Key Points
Question
Is the use of sacubitril-valsartan associated with reduced risk of all-cause mortality and hospitalization among individuals requiring hemodialysis for heart failure with reduced ejection fraction (HFrEF)?
Findings
In this comparative effectiveness research study of 1434 patient pairs, initiation of sacubitril-valsartan therapy was associated with a statistically significant 18% reduction in all-cause mortality and 14% reduction in all-cause hospitalization.
Meaning
These findings suggest sacubitril-valsartan therapy may benefit individuals with HFrEF requiring hemodialysis.
Abstract
Importance
Randomized clinical trials have shown that sacubitril-valsartan reduces the risks of mortality and hospitalization in patients with heart failure with reduced ejection fraction (HFrEF), but patients with kidney failure requiring dialysis were excluded.
Objective
To investigate the comparative effectiveness of sacubitril-valsartan vs angiotensin-converting enzyme inhibitors or angiotensin receptor blockers (ACEIs or ARBs) in patients with HFrEF requiring hemodialysis.
Design, Setting, and Participants
This retrospective, 1:1 propensity score–matched comparative effectiveness study included patients who were 18 years or older with HFrEF, enrolled in Medicare Parts A, B, and D, and survived at least 90 days receiving in-center hemodialysis from July 8, 2015, to December 31, 2020. Patients were excluded for less than 180 days of continuous Medicare Parts A, B, and D primary payer coverage or prior dispensing of sacubitril-valsartan. Data analysis was conducted from September 23, 2023, to June 25, 2024.
Exposures
New use of sacubitril-valsartan vs new or continued use of ACEIs or ARBs.
Main Outcomes and Measures
The associations between initiation of sacubitril-valsartan therapy and all-cause mortality, cardiovascular mortality, all-cause hospitalization, and HF hospitalization were assessed using Cox proportional hazards regression models in a propensity score–matched sample.
Results
Participants included 1:1 matched pairs of 1434 sacubitril-valsartan users and 1434 ACEI or ARB users (mean [SD] age, 64 [13] years). Of the 2868 matched participants, 996 (65%) were male; 987 (34%) were Black or African American and 1677 (58%) were White; and median dialysis vintage was 3.8 (IQR, 1.8-6.3) years. The median follow-up was 0.9 (IQR, 0.4-1.7) years. Sacubitril-valsartan (vs ACEI or ARB) therapy was associated with a reduction in all-cause mortality (hazard ratio [HR], 0.82 [95% CI, 0.73-0.92]) and all-cause hospitalization (HR, 0.86 [95% CI, 0.79-0.93]) but not cardiovascular mortality (HR, 1.01 [95% CI, 0.86-1.19]) or HF hospitalization (HR, 0.91 [95% CI, 0.82-1.02]). There was a decrease in hyperkalemia (HR, 0.71 [95% CI, 0.62-0.81]) and no difference in hypotension (HR, 0.99 [95% CI, 0.83-1.19]). Only 195 participants (14%) ever received the maximum combination dose of sacubitril (97 mg twice daily) and valsartan (103 mg twice daily).
Conclusions and Relevance
In this comparative effectiveness study of patients with HFrEF requiring hemodialysis, sacubitril-valsartan therapy was associated with beneficial effects in all-cause mortality and all-cause hospitalization.
This comparative effectiveness study assesses the risk of all-cause mortality, cardiovascular mortality, all-cause hospitalization, and heart failure hospitalization among patients with heart failure with reduced ejection fraction requiring dialysis who receive combined sacubitril-valsartan vs angiotensin-converting enzyme inhibitors or angiotensin receptor blockers.
Introduction
Cardiovascular (CV) disease causes 50% of deaths in individuals in the US who require kidney replacement therapy, and heart failure (HF) is the most common CV diagnosis, with a prevalence of 40% in this population.1 Multiple medical therapies in randomized clinical trials have shown to be effective in reducing CV mortality in HF (such as angiotensin receptor–neprilysin inhibitors [ARNIs], sodium-glucose cotransporter-2 inhibitors, and spironolactone), but patients receiving dialysis were excluded from these trials.2,3,4 Thus, studies using observational data in this population are helpful to quantify the effect of medication interventions.
Sacubitril-valsartan, a first-in-class ARNI, was shown to be superior to angiotensin-converting enzyme inhibitors (ACEIs) in the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial,2 and for those with HF with reduced ejection fraction (HFrEF), initiation of sacubitril-valsartan therapy is a class I recommendation by the American Heart Association, American College of Cardiology, and Heart Failure Society of America guidelines.5 In addition to improvements in CV mortality, sacubitril-valsartan has been associated with reductions in all-cause mortality, HF hospitalization, and all-cause hospitalization.2,6,7 Despite the high annual price of $5576 for individuals with Medicare Part D,8 sacubitril-valsartan has been shown to be associated with overall decreases in health care expenditure in a population not receiving dialysis.9 Thus, sacubitril-valsartan may have therapeutic potential with significant clinical implications for patients with HFrEF requiring dialysis. Data regarding clinical benefit, however, are limited, and randomized clinical trials in Asia are ongoing.10,11
Two observational studies12,13 have examined clinical outcomes with sacubitril-valsartan in patients requiring dialysis, and no mortality benefit was seen. Furthermore, outcomes regarding HF hospitalization were mixed (one showing benefit; the other showing harm). Given limited sample sizes of published data from only Asian populations (range, 7-110 patients with total numbers <400),12,13,14,15,16,17,18,19 we sought to examine the effectiveness of sacubitril-valsartan using the US Renal Data System (USRDS). We assessed the risk of all-cause mortality, CV mortality, all-cause hospitalization, and HF hospitalization.
Methods
Data Source
The USRDS database1 is a national surveillance system that captures most US patients requiring dialysis and kidney transplant. The USRDS includes Medicare Part A, B, and D claims (consisting of hospitalizations, outpatient care, billing diagnoses, and medications dispensed) and Medicare enrollment history, which can be combined with USRDS data. The Johns Hopkins School of Public Health Institutional Review Board approved the study and waived the need for informed consent owing to the use of deidentified registry data. We followed the International Society for Pharmacoeconomics and Outcomes Research (IPSOR) reporting guidelines for comparative effectiveness research (eAppendix in Supplement 1).
Target Trial Emulation
Eligibility Criteria and Study Population
We used the target trial emulation framework comparing new users of sacubitril-valsartan vs new or continued users of ACEIs or angiotensin receptor blockers (ARBs) among patients with HFrEF receiving hemodialysis (eTable 1 in Supplement 1). Enrollment started July 8, 2015 (after the US Food and Drug Administration approval date for sacubitril-valsartan), and participants were eligible at treatment strategy initiation (ie, baseline) if they had a history of HFrEF, were 18 years or older, and survived at least 90 days receiving in-center hemodialysis (per USRDS recommendations).1 Heart failure with reduced ejection fraction was ascertained from Medicare records by the presence of at least 1 inpatient or 2 outpatient codes (428.2X from the International Classification of Diseases, Ninth Revision, or I50.2X from the International Statistical Classification of Diseases, Tenth Revision) any time prior to baseline, which have 97.7% specificity for individuals with HFrEF (<45% in patients not receiving dialysis).6,20,21
Exclusion criteria at enrollment consisted of not receiving in-center hemodialysis (eg, kidney recovery without dialysis, kidney transplant, transition to other dialysis modality, or unconfirmed dialysis modality), less than 180 days of continuous Medicare Parts A, B, and D primary payer coverage, prior dispensing of sacubitril-valsartan, and missing baseline information on the Centers for Medicare & Medicaid Services (CMS) Form 2728 (eFigure 1 in Supplement 1).
