 in the serum of patients to achieve the antitumor effect [68]. In addition, β-carboline enantiomers extracted from P. quassioides were able to reduce the expression of H-Ras, inhibit the viability of hepatocellular carcinoma cells, and affect ROS accumulation and mitochondrial function in HepG2 cells with H-ras mutations [69]. The compounds isolated from P. quassioides can have antitumor effects by significantly and selectively inhibiting hepatocellular carcinoma cell activity and inducing apoptosis leading to hepatocellular carcinoma cell death [16,70].
Picrasidine F, which also has therapeutic effects in cervical cancer, can exert antitumor effects by mediating the miR-331-3p/PSME3 axis to inhibit the proliferation of cervical cancer HeLa cells and promote apoptosis [71]. P. quassioides ethanol extract can inhibit ATP synthesis and induce apoptosis in cervical cancer SiHa cells, and the P38MAPK signaling pathway is key to the induction of apoptosis [72]. β-CCE (a β-carboline alkaloid in P. quassioides) can increase ROS levels in cervical cancer cells, damage mitochondria, induce apoptosis, and activate p38/MAPK and mitochondria-dependent apoptotic signaling pathways [73]. Furthermore, the three β-carboline alkaloid components isolated from the ethanolic extract of the stem of P. quassioides showed inhibitory effects on both cervical and other cancer cells, further confirming the anticancer effects of P. quassioides [8].
Dehydrohumanoside extracted from P. quassioides induces cell cycle arrest and apoptosis in nasopharyngeal carcinoma cells by regulating JNK and ERK signaling pathways [74]. Picrasidine G (PG), a natural dimeric alkaloid of P. quassioides, reduces the viability of the MDA-MB468 cell line (TNBCEGFR+), increases apoptotic markers, and inhibits transcription of STAT3 target gene survivin, which may contribute to targeted therapy in patients with triple-negative breast cancer (TNBC) [75]. In addition, the P. quassioides alkaloid 1-methoxycarbonyl-β-carboline (MCC) inhibited the viability, migration, invasion, and lumen formation of human umbilical vein endothelial cells (HUVECs) and suppressed the growth of tumor masses and metastasis of DU145 tumor cells [76].
2.2. Anti-inflammatory
The alkaloid component of P. quassioides is the main effective anti-inflammatory substance, and a large number of β-carboline alkaloid compounds isolated from the branches, stems, and roots of P. quassioides exhibited good anti-inflammatory biological activity [77]. For example, the alkaloid picrasidine I in P. quassioides can inhibit osteoclastogenesis and exert anti-inflammatory effects [78]. The carboline alkaloids in P. quassioides also have potential anti-inflammatory and antimicrobial activities, with studies finding the highest inhibitory effect of 1-formyl-4-methoxy-β-carboline and 1-formyl-β-carboline [79]. In addition, P. quassioides lipotropin A, boldenolA, boldenolC, and Fustin also inhibit the release of inflammatory factors and achieve anti-inflammatory effects [80,81]. It was also found that total P. quassioides alkaloids had a significant effect on collagen-induced arthritis, reducing the degree of swelling and joint damage. It reduced TNF-α, IL-6, foot and plantar swelling and joint damage in rats [82].
Clinical trials on the anti-inflammatory properties of P. quassioides are as follows: P. quassioides injection combined with azithromycin provides synergistic and additive inhibition of the inflammatory factors NO and TNF-α [63]. The combination of oral compound P. quassioides anti-inflammatory tablets with topical Lankefuning can safely and effectively treat acne vulgaris with an efficiency of 95 % in the treatment group [83]. P. quassioides injection nebulized inhalation adjunct to conventional treatment significantly increased the overall efficiency of acute upper respiratory tract infections in pediatric patients [84]. Intramuscular injection of P. quassioides injection in the treatment of acute upper respiratory tract infections in pediatric patients has an overall efficiency of 97.8 % and a high safety profile [4].
Lotus gall injection can relieve fever caused by mixed typhoid and paratyphoid bacteria in rabbits [85,86], and also has a counteracting and antipyretic effect on fever caused by tripterygium vaccine [87]. In addition, the active ingredients in P. quassioides have analgesic effects, among which the β-carboline alkaloid Dehydrocrenatidine can achieve analgesic effects by inhibiting neuronal excitability [88]. In addition, the β-carboline alkaloid dehydrocretidine (DHCT) also has analgesic effects and has demonstrated a reduction in mechanical nociceptive hypersensitivity in a rat model of neuropathic pain from chronic compression injury of the sciatic nerve [89].
2.3. Antibacterial
P. quassioides alkaloids have shown varying degrees of inhibition in vitro against a variety of bacteria, such as Streptococcus haemolyticus B, Staphylococcus aureus, Bacillus sonnei dysentery, Bacillus subtilis, and Bacillus octococci [90,91].
P. quassioides aqueous decoction and lipid-soluble total alkaloids showed antibacterial effects against four experimental bacteria in vitro, and topical application of P. quassioides aqueous decoction relieved ear redness and lowered serum IgE and IL-6 levels in mice with atopic dermatitis, indicating its antibacterial and anti-inflammatory effects [92]. One study determined that lipid-soluble P. quassioides alkaloids had a strong inhibitory effect on E. coli strains C249, WM, and YL in vitro [93]. Chen M. et al. [94] found that 4,5-Dimethoxycanthin-6-one had a significant inhibitory effect on Pneumococcus pneumoniae, and 5-Hydroxy-4-methoxycanthin-6-one and 3-methyl-canthin-2,6-dione also had an inhibitory effect. Methanolic substances extracted from the leaves, seeds, stems and root bark of P. quassioides, as well as fractions petroleum ether, methylene chloride, ethyl acetate, and n-butanol, all have broad-spectrum antibacterial activity against a variety of fungi [95]. In an in vitro evaluation of the anti-tuberculosis activity, foreign scholars found that the methanolic extract of P. quassioides also had an inhibitory effect on Mycobacterium tuberculosis. Shi G. et al. [8] isolated three β-carboline alkaloid components picrasidine F, G and S from the ethanol extract of P. quassioides stem, which showed significant antibacterial and cytotoxic activity against Staphylococcus aureus.
The combination of P. quassioides injection and azithromycin may synergistically inhibit a variety of bacteria such as Staphylococcus aureus, including drug-resistant strains, by activating NF-κB and MAPKs signaling pathways and inhibiting the release of NO, TNF-α, and IL-6. In addition, combination therapy improves Klebsiella pneumoniae induced lung histopathy and alleviates clinical symptoms [63,64].
