Abstract
The increasing emergence and spread of antibiotic resistance accelerate the desire for antibiotic alternatives. Plant extracts have emerged as a promising and relatively unexplored area of research as potential substitutes. Herein, we investigated the prevalence and distribution patterns of bacteria on egg surfaces and evaluated the inhibitory effects of mangosteen extract on these surface bacteria. In addition, we examined the antioxidant activity and egg quality in improving the ability of mangosteen extract. The results showed that the predominant bacteria isolated from eggshells were Gram-positive, with Staphylococcus and Micrococcus as the dominant genera. Notably, mangosteen extract exhibited significant bactericidal activity, effectively inhibiting Gram-positive bacteria on the surface of chicken eggshells. Moreover, the supplementation of mangosteen extract in the feed of laying hens yielded a noteworthy improvement in egg quality, accompanied by positively shaped structure and function of microbial communities on the egg surface and in the feces. Collectively, our findings suggested that mangosteen extract was an effective alternative to traditional antibiotics, offering valuable insights for animal husbandry development.
Keywords: Antibiotic substitute, Mangosteen extract, Bacteria inhibition, Egg quality enhancement, Microbial communities
1. Introduction
The widespread use of antibiotics promotes the emergence of resistant bacteria, which not only threatens animal health but also poses significant risks to human health through foodborne pathogens. This urgency underlines the need for alternative strategies to combat bacterial infections effectively without exacerbating antibiotic resistance [1,2]. Mangosteen, a tropical fruit cultivated primarily in Southeast Asia, is renowned for its rich content of bioactive compounds such as xanthones and polyphenols [3]. These compounds have been reported to possess potent antibacterial properties and exhibit antioxidant activity [[4], [5], [6]]. Additionally, dietary supplementation with mangosteen extract has shown beneficial effects in improving the growth performance of different animal species [7,8]. However, the potential of mangosteen extract as an antibiotic substitute has not been extensively studied. Therefore, we focused on investigating the inhibitory effects of mangosteen extract on bacteria present on egg surfaces, as well as its antioxidant activity and its potential to enhance egg quality.
Eggs serve as a suitable environment for bacterial colonization, and bacteria on egg surfaces can lead to contamination and compromised egg quality. The contamination of eggs occurs through two primary pathways: vertical transmission, where pathogens infect the ovaries or oviduct, often attributed to specific microorganisms [9,10], and horizontal transmission, which involves eggshell contamination from external sources like fecal matter or environmental vectors [11,12]. The eggshell, acting as a potential breeding ground for bacteria, is particularly vulnerable to the colonization of Gram-positive bacteria such as Bacillus cereus and Staphylococcus aureus [13,14]. These bacteria pose a significant risk due to their adaptability, survival mechanisms, and capacity to form biofilms [[15], [16], [17]]. To reduce the use of antibiotics in food-producing animals, it is essential to analyze bacterial distribution patterns on eggshell surfaces and develop effective technologies to prevent and control pathogenic contamination.
Recent studies have highlighted the remarkable potential of α-mangostin derived from mangosteen extract in effectively inhibiting various Gram-positive bacteria commonly found in poultry and livestock, including Staphylococcus aureus and Clostridium perfringens [18,19]. In this study, we investigated the prevalence and distribution patterns of bacteria on egg surfaces, with a particular focus on Gram-positive bacteria. We also examined the inhibitory effects of mangosteen extract on these surface bacteria and evaluated its antioxidant activity. Furthermore, we assessed the supplementation effects of mangosteen extract in the diets on the egg quality of laying hens. In addition, we explored the structural and functional modifications in microbial communities present on the egg surface and the feces following mangosteen extract supplementation. The findings provided valuable insights into the potential application of mangosteen extract as an effective alternative to traditional antibiotics in animal husbandry, contributing to the development of sustainable and antibiotic-free farming practices.
2. Materials and methods
2.1. Plant materials
Mangosteen was obtained from a large-scale fruit wholesale market, with a preference for fruit that had a deep red outer skin. The fresh fruit was thoroughly cleaned and the edible flesh was separated. The fruit peel was then cut into small pieces and subjected to a 72 h drying process in an oven set at 45 °C. Once dried, the fruit peel was finely ground into a powder and sieved through a 10-mesh sieve. The resulting powder was stored in a sealed container, protected from light.
2.2. Extraction and isolation
Fruit peel (400 g) was taken and subjected to cold maceration extraction with 10 times the volume of 95 % ethanol, performed three times, with each extraction lasting 24 h. The ethanol extracts were combined and concentrated under reduced pressure to obtain a crude extract. The crude extract was dispersed in eight times the ethyl acetate volume, resulting in a brown solution. The extraction liquids were combined and further concentrated. Silica gel was added to the concentrated solution in a 1:1.5 ratio, forming a slurry, which was then loaded onto a column with silica gel (100–200 mesh) in a 1:10 ratio by mass. The ethyl acetate extract was subjected to silica gel column chromatography using a petroleum ether-ethyl acetate system, with increasing polarity (from 100:1 to 1:1) to elute the active components, monitored using thin-layer chromatography (TLC), and the eluates containing the active ingredients were collected. The eluates were concentrated and allowed to crystallize for 20 h, and the crude crystals obtained were collected. The crude crystals were dissolved in twice the volume of 95 % ethanol, subjected to hot dissolution, and crystallized for 24 h. The resulting crude extract was dried under a vacuum.
