Abstract
Background
Alpha blockers are occasionally prescribed for hypertension so it is important to determine and compare their effects on blood pressure (BP), heart rate and withdrawals due to adverse effects (WDAE).
Objectives
To quantify the dose‐related systolic and/or diastolic BP lowering efficacy of alpha blockers versus placebo in the treatment of primary hypertension.
Search methods
For the updated review, we searched CENTRAL (The Cochrane Library 2012, Issue 4), MEDLINE (1946 to May 2012), EMBASE (1980 to May 2012) and reference lists of articles.
Selection criteria
Double‐blind, randomized, controlled trials evaluating the BP lowering efficacy of fixed‐dose monotherapy with an alpha blocker compared with placebo for a duration of 3 to 12 weeks in patients with primary hypertension.
Data collection and analysis
Two authors independently assessed the risk of bias and extracted data. Study authors were contacted for additional information. WDAE information was collected from the trials.
Main results
Only 10 trials evaluated the dose‐related trough BP lowering efficacy of 4 different alpha blockers in 1175 participants with a baseline BP of 155/101 mm Hg. The data do not suggest that any one alpha blocker is better or worse at lowering BP. The best but unsatisfactory estimate of the trough BP lowering efficacy for alpha blockers is ‐8/‐5 mmHg.
Authors' conclusions
Based on the limited number of published RCTs, the BP lowering effect of alpha blockers is modest; the estimate of the magnitude of trough BP lowering of ‐8/‐5 mmHg is likely an overestimate. There are no clinically meaningful BP lowering differences between different alpha blockers. The review did not provide a good estimate of the incidence of harms associated with alpha blockers because of the short duration of the trials and the lack of reporting of adverse effects in many of the trials.
Keywords: Humans, Adrenergic alpha‐Antagonists, Adrenergic alpha‐Antagonists/administration & dosage, Antihypertensive Agents, Antihypertensive Agents/administration & dosage, Blood Pressure, Blood Pressure/drug effects, Hypertension, Hypertension/drug therapy, Randomized Controlled Trials as Topic
Plain language summary
Alpha blockers have a modest BP lowering effect
The class of drugs called alpha blockers is sometimes used to lower elevated blood pressure. This class includes drugs such as doxazosin (brand name: Cardura), prazosin (Minipress) and terazosin (Hytrin). We asked how much this class of drugs lowers blood pressure and whether there is a difference between individual drugs within the class. The available scientific literature was searched to find all the trials that had assessed this question. We found only 10 trials studying the blood pressure lowering ability of 4 different alpha blockers in 1175 participants. The blood pressure lowering effect was modest. There was an 8‐point reduction in the upper number that signifies the systolic pressure and a 5‐point reduction in the lower number that signifies the diastolic pressure. No alpha blocker drug appears to be any better or worse than others in terms of blood pressure lowering ability. Due to incomplete reporting of the number of participants who dropped out of the trials due to adverse drug reactions, as well as the short duration of these trials, this review could not provide an estimate of the harms associated with this class of drugs.
Background
Hypertension is an important health problem and it is associated with an increased risk of death, stroke, and heart disease. Considerable scientific evidence shows that blood pressure reduction with different drug treatments reduces death, stroke, and heart disease. However, evidence also suggests the blood pressure lowering effects of different classes of antihypertensive agents may not always parallel the reductions in mortality or cardiovascular morbidity (Wright 2009). In other words, blood pressure lowering does not always predict the same magnitude of improvement in health outcomes. Other factors may contribute to the reductions in mortality and vascular morbidity with antihypertensive drugs. Such factors may be independent of the blood pressure lowering effect of the drug, or the mechanism by which these drugs lower blood pressure. Nevertheless, blood pressure reduction remains an important factor.
Alpha blockers are used as pharmacological agents for the treatment of hypertension. In light of the findings of the ALLHAT hypertension trial (ALLHAT 2000), where the alpha blocker (doxazosin) treatment arm of the trial was stopped early due to a statistically significant increase in congestive heart failure, angina and stroke compared to the thiazide‐like diuretic, chlorthalidone, alpha blockers are rarely used as first‐line antihypertensive therapy. Nevertheless, alpha blockers are still widely used as second‐ or third‐line agents and perhaps as first‐line agents in hypertensive patients with concomitant benign prostatic hyperplasia.
One of the main difficulties of managing a patient with hypertension using alpha blockers is deciding which dose should be prescribed. This decision should be made primarily on the basis of the best available evidence of effectiveness. Despite nearly 30 years of research evidence and clinical use of alpha blockers, the dose‐related blood pressure lowering effect of this antihypertensive drug class is still not known.
A systematic review of the dose‐related blood pressure lowering efficacy of alpha blockers has not been previously performed. The aims of this systematic review are: 1) to quantify the dose‐related blood pressure lowering efficacy of alpha blockers in patients with primary hypertension; and 2) to establish dose equivalencies of different drugs within the alpha blocker class. The information derived from this review should facilitate future reviews of head‐to‐head comparisons with other drug classes and assist clinicians in choosing optimal doses of alpha blockers.
Objectives
Primary objective:
To quantify the dose‐related systolic and/or diastolic blood pressure lowering efficacy of alpha blockers versus placebo in the treatment of primary hypertension.
Secondary objectives:
To determine the effects of alpha blockers on variability of blood pressure.
To determine the effects of alpha blockers on pulse pressure.
To quantify the dose‐related effect of alpha blockers on heart rate.
To quantify the dose‐related effect of alpha blockers on withdrawals due to adverse effects.
Methods
Criteria for considering studies for this review
Types of studies
Included studies were randomized controlled trials (RCTs) and their design must have met the following criteria:
double‐blind
random allocation to alpha blocker group(s) and parallel placebo group
duration of follow‐up of at least three weeks
office blood pressure measurements at baseline (following washout) and at one or more time points between 3 and 12 weeks post‐treatment
Types of participants
Participants with an office baseline blood pressure of at least 140 mmHg systolic and/or a diastolic blood pressure of at least 90 mmHg were included. Participants were not restricted by age, gender, baseline risk or any other co‐morbid conditions.
Patients with creatinine levels greater than 1.5 times the normal level were excluded. Participants who were taking medications that affect blood pressure other than the study medications were excluded.
Types of interventions
Monotherapy with any alpha blocker, including alfuzosin, bunazosin, doxazosin, prazosin, tamsulosin, terazosin, trimazosin and indoramin.
Trials in which titration to a higher dose was based on blood pressure response were not eligible if the titration occurred before 3 weeks of treatment because dose‐response relationships cannot be analyzed if patients within each randomized group are taking different doses. However, trials in which a response‐dependent titration took place during or after the 3‐12 week interval were eligible if pre‐titration data were given. For forced titration trials, data at each dose level were extracted, provided this dose was given for a 3 to 12 week period.
Types of outcome measures
Primary:
Change from baseline of trough and/or peak systolic and diastolic blood pressure at 3 to 12 weeks, compared with placebo. If blood pressure measurements were available at more than one time within the accepted window, the weighted means of blood pressures taken in the 3 to 12 week range were used.
Secondary:
Standard deviation of the change in blood pressure compared with placebo.
Change in standard deviation of blood pressure compared with placebo.
Change in pulse pressure compared with placebo.
Change in heart rate compared with placebo.
Number of patient withdrawals due to adverse effects compared with placebo.
Search methods for identification of studies
Electronic searches
The following electronic databases were searched for primary studies:
Cochrane Central Register of Controlled Trials (CENTRAL), The Cochrane Library 2012, Issue 4
Ovid MEDLINE (1946 to May 2012)
EMBASE (1974 to May 2012)
Electronic databases were searched using a strategy combining the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐maximizing version (Cochrane Handbook 2008) with selected MeSH and free text terms relating to alpha blockers and hypertension. No language restrictions were used. The MEDLINE search strategy (Appendix 1) was translated into Embase (Appendix 2) and CENTRAL (Appendix 3) using the appropriate controlled vocabulary as applicable. Search strategies from the orginal review are listed in Appendix 4.
Searching other resources
Previously published meta‐analyses on the dose‐response of alpha blockers, as well as narrative reviews, were used to help identify references to trials.
Data collection and analysis
Selection of studies
The databases listed above were searched and potentially relevant citations were identified. The initial screen of these abstracts excluded articles whose titles and/or abstracts were clearly irrelevant. The full text of remaining articles was then retrieved (and translated into English where required) to assess whether the trials met the prespecified inclusion criteria. The bibliographies of pertinent articles, reviews and texts were searched for additional citations. Two independent reviewers (BSH and BPG) assessed the eligibility of the trials using a standardized trial inclusion form. A third reviewer (JMW) resolved discrepancies.
Data extraction and management
Data from included studies were extracted by one reviewer (BSH or BPG) using standardized data extraction forms and checked by a second reviewer (BPG or BSH). If data were presented numerically (in tables or text) and graphically (in figures), the numeric data were preferred because of possible measurement error when estimating from graphs. All numeric calculations and extractions from graphs or figures were confirmed by a second reviewer. Any discrepancies were resolved by consensus.
The position of the patient during blood pressure measurement may affect the blood pressure lowering effect. However, in order not to lose valuable data, if only one position was reported, data from that position were extracted. When blood pressure measurement data were available in more than one position, data were extracted in accordance with the following order of preference: 1) sitting; 2) standing; and 3) supine.
Assessment of risk of bias in included studies
Two reviewers (BSH and BPG) independently assessed the risk of bias in included studies using the Cochrane Collaboration’s recommended tool, which is a domain‐based critical evaluation of the following domains: sequence generation; allocation concealment; blinding; incomplete outcome data; selective outcome reporting; and other sources of bias (Cochrane Handbook 2008). Assessments of risk of bias are provided in the ‘Risk of bias’ table for each study.
Dealing with missing data
If there were multiple reports from the same study, the duplicate publications were scanned for additional data. If necessary, investigators were contacted (by email, letter and/or fax) to obtain the missing information.
In the case of missing values for standard deviation of the change in blood pressure or heart rate, the standard deviation was imputed based on the information in the same trial or from other trials using the same dose. The following hierarchy (listed from high to low preference) was used to impute standard deviation values:
Pooled standard deviation calculated either from the t‐statistic corresponding to an exact p‐value reported or from the 95% confidence interval of the mean difference between treatment group and placebo.
Standard deviation of change in blood pressure/heart rate from a different position than that of the blood pressure data/heart rate used.
Standard deviation of blood pressure/heart rate at the end of treatment.
Standard deviation of blood pressure/heart rate at the end of treatment measured from a different position than that of the blood pressure/heart rate data used.
Standard deviation of blood pressure/heart rate at baseline (except if this measure was used for entry criteria).
Weighted mean standard deviation of change in blood pressure/heart rate from other trials using the same class of drug (at any dose).
Assessment of heterogeneity
If there was significant statistical heterogeneity (P‐value <0.10} associated with an effect estimate, a random effects model was applied. This model provides a more conservative statistical comparison of the difference between intervention and control because a confidence interval around the effect estimate is wider than a confidence interval around a fixed effect estimate. If a statistically significant difference was still present using the random effects model, the fixed effect pooled estimate and 95% CI was reported because of the tendency of smaller trials, which are more susceptible to publication bias, to be overweighted with a random effects analysis.
