SUMMARY.
Wild birds, particularly Anseriformes and Charadriiformes, are considered the natural reservoir of low pathogenic avian influenza (LPAI) viruses. The high prevalence and subtype diversity of avian influenza viruses at premigrational staging areas provide the perfect opportunity for multiple exposures to different LPAI virus subtypes. Natural consecutive and concurrent infections of sentinel ducks with different LPAI virus subtypes have been reported. The protective immune response from different LPAI virus infections is not understood nor is the effect of such repeated exposures. This study experimentally evaluated the effect of a prior exposure to a LPAI virus on the outcome of a heterosubtypic LPAI virus infection in mallards (Anas platyrhynchos). The results of this investigation suggest that recent prior exposure to a LPAI virus may affect the outcome of a subsequent heterosubtypic LPAI infection in mallards by reducing the duration of cloacal and oropharyngeal viral shedding as well as the viral load excreted via the cloaca. Wild mallards are likely exposed to multiple subtypes of LPAI virus during the periods of peak viral circulation, and the results of this study suggest that the duration of viral shedding in subsequent exposures might be reduced.
Keywords: Anas platyrhynchos, avian influenza, heterosubtypic infection, LPAI, mallard, prior exposure
RESUMEN.
Efecto de una exposición previa al virus de influenza aviar de baja patogenicidad en el resultado de una infección con un subtipo heterólogo del virus de la influenza aviar de baja patogenicidad en patos silvestres (Anas platyrhynchos).
Las aves silvestres, en particular las Anseriformes y Charadriiformes, son consideradas los reservorios naturales de la influenza aviar de baja patogenicidad. La alta prevalencia y la diversidad de los subtipos del virus de la influenza aviar en las zonas de estancia premigratoria proporcionan las condiciones perfectas para que se desarrollen exposiciones múltiples con diferentes subtipos del virus de la influenza aviar de baja patogenicidad. Se han reportado en patos centinelas, infecciones naturales consecutivas y simultáneas con diferentes subtipos de virus de influenza baja patogenicidad. No se conoce completamente la respuesta inmune protectora contra infecciones por diferentes virus de baja patogenicidad, ni tampoco el efecto de tales exposiciones repetidas. Este estudio experimental evaluó el efecto de una exposición previa a un virus de baja patogenicidad sobre el resultado de una infección en patos de collar (Anas platyrhynchos) con un virus de la influenza aviar de baja patogenicidad de subtipo heterólogo. Los resultados de esta investigación sugieren que la exposición previa y reciente a un virus de baja patogenicidad puede afectar el resultado de una infección posterior con un subtipo heterólogo del virus de la influenza de baja patogenicidad en patos de collar, mediante la reducción en la duración de la eliminación viral por las vías cloacal y orofaríngea así como en la carga viral excretada a través de la cloaca. Probablemente, los patos de collar silvestres están expuestos a múltiples subtipos del virus de baja patogenicidad durante los períodos de mayor circulación viral y los resultados de este estudio sugieren que la duración de la excreción del virus en las exposiciones posteriores podría reducirse.
Wild birds, particularly Anseriformes and Charadiiformes, are known to be the natural reservoirs of low pathogenic avian influenza (LPAI) viruses. Within Anseriformes, the prevalence of natural infections is particularly high in members of the subfamily Anatinae (dabbling and diving ducks), and the vast majority of the isolations, globally, have been from mallards (Anas platyrhynchos) (17). In mallards, LPAI viruses replicate in epithelial cells lining the intestinal tract (8) and are excreted in high concentration in feces (4,25). Viral transmission between waterfowl is dependent on a fecal/oral route (24). The prevalence of LPAI in wild duck populations in North America peaks in late summer/early fall, when susceptible hatch-year birds and adult birds congregate at staging areas prior to south migration (5). Avian influenza (AI) virus subtypes do not circulate equally among wild bird populations, and variation can occur between host species, geographic location, and years (17). Among North American ducks, H3, H4, and H6 are the most common hemagglutinin (HA) subtypes isolated (3,9,15,19), while the H5, H7, H8, and H9 subtypes are generally isolated at a lower rate (9,18).