2.3. Determination of the purity of α-mangostin
The analytical method for measuring α-mangostin was modified from a previous report [20]. The sample and standard compound were dissolved in acetonitrile and filtered with 0.22 μm pore filter membranes. Subsequently, 20 μL of the supernatant was directly injected into a reversed-phase C18 column (Hypersil™ BDS-C18, 4.6 mm × 100 mm i.d., 2.4 μm particle size; ThermoFisher, Switzerland) and kept at 4 °C until injection. These analytes were separated on the column with a gradient elution of 0.1 % formic acid in acetonitrile (A phase) and 0.1 % phosphoric acid aqueous solution (B phase) at a 1.0 mL/min flow rate. Gradient elution was employed over 15 min, with the concentration of Phase A gradually decreasing from 90 % to 0 %. All analytical data were processed using the Shimadzu lab solutions (Version 5.106).
2.4. Isolation and identification of bacteria
During May and June 2022, 12 eggs were collected for the study. The test group consisted of 6 eggs obtained from the Beijing Pinggu District Shuangyin Farm, while the control group comprised 6 eggs purchased from the market. The eggs were placed in sterile sampling bags with 40 mL of sterile phosphate-buffered saline (PBS) and underwent 5–7 min ultrasonic treatment. Dilutions (101, 102, and 103) were prepared by combining 100 μL of the egg wash solution with 900 μL of sterile PBS. Each dilution was spread onto 5 % sterile sheep blood agar plates and incubated at 37 °C for 24 h. After incubation, single colonies were selected and streaked onto brain heart infusion (BHI) agar (Land Bridge, Beijing, China) for isolation. MALDI-TOF MS (AXIMA Performance, Shimadzu, Japan) was used for initial species identification. Subsequently, 16S rRNA sequence analysis was performed following the methodology described in a previous study [21]. The identified strains were cultured and preserved for future research.
2.5. Antimicrobial susceptibility testing
Minimum inhibitory concentrations (MICs) of mangosteen extract and α-mangostin were determined by the broth microdilution method, following the CLSI 2023 guideline. Briefly, α-mangostin was twofold diluted in Mueller-Hinton Broth (MHB, Land Bridge, Beijing, China) and mixed with an equal volume of bacterial suspensions in MHB containing approximately 1.5 × 106 CFU/mL in a clear UV-sterilized 96-well microtiter plate (Corning, USA). After incubation at 37 °C for 18 h, the MICs were determined as the lowest concentrations of α-mangostin at which no visible bacterial growth was observed. Vancomycin was used as the control antimicrobial drug.
2.6. Evaluation of antioxidant activity
The antioxidant activity of the plant extracts was evaluated using three different methods: the DPPH (2,2-diphenyl-1-picrylhydrazyl) assay based on Alzagameem et al. [22], the FRAP (ferric ion reducing antioxidant potential) assay based on Yang et al. [23], and the ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) assay based on García et al. [24]. In the DPPH assay, a sample solution containing lignin was mixed with a DPPH radical solution, and the absorbance was measured after 30 min. The FRAP assay involved mixing the extracts with a FRAP reagent and measuring the absorbance after a 30-min incubation. For the ABTS assay, the ABTS radical cation was generated by reacting ABTS with potassium persulfate, and the decolorization was measured after adding the plant extracts. These methods provided valuable insights into the antioxidant potential of the plant extracts, and the results were expressed in terms of Trolox equivalent antioxidant capacity value or μM of Fe(II)/g DM.
2.7. Folin-Ciocalteu assay
The total phenolic content in mangosteen extract was determined using the Folin-Ciocalteu colorimetric method [22]. In brief, the obtained extract samples were diluted in ethanol and transferred to a 96-well plate. Then, Folin-Ciocalteu reagent and sodium carbonate were added to each well. The mixture was incubated at room temperature in the dark for 2 h. The resulting blue color was measured using a microplate reader. Gallic acid was used for calibration. The phenolic content of the extract was expressed as gallic acid equivalent concentration (mg/mL).
2.8. Animals and ethics statement
The animal study protocol was approved by the State Council of the People's Republic of China (November 14, 1988) and by the China Agricultural University (CAU) Institutional Animal Care and Use Committee guidelines (ID: SKLAB-B-2010-003) approved by the Animal Welfare Committee of CAU.
2.9. Egg quality analyses
In a 56-day experiment, 3000 Hy-Line Brown laying hens aged 52 weeks were randomly assigned to three groups: Group A (control), Group B (200 mg/kg mangosteen extract of diet), Group C (500 mg/kg mangosteen extract of diet), and Group D (a composite probiotic preparation, including Bacillus subtilis, lactic acid bacteria, etc.). The hens were housed in wire-floored cages, provided with mash feed and water ad libitum, and exposed to 14–17 h of light daily. The temperature was maintained at approximately 21–22 °C, and routine immunization procedures were followed. Thirty cages from each group were selected to measure parameters such as feed consumption, number of laying hens, egg production, rotten eggs, and hen fatalities. Feed intake was recorded weekly using an electronic balance with a sensitivity of 0.1 g. The feed conversion ratio was calculated by dividing the total feed intake by the total egg mass in the same week. Egg quality analysis was conducted monthly using 6 eggs collected from each group during the last three days of the month. The breaking strength of the eggshells (measured in kg/cm2) was determined using a specialized device. The collected eggs were individually marked, weighed, and their eggshell thickness was measured with a micrometer. The weights of the eggshells and egg yolks were recorded. Fecal and egg samples were collected from 12 cages in each group, with fecal samples stored at −80 °C and eggs refrigerated. Bacterial genomic DNA was extracted using the Hipure Bacterial DNA Kit (Magen, Guangzhou, China), and microbial diversity analysis was conducted through sequencing at Beijing Saimo Baihe Biotechnology Co., Ltd.
2.10. Statistical analysis
Experimental results were analyzed for statistical significance using GraphPad Prism 8.0.1 (GraphPad Software Inc.). Differences were analyzed by Student's t-test and one-way ANOVA. P values were indicated in the figures, P ≤ 0.05 was considered to be significant. All data were presented as the mean ± SD.