Assessment of reporting biases
No language restrictions were applied.
Data synthesis
Data were processed in accordance with the Cochrane Handbook 2008 for Systematic Reviews of Interventions. Data synthesis and analyses were done using Review Manager 5.0 software.
Blood pressure and heart rate (continuous outcomes) were expressed as the mean (±SD) change from baseline to follow‐up. Otherwise continuous outcomes were pooled as weighted mean difference (WMD). Withdrawals due to adverse effects (dichotomous outcome) for each comparison were expressed as relative risks with 95% confidence intervals (CI). If there was a statistically significant relative risk difference, the associated number needed to treat/harm was also calculated.
Direct and indirect comparisons
Where possible, direct and indirect comparisons of effect sizes between doses were performed for each alpha blocker. In the direct method, only trials that randomized participants to different doses were included in the analysis. In the indirect method, an "adjusted indirect comparison" and the associated standard error were calculated using the method described by Bucher 1997 and Song 2003. A P‐value <0.05 was considered statistically significant for all comparisons.
Subgroup analysis and investigation of heterogeneity
Where possible, subgroup analyses were used to examine the results for specific categories of participants. Possible subgroup analyses included:
Race: black, white, other.
Age: adults (18‐69 years), older people (70 years and older).
Baseline severity of hypertension: mild, moderate, severe.
The robustness of the results was tested using several sensitivity analyses, including:
Trials that are industry‐sponsored versus non‐industry sponsored.
Trials that assess drug as primary drug of investigation versus trials that assess drug as comparator.
Trials with blood pressure data measured in the sitting position versus other measurement positions.
Trials with published standard deviations of blood pressure change versus imputed standard deviations.
Heterogeneity amongst included studies was explored qualitatively (by comparing the characteristics of included studies) and quantitatively (using the chi‐squared test of heterogeneity and I2 statistic). Where appropriate, data from each study was pooled using a fixed effect model, except where substantial heterogeneity exists.
The funnel plot was used to examine small study bias.
Results
Description of studies
Results of the search
The search strategy identified 1496 citations, of which only 10 (0.7%) trials (22 publications) met the inclusion criteria and had extractable data to evaluate the dose‐related blood pressure lowering efficacy of 4 alpha blockers (Figure 1). Each included study is summarized in the Characteristics of included studies table.
1.
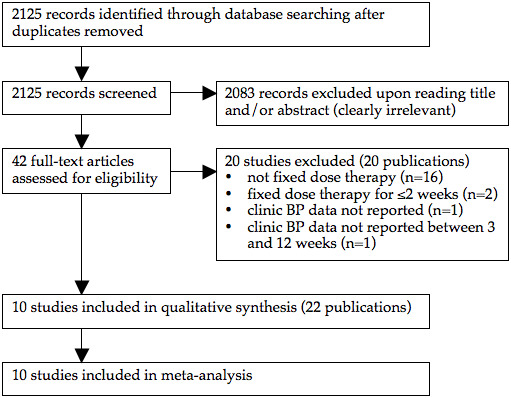
PRISMA flow diagram
Included studies
The earliest study evaluating the antihypertensive efficacy of alpha blocker monotherapy using office blood pressure measurements was published in 1975. The other 9 studies were published during the 1980s and 90s. No further studies have been published since 1999. All 10 included studies were published in English. Four of the included studies were industry‐sponsored while the remaining 6 did not report the source of funding. One duplicate publication of a study evaluating doxazosin (Os 1999) and a total of 11 duplicate publications of 3 terazosin studies (Abraham 1985, Dauer 1986, Mersey 1984) were identified.
Eight of the included studies randomized patients to fixed‐dose monotherapy during double‐blind treatment. Six of these studies consisted of a dose titration period (initial dose usually 1 mg once daily) followed by a "maintenance" period at the pre‐assigned fixed dose. One was a forced titration study and another study titrated to BP response at pre‐specified intervals during the double‐blind treatment period. For 5 of the included studies, the number of patients treated with an alpha blocker was larger than the number of placebo‐treated patients because they had multiple treatment arms comparing different doses of an alpha blocker with a single placebo arm.
Baseline characteristics of the 10 included studies are provided in Table 1. A total of 1175 participants with a weighted mean age of 57.4 years and a baseline BP of 155.4/101.4 mm Hg were treated at a fixed dose for a weighted mean duration of 5.9 weeks.
1. Overview of the 10 studies investigating alpha blockers as monotherapy.
| Alpha blocker | Dose range (mg/day) | Number of studies | Alpha patients (n) | Placebo patients (n) | Mean duration (weeks) | Mean age (years) | Baseline BP (mm Hg) |
| Bunazosin | 3‐12 | 1 | 12 | 4 | 8.0 | Not reported | 163.3/102.8 |
| Doxazosin | 2‐12 | 3 | 347 | 125 | 7.1 | 58.1 | 157.8/101.4 |
| Prazosin | 2.5‐20 | 2 | 108 | 32 | 4.2 | 55.1 | 151.9/99.9 |
| Terazosin | 5‐20 | 4 | 203 | 159 | 4.0 | Not reported | 155.4/101.4 |
Imputation of missing variance data
Standard deviation of blood pressure change
Four of the included trials reported the standard deviation of the change in blood pressure (Abraham 1985, Cubeddu 1988, Dauer 1986, Mersey 1984). These values were pooled for the alpha blocker and placebo groups and weighted mean estimates of the standard deviation of the change in SBP and DBP were determined. The weighted mean standard deviations of the change in SBP and DBP were 12.4 (SD 3.7) mm Hg and 7.1 (SD 1.0) mm Hg for the alpha blocker group, respectively. For the placebo group, the standard deviation of the change was 13.6 (SD 3.0) mm Hg for SBP and 7.4 (SD 0.9) mm Hg for DBP. There was no statistically significant difference between the alpha blocker and placebo groups for SD of SBP change or SD of DBP change. These values were used according to the imputation hierarchy for trials that did not report SD of BP change or reported an outlier SD value. The SD of BP change was imputed for 5 of the included studies. Of these studies, 1 was imputed using endpoint SD, 1 was imputed using baseline SD for SBP, 2 were imputed using the weighted mean SD of SBP change from other trials, and 4 were imputed using the weighted mean SD of DBP change from other trials.
Excluded studies
Twenty studies were excluded because they did not meet the pre‐specified inclusion criteria and the reasons for exclusion are listed in the Characteristics of excluded studies table. The main reason for exclusion is that titration to higher doses occurred.
An additional 2 papers met the inclusion criteria but did not have extractable data (Achari 2000, Yasunari 2006). In order to obtain the missing data, the lead authors were contacted by email. The lead author of one of the papers (Achari 2000) replied that their paper reported on a subset of patients from a terazosin study conducted around 1990 that had 24‐hour blood pressure measurements taken and that he no longer has access to the full trial data. Therefore, this trial has been excluded from our review and is listed in the Characteristics of excluded studies table. We did not receive a reply from the authors of Yasunari 2006 so this trial has also been excluded.
Two fixed titration studies were excluded because any given dose of an alpha blocker was only administered for 2 weeks or less (Aronow 1977, Aronow 1978).
Risk of bias in included studies
Sequence generation, allocation concealment
All the trial publications simply reported that the trial was "randomized" but did not provide any details about the randomization method. Details of the method of allocation concealment were reported in only 1 of the 10 included studies (Schnaper 1975, Study 2). Such vague reporting is insufficient to be confident that the allocation sequence was properly randomized and adequately concealed given the fact that many investigators use the term "randomized" when it is not justified. Authors should report their methods of sequence generation and allocation concealment clearly.
Blinding bias
Nearly all the trial publications simply reported that the trial was "double‐blind" but did not provide any details about the blinding methods. Only 2 trials described the blinding method as using "identical" placebo capsules. The potential for loss of blinding was reduced because 9 of the 10 trials included a dose titration period up to the randomized dose to minimize the characteristic hypotensive effects associated with alpha blockers. The success of blinding in patients or investigators was not assessed in any of the included trials.
Attrition bias
It is unlikely that attrition bias would have had an impact on the systematic review since most of the patients randomized to fixed‐dose monotherapy in each trial completed the double blind treatment period.
Selective reporting bias
This would not affect the blood pressure measurements as these were the primary outcome of most of these trials. There is a potential for selective reporting bias for heart rate and withdrawals due to adverse effects since only half of the included trials reported these outcomes.
Other potential sources of bias
Selection Bias
Another potential source of bias that we became aware of in working on this review is patient selection bias. One of the exclusion criteria reported in 3 trials was participants with a known hypersensitivity or a history of failure to respond to alpha blockers (Abraham 1985; Gillenwater 1995; Os 1999). This suggests that investigators have knowledge of each participant's prior experience with this drug class and thus may select for patients who have responded favorably to alpha blockers in terms of BP lowering or have been found to tolerate treatment with alpha blockers. However, it was not possible to prove selection bias as none of the included trials described in detail these details of patient recruitment.
Publication Bias
Although trials must have been completed and provided to the regulators in order for the drug to be approved for the treatment of primary hypertension, only 10 trials were identified that met the inclusion criteria for our review. Furthermore, many of the doses that have been approved by regulators do not have sufficient published trial evidence to support their use. In fact, for some doses there were no published data available. For example, doxazosin has been approved up to a maximum daily dose of 16 mg. However, we only found data for the effect of doxazosin up to 12 mg and we know that trials must have been completed and provided to the regulators for doxazosin at 16 mg daily.
Another source of bias that is likely to have a significant impact on this review is the selective publication of trials with positive results. This review was evaluated for the existence of publication bias since it only included and appraised published trial evidence. In the absence of bias, the funnel plot should resemble a symmetrical inverted funnel since the precision in the estimation of the true blood pressure lowering decreases as the study size decreases. Thus small studies will scatter more widely at the bottom of the graph (Cochrane Handbook 2008). The most common way to investigate whether or not a review is subject to publication bias is to examine for funnel plot asymmetry as smaller studies with null results remained unpublished. However, due to the small number of included trials, funnel plots could not be generated to adequately assess whether publication bias is likely. Despite this limitation, there was still evidence of selective publication of positive trials. One publication (Harder 1994) only reported on a subset of patients (from a single center) randomized in a multicenter study. The full data for this multicenter study has not been published elsewhere. A report of another study provided "only a preliminary analysis of the blood pressure data obtained", with only the DBP data reported for one of the three groups receiving terazosin (Weber 1991). A published report of the complete BP data for all treatment groups was not identified by our comprehensive search.
A total of 11 connected papers published between 1984 and 1990 summarized a series of trials conducted by the manufacturer that assessed the clinical profile of terazosin in hypertensive patients. Only after close scrutiny of these 11 papers were we able to determine that they were duplicate publications of the same trials. Three of these trials (Study M81‐059, M81‐060 and M81‐061) were fixed dose studies that met our inclusion criteria, of which 2 have been published elsewhere in abstract form only (Mersey 1984) or as a report on only a subset of the total randomized patients (Abraham 1985). A review article by Dauer 1986 summarized the BP data for all patients randomized in these 3 studies and, therefore, was used as the primary reference. The potential for mistaking these connected papers as separate trials as opposed to duplicate publications is high and thus could have led us to incorrectly believe there were more trials assessing terazosin if we had not conducted such a thorough examination. The results of this review underscore the need for all studies, regardless of the findings, to be published and accessible for secondary analysis. Trial registration has been recognized in order to improve transparency in research and knowledge sharing. In recent years, regulatory bodies around the world, led by the World Health Organization (WHO), have set standards for trial registration and reporting and are urging research institutions and companies to register all medical studies that test treatments on humans. Initiatives such as the WHO's International Clinical Trials Registry Platform will help improve transparency and reduce the risk of publication bias skewing the results of future systematic reviews.