2.4. Treatment of cardiovascular diseases
P. quassioides increased serum nitric oxide and superoxide dismutase SOD levels in essential hypertensive rats and dilated blood vessels and lowered blood pressure by increasing the content of vasodilatory factor NO [96]. Several studies have shown that total alkaloids of P. quassioides have hypotensive effects in both normal and renal hypertensive rats, while slowing heart rate, improving myocardial blood flow and inhibiting sympathetic discharge [97]. The study of acute hypotensive and rat hypotensive experiments revealed that the total alkaloids of P. quassioides have obvious hypotensive effects and certain detoxifying effects, with good long-term efficacy and mild side effects [98]. The Chinese patent medicine of P. quassioides has also been shown to have antihypertensive effects similar to those of hypocretin, while P. quassioides lactone A may achieve hypotension through central α receptors [99,100]. Researchers isolated the quassinoid compound P. quassioides lactone A (I) from the stems of P. quassioides by aqueous extraction and found it to have significant antihypertensive effects and also showed better efficacy in clinical trials [101].
Total alkaloids of P. quassioides have significant hypotensive and heart rate-slowing effects in anesthetized dogs. Intravenous administration of total P. quassioides alkaloids to anesthetized dogs, significantly lowered blood pressure, slowed heart rate, reduced coronary blood flow, decreased cardiac output, reduced left ventricular work, and decreased myocardial oxygen consumption [102]. Perfusion experiments on the isolated heart of toads showed a slowing of heart rate, but did not affect myocardial contractility; perfusion experiments on the isolated rabbit ear and toad vessels both showed vasodilatation; different doses of total alkaloids had different degrees of heart rate slowing effects on anesthetized dogs by sedation while prolonging the P–R interval and slightly slowing atrioventricular conduction [103].
Therefore, the comprehensive literature shows that the antihypertensive mechanism of P. quassioides is multi-fold, including the direct action, blocking of a receptor, inhibiting superoxide dismutase activity, increasing the expression of nitric oxide synthase and NO production, and inhibiting the sympathetic nerve center.
2.5. Effects on the gastrointestinal tract
It was found that Canthin-6-one and β-Carboline-1-propanoic acid from P. quassioides increased gastrointestinal blood flow rate in rabbits, whereas 4,5-Dimethoxycanthin-6-one, 5-Hydroxy-4-methoxycanthin-6-one, and Methyl β-carboline-1-carboxylate increased intestinal blood flow rate only [104]. In addition, the active components of P. quassioides have a protective effect on the gastric mucosa and are effective against gastric ulcers and mucosal damage. The alkaloids in P. quassioides can reduce the inflammatory reaction of gastric mucosa by inhibiting the production and release of inflammatory factors, so as to achieve the purpose of protecting gastric mucosa. The picrogens in P. quassioides have anti-inflammatory and antibacterial effects, which can reduce the damage and death of gastric mucosa cells, promote the repair and regeneration of gastric mucosa cells, and thus improve the symptoms of gastric mucosa damage and gastric ulcer [105]. Wei Mingqiang et al. [5] showed that P. quassioides injection was significantly more effective than traditional methods in the treatment of pediatric enteritis. Also, P. quassioides injection at acupuncture points is an effective method for the treatment of pediatric diarrhea, especially for autumn and winter diarrhea in children over 1 year of age [106,107].
2.6. Antiviral
Clinical trials have shown that P. quassioides injection has the effect of clearing heat and detoxifying, dispelling dampness, and relieving pain, and is more effective than virazole injection in the treatment of blistering, pain relief, crusting, and healing time [108]. In addition, P. quassioides injection has been suggested to have a possible inhibitory effect on novel coronaviruses and is expected to be a new drug against novel coronaviruses [109].
2.7. Protective effect on poisoned animals
A study by Du [110] et al. showed that P. quassioides alkaloids had no effect on normal rabbits, but could significantly reduce the serum alanine aminotransferase and mortality of rabbits with toxic hepatitis induced by severe subcutaneous injection of carbon tetrachloride. The experiments of Liang Wen [111] found that P. quassioides injection had significant protective effects on both mice and dogs injected with lethal doses of snake venom, reaching 75.6 % protection in mice and up to 100 % protection in dogs.
2.8. Anti-malarial
Saiin et al. [112] found that the n-hexane extract of P. quassioides has anti-malarial activity. Pavanand et al. [113], on the other hand, found that compounds 6-hydroxy-4-methoxy-1-vinyl-B-carboline and 4-methoxy-1-6-carboline extracted from the stem bark of Thai P. quassioides produced inhibitory activity against a variety of Plasmodium falciparum species.
2.9. Antioxidant
The roots, stems, branches, and leaves of P. quassioides have antioxidant effects, with the leaves being the most effective and can be used as an antioxidant stress agent in animal production [114].Yang et al. [115] found that P. quassioides had significant antioxidant activity, demonstrating that 4-methoxy-5-hydroxy-6-one was the only compound with antioxidant activity, which provides a basis for further development of P. quassioides as an antioxidant. 4-methoxy-5-hydroxycanthin-6-one exhibited obvious 1,1-diphenyl-2-picryl-hydrazyl free radical scavenging activity with an IC50 value of 84.037 μM.
2.10. Anti-metabolic disorder effects
Naturally occurring compounds dimeric alkaloid picrasidine N in P. quassioides have been reported to stimulate receptors such as PPARγ and PPARβ/δ, which play important roles in the regulation of energy metabolism, inflammation, and other physiological processes such as obesity, diabetes, and hyperlipidemia [116]. In addition, the dimeric β-carboline type alkaloid compound Picrosinine C from P. quassioides root is also a PPARα activator with potential applications for the treatment of metabolic diseases such as hyperlipidemia [117].
2.11. Protective effects on the nervous system
The Canthin-6-one alkaloids (CO) in P. quassioides have potential anti-neuroinflammatory effects and may be protective against astrocyte-mediated pro-inflammatory responses and associated endothelial barrier disruption [118]. In addition, the ethyl acetate extract of P. quassioides stem improved memory and cognition in mice with amyloid β-peptide-induced AD and was neuroprotective in a neuronal cell model, and its anti-AD mechanism may involved inhibition of neuroinflammation and reduction of Aβ1-42 deposition [119].
3. Toxicity
Many studies have demonstrated that total P. quassioides alkaloids have no significant effects on the growth and development of rats, nor do they cause significant adverse effects on the blood indicators and organs of rats [120].However, a study by Gong et al. [9] showed that the 5-hydroxy-4-methoxycanthin-6-one (CAN) component of P. quassioides has toxic effects on zebrafish embryos, which may lead to reduced survival, delayed hatching, and malformation of zebrafish embryos, as well as causing oxidative stress. And a series of derivatives containing the CAN1 framework were designed, synthesized, and evaluated for anti-proliferative activity against two human cancer cell lines, HepG2 and HCT116, IC50 values of 5.05 μM (HepG2) and 6.65 μM (HCT116).