3. Results
3.1. Isolation of mangosteen extract from mangosteen pericarp
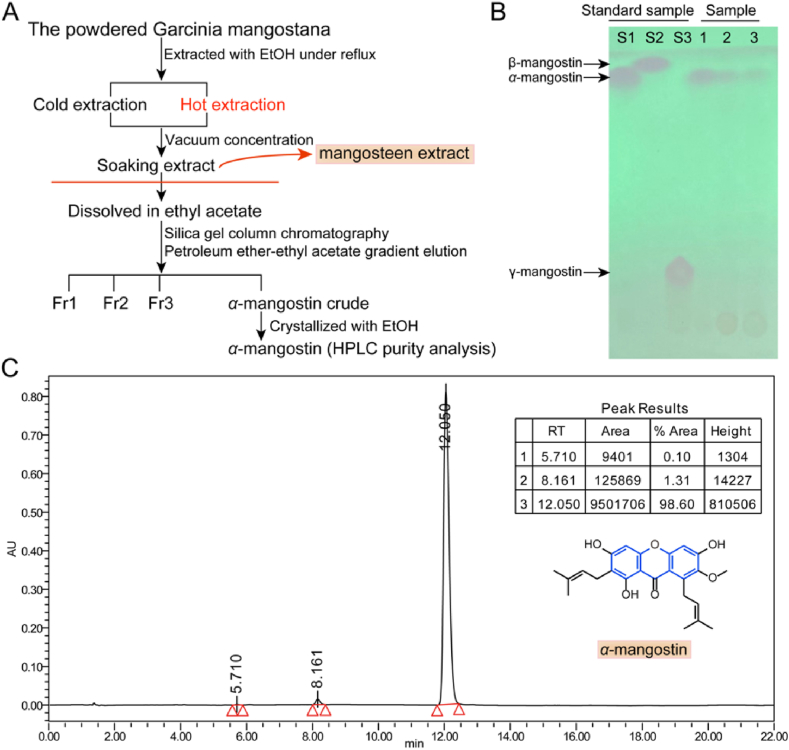
The mangosteen extract was obtained using the solvent extraction method, which was chosen due to its efficacy in extracting α-mangostin and total polyphenols, as highlighted in previous study [[25], [26], [27], [28]] (Fig. 1A).The mangosteen extract obtained from the processed dry powder demonstrated a yield of 17.50 %, and the presence of α-mangostin in the mangosteen extract was confirmed using TLC analysis, consistent with the standard α-mangostin (Fig. 1B). Furthermore, the content of α-mangostin was accurately determined through HPLC analysis, revealing a concentration of 20.67 % with a retention time of 12.05 min, corresponding to the peak of the standard α-mangostin (Fig. 1C).
Fig. 1.
Isolation of mangosteen extract from mangosteen pericarp. (A) Flow chart of extraction and isolation of mangosteen extract and α-mangostin, as well as the optimization of mangosteen extract isolation process using cold-soaking and hot-extraction methods. (B) The thin layer chromatography tracking of α-mangostin during the elution process, where S1, S2, and S3 represent the standards of α-mangostin (track 1), β-mangostin (track 2), and γ-mangostin (track 3), respectively. (C) The exact amount of α-mangostin was measured using the HPLC technique. The α-mangostin peak was observed at a retention time of 12.05 min.
3.2. Composition analysis of mangosteen extract
To analyze the components of mangosteen extract, ESI–MS spectrometry was employed. The ESI-MS spectrum exhibited a diverse array of ions within the observed m/z range of 120 to 1200 (Fig. 2A). Through this spectrum, we identified the following target compounds: α-mangostin, depicted by the peak at 13.87 min and corresponding to an m/z of 409 (Fig. 2B); γ-mangostin, represented by the peak at 13.28 min and corresponding to m/z 395, which is the O-demethylated product of the target compound (Fig. 2C). Moreover, we observed an additional peak between 13.28 min and 13.87 min, suggesting the presence of a structurally similar compound to the target compound at 13.28 min, possibly with variations in hydroxylation positions. In addition, peaks at 11.81 min and 12.02 min corresponded to the hydrogenated and further O-methylated products of the target compound at 13.87 min. In conclusion, the primary constituents of the mangosteen extract were identified as α-mangostin (the target compound) and γ-mangostin (the O-demethylated product).
Fig. 2.
Principal component analysis of mangosteen extract. (A) ESI-MS spectra of mangosteen extract recorded in the negative ion mode and possible fragmentation pathway. ESI conditions: 320 °C and 3.5 kV, full scan m/z 120-1200. Solvent, a mixture of acetonitrile and water in a ratio of 2.2:1. (B) Mass spectrum extraction at tR = 13.87 min of mangosteen extract. (C) Mass spectrum extraction at tR = 13.28 min of mangosteen extract.
Moreover, a comprehensive composition analysis was conducted on the mangosteen extract, revealing its constituent percentages (Table 1). The results demonstrated that the extract contained 22.63 % mangostin, 19.58 % total polyphenols, 24.51 % total polysaccharides, 4.37 % protein content, and 2.87 % moisture content. These findings further supported the dominant presence of α-mangostin, γ-mangostin, and other bioactive compounds such as polyphenols and polysaccharides within the mangosteen extract.
Table 1.