Because of the high likelihood of publication bias outlined above we think that the estimates of blood pressure lowering effect that we have calculated is likely an overestimate of the real effect.
Effects of interventions
Dose‐ranging BP lowering efficacy of individual alpha blocker drugs
Summarized below are the dose‐related trough blood pressure lowering efficacy estimates of 4 alpha blockers that were administered once daily in the included studies. The weighted mean placebo effect across all trials was ‐1.7 (95% CI ‐7.0, 3.5; range ‐5.5 to 5.5) mm Hg and ‐3.9 (95% CI ‐7.3, ‐0.5; range ‐5.6 to 3.5) mm Hg for SBP and DBP, respectively. Therefore, to determine the magnitude of the BP lowering efficacy of each alpha blocker, a weighted mean difference from placebo (alpha blocker effect size minus placebo effect size) with a 95% confidence interval (in parentheses) was calculated.
Dose‐ranging BP lowering efficacy of bunazosin
Only one small trial (n=16) assessed bunazosin at doses of 3, 6 and 12 mg/day (Harder 1994). The risk of publication bias is high because this study was performed in one center as part of a multicenter trial that has not been published. Furthermore, the baseline BP differed between the bunazosin 12 mg and placebo groups by 31.3 mm Hg for SBP and 7.5 mm Hg for DBP, which brings into question the quality of randomization in this trial. For these reasons, we did not attempt to estimate the BP lowering efficacy of bunazosin based on this published report and have not included this trial in the Data and analysis section.
Dose‐ranging BP lowering efficacy of doxazosin
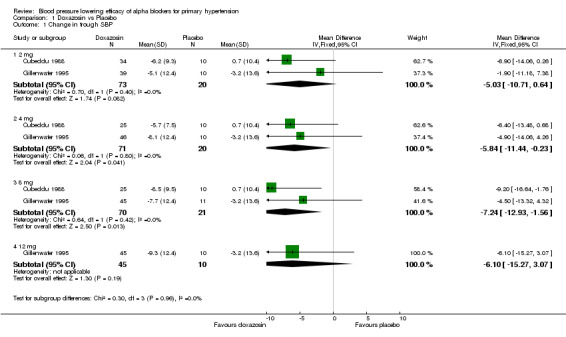
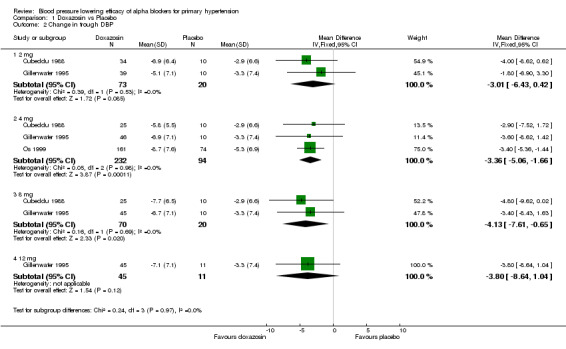
Three of the included studies (Cubeddu 1988, Gillenwater 1995, Os 1999) assessed the BP lowering efficacy of doxazosin from 2 to 12 mg/day but there were no data available at 16 mg/day, the manufacturer's maximum recommended daily dosage. Doxazosin at 2 mg/day did not significantly reduce BP compared with placebo. Doxazosin at 4 mg/day was the lowest effective dose, demonstrating a significantly greater reduction in SBP and DBP as compared to placebo. Compared with placebo, the 8 mg/day group also had a statistically significant reduction in SBP and DBP. The reduction in the 12 mg/day group did not reach statistical significance compared with placebo but this is likely due to a paucity of data. Since there were few studies at each dose, there were insufficient data to demonstrate a statistically significant difference between any of the doses using indirect comparisons and thus very limited information regarding the dose‐response of doxazosin. Based on the available data, the best estimate of the blood pressure lowering efficacy for doxazosin 4 to 12 mg/day is ‐6.42 (95% CI ‐10.12, ‐2.80) mm Hg for SBP and ‐3.53 (95% CI ‐4.99, ‐2.07) mm Hg for DBP.
Dose‐ranging BP lowering efficacy of prazosin
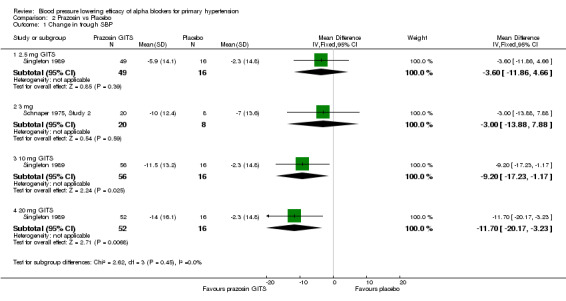
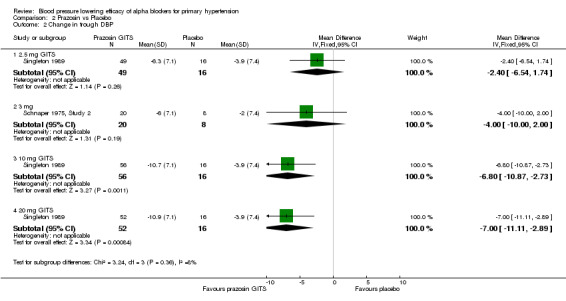
One of the included trials (Singleton 1989), sponsored by the manufacturer, evaluated the BP lowering efficacy of a controlled‐release formulation of prazosin (prazosin GITS) 2.5, 10 and 20 mg/day and one additional trial (Schnaper 1975, Study 2) reported the change in BP with standard prazosin 3 mg/day. Prazosin at 2.5 and 3 mg/day did not statistically significantly lower BP compared with placebo. The lowest effective dose is 10 mg/day. Due to the wide confidence intervals, an indirect comparison of 20 mg/day with 10 mg/day did not show a statistically significant difference. Because of the lack of data, there is very limited information regarding the dose‐response of prazosin. Based on the limited available data, the best estimate of the blood pressure lowering efficacy for prazosin GITS 10 to 20 mg/day is ‐10.38 (95% CI ‐16.21, ‐4.56) mm Hg and ‐6.90 (95% CI ‐9.79, ‐4.01) mm Hg for SBP and DBP, respectively.
Dose‐ranging BP lowering efficacy of terazosin
Four of the included trials (Abraham 1985, Dauer 1986, Study M81‐059, Mersey 1984, Weber 1991) evaluated terazosin from 5 to 20 mg/day but, with exception to the two trials that contributed to the DBP effect estimate of terazosin at 5 mg/day, there was only one study at each dose and therefore insufficient data to demonstrate a statistically significant difference between any of the doses using indirect comparisons. Based on the available evidence, terazosin at 5 mg/day was the lowest dose that demonstrated a significantly greater reduction in SBP and DBP as compared to placebo. It is possible the lowest effective dose may be lower than 5 mg/day but there are no available data. Efficacy data for 10 and 20 mg/day did not demonstrate a statistically significant reduction in BP compared with placebo. However, this is likely due to the wide confidence intervals associated with the effect size estimate. Due to a lack of data for each dose, a dose‐response relationship with terazosin could not be statistically established.
When all doses were combined to establish an overall effect with terazosin, there was a statistically significant reduction in SBP and DBP compared with placebo. The best estimate of the BP lowering effect of terazosin at 5 to 20 mg/day is ‐6.59 (95% CI ‐10.22, ‐2.96) mm Hg for SBP and ‐4.40 (95% CI ‐5.95, ‐2.84) mm Hg for DBP.
Summary of the blood pressure lowering efficacy of alpha blockers
Table 2 provides an overview of the lowest effective dose, the lowest dose with near maximal blood pressure lowering and the near maximal blood pressure lowering effect of each alpha blocker studied in this review. The lowest effective dose is defined as the lowest dose for which there is a statistically significant difference from placebo. The lowest dose with near maximal blood pressure lowering efficacy is defined as the dose that demonstrates a statistically significantly greater response than doses below it, but does not exhibit a statistically significant difference in effect size compared with higher doses.
2. Summary of the blood pressure lowering efficacy of alpha blockers.
| Alpha blocker | Lowest effective dose (mg/day) | Lowest dose with near maximal BP lowering (mg/day) | Near maximal trough SBP lowering | Near maximal trough DBP lowering | Trough SBP lowering (mm Hg), 95% CI | Trough DBP lowering (mm Hg), 95% CI |
| Bunazosin | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable |
| Doxazosin | 4 | Not estimable | Not estimable | Not estimable | ‐6.42 (‐10.12, ‐2.80) | ‐3.53 (‐4.99, ‐2.07) |
| Prazosin | 10 | Not estimable | Not estimable | Not estimable | ‐10.38 (‐16.21, ‐4.56) | ‐6.90 (‐9.79, ‐4.01) |
| Terazosin | 5 | Not estimable | Not estimable | Not estimable | ‐6.59 (‐10.22, ‐2.96) | ‐4.40 (‐5.95, ‐2.84) |
Due to a lack of data, neither an estimate of the lowest dose with near maximal blood pressure lowering efficacy nor an estimate of the near maximal blood pressure lowering efficacy could be determined for any of the individual alpha blocker drugs. Instead, the best estimate of the trough blood pressure lowering efficacy of each alpha blocker is based on the limited data that were available.
Blood pressure variability
The variability of blood pressure at both baseline and endpoint was not reported in any of the included trials. Therefore, the effect of alpha blockers on blood pressure variability could not be assessed.
Dose‐ranging peak blood pressure lowering efficacy
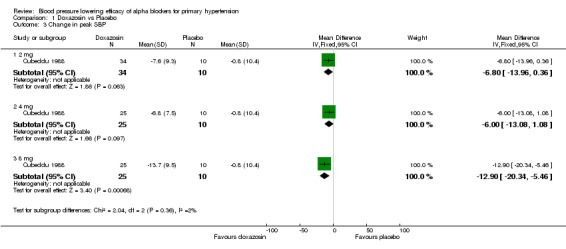
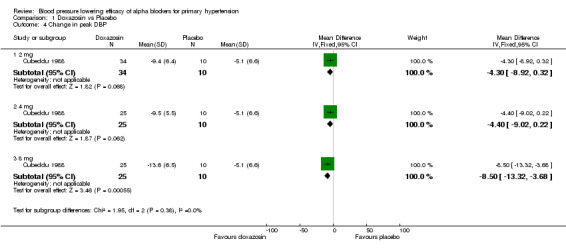
One of the included trials reported the peak blood pressure lowering efficacy of doxazosin 2‐8 mg/day (Cubeddu 1988). Only doxazosin at 8 mg/day demonstrated a statistically significant difference compared with placebo, ‐12.90 (95% CI ‐20.34, ‐5.46) mm Hg for SBP and ‐8.50 (95% CI ‐13.32, ‐3.68) mm Hg for DBP. This effect was larger than the trough effect but the limited data does not allow one to make any conclusion as to whether there is a difference in peak and trough effect.
