Key Points
Question
What is the effect of once-weekly subcutaneous tirzepatide injection on body weight reduction in Chinese adults with obesity or overweight without diabetes?
Findings
In this randomized clinical trial (N = 210), mean percent change in body weight at week 52 was −13.6% and −17.5% with tirzepatide 10 mg and 15 mg, respectively, compared with −2.3% with placebo; the differences between each tirzepatide group and the placebo group were statistically significant.
Meaning
Among Chinese adults with obesity or overweight, tirzepatide resulted in greater reduction in body weight than placebo.
Abstract
Importance
Obesity has become a global public health concern and China has the largest number of affected people worldwide.
Objective
To assess the efficacy and safety of treatment with tirzepatide for weight reduction in Chinese adults with obesity or overweight and weight-related comorbidities.
Design, Setting, and Participants
This randomized, double-blind, placebo-controlled, phase 3 clinical trial conducted at 29 centers in China from September 2021 to December 2022 included Chinese adults (aged ≥18 years) with a body mass index (BMI) greater than or equal to 28 or greater than or equal to 24 and at least 1 weight-related comorbidity, excluding diabetes.
Interventions
Participants were randomly assigned (1:1:1) to receive once-weekly, subcutaneous 10-mg (n = 70) or 15-mg (n = 71) tirzepatide or placebo (n = 69), plus a lifestyle intervention, for 52 weeks.
Main Outcomes and Measures
Co–primary end points were the percent change in body weight from baseline and weight reduction of at least 5% at week 52. Efficacy and safety analyses were performed on an intention-to-treat population.
Results
Of 210 randomized participants (103 [49.0%] female; mean [SD] age, 36.1 [9.1] years; body weight, 91.8 [16.0] kg; BMI, 32.3 [3.8]), 201 (95.7%) completed the trial. The mean change in body weight at week 52 was −13.6% (95% CI, −15.8% to −11.4%) with tirzepatide 10 mg, −17.5% (95% CI, −19.7% to −15.3%) with tirzepatide 15 mg, and −2.3% with placebo (difference between 10 mg and placebo, −11.3% [95% CI, −14.3% to −8.3%; P < .001]; difference between 15 mg and placebo, −15.1% [95% CI, −18.2% to −12.1%; P < .001]). The percentage of participants achieving body weight reductions of 5% or greater was 87.7% with tirzepatide 10 mg, 85.8% with tirzepatide 15 mg, and 29.3% with placebo (P < .001 for comparisons with placebo). The most frequent treatment-emergent adverse events with tirzepatide were gastrointestinal. Most were mild to moderate in severity, with few events leading to treatment discontinuation (<5%).
Conclusions and Relevance
In Chinese adults with obesity or overweight, once-weekly treatment with tirzepatide 10 mg or 15 mg resulted in statistically significant and clinically meaningful weight reduction with an acceptable safety profile.
Trial Registration
ClinicalTrials.gov Identifier: NCT05024032
This randomized clinical trial investigates the safety and efficacy of treatment with once-weekly tirzepatide for weight reduction in Chinese adults with overweight or obesity without diabetes over a 52-week period.
Introduction
Obesity is a chronic, relapsing, progressive disease and a public health problem associated with serious complications and comorbidities.1,2 The prevalence of obesity or overweight among Chinese adults is anticipated to rise to 70.5% by 2030, impacting the lives of approximately 810 million individuals.3
The Working Group on Obesity in China recommended body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) cutoffs of 24.0 and 28.0 to define overweight and obesity, respectively.4 These cutoffs are lower than the World Health Organization criteria for the US population because Chinese individuals are likely to have higher percentages of body fat and an elevated cardiometabolic risk at similar BMI levels.5,6 Pharmacotherapy as an adjunct to lifestyle intervention is recommended by current Chinese guidelines in individuals with BMI of 28 or greater or BMI of 24 or greater with weight-related comorbidities who do not achieve at least 5% weight reduction within the first 3 to 6 months of lifestyle interventions.7
Tirzepatide is a first-in-class once-weekly glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 receptor agonist approved for the treatment of type 2 diabetes and under review for chronic weight management in China.8 In the SURMOUNT clinical trial program, once-weekly tirzepatide has demonstrated sustained reductions in body weight among adults with obesity or overweight, accompanied by improvements in cardiometabolic risk factors.9,10,11,12
The SURMOUNT-CN study aimed to evaluate the efficacy and safety of tirzepatide for weight reduction in Chinese participants with obesity or overweight without diabetes.
Methods
Study Design
This study was a multicenter, randomized, double-blind, placebo-controlled phase 3 trial conducted at 29 centers in China. The protocol (Supplement 1) was approved by the institutional review board of each participating center and the trial was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice Guidelines. All participants provided written informed consent.
Participants
Key inclusion criteria were adults (aged 18 years or older) with a BMI greater than or equal to 28, or greater than or equal to 24 with at least 1 weight-related comorbidity (eg, hypertension, dyslipidemia, cardiovascular disease), and who reported at least 1 unsuccessful dietary effort to lose weight. Key exclusion criteria included diabetes, a self-reported change in body weight of 5 kg or more within 3 months before screening, a previous or planned surgical treatment for obesity, and treatment with medications or alternative remedies intended for weight reduction within 3 months before randomization. Full eligibility criteria are provided in eAppendix 1 in Supplement 2.
Randomization and Masking
Participants were randomly assigned (1:1:1) to receive tirzepatide 10 mg, tirzepatide 15 mg, or placebo by a computer-generated random sequence using an interactive web response system. Randomization was stratified by self-reported sex (female, male) and the presence of weight-related comorbidities (yes, no). Participants, investigators, and the sponsor were masked to treatment assignments until study completion.
Interventions
After a 2-week screening period, eligible participants performed all required baseline study procedures, including laboratory measures and questionnaires, prior to randomization and to receiving the first dose of the study drug. Participants received self-administered subcutaneous injections of tirzepatide 10 mg, tirzepatide 15 mg, or placebo once a week, plus a lifestyle intervention, for 52 weeks, followed by a 4-week safety follow-up period without treatment (eFigure 1 in Supplement 2). Participants who were unable to perform the injections were required to have the assistance of an individual trained to inject the study drug. Tirzepatide was initiated at 2.5 mg once weekly and increased by 2.5 mg every 4 weeks until the maintenance dose of 10 mg or 15 mg was reached at week 12 and week 20, respectively. Participants were advised to administer study treatment on the same day and at the same time each week. Lifestyle intervention included regular lifestyle counseling with a dietitian or qualified health care professional to help the participants adhere to healthy, balanced meals with a 500-kcal deficit per day relative to estimated total daily energy expenditure and at least 150 minutes of physical activity per week. Concomitant medications were permitted except for those that might interfere with the assessment of the efficacy and safety characteristics of the study treatments.