Treatment Strategies
We compared new users of sacubitril-valsartan with new or continued users of ACEIs or ARBs between July 8, 2015, and December 31, 2020. New users of sacubitril-valsartan had 180 days prior to enrollment without a previous dispense for sacubitril-valsartan. Similarly, new users of ACEIs or ARBs had 180 days prior to enrollment without a previous dispense of ACEIs or ARBs, and for continued users of ACEIs or ARBs (dispensed in the previous 180 days), we randomly selected an enrollment date from eligible dispenses.22
We included individuals with a history of ACEI or ARB use, as sacubitril-valsartan is a first-in-class medication, and landmark trials of sacubitril-valsartan (PARADIGM-HF and Comparison of Sacubitril-Valsartan vs Enalapril on Effect on NT-proBNP [N-terminal pro–brain-type natriuretic peptide] in Patients Stabilized from an Acute Heart Failure Episode [PIONEER-HF]) enrolled patients with2 and without23 recent ACEI or ARB use. Prescription of ACEIs or ARBs for US patients receiving hemodialysis is also common1 and higher among those with HF.
Covariate Ascertainment
Patient demographic characteristics (including race), initial dialysis access, comorbidities at dialysis initiation (ie, atherosclerotic heart disease, cancer, chronic pulmonary disease, cerebrovascular disease, diabetes, dysrhythmia, hypertension, myocardial infarction, other cardiac disease, and peripheral vascular disease), smoking status, ESRD (end-stage renal disease or kidney failure) network region (ie, geographic region), and etiology of kidney failure (diabetes or not) were abstracted from CMS Medical Evidence Form 2728. Race was clinician reported from preselected categories that included Black or African American and White. We collapsed American Indian or Alaska Native, Asian, Hawaiian or Other Pacific Islander, Middle Eastern or Arabian, Other, and unknown to a single category of Other due to limited sample size. Certain comorbidities (ie, atherosclerotic heart disease, cerebrovascular disease, peripheral vascular disease, other cardiac disease, respiratory disease, liver disease, dysrhythmia, cancer, and diabetes)24 were defined by the presence of 1 inpatient or 2 outpatient ICD-9 or ICD-10 claims in the 180 days prior to baseline (eTable 3 in Supplement 1). Number of hospitalizations and primary care visits (defined as office visits to Internal Medicine, Family Practice, or General Medicine) in the previous 180 days were captured in Medicare Part A and B, respectively. We used the most updated body mass index, KT/V (dialyzer clearance of urea × dialysis time divided by volume of distribution of urea [a measure of dialysis adequacy]), and erythropoietin stimulating agent use from dialysis records. Prior medications (<90 days prior to baseline) (eTable 2 in Supplement 1), Medicare Part D subsidy status, dialysis vintage (baseline minus dialysis start date), baseline calendar year, strength of historic ACEI or ARB dose (none, low, medium, or high) (eTable 4 in Supplement 1),6 and number of ACEI or ARB dispenses in the past 180 days were also noted. Dose of ACEIs or ARBs was determined by the National Drug Code of medication dispensed.
Treatment Assignment: Randomization Emulation by 1:1 Propensity Score Match
We used the MatchIt package25 to perform nearest neighbor propensity score matching without replacement to identify the 1:1 matched pairs of sacubitril-valsartan and ACEI or ARB users. The propensity score model included all baseline covariates,26 and we used exact matching on history of ACEI or ARB use, meaning patients who received ACEIs or ARBs in the 180 days prior to sacubitril-valsartan therapy initiation were matched against ACEI or ARB users who also had ACEI or ARB use in the 180 days prior (ie, continued users of ACEIs or ARB). Similarly, sacubitril-valsartan users without recent ACEI or ARB use were matched against new users of ACEI or ARB therapy.6
Follow-Up, Outcomes, and Causal Contrasts
Individuals were followed up until study outcome, kidney transplant, renal recovery without dialysis, loss of Medicare Primary Payer status, death, or December 31, 2020, whichever came first. The primary study outcome was all-cause mortality, and secondary outcomes included CV mortality, index hospitalization, and index HF hospitalization.
Date and cause of death was determined by the USRDS.1 We categorized CV mortality as causes of death that the USRDS determined as arrhythmia, atherosclerosis, cardiac arrest, cardiomyopathy, congestive HF, myocardial, pericardial, or valvular cause of death (CMS Form 2746). During follow-up, index hospitalization was ascertained by the first inpatient billing code date. Index HF hospitalization was abstracted from Medicare Part A in any billing position (eTable 3 in Supplement 1).
For safety outcomes, we examined hyperkalemia and hypotension using either 1 hospitalization claim or 2 outpatient claims (eTable 3 in Supplement 1). For outpatient claims, we used the earlier date. We categorized adverse events as any for both outpatient and inpatient claims and hospitalization for inpatient claims.
We estimated both intention-to-treat (ITT) and as-treated (AT) effects. In the AT analysis, individuals were additionally censored at end of medication dispense, defined as no refill within 30 days after end date of last dispense. Sacubitril-valsartan users were additionally censored for switching to an ACEI or ARB.
Statistical Analysis
Main Analysis
Data were analyzed from September 23, 2023, to June 25, 2024. Baseline characteristics between sacubitril-valsartan and ACEI or ARB users were compared before and after matching with absolute standardized mean differences (SMDs) less than 0.10 considered good balance.27
We used both Cox proportional hazards regression and Poisson regression on the 1:1 matched cohort to assess sacubitril-valsartan therapy initiation and risk of all-cause mortality, CV mortality, all-cause hospitalization, and HF hospitalization. For each outcome, we estimated the hazard ratio (HR), incidence rate ratio, and incident rate difference. We repeated this analysis for the safety outcomes of hyperkalemia and hypotension. Kaplan-Meier plots are shown for all-cause mortality, CV mortality, all-cause hospitalization, and HF hospitalization.
We looked at subgroups by age (≥65 years), sex, race, smoking status, diabetic kidney disease, atherosclerotic heart disease, dysrhythmia, history of diabetes, diuretic use, and dialysis vintage (by quartile) with all-cause mortality and all-cause hospitalization using the 1:1 matched cohort. In each subgroup, we included an interaction term between the treatment variable and subgroup indicator variable in the regression model. We examined the SMDs within subgroups and included variables with SMDs of at least 0.10 as covariates.28 Given that we hypothesized there would be a significant interaction with dialysis vintage (with lower vintage associated with more benefit), we report baseline demographics by dialysis vintage.
We also examined sacubitril-valsartan titration patterns after the first dose. Dispensing patterns were defined using the starting, maximum, and final doses. To evaluate for a dose effect, we used multivariable Cox proportional hazards regression (adjusted for all covariates) to examine all-cause mortality and all-cause hospitalization among sacubitril-valsartan users using an ITT approach across starting dose (with 24 and 26 mg, respectively, as reference). Statistical significance was defined by a linear trend across doses. Analyses were performed using the survival package in R, version 4.2.2 (R Program for Statistical Computing)31 and Stata, version 17 (StataCorp LLC).32 Significant results were determined by a 2-tailed P < .05.