Dose‐ranging effect on pulse pressure
Pulse pressure was not reported as an outcome in any of the included trials and there were insufficient data to adequately determine the effect of each alpha blocker on pulse pressure.
Dose‐ranging effect on heart rate
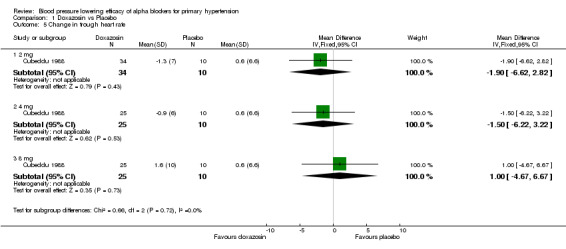
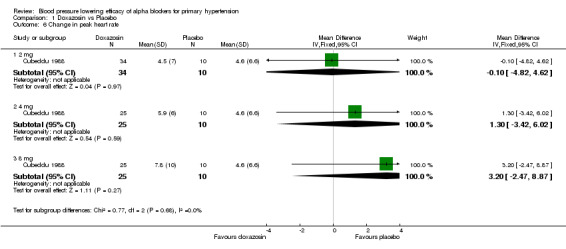
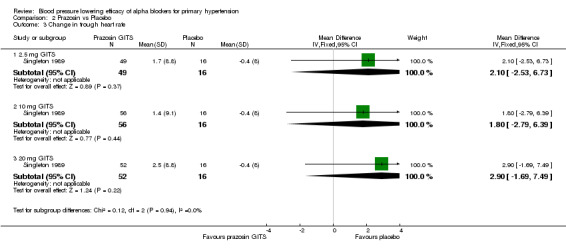
Only three of the included trials provided dose‐related heart rate data (Cubeddu 1988, Harder 1994, Singleton 1989). All trials reported changes in heart rate at trough and one trial reported changes in heart rate at peak (Cubeddu 1988). The available data suggests no effect on heart rate; however, there were insufficient data to evaluate the dose‐related effect of the individual alpha blockers on heart rate.
Dose‐ranging effect on withdrawals due to adverse effects
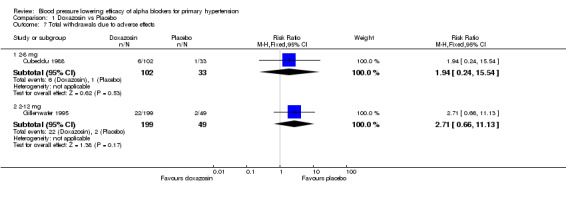
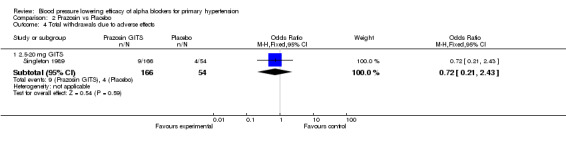
An analysis of withdrawals due to adverse effects during 3 to 12 weeks of treatment with alpha blockers was only reported in 4 of the included trials (Cubeddu 1988, Gillenwater 1995, Harder 1994, Singleton 1989). There were insufficient data to evaluate the dose‐related effect of the individual alpha blockers on withdrawals due to adverse effects. These 4 trials were combined to assess the effects of alpha blockers as a class on WDAE and there was no statistically significant difference versus placebo.
Discussion
Only 10 trials with a mean duration of 5.9 weeks at a fixed dose of alpha blocker met the pre‐specified inclusion criteria and reported data on 1175 participants (811 treated with alpha blockers and 364 treated placebo) with a weighted mean age of 57 years, weighted mean baseline blood pressure of 155/101 mm Hg and a mean pulse pressure of 54 mm Hg.
Due to the limited number of published studies, there is insufficient evidence for the various alpha blockers to generate dose‐response curves for systolic and diastolic BP reduction as well as accomplish the secondary goals of this review. At any given dose of alpha blocker, there were only 1 or 2 studies contributing BP data, with the only exception being 3 studies that measured the trough DBP lowering efficacy of doxazosin at 4 mg once daily.
What is the magnitude of the effect of alpha blockers on BP?
When the different alpha blockers are compared, there is a remarkable similarity in the trough BP lowering effects of doxazosin and terazosin (Table 2). Although numerically higher, the trough BP lowering effect of prazosin is not significantly different than either doxazosin or terazosin using indirect comparisons. When the best estimate of the BP lowering efficacy of these 3 drugs is combined, the overall estimate is ‐8/‐5 mmHg. Because of the high likelihood of publication bias as discussed above this is most likely an overestimate of the real effect. If that is the case, then alpha blockers probably have a lesser ability to lower blood pressure than drugs inhibiting the renin angiotensin system, which also have been estimated to lower trough BP by 8/5 mmHg (Heran 2008a, Heran 2008b, Musini 2008). If it is not an overestimate then the BP lowering effect could be similar. Complete reporting of all the alpha blocker trials that have been completed are needed.
Given the limited data available, it is impossible with this analysis to be certain that there are any BP lowering differences between one or more of the drugs. It would require head‐to‐head trials of different alpha blockers at equivalent BP lowering doses to assess whether or not there are differences between different drugs. This review will provide useful information for estimating equivalent doses and thereby designing trials to compare different alpha blockers. However, at the present time given that all the drugs are working by the same mechanism and the similarities in the blood pressure lowering effect it is most likely that the near maximal BP lowering of the different alpha blockers is the same.
For each alpha blocker, do the manufacturer's dosage recommendations coincide with the findings of this review?
There is insufficient evidence of the dose‐related BP lowering efficacy for each alpha blocker and thus no comparison could be made with the manufacturer's recommendations.
What is the effect of alpha blockers on BP variability?
The variability of blood pressure at both baseline and endpoint was not reported in any of the included trials so the effect of alpha blockers on blood pressure variability could not be assessed.
Is there evidence of a dose‐response relationship for heart rate?
There is a possibility of selective reporting bias of resting heart rate only 3 trials reported data for this outcome. Based on the 3 trials for which data were available, alpha blockers do not appear to affect heart rate. However, there were insufficient data to determine a dose‐related effect on heart rate.
Is there evidence of a dose‐response relationship for withdrawals due to adverse effects?
There were not enough data to construct a meaningful dose‐response relationship for individual alpha blockers to determine a dose‐related effect on WDAE. The available data demonstrate that for all doses alpha blockers did not change WDAE compared with placebo. However, only 4 included trials reported the number of WDAE, so selective reporting bias is a distinct possibility. A description of the type and severity of the adverse effects that led to premature withdrawal was rarely reported. Short‐term trials are not the best type of trial to assess adverse effects and longer trials and other types of data can assist, such as non‐randomized trials or post‐marketing surveillance studies. However, there is no justification for not reporting all withdrawals due to adverse effects in all completed trials.
Limitations of the review
Given that alpha blockers are commonly prescribed as second‐ or third‐line antihypertensive agents or even as first‐line agents in hypertensive males with benign prostatic hyperplasia, the lack of published RCT evidence of the dose‐ranging BP lowering efficacy for this class of drugs is alarming. It is clear that not all the trials assessing the efficacy of alpha blockers have been published. We know that because many of the doses that have been approved by regulators are not included in this review. For example, there were no trials assessing maximum recommended dose of doxazosin, 16 mg daily. Also, the usual recommended dose range of terazosin is 1 mg to 5 mg administered once a day; however, there are no available data for 1 mg per day. Furthermore, efficacy data are available for 3 mg of standard prazosin only, yet it is recommended from an initial dose of 1 mg two or three times a day up to a total daily dose of 20 mg. We know that trials must have been completed and provided to the regulators for the other doses.
Authors' conclusions
Implications for practice.
Specific findings of the review
The review provides very limited data on the dose‐related blood pressure lowering efficacy of 4 different alpha blockers at trough. A dose‐response relationship for the blood pressure lowering effect of the alpha blockers could not be established. The best estimate of the trough blood pressure lowering efficacy of these drugs is ‐8/‐5 mm Hg; however, this is likely an overestimate of the real effect. The data do not suggest that any one alpha blocker is better or worse at lowering blood pressure.
The effect of alpha blockers on blood pressure variability, pulse pressure, or heart rate could not be determined.
All doses of alpha blockers, whether analyzed individually or combined, did not change WDAE as compared to placebo; however, this outcome was not reported for 6 of the 10 trials so this lack of demonstrated effect is unlikely to be real.
Implications of these findings
The major limitation of this review is that it is limited to available trials and it is evident that a lot of trials that manufacturers would have needed to gain marketing approval have not been published and otherwise available. Thus, even though there was no evidence of publication bias using standard methods to assess this, there remains a high risk for publication bias. It is also estimated that there is a risk of patient selection bias that could have led to overestimation of the blood pressure lowering effect. For these reasons, the magnitude of blood pressure lowering found is this review is probably an overestimate of the true effect. This observation makes even more surprising that the estimates of trough and peak blood pressure lowering effects of the alpha blockers are modest at best and lower than commonly believed can be achieved by this class of drugs. Furthermore, the dose‐related blood pressure lowering effect of these drugs could not be established and thus it is unclear whether higher doses have a greater effect than demonstrated here. This review did not provide any evidence of an increase in withdrawals due to adverse effects. However, this finding is severely limited by the short duration of the included trials and a high risk of both selective reporting bias and patient selection bias. Therefore, this systematic review is not a good measure of the incidence of adverse effects of this class of drugs.
Therefore, due to limited published RCT evidence, this review is only able to provide physicians with limited information necessary to optimize prescribing of alpha blockers in patients with elevated blood pressure.
Implications for research.
It is evident that for some of the alpha blockers studied, trials reporting data on doses recommended for use are not published. It should be mandatory that all clinical trials be registered and the results of these trials be published or otherwise made available in full detail.
Full dose‐response data for doses within the recommended and beyond the recommended dose range are needed to properly appreciate the dose‐response relationship for each alpha blocker.
Trials should measure and report blood pressure data for peak effects as well as trough effects.
All trials should report both systolic and diastolic BP and heart rate plus all withdrawals due to adverse effects and serious adverse events.
What's new
| Date | Event | Description |
|---|---|---|
| 9 July 2012 | New citation required but conclusions have not changed | updated |
| 9 July 2012 | New search has been performed | The electronic search for new studies was updated to May 2012. No new studies were identified that met the inclusion criteria of this review. |
Acknowledgements
The authors would like to thank Douglas Salzwedel for developing and running the updated searches. The authors would like to acknowledge the assistance provided by Mr. Stephen Adams who retrieved the papers for this review. The authors would also like to acknowledge the contribution of Dr. Gurdial Mattu and Mr. Andrew Desouza to the protocol.