Outcomes
The co–primary end points were the percent change in body weight from baseline to week 52 and the percentage of participants with weight reduction of at least 5% at week 52. Key secondary end points controlled for type I error rate included the change in body weight from baseline to week 20; weight reduction of at least 10% and 15% at week 52; and the change in waist circumference from baseline to week 52. Additional secondary end points included the changes from baseline to week 52 in body weight, BMI, glycated hemoglobin (HbA1c), blood pressure, fasting glucose, insulin and lipids, the 36-Item Short Form Health Survey version 2 (SF-36v2) acute form physical functioning domain score, and the Impact of Weight on Quality of Life-Lite Clinical Trials Version (IWQOL-Lite-CT) physical function composite score. Efficacy end points were assessed for tirzepatide 10 mg or tirzepatide 15 mg vs placebo, with the exception of diastolic blood pressure, systolic blood pressure, fasting lipids, and insulin, which were assessed for pooled tirzepatide (10 mg and 15 mg combined) vs placebo.
Safety assessments included treatment-emergent adverse events, serious adverse events, vital signs, and laboratory measures. Deaths, major adverse cardiovascular events, and pancreatitis were adjudicated by an independent external adjudication committee.
Sample Size Calculation
For the co–primary end points, a sample size of 210 provided a power of greater than 90% to demonstrate the superiority of tirzepatide 10 mg and 15 mg over placebo, each at a 2-sided significance level of .025. The sample size calculation assumed at least an 11% difference in the mean percent body weight reduction from baseline at week 52 for each tirzepatide dose (10 mg and 15 mg) compared with placebo, a common standard deviation of 11%, and 25% of placebo-treated participants and 90% of tirzepatide-treated participants achieving a weight reduction of at least 5% at week 52 and a dropout rate of 25%.
Statistical Analyses
Efficacy end points were evaluated for 2 estimands (treatment regimen and efficacy) in the intention-to-treat population, comprising all randomly assigned participants with at least 1 dose of the study treatment. The type I error rate was controlled at .05 for evaluation of primary and key secondary end points with a graphical approach. Safety analyses were performed in the intention-to-treat population using the data collected during the treatment period and safety follow-up period, regardless of adherence to study treatment. All treatment difference results from statistical analyses were accompanied with 2-sided 95% CIs and P values, with statistical significance defined as P < .05. Statistical analyses were performed using SAS version 9.4 (SAS Institute).
The treatment regimen estimand represented the mean treatment effect of tirzepatide relative to placebo at 52 weeks for all participants who had undergone randomization, regardless of adherence to study treatment. For continuous end points, an analysis of a covariance model with randomized treatment and stratification factors as fixed effects and baseline measure as a covariate was used. For categorical end points, a logistic regression model (treatment difference was assessed by odds ratio) with the same fixed effects and covariate as continuous end points was used. Missing values at week 52 were imputed 100 times using the method of multiple imputation based on the same treatment group. Statistical inference over imputed data was guided by the Rubin Rule.
The efficacy estimand represented the average treatment effect of tirzepatide relative to placebo for all participants who had undergone randomization if they had remained on their randomized treatment for the entire 52-week treatment period (excluding data obtained after discontinuation of study treatment). Continuous end points were analyzed using a mixed model for repeated measures, with randomized treatment, visit, treatment × visit interaction, and stratification factors as fixed effects and baseline measure as a covariate. Categorical end points were analyzed by a logistic regression model with the same factors and covariate (excluding visit effects) as continuous end points, where missing end points were dichotomized after they were predicted from the continuous end point mixed model for repeated measures analysis explained above.
Additional details on estimands, handling of missing values, and statistical analysis methods are provided in eAppendix 2 in Supplement 2.
Results
Baseline Characteristics
Between September 1, 2021, and December 27, 2022, a total of 248 participants were assessed for eligibility and 210 were randomized and received at least 1 dose of the study drug (Figure 1). A total of 201 participants (95.7%) completed the study and 182 (86.7%) completed the treatment. Reasons for premature study treatment discontinuation across treatment groups were withdrawal by participants and adverse events. Baseline demographics and clinical characteristics were similar across treatment groups (Table 1). Overall, participants had a mean age of 36.1 years, mean body weight of 91.8 kg, mean BMI of 32.3, and mean waist circumference of 104.8 cm; 103 participants (49.0%) were female and 91.4% had weight-related comorbidities.
Figure 1. Flow of Participants in the SURMOUNT-CN Trial.
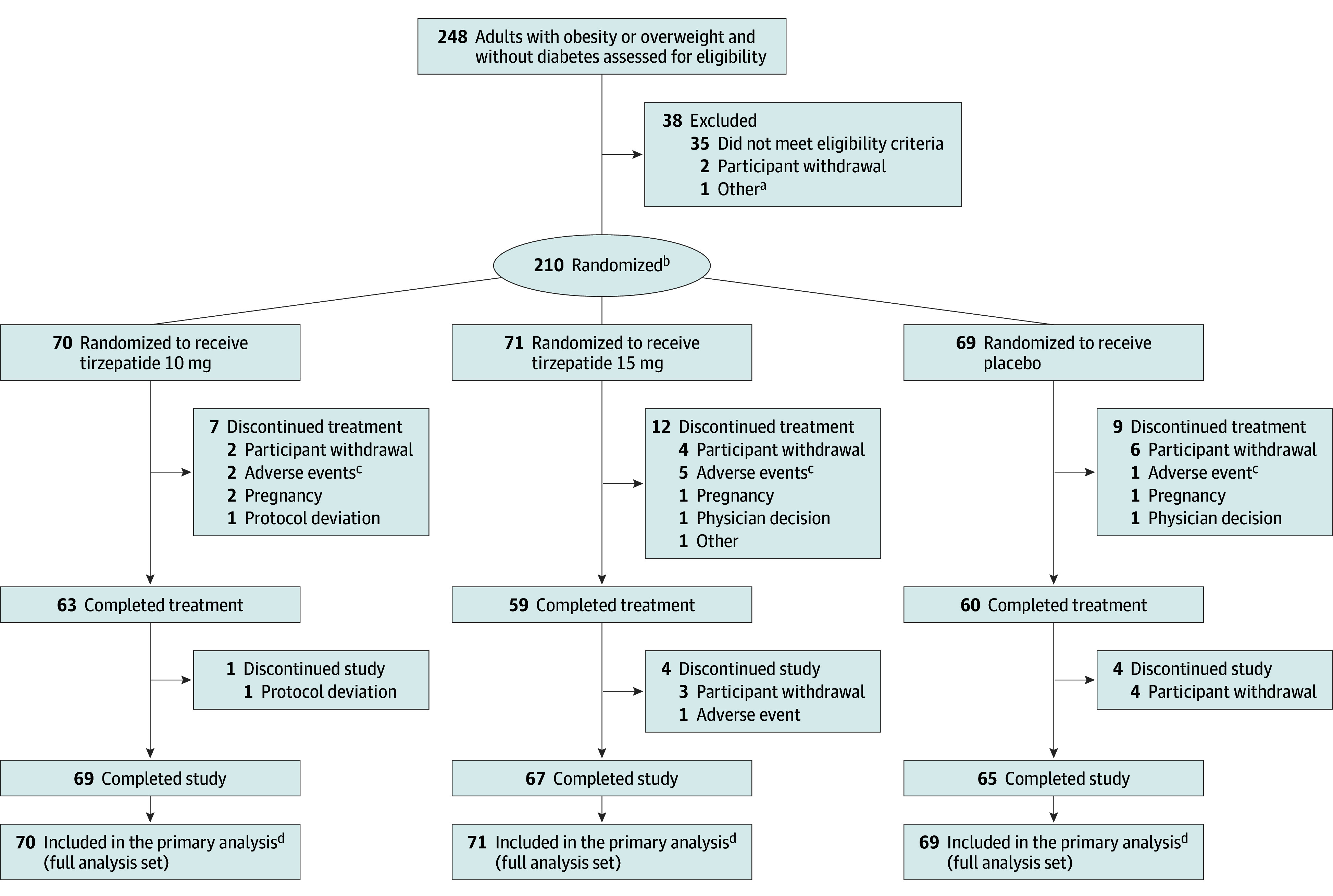
aIncludes 1 participant who was not able to commit to the study visit schedule due to time conflicts and failed screening.