The high prevalence and subtype diversity of AI viruses circulating in ducks at staging areas provides the perfect opportunity for multiple exposures to different LPAI virus subtypes. Natural consecutive infections of sentinel ducks with different LPAI virus subtypes have been reported, providing evidence that a prior exposure does not fully protect against a subsequent AI virus infection (16,20). Few studies have experimentally addressed the outcome of concurrent or subsequent LPAI infections in its natural waterfowl host. Viral replication and shedding was suppressed in Pekin ducks after a repeated exposure to the same LPAI isolate (8). In this study, the reduced viral shedding was associated with a secondary immune response if the challenge occurs 46 days after the primary inoculation or later (8). Another recent study concluded that infection by a LPAI virus in mallards was limited by prior infection with a homosubtypic (HA homologous) LPAI strain, and it may be prevented by prior infection with a heterosubtypic (HA heterologous) LPAI strain (7). The cocirculation of many AI virus subtypes and the possibility of concurrent and/or subsequent infection of wild ducks with different subtypes are a testament to the complex natural history of AI (1).
The goal of this study was to experimentally evaluate the viral shedding and antibody response associated with LPAI virus challenge in mallards pre-exposed to a heterosubtypic LPAI virus. Mallards were chosen as the model species for this trial because of the species’ worldwide distribution and importance as a LPAI virus reservoir (9,23).
MATERIALS AND METHODS
Viruses.
The LPAI viruses A/mallard/MN/355779/00 (H5N2) and A/mallard/MN/199106/99 (H3N8) were used in this study. The viruses were originally isolated in specific-pathogen-free (SPF) embryonating chicken eggs (ECEs) from cloacal swab material collected from wild mallards. Viral stocks were propagated by second passage in 9-to-11-day-old SPF ECEs and titrated using previously described techniques (13,26). Infective allantoic fluid was diluted in sterile brain-heart-infusion (BHI) medium to yield 106 median egg infectious dose (EID50) per 0.1 ml (single-bird inoculum). Back-titers determined immediately after inoculation of the birds varied from 105.36 to 106.27 EID50/0.1 ml. A sham inoculum was prepared using uninfected sterile BHI medium.
Animals.
One-day-old mallards were purchased from a commercial source (Murray McMurray Hatchery, Webster City, IA) and raised under confined conditions until they were 16 weeks old. Both males and females were included in approximately equal numbers and equally distributed between groups. Birds were housed in groups of five in self-contained isolation units ventilated under negative pressure with high-efficiency particulate air filters. Food and water were provided ad libitum. General animal care was provided under an animal use protocol approved by the Institutional Animal Care and Use Committee at the University of Georgia, Athens, GA.
Experimental design.
Twenty-five mallards were evenly divided in four virus-exposed and one negative control groups. Each bird in the negative control group was inoculated intrachoanally with 0.1 ml of sham inoculum. The birds in the two heterosubtypic challenge groups were inoculated sequentially with both of the two LPAI strains, allowing for a 21-day interval between inoculations (consecutive infection groups). Therefore, one group was first inoculated with A/mallard/MN/355779/00 (H5N2) (primary inoculation) at 0 days post–primary inoculation (dpi) and challenged with A/mallard/MN/199106/99 (H3N8) (heterosubtypic challenge) at 21 dpi (H5N2×H3N8 group); the other group was initially inoculated with A/mallard/MN/199106/99 (H3N8) at 0 dpi and challenged with A/mallard/MN/355779/00 (H5N2) at 21 dpi (H3N8×H5N2 group).
Birds in the two single LPAI virus challenge groups (single-infection groups) were inoculated with only one of the two LPAI viruses used in this study, either A/mallard/MN/355779/00 (H5N2) (H5N2 group) or A/mallard/MN/199106/99 (H3N8) (H3N8 group). These groups served as positive controls. To allow data comparison and ensure that all the treatment groups were at the same age, the birds in the single-infection groups were inoculated on the same date that the consecutive infection groups were challenged with the heterosubtypic LPAI virus; therefore, the trials for the two single-infection groups started 21 days after the trials for the consecutive infection groups.