4. Metabolic studies
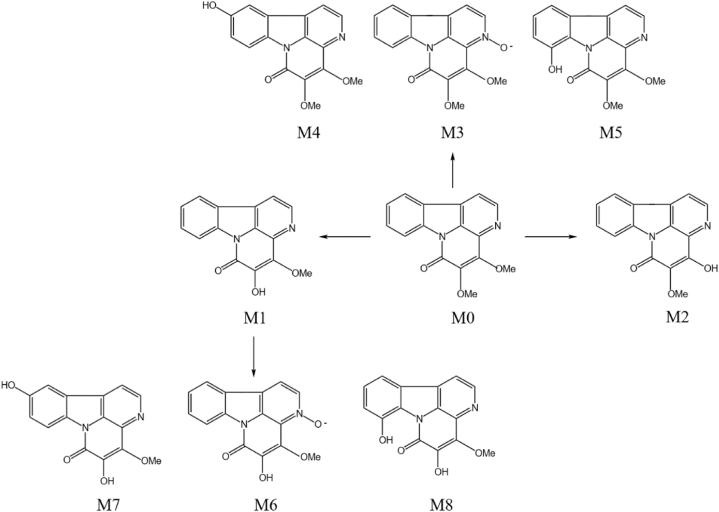
In recent years, there have been new developments in metabolic studies on P. quassioides. Miao et al. [[121], [122], [123]] studied the metabolites (M1-M8, as shown in Fig. 6) of 4,5-Dimethoxycanthin-6-one in liver microsomes of different animals (rats, mice, dogs and humans) and analyzed the tissue distribution and pharmacokinetic characteristics of the major metabolites in rats. In addition, the results showed that 4,5-dimethoxycanthin-6-one could uncompetitively inhibit CYP1A2 mediated phenacetin O-deethylation with an IC50 value of 1.7 μM and a Ki value of 2.6 μM. Shi et al. [124,125] studied the concentrations of 4,5-Dimethoxycanthin-6-one and 5-Hydroxy-4-methoxycanthin-6-one in rat plasma after oral administration of P. quassioides by LC-MS and their pharmacokinetic characteristics, and found that 4,5-Dimethoxycanthin-6-one was eliminated faster in rats (as shown in Fig. 7). A total of 17 metabolites were identified, and a new CAN metabolic pathway was identified using LC-Q-TOF-MS and other techniques to screen and characterize CAN metabolites in rats. Zhao et al. [126] determined the content of 5-Methoxycanthin-6-one in rat plasma by LC-MS/MS and investigated its pharmacokinetic characteristics, which showed that 5-Methoxycanthin-6-one was rapidly absorbed and had a moderate elimination half-life in rats, but had low bioavailability (as shown in Table 1). Meixiang Cai et al. [127] analyzed the alkaloid chemical composition of P. quassioides and its conversion pattern in SD rats and found that the 4,5-carbon double bond and 6,7-lactam bond could be broken in vivo to form β-carboline type alkaloids. It was demonstrated that 5-hydroxy-4-methoxycanthin-6-one could be converted to 1-Methoxycarbonyl-β-carboline in rats. Fan et al. [128] used Cunninghamella blakesleeana CGMCC was applied as a microbial system to mimic mammalian metabolism of 4,5-Dimethoxylcanthin-6-one, and seven metabolites of compound 1 were successfully metabolized. The metabolic pathways of 1 were proposed, and the metabolic processes involved phase I and phase II reactions. In addition, P. quassioides promotes the metabolism of azithromycin in a rat pneumonia model, reduces tissue accumulation and avoids liver damage, and improves the efficiency of drug utilization [129]. Chen Fengzheng et al. [130] obtained the total alkaloid P. quassioides ketone from the ethanol extract of P. quassioides by acid solubilization and alkali precipitation, and found that P. quassioides ketone undergoes structural changes to the highly active 4,5-Dimethoxycanthin-6-one by the action of human-derived intestinal microorganisms and enzymes secreted by microorganisms.
Fig. 6.
Metabolic pathway of 4,5-Dimethoxylcanthin-6-one in liver microsomes in vitro.
Fig. 7.
Blood concentration and pharmacokinetic characteristics of 5-Hydroxy-4-methoxycanthin-6-one and 4,5-Dimethoxycanthin-6-one in rats.
Table 1.
Pharmacokinetic parameters of 5 -hydroxy-4 -methoxy-ferrocinone in rats after oral and intravenous administration.
|
Parameters |
Unit | 10 mg/kg | Oral 25 mg/kg |
50 mg/kg | Intravenous 5 mg/kg |
|---|---|---|---|---|---|
| AUC0-t | μg h/L | 294.86 ± 62.40 | 564.98 ± 117.16 | 1003.28 ± 316.08 | 603.55 ± 119.80 |
| AUC0-∞ | μg h/L | 315.53 ± 72.62 | 593.78 ± 134.58 | 1059.62 ± 345.36 | 645.86 ± 108.04 |
| MRT0-t | h | 1.74 ± 0.33 | 1.92 ± 0.33 | 2.03 ± 0.34 | 0.69 ± 0.12 |
| T1/2 | h | 1.37 ± 0.44 | 2.11 ± 1.09 | 1.55 ± 0.80 | 0.85 ± 0.14 |
| Tmax | min | 33.00 ± 6.71 | 36.00 ± 8.22 | 42.00 ± 6.71 | 2.00 ± 0.00 |
| VZ/F | L/kg | 59.27 ± 32.54 | 132.22 ± 51.12 | 92.57 ± 36.40 | 9.67 ± 1.98 |
| CLZ/F | L kg/h | 30.12 ± 13.07 | 50.97 ± 25.95 | 55.03 ± 26.25 | 7.94 ± 1.52 |
| Cmax | ng/mL | 232.80 ± 54.44 | 353.40 ± 91.17 | 484.35 ± 161.31 | 1745.51 ± 109.93 |
| F | % | 24.42 | 18.72 | 16.62 |
5. Summary and outlook
In recent years, significant progress has been made in the study of the chemical composition, biological activity, and metabolism of the Chinese herbal medicine P. quassioides. The chemical composition of P. quassioides has been continuously discovered and new techniques have been used to isolate new major constituent substances such as alkaloids, quassinoids, triterpenoids and other substances. Studies have shown that the active substances in P. quassioides have anti-cancer, anti-inflammatory and antihypertensive effects, in addition to antiviral, analgesic, and neuroprotective effects, which have potential applications in the treatment of a variety of diseases. And a precise understanding of the pharmacological effects of P. quassioides and a solid scientific basis for its application in clinical therapy. Studying the formation, transformation and elimination of its metabolites, we can profounder understand the dynamics of P. quassioides drugs in vivo, providing strong support for optimizing drug design and improving therapeutic efficacy. Meanwhile, the study of P. quassioides alkaloid metabolites can deeper understand its mechanism of action and provide an important theoretical basis for the drug development of P. quassioides. In the future, we need to continue to study the chemical composition, pharmacological effects, and pharmacokinetics of P. quassioides in depth to develop new safe and effective drugs.
Data availability statement
No data are available.
Fund projects
Natural Science Foundation of Hubei Province (2018CFB361); Open Project Fund of National and Local Joint Engineering Research Center for Drug High-throughput Screening Technology (K20201006); Hubei University of Science and Technology Doctoral Start-up Project Fund (BK201810).