bRandomization was stratified by sex (female, male) according to fixed selection categories and the presence of weight-related comorbidities (yes, no).
cSee Table 3 for details of the adverse events that led to treatment discontinuation.
dGuided by the treatment regimen estimand.
Table 1. Demographics and Clinical Characteristics of the Randomized Population.
| Characteristic | Mean (SD) | ||
|---|---|---|---|
| Tirzepatide 10 mg (n = 70) | Tirzepatide 15 mg (n = 71) | Placebo (n = 69) | |
| Age, y | 34.7 (7.2) | 35.8 (9.3) | 37.8 (10.2) |
| Sex, No. (%)a | |||
| Female | 35 (50.0) | 35 (49.3) | 33 (47.8) |
| Male | 35 (50.0) | 36 (50.7) | 36 (52.2) |
| Body weight, kg | 92.2 (16.2) | 91.3 (16.2) | 92.0 (15.8) |
| BMI | 32.6 (4.1) | 32.0 (3.7) | 32.4 (3.6) |
| BMI category-1, No. (%) | |||
| ≥24 to <28 | 4 (5.7) | 6 (8.5) | 6 (8.7) |
| ≥28 | 66 (94.3) | 65 (91.5) | 63 (91.3) |
| BMI category-2, No. (%) | |||
| 24 to <25 | 0 | 0 | 1 (1.4) |
| ≥25 to <30 | 21 (30.0) | 23 (32.4) | 17 (24.6) |
| ≥30 to <35 | 30 (42.9) | 35 (49.3) | 35 (50.7) |
| ≥35 to <40 | 14 (20.0) | 10 (14.1) | 15 (21.7) |
| ≥40 | 5 (7.1) | 3 (4.2) | 1 (1.4) |
| Waist circumference, cm | 105.0 (10.4) | 104.2 (10.9) | 105.3 (10.4) |
| Blood pressure, mm Hg | |||
| Systolic | 120 (12) | 119 (12) | 121 (13) |
| Diastolic | 82 (9) | 82 (9) | 83 (8) |
| Pulse rate, beats/min | 77 (10) | 76 (9) | 77 (9) |
| HbA1c, %b | 5.60 (0.35) | 5.57 (0.32) | 5.65 (0.29) |
| Fasting serum glucose, mg/dLc | 91.4 (8.5) | 92.4 (9.3) | 92.7 (7.6) |
| Fasting serum insulin, mU/Lc | 16.2 (8.9) | 18.3 (15.5) | 16.1 (8.0) |
| Lipid parameters, geometric mean (coefficient of variation, %), mg/dLd | |||
| Total cholesterol | 187.0 (18.1) | 184.8 (14.4) | 194.5 (17.6) |
| Triglycerides | 155.1 (53.6) | 141.4 (54.5) | 159.3 (61.8) |
| HDL-C | 41.7 (24.9) | 42.4 (20.1) | 42.2 (21.9) |
| LDL-C | 108.7 (28.1) | 108.8 (25.1) | 112.9 (31.1) |
| VLDL-C | 30.7 (50.4) | 28.1 (52.2) | 31.3 (56.3) |
| Free fatty acids, mEq/L | 0.58 (33.55) | 0.49 (33.59) | 0.59 (39.96) |
| eGFR, mL/min/1.73 m2e | 114.7 (12.3) | 111.1 (13.4) | 112.3 (13.1) |
| Comorbidities, No. (%)f | |||
| Dyslipidemia | 48 (68.6) | 54 (76.1) | 51 (73.9) |
| Hyperuricemia | 42 (60.0) | 38 (53.5) | 39 (56.5) |
| NAFLD (MASLD) | 38 (54.3) | 22 (31.0) | 22 (31.9) |
| Hypertension | 19 (27.1) | 14 (19.7) | 20 (29.0) |
| Osteoarthritis | 7 (10.0) | 4 (5.6) | 4 (5.8) |
| Obstructive sleep apnea | 5 (7.1) | 2 (2.8) | 1 (1.4) |
| Atherosclerotic cardiovascular disease | 5 (7.1) | 1 (1.4) | 0 |
| Gout | 5 (7.1) | 3 (4.2) | 2 (2.9) |
| Asthma or COPD | 4 (5.7) | 0 | 2 (2.9) |
| Polycystic ovary syndromeg | 2/35 (5.7) | 4/35 (11.4) | 1/33 (3.0) |
| Anxiety or depression | 1 (1.4) | 0 | 1 (1.4) |
| Weight-related comorbidities, No. (%) | |||
| 1 | 8 (11.4) | 20 (28.2) | 14 (20.3) |
| 2 | 21 (30.0) | 26 (36.6) | 20 (29.0) |
| 3 | 17 (24.3) | 20 (28.2) | 25 (36.2) |
| 4 | 10 (14.3) | 2 (2.8) | 3 (4.3) |
| ≥5 | 6 (8.6) | 0 | 0 |
| SF-36v2 physical functioning domain scoreh | 55.1 (3.3) | 53.7 (4.3) | 54.0 (4.4) |
| IWQOL-Lite-CT physical function composite scorei | 83.8 (15.6) | 82.7 (15.3) | 80.9 (19.8) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; IWQOL-Lite-CT, Impact of Weight on Quality of Life-Lite Clinical Trials Version; LDL-C, low-density lipoprotein cholesterol; MASLD, metabolic dysfunction-associated steatotic liver disease; NAFLD, nonalcoholic fatty liver disease; SF-36v2, 36-Item Short Form Health Survey version 2 acute form; VLDL-C, very low-density lipoprotein cholesterol.