All birds were evaluated twice daily for behavioral changes and clinical signs. Oropharyngeal (OP) and cloacal swabs were collected on 0, 1, 2, 4, 7, 9, 11, 14, 16, and 21 dpi, and at the same interval of days post–heterosubtypic challenge (dpc). Blood samples were collected on 0, 14, and 21 dpi and on 14 and 21 dpc. At 21 dpc, all birds were humanely euthanatized by CO2 inhalation, and full necropsies were performed. Experimental infections were performed in a BSL-Ag2+ facility at the Poultry Diagnostic and Research Center, College of Veterinary Medicine, University of Georgia, Athens, GA.
Virus isolation.
Cloacal and OP swabs were collected in vials containing 2 ml of sterile BHI medium with antimicrobial drugs (100 μg/ml gentamicin, 100 units/ml penicillin, and 5 μg/ml amphotericin B) and were stored at −70 C until further testing. Virus isolations were performed in 9-to-11-day-old SPF ECEs using standard protocols (21). The mean duration of viral shedding was calculated based on the last day a swab was positive on virus isolation. Positive cloacal swabs samples collected on 2 dpc were also titrated in 9-to-11-day-old SPF ECEs to determine the EID50/ml (13). The titrations of samples collected on 2 dpc were performed after two freeze-thaw cycles.
Subtyping.
Cloacal samples from the consecutive infection groups collected at 2 dpc were further tested for the H3 and H5 subtypes. RNA was extracted from cloacal swabs by using the QIAamp® Viral RNA Mini Kit (Qiagen, Valencia, CA) and tested for AI virus nucleic acid by standard one-step RT-PCR targeted to the influenza A matrix gene. The RT-PCR mixture for each reaction contained 1× Green GoTaq™ Buffer (Promega, Madison, WI), 2.5 mM MgCl2, 0.25 mM dNTPs, 200 μM of forward primer, 200 μM of reverse primer, 2.5 units of AMV Reverse Transcripase (Promega), 1.25 units Taq DNA Polymerase (Promega), and nuclease-free water to a final volume of 50 μl. The H3 and H5 primers utilized in the reaction have been previously described (22). PCR amplification was performed as follows: reverse transcription for 30 min at 42 C; denaturation for 2 min at 94 C; followed by 39 cycles of PCR amplification, with each cycle consisting of 40 sec of denaturation at 94 C, 60 sec of annealing at 50 C, and 60 sec of elongation at 72 C; and one final cycle of elongation for 10 min at 72 C. The amplified PCR products were analyzed on a 2% agarose gel electrophoresis.
Serologic assay.
Blood samples were collected from the right jugular vein, and serum samples were stored at −20 C until they were tested. Serologic testing was performed on all samples via the hemagglutination inhibition (HI) test by using standard procedures (11). Reference antigens were prepared using A/mallard/MN/355779/00 (H5N2) and A/mallard/MN/199106/99 (H3N8) LPAI viruses. Samples with HI titer ≥ 8 were considered positive.
Microscopic analyses.
Samples of cerebrum, cerebellum, heart, lung, trachea, liver, spleen, esophagus, proventriculus, ventriculus, small intestine, large intestine, pancreas, adrenal gland, ovaries/testis, kidney, bursa, pectoral muscle, nasal turbinates, and sinus were collected from all birds during necropsy and fixed in 10% neutral buffered formalin. After fixation, tissues were processed and embedded in paraffin, and 5 mm sections were stained with hematoxylin and eosin using standard histopathology protocols. Nasal turbinates were decalcified with Kristensen’s decalcifying solution (10) before being processed for microscopic examination as described above for other tissues.
RESULTS
Behavioral changes or clinical signs were not observed in any of the birds during the entire length of the trial. Neither seroconversion nor virus isolation were detected in any of the birds in the negative control group.
Antibodies against AI viruses were detected in all the birds in all four virus-exposed groups at 14 days after inoculation (Table 1). Overall, the HI titers against the A/mallard/MN/199106/99 (H3N8) ranged from 8 to 16 (mean 10). All the birds in the H3N8×H5N3 and the H3N8 groups seroconverted after the primary H3N8 exposure, but only 4/5 birds in the H5N2×H3N8 group developed H3 antibodies after being challenged with H3N8 virus (Table 1). In addition, a loss of antibodies against H3 after the heterosubtypic challenge was observed in both consecutive infection groups, as only 2/5 birds at the H5N2×H3N8 group (birds 1 and 5) and 3/5 birds at the H3N8×H5N2 group (birds 6, 8, and 9) had detectable HI titers against H3 at 21 dpc. Antibody titers against the A/mallard/MN/355779/00 (H5N2) ranged from 8 to 128 (mean 25) and persisted longer than antibodies against the H3. For example, all the birds in the H5N2×H3N8 group had detectable levels of antibodies until 42 days after primary inoculation (21 dpc), when the trial was terminated.