Ethics approval
Review and approval by an ethics committee was not needed for this study because this is a review, there are no experiments and their ethical conflicts.
CRediT authorship contribution statement
Yiye Zhao: Writing – original draft, Investigation. Dan Ye: Supervision, Formal analysis. Chen Xie: Methodology, Conceptualization. Haoyang Quan: Investigation, Data curation. Min Zheng: Supervision, Resources. Xiaolei Miao: Writing – review & editing, Validation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Min Zheng, Email: zhengminsci@163.com.
Xiaolei Miao, Email: xlmiao1013@163.com.
References
- 1.Chinese Pharmacopoeia [S] Part. 2020;1 [Google Scholar]
- 2.Wenna Zhao, Zhang Xinxin, Xie Renming, et al. Research progress on chemical constituents and pharmacological effects of Picrasma quassioides. Sinobai. 2011;34(7):1149–1152. [Google Scholar]
- 3.Gu Hua, Wang Mei, Wang Zhiyang. Modern pharmacology and clinical application of Picrasma quassioides. Research of Traditional Chinese Medicine. 2001;14(5):55–56. [Google Scholar]
- 4.Wu Wanhui. Clinical observation on the treatment of acute upper respiratory tract infection by atomizing inhalation of Picrasma quassioides injection in children. Chinese Community Physician. 2016;32(22):119–120. [Google Scholar]
- 5.Mingqiang Wei, Chen Bin, Wu Qianyong. Clinical study of Hai Lixin (Picrasma quassioides Injection) in treatment of infantile enteritis. Chinese Medical Guide. 2018;16(34):170–171. [Google Scholar]
- 6.Pei Shufei, Zhang Xiangdong, Zhao Xianqun, et al. Clinical study of Xiaoyanlidan Tablet combined with ceftriaxone sodium in the treatment of chronic cholecystitis [J]. New Chinese Medicine,202,54(22):76-79.
- 7.Deng Yanqiu, Lichun Zhao, Tang Hongzhen, et al. Research progress on chemical constituents and biological activities of Picrasma quassioides. Chinese Patent Medicine. 2020;42(5):1291–1296. [Google Scholar]
- 8.Shi Guohua, Jiao Weihua, Fan Yang, et al. Study on three dimerized β-cabalin alkaloids and their bioactivity in Balsam pear. Chinese Herbal Medicine. 2015;46(6):803–807. [Google Scholar]
- 9.Gong G., Jiang L., Lin Q., et al. In vivo toxic effects of 4-methoxy-5-hydroxy-canthin-6-one in zebrafish embryos via copper dyshomeostasis and oxidative stress. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2018 Jan;204:79–87. doi: 10.1016/j.cbpc.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Wenna Zhao. Northwest University; 2013. Study on Chemical Constituents and Pharmacological Activities of Picrasma Quassioides [D] [Google Scholar]
- 11.Zhang Qiuyu, Lin Zhaozhan, Yuan Yue, et al. Study on chemical constituents of Picrasma quassioides [J]. Chinese Herbal Medicine,20,51(19):4884-4890.
- 12.Lin Qinghua, Xu Jian, Feng Feng. Chemical constituents of stem of Picrasma quassioides. J. China Pharm. Univ. 2017;48(6):675–679. [Google Scholar]
- 13.Zhang J., Zhao S.S., Xie J., et al. N-methoxy-β-carboline alkaloids with inhibitory activities against Aβ42 aggregation and acetylcholinesterase from the stems of Picrasma quassioides. Bioorg. Chem. 2020 Aug;101 doi: 10.1016/j.bioorg.2020.104043. [DOI] [PubMed] [Google Scholar]
- 14.Kwon H.S., Lee H., Lee J.S., et al. Two new β-carboline alkaloids from the stems of Picrasma quassioides. Arch Pharm. Res. (Seoul) 2018 May;41(5):513–518. doi: 10.1007/s12272-018-1034-2. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J., Wang C.X., Song X.J., et al. A new cinnamamide derivative and two new β-carboline alkaloids from the stems of Picrasma quassioides. Fitoterapia. 2019 Nov;139 doi: 10.1016/j.fitote.2019.104375. [DOI] [PubMed] [Google Scholar]
- 16.Zhao W.Y., Shang X.Y., Zhao L., et al. Bioactivity-guided isolation of β-Carboline alkaloids with potential anti-hepatoma effect from Picrasma quassioides (D. Don) Benn. Fitoterapia. 2018 Oct;130:66–72. doi: 10.1016/j.fitote.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Kondo Y., Takemoto T. The structure of a New β-carboline alkaloid from Picrasma ailanthoides Planchon. Chem Pharm Bull. 1973;21(4):837–839. [Google Scholar]
- 18.Yang Junshan, Luo Shurong, Shen Xiulan, et al. Chemical study on alkaloids of Picrasma quassioides. Acta Pharmacy. 1979;14(3):167–177. [PubMed] [Google Scholar]
- 19.Yang J.S., Gong D. Kumujancine and kumujanrine, two new β-carboline alkaloids from Picrasma quassioides (D. DON.) Benn. Acta Chim Sinica. 1984;42(7):679–683. [Google Scholar]
- 20.Matsumura S., Enomoto H. CODEN: GWXXBX DE 2941449 19800417. 1980. Indole derivatives [P]. Ger often; p. 21pp. (German) [Google Scholar]
- 21.Chen Meng. Yantai University; Yantai: 2007. Study on Active Constituents of Alkaloids from Bittern [D] (in Chinese) [Google Scholar]
- 22.Ohmoto T., Koike K. Studies on the constituents of Picrasma quassioides Bennet.I. On the alkaloidal constituents. Chem Pharm Bull. 1982;30(4):1204–1209. [Google Scholar]