SI conversion factors: To convert HbA1c to mmol/mol, use the following equation: 10.93 × HbA1c − 23.50; glucose to mmol/L, multiply by 0.0555; insulin to pmol/L, multiply by 6.945; HDL-C, LDL-C, VLDL-C, and total cholesterol to mmol/L, multiply by 0.0259; triglycerides to mmol/L, multiply by 0.0113; and free fatty acids to mmol/L, multiply by 1.0.
Sex was self-reported according to fixed selection categories.
Reference value for HbA1c was <6.5%.
Reference value for fasting serum glucose was 74-106 mg/dL for age 60 years and younger, 83-115 mg/dL for age 60 to 90 years, and 76-121 mg/dL for age 90 years and older; for fasting serum insulin, 3.0-25.0 mU/L.
Reference values for total cholesterol, <199.99 mg/dL; LDL, <129.99 mg/dL; HDL, >40 mg/dL; VLDL-C, 10-50 mg/dL; free fatty acids, 0.28-0.89 mEq/L; and triglycerides, <149.99 mg/dL.
eGFR was calculated with use of the serum creatinine–based Chronic Kidney Disease Epidemiology Collaboration equation. Reference value for eGFR was ≥60 mL/min/1.73 m2.
Baseline medical conditions were assessed through a review of participant’s medical history.
Percentage is based on total number of female participants in the respective treatment group.
SF-36v2 measures health-related quality of life and general health status. SF-36v2 scores are norm-based (ie, scores are transformed to a scale in which the 2009 US general population has a mean score of 50 and SD of 10). An increase in score represents an improvement in health status.
IWQOL-Lite-CT measures weight-specific, health-related quality of life. All items are rated on either a 5-point frequency scale (“never” to “always”) or a 5-point truth scale (“not at all true” to “completely true”). Scores are transformed to a scale of 0 to 100, with higher scores reflecting better levels of functioning.
Co–Primary End Points
For the treatment regimen estimand, the mean percent change in body weight from baseline to week 52 was −13.6% (95% CI, −15.8% to −11.4%) with tirzepatide 10 mg, −17.5% (95% CI, −19.7% to −15.3%) with tirzepatide 15 mg, and −2.3% with placebo (Figure 2A, Table 2; eFigure 3A in Supplement 2). Both tirzepatide doses were superior to placebo, with estimated treatment differences of −11.3% (95% CI, −14.3% to −8.3%) for tirzepatide 10 mg and −15.1% (95% CI, −18.2% to −12.1%) for tirzepatide 15 mg (P < .001 for both; Table 2). For the efficacy estimand, the corresponding change was −14.4% and −19.9% with tirzepatide 10 mg and 15 mg, respectively, vs −2.4% with placebo (10 mg difference, −12.0% [95% CI, −14.8% to −9.3%]; 15 mg difference, −17.5% [95% CI, −20.3% to −14.8%]; P < .001 for both; eTable 1, eFigure 3C in Supplement 2).
Figure 2. Effect of Tirzepatide vs Placebo on Body Weight and Waist Circumference.
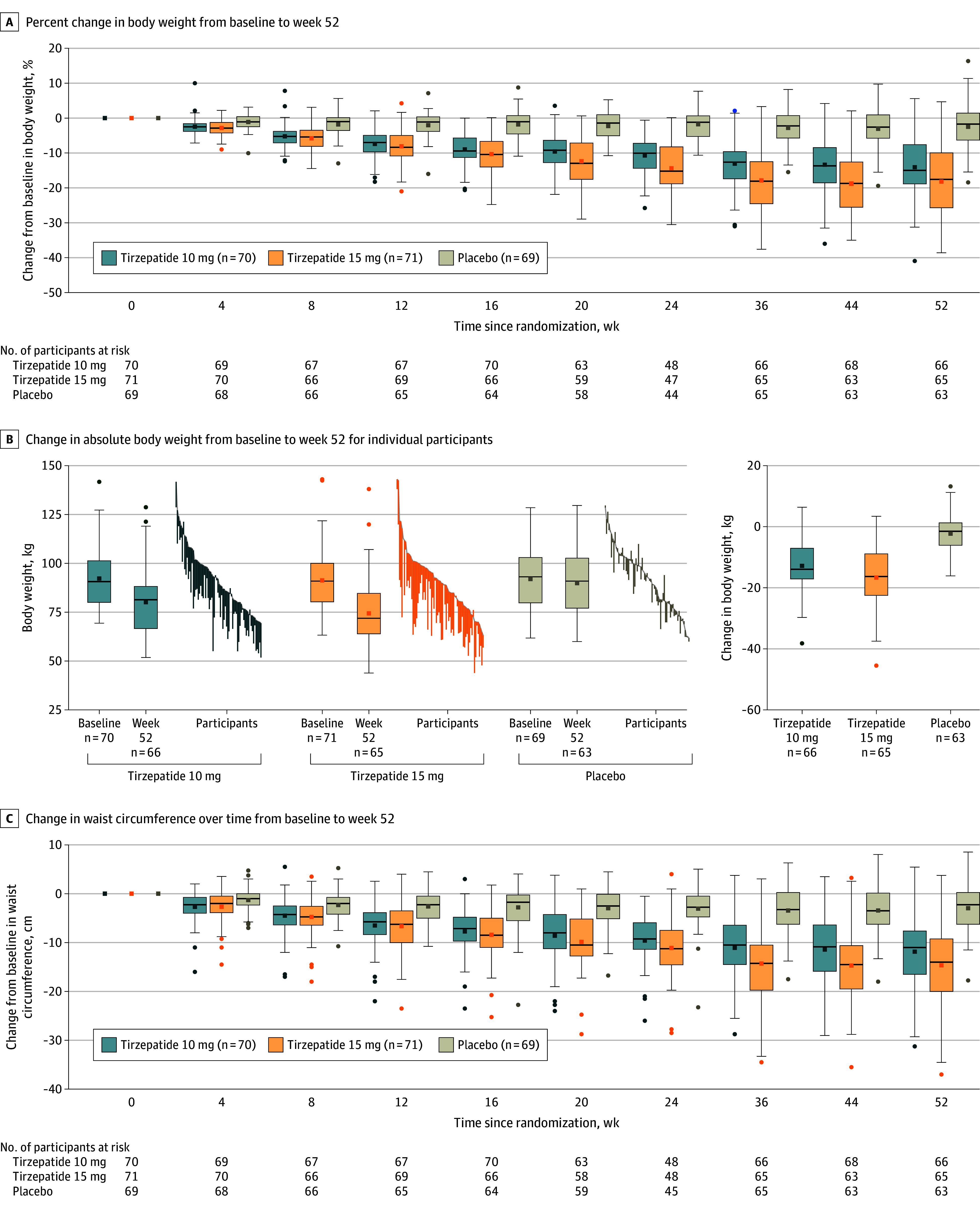
Data are observed (as-measured) changes during treatment (time from randomization to last contact with trial site, irrespective of treatment discontinuation) for each participant in the full analysis set. The lines in each box represent the median; small squares, the mean. Box tops and bottoms represent the IQR; whiskers extend to most extreme observed values with 1.5× the IQR of the nearer quartile; and symbols beyond these points are values outside that range.