Sensitivity Analyses
We repeated our primary and secondary outcome analysis using 1:4 matching, and we repeated the AT analysis using a 60-day (instead of 30-day) refill window to define medication discontinuation. To address residual and/or unmeasured confounding, we examined fracture hospitalization as a negative control outcome, as risk of fracture is not expected to differ by sacubitril-valsartan vs ACEI or ARB use, and we estimated the E-value,29 which is the minimum relative risk that an unmeasured confounder would need to have with both the exposure and the outcome to explain the observed association between sacubitril-valsartan use and all-cause mortality. To estimate the relative risk of study covariates and all-cause mortality, we used multivariate logistic regression adjusted for all covariates in Table 1. Finally, we repeated the analysis of secondary outcomes using competing risk models to take into account competing risks (non-CV death for CV mortality; all-cause mortality for both hospitalization and safety outcomes [hypotension or hyperkalemia].30
Table 1. Baseline Characteristics of Sacubitril-Valsartan Users vs ACEI or ARB Users Before and After 1:1 Matcha.
| Characteristic | Entire cohort | 1:1 Matched cohort | ||||
|---|---|---|---|---|---|---|
| Sacubitril-valsartan (n = 1434) | ACEI or ARB (n = 29 654) | SMD | Sacubitril-valsartan (n = 1434) | ACEI or ARB (n = 1434) | SMD | |
| Age, mean (SD), y | 64 (13) | 65 (13) | 0.05 | 64 (13) | 64 (14) | 0.05 |
| Sex | ||||||
| Female | 490 (34) | 12 667 (43) | 0.18 | 490 (34) | 506 (35) | 0.02 |
| Male | 944 (66) | 16 987 (57) | 944 (66) | 928 (65) | ||
| Raceb | ||||||
| Black or African American | 500 (35) | 11 097 (37) | 0.05 | 500 (35) | 487 (34) | 0.04 |
| White | 840 (59) | 16 622 (56) | 840 (59) | 837 (58) | ||
| Otherc | 94 (7) | 1935 (7) | 94 (7) | 110 (8) | ||
| Year of medication therapy initiation | ||||||
| 2015 | <10d | 6407 (22) | 1.08 | <10d | <10d | 0.05 |
| 2016 | 80sd | 6214 (21) | 80sd | 70sd | ||
| 2017 | 179 (12) | 4801 (16) | 179 (12) | 188 (13) | ||
| 2018 | 270 (19) | 4251 (14) | 270 (19) | 276 (19) | ||
| 2019 | 464 (32) | 4432 (15) | 464 (32) | 463 (32) | ||
| 2020 | 433 (30) | 3549 (12) | 433 (30) | 433 (30) | ||
| Dialysis vintage, median (IQR), y | 3.9 (1.9-6.3) | 3.7 (1.8-6.0) | 0.05 | 3.9 (1.9-6.3) | 3.5 (1.8-6.3) | 0.03 |
| KT/V | 1.6 (1.4-1.7) | 1.6 (1.4-1.8) | 0.06 | 1.6 (1.4-1.7) | 1.6 (1.4-1.7) | 0.01 |
| Current smoker | 110 (8) | 2511 (8) | 0.03 | 110 (8) | 108 (8) | 0.01 |
| Part D subsidy | 978 (68) | 21 418 (72) | 0.08 | 978 (68) | 1015 (71) | 0.05 |
| History of ESA use | 1201 (84) | 25 288 (85) | 0.04 | 1201 (84) | 1198 (84) | 0.01 |
| Kidney failure not from diabetes | 751 (52) | 13 744 (46) | 0.12 | 751 (52) | 731 (51) | 0.03 |
| US geographic region | ||||||
| Midwest | 302 (21) | 7071 (24) | 0.09 | 302 (21) | 298 (21) | 0.02 |
| Northeast | 364 (25) | 7194 (24) | 364 (25) | 356 (25) | ||
| South | 522 (36) | 9852 (33) | 522 (36) | 525 (37) | ||
| West | 246 (17) | 5537 (19) | 246 (17) | 255 (18) | ||
| Started dialysis via catheter | 1109 (77) | 22 824 (77) | 0.01 | 1109 (77) | 1134 (79) | 0.04 |
| BMI, median (IQR) | 26 (23-30) | 26 (22-31) | 0.01 | 26 (23-30) | 27 (23-31) | 0.05 |
| Atherosclerotic heart disease | 825 (58) | 17 708 (60) | 0.04 | 825 (58) | 802 (56) | 0.03 |
| Cancer | 157 (11) | 2970 (10) | 0.03 | 157 (11) | 172 (12) | 0.03 |
| Dysrhythmia | 700 (49) | 13 535 (46) | 0.06 | 700 (49) | 685 (48) | 0.02 |
| Cerebrovascular disease | 308 (21) | 7978 (27) | 0.13 | 308 (21) | 303 (21) | 0.01 |
| Other cardiac disease | 786 (55) | 14 216 (48) | 0.14 | 786 (55) | 770 (54) | 0.02 |
| Diabetes | 1023 (71) | 22 475 (76) | 0.10 | 1023 (71) | 1021 (71) | 0.003 |
| History of gastrointestinal tract bleed | 171 (12) | 3874 (13) | 0.03 | 171 (12) | 147 (10) | 0.05 |
| Hypertension | 1263 (88) | 26 527 (89) | 0.04 | 1263 (88) | 1273 (89) | 0.02 |
| Inability to ambulate | 40 (3) | 1301 (4) | 0.09 | 40 (3) | 50 (3) | 0.04 |
| Liver disease | 161 (11) | 5424 (18) | 0.20 | 161 (11) | 161 (11) | <0.001 |
| Peripheral vascular disease | 717 (50) | 17 315 (58) | 0.17 | 717 (50) | 718 (50) | 0.001 |
| Respiratory disease | 370 (26) | 8904 (30) | 0.09 | 370 (26) | 376 (26) | 0.01 |
| No. of hospitalizations within 6 mo, mean (SD) | 1.79 (1.95) | 1.93 (2.07) | 0.07 | 1.79 (1.95) | 1.78 (1.83) | 0.01 |
| No. of primary care visits within 6 mo, mean (SD) | 2.25 (3.12) | 1.73 (2.53) | 0.18 | 2.25 (3.12) | 2.35 (3.60) | 0.03 |
| No. of medications <90 d, mean (SD) | 10.76 (5.02) | 10.91 (4.95) | 0.03 | 10.76 (5.02) | 10.72 (5.07) | 0.01 |
| Medications <90 d | ||||||
| Aldosterone antagonist | 82 (6) | 734 (2) | 0.16 | 82 (6) | 82 (6) | <0.001 |
| Antiplatelets | 430 (30) | 8207 (28) | 0.05 | 430 (30) | 417 (29) | 0.02 |
| β-blockers | 1124 (78) | 22 377 (75) | 0.07 | 1124 (78) | 1104 (77) | 0.03 |
| CCBs | 401 (28) | 12 829 (43) | 0.32 | 401 (28) | 422 (29) | 0.03 |
| Diuretics | 396 (28) | 6879 (23) | 0.10 | 396 (28) | 402 (28) | 0.01 |
| DOAC | 184 (13) | 1809 (6) | 0.23 | 184 (13) | 198 (14) | 0.03 |
| GLP-1RA | 14 (1) | 113 (8) | 0.07 | 14 (1) | 12 (1) | 0.01 |
| Insulin | 351 (24) | 8235 (28) | 0.08 | 351 (24) | 352 (25) | 0.002 |
| Phosphate binder | 796 (56) | 17 236 (58) | 0.05 | 796 (56) | 846 (59) | 0.07 |
| SGLT2 | <10d | <10d | 0.03 | <10d | <10d | <0.001 |
| Warfarin | 132 (9) | 3390 (11) | 0.07 | 132 (9) | 137 (10) | 0.01 |
| Prior ACEI or ARB use | ||||||
| Low dose | 235 (16) | 4470 (15) | 0.24 | 235 (16) | 232 (16) | 0.01 |
| Moderate dose | 271 (19) | 7039 (24) | 271 (19) | 270 (19) | ||
| High dose | 190 (13) | 5736 (19) | 190 (13) | 194 (14) | ||
| No use | 738 (51) | 12 409 (42) | 738 (51) | 738 (51) | 0.01 | |
| No. of ACEI or ARB refills within 6 mo, mean (SD) | 1.28 (1.84) | 1.80 (2.30) | 0.18 | 1.28 (1.84) | 1.28 (1.88) | 0.004 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index (calculated as the weight in kilograms divided by the height in meters squared); CCB, calcium channel blocker; DOAC, direct oral anticoagulant; ESA, erythropoietin-stimulating agent; GLP-1RA, glucagonlike peptide–1 receptor agonist; KT/V, dialyzer clearance of urea × dialysis time divided by volume of distribution of urea (a quantitative measurement of dialysis adequacy); SGLT2, sodium-glucose cotransporter-2 inhibitor; SMD, absolute standardized mean difference.