- 23.Bennet I.V. Structures of picrasidines I, J, and K. Chem Pharm Bull. 1985;33(8):3356–3360. [Google Scholar]
- 24.Ohmoto T., Koike K. Studies on the constituents of Picrasma quassioides Bennet. II. On the alkaloidal constituents. Chem Pharm Bull. 1983;31(9):3198–3204. [Google Scholar]
- 25.Ohmoto T., Koike K. Studies on the constituents of Picrasma quassioides Bennet. III. The alkaloidal constituents. Chem Pharm Bull. 1984;32(9):3579–3583. [Google Scholar]
- 26.Ohmoto T., Koike K. Studies on the alkaloids from Picrasma quassioides bennet. V: structures of picrasidines L, M, and P. Chem Pharm Bull. 1985;33(9):3847–3851. [Google Scholar]
- 27.Li H.Y., Ohmoto T., Koike K., et al. New alkaloids, picrasidines W, X and Y, from Picrasma quassioides and X-ray crystallographic analysis of picrasidine Q. Chem Pharm Bull. 1993;41(10):1807–1811. [Google Scholar]
- 28.Jiao W.H., Gao H., Li C.Y., et al. β-Carboline alkaloids from the stems of Picrasma quassioides. Magn. Reson. Chem. 2010;48(6):490–495. doi: 10.1002/mrc.2602. [DOI] [PubMed] [Google Scholar]
- 29.Koike K., Ohmoto T., Ikeda K. β-Carboline alkaloids from Picrasma quassioides. Phytochemistry. 1990;29(9):3060–3061. [Google Scholar]
- 30.Liu J., Davidson R.S., Heijden R.V.D., et al. Isolation of 4-Hydroxy-5-methoxycanthin-6-one from Picrasma quassioides and revision of a previously reported structure. Liebigs Ann. Chem. 1992;1992(9):987–988. [Google Scholar]
- 31.Ohmoto T., Koike K. Studies on the alkaloids from Picrasma quassioides bennet. VI. Structures of picrasidines N, O, and Q. Chem Pharm Bull. 1985;33(11):4901–4905. [Google Scholar]
- 32.Koike K., Ohmoto T. Studies on the alkaloids from Picrasma quassioides bennet. VII. Structures of. BETA.-carboline dimer alkaloids, picrasidines-H and-R [J]. Chem Pharm Bull. 1986;34(5):2090–2093. [Google Scholar]
- 33.Koike K., Ohmoto T. Picrasidine-U, dimeric alkaloid from Picrasma quassioides. Phytochemistry. 1988;27(9):3029–3030. [Google Scholar]
- 34.Koike K., Ohmoto T. Studies on the alkaloids of Picrasma quassioides Bennet. VIII X-ray crystal structure analysis of picrasidine-F. Chem Pharm Bull. 1986;34(8):3228–3236. [Google Scholar]
- 35.Koike K., Ohmoto T., Higuchi T. Picrasidine-T, a dimeric β-carboline alkaloid from Picrasma quassioides. Phytochemistry. 1987;26(12):3375–3377. [Google Scholar]
- 36.Koike K., Ohmoto T. Studies on the alkaloids from Picrasma quassioides Bennet. IX. Structures of two. BETA.-carboline dimeric alkaloids, picrasidines-G and-S. Chem Pharm Bull. 1987;35(8):3305–3308. [Google Scholar]
- 37.Xu J., Xiao D., Song W.W., et al. Quassinoids from the stems of Picrasma quassioides and their cytotoxic and NO production-inhibitory activities. Fitoterapia. 2016 Apr;110:13–19. doi: 10.1016/j.fitote.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 38.He C., Wang Y., Yang T., et al. Quassinoids with insecticidal activity against diaphorina citri kuwayama and neuroprotective activities from Picrasma quassioides. J. Agric. Food Chem. 2020 Jan 8;68(1):117–127. doi: 10.1021/acs.jafc.9b05796. [DOI] [PubMed] [Google Scholar]
- 39.Zhao W.Y., Song X.Y., Zhao L., et al. Quassinoids from Picrasma quassioides and their neuroprotective effects. J. Nat. Prod. 2019 Apr 26;82(4):714–723. doi: 10.1021/acs.jnatprod.8b00470. [DOI] [PubMed] [Google Scholar]
- 40.Liu C., Cheng R.R., Yang L., et al. Inhibition of CYP450 enzymes by quassinoids from Picrasma quassioides leaves. Phytochem. Lett. 2019;30:138–142. [Google Scholar]
- 41.Okano M., Fujita T., Fukamiya N., et al. New quassinoid glycosides and hemiacetals from Picrasma ailanthoides planchon. Picrasinoside-B,-C,-D,-E,-F, and-G, and picrasinol-A and-B. Chem. Lett. 1984;13(2):221–224. [Google Scholar]
- 42.Daido M., Fukamiya N., Okano M., et al. Picrasinol D, a new quassinoid from the stem wood of Picrasma ailanthoides. J. Nat. Prod. 1995;58(4):605–608. doi: 10.1021/np50075a014. [DOI] [PubMed] [Google Scholar]
- 43.Okano M., Fujita T., Fukamiya N., et al. New quassinoid glucosides, picrasinoside-A,-B,-C,-D,-E,-F, and-G and new hemiacetals, picrasinol-A and-B, from the stem bark of Picrasma ailanthoides Planchon. Bull. Chem. Soc. Jpn. 1985;58(6):1793–1800. [Google Scholar]
- 44.Matsuzaki T., Fukamiya N., Okano M., et al. Picrasinoside H, a new quassinoid glucoside, and related compounds from the stem wood of Picrasma ailanthoides. J. Nat. Prod. 1991;54(3):844–848. doi: 10.1021/np50075a014. [DOI] [PubMed] [Google Scholar]
- 45.Murae T., Tsuyuki T., Ikeda T., et al. Bitter principles of Picrasma ailanthoides planchon. Nigakilactones A, B, C, D, E and F. Tetrahedron. 1971;27(7):1545–1555. [Google Scholar]
- 46.Thaiwei, Chen Zhongliang. Chemistry, biogenesis and anticancer activity of Picrin from momordica armigera [J]. Foreign Medicine. Journal of Pharmaceutical Sciences,1979(1):10-19.