A and C, More negative values indicate greater reductions. Numbers in the key represent the full analysis set. B shows participants at baseline (gray lines) and with measurement at 52 weeks. Overall n’s are the same as in panels A and C.
Table 2. Primary and Secondary End Points (Treatment Regimen Estimand)a.
| Efficacy end point | LSM (95% CI) | Tirzepatide 10 mg vs placebo | Tirzepatide 15 mg vs placebo | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Tirzepatide 10 mg (n = 70) | Tirzepatide 15 mg (n = 71) | Placebo (n = 69) | Difference (95% CI)b | OR (95% CI) | P value | Difference (95% CI)b | OR (95% CI) | P value | |
| Co–primary end pointsc | |||||||||
| Percent change in weight from baseline to week 52, % | −13.6 (−15.8 to −11.4) | −17.5 (−19.7 to −15.3) | −2.3 (−4.4 to −0.3) | −11.3 (−14.3 to −8.3) | <.001 | −15.1 (−18.2 to −12.1) | <.001 | ||
| Participants with weight reduction ≥5% at week 52, No. (%)d | 61 (87.7) | 61 (85.8) | 20 (29.3) | 56.8 (45.7 to 67.8) | 15.4 (6.4 to 37.4) | <.001e | 54.8 (43.2 to 66.4) | 13.2 (5.5 to 31.6) | <.001e |
| Key secondary end pointsc | |||||||||
| Body weight, change from baseline to week 20, kg | −8.7 (−9.9 to −7.5) | −10.4 (−11.6 to −9.2) | −2.0 (−3.2 to −0.8) | −6.7 (−8.4 to −5.0) | <.001 | −8.4 (−10.1 to −6.7) | <.001 | ||
| Participants with weight reduction ≥10% at week 52, No. (%)d | 45 (64.1) | 51 (71.9) | 10 (14.5) | 48.2 (36.6 to 59.8) | 9.8 (4.3 to 22.4) | <.001e | 55.9 (44.5 to 67.2) | 13.9 (5.9 to 32.5) | <.001e |
| Participants with weight reduction ≥15% at week 52, No. (%)d | 32 (46.4) | 44 (61.4) | 2 (2.9) | 42.7 (32.4 to 52.9) | 23.1 (6.0 to 88.4) | <.001e | 57.3 (47.2 to 67.4) | 42.2 (11.0 to 162.4) | <.001e |
| Waist circumference, change from baseline to week 52, cm | −11.4 (−13.2 to −9.6) | −14.5 (−16.3 to −12.6) | −2.6 (−4.4 to −0.9) | −8.7 (−11.2 to −6.2) | <.001 | −11.8 (−14.4 to −9.3) | <.001 | ||
Abbreviations: LSM, least-squares mean; OR, odds ratio.
All parameters presented as LSM unless otherwise indicated. Treatment regimen estimand (corresponding analyses used the full analysis set) evaluated treatment effects regardless of treatment adherence. Missing values were imputed using method of multiple imputation guided by hybrid approach. For continuous variables, analysis of covariance model was used and logistic regression was used for categorical end points, including terms of treatment, stratification factors (sex and the presence of weight-related comorbidities at randomization), and body weight at randomization as covariate. See eTable 1 in Supplement 2 for corresponding data for the efficacy estimand and eTable 4 in Supplement 2 for actual values of all continuous variables at baseline and week 52 (and body weight at week 20).
Data are absolute differences between mean changes unless otherwise indicated. The differences between mean percent changes in body weight are expressed in percentage points. The differences between proportions of participants achieving certain criteria are unconditional risk difference from logistic regression model using imputed data with treatment, stratification factors (sex and the presence of weight-related comorbidities at randomization), and body weight at randomization as factors.
Tested for superiority, controlled for type I error.
Actual measurements are No. (%) based on imputed data and treatment difference is OR (95% CI).
Both the P value for absolute difference and OR are < .001.
For the treatment regimen estimand, the percentage of participants achieving weight reduction of at least 5% at week 52 was 87.7% with tirzepatide 10 mg and 85.8% with tirzepatide 15 mg vs 29.3% with placebo (P < .001 for comparisons with placebo) (Table 2; eFigure 4A in Supplement 2). For the efficacy estimand, the percentages were 91.4% and 92.7% with tirzepatide 10 mg and 15 mg, respectively, vs 29.4% with placebo (P < .001 for comparisons with placebo) (eTable 1 and eFigure 4B in Supplement 2).
Key Secondary End Points
A greater proportion of participants receiving tirzepatide 10 mg and 15 mg vs placebo achieved weight reductions of at least 10% and 15% at week 52 (eFigure 4A and eFigure 4B in Supplement 2). For the treatment regimen estimand, the 10% weight reduction threshold was achieved by 64.1% of participants in the 10 mg group and 71.9% of participants in the 15 mg group compared with 14.5% of participants in the placebo group (Table 2). The 15% weight reduction threshold was achieved by 46.4% of participants in the 10 mg group and 61.4% of participants in the 15 mg group compared with 2.9% of participants in the placebo group (Table 2).
The mean change in body weight from baseline to week 20 was −8.7 kg with tirzepatide 10 mg and −10.4 kg with tirzepatide 15 mg vs −2.0 kg with placebo for the treatment regimen estimand (see Figure 2B for absolute body weight change for individual participants). Tirzepatide was superior to placebo, with estimated treatment differences of −6.7 kg (95% CI, −8.4 to −5.0) for tirzepatide 10 mg and −8.4 kg (95% CI, −10.1 to −6.7) for tirzepatide 15 mg (P < .001 for both; Table 2).