Unless otherwise indicated, data are expressed as No. (%) of participants. Percentages have been rounded and may not total 100.
Race was ascertained via Centers for Medicaid & Medicare Services Medical Evidence Form 2728 (completed at dialysis initiation).
Other race was defined as American Indian or Alaska Native, Asian, Hawaiian or Other Pacific Islander, Middle Eastern or Arabian, Other, and unknown.
Cells with fewer than 10 participants were censored per US Renal Data System reporting policy or rounded down (if multiple categories).
Results
Patient Characteristics
We identified 1434 sacubitril-valsartan and 29 654 ACEI or ARB users who met study criteria for a total cohort of 31 088 participants (Figure 1), of whom 17 931 (58%) were male, 13 157 (42%) were female, and mean (SD) age was 65 (13) years. In terms of race, 11 597 participants (37%) were Black or African American, 17 462 (56%) White, and 2029 (7%) Other race. Prior to matching, sacubitril-valsartan users (compared with ACEI or ARB users) were more likely to be male, have nondiabetic kidney disease, be seen by their primary care physician, use aldosterone antagonists, and use direct oral anticoagulants. They were less likely to have atherosclerotic heart disease, history of transient ischemic attack or stroke, diabetes, recent hospitalization, peripheral vascular disease, liver disease, respiratory disease, recent calcium channel blocker dispensed, and recent ACEI or ARB dispensed (696 [48%] vs 17 245 [58%]). After 1:1 matching, all variables were balanced with SMDs of less than 0.10 (Table 1 and eFigure 2 in Supplement 1).
Figure 1. Study Flow Diagram.
Medication dispensed history and heart failure with reduced ejection fraction (HFrEF) were ascertained using Medicare records. Dialysis vintage was calculated at the time of medication therapy initiation. ACEI indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CMS, Centers for Medicare & Medicaid Services; KT/V, dialyzer clearance of urea × dialysis time divided by volume of distribution of urea (a quantitative measurement of dialysis adequacy); and USRDS, US Renal Data System.
In the matched cohort of 2868 participants, mean (SD) age of the study population was 64 (13) years; 996 (35%) were female and 1872 (65%) were male; 987 (34%) were Black or African American, 1677 (58%) were White, and 204 (7%) were Other race. Median dialysis vintage was 3.8 (IQR, 1.8-6.3) years and 1386 (48%) had kidney failure due to diabetes (Table 1).
Follow-Up and Outcomes in the Matched Cohort
The median follow-up was 0.9 (IQR, 0.4-1.7) years. The primary outcome of death occurred in 554 individuals (39%) receiving sacubitril-valsartan and in 618 (43%) receiving ACEIs or ARBs. The cumulative incidence of mortality was 28% at 1 year and 46% at 2 years for the sacubitril-valsartan group vs 34% and 53%, respectively for the ACEI or ARB group (Figure 2). Compared with ACEIs or ARBs, sacubitril-valsartan use was associated with a lower risk of all-cause mortality (HR, 0.82 [95% CI, 0.73-0.92]) and all-cause hospitalization (HR, 0.86 [95% CI, 0.79-0.93]). There was no association with CV mortality (HR, 1.01 [95% CI, 0.86-1.19]) or HF hospitalization (HR, 0.91 [95% CI, 0.82-1.02]) (Table 2).
Figure 2. Kaplan-Meier Curves for Primary and Secondary Outcomes.
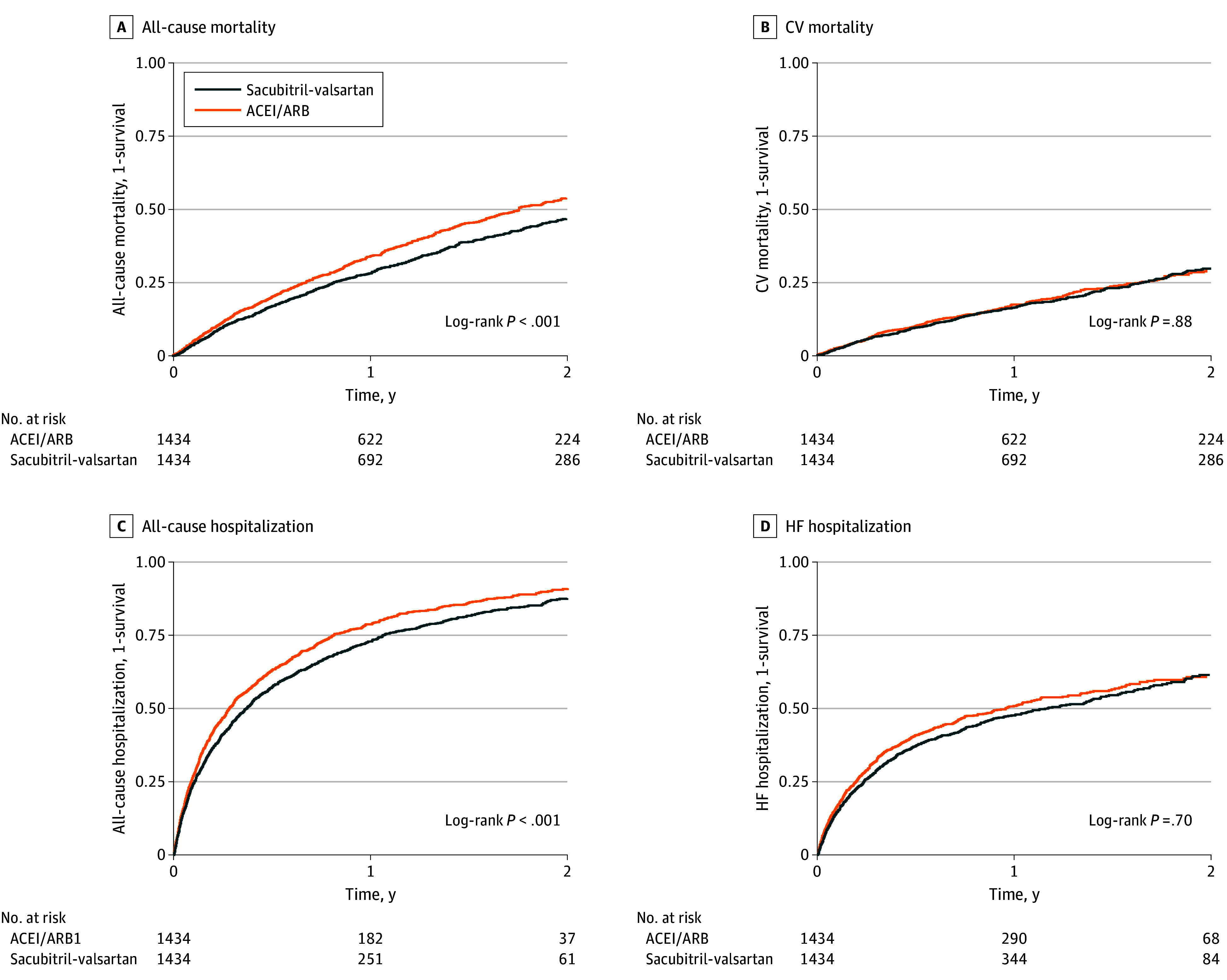
Participants were eligible for enrollment from 2015 to 2020, and analysis used an intention-to-treat approach. ACEI indicates angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; CV, cardiovascular; and HF, heart failure.