Chemical composition analysis of mangosteen extract.
| Component | Per 100 g (g) | Percentage (%) |
|---|---|---|
| Mangostin (α-, γ-) | 22.63 | 22.63 |
| Polyphenols | 19.58 | 19.58 |
| Polysaccharides | 24.51 | 24.51 |
| Protein | 4.37 | 4.37 |
| Moisture | 2.87 | 2.87 |
| Other | 26.04 | 26.04 |
3.3. Efficacy of mangosteen extract against Gram-positive bacteria on eggshell surface
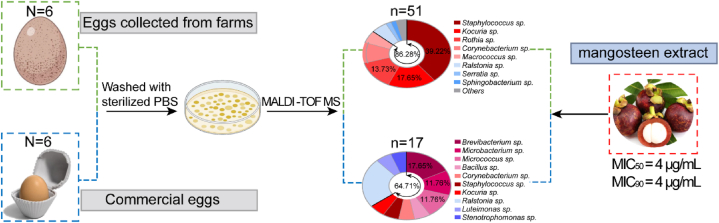
A total of 68 isolates were obtained belonging to 14 genera, including 51 and 17 isolates from the farm and commercial egg samples, respectively. Among the bacteria isolated from farm eggs, 44 isolates belonged to Gram-positive bacteria, accounting for 86.26 % of the microbial composition. The dominant genus within this group was Staphylococcus, indicating its prevalence in the farm eggs. In contrast, the commercially available eggs exhibited a lower diversity of microbial species, with 17 bacterial strains falling into 10 genera. Out of 17 strains from commercial eggs, 11 isolates belonged to Gram-positive bacteria (11/17, 64.71 %), and the primary genus observed was Ralstonia (Fig. 3).
Fig. 3.
Mangosteen extract exhibits excellent antibacterial activity against various bacterial genera present on the eggshells of farm eggs and commercial eggs.
Mangosteen has a well-established history in traditional medicine for its efficacy in infection control and has recently gained recognition for its potential in treating bacterial infections [29]. Research has demonstrated the significant advantages of mangosteen extract, particularly α-mangostin, in combating bacterial infections [30]. Mangosteen extract exhibited excellent antibacterial effects against the Staphylococcus genera, with MICs of 4 μg/mL (Table 2). Furthermore, an evaluation of 55 strains of Gram-positive isolates revealed that the MIC50 and MIC90 of mangosteen extract were 4 μg/mL (Fig. 3). These findings suggested that mangosteen extract had potential as a candidate disinfectant for eggshell surfaces.
Table 2.
Antimicrobial potency of α-mangostin against eggshell surface isolates.
| Strains | MIC (μg/mL) |
||
|---|---|---|---|
| mangosteen extract | α-mangostin | vancomycin | |
| Bacillus licheniformis | 4 | 0.5 | 0.25 |
| Brevibacterium luteolum | 1 | 0.125 | <0.125 |
| Corynebacterium xerosis | 8 | 2 | 0.5 |
| Kocuria artinae | 4 | 1 | <0.25 |
| Kocuria indica | 4 | 0.5 | 0.5 |
| Kocuria massiliensis | 4 | 1 | <0.25 |
| Macrococcus caseolyticus | 4 | 0.5 | <0.25 |
| Macrococcus epidermidis | 2 | 0.25 | <0.25 |
| Microbacterium lacticum | 2 | 0.25 | <0.125 |
| Micrococcus luteus | 4 | 0.5 | <0.125 |
| Rothia terrae | 4 | 0.5 | 1 |
| Staphylococcus agnetis | 4 | 0.5 | 1 |
| Staphylococcus aureus | 4 | 0.5 | <0.25 |
| Staphylococcus chromogenes | 4 | 0.5 | 1 |
| Staphylococcus saprophyticus | 4 | 0.5 | 1 |
3.4. Mangosteen extract exhibits antioxidant effects
The antioxidant capabilities of mangosteen extract were assessed by measuring its ability to scavenge DPPH and ABTS radicals, as well as its chelating effect on Fe2+. The results showed that the mangosteen extract displayed superior antioxidant activity compared with high purity α-mangostin (Fig. 4A–C). This could be attributed to the potent free radical scavenging ability of plant polyphenolic compounds, which effectively inhibited the cascade of radical reactions and made them highly efficient antioxidants. In addition, the Folin-Ciocalteu method was used to quantify the total phenolic content in the mangosteen extract from different extraction techniques. This analysis revealed a direct correlation between the antioxidant capacity and the content of polyphenolic compounds in the mangosteen extract (Fig. 4D). Therefore, the presence of polyphenols in the mangosteen extract acted as an effective constituent responsible for its antioxidant effects, consistent with previous research [31,32].
Fig. 4.
Antioxidant activity of mangosteen extracts evaluated through DPPH, ABTS, and FRAP assays. (A) DPPH. (B) ABTS. (C) FRAP. (D) Total phenolic contents of mangosteen extracts. All experiments were performed as three biologically independent experiments, with data presented as mean ± SD, n = 3. P-values were calculated using nonparametric one-way ANOVA.
3.5. Trend of improvement in egg quality with mangosteen extract
The addition of mangosteen extract did not significantly impact the average weight and quantity of eggs produced. Similarly, there were no notable differences observed in shell strength, albumen height, and yolk color among the groups (Fig. 5A–D). However, an interesting correlation was observed between the Haugh units of the eggs and the dosage of mangosteen extract, indicating a potential improvement in egg quality with higher extract supplementation. In addition, as the concentration of mangosteen extract in the feed increased, there was a decrease in the number of substandard eggs, suggesting an overall enhancement in egg quality (Table 3). These results highlighted the potential benefits of incorporating mangosteen extract as a feed additive for laying hens, particularly in terms of improving egg quality. Further research is warranted to explore the underlying mechanisms and optimize the dosage for maximum effectiveness.
Fig. 5.
The effects of mangosteen extract supplementation on laying hen production performance and egg quality. (A) Egg weight and yolk weight. (B) Shell weight and shell strength. (C) Albumen height and yolk color. (D) Daily production per laying hen (pieces/hen). Additive used as a control is a composite probiotic preparation (including Bacillus subtilis, lactic acid bacteria, etc.), with an addition amount of 0.2 %. Data presented as mean ± s.d, n = 6. P-values were calculated using one-way ANOVA.