The change in waist circumference from baseline to week 52 was −11.4 cm with tirzepatide 10 mg and −14.5 cm with tirzepatide 15 mg, compared with −2.6 cm with placebo for the treatment regimen estimand (10 mg: difference from placebo, −8.7 cm [95% CI, −11.2 to −6.2]; 15 mg: difference from placebo, −11.8 cm [95% CI, −14.4 to −9.3]; P < .001 for both; Figure 2C; Table 2; eFigure 3B in Supplement 2).
Consistent results were observed in key secondary end points when using the efficacy estimand (eFigure 3D and eTable 1 in Supplement 2).
Additional Secondary End Points
Improvements with tirzepatide 10 mg and 15 mg were significantly greater compared with placebo in body weight reduction (−12.3 kg and −16.1 kg vs −2.1 kg; P < .001), BMI (−4.4 and −5.7 vs −0.7; P < .001), and other cardiometabolic factors (including fasting glucose, insulin, very low-density lipoprotein cholesterol, triglycerides, and systolic and diastolic blood pressure), from baseline to week 52 for the treatment regimen estimand (eTable 2 in Supplement 2). Both the SF-36v2 physical functioning domain score and IWQOL-Lite-CT physical function composite score increased more with tirzepatide 10 mg and 15 mg compared with placebo from baseline to week 52. Results from the efficacy estimand were consistent with the treatment regimen estimand, showing greater improvements with tirzepatide treatment compared with placebo for all additional secondary end points (eTable 3 in Supplement 2). Additional efficacy data for the treatment regimen estimand and the efficacy estimand are presented in eTables 1-3 and eFigures 5-8 in Supplement 2.
Adverse Events and Tolerability
Treatment-emergent adverse events were reported by 95.7% of participants in the tirzepatide 10 mg group, 90.1% in the tirzepatide 15 mg group, and 82.6% with placebo (Table 3). The most frequently reported adverse events with tirzepatide were gastrointestinal (diarrhea, nausea, and vomiting). Most gastrointestinal events were mild to moderate in severity and occurred primarily during the dose escalation period (eFigure 9 in Supplement 2).
Table 3. Adverse Events During the Treatment and Safety Follow-Up Period (Safety Analysis Set).
| Adverse events | No. (%) | ||
|---|---|---|---|
| Tirzepatide 10 mg (n = 70) | Tirzepatide 15 mg (n = 71) | Placebo (n = 69) | |
| Participants with ≥1 treatment-emergent adverse eventa | 67 (95.7) | 64 (90.1) | 57 (82.6) |
| Serious adverse eventsb | 3 (4.3) | 8 (11.3) | 6 (8.7) |
| Death | 0 | 0 | 0 |
| Adverse events leading to treatment discontinuationc | 2 (2.9) | 5 (7.0) | 1 (1.4) |
| Gastrointestinal disorders | 2 (2.9) | 3 (4.2) | 0 |
| Nausea | 1 (1.4) | 1 (1.4) | 0 |
| Diarrhea | 1 (1.4) | 0 | 0 |
| Abdominal pain | 0 | 1 (1.4) | 0 |
| Vomiting | 0 | 1 (1.4) | 0 |
| Treatment-emergent adverse events occurring in ≥10% of participants in any treatment groupc | |||
| Diarrhea | 28 (40.0) | 29 (40.8) | 6 (8.7) |
| Nausea | 21 (30.0) | 23 (32.4) | 4 (5.8) |
| Decreased appetite | 19 (27.1) | 20 (28.2) | 8 (11.6) |
| Upper respiratory tract infection | 16 (22.9) | 17 (23.9) | 12 (17.4) |
| Abdominal distension | 13 (18.6) | 9 (12.7) | 1 (1.4) |
| Vomiting | 8 (11.4) | 14 (19.7) | 3 (4.3) |
| Gastroenteritis | 8 (11.4) | 2 (2.8) | 0 |
| Hyperuricemia | 3 (4.3) | 5 (7.0) | 8 (11.6) |
| Adverse events of special interest | |||
| Hepatic events | 7 (10.0) | 2 (2.8) | 11 (15.9) |
| Hypersensitivityd | 4 (5.7) | 11 (15.5) | 7 (10.1) |
| Cardiac disorders | 3 (4.3) | 4 (5.6) | 1 (1.4) |
| Severe or serious gastrointestinal events | 2 (2.9) | 4 (5.6) | 1 (1.4) |
| Gallbladder disease | 2 (2.9) | 1 (1.4) | 1 (1.4) |
| Malignancies | 0 | 1 (1.4) | 0 |
| Adjudicated pancreatitis | 0 | 0 | 0 |
| Adjudicated major adverse cardiovascular events | 0 | 0 | 0 |
| Kidney events | 0 | 1 (1.4) | 0 |
| Major depressive disorder or suicidal ideation | 0 | 0 | 0 |
| Hypoglycemia (blood glucose <54 mg/dL) | 0 | 0 | 1 (1.4) |
| Other adverse events of interestc | |||
| Cholelithiasis | 2 (2.9) | 1 (1.4) | 1 (1.4) |
| Acute cholecystitis | 0 | 1 (1.4) | 0 |
| Chronic cholecystitis | 0 | 1 (1.4) | 0 |
| Cholecystectomy | 0 | 1 (1.4) | 0 |
A treatment-emergent adverse event was defined as an adverse medical occurrence that emerged during the defined treatment period, having been absent before treatment, or worsening relative to the pretreatment state and not necessarily having a causal relationship with this treatment.
A serious adverse event was defined as any adverse event that resulted in death, was life-threatening, required inpatient hospitalization or prolongation of existing hospitalization, resulted in persistent disability or incapacity, was a congenital anomaly or birth defect, or a medical event that may not be immediately life threatening or result in death or hospitalization but may jeopardize the participant or require medical or surgical intervention to prevent 1 of the other outcomes previously listed.
Adverse events defined as system organ class (gastrointestinal disorders) or preferred term according to the Medical Dictionary for Regulatory Activities, version 25.1.
Hypersensitivity included immediate (≤24 hours after administration of tirzepatide or placebo) and nonimmediate (>24 hours after administration of tirzepatide or placebo) hypersensitivity events.
Serious adverse events were reported by 3 participants (4.3%) treated with tirzepatide 10 mg, 8 (11.3%) with tirzepatide 15 mg, and 6 (8.7%) with placebo (Table 3). No deaths were reported during the study. Papillary thyroid cancer occurred in 1 (1.4%) participant with a history of thyroid nodules who received tirzepatide 15 mg, which was considered unrelated to study treatment by the investigator. There were no cases of medullary thyroid cancer, adjudicated pancreatitis, or major adverse cardiovascular events.