Table 2. Outcome Events Associated With Sacubitril-Valsartan Use in Medicare Recipients With HFrEF on Hemodialysis.
| Outcome | No. of events by treatment | IR (95% CI) per 1000 person-years by treatment | IRD (95% CI) | HR (95% CI) | ||
|---|---|---|---|---|---|---|
| Sacubitril-valsartan | ACEI or ARB | Sacubitril-valsartan | ACEI or ARB | |||
| Intention to treat | ||||||
| All-cause mortality | 554 | 618 | 318 (293 to 346) | 391 (361 to 423) | −74 (−110 to −35) | 0.82 (0.73 to 0.92) |
| CV mortality | 299 | 272 | 172 (153 to 192) | 172 (153 to 194) | 0 (−26 to 29) | 1.01 (0.86 to 1.19) |
| Any hospitalization | 1057 | 1083 | 1379 (1297 to 1464) | 1694 (1595 to 1797) | −322 (−424 to −186) | 0.86 (0.79 to 0.93) |
| HF hospitalization | 631 | 649 | 653 (604 to 706) | 748 (692 to 807) | −97 (−165 to −22) | 0.91 (0.82 to 1.02) |
| As treated | ||||||
| All-cause mortality | 232 | 286 | 242 (212 to 274) | 317 (281 to 355) | −76 (−114 to −29) | 0.80 (0.67 to 0.95) |
| CV mortality | 132 | 135 | 138 (115 to 162) | 149 (126 to 176) | −12 (−42 to 25) | 0.97 (0.76 to 1.23) |
| Any hospitalization | 808 | 889 | 1518 (1415 to 1625) | 1927 (1803 to 2056) | −405 (−539 to −250) | 0.84 (0.77 to 0.93) |
| HF hospitalization | 488 | 539 | 771 (705 to 842) | 939 (861 to 1020) | −169 (−253 to −66) | 0.89 (0.79 to 1.01) |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CV, cardiovascular; HF, heart failure; HR, hazard ratio; IR, incidence rate; IRD, incidence rate difference.
In the AT analysis, censoring due to medication discontinuation was high, with 809 patients (56%) stopping sacubitril-valsartan therapy after a median of 85 (IQR, 60-214) days, and 787 (55%) stopped ACEI or ARB therapy after 120 (IQR, 60-278) days, with 330 (23%) receiving sacubitril-valsartan for at least 1 year and 288 (20%) receiving ACEIs or ARBs. There were 232 deaths (16%) among participants receiving sacubitril-valsartan and 286 (20%) receiving ACEIs or ARBs with estimates similar to ITT analyses (Table 2).
Results of subgroup analysis for all-cause mortality and all-cause hospitalization in both ITT and AT analyses were generally consistent across age, sex, race, smoking status, dysrhythmia, atherosclerotic heart disease, diabetic kidney disease, history of diabetes, and diuretic use (eFigures 3 and 4 in Supplement 1) with some significant interaction effects. None, however, were consistent across both all-cause mortality and all-cause hospitalization. Similarly, we did not observe increased benefit in those with lower dialysis vintage. Instead, we observed that patients with higher dialysis vintage tended toward more benefit, though they were younger and healthier compared with those with lower dialysis vintage (eTable 5 in Supplement 1).
For safety outcomes, sacubitril-valsartan therapy was associated with a lower risk of hyperkalemia (HR, 0.71 [95% CI, 0.62-0.81]) and similar risk of hypotension (HR, 0.99 [95% CI, 0.83-1.19]) (Table 3). As-treated estimates were similar.
Table 3. Hyperkalemia and Hypotension Associated With Sacubitril-Valsartan Use in Medicare Recipients With HFrEF Receiving Hemodialysis.
| Safety outcomea | No. of events by treatment | IR (95% CI) per 1000 person-years by treatment | IRD (95% CI) | HR (95% CI) | ||
|---|---|---|---|---|---|---|
| Sacubitril-valsartan | ACEI or ARB | Sacubitril-valsartan | ACEI or ARB | |||
| Intention to treat | ||||||
| Hyperkalemia (any) | 367 | 456 | 276 (249 to 305) | 414 (377 to 453) | −136 (−174 to −95) | 0.71 (0.62 to 0.81) |
| Hyperkalemia (hospitalization claim) | 295 | 381 | 214 (190 to 239) | 331 (299 to 365) | −116 (−149 to −83) | 0.69 (0.59 to 0.80) |
| Hypotension (any) | 246 | 232 | 163 (143 to 184) | 169 (148 to 191) | −5 (−32 to 27) | 0.99 (0.83 to 1.19) |
| Hypotension (hospitalization claim) | 11 | <10b | 6 (3 to 11) | 5 (2 to 9) | 1 (−3 to 11) | 1.30 (0.52 to 3.23) |
| As treated | ||||||
| Hyperkalemia (any) | 241 | 338 | 299 (263 to 339) | 482 (433 to 536) | −183 (−227 to −130) | 0.67 (0.57 to 0.80) |
| Hyperkalemia (hospitalization claim) | 201 | 286 | 242 (210 to 278) | 396 (352 to 444) | −155 (−194 to −107) | 0.67 (0.56 to 0.80) |
| Hypotension (any) | 131 | 149 | 149 (125 to 176) | 177 (150 to 207) | −28 (−58 to 12) | 0.90 (0.71 to 1.13) |
| Hypotension (hospitalization claim) | <10b | <10b | 4 (1 to 10) | 7 (3 to 13) | −2 (−5 to 8) | 0.67 (0.19 to 2.38) |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; IR, incidence rate; IRD, incidence rate difference.
Defined by codes from International Classification of Diseases, Ninth Revision, and International Statistical Classification of Diseases, Tenth Revision.
Cells with less than 10 participants were censored per US Renal Data System reporting policy.
Of 1434 sacubitril-valsartan users, 70 (5%) started maximum dose of sacubitril-valsartan (97 mg twice a day [BID] and 103 mg BID, respectively); 249 (17%) started at 49 mg and BID and 51 mg BID, respectively; 1115 (78%) started at the lowest dose (24 mg BID and 26 mg BID, respectively). Compared with those receiving the lowest dose, individuals starting with the maximum dose were younger, less likely to have a recent hospitalization, and were generally healthier (eTable 6 in Supplement 1). When we examined for a dose-response relationship with starting dose among new users of sacubitril-valsartan, there was no association with all-cause mortality or hospitalization (eFigure 4 in Supplement 1). Titration was low with only 195 participants (14%) receiving the maximum dose and 900 (63%) receiving the lowest dose (eFigure 5 in Supplement 1). For comparison, 365 ACEI or ARB users (25%) received a maximum dose.
Sensitivity Analysis
Results with 1:4 matching were consistent with 1:1 matching, and results with a 60-day (instead of 30-day) refill window were similar. There was no difference in fracture hospitalization in ITT (HR, 0.96 [95% CI, 0.76-1.21]) or AT (HR, 0.89 [95% CI, 0.67-1.18]) analysis. The E-value for mortality was 1.74, and the highest adjusted odds ratios (OR) for major study risk factors and all-cause mortality (n = 31 088) were current smoking status (OR 1.25) and diabetes (OR 1.15). Finally, estimates from competing risk models were similar (eTable 7 in Supplement 1).