Table 3.
Effect of mangosteen extract on egg quality and production performance.
| Group | Haugh units | Egg grading | Number of defective eggs | Number of deaths or eliminations |
|---|---|---|---|---|
| Untreated group | 53.87 ± 27.30 | AA (2), B (3), C (1) | 0.27 % (19/6989) | 0/1000 |
| 200 mg/kg of diet | 59.17 ± 29.43 | AA (2), A (1), B (2), C (1) | 0.23 % (16/6918) | 0/1000 |
| 500 mg/kg of diet | 69.47 ± 14.10 | AA (3), A (2), B (1) | 0.15 % (10/6616) | 1/1000 |
3.6. Impact of mangosteen extract on the microbial distribution on eggshell surfaces and in hen feces
To evaluate the impact of mangosteen extract on microbial communities present on eggshell surfaces and in hen feces, a 54-day feeding experiment was conducted. This experiment involved the collection of eggs and feces for subsequent analysis using 16S rRNA gene sequencing. Diversity analysis revealed notable findings at the phylum level. The relative abundance of Actinobacteria and Firmicutes demonstrated a negative correlation with the quantity of α-mangostin added. By contrast, the relative abundance of Bacteroidetes, Fusobacteria, and Proteobacteria displayed a positive correlation with the amount of α-mangostin introduced. The Firmicutes to Bacteroidetes (F/B) ratio is commonly employed as an indicator of microbial dysbiosis [33]. In this study, the introduction of α-mangostin resulted in a decrease in the F/B ratio, suggesting a shift toward microbial stability.
At the genus level, the relative abundance of Bacteroides, Fusobacterium, Megamonas, and Pseudomonas increased as the amount of α-mangostin increased on the eggshell surface. Conversely, the relative abundance of Kocuria, Lactobacillus, and Rothia decreased. In fecal samples, the presence of Acinetobacter, Faecalibaculum, Fusobacterium, and Phocaeicola exhibited a positive correlation with the increase in α-mangostin amount. Conversely, the presence of Bacteroides, Enterococcus, and Erysipelothrix showed a negative correlation (Fig. 6A). Consequently, the inclusion of mangosteen extract stimulated the growth of beneficial bacteria while reducing the relative abundance of pathogens.
Fig. 6.
Impact of different doses of mangosteen extract on the microbial community composition of laying hens. (A) Effect of mangosteen extract on relative abundance at the phylum and species levels in eggshell surface (n = 7) and feces (n = 6). The experimental groups included the untreated group (without mangosteen extract supplementation), a group supplemented with 200 mg/kg of diet, and a group supplemented with 500 mg/kg of diet. The abbreviations "S" and "F" represent the eggshell surface and feces, respectively. (B) Assessment of alpha diversity in eggshell and feces samples from hens fed with mangosteen extract for 54 days. The three commonly used indices for evaluating richness are Chao1, Shannon, and Simpson. Higher index values indicate greater species richness in the sample. (C) Principal Coordinates Analysis of the microbial community. The analysis is based on the UniFrac distance, where each point represents a sample. The distance between points reflects the degree of difference, and samples from the same group are represented by the same color.
In addition, the α-diversity analysis revealed that the inclusion of mangosteen extract effectively maintained species abundance and diversity when compared with the control group (Fig. 6B). Moreover, the β-diversity analysis indicated that the presence of mangosteen extract primarily influenced the composition of microbial communities in hen feces, while exerting minimal influence on the microbial community structure on the eggshell surface (Fig. 6C). These findings suggested that the incorporation of mangosteen extract had an impact on both the structure and function of gut communities, potentially contributing to the restoration of stability within the gut microbial ecosystems.
4. Discussion
Plant-derived bioactive compounds have been widely applied in various fields, particularly in the food industry [[34], [35], [36]]. Consumers have become increasingly worried about the quality and safety of eggs and egg products, which has brought issues such as drug residues and resistance related to the disinfection techniques used for commercially available eggs [37,38]. This study demonstrated that mangosteen extract exhibited remarkable antibacterial properties on eggshell surfaces, showing exceptional efficacy without adverse effects. Therefore, it held potential as a food disinfectant. However, this research solely focused on the effects of mangosteen extract on the external microbial environment of eggshells, whereas further investigation is required to determine its impact on the internal microbial environment of eggs. Besides, the increasing occurrence of stress-related issues in livestock and poultry due to intensive farming poses a significant challenge. It is widely recognized that the best approach to alleviate oxidative stress is the use of diverse exogenous antioxidants [39]. This study highlights the substantial antioxidant activity of mangosteen extract, emphasized by its ability to scavenge free radicals and chelate iron ions effectively.
After incorporating mangosteen extract into the feed of laying hens, it was observed that there was no significant impact on the hens’ production performance. Despite the disparity between these results and those reported by other researchers [40], it is essential to acknowledge that the inclusion of specific components and dosage of mangosteen extract in the feed could potentially influence the outcomes. In addition, we noticed an improvement in egg quality upon the addition of mangosteen extract, possibly due to increased protein levels [41]. These findings suggested that mangosteen extract holds promise as a natural supplement for enhancing egg quality. Future studies should investigate the optimal dosage and composition of mangosteen extract in laying hen feed, and explore its potential application in various poultry and livestock industries.