Acute cholecystitis occurred in 1 participant (1.4%) treated with tirzepatide 15 mg. No participants reported serious or severe treatment-emergent hepatic events (eTable 6 in Supplement 2). Additional safety measures are presented in Table 3 and eTable 5 in Supplement 2.
Discussion
This study demonstrated that once-weekly tirzepatide 10 mg and 15 mg was superior to placebo for weight reduction in Chinese adults with a BMI greater than or equal to 28 or greater than or equal to 24 with at least 1 weight-related comorbidity, excluding diabetes. In the present study, a clinically meaningful reduction of up to 17.5% in body weight from baseline to week 52 was observed with tirzepatide, with 85.8% to 87.7% of participants achieving weight reduction of at least 5%. These results are consistent with findings in previous SURMOUNT trials, conducted in predominantly White populations, with tirzepatide 10 mg and 15 mg in participants with a BMI of 30 or more or 27 or more with at least 1 weight-related comorbidity, excluding diabetes.9,10,11,13
A weight reduction of 5% or more has long been considered clinically meaningful. Studies have demonstrated that achieving weight reduction of at least 10% or 15% can yield additional clinical benefits and may serve as therapeutic goals for improving certain weight-related comorbidities.14 In the present study, participants treated with tirzepatide 10 mg and 15 mg were significantly more likely to achieve these 2 higher weight reduction thresholds compared to participants who received placebo.
Well-designed clinical trials investigating antiobesity medications have rarely been reported in the Chinese population. In 2 studies, participants with obesity or overweight who were treated with orlistat for 24 weeks experienced approximately 5.0 kg of weight reduction.15,16 In a 44-week study, a mean weight reduction of 12.1%, with 85.3% of 238 participants achieving at least 5% reduction in body weight, was seen with semaglutide 2.4 mg in predominantly Chinese adults with a BMI of 30 or greater or 27 or greater with at least 1 weight-related comorbidity.17 The present study provides evidence suggesting that tirzepatide could be a promising therapeutic option for addressing obesity and overweight in Chinese individuals.
For participants receiving tirzepatide 15 mg in this study, mean body weight decreased progressively from baseline to week 44 and did not significantly decrease further. However, in the SURMOUNT-1 study, body weight continued to decrease through week 72.9 The mean percent weight reduction in this study was less for the 10-mg and 15-mg doses than in the SURMOUNT-1 study. The explanation for these differences remains unknown, though sex differences or degree of baseline obesity could contribute to variations in weight reduction outcomes. The proportion of females differed between this study and the SURMOUNT-1 study; 49.0% of participants in this study were female, compared with 67.5% in SURMOUNT-1.9 The mean baseline BMI (32.3) in this study was lower than that in the SURMOUNT-1 study (38.0).9
Notably, waist circumference progressively decreased from baseline until week 52 with both doses of tirzepatide, suggesting a potential sustained improvement in adipose distribution. Further research may provide more insights into the effect of tirzepatide on visceral adipose distribution. Treatment with tirzepatide not only led to decreased body weight and reduced waist circumference, but it was also associated with improvements in other cardiometabolic factors, including blood pressure, fasting glucose, insulin, and lipid profiles. The effects of tirzepatide on these cardiometabolic factors may hold even greater clinical significance in Chinese participants, given the population’s heightened risk of cardiometabolic complications associated with obesity.18
Overall, the safety profile of tirzepatide in this study was consistent with that previously reported in SURMOUNT and SURPASS trials, as well as other studies involving glucagon-like peptide-1 receptor agonists.9,10,11,12,19,20,21 Gastrointestinal events, occurring primarily during the dose escalation period, were the most common adverse events observed with tirzepatide. Most of these events were mild to moderate in severity and few participants (<5%) discontinued from treatment due to adverse events, which is in line with findings from the global SURMOUNT-1 study.9
The present study has several strengths. This is the first study to assess tirzepatide for weight management in Chinese participants, who account for the largest number of individuals living with obesity and overweight worldwide.1 This study is 1 of the few phase 3 weight management studies that applied Chinese population–specific BMI criteria,1 which allowed for more representation of individuals with obesity and overweight. In addition, this study enrolled a balanced proportion of both males and females.
Limitations
This study has several limitations. First, this study utilized waist circumference as a surrogate parameter to assess visceral adiposity. A more precise evaluation could be achieved by directly measuring visceral fat area using imaging techniques and may provide more insights into the effect of tirzepatide on visceral fat distribution. Second, the treatment period of 52 weeks is relatively short. Third, the number of participants with BMI greater than or equal to 24 to less than 28 was low. Fourth, these findings may not be directly generalizable to other populations outside of China.
Conclusions
In Chinese adults with obesity or overweight, once-weekly treatment with tirzepatide resulted in statistically significant and clinically meaningful weight reduction with an acceptable safety profile.