- 47.Yang S.P., Yue J.M. Five new quassinoids from the bark of Picrasma quassioides. Helv. Chim. Acta. 2004;87(6):1591–1600. [Google Scholar]
- 48.Hikino H., Ohta T., Takemoto T. Picrasins, simaroubolides of Japanese quassia tree Picrasma quassioides. Phytochemistry. 1975;14(11):2473–2481. [Google Scholar]
- 49.Yang J.S., Gong D. A new bitter principle, kusulactone, from Indian quassiawood(Picrasma quassioides) Zhongcaoyao. 1984;15(12):531–533. [Google Scholar]
- 50.Hirota H., Yokoyama A., Tsuyuki T., et al. Structure of Nigakilactone O, a new quassinoid from Picrasma ailanthoides. Chem. Lett. 1988;17(4):651–652. [Google Scholar]
- 51.Zhao W.Y., Chen J.J., Zou C.X., et al. New tirucallane triterpenoids from Picrasma quassioides with their potential antiproliferative activities on hepatoma cells. Bioorg. Chem. 2019;84:309–318. doi: 10.1016/j.bioorg.2018.11.049. [DOI] [PubMed] [Google Scholar]
- 52.Xu J., Xiao D., Lin Q.H., et al. Cytotoxic tirucallane and Apotirucallane triterpenoids from the stems of Picrasma quassioides. J. Nat. Prod. 2016;79(8):1899–1910. doi: 10.1021/acs.jnatprod.5b01137. [DOI] [PubMed] [Google Scholar]
- 53.Zhang J., Wang C.X., Song X.J., et al. A pair of new tirucallane triterpenoid epimers from the stems of Picrasma quassioides. Chin. J. Nat. Med. 2019 Dec;17(12):906–911. doi: 10.1016/S1875-5364(19)30111-6. [DOI] [PubMed] [Google Scholar]
- 54.Yang P.Y., Zhao P., Bai M., et al. Structure elucidation and absolute configuration determination of C26, C27 and C30 tirucallane triterpenoids from the leaves of Picrasma quassioides (D. Don) Benn. Phytochemistry. 2021 Apr;184 doi: 10.1016/j.phytochem.2021.112675. [DOI] [PubMed] [Google Scholar]
- 55.Niimi Y., Hirota H., Tsuyuki T., et al. New 7, 24-tirucalladiene-type triterpenoids from Picrasma quassioides bennett. Chem Pharm Bull. 1989;37(1):57–60. [Google Scholar]
- 56.Yoshikawa K., Sugawawa S., Arihara S. Phenylpropanoids and other secondary metabolites from fresh fruits of Picrasma quassioides. Phytochemistry. 1995;40(1):253–256. [Google Scholar]
- 57.Sugimoto Y., Sakita T., Moriyam Y., et al. Structure of nigakialcohol, a new ionone derivative from picrasma ailanthoides planchon. Tetrahedron Lett. 1978;19(44):4285–4288. [Google Scholar]
- 58.Zhu Chenlan, Guihua Deng. Lin Study on chemical constituents of bitter wood. Natural Products Research and Development. 2012;24(4):476–478. (in Chinese) [Google Scholar]
- 59.Lou L.L., Yao G.D., Wang J., et al. Enantiomeric neolignans from Picrasma quassioides exhibit distinctive cytotoxicity on hepatic carcinoma cells through ROS generation and apoptosis induction. Bioorg. Med. Chem. Lett. 2018 May 1;28(8):1263–1268. doi: 10.1016/j.bmcl.2018.03.043. [DOI] [PubMed] [Google Scholar]
- 60.Yao G.D., Wang J., Song X.Y., et al. Stereoisomeric guaiacylglycerol-β-coniferyl aldehyde ether induces distinctive apoptosis by downregulation of MEK/ERK pathway in hepatocellular carcinoma cells. Bioorg. Chem. 2018 Dec;81:382–388. doi: 10.1016/j.bioorg.2018.08.033. [DOI] [PubMed] [Google Scholar]
- 61.Xu R., Zhang D., Shu F., et al. Picraquanines A-C, three new phenolic derivatives from the stems of Picrasma quassioides. Nat. Prod. Res. 2021 Nov;35(21):3687–3693. doi: 10.1080/14786419.2020.1727475. [DOI] [PubMed] [Google Scholar]
- 62.Liu C., Cheng R.R., Han Z.Z., et al. A new ionone derivative from the leaves of Picrasma quassioides. J. Asian Nat. Prod. Res. 2019 Jul;21(7):652–658. doi: 10.1080/10286020.2018.1464561. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Jie, Li Qiao, Guo Wenna, et al. Effects of Kumu Injection and Azithromycin on the release of inflammatory cytokines in vitro [J]. J. Yantai Univ. (Nat. Sci. Eng.),20,33(1):115-119.
- 64.Yu M., Gu X., Qu Y., et al. Combination therapy with TCM preparation kumu injection and azithromycin against bacterial infection and inflammation: in vitro and in vivo. Evid Based Complement Alternat Med. 2022 Mar 16;2022 doi: 10.1155/2022/8533005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jin Yongzhe, Xie Danping, Sun Hunan. Effect of extract of Picrasma quassioides on apoptosis of drug-resistant lung cancer cells [J]. Medical Journal of Yanbian University, 20,43(4):243-250.
- 66.Dai W., He J., Ye F., et al. Design, synthesis and in vitro biological evaluation of novel 4-methoxy-5-hydroxycanthin-6-one derivatives as potential anti-tumor agents. Nat. Prod. Res. 2020 Aug;34(16):2289–2294. doi: 10.1080/14786419.2018.1536708. [DOI] [PubMed] [Google Scholar]
- 67.Zhu Zhengri, Liu Wanlu, Chen Aidong. Study on related factors of gastric cancer mice by extract of Picrasma quassioides. Foreign Medicine (Antibiotics) 2015;36(3):126–127. [Google Scholar]
- 68.Yue Li, Luo Xin, Yufeng Li, Jing Li. Clinical research of traditional. Chin. Med. 2017;9(20):41–42. [Google Scholar]
- 69.Xie D.P., Gong Y.X., Jin Y.H., et al. Anti-tumor properties of Picrasma quassioides extracts in H-RasG12V liver cancer are mediated through ROS-dependent mitochondrial dysfunction. Anticancer Res. 2020 Jul;40(7):3819–3830. doi: 10.21873/anticanres.14371. [DOI] [PubMed] [Google Scholar]
- 70.Zhao W.Y., Chen J.J., Zou C.X., et al. Effects of enantiomerically pure β-carboline alkaloids from Picrasma quassioides on human hepatoma cells. Planta Med. 2019 May;85(8):648–656. doi: 10.1055/a-0879-4721. [DOI] [PubMed] [Google Scholar]
- 71.Zhang Lin, Deng Guihua, Yang Liang, et al. Study on the mechanism of regulating the proliferation and apoptosis of cervical cancer HeLa cells through the regulation of miR-331-3p [J]. Shaanxi J. Tradit. Chin. Med.,202,43(1):17-22.