Table 1.
Serological status, as determined by hemagglutination inhibition, of mallards at 14 and 21 dpi and at 14 and 21 dpc with low pathogenicity avian influenza viruses.A
| Hemagglutination inhibition resultC |
||||||||
|---|---|---|---|---|---|---|---|---|
| GroupB and bird ID | 14 dpi |
21 dpi |
14 dpc |
21 dpc |
||||
| H5N2 | H3N8 | H5N2 | H3N8 | H5N2 | H3N8 | H5N2 | H3N8 | |
|
| ||||||||
| H5N2×H3N8 | ||||||||
| 1 | 128 | — | 64 | — | 32 | 16 | 16 | 8 |
| 2 | 32 | — | 32 | — | 16 | — | 8 | — |
| 3 | 16 | — | 16 | — | 16 | 8 | 16 | — |
| 4 | 16 | — | 16 | — | 8 | 8 | 8 | — |
| 5 | 32 | — | 32 | — | 16 | 8 | 32 | 8 |
| H3N8×H5N2 | ||||||||
| 6 | — | 16 | — | 8 | 16 | 16 | 16 | 8 |
| 7 | — | 8 | — | 8 | 16 | 8 | 16 | — |
| 8 | — | 16 | — | 16 | 16 | 8 | 32 | 8 |
| 9 | — | 16 | — | 16 | 8 | 16 | 8 | 16 |
| 10 | — | 8 | — | 8 | 16 | 8 | 16 | — |
| H5N2 control | ||||||||
| 11 | NA | NA | — | — | 16 | — | 16 | — |
| 12 | NA | NA | — | — | 32 | — | 16 | — |
| 13 | NA | NA | — | — | 32 | — | 16 | — |
| 14 | NA | NA | — | — | 32 | — | 32 | — |
| 15 | NA | NA | — | — | 32 | — | 64 | — |
| H3N8 control | ||||||||
| 16 | NA | NA | — | — | — | 16 | — | 16 |
| 17 | NA | NA | — | — | — | 16 | — | 16 |
| 18 | NA | NA | — | — | — | 16 | — | 16 |
| 19 | NA | NA | — | — | — | 16 | — | 16 |
| 20 | NA | NA | — | — | — | 16 | — | 16 |
Birds were inoculated via choanal cleft with a dose of 106 EID50 of either A/mallard/MN/355779/00 (H5N2) and/or A/mallard/MN/199106/99 (H3N8). Serologic data of the negative control group were omitted.
H5N2×H3N8 group: exposed to H5N2 at 0 dpi, and subsequently challenged with H3N8 at 21 dpi; H3N8×H5N2: exposed to H3N8 at 0 dpi, and subsequently challenged with H5N2 at 21 dpi; H5N2 control group: exposed to H5N2 only; H3N8 control group: exposed to H3N8 only.
Hemagglutination inhibition test using antigen against A/mallard/MN/355779/00 (H5N2) or A/mallard/MN/199106/99 (H3N8). Samples with HI titer ≥ 8 were considered positive. NA = nonapplicable; − = negative.