Trial Protocol
eAppendix 1. Eligibility Criteria
eAppendix 2. Additional statistical analysis methods
eTable 1. Primary and Secondary End Points (Efficacy Estimand)
eTable 2. Additional Secondary End Points (Treatment Regimen Estimand)
eTable 3. Additional Secondary End Points (Efficacy Estimand)
eTable 4. Actual Value of Efficacy End Points
eTable 5. Additional safety measures (Safety Analysis Set)
eTable 6. Serious adverse events by decreasing frequency (Safety Analysis Set)
eFigure 1. Study design
eFigure 2. Cumulative distribution plot of the percent change in body weight from baseline to week 52 (Efficacy Estimand)
eFigure 3. Effect of tirzepatide versus placebo on body weight and waist circumference
eFigure 4. Effect of tirzepatide versus placebo on the proportion of participants achieving weight reduction thresholds
eFigure 5. Bodyweight from baseline to week 52 (Efficacy Estimand)
eFigure 6. Body mass index over time from baseline to week 52 (Efficacy Estimand)
eFigure 7. Changes in blood pressure over time from baseline to week 52 (Safety Analysis Set)
eFigure 8. Percent change in lipid parameters by pooled analysis of tirzepatide groups from baseline to week 52 (Efficacy Estimand)
eFigure 9. Incidence of nausea, vomiting, and diarrhea over time (Safety Analysis Set)
Data Sharing Statement
References
- 1.Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021;9(6):373-392. doi: 10.1016/S2213-8587(21)00045-0 [DOI] [PubMed] [Google Scholar]
- 2.Koliaki C, Liatis S, Kokkinos A. Obesity and cardiovascular disease: revisiting an old relationship. Metabolism. 2019;92:98-107. doi: 10.1016/j.metabol.2018.10.011 [DOI] [PubMed] [Google Scholar]
- 3.Sun X, Yan AF, Shi Z, et al. Health consequences of obesity and projected future obesity health burden in China. Obesity (Silver Spring). 2022;30(9):1724-1751. doi: 10.1002/oby.23472 [DOI] [PubMed] [Google Scholar]
- 4.Zhou BF; Cooperative Meta-Analysis Group of the Working Group on Obesity in China . Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults—study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15(1):83-96. [PubMed] [Google Scholar]
- 5.He W, Li Q, Yang M, et al. Lower BMI cutoffs to define overweight and obesity in China. Obesity (Silver Spring). 2015;23(3):684-691. doi: 10.1002/oby.20995 [DOI] [PubMed] [Google Scholar]
- 6.Tan K; WHO Expert Consultation . Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157-163. doi: 10.1016/S0140-6736(03)15268-3 [DOI] [PubMed] [Google Scholar]
- 7.Chinese Society of Health Management, Chinese Nutrition Society, Reproductive Medicine Branch of China International Exchange and Promotion Association for Medicine and Healthcare, China Health Promotion Foundation, Zhejiang Provincial Clinical Nutrition Center . Expert consensus & standard on weight management for overweight or obese people. Article in Chinese. Zhonghua Jiankang Guanlixue Zazhi. 2018;12:200-207. [Google Scholar]
- 8.Coskun T, Sloop KW, Loghin C, et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: from discovery to clinical proof of concept. Mol Metab. 2018;18:3-14. doi: 10.1016/j.molmet.2018.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jastreboff AM, Aronne LJ, Ahmad NN, et al. ; SURMOUNT-1 Investigators . Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205-216. doi: 10.1056/NEJMoa2206038 [DOI] [PubMed] [Google Scholar]
- 10.Wadden TA, Chao AM, Machineni S, et al. Tirzepatide after intensive lifestyle intervention in adults with overweight or obesity: the SURMOUNT-3 phase 3 trial. Nat Med. 2023;29(11):2909-2918. doi: 10.1038/s41591-023-02597-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aronne LJ, Sattar N, Horn DB, et al. ; SURMOUNT-4 Investigators . Continued treatment with tirzepatide for maintenance of weight reduction in adults with obesity: the SURMOUNT-4 randomized clinical trial. JAMA. 2024;331(1):38-48. doi: 10.1001/jama.2023.24945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garvey WT, Frias JP, Jastreboff AM, et al. ; SURMOUNT-2 investigators . Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2023;402(10402):613-626. doi: 10.1016/S0140-6736(23)01200-X [DOI] [PubMed] [Google Scholar]
- 13.le Roux CW, Zhang S, Aronne LJ, et al. Tirzepatide for the treatment of obesity: rationale and design of the SURMOUNT clinical development program. Obesity (Silver Spring). 2023;31(1):96-110. doi: 10.1002/oby.23612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan DH, Yockey SR. Weight loss and improvement in comorbidity: differences at 5%, 10%, 15%, and over. Curr Obes Rep. 2017;6(2):187-194. doi: 10.1007/s13679-017-0262-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi YF, Pan CY, Hill J, Gao Y. Orlistat in the treatment of overweight or obese Chinese patients with newly diagnosed type 2 diabetes. Diabet Med. 2005;22(12):1737-1743. doi: 10.1111/j.1464-5491.2005.01723.x [DOI] [PubMed] [Google Scholar]
- 16.Zhu HJ, Pan H, Gong FY, et al. A comparison of the efficacy and safety of domestic orlistat and imported orlistat in Chinese overweight and obese patients. Article in Chinese. Zhonghua Nei Ke Za Zhi. 2009;48(10):825-829. [PubMed] [Google Scholar]
- 17.Mu Y, Bao X, Eliaschewitz FG, et al. ; STEP 7 Study Group . Efficacy and safety of once weekly semaglutide 2·4 mg for weight management in a predominantly east Asian population with overweight or obesity (STEP 7): a double-blind, multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2024;12(3):184-195. doi: 10.1016/S2213-8587(23)00388-1 [DOI] [PubMed] [Google Scholar]
- 18.Adair LS, Gordon-Larsen P, Du SF, Zhang B, Popkin BM. The emergence of cardiometabolic disease risk in Chinese children and adults: consequences of changes in diet, physical activity and obesity. Obes Rev. 2014;15(0 1)(suppl 1):49-59. doi: 10.1111/obr.12123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frías JP. An update on tirzepatide for the management of type 2 diabetes: a focus on the phase 3 clinical development program. Expert Rev Endocrinol Metab. 2023;18(2):111-130. doi: 10.1080/17446651.2023.2184796 [DOI] [PubMed] [Google Scholar]
- 20.Gao L, Lee BW, Chawla M, et al. Tirzepatide versus insulin glargine as second-line or third-line therapy in type 2 diabetes in the Asia-Pacific region: the SURPASS-AP-Combo trial. Nat Med. 2023;29(6):1500-1510. doi: 10.1038/s41591-023-02344-1 [DOI] [PubMed] [Google Scholar]
- 21.Bettge K, Kahle M, Abd El Aziz MS, Meier JJ, Nauck MA. Occurrence of nausea, vomiting and diarrhoea reported as adverse events in clinical trials studying glucagon-like peptide-1 receptor agonists: a systematic analysis of published clinical trials. Diabetes Obes Metab. 2017;19(3):336-347. doi: 10.1111/dom.12824 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix 1. Eligibility Criteria
eAppendix 2. Additional statistical analysis methods
eTable 1. Primary and Secondary End Points (Efficacy Estimand)
eTable 2. Additional Secondary End Points (Treatment Regimen Estimand)
eTable 3. Additional Secondary End Points (Efficacy Estimand)
eTable 4. Actual Value of Efficacy End Points
eTable 5. Additional safety measures (Safety Analysis Set)
eTable 6. Serious adverse events by decreasing frequency (Safety Analysis Set)
eFigure 1. Study design
eFigure 2. Cumulative distribution plot of the percent change in body weight from baseline to week 52 (Efficacy Estimand)
eFigure 3. Effect of tirzepatide versus placebo on body weight and waist circumference
eFigure 4. Effect of tirzepatide versus placebo on the proportion of participants achieving weight reduction thresholds
eFigure 5. Bodyweight from baseline to week 52 (Efficacy Estimand)
eFigure 6. Body mass index over time from baseline to week 52 (Efficacy Estimand)
eFigure 7. Changes in blood pressure over time from baseline to week 52 (Safety Analysis Set)
eFigure 8. Percent change in lipid parameters by pooled analysis of tirzepatide groups from baseline to week 52 (Efficacy Estimand)
eFigure 9. Incidence of nausea, vomiting, and diarrhea over time (Safety Analysis Set)
Data Sharing Statement