Appendices
Appendix 1. MEDLINE search strategy
Database: Ovid MEDLINE(R) 1946 to Present with Daily Update Search Date: 8 May 2012 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp adrenergic alpha antagonists/ 2 (alfuzosin or bunazosin or doxazosin or indoramin or metazosin or neldazosin or prazosin or silodosin or tamsulosin or terazosin or tiodazosin or trimazosin).mp. 3 (alpha adrenergic antagonist? or alpha adrenergic receptor antagonist? or adrenergic alpha antagonist?).tw. 4 ((alpha or alpha‐adrenergic) adj2 block$).tw. 5 or/1‐4 6 hypertension/ 7 hypertens$.tw. 8 exp blood pressure/ 9 blood pressure.mp. 10 or/6‐9 11 randomized controlled trial.pt. 12 controlled clinical trial.pt. 13 randomi?ed.ab. 14 placebo.ab. 15 clinical trials as topic/ 16 randomly.ab. 17 trial.ti. 18 or/11‐17 19 animals/ not (humans/ and animals/) 20 18 not 19 21 5 and 10 and 20
Appendix 2. EMBASE search strategy
Database: Embase <1974 to 2012 Week 18> Search Date: 8 May 2012 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp alpha adrenergic receptor blocking agent/ 2 (alfuzosin or bunazosin or doxazosin or indoramin or metazosin or neldazosin or prazosin or silodosin or tamsulosin or terazosin or tiodazosin or trimazosin).mp. 3 (alpha adrenergic antagonist? or alpha adrenergic receptor antagonist? or adrenergic alpha antagonist?).tw. 4 ((alpha or alpha‐adrenergic) adj2 block$).tw. 5 or/1‐4 6 exp hypertension/ 7 hypertens$.tw. 8 exp blood pressure/ 9 (blood pressure or bloodpressure).tw. 10 or/6‐9 11 randomized controlled trial/ 12 crossover procedure/ 13 double‐blind procedure/ 14 (randomi?ed or randomly).tw. 15 (crossover$ or cross‐over$).tw. 16 placebo$.tw. 17 (doubl$ adj blind$).tw. 18 allocat$.tw. 19 comparison.ti. 20 trial.ti. 21 or/11‐20 22 (animal$ not (human$ and animal$)).mp. 23 21 not 22 24 5 and 10 and 23
Appendix 3. CENTRAL search strategy
Database: EBM Reviews ‐ Cochrane Central Register of Controlled Trials <April 2012> Search Date: 8 May 2012 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp adrenergic alpha antagonists/ 2 (alfuzosin or bunazosin or doxazosin or indoramin or metazosin or neldazosin or prazosin or silodosin or tamsulosin or terazosin or tiodazosin or trimazosin).mp. 3 (alpha adrenergic antagonist? or alpha adrenergic receptor antagonist? or adrenergic alpha antagonist?).tw. 4 ((alpha or alpha‐adrenergic) adj2 block$).tw. 5 or/1‐4 6 hypertension/ 7 hypertens$.tw. 8 exp blood pressure/ 9 blood pressure.mp. 10 or/6‐9 11 5 and 10
Appendix 4. Search strategies used in original review
MEDLINE
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
exp double blind method/
(double adj blind$).ti,ab.
(double adj mask$).ti,ab.
or/1‐11
(animals not (human and animals)).sh.
12 not 13
exp hypertension/
hypertens$.ti,ab.
exp blood pressure/
(blood adj pressure).ti,ab.
or/15‐18
exp adrenergic alpha‐antagonists/
alfuzosin.ti,ab.
bunazosin.ti,ab.
exp doxazosin/
doxazosin.ti,ab.
exp prazosin/
prazosin.ti,ab.
tamsulosin.ti,ab.
terazosin.ti,ab.
trimazosin.ti,ab.
exp indoramin/
indoramin.ti,ab.
or/20‐31
exp placebos/
placebo$.ti,ab.
or/33‐34
14 and 19 and 32 and 35
EMBASE
random$.mp.
factorial$.mp.
crossover$.mp.
cross over$.mp.
cross‐over$.mp.
placebo$.mp.
(doubl$ adj blind$).mp.
(singl$ adj blind$).mp.
assign$.mp.
allocat$.mp.
volunteer$.mp.
or/1‐11
Crossover Procedure/
Double Blind Procedure/
Randomized Controlled Trial/
Single Blind Procedure/
or/13‐16
12 or 17
exp hypertension/
hypertens$.ti,ab.
exp blood pressure/
blood pressure.ti,ab.
or/19‐22
exp alfuzosin/
alfuzosin.ti,ab.
exp bunazosin/
bunazosin.ti,ab.
exp doxazosin/
doxazosin.ti,ab.
exp prazosin/
prazosin.ti,ab.
exp tamsulosin/
tamsulosin.ti,ab.
exp terazosin/
terazosin.ti,ab.
exp trimazosin/
trimazosin.ti,ab.
exp indoramin/
indoramin.ti,ab.
or/24‐39
exp placebos/
placebo$.ti,ab.
41 or 42
18 and 23 and 40 and 43
CENTRAL
((doubl*) NEXT (blind* or mask*)):ti,ab
alpha* NEAR/5 antagonist*:ti,ab
alpha* NEAR/5 blocker*:ti,ab
alfuzosin:ti,ab
bunazosin:ti,ab
doxazosin:ti,ab
indoramin:ti,ab
prazosin:ti,ab
tamsulosin:ti,ab
terazosin:ti,ab
trimazosin:ti,ab
#2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11
hypertens*:ti,ab
"blood pressure*":ti,ab
#13 or #14
placebo*:ti,ab
#1 and #12 and #15 and #16
Data and analyses
Comparison 1. Doxazosin vs Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in trough SBP | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 2 mg | 2 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐5.03 [‐10.71, 0.64] |
| 1.2 4 mg | 2 | 91 | Mean Difference (IV, Fixed, 95% CI) | ‐5.84 [‐11.44, ‐0.23] |
| 1.3 8 mg | 2 | 91 | Mean Difference (IV, Fixed, 95% CI) | ‐7.24 [‐12.93, ‐1.56] |
| 1.4 12 mg | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐6.1 [‐15.27, 3.07] |
| 2 Change in trough DBP | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 2 mg | 2 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐3.01 [‐6.43, 0.42] |
| 2.2 4 mg | 3 | 326 | Mean Difference (IV, Fixed, 95% CI) | ‐3.36 [‐5.06, ‐1.66] |
| 2.3 8 mg | 2 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐4.13 [‐7.61, ‐0.65] |
| 2.4 12 mg | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐3.8 [‐8.64, 1.04] |
| 3 Change in peak SBP | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 2 mg | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐6.8 [‐13.96, 0.36] |
| 3.2 4 mg | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐6.0 [‐13.08, 1.08] |
| 3.3 8 mg | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐12.90 [‐20.34, ‐5.46] |
| 4 Change in peak DBP | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 2 mg | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐4.30 [‐8.92, 0.32] |
| 4.2 4 mg | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐4.4 [‐9.02, 0.22] |
| 4.3 8 mg | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐8.5 [‐13.32, ‐3.68] |
| 5 Change in trough heart rate | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 2 mg | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐6.62, 2.82] |
| 5.2 4 mg | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐6.22, 3.22] |
| 5.3 8 mg | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐4.67, 6.67] |
| 6 Change in peak heart rate | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 2 mg | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐4.82, 4.62] |
| 6.2 4 mg | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐3.42, 6.02] |
| 6.3 8 mg | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 3.2 [‐2.47, 8.87] |
| 7 Total withdrawals due to adverse effects | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 2‐8 mg | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [0.24, 15.54] |
| 7.2 2‐12 mg | 1 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [0.66, 11.13] |
1.1. Analysis.
Comparison 1 Doxazosin vs Placebo, Outcome 1 Change in trough SBP.
1.2. Analysis.
Comparison 1 Doxazosin vs Placebo, Outcome 2 Change in trough DBP.
1.3. Analysis.
Comparison 1 Doxazosin vs Placebo, Outcome 3 Change in peak SBP.
1.4. Analysis.
Comparison 1 Doxazosin vs Placebo, Outcome 4 Change in peak DBP.
1.5. Analysis.
Comparison 1 Doxazosin vs Placebo, Outcome 5 Change in trough heart rate.
1.6. Analysis.
Comparison 1 Doxazosin vs Placebo, Outcome 6 Change in peak heart rate.
1.7. Analysis.
Comparison 1 Doxazosin vs Placebo, Outcome 7 Total withdrawals due to adverse effects.
Comparison 2. Prazosin vs Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in trough SBP | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 2.5 mg GITS | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐3.60 [‐11.86, 4.66] |
| 1.2 3 mg | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐13.88, 7.88] |
| 1.3 10 mg GITS | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐9.20 [‐17.23, ‐1.17] |
| 1.4 20 mg GITS | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | ‐11.7 [‐20.17, ‐3.23] |
| 2 Change in trough DBP | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 2.5 mg GITS | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐2.4 [‐6.54, 1.74] |
| 2.2 3 mg | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐4.0 [‐8.00, 2.00] |
| 2.3 10 mg GITS | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐6.80 [‐10.87, ‐2.73] |
| 2.4 20 mg GITS | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | ‐7.0 [‐11.11, ‐2.89] |
| 3 Change in trough heart rate | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 2.5 mg GITS | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | 2.1 [‐2.53, 6.73] |
| 3.2 10 mg GITS | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | 1.80 [‐2.79, 6.39] |
| 3.3 20 mg GITS | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 2.9 [‐1.69, 7.49] |
| 4 Total withdrawals due to adverse effects | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 2.5‐20 mg GITS | 1 | 220 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.21, 2.43] |
2.1. Analysis.
Comparison 2 Prazosin vs Placebo, Outcome 1 Change in trough SBP.
2.2. Analysis.
Comparison 2 Prazosin vs Placebo, Outcome 2 Change in trough DBP.
2.3. Analysis.
Comparison 2 Prazosin vs Placebo, Outcome 3 Change in trough heart rate.
2.4. Analysis.
Comparison 2 Prazosin vs Placebo, Outcome 4 Total withdrawals due to adverse effects.
Comparison 3. Terazosin vs Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in trough SBP | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 5 mg | 1 | 79 | Mean Difference (IV, Fixed, 95% CI) | ‐6.9 [‐12.20, ‐1.60] |
| 1.2 10 mg | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐6.10 [‐12.08, ‐0.12] |
| 1.3 20 mg | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐6.80 [‐15.73, 2.13] |
| 2 Change in trough DBP | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 5 mg | 2 | 205 | Mean Difference (IV, Fixed, 95% CI) | ‐4.92 [‐6.87, ‐2.97] |
| 2.2 10 mg | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐3.2 [‐6.54, 0.14] |
| 2.3 20 mg | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐3.9 [‐7.95, 0.15] |
3.1. Analysis.
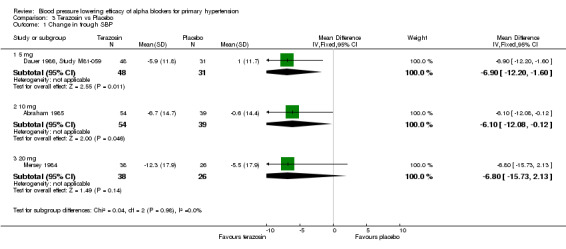
Comparison 3 Terazosin vs Placebo, Outcome 1 Change in trough SBP.