- 72.Gong Y.X., Liu Y., Jin Y.H., et al. Picrasma quassioides extract elevates the cervical cancer cell apoptosis through ROS-mitochondrial Axis activated p38 MAPK signaling pathway. In Vivo. 2020 Jul-Aug;34(4):1823–1833. doi: 10.21873/invivo.11977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun H.N., Xie D.P., Ren C.X., et al. Ethyl β-carboline-3-carboxylate increases cervical cancer cell apoptosis through ROS-p38 MAPK signaling pathway. In Vivo. 2022 May-Jun;36(3):1178–1187. doi: 10.21873/invivo.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hsieh M.C., Lo Y.S., Chuang Y.C., et al. Dehydrocrenatidine extracted from Picrasma quassioides induces the apoptosis of nasopharyngeal carcinoma cells through the JNK and ERK signaling pathways. Oncol. Rep. 2021 Aug;46(2):166. doi: 10.3892/or.2021.8117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yamashita N., Kondo M., Zhao S., et al. Picrasidine G decreases viability of MDA-MB 468 EGFR-overexpressing triple-negative breast cancer cells through inhibition of EGFR/STAT3 signaling pathway. Bioorg. Med. Chem. Lett. 2017 Jun 1;27(11):2608–2612. doi: 10.1016/j.bmcl.2017.03.061. [DOI] [PubMed] [Google Scholar]
- 76.Lin Q.H., Qu W., Xu J., et al. 1-Methoxycarbony-β-carboline from Picrasma quassioides exerts anti-angiogenic properties in HUVECs in vitro and zebrafish embryos in vivo. Chin. J. Nat. Med. 2018 Aug;16(8):599–609. doi: 10.1016/S1875-5364(18)30097-9. [DOI] [PubMed] [Google Scholar]
- 77.Zhao F., Gao Z., Jiao W., et al. 2012. PlantaMed [M] p. 1906. 78. [Google Scholar]
- 78.Kong L., Wang B., Yang X., et al. Picrasidine I from picrasma quassioides suppresses osteoclastogenesis via inhibition of RANKL induced signaling pathways and attenuation of ROS production. Cell. Physiol. Biochem. 2017;43(4):1425–1435. doi: 10.1159/000481874. [DOI] [PubMed] [Google Scholar]
- 79.Chen Meng. Yantai University; 2007. Study on Active Constituents of Alkaloids from Picrasma quassioides [D] [Google Scholar]
- 80.Jiao Weihua, Gao Hao, Feng Zhao, et al. Proceedings of the 8th Symposium on Natural Organic Chemistry of the Chinese Chemical Society. 2010. Study on anti-inflammatory active constituents of Picrasma quassioides [C]//Chinese chemical Society, National natural science Foundation of China; p. 230. [Publisher unknown] [Google Scholar]
- 81.Weihua Chiao. Shenyang Pharmaceutical University; 2010. Study on Anti-inflammatory Active Constituents of Chinese Herb Picrasma quassioides [D] [Google Scholar]
- 82.Liu Bowen, Liu Fulei, Wang Ruyi, et al. Analysis of Chemical constituents of total alkaloids from Picrasma quassioides and their effects on Collagen-induced arthritis in rats. J. China Pharm. Univ. 2019;50(5):585–592. [Google Scholar]
- 83.Ning Mi, Chen Huanying. Effect of Compound Picrasma quassioides anti-inflammatory tablets combined with Lankefuning in the treatment of acne vulgaris. Dermatology and Venereal Diseases. 2016;38(3):217–219. [Google Scholar]
- 84.Chen Ci Cheng, Zhenrong Li, Liu Xiaochun. Therapeutic effect of Picrasma quassioides injection on acute upper respiratory tract infection in children. China Prescription Drugs. 2016;14(5):40–41. [Google Scholar]
- 85.Chinese Herbal Medicine Information Station . In: Active Ingredient Manual of Botanical Medicine [M] Administration National Medicine., editor. People's Medical Publishing House; Beijing: 1986. pp. 875–876. [Google Scholar]
- 86.Liandan injection Chinese pharmaceutical. Journal. 1988;(7):440. [Google Scholar]
- 87.Zheng Kunqin. Experimental study on liandan injection. Guangdong Medical Journal. 1987;(6):50. [Google Scholar]
- 88.Ho H.Y., Lin C.C., Chuang Y.C., et al. Apoptotic effects of dehydrocrenatidine via JNK and ERK pathway regulation in oral squamous cell carcinoma. Biomed. Pharmacother. 2021 May;137 doi: 10.1016/j.biopha.2021.111362. [DOI] [PubMed] [Google Scholar]
- 89.Zhao F., Tang Q., Xu J., et al. Dehydrocrenatidine inhibits voltage-gated sodium channels and ameliorates mechanic allodia in a rat model of neuropathic pain. Toxins. 2019 Apr 18;11(4):229. doi: 10.3390/toxins11040229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Song Zhenyu. vol. I. Beijing Medical University, Peking Union Medical University Associated Press; Beijing: 1995. p. 100. (Modern Study of Chinese Herbal Medicine [J]). [Google Scholar]
- 91.Study on the toxicity of total alkaloids of Picrasma quassioides Chinese Herbal Medicine Bulletin. 1977;(6):34–35+49. [Google Scholar]
- 92.Wenna Zhao, Ying Cui, Bai Jing, et al. Study on antibacterial activity and anti-inflammatory effect of extracts of Picrasma quassioides in vitro. Northwest Pharmaceutical Journal. 2019;34(4):505–508. [Google Scholar]
- 93.Ying He, Liu Wei, Chen Zhongwei, et al. Study on the inhibitory effect of alkaloids from Picrasma quassioides on Escherichia coli in vitro. Journal of Anhui Agricultural Sciences. 2008;(7):2777–2778. [Google Scholar]
- 94.Chen Meng, Fan Huaying, Dai Shengjun, et al. Proceedings of the Symposium on Research and Development of Innovative Drugs and New Varieties. 2006. Study on antibacterial active components of alkaloids of Picrasma quassioides [C] pp. 315–321. [Publisher unknown] [Google Scholar]
- 95.Khan M.R., Kihara M., Omoloso A.D. Antibacterial activity of Picrasma javanica. Fitoterapia. 2001 May;72(4):406–408. doi: 10.1016/s0367-326x(00)00274-4. [DOI] [PubMed] [Google Scholar]
- 96.Wenna Zhao, Qi Su, He Jiao, et al. Study on antihypertensive effect of extract of Picrasma quassioides on essential hypertension rats. Pharmacology and Clinic of Traditional Chinese Medicine. 2012;28(5):108–111. [Google Scholar]
- 97.Ma Shude, Xie Renming, Miao Airong, et al. Effects of total alkaloids of Picrasma quassioides on cardiovascular system [J]. Acta Medica Sinica,1982(5):327-330.
- 98.Du Zhide, Zhang Aiwu, Wang Yufeng. Study on antihypertensive effect and toxicity of total alkaloids of Picrasma quassioide. Chinese Journal of Pharmacy. 1981;(6):5–7. [Google Scholar]
- 99.Cheng Zhuan-quan, Yin Chuan-xiu, Wang Su-xia, et al. Clinical observation of 52 cases of hypertension treated with Balsam herb [J]. Shaanxi New Medicine,1980(7):9-10.
- 100.Cheng Zhunquan, Wang Suxia, Wang Zonghe, et al. Treatment of hypertension with picrolactone A in 136 patients [J]. New Drug and Clinical,1987(5):275-278.
- 101.Zhang Zhenjie, Li Suiying, Guo Li, et al. Isolation and identification of antihypertensive components of Picrasma quassioide [J]. Acta Botanica Sinica of Northwest China,1986(2):138-140.
- 102.Xie Renming, Miao Airong, Shen Yaqin, et al. Effects of total alkaloids of Picrasma quassioide on coronary circulation and cardiac hemodynamics in dogs [J]. Chinese Journal of Traditional Chinese Medicine,1982,13(11):30-32+34.