The research conducted a continuous 54-day feeding experiment to evaluate the impact of mangosteen extract on the microbial community on eggshell surfaces and the excrement of laying hens. This assessment utilized 16S rRNA gene sequencing analysis. The findings indicated that the incorporation of mangosteen extract promoted the presence of beneficial bacteria, specifically the genera Bacteroides and Erysipelothrix [42]. Certain strains of Bacteroides can decompose cellulose in food, thereby producing beneficial short-chain fatty acids (SCFAs) that provide energy and nutrients. In addition, these SCFAs play a crucial role in maintaining the integrity of the intestinal mucosal barrier [43]. In contrast, some strains of Rothia are known pathogens in both humans and animals, leading to gastrointestinal infections and other infectious diseases [44]. Furthermore, mangosteen extract primarily affected the microbial community composition in the excrement of laying hens, while exerting minimal influence on the microbial community structure on the eggshell surface. The safety of mangosteen extract was also investigated as part of the research. Laying hens fed with mangosteen extract showed no significant adverse effects or mortality rate compared with the control group, aligning with the previously documented LD50 value of approximately 1000 mg/kg for mangosteen extract in BALB/c mice [45]. Thus, the mangosteen extract obtained by our extraction method is safe and effective when supplemented at dosages of 200 mg/kg and 500 mg/kg in laying hens.
From various perspectives, this study investigated the impact of mangosteen extract on bacteria inhibition and egg quality enhancement. Mangosteen extract effectively eliminated the Gram-positive bacteria on the eggshell surface. Moreover, the addition of mangosteen extract in feed improved egg quality and reduced the presence of harmful bacteria, indicating its potential as a substitute for antibiotics. These findings provided valuable insights for the prevention and regulation of foodborne pathogens, thereby contributing to the sustainable development of food safety and the healthcare industry.
5. Conclusions
In summary, this study successfully extracted and isolated mangosteen extract, demonstrating its remarkable antimicrobial properties against Gram-positive bacteria present on the surface of eggshells. In addition, the supplementation of mangosteen extract in the diet of laying hens resulted in significant enhancements in egg quality while effectively managing microbial populations on both fecal and eggshell surfaces. These findings highlighted the promising potential of mangosteen extract as a viable substitute for conventional antibiotics in terms of inhibiting bacterial growth and improving egg quality. Consequently, the results suggested that mangosteen extract was an effective alternative to traditional antibiotics, providing valuable insights for the advancement of animal husbandry practices.
Data availability statement
Data will be made available on request.
CRediT authorship contribution statement
Jianfei Zhu: Methodology, Conceptualization. Qing Liu: Validation, Software. Yongqiang Wang: Visualization, Formal analysis. Kui Zhu: Writing – review & editing, Investigation. Jiangpeng Guo: Supervision, Resources. Yinji Jin: Project administration, Data curation. Ying Liu: Writing – original draft, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was supported by the Science Foundation for Young Scholars of Beijing Academy of Agriculture and Forestry Sciences (QNJJ202431), the Reform and Development Project of Beijing Academy of Agricultural and Forestry Sciences (XMS202419), and the Beijing Innovation Consortium of Peking Poultry Research System (BAIC 06–2024).
Contributor Information
Jianfei Zhu, Email: zhujf@cau.edu.cn.
Qing Liu, Email: lq@cau.edu.cn.
Yongqiang Wang, Email: vetwyq@cau.edu.cn.
Kui Zhu, Email: zhuk@cau.edu.cn.
Jiangpeng Guo, Email: guojp72@163.com.
Yinji Jin, Email: jinyinji@sina.com.
Ying Liu, Email: liuying@baafs.net.cn.
References
- 1.Larsson D.G.J., Flach C.F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022;20(5):257–269. doi: 10.1038/s41579-021-00649-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laxminarayan R., Duse A., Wattal C., Zaidi A.K.M., Wertheim H.F.L., Sumpradit N., Vlieghe E., Hara G.L., Gould I.M., Goossens H., Greko C., So A.D., Bigdeli M., Tomson G., Woodhouse W., Ombaka E., Peralta A.Q., Qamar F.N., Mir F., Kariuki S., Bhutta Z.A., Coates A., Bergstrom R., Wright G.D., Brown E.D., Cars O. Antibiotic resistance—the need for global solutions. Lancet Infect. Dis. 2013;13(12):1057–1098. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- 3.Ovalle-Magallanes B., Eugenio-Perez D., Pedraza-Chaverri J. Medicinal properties of mangosteen (Garcinia mangostana L.): a comprehensive update. Food Chem. Toxicol. 2017;109(Pt 1):102–122. doi: 10.1016/j.fct.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 4.Albuquerque B.R., Dias M.I., Pinela J., Calhelha R.C., Pires T., Alves M.J., Correa R.C.G., Ferreira I., Oliveira M., Barros L. Insights into the chemical composition and in vitro bioactive properties of mangosteen (Garcinia mangostana L.) pericarp. Foods. 2023;12(5) doi: 10.3390/foods12050994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tatiya-Aphiradee N., Chatuphonprasert W., Jarukamjorn K. Anti-inflammatory effect of Garcinia mangostana Linn. pericarp extract in methicillin-resistant Staphylococcus aureus-induced superficial skin infection in mice. Biomed. Pharmacother. 2019;111:705–713. doi: 10.1016/j.biopha.2018.12.142. [DOI] [PubMed] [Google Scholar]