3.2. Analysis.
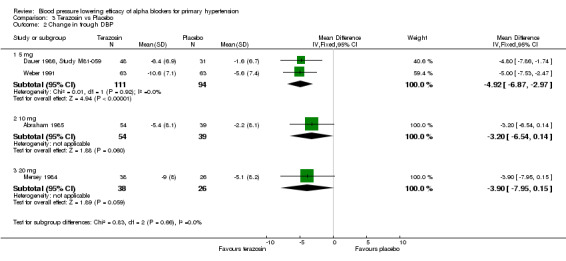
Comparison 3 Terazosin vs Placebo, Outcome 2 Change in trough DBP.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abraham 1985.
| Methods | Minimum 1‐week washout; 3‐week single‐blind placebo baseline period; inclusion criteria=supine DBP 95‐114 mm Hg; 6‐week double‐blind treatment, consisting of 2‐week dose titration period (1 mg once daily for 3 days, 2 mg once daily for 4 days and 5 mg once daily for 7 days) followed by 4‐week "maintenance" period at fixed dose of 10 mg | |
| Participants |
Reported in Abraham 1985: All patients: n=38 patients randomized to double‐blind treatment; n=32 completed 6 weeks of double‐blind treatment and included in efficacy analysis Terazosin 10 mg: n=17 (12 males, 5 females); 15 white, 2 black; mean age=51.7(10.5) years; baseline standing SBP=156.6(15.4) mm Hg, DBP=111.7(6.7) mm Hg, HR=79.9(9.0) bpm; baseline supine SBP=155.6(12.5) mm Hg, DBP=101.9(5.7) mm Hg, HR=71.9(8.4) bpm Placebo: n=15 (12 males, 3 females); 14 white, 1 black; mean age=46.6(10.5) years; baseline standing SBP=155.3(17.0) mm Hg, DBP=109.2(6.8) mm Hg; HR=82.1(7.5) bpm; baseline supine SBP=153.9(15.7) mm Hg, DBP=100.0(3.6) mm Hg; HR=73.9(7.3) bpm Reported in Dauer 1986 (Study M81‐060): Terazosin 10 mg: n=54; baseline standing SBP=155.5(17.6) mm Hg, DBP=105.4(7.4) mm Hg; baseline supine SBP=157.2(15.4) mm Hg, DBP=101.8(5.1) mm Hg Placebo: n=39; baseline standing SBP=154.7(16.2) mm Hg, DBP=106.1(6.9) mm Hg; baseline supine SBP=156.5(15.6) mm Hg, DBP=101.9(5.0) mm Hg |
|
| Interventions | Terazosin 10 mg once daily Placebo Taken in the morning between 7 and 10 AM |
|
| Outcomes |
Reported in Abraham 1985: Trough standing SBP/DBP using mercury sphygmomanometer Trough supine SBP/DBP using mercury sphygmomanometer Trough standing HR Trough supine HR Peak standing SBP/DBP using mercury sphygmomanometer Peak supine SBP/DBP using mercury sphygmomanometer Peak standing HR Peak supine HR WDAE Reported in Dauer 1986 (Study M81‐060): Change from baseline in trough standing SBP/DBP using mercury sphygmomanometer Change from baseline in trough supine SBP/DBP using mercury sphygmomanometer |
|
| Notes | Used BP data reported in Dauer 1986 instead of Abraham 1985 Reported in Abraham 1985: BP change and SD of change not reported, endpoint BP and SD reported; baseline BP and SD reported; imputed endpoint SD for SD of change; trough BP and HR data from Table 1, p. 287; peak BP and HR data from Table 1, p. 287 Reported in Dauer 1986 (Study M81‐060): BP change and SE of change reported, endpoint BP and SE not reported; baseline BP and SE reported; calculated SD of change from N and change SE; BP data from Table IV, p. 32 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "...would receive an identical‐appearing placebo capsule (medications were provided by Abbott Laboratories, North Chicago, IL)." Comment: Probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5/22 missing from intervention group (3 due to 'uncontrolled hypertension'); 1/16 missing from control group (due to 'uncontrolled hypertension'). |
| Selective reporting (reporting bias) | High risk | BP lowering efficacy was primary outcome. HR and safety/tolerability reported in Abraham 1985 but not separately for Study M81‐060 in Dauer 1986. |
| Other bias | High risk | Abraham 1985 reported on a subgroup of patients from a larger trial (n=93) trial that has not been published, only summarized in two review articles (Dauer 1986, Titmarsh 1987). Comment: Publication bias. Full trial data from Dauer 1986 used instead. Quote: None had a history of failure to respond to prazosin; orthostatic hypotension, fainting spells or blackouts..." Comment: Patient selection bias. Funding source not reported. |
Cubeddu 1988.
| Methods | 2‐week screening period; 4‐week single‐blind placebo run‐in period; inclusion criteria=supine and standing DBP >95 mm Hg during screening period, and supine and standing DBP >90 mm Hg during screening period and placebo run‐in; 9‐week double‐blind treatment, consisting of 3 upward titration schedules up to pre‐assigned dose of doxazosin (2, 4, or 8 mg) or placebo; this titration schedule was followed until "goal" BP was achieved, defined as reduction in supine or standing diastolic BP of ≥10 mm Hg from baseline and to less than 90 mm Hg at 24h postdose; if adverse effects (limiting or persisting side effects) or significant laboratory toxicity prevented further upward titration, then patient was discontinued from study | |
| Participants | Doxazosin 2 mg: n=38 (25 males, 13 females); 26 white, 11 black, 1 other; mean age=52.4 (range 28‐68) years; n=34 with baseline BP reported; baseline standing SBP=150.5 mm Hg, DBP=102.2 mm Hg, HR=79.6 bpm; baseline supine BP=152.7 mm Hg, DBP=98.8 mm Hg, HR=73.1 bpm Doxazosin 4 mg: n=31 (20 males, 11 females); 22 white, 9 black; mean age=50.6 (range 32‐66) years; n=25 with baseline BP reported; baseline standing SBP=147.4 mm Hg, DBP=102.2 mm Hg, HR=80.0 bpm; baseline supine BP=149.4 mm Hg, DBP=99.8 mm Hg, HR=71.9 bpm Doxazosin 8 mg: n=33 (17 males, 16 females); 19 white, 13 black, 1 not recorded; mean age=49.4 (range 26‐66) years; n=25 with baseline BP reported; baseline baseline standing SBP=147.5 mm Hg, DBP=102.4 mm Hg, HR=79.6 bpm; baseline supine BP=150.3 mm Hg, DBP=99.9 mm Hg, HR=72.9 bpm Placebo: n=33 (22 males, 11 females); 18 white, 15 black; mean age=50.5 (range 33‐71) years; n=30 with baseline BP reported; baseline standing SBP=140.9 mm Hg, DBP=99.7 mm Hg, HR=84.5 bpm; baseline supine BP=145.8 mm Hg, DBP=98.8 mm Hg, HR=76.5 bpm |
|
| Interventions | Doxazosin 2 mg once daily Doxazosin 4 mg once daily Doxazosin 8 mg once daily Placebo Taken upon awakening |
|
| Outcomes | Trough standing SBP/DBP using mercury sphygmomanometer Trough supine SBP/DBP using mercury sphygmomanometer Peak standing SBP/DBP using mercury sphygmomanometer Trough standing HR Trough supine HR Peak standing HR WDAE |
|
| Notes | BP change and SE of change reported, endpoint BP reported, endpoint SD not reported, baseline BP reported, baseline SD not reported; calculated SD of change from N and SE of change; BP and HR data from Table 5, p. 163 Mean doxazosin doses prior to final BP observation were 2, 4, and 7.7 mg for 2, 4, and 8 mg groups, respectively; 1 of 34 patients in 2 mg group was receiving 1 mg doxazosin and only 2 of 25 patients in 8 mg group were receiving a lower dose (4 mg) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 21/135 patients (4/38 doxazosin 2 mg; 6/31 doxazosin 4 mg; 8/33 doxazosin 8 mg; 3/33 placebo) who "discontinued during the double‐blind period" not included in efficacy analysis. Reasons for discontinuations not described. |
| Selective reporting (reporting bias) | Low risk | BP lowering efficacy was primary outcome. HR and safety/tolerability data reported. |
| Other bias | Unclear risk | Funding source not reported. |
Dauer 1986, Study M81‐059.
| Methods | Minimum 1‐week washout; 3‐week single‐blind placebo baseline period; inclusion criteria=supine DBP 95‐114 mm Hg; 5‐week double‐blind treatment, consisting of 1‐week dose titration period (1 mg once daily increased by steps to 5 mg once daily, regardless of BP) followed by 4‐week "maintenance" period at fixed dose | |
| Participants | Terazosin 5 mg: n=48; baseline standing SBP=149.8(13.9) mm Hg, DBP=100.4(6.2) mm Hg, baseline supine SBP=156.0(13.2) mm Hg, DBP=100.1(3.5) mm Hg Placebo: n=31; baseline standing SBP=148.7(13.9) mm Hg, DBP=101.6(6.1) mm Hg, baseline supine SBP=154.2(13.4) mm Hg, DBP=100.1(3.3) mm Hg |
|
| Interventions | Terazosin 5 mg once daily Placebo |
|
| Outcomes | Change from baseline in trough standing SBP/DBP using mercury sphygmomanometer Change from baseline in trough supine SBP/DBP using mercury sphygmomanometer |
|
| Notes | BP change and SE of change reported, endpoint BP and SE not reported; baseline BP and SE reported; calculated SD of change from N and SE of change; BP data from Table IV, p. 32 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses to follow‐up and withdrawals not reported separately for M81‐059. Unclear how dropouts were dealt with. |
| Selective reporting (reporting bias) | High risk | BP lowering efficacy was primary outcome. HR and safety/tolerability not reported separately for Study M81‐059. |
| Other bias | High risk | Full trial data has not been published separately, only summarized in this review article. Funding source not reported. |
Gillenwater 1995.
| Methods | 0 to 4‐week screening/washout period; 2‐week placebo run‐in period; inclusion criteria=sitting DBP 90‐114 mm Hg; 14‐week double‐blind treatment, consisting of 5‐week dose titration period (initial dose of 1 mg once daily, increased sequentially at weekly intervals to randomized, fixed dose level) followed by 9‐week fixed dose period | |
| Participants | All patients: n=248 (199 doxazosin, 49 placebo) male patients ≥45 years old with benign prostatic hyperplasia and mild to moderate hypertension; baseline SBP=163(23) mm Hg, DBP=103(8) mm Hg Doxazosin 2 mg: n=47; mean age=64.8(8.5) years; baseline SBP/DBP not reported Doxazosin 4 mg: n=52; mean age=64.4(7.5) years; baseline SBP/DBP not reported Doxazosin 8 mg: n=50; mean age=63.9(8.4) years; baseline SBP/DBP not reported Doxazosin 12 mg: n=50; mean age=62.8(8.9) years; baseline SBP/DBP not reported Placebo: n=49; mean age=64.5(7.7) years; baseline SBP/DBP not reported |
|
| Interventions | Doxazosin 2 mg once daily Doxazosin 4 mg once daily Doxazosin 8 mg once daily Doxazosin 12 mg once daily Placebo Taken in the morning |
|
| Outcomes | Change from baseline in trough sitting SBP/DBP using sphymomanometer Change from baseline in trough standing SBP/DBP using sphymomanometer WDAE |
|
| Notes | BP change reported; SD of change not reported; endpoint BP and SD not reported; baseline BP and SD not reported; imputed overall trial mean SD of change for SBP and DBP; BP data from Figure 3, p. 114 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 32/248 patients (8/47 doxazosin 2 mg; 6/52 doxazosin 4 mg; 5/50 doxazosin 8 mg; 5/50 doxazosin 12 mg; 8/49 placebo) not included in efficacy analysis. 7/32 patients had no follow‐up efficacy measurements and 25/32 did not meet inclusion criterion for maximum urinary flow rate |
| Selective reporting (reporting bias) | High risk | Baseline BP data not reported. Peak sitting/standing BP not fully reported. Quote: "Changes in heart rate were slight and comparable in both treatment groups." Comment: Quantitative HR data not reported. Safety/tolerability data reported. |
| Other bias | High risk | Quote: "Other reasons for exclusion were intolerance/sensitivity to quinazoline derivatives..." Comment: Patient selection bias. Funding source is manufacturer of doxazosin (Pfizer). |
Harder 1994.