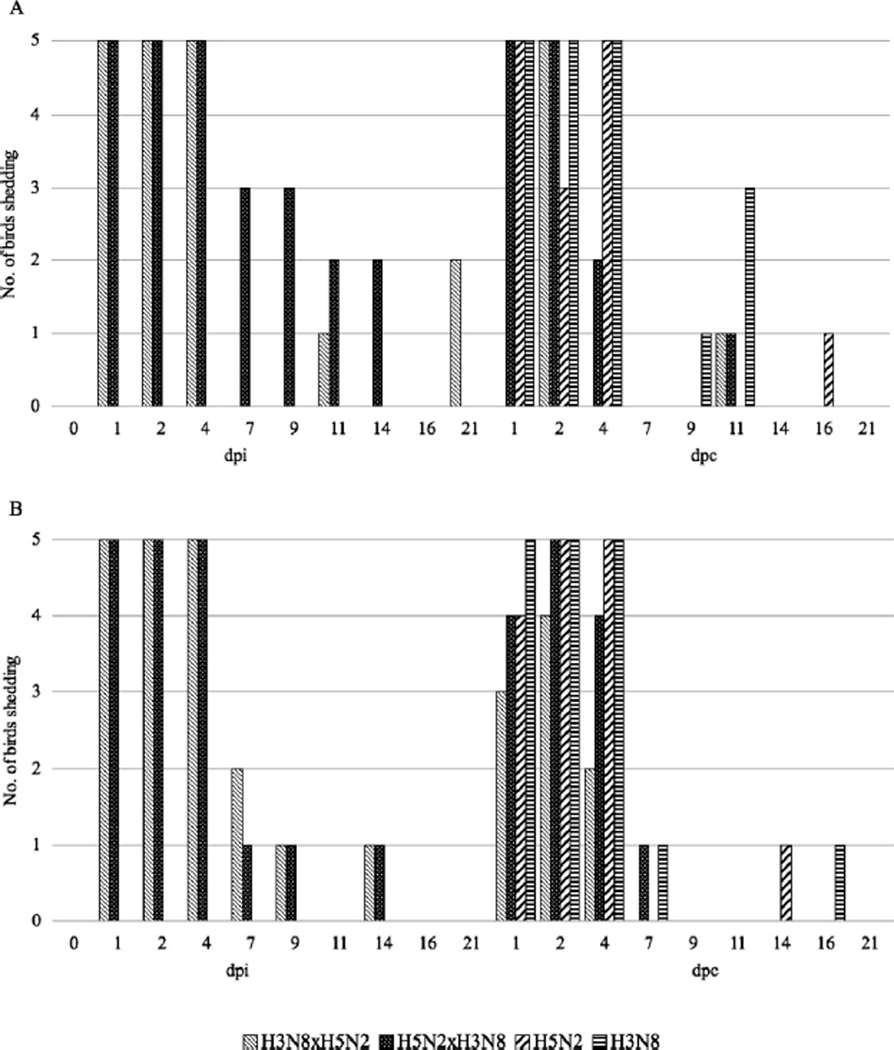
Viral shedding via the oropharynx and cloaca was consistent during the first four days after the primary inoculation (in both single-infection and consecutive infection groups). After that, viral shedding was intermittent, but often was prolonged; that is, virus was recovered from OP swabs up to 21 dpi in the H3N8×H5N2 group (Fig. 1). Both consecutive infection groups had a reduced duration in OP and cloacal swabs after the heterosubtypic LPAI virus challenge, when compared to single-infection groups (Table 2; Fig. 1). Mean duration of viral shedding via OP and cloaca for the consecutive infection groups after heterosubtypic challenge was 4.2 (range 2–11) and 4.2 (range 2–7) days for the H5N3×H3N8, and 3.8 (range 2–11) and 2.4 (range 0–4) days for the H3N8×H5N2 groups, respectively. In comparison, mean duration of viral shedding via OP and cloaca for the single infection groups was 6.4 (range 4–16) and 6.0 (range 4–14) days for the H5N3 group, and 9.2 (range 4–11) and 4.4 (range 4–16) days for the H3N8 group, respectively (Table 2).
Fig. 1.
Oropharyngeal (A) and cloacal (B) viral shedding of mallards (Anas platyrhynchos) experimentally inoculated with 106 EID50 of either A/mallard/MN/355779/00 (H5N2) and/or A/mallard/MN/199106/99 (H3N8), at 21 dpi and 21 dpc. Co-infection groups: H5N2×H3N8—exposed to H5N2 at 0 dpi, and subsequently challenged to H3N8 at 21 dpi; H3N8×H5N2—exposed to H3N8 at 0 dpi, and subsequently challenged to H5N2 at 21 dpi. Single inoculation groups: H5N2—exposed to H5N2 only; H3N8—exposed to H3N8 only (these single inoculation groups were included in the trial at 21 dpi).
Table 2.