- 6.Nawawi N.I.M., Ijod G., Abas F., Ramli N.S., Mohd Adzahan N., Mohamad Azman E. Influence of different drying methods on anthocyanins composition and antioxidant activities of mangosteen (Garcinia mangostana L.) pericarps and LC-MS analysis of the active extract. Foods. 2023;12(12) doi: 10.3390/foods12122351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuo H.W., Li C.Y., Chieng Z.X., Cheng W. Dietary administration of mangosteen, Garcinia mangostana, peel extract enhances the growth, and physiological and immunoendocrinological regulation of prawn, Macrobrachiumrosenbergii. Fish Shellfish Immunol. 2023;140 doi: 10.1016/j.fsi.2023.108982. [DOI] [PubMed] [Google Scholar]
- 8.Ban C., Paengkoum S., Yang S., Tian X., Thongpea S., Purba R.A.P., Paengkoum P. Feeding meat goats mangosteen (Garcinia mangostana L.) peel rich in condensed tannins, flavonoids, and cinnamic acid improves growth performance and plasma antioxidant activity under tropical conditions. J. Appl. Anim. Res. 2022;50(1):307–315. [Google Scholar]
- 9.Fernández Márquez M.L., Burgos M.J.G., Pulido R.P., Gálvez A., López R.L. Biocide tolerance and antibiotic resistance in Salmonella isolates from hen eggshells. Foodb. Pathog. Dis. 2017;14(2):89–95. doi: 10.1089/fpd.2016.2182. [DOI] [PubMed] [Google Scholar]
- 10.Pande V.V., Gole V.C., McWhorter A.R., Abraham S., Chousalkar K.K. Antimicrobial resistance of non-typhoidal Salmonella isolates from egg layer flocks and egg shells. Int. J. Food Microbiol. 2015;203:23–26. doi: 10.1016/j.ijfoodmicro.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 11.Yang N. Egg production in China: current status and outlook. Frontiers of Agricultural Science and Engineering. 2021;8(1) [Google Scholar]
- 12.Paramithiotis S., Drosinos E.H., Skandamis P.N. Food recalls and warnings due to the presence of foodborne pathogens — a focus on fresh fruits, vegetables, dairy and eggs. Curr. Opin. Food Sci. 2017;18:71–75. [Google Scholar]
- 13.Dearborn D.C., Page S.M., Dainson M., Hauber M.E., Hanley D. Eggshells as hosts of bacterial communities: an experimental test of the antimicrobial egg coloration hypothesis. Ecol. Evol. 2017;7(22):9711–9719. doi: 10.1002/ece3.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grizard S., Versteegh M.A., Ndithia H.K., Salles J.F., Tieleman B.I. Shifts in bacterial communities of eggshells and antimicrobial activities in eggs during incubation in a ground-nesting passerine. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0121716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee B.H., Cole S., Badel-Berchoux S., Guillier L., Felix B., Krezdorn N., Hebraud M., Bernardi T., Sultan I., Piveteau P. Biofilm Formation of Listeria monocytogenes strains under food processing environments and pan-genome-wide association study. Front. Microbiol. 2019;10:2698. doi: 10.3389/fmicb.2019.02698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colagiorgi A., Di Ciccio P., Zanardi E., Ghidini S., Ianieri A. A look inside the Listeria monocytogenes biofilms extracellular matrix. Microorganisms. 2016;4(3) doi: 10.3390/microorganisms4030022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reuter M., Mallett A., Pearson B.M., van Vliet A.H. Biofilm formation by Campylobacter jejuni is increased under aerobic conditions. Appl. Environ. Microbiol. 2010;76(7):2122–2128. doi: 10.1128/AEM.01878-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song M., Liu Y., Li T., Liu X., Hao Z., Ding S., Panichayupakaranant P., Zhu K., Shen J. Plant natural flavonoids against multidrug resistant pathogens. Adv. Sci. 2021;8(15) doi: 10.1002/advs.202100749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu S.C., Yang Z.Q., Liu F., Peng W.J., Qu S.Q., Li Q., Song X.B., Zhu K., Shen J.Z. Antibacterial effect and mode of action of flavonoids from licorice against methicillin-resistant Staphylococcus aureus. Front. Microbiol. 2019;10:2489. doi: 10.3389/fmicb.2019.02489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naing S., Sandech N., Maiuthed A., Chongruchiroj S., Pratuangdejkul J., Lomarat P., Garcinia mangostana L. Pericarp extract and its active compound alpha-mangostin as potential inhibitors of immune checkpoint programmed death ligand-1. Molecules. 2023;28(19) doi: 10.3390/molecules28196991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Das S., Dash H.R., Mangwani N., Chakraborty J., Kumari S. Understanding molecular identification and polyphasic taxonomic approaches for genetic relatedness and phylogenetic relationships of microorganisms. J. Microbiol. Methods. 2014;103:80–100. doi: 10.1016/j.mimet.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 22.Alzagameem A., Khaldi-Hansen B.E., Buchner D., Larkins M., Kamm B., Witzleben S., Schulze M. Lignocellulosic biomass as source for lignin-based environmentally benign antioxidants. Molecules. 2018;23(10) doi: 10.3390/molecules23102664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang L., Wang D., Zhou D., Zhang Y. Effect of different isolation methods on structure and properties of lignin from valonea of Quercus variabilis. Int. J. Biol. Macromol. 2016;85:417–424. doi: 10.1016/j.ijbiomac.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 24.García A., González Alriols M., Spigno G., Labidi J. Lignin as natural radical scavenger. Effect of the obtaining and purification processes on the antioxidant behaviour of lignin. Biochem. Eng. J. 2012;67:173–185. [Google Scholar]
- 25.Pothitirat W., Chomnawang M.T., Supabphol R., Gritsanapan W. Free radical scavenging and anti-acne activities of mangosteen fruit rind extracts prepared by different extraction methods. Pharmaceut. Biol. 2010;48(2):182–186. doi: 10.3109/13880200903062671. [DOI] [PubMed] [Google Scholar]