Discussion
In this active comparator, 1:1 propensity score–matched cohort of US patients receiving hemodialysis from 2015 to 2020, initiation of sacubitril-valsartan therapy among patients with HFrEF was associated with decreased all-cause mortality and all-cause hospitalization (compared with ACEI or ARB initiation or continued use). There was no association with CV mortality or HF hospitalization, and there were no consistent subgroup associations for both all-cause mortality and all-cause hospitalization. Sacubitril-valsartan was associated with a decreased risk of hyperkalemia and a similar risk of hypotension. There was a high rate of discontinuation of both sacubitril-valsartan therapy (56%) and ACEI or ARB therapy (55%), but AT point estimates were consistent with ITT analyses. Taken together, our results demonstrate that sacubitril-valsartan may have beneficial effects in all-cause mortality and hospitalization among patients with HFrEF requiring hemodialysis.
Our findings expand on studies in Asian patients with HF requiring dialysis14,15,16,17,18,19 where sacubitril-valsartan therapy was associated with biochemical (levels of high-sensitivity troponin T, BNP, and NT-proBNP) and echocardiographic improvement suggestive of clinical benefit. Given that Asian patients receiving hemodialysis have higher renal reserve (mean urine output of 500 [IQR, 100-1000] mL daily)16,33 and may benefit more from the diuretic effects of sacubitril,34 we looked at a subgroup interaction by diuretic use (as a proxy for residual renal function), which was negative. Only 2 prior observational studies12,13 have examined sacubitril-valsartan and clinical outcomes in patients receiving dialysis, and both studies were conducted in Asia. These studies, however, included patients with HF with preserved EF12 as well as those not requiring dialysis.13 Both studies did not observe a mortality benefit, but power was limited with a combined study population receiving sacubitril-valsartan of less than 200 (vs 1434 for this study and 4187 in PARADIGM-HF).13,35 Additionally, one of these studies compared sacubitril-valsartan use vs no medication use, raising concern for bias (ie, confounding by indication).12,36 Importantly, we saw a beneficial association despite the high morbidity and mortality among patients receiving dialysis, with 1-year mortality at 30% vs 10% to 15% in Asia12,13 (and <10% in PARADIGM-HF).2 We additionally examined dialysis vintage as those newly receiving dialysis may have less cardiac remodeling, thereby allowing more benefit.37 There was no significant interaction, but paradoxically, those with higher dialysis vintage tended toward more benefit, potentially due to confounding from younger age and less comorbidity.
We did not observe a significant difference in CV mortality or HF hospitalization, which contrasts with the PARADIGM-HF trial. Importantly, our cause-specific outcomes (HF hospitalization [defined by ICD-9 and ICD-10 codes] and CV mortality [defined via the USRDS]) may be susceptible to misclassification. Sacubitril-valsartan (vs ACEI or ARB) users may have different ICD billing practices (such as increased number of ICD-9 or ICD-10 billed codes),6 and this may help explain the inconsistent association with HF hospitalization among individuals requiring dialysis (ie, increased,13 decreased,12 or neutral [in this study]). Similarly, cause of death in the USRDS has been shown to have only moderate agreement with an integrated health system.38 We saw reductions in all-cause mortality and all-cause hospitalization, which is immune to this type of misclassification. Conversely, we may have lacked power for cause-specific outcomes (ie, HF hospitalization HR, 0.91 [95% CI, 0.82-1.02]). Further studies are needed to confirm our findings (like ongoing Asian clinical trials)10,11 and should consider prospective adjudication of outcomes similar to PARADIGM-HF.2
Alternatively, the reduction in all-cause mortality but not cardiovascular mortality with sacubitril-valsartan (vs ACEIs or ARBs) could be related to decreases in hyperkalemia,39,40,41,42 which is common in patients receiving dialysis. Previous studies have suggested that patient factors (and not dialysis prescription) have stronger associations with serum potassium levels and that potassium-specific interventions (like medications) could improve clinical outcomes.39 Considering that the interplay among potassium level,43,44,45,46 renin-angiotensin-aldosterone system inhibition (via ACEI, ARB, or ARNI), and clinical outcomes is complex,47,48 further investigation is warranted as it may contribute to our positive findings.
For hypotension, there was not a significant difference, but 63% of patients continued to receive the starting dose of sacubitril-valsartan (ie, 24 and 26 mg, BID, respectively). Additionally, the rate of hypotensive events was 10-fold higher than the general population6 and may have contributed to low dose titration. Given that less benefit for sacubitril-valsartan has been seen in individuals with lower blood pressures,49,50,51 we examined the association between the starting dose and clinical outcomes among sacubitril-valsartan new users, which was negative. This observation is similar to those of other studies that specifically examined sacubitril-valsartan doses and clinical outcomes.52,53
Strengths and Limitations
Strengths of this study include the active comparator design, complete outcome ascertainment due to Medicare and USRDS, the largest sample, to our knowledge, of patients with HFrEF undergoing hemodialysis to date, and the first non-Asian observational data. Our study also has limitations. First, we included participants with prevalent use of ACEs or ARBs, which can introduce selection bias (as individuals with prevalent ACEI or ARB use already tolerate therapy and may be “healthier” compared with those with incident ACEI or ARB use). If this bias was present after propensity score matching, however, it would bias our results toward the null and not explain our positive finding.54 Next, we were unable to account for residual or unmeasured confounding seen in observational studies (ie, New York Heart Association class or echocardiographic measurements). Using the E-value method, an unmeasured confounder would need to be associated with both sacubitril-valsartan and all-cause mortality by a risk ratio of at least 1.74 to explain our observed benefit. Within our eligible patients, the highest OR of mortality and major risk factors was less than 1.3 (ie, smoking OR, 1.25). Therefore, the possibility of an unknown confounder having an association of greater magnitude is low. There could also be confounding by indication where healthier patients received sacubitril-valsartan despite our propensity score match and negative control outcome. We used medication dispensing history, which is better than prescription data, but misclassification risk remains. Due to limited power, we were unable to explore sacubitril-valsartan titration and outcomes. Individuals also had at least 180 days of Medicare primary payer status, which limits generalizability. Finally, multiple covariates and outcomes (like HFrEF and hypotension) were defined by ICD codes that are susceptible to measurement error.
Conclusions
In this comparative effectiveness study with a 1:1 matched cohort of US Medicare beneficiaries in the USRDS registry from 2015 to 2020 with HFrEF requiring hemodialysis, initiation of sacubitril-valsartan (vs ACEI or ARB) therapy was associated with improved all-cause mortality and hospitalization but not with improved cardiovascular mortality or HF hospitalization. Therapy was well tolerated without an increase in hyperkalemia or hypotension, and AT effect estimates were similar despite at least 50% of patients discontinuing therapy during follow-up. Our study shows that sacubitril-valsartan may have significant therapeutic potential among patients with HFrEF requiring hemodialysis, but further studies are needed before changes in clinical practice.