| Methods | 2‐week washout; 2‐week placebo run‐in period; inclusion criteria=sitting DBP 95‐115 mm Hg; 8‐week double‐blind treatment | |
| Participants | All patients: n=16 (11 males, 5 females); all white; age range=42‐68 years; baseline SBP=163(23) mm Hg, DBP=103(8) mm Hg Bunazosin 3 mg: n=4; baseline supine SBP=155.3(23.9) mm Hg, DBP=100.0(7.1) mm Hg, HR=87.8(12.2) bpm Bunazosin 6 mg: n=4; baseline supine SBP=168.5(9.3) mm Hg, DBP=98.8(4.8) mm Hg; HR=76.0(13.5) bpm Bunazosin 12 mg: n=4; baseline supine SBP=180.3(28.4) mm Hg, DBP=110.0(8.6) mm Hg; HR=81.0(13.1) bpm Placebo: n=4; baseline supine SBP=149.0(21.0) mm Hg, DBP=102.5(6.5) mm Hg, HR=85.0(16.1) bpm |
|
| Interventions | Bunazosin 3 mg once daily Bunazosin 6 mg once daily Bunazosin 12 mg once daily Placebo |
|
| Outcomes | Trough supine SBP/DBP using mercury sphygmomanometer Trough supine HR WDAE |
|
| Notes | Individual patient BP and HR reported at baseline and endpoint; calculated BP change and SD of change for each group; BP and HR data from Table 1, p. 39 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One patient on 6 mg bunazosin was withdrawn from the study after the occurrence of nycturia due to mild heart failure." |
| Selective reporting (reporting bias) | Low risk | BP lowering efficacy was primary outcome. HR and safety/tolerability data reported. |
| Other bias | High risk | Quote: "The study was performed in one center as a part of a double‐blind, placebo controlled multicenter trial comparing the efficacy of three different dose levels of bunazosin and placebo in a parallel‐group design." Comment: Publication of the full trial results has not been identified. Risk of publication bias is high. Funding source not reported. |
Mersey 1984.
| Methods | Minimum 2‐week washout period; 3‐week single‐blind placebo lead‐in period; inclusion criteria=supine DBP 95‐114 mm Hg; 7‐week double‐blind treatment, consisting of 3‐week dose titration period (initial dose of 1 mg once daily, increased by steps to 20 mg once daily, regardless of BP) followed by 4‐week fixed dose period | |
| Participants |
Reported in Mersey 1984: All patients: n=64(41 males, 23 females); 35 white, 29 other; age range 21‐72 years; Terazosin 20 mg: n=38; baseline supine SBP=149.7 mm Hg, DBP=100.4 mm Hg Placebo: n=26; baseline supine SBP=147.1 mm Hg, DBP=99.2 mm Hg Reported in Dauer 1986 (Study M81‐061): Terazosin 20 mg: n=38; baseline standing SBP=147.9(15.4) mm Hg, DBP=103.3(6.2) mm Hg; baseline supine SBP=152.2(13.6) mm Hg, DBP=101.0(3.7) mm Hg Placebo: n=26; baseline standing SBP=143.8(15.3) mm Hg, DBP=100.4(6.1) mm Hg; baseline supine SBP=147.1(13.8) mm Hg, DBP=99.2(3.6) mm Hg |
|
| Interventions | Terazosin 20 mg once daily Placebo |
|
| Outcomes |
Reported in Mersey 1984: Change from baseline in trough supine SBP/DBP using mercury sphygmomanometer Change from baseline in peak supine SBP/DBP using mercury sphygmomanometer Reported in Dauer 1986 (Study M81‐061): Change from baseline in trough standing SBP/DBP using mercury sphygmomanometer Change from baseline in trough supine SBP/DBP using mercury sphygmomanometer |
|
| Notes | Used BP data reported in Dauer 1986 instead of Mersey 1984 Reported in Mersey 1984: BP change reported, SD of change not reported, endpoint BP reported, endpoint SD not reported; baseline BP reported, baseline SD not reported Reported in Dauer 1986 (Study M81‐061): BP change and SE of change reported, endpoint BP and SD not reported; baseline BP and SE reported; calculated SD of change from N and SE of change; BP data from Table IV, p. 32 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses to follow‐up and withdrawals not reported in Mersey 1984 or separately for M81‐061 in Dauer 1986. Unclear how dropouts were dealt with. |
| Selective reporting (reporting bias) | High risk | BP lowering efficacy was primary outcome. Quote: "...pulse rate did not increase with P or T." Comment: Quantitative HR and safety/tolerability not reported in abstract or separately for Study M81‐059 in Dauer 1986. |
| Other bias | High risk | Full trial data has not been published separately, only summarized in abstract and Dauer 1986 review article. Funding source not reported. |
Os 1999.
| Methods | 2‐week single‐blind placebo run‐in period; inclusion criteria=DBP 95‐105 mm Hg and SBP ≤180 mm Hg; 12‐week double‐blind treatment, consisting of dose titration period (to achieve target BP ≤90 mm Hg and a 10 mm Hg decrease from baseline 24 h postdose) at 5 weeks | |
| Participants | All patients: n=392; all white; mean age=55.9 (range 24‐80) years Doxazosin GITS: n=161 (75 males, 86 females); mean age=56.4 (range 29‐79) years; baseline sitting SBP=157(14) mm Hg, DBP=99(3) mm Hg, HR=72(8) bpm; baseline standing SBP=158(14) mm Hg, DBP=102(5) mm Hg, HR=76(9) bpm Doxazosin Standard: n=155(72 males, 83 females); mean age=55.6 (range 26‐80) years; baseline sitting SBP=157(14) mm Hg, DBP=99(3) mm Hg, HR=73(9) bpm; baseline standing SBP=158(15) mm Hg, DBP=102(5) mm Hg, HR=77(10) bpm Placebo: n=74(40 males, 34 females); mean age=55.4 (range 24‐78) years; baseline sitting SBP=157(14) mm Hg, DBP=99(3) mm Hg, HR=72(9) bpm; baseline standing SBP=158(14) mm Hg, DBP=101(5) mm Hg, HR=76(10) bpm |
|
| Interventions | Doxazosin GITS 4‐8 mg once daily; patients received doxazosin GITS 4 mg for at least 5 weeks; at week 5, dose was increased to 8 mg once daily if target BP not achieved Doxazosin Standard 1‐8 mg once daily; patients received initial dose of 1 mg; dose was increased at 1 week to 2 mg, at 3 weeks to 4 mg, and at 5 weeks to 8 mg if target BP not achieved Placebo Taken at breakfast |
|
| Outcomes | Change from baseline in trough sitting SBP/DBP using sphymomanometer Change from baseline in trough standing SBP/DBP using sphymomanometer Change from baseline in trough sitting HR Change from baseline in trough standing HR WDAE |
|
| Notes | Used only week 5 BP data for doxazosin GITS; SBP at week 5 not reported; DBP at week 5 reported; DBP SD of change at week 5 not reported; BP change and SD of change reported at endpoint; baseline BP and SD reported; imputed endpoint DBP SD of change; week 5 DBP data from Figure 5, p. 189 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Two patients randomized to placebo were not eligible for inclusion in the ITT population (n=390) because they had no on‐treatment efficacy assessment." |
| Selective reporting (reporting bias) | High risk | SBP and safety/tolerability data at week 5 not reported. Quote: "No significant changes in sitting or standing HR were observed in any of the treatment groups." Comment: Quantitative HR data not reported. |
| Other bias | High risk | Quote: "...those with a known sensitivity to α‐blocking drugs...were also ineligible." Comment: Patient selection bias. Funding source is manufacturer of doxazosin GITS (Pfizer). |
Schnaper 1975, Study 2.
| Methods | 2‐week screening/observation period; 2‐week placebo period; inclusion criteria=supine DBP 95‐115 mm Hg; 6‐week double‐blind treatment period, followed by 1‐week placebo period, followed by 4‐week double‐blind continuation of fixed dosage of medication to responding patients Responding patients included: 1) those whose mean arterial pressure (DBP plus 1/3 PP), either supine or standing, had dropped by ≥15 mm Hg since end of preceding placebo period; and 2) patients whose supine or standing DBP decreased to ≤90 mm Hg. Patients who did not respond were dropped from study and were started on a titration regimen of 1 of 2 active drugs. |
|
| Participants | Prazosin 3 mg: n=20; baseline SBP/DBP not reported Placebo: n=10; baseline SBP/DBP not reported |
|
| Interventions | Prazosin 3 mg once daily Placebo |
|
| Outcomes | Change from baseline in trough standing SBP/DBP using mercury sphygmomanometer Change from baseline in trough standing SBP/DBP using mercury sphygmomanometer |
|
| Notes | BP change reported; SD of change not reported; endpoint BP and SD not reported; baseline BP and SD not reported; imputed overall trial mean SD of change for SBP and DBP; BP data from Table 4, p. 85 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Low risk | Quote: "...and each patient was given an individually coded bottle. A sealed envelope containing the code was attached to the bottle so the investigator could selectively break the code in an emergency." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Medication was dispensed in identical capsules..." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "In two patients, pressure rose to an unacceptable level after six weeks and four weeks, respectively. The code was broken, and they were given active therapy with prazosin. These patients are clearly failures on placebo and as such are significant to this trial. For this reason, they were included in the efficacy analysis as placebo patients, with the last pressures recorded during the double‐blind phase as their final values." |
| Selective reporting (reporting bias) | High risk | BP lowering efficacy was primary outcome. Safety/tolerability data not reported |
| Other bias | Unclear risk | Funding source not reported. |
Singleton 1989.