Oropharyngeal and cloacal viral shedding pattern of mallards after challenge with heterosubtypic low pathogenicity avian influenza viruses.A
| Viral shedding, range (mean) days |
||
|---|---|---|
| GroupB | Oropharyngeal | Cloacal |
|
| ||
| H5N2×H3N8 | 2–11 (4.2) | 2–7 (4.2) |
| H3N8×H5N2 | 2–11 (3.8) | 0−4 (2.4) |
| H5N2 | 4–16 (6.4) | 4–14 (6.0) |
| H3N8 | 4–11 (9.2) | 4–16 (4.4) |
Birds were inoculated via choanal cleft with a dose of 106 EID50 of either A/mallard/MN/355779/00 (H5N2) and/or A/mallard/MN/199106/99 (H3N8).
H5N2×H3N8 group: exposed to H5N2 at 0 dpi, and subsequently challenged with H3N8 at 21 dpi; H3N8×H5N2: exposed to H3N8 at 0 dpi, and subsequently challenged with H5N2 at 21 dpi; H5N2 control group: exposed to H5N2 only; H3N8 control group: exposed to H3N8 only.
Cloacal viral shedding was completely suppressed in one bird in the consecutive infection group (H3N8×H5N2 group, bird 9). Viral titrations of positive cloacal swabs collected at 2 dpc were performed after two freeze-thaw cycles, and mean viral titers were 101.6 EID50/ml for the H5N2×H3N8 group, 101.5 EID50/ml for the H3N8×H5N2 group, 102.9 EID50/ml for the H5N2 group, and 102.7 EID50/ml for the H3N8 group. The viral subtyping of cloacal swab samples collected at 2 dpc confirmed that the virus being shed was of the same HA subtype as the viruses used for the heterosubtypic challenge.
Gross or histopathological lesions associated with AI virus infection were not observed in any birds in the treatment or negative control groups.
DISCUSSION
A primary exposure to a LPAI virus did not fully protect mallards against a subsequent infection with a heterosubtypic LPAI virus, as observed by seroconversion to and viral excretion of the heterosubtypic challenge virus (Table 1; Fig. 1). Nevertheless, ducks in the consecutive infection groups had reduced duration and concentration of viral shedding as detected by cloacal and oropharyngeal swabs when compared to the single-infection groups. The reduction in duration of viral shedding varied between virus-exposed groups and route of viral shedding, being most evident in OP swabs collected from the H3N8×H5N2 group (Fig. 1). Cloacal viral shedding after heterosubtypic challenge was not detected in one bird in the H3N8×H5N2 group (bird 9), although seroconversion was detected in this bird (HI titer of 8 against the H5 virus at 14 and 21 dpc; Table 1).
At 2 dpc, an average of one-log reduction in viral titer was observed between cloacal swab samples collected from the two single-infection groups and the two consecutive infection groups. Viral titrations were performed after the samples underwent two freeze-thaws cycles, which is known to negatively affect the infectivity of influenza virus in a sample (2,6,12). Consequently, the viral titers obtained may not reflect the actual viral load being shed by the birds at 2 dpc; rather they were used as a comparative measure to compare infectious titers being excreted by the different groups (all samples underwent the same number of freeze-thaw cycles). The reduction in viral titers was consistent among birds in the consecutive infection groups relative to the single-infection groups. These observations suggest that the viral load shed via cloaca after a LPAI infection may be reduced by recent prior infection with a heterosubtypic LPAI strain in mallards.
A difference in immunogenicity was observed between the two LPAI viruses used in this experimental trial, as demonstrated by consistently higher HI titers produced against the H5N2 virus (8 to 128, mean 25) than the H3N8 virus (8 to 16, mean 10). A loss of detectable HI antibodies against H3 at 21 dpc was observed in two birds in the H3N8×H5N2 group (birds 7 and 10), which had an HI titer of 8 until 21 dpc, when a negative HI result was obtained. Furthermore, based on HI results of the H5N2×H3N8 group (Table 1), the production of antibodies against H3 was negatively affected by the pre-existing heterosubtypic immunity, as only 4/5 birds had detectable HI titers against H3 at 14 dpc (birds 1, 3, 4, and 5), and only 2/5 at 21 dpc (birds 1 and 5). Although no antibodies against H3 were detected in bird 2 (Table 1), cloacal viral shedding was observed from 1 to 4 dpc.