eTable 1. Specification and Emulation of a Target Trial for Sacubitril-Valsartan vs ACEI or ARB in US Medicare Patients
eTable 2. Specific Medications Assessed Using Medicare Part D Dispense Records
eTable 3. ICD-9, ICD-10, and CPT Codes for Comorbidity and Outcome Assessment
eTable 4. ACEI or ARB Stratification by Total Daily Dose
eTable 5. Baseline Characteristics of Sacubitril-Valsartan Users by Quartile of Dialysis Vintage
eTable 6. Baseline Characteristics of Sacubitril-Valsartan Users by Initial Dispensed Dose
eTable 7. HR of All-Cause Mortality, CV Mortality, Any Hospitalization, HF Hospitalization, Hyperkalemia, and Hypotension Associated With Sacubitril-Valsartan Use (vs ACEI or ARB) in Medicare Recipients With HFrEF Receiving Hemodialysis
eFigure 1. Covariate Balance and Post 1:1 Propensity Score Matching Without Replacement
eFigure 2. Subgroup Analyses of the HR for All-Cause Mortality Comparing Sacubitril-Valsartan vs ACEI or ARB After 1:1 Match Across Demographics, Medical Comorbidities, and Medication Dispensed History
eFigure 3. Subgroup Analyses of the HR for All-Cause Hospitalization Comparing Sacubitril-Valsartan vs ACEI or ARB
eFigure 4. Dose-Response Relationship Comparing Both All-Cause Mortality and All-Cause Hospitalization With Sacubitril-Valsartan Initiation Dose
eFigure 5. Sacubitril/Valsartan Dosing Pattern During Follow-Up Defined Using the Starting Dispenses Dose, Maximum Dispensed Dose, and Most Recent Dispensed Dose
Data Sharing Statement
References
- 1.U.S. Renal Data System . 2021 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2021. [Google Scholar]
- 2.McMurray JJV, Packer M, Desai AS, et al. ; PARADIGM-HF Investigators and Committees . Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993-1004. doi: 10.1056/NEJMoa1409077 [DOI] [PubMed] [Google Scholar]
- 3.Pitt B, Zannad F, Remme WJ, et al. ; Randomized Aldactone Evaluation Study Investigators . The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341(10):709-717. doi: 10.1056/NEJM199909023411001 [DOI] [PubMed] [Google Scholar]
- 4.McMurray JJV, Solomon SD, Inzucchi SE, et al. ; DAPA-HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995-2008. doi: 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 5.Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e895-e1032. doi: 10.1161/CIR.0000000000001063 [DOI] [PubMed] [Google Scholar]
- 6.Tan NY, Sangaralingham LR, Sangaralingham SJ, Yao X, Shah ND, Dunlay SM. Comparative effectiveness of sacubitril-valsartan versus ACE/ARB therapy in heart failure with reduced ejection fraction. JACC Heart Fail. 2020;8(1):43-54. doi: 10.1016/j.jchf.2019.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solomon SD, Vaduganathan M, L Claggett B, et al. Sacubitril/valsartan across the spectrum of ejection fraction in heart failure. Circulation. 2020;141(5):352-361. doi: 10.1161/CIRCULATIONAHA.119.044586 [DOI] [PubMed] [Google Scholar]
- 8.DeJong C, Kazi DS, Dudley RA, Chen R, Tseng CW. Assessment of national coverage and out-of-pocket costs for sacubitril/valsartan under Medicare Part D. JAMA Cardiol. 2019;4(8):828-830. doi: 10.1001/jamacardio.2019.2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaziano TA, Fonarow GC, Claggett B, et al. Cost-effectiveness analysis of sacubitril/valsartan vs enalapril in patients with heart failure and reduced ejection fraction. JAMA Cardiol. 2016;1(6):666-672. doi: 10.1001/jamacardio.2016.1747 [DOI] [PubMed] [Google Scholar]
- 10.Efficacy and Safety of Sacubitril/Valsartan in Maintenance Hemodialysis Patients With Heart Failure (ESARHD-HF). ClinicalTrials.gov Identifier NCT04458285. Updated October 6, 2020. Accessed December 31, 2022. https://clinicaltrials.gov/study/NCT04458285
- 11.The Effect of Sacubitril/Valsartan on Cardiovascular Events Outcome in Maintenance Dialysis Patients With Heart Failure and Efficacy Prediction of Baseline LVEF Value. ClinicalTrials.gov Identifier NCT4572724. Updated October 8, 2020. Accessed December 31, 2022. https://clinicaltrials.gov/study/NCT04572724
- 12.Liu X, Huang L, Tse G, Liu T, Che J. Effects of sacubitril-valsartan in the treatment of chronic heart failure patients with end-stage renal disease undergoing dialysis. Clin Cardiol. 2023;46(8):930-936. doi: 10.1002/clc.24075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsiao FC, Lin CP, Yu CC, Tung YC, Chu PH. Angiotensin receptor-neprilysin inhibitors in patients with heart failure with reduced ejection fraction and advanced chronic kidney disease: a retrospective multi-institutional study. Front Cardiovasc Med. 2022;9:794707. doi: 10.3389/fcvm.2022.794707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee S, Oh J, Kim H, et al. Sacubitril/valsartan in patients with heart failure with reduced ejection fraction with end-stage of renal disease. ESC Heart Fail. 2020;7(3):1125-1129. doi: 10.1002/ehf2.12659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daimon S, Yasuda M, Maeda K. Effect of sacubitril-valsartan on cardiac function in hemodialysis patients. Ther Apher Dial. 2022;26(1):244-245. doi: 10.1111/1744-9987.13715 [DOI] [PubMed] [Google Scholar]
- 16.Ding Y, Wan L, Zhang ZC, et al. Effects of sacubitril-valsartan in patients undergoing maintenance dialysis. Ren Fail. 2023;45(1):2222841. doi: 10.1080/0886022X.2023.2222841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niu CY, Yang SF, Ou SM, et al. Sacubitril/valsartan in patients with heart failure and concomitant end-stage kidney disease. J Am Heart Assoc. 2022;11(18):e026407. doi: 10.1161/JAHA.122.026407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu S, Xu Z, Lin B, et al. Effects of sacubitril-valsartan in heart failure with preserved ejection fraction in patients undergoing peritoneal dialysis. Front Med (Lausanne). 2021;8:657067. doi: 10.3389/fmed.2021.657067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheng Y, Ma X, Liu Y, Yang X, Sun F. Study on the efficacy of sacubitril/valsartan in patients with heart failure with preserved ejection fraction undergoing peritoneal dialysis. Cardiology. 2023;148(5):385-394. doi: 10.1159/000531217 [DOI] [PubMed] [Google Scholar]
- 20.Li Q, Glynn RJ, Dreyer NA, Liu J, Mogun H, Setoguchi S. Validity of claims-based definitions of left ventricular systolic dysfunction in Medicare patients. Pharmacoepidemiol Drug Saf. 2011;20(7):700-708. doi: 10.1002/pds.2146 [DOI] [PubMed] [Google Scholar]
- 21.Sangaralingham LR, Sangaralingham SJ, Shah ND, Yao X, Dunlay SM. Adoption of sacubitril/valsartan for the management of patients with heart failure. Circ Heart Fail. 2018;11(2):e004302. doi: 10.1161/CIRCHEARTFAILURE.117.004302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183(8):758-764. doi: 10.1093/aje/kwv254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Velazquez EJ, Morrow DA, DeVore AD, et al. ; PIONEER-HF Investigators . Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380(6):539-548. doi: 10.1056/NEJMoa1812851 [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Huang Z, Gilbertson DT, Foley RN, Collins AJ. An improved comorbidity index for outcome analyses among dialysis patients. Kidney Int. 2010;77(2):141-151. doi: 10.1038/ki.2009.413 [DOI] [PubMed] [Google Scholar]
- 25.Ho D, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42:1-28. doi: 10.18637/jss.v042.i08 [DOI] [Google Scholar]