- 103.Zhi-De Du, Ai-wu Zhang. Pharmacologic study on total alkaloids of Picrasma quassioide. Pharmaceutical Industry. 1982;(6):21–26. [Google Scholar]
- 104.Ohmoto T., Sung Y.I., Koike K., et al. Effect of alkaloids of simaroubaceous plants on the local blood flow rate. J. Pharmaceut. Sci. 1985;39(1):28–34. [Google Scholar]
- 105.Yujiro N., Koike K., et al. Gastric aniulcer components from the woods of Picrasma quassioides (Simaroubaceae) Nat. Med. 1994;48(2):116–121. [Google Scholar]
- 106.Liu Wenxing. Report of 60 cases of autumn and winter diarrhea treated by acupoint injection of hailixin brand Picrasma quassioides injection. World Latest Medical Information Abstracts. 2015;15(14):125. [Google Scholar]
- 107.Zhang Chunxia. Clinical observation of acupoint injection of Picrasma quassioides injection in the treatment of diarrhea in children. Inner Mongolia Traditional Chinese Medicine. 2015;34(6):81. [Google Scholar]
- 108.Xie Weixuan. Effect of Picrasma quassioides injection on herpes zoster. Journal of Youjiang Medical College for Nationalities. 2000;(6):967. [Google Scholar]
- 109.Guangjian Zhao, Zhai Deshe, Rilei Yu, et al. Study on the inhibition of SARS-CoV-2 by the composition of Picrasma quassioides Injection. J. Ocean Univ. China. 2021;51(1):70–75. [Google Scholar]
- 110.Zhi-De Du, Ai-wu Zhang. Pharmacologic study on total alkaloids of Picrasma quassioides. Pharmaceutical Industry. 1982;(6):21–26. [Google Scholar]
- 111.Liang Jianhua. Study on antivenom of Picrasma quassioides [J]. Bulletin of Chinese Traditional Medicine,1987(4):56.
- 112.Saiin C., Rattanajak R., Kamchonwongpaisan S., et al. Isolation and in vitro antimalarial activity of hexane extract from Thai Picrasma javanica B1 stembark. Southeast Asian J Trop Med Public Health. 2003;34(Suppl 2):51–55. [PubMed] [Google Scholar]
- 113.Pavanand K., Yongvanitchit K., Webster H.K., et al. In vitro antimalarial activity of a Thai medicinal plant Picrasma javanica Bl. Phytother Res. 1988;2(1):33–36. [Google Scholar]
- 114.Huang Huaxi, Feng Lixin, Li Jinhua, et al. Study on antioxidant activity of different medicinal parts of Picrasma quassioides [J]. Feed Res.,202,45(15):67-70.
- 115.Yang N., Shi Y., Xiong A., et al. Preparation and purification of canthinone and β-carboline alkaloids from Picrasma quassioides based on bioautography and mass-spectrometry-directed autopurification system. J Sep Sci. 2018 Aug;41(15):3014–3021. doi: 10.1002/jssc.201800127. [DOI] [PubMed] [Google Scholar]
- 116.Zhao S., Kanno Y., Li W., et al. Picrasidine N is a subtype-selective pparβ/δ agonist. J. Nat. Prod. 2016 Apr 22;79(4):879–885. doi: 10.1021/acs.jnatprod.5b00909. [DOI] [PubMed] [Google Scholar]
- 117.Li F., Wang H., Wang Y., et al. Computational investigation reveals Picrasidine C as selective PPARα lead: binding pattern, selectivity mechanism and ADME/tox profile. J. Biomol. Struct. Dyn. 2020 Nov;38(18):5401–5418. doi: 10.1080/07391102.2019.1699861. [DOI] [PubMed] [Google Scholar]
- 118.Yue Q., Xu Y., Lin L., et al. Canthin-6-one (CO) from Picrasma quassioides (D.Don) Benn. ameliorates lipopolysaccharide (LPS)-induced astrocyte activation and associated brain endothelial disruption. Phytomedicine. 2022;101 doi: 10.1016/j.phymed.2022.154108. [DOI] [PubMed] [Google Scholar]
- 119.Guo E., Hu Y., Du T., et al. Effects of Picrasma quassioides and its active constituents on Alzheimer's disease in vitro and in vivo. Bioorg. Chem. 2019 Nov;92 doi: 10.1016/j.bioorg.2019.103258. [DOI] [PubMed] [Google Scholar]
- 120.Xiaozhuang Guo, Wanying La, Zhang Shufeng, et al. Tianjin Science and Technology Translation and Publishing Company; Tianjin: 1992. The Dictionary of Toxic Chinese Herbal Medicine [M] p. 290. [Google Scholar]
- 121.Miao Xiaolei . Hubei University; 2016. Study on Metabolism and Mechanism of 4,5-Dimethoxycanthin-6-One in Vitro and in Vivo [D] [Google Scholar]
- 122.Miao X., Wang J., Chen L., et al. Identification of in vivo and in vitro metabolites of 4,5-dimethoxycanthin-6-one by HPLC-Q-TOF-MS/MS. J Chomatogr B Analyt Technol Biomed Life Sci. 2016 May 1;1020:78–84. doi: 10.1016/j.jchromb.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 123.Miao X., Wang J., Chen Y. Pharmacokinetics and tissue distribution of 4,5-dimethoxycanthin-6-one and its major metabolites in rats. J. Pharm. Biomed. Anal. 2017 May 30;139:22–29. doi: 10.1016/j.jpba.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 124.Shi Y., Xia Y., Wang J., et al. Metabolic profile of 5-hydroxy-4-methoxycanthin-6-one, a typical canthinone alkaloid, in rats determined by liquid chromatography-quadrupole time-of-flight tandem mass spectrometry together with multiple data processing techniques. J. Pharm. Biomed. Anal. 2016 Sep 10;129:60–69. doi: 10.1016/j.jpba.2016.06.047. [DOI] [PubMed] [Google Scholar]
- 125.Shi Y., Hong C., Xu J., et al. Simultaneous quantification of two canthinone alkaloids of Picrasma quassioides in rat plasma by liquid chromatography-tandem mass spectrometry and its application to a rat pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci. 2015 Apr 1;986–987:100–107. doi: 10.1016/j.jchromb.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 126.Zhao L., Zhao Y., Guo L., et al. Pharmacokinetic and bioavailability study of 5-hydroxy-4-methoxycanthin-6-one, a typical canthinone alkaloid, in rats using ultra-high performance liquid chromatography/electrospray ionization tandem mass spectrometry. Biomed. Chromatogr. 2020 Jul;34(7) doi: 10.1002/bmc.4830. [DOI] [PubMed] [Google Scholar]
- 127.Jiang Meixiang, Zhou Yingjun, Mao Qunfang. Study on chemical composition and biotransformation of Picrasma quassioides alkaloids. J. Tradit. Chin. Med. 2022;28(1):56–58+76. [Google Scholar]
- 128.Fan H.X., Zhou Z.Q., Peng J., et al. A microbial model of mammalian metabolism: biotransformation of 4,5-dimethoxyl-canthin-6-one using Cunninghamella blakesleeana CGMCC 3.970. Xenobiotica. 2017 Apr;47(4):284–289. doi: 10.1080/00498254.2016.1184774. [DOI] [PubMed] [Google Scholar]
- 129.Sun Faxin, Xu Xiaomeng, Feng Yingying, et al. Effects of kumu injection and azithromycin distribution in rats with pneumonia [J]. J. Yantai Univ. (Nat. Sci. Eng.), 201,34(3):295-301.
- 130.Chen Fengzheng, Long Jie, Sun Guofeng, et al. Isolation of main alkaloid components from Picrasma quassioides and transformation of intestinal microorganisms [J]. Journal of Leshan Normal University, 201,36(8):14-19.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data are available.