The observations of this study suggest that a previous exposure to a heterosubtypic LPAI virus may negatively affect the outcome of a second LPAI exposure in mallards, by reducing the duration of OP and cloacal viral shedding, and also the concentration of virus excreted via cloaca. These results are in accordance with previous experimental cross-protective studies with LPAI virus in mallards (7). The immune mechanisms responsible for such suppression have not been defined in ducks, but may been have related to a cross-protective humoral response that was below our threshold of antibody detection or to a cell-mediated immune response as described in chickens (14).
The heterosubtypic LPAI virus-induced partial protection described herein might be of great significance for the ecology of these viruses in wild avian populations. Based on these results an infected duck with heterosubtypic immunity may shed the LPAI virus at lower concentrations in feces for a shorter duration, and, as a result, environmental contamination would be reduced. These observations, however, should be interpreted with caution, as the magnitude of heterosubtypic LPAI virus-induced protective effects for longer durations than 3 weeks post–primary infection have not been evaluated and could be reduced. In addition, based on the constant circulation of different LPAI virus subtypes in wild duck populations, it is improbable that a total protective immunity is mounted as an effect of multiple exposures. In conclusion, the protective effects of subsequent heterosubtypic LPAI virus infections may limit the pattern of AI infection in wild birds but do not appear to completely suppress it.
ACKNOWLEDGMENTS
We would like to express our gratitude to the personnel of the Southeastern Cooperative Wildlife Disease Study (SCWDS) for their technical assistance, especially Ginger Goekjian, Rebecca Poulson, Jennifer Smith, and Deb Carter. Funding for this work was provided through Specific Cooperative Agreement 58-612-0220 between the Southeast Poultry Research Laboratory and SCWDS, and by the National Institutes of Health (NIH), Department of Health and Human Services, under contract no. HHSN266200700007C. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH.
Abbreviations:
- AI
avian influenza
- BHI
brain-heart-infusion
- dpc
days post-heterosubtypic challenge
- dpi
days post-primary inoculation
- ECE
embryonating chicken egg
- EID50
median egg infectious dose
- HA
hemagglutinin
- HI
hemagglutination inhibition
- LPAI
low pathogenic avian influenza
- OP
oropharyngeal
- SPF
specific-pathogen-free
REFERENCES
- 1.Dugan VG, Chen R, Spiro DJ, Sengamalay N, Zaborsky J, Ghedin E, Nolting J, Swayne DE, Runstadler JA, Happ GM, Senne DA, Wang R, Slemons RD, Holmes EC, and Taubenberger JK. The evolutionary genetics and emergence of avian influenza viruses in wild birds. PLoS Pathog. 4:e1000076. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greiff D, Blumenthal H, Chiga M, and Pinkerton H. The effects on biological materials of freezing and drying by vacuum sublimation. II. Effect on influenza virus. J. Exp. Med. 100:89–101. 1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanson BA, Stallknecht DE, Swayne DE, Lewis LA, and Senne DA. Avian influenza viruses in Minnesota ducks during 1998–2000. Avian Dis. 47:867–871. 2003. [DOI] [PubMed] [Google Scholar]
- 4.Hinshaw VS, Air GM, Gibbs AJ, Graves L, Prescott B, and Karunakaran D. Antigenic and genetic characterization of a novel hemagglutinin subtype of influenza A viruses from gulls. J. Virol. 42:865–872. 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinshaw VS, Wood JM, Webster RG, Deibel R, and Turner B. Circulation of influenza viruses and paramyxoviruses in waterfowl originating from two different areas of North America. Bull. World Health Organ. 63:711–719. 1985. [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson GG, and Muldoon RL. Viruses causing common respiratory infections in man. V. Influenza A (Asian). J. Infect. Dis. 131: 308–357. 1975. [DOI] [PubMed] [Google Scholar]