- 26.Eukun Sage E., Jailani N., Md Taib A.Z., Mohd Noor N., Mohd Said M.I., Abu Bakar M., Mackeen M.M. From the Front or Back Door? Quantitative analysis of direct and indirect extractions of alpha-mangostin from mangosteen (Garcinia mangostana) PLoS One. 2018;13(10) doi: 10.1371/journal.pone.0205753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsuprom L., Rungroj N., Lekcharoensuk C., Pruksakorn C., Kongkiatpaiboon S., Chen C., Sukatta U. In vitro antibacterial activity of mangosteen (Garcinia mangostana Linn.) crude extract against Staphylococcus pseudintermedius isolates from canine pyoderma. Vet. Dermatol. 2019;30(6) doi: 10.1111/vde.12783. 487-e145. [DOI] [PubMed] [Google Scholar]
- 28.Chaiwarit T., Kantrong N., Sommano S.R., Rachtanapun P., Junmahasathien T., Kumpugdee-Vollrath M., Jantrawut P. Extraction of tropical fruit peels and development of HPMC film containing the extracts as an active antibacterial packaging material. Molecules. 2021;26(8) doi: 10.3390/molecules26082265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Do H.T.T., Cho J. Mangosteen pericarp and its bioactive xanthones: potential therapeutic value in alzheimer's disease, Parkinson's disease, and depression with pharmacokinetic and safety profiles. Int. J. Mol. Sci. 2020;21(17) doi: 10.3390/ijms21176211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koh J.J., Qiu S., Zou H., Lakshminarayanan R., Li J., Zhou X., Tang C., Saraswathi P., Verma C., Tan D.T., Tan A.L., Liu S., Beuerman R.W. Rapid bactericidal action of alpha-mangostin against MRSA as an outcome of membrane targeting. Biochim. Biophys. Acta. 2013;1828(2):834–844. doi: 10.1016/j.bbamem.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Yoshimura M., Ninomiya K., Tagashira Y., Maejima K., Yoshida T., Amakura Y. Polyphenolic constituents of the pericarp of mangosteen (Garcinia mangostana L.) J. Agric. Food Chem. 2015;63(35):7670–7674. doi: 10.1021/acs.jafc.5b01771. [DOI] [PubMed] [Google Scholar]
- 32.Li G., Thomas S., Johnson J.J. Polyphenols from the mangosteen (Garcinia mangostana) fruit for breast and prostate cancer. Front. Pharmacol. 2013;4:80. doi: 10.3389/fphar.2013.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu H., Wang G., Zhang J., Lu B., Li D., Chen J. Inhalation of diesel exhaust particulate matter accelerates weight gain via regulation of hypothalamic appetite-related genes and gut microbiota metabolism. J. Hazard Mater. 2024;466 doi: 10.1016/j.jhazmat.2024.133570. [DOI] [PubMed] [Google Scholar]
- 34.Mahfuz S., Shang Q., Piao X. Phenolic compounds as natural feed additives in poultry and swine diets: a review. J. Anim. Sci. Biotechnol. 2021;12(1):48. doi: 10.1186/s40104-021-00565-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang Z., Dai H., Jiang J., Ye N., Zhu S., Wei Q., Lv Z., Shi F. Dietary mulberry-leaf flavonoids improve the eggshell quality of aged breeder hens. Theriogenology. 2022;179:177–186. doi: 10.1016/j.theriogenology.2021.11.019. [DOI] [PubMed] [Google Scholar]
- 36.Li X.L., He W.L., Yang M.L., Yan Y.M., Xue Y.H., Zhao S.T. Effect of dietary supplementation of Ligustrum lucidum on performance, egg quality and blood biochemical parameters of Hy-Line Brown hens during the late laying period. Animal. 2017;11(11):1899–1904. doi: 10.1017/S1751731117000532. [DOI] [PubMed] [Google Scholar]
- 37.Manyi-Loh C., Mamphweli S., Meyer E., Okoh A. Antibiotic use in agriculture and its consequential resistance in environmental sources: potential public health implications. Molecules. 2018;23(4) doi: 10.3390/molecules23040795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melo E.F., Clímaco W.L.S., Triginelli M.V., Vaz D.P., de Souza M.R., Baião N.C., Pompeu M.A., Lara L.J.C. An evaluation of alternative methods for sanitizing hatching eggs. Poultry Sci. 2019;98(6):2466–2473. doi: 10.3382/ps/pez022. [DOI] [PubMed] [Google Scholar]
- 39.Forman H.J., Zhang H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021;20(9):689–709. doi: 10.1038/s41573-021-00233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sriboonyong P., Poommarin P., Sittiya J., Opanasopit P., Ngawhirunpat T., Patrojanasophon P., Pornpitchanarong C. The utilization of mangosteen pericarp extract for anticoccidial drug replacement in broiler feed. Int J Vet Sci Med. 2022;10(1):90–99. doi: 10.1080/23144599.2022.2128271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kusmayadi A., Bachtiar K.R., Prayitno C.H. The effects of mangosteen peel (Garcinia mangostana L.) and Turmeric (Curcuma domestica Val) flour dietary supplementation on the growth performance, lipid profile, and abdominal fat content in Cihateup ducks. Vet. World. 2019;12(3):402–408. doi: 10.14202/vetworld.2019.402-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olsen R., Kudirkiene E., Thøfner I., Pors S., Karlskov-Mortensen P., Li L., Papasolomontos S., Angastiniotou C., Christensen J. Impact of egg disinfection of hatching eggs on the eggshell microbiome and bacterial load. Poultry Sci. 2017;96(11):3901–3911. doi: 10.3382/ps/pex182. [DOI] [PubMed] [Google Scholar]
- 43.Zafar H., Saier M.H., Jr. Gut Bacteroides species in health and disease. Gut Microb. 2021;13(1):1–20. doi: 10.1080/19490976.2020.1848158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramanan P., Barreto J.N., Osmon D.R., Tosh P.K. Rothia bacteremia: a 10-year experience at mayo clinic, rochester, Minnesota. J. Clin. Microbiol. 2014;52(9):3184–3189. doi: 10.1128/JCM.01270-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kosem N., Ichikawa K., Utsumi H., Moongkarndi P. In vivo toxicity and antitumor activity of mangosteen extract. J. Nat. Med. 2013;67(2):255–263. doi: 10.1007/s11418-012-0673-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.