- 26.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163(12):1149-1156. doi: 10.1093/aje/kwj149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stuart EA, Lee BK, Leacy FP. Prognostic score-based balance measures can be a useful diagnostic for propensity score methods in comparative effectiveness research. J Clin Epidemiol. 2013;66(8)(suppl):S84-S90, 90.e1. doi: 10.1016/j.jclinepi.2013.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen TL, Collins GS, Spence J, et al. Double-adjustment in propensity score matching analysis: choosing a threshold for considering residual imbalance. BMC Med Res Methodol. 2017;17(1):78. doi: 10.1186/s12874-017-0338-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268-274. doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 30.Austin PC, Fine JP. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med. 2017;36(27):4391-4400. doi: 10.1002/sim.7501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.R Core Team . R: A language and environment for statistical computing. R Program for Statistical Computing; 2020. Accessed September 23, 2023. https://www.R-project.org/
- 32.StataCorp . Stata Statistical Software: Release 17. StataCorp LLC; 2021. [Google Scholar]
- 33.Shafi T, Jaar BG, Plantinga LC, et al. Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Am J Kidney Dis. 2010;56(2):348-358. doi: 10.1053/j.ajkd.2010.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vardeny O, Claggett B, Kachadourian J, et al. Reduced loop diuretic use in patients taking sacubitril/valsartan compared with enalapril: the PARADIGM-HF trial. Eur J Heart Fail. 2019;21(3):337-341. doi: 10.1002/ejhf.1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raad H, Cornelius V, Chan S, Williamson E, Cro S. An evaluation of inverse probability weighting using the propensity score for baseline covariate adjustment in smaller population randomised controlled trials with a continuous outcome. BMC Med Res Methodol. 2020;20(1):70. doi: 10.1186/s12874-020-00947-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshida K, Solomon DH, Kim SC. Active-comparator design and new-user design in observational studies. Nat Rev Rheumatol. 2015;11(7):437-441. doi: 10.1038/nrrheum.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaesler N, Babler A, Floege J, Kramann R. Cardiac remodeling in chronic kidney disease. Toxins (Basel). 2020;12(3):161. doi: 10.3390/toxins12030161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhandari SK, Zhou H, Shaw SF, et al. Causes of death in end-stage kidney disease: comparison between the United States Renal Data System and a large integrated health care system. Am J Nephrol. 2022;53(1):32-40. doi: 10.1159/000520466 [DOI] [PubMed] [Google Scholar]
- 39.Karaboyas A, Zee J, Brunelli SM, et al. Dialysate potassium, serum potassium, mortality, and arrhythmia events in hemodialysis: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2017;69(2):266-277. doi: 10.1053/j.ajkd.2016.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, Sakhuja A, Mao MA, Erickson SB. Impact of admission serum potassium on mortality in patients with chronic kidney disease and cardiovascular disease. QJM. 2017;110(11):713-719. doi: 10.1093/qjmed/hcx118 [DOI] [PubMed] [Google Scholar]
- 41.Aldahl M, Jensen AC, Davidsen L, et al. Associations of serum potassium levels with mortality in chronic heart failure patients. Eur Heart J. 2017;38(38):2890-2896. doi: 10.1093/eurheartj/ehx460 [DOI] [PubMed] [Google Scholar]
- 42.Collins AJ, Pitt B, Reaven N, et al. Association of serum potassium with all-cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am J Nephrol. 2017;46(3):213-221. doi: 10.1159/000479802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagasu H, Tokuyama A, Kanda E, et al. The impact of potassium binders on mortality in patients with hyperkalemia: a single-center study. Kidney Dial. 2023;3(3):244-254. doi: 10.3390/kidneydial3030022 [DOI] [Google Scholar]
- 44.Butler J, Anker SD, Lund LH, et al. Patiromer for the management of hyperkalemia in heart failure with reduced ejection fraction: the DIAMOND trial. Eur Heart J. 2022;43(41):4362-4373. doi: 10.1093/eurheartj/ehac401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brunelli SM, Du Mond C, Oestreicher N, Rakov V, Spiegel DM. Serum potassium and short-term clinical outcomes among hemodialysis patients: impact of the long interdialytic interval. Am J Kidney Dis. 2017;70(1):21-29. doi: 10.1053/j.ajkd.2016.10.024 [DOI] [PubMed] [Google Scholar]
- 46.Chen Y, Sang Y, Ballew SH, et al. Race, serum potassium, and associations with ESRD and mortality. Am J Kidney Dis. 2017;70(2):244-251. doi: 10.1053/j.ajkd.2017.01.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rossignol P, Lainscak M, Crespo-Leiro MG, et al. ; Heart Failure Long-Term Registry Investigators Group . Unravelling the interplay between hyperkalaemia, renin-angiotensin-aldosterone inhibitor use and clinical outcomes: data from 9222 chronic heart failure patients of the ESC-HFA-EORP Heart Failure Long-Term Registry. Eur J Heart Fail. 2020;22(8):1378-1389. doi: 10.1002/ejhf.1793 [DOI] [PubMed] [Google Scholar]
- 48.Ferreira JP, Mogensen UM, Jhund PS, et al. Serum potassium in the PARADIGM-HF trial. Eur J Heart Fail. 2020;22(11):2056-2064. doi: 10.1002/ejhf.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang HY, Feng AN, Fong MC, et al. Sacubitril/valsartan in heart failure with reduced ejection fraction patients: real world experience on advanced chronic kidney disease, hypotension, and dose escalation. J Cardiol. 2019;74(4):372-380. doi: 10.1016/j.jjcc.2019.03.010 [DOI] [PubMed] [Google Scholar]
- 50.Raphael CE, Whinnett ZI, Davies JE, et al. Quantifying the paradoxical effect of higher systolic blood pressure on mortality in chronic heart failure. Heart. 2009;95(1):56-62. doi: 10.1136/hrt.2007.134973 [DOI] [PubMed] [Google Scholar]
- 51.Gheorghiade M, Abraham WT, Albert NM, et al. ; OPTIMIZE-HF Investigators and Coordinators . Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA. 2006;296(18):2217-2226. doi: 10.1001/jama.296.18.2217 [DOI] [PubMed] [Google Scholar]
- 52.Mohebi R, Liu Y, Piña IL, et al. Dose-response to sacubitril/valsartan in patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2022;80(16):1529-1541. doi: 10.1016/j.jacc.2022.08.737 [DOI] [PubMed] [Google Scholar]
- 53.Vardeny O, Claggett B, Packer M, et al. ; Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) Investigators . Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: the PARADIGM-HF trial. Eur J Heart Fail. 2016;18(10):1228-1234. doi: 10.1002/ejhf.580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Danaei G, Tavakkoli M, Hernán MA. Bias in observational studies of prevalent users: lessons for comparative effectiveness research from a meta-analysis of statins. Am J Epidemiol. 2012;175(4):250-262. doi: 10.1093/aje/kwr301 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Specification and Emulation of a Target Trial for Sacubitril-Valsartan vs ACEI or ARB in US Medicare Patients
eTable 2. Specific Medications Assessed Using Medicare Part D Dispense Records
eTable 3. ICD-9, ICD-10, and CPT Codes for Comorbidity and Outcome Assessment
eTable 4. ACEI or ARB Stratification by Total Daily Dose
eTable 5. Baseline Characteristics of Sacubitril-Valsartan Users by Quartile of Dialysis Vintage
eTable 6. Baseline Characteristics of Sacubitril-Valsartan Users by Initial Dispensed Dose
eTable 7. HR of All-Cause Mortality, CV Mortality, Any Hospitalization, HF Hospitalization, Hyperkalemia, and Hypotension Associated With Sacubitril-Valsartan Use (vs ACEI or ARB) in Medicare Recipients With HFrEF Receiving Hemodialysis
eFigure 1. Covariate Balance and Post 1:1 Propensity Score Matching Without Replacement
eFigure 2. Subgroup Analyses of the HR for All-Cause Mortality Comparing Sacubitril-Valsartan vs ACEI or ARB After 1:1 Match Across Demographics, Medical Comorbidities, and Medication Dispensed History
eFigure 3. Subgroup Analyses of the HR for All-Cause Hospitalization Comparing Sacubitril-Valsartan vs ACEI or ARB
eFigure 4. Dose-Response Relationship Comparing Both All-Cause Mortality and All-Cause Hospitalization With Sacubitril-Valsartan Initiation Dose
eFigure 5. Sacubitril/Valsartan Dosing Pattern During Follow-Up Defined Using the Starting Dispenses Dose, Maximum Dispensed Dose, and Most Recent Dispensed Dose
Data Sharing Statement