- 7.Jourdain E, Gunnarsson G, Wahlgren J, Latorre-Margalef N, Bröjer C, Sahlin S, Svensson L, Waldenström J, Lundkvist A, and Olsen B. Influenza virus in a natural host, the mallard: experimental infection data. PLoS ONE 5:e8935. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kida H, Yanagawa R, and Matsuoka Y. Duck influenza lacking evidence of disease signs and immune response. Infect. Immun. 30:547–553. 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krauss S, Walker D, Pryor SP, Niles L, Chenghong L, Hinshaw VS, and Webster RG. Influenza A viruses of migrating wild aquatic birds in North America. Vector Borne Zoonotic Dis. 4:177–189. 2004. [DOI] [PubMed] [Google Scholar]
- 10.Kristensen HK An improved method of decalcification. Biotech. Histochem. 23:151–154. 1948. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen JC Hemagglutination-inhibition test for avian influenza virus subtype identification and the detection and quantitation of serum antibodies to the avian influenza virus. In: Avian influenza virus. Spackman E, ed. Humana Press, Totowa, NJ. pp. 53–66. 2008. [DOI] [PubMed] [Google Scholar]
- 12.Quinlivan M, Cullinane A, Nelly M, Van Maanen K, Heldens J, and Arkins S. Comparison of sensitivities of virus isolation, antigen detection, and nucleic acid amplification for detection of equine influenza virus. J. Clin. Microbiol. 42:759–763. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reed LJ, and Muench H. A simple method of estimating fifty percent endpoint. Am. J. Epidemiol. 27:493–497. 1938. [Google Scholar]
- 14.Seo SH, and Webster RG. Cross-reactive, cell-mediated immunity and protection of chickens from lethal H5N1 influenza virus infection in Hong Kong poultry markets. J. Virol. 75:2516–2525. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharp GB, Kawaoka Y, Wright SM, Turner B, Hinshaw V, and Webster RG. Wild ducks are the reservoir for only a limited number of influenza A subtypes. Epidemiol. Infect. 110:161–176. 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinnecker H, Sinnecker R, Zilske E, and Koehler D. Detection of influenza A viruses and influenza epidemics in wild pelagic birds by sentinels and population studies. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 253: 297–304. 1982. [PubMed] [Google Scholar]
- 17.Stallknecht DE, and Brown JD. Ecology of avian influenza in wild birds. In: Avian influenza. Swayne DE, ed. Blackwell Publishing, Ames, IA. pp. 43–58. 2008. [Google Scholar]
- 18.Stallknecht DE, and Shane SM. Host range of avian influenza virus in free-living birds. Vet. Res. Commun. 12:125–141. 1988. [DOI] [PubMed] [Google Scholar]
- 19.Stallknecht DE, Shane SM, Zwank PJ, Senne DA, and Kearney MT. Avian influenza viruses from migratory and resident ducks of coastal Louisiana. Avian Dis. 34:398–405. 1990. [PubMed] [Google Scholar]
- 20.Süss J, Schäfer J, Sinnecker H, and Webster RG. Influenza virus subtypes in aquatic birds of eastern Germany. Arch. Virol. 135:101–114. 1994. [DOI] [PubMed] [Google Scholar]
- 21.Swayne DE, Senne DA, and Suarez DL. Avian influenza. In: Isolation, identification, and characterization of avian pathogens, 5th ed. Dufour-Zavala L, ed. American Association of Avian Pathologists, Jackson-ville, FL. pp. 128–134. 2008. [Google Scholar]
- 22.Tsukamoto K, Ashizawa H, Nakanishi K, Kaji N, Suzuki K, Okamatsu M, Yamaguchi S, and Mase M. Subtyping of avian influenza viruses H1 to H15 on the basis of hemagglutinin genes by PCR assay and molecular determination of pathogenic potential. J. Clin. Microbiol. 46:3048–3055. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallensten A, Munster VJ, Latorre-Margalef N, Brytting M, Elmberg J, Fouchier RA, Fransson T, Haemig PD, Karlsson M, Lundkvist A, Osterhaus AD, Stervander M, Waldenstrom J, and Bjorn O. Surveillance of influenza A virus in migratory waterfowl in northern Europe. Emerg. Infect. Dis. 13:404–411. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Webster RG, Bean WJ, Gorman OT, Chambers TM, and Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 56:152–179. 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Webster RG, Yakhno M, Hinshaw VS, Bean WJ, and Murti KG. Intestinal influenza: replication and characterization of influenza viruses in ducks. Virology 84:268–278. 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woolcock PR Avian influenza virus isolation and propagation in chicken eggs. In: Avian influenza virus. Spackman E, ed. Humana Press, Totowa, NJ. pp. 35–46. 2008. [DOI] [PubMed] [Google Scholar]