| Methods | 1‐week washout; 2‐week single‐blind placebo run‐in period; inclusion criteria=sitting DBP 95‐105 mm Hg for 2 consecutive weeks; 7‐week double‐blind treatment, consisting of 3‐week dose titration period (to preassigned dose group) followed by 4‐week "maintenance" period at preassigned fixed dose unless limited by side effects | |
| Participants | All patients: n=220 (166 prazosin GITS, 54 placebo); n=205 patients included in efficacy analysis; Prazosin GITS 2.5 mg: n=49 (32 males, 17 females); 35 white, 12 black, 2 other; mean age=54.4(11.3) years; baseline sitting SBP=150.3(14.1) mm Hg, DBP=99.4(3.2) mm Hg, HR=76.5(8.8) bpm; baseline standing SBP=150.6(13.6) mm Hg, DBP=100.5(5.8) mm Hg, HR=80.1(9.0) bpm Prazosin GITS 10 mg; n=56 (38 males, 18 females); 44 white, 10 black, 2 other; mean age=54.1(12.2) years; baseline sitting SBP=150.0(13.2) mm Hg, DBP=100.6(3.0) mm Hg, HR=76.0(9.1) bpm; baseline standing SBP=149.5(14.1) mm Hg, DBP=101.8(5.4) mm Hg, HR=77.5(8.8) bpm Prazosin GITS 20 mg; n=52 (32 males, 20 females); 40 white, 10 black, 2 other; mean age=57.1(10.5) years; baseline sitting SBP=155.6(16.1) mm Hg, DBP=100.1(3.0) mm Hg, HR=75.7(8.8) bpm; baseline standing SBP=154.6(16.5) mm Hg, DBP=101.8(5.0) mm Hg, HR=78.6(8.7) bpm Placebo: n=48 (28 males, 20 females); 42 white, 5 black, 1 other; mean age=55.0(10.6) years; baseline sitting SBP=151.9(14.8) mm Hg, DBP=99.5(3.2) mm Hg, HR=77.6(8.0) bpm; baseline standing SBP=150.8(17.1) mm Hg, DBP=101.3(5.9) mm Hg, HR=81.2(8.4) bpm |
|
| Interventions | Prazosin GITS 2.5 mg once daily Prazosin GITS 10 mg once daily Prazosin GITS 20 mg once daily Placebo |
|
| Outcomes | Change from baseline in trough sitting SBP/DBP using sphymomanometer Change from baseline in trough standing SBP/DBP using sphymomanometer Change from baseline in trough sitting HR Change from baseline in trough standing HR WDAE |
|
| Notes | GITS=gastrointestinal therapeutic system; BP change reported, SD of change not reported, endpoint BP and SD not reported; baseline BP and SD reported; imputed baseline SBP SD for SBP SD of change, imputed overall trial mean DBP SD of change; trough BP data from Table IV, p. 47S | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 15/220 patients (9/166 receiving prazosin GITS; 6/54 receiving placebo) who were lost to follow‐up or withdrew prematurely not included in efficacy analysis. |
| Selective reporting (reporting bias) | Low risk | BP lowering efficacy was primary outcome. HR and safety/tolerability data reported. |
| Other bias | High risk | Funding source is manufacturer of prazosin GITS (Pfizer). |
Weber 1991.
| Methods | 4‐week placebo phase; inclusion criteria=supine DBP 100‐114 mm Hg; 14‐week double‐blind treatment consisting of 2‐week titration period followed by three 1‐month fixed‐dose treatment periods; after each 1‐month period, patients were advanced to next highest dosage level even if their BP had already responded well to treatment (i.e. forced titration design) | |
| Participants | All patients: n=256 (179 males, 77 females); 102 white, 86 black, 65 hispanic, 3 other; mean age=58 (range 27‐83) years; baseline SBP/DBP not reported Group 1 (terazosin 1, 2, 5 mg): n=63; baseline SBP/DBP not reported Group 2 (terazosin 5, 10, 20 mg): n=64; baseline SBP/DBP not reported Group 3( terazosin 20, 40, 80 mg): n=66; baseline SBP/DBP not reported Placebo: n=63; baseline SBP/DBP not reported |
|
| Interventions | Terazosin 1 mg during first month, 2 mg during second month, 5 mg during third month Terazosin 5 mg during first month, 10 mg during second month, 20 mg during third month Terazosin 20 mg during first month, 40 mg during second month, 80 mg during third month Placebo during each of the three 1‐month segments Administered as single oral dose between 8 and 10 AM each morning |
|
| Outcomes | Mean change from baseline in trough standing SBP/DBP using mercury sphygmomanometer Mean change from baseline in trough supine SBP/DBP using mercury sphygmomanometer |
|
| Notes | Data for Group 1 after third month (terazosin 5mg) reported only; SBP change and SD not reported; DBP change reported; DBP SD of change not reported, endpoint BP and SD not reported; baseline BP and SD not reported; imputed overal trial mean SD of change for DBP; BP data from text, pp. 907‐8 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Method of analysis (intention‐to‐treat or per protocol) not reported. Losses to follow‐up and withdrawals from study not reported. Unclear how dropouts were dealt with. |
| Selective reporting (reporting bias) | High risk | Quote: "This report provides only a preliminary analysis of the blood pressure data obtained in this study." BP data for Groups 1‐3 not extractable from Figure 2, p. 907. BP data for placebo group not provided in Figure 2. Only DBP data for Group 1 and placebo after third month reported in text; BP data for Groups 2 and 3 not reported in text. Safety/tolerability data not reported. |
| Other bias | High risk | Funding source is manufacturer of terazosin (Abbott). |
BP = blood pressure; SBP = systolic blood pressure; DBP = diastolic blood pressure; PP = pulse pressure; HR = heart rate; bpm = beats per minute; WDAE = withdrawals due to adverse effects; SD = standard deviation; SE = standard error; 95% CI = 95% confidence interval
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Achari 2000 | Published paper states BP measurements were taken using standard sphygmomanometer but clinic BP data not reported, only 24‐hour ambulatory BP data. Authors were contacted for clinic BP data. Lead author replied that data for their paper were taken from a terazosin study conducted around 1990 in which a subset of patients had 24‐hour blood pressure monitoring and that he will not be able to provide any additional data as he no longer has access to original terazosin study. |
| Ames 1989 | Alpha blocker dose titrated to BP response every 2 weeks during double‐blind treatment period. Participants were not receiving same dose of alpha blocker. |
| Ames 1989a | Alpha blocker dose titrated to BP response every 2 weeks during double‐blind treatment period. |
| Aronow 1977 | Fixed titration study but any given dose of alpha blocker was only administered for 2 weeks or less. |
| Aronow 1978 | Fixed titration study but any given dose of alpha blocker was only administered for 2 weeks or less. |
| Baez 1986 | Alpha blocker dose titrated to BP response during double‐blind treatment period. |
| Chrysant 1981 | Alpha blocker dose was being up‐titrated once weekly or every 2 weeks (not clear) during double‐blind treatment period. Participants were not receiving same dose of alpha blocker. |
| Cohen 1987 | Alpha blocker dose titrated to BP response at weekly intervals during double‐blind treatment period. Participants were not receiving same dose of alpha blocker. |
| Courtney 2003 | Alpha blocker dose titrated to BP target during double‐blind treatment period. Participants were not receiving same dose of alpha blocker. |
| Gould 1981 | Alpha blocker dose titrated to BP response during double‐blind treatment period. Participants were not receiving same dose of alpha blocker. |
| Materson 1993 | Alpha blocker dose titrated to BP response every 2 weeks during double‐blind treatment period. Participants were not receiving same dose of alpha blocker. |
| Mroczek 1974 | Alpha blocker dose titrated to BP response during double‐blind treatment period. Participants were not receiving same dose of alpha blocker. |
| Nash 1987 | Alpha blocker dose titrated to BP response during double‐blind treatment period. Participants were not receiving same dose of alpha blocker. |
| Romero 1992 | Alpha blocker dose titrated to BP response at week 1 of double‐blind treatment period. Participants were not receiving same dose of alpha blocker. |
| Shionoiri 1997 | Alpha blocker dose titrated to BP response during double‐blind treatment period. Participants were not receiving same dose of alpha blocker. |
| Smyth 1988 | Alpha blocker dose titrated to BP response during double‐blind treatment period. Participants were not receiving same dose of alpha blocker. |
| Svetkey 1988 | Alpha blocker dose titrated to BP response at week 4 of double‐blind treatment period. Participants were not receiving same dose of alpha blocker. |
| Torvik 1986 | Alpha blocker dose titrated to BP response during double‐blind treatment period. Participants were not receiving same dose of alpha blocker. |
| Venter 1991 | Alpha blocker dose titrated to BP response during double‐blind treatment period. Participants were not receiving same dose of alpha blocker. |
| Yasunari 2006 | BP measured was measured at least once per month but only BP data at 6 months (week 24) reported. Email sent to lead author (yasunari@med.osaka‐cu.ac.jp) on June 22, 2009 requesting 3 to 12‐week post‐randomization BP data but no reply. |
Differences between protocol and review
There are no differences between the protocol and the published review.
Contributions of authors
Dr. James M. Wright conceived and designed the review, assisted with the analysis and interpretation of data, as well as provided a clinical perspective. Dr. Balraj S. Heran designed the original search strategy, undertook the search, screened search results, collected data for the review, screened retrieved papers against eligibility criteria, appraised the risk of bias of papers, extracted data from papers, wrote to authors of papers for additional information, entered data into RevMan, analyzed and interpreted data, and wrote the review. Brandon P. Galm screened the retrieved papers against the eligibility criteria and extracted data from papers.
Sources of support
Internal sources
Departments of Anesthesiology, Pharmacology & Therapeutics and Medicine, Faculty of Medicine, University of British Columbia, Canada.
External sources
British Columbia Ministry of Health Grant to the Therapeutics Initiative, Canada.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Abraham 1985 {published data only}
- Abraham P A, Halstenson C E, Matzke G R, Napier J L, Keane W F. Antihypertensive therapy with once‐daily administration of terazosin, a new alpha 1‐adrenergic‐receptor blocker. Pharmacotherapy 1985;5(5):285‐9. [DOI] [PubMed] [Google Scholar]
- Dauer A D. Terazosin: an effective once‐daily monotherapy for the treatment of hypertension. American Journal of Medicine 1986;80(5B):29‐34. [DOI] [PubMed] [Google Scholar]
- Deger G. Comparison of the safety and efficacy of once‐daily terazosin versus twice‐daily prazosin for the treatment of mild to moderate hypertension. American Journal of Medicine 1986;80(5B):62‐7. [DOI] [PubMed] [Google Scholar]
- Deger G. Effect of terazosin on serum lipids. American Journal of Medicine 1986;80(5B):82‐5. [DOI] [PubMed] [Google Scholar]
- Luther R R. Terazosin: a new antihypertensive agent with favorable effects on lipids. International Journal of Clinical Pharmacology, Therapy, & Toxicology 1989;27(7):313‐9. [PubMed] [Google Scholar]
- Luther R R, Glassman H N, Jordan D C, Sperzel W D. Efficacy of terazosin as an antihypertensive agent. American Journal of Medicine 1986;80(5B):73‐6. [DOI] [PubMed] [Google Scholar]
- Luther R R, Glassman H N, Sperzel W D, Steinberg F J, Horton J K, Jordan D C. Terazosin: a new alpha 1‐blocker for the treatment of hypertension: a review of randomized, controlled clinical trials of once‐daily administration as monotherapy. Journal of Hypertension ‐ Supplement 1986;4(5):S494‐7. [PubMed] [Google Scholar]
- Luther R R, Klepper M J, Maurath C J, Glassman H N, Achari R, Laddu A R. Effects of terazosin on serum lipid levels in hypertensive blacks. Journal of Human Hypertension 1990;4(2):154‐6. [PubMed] [Google Scholar]
- Luther R R, Klepper M J, Maurath C J, Glassman H N, Achari R, Laddu A R. Efficacy of terazosin in the treatment of essential hypertension in blacks. Journal of Human Hypertension 1990;4(2):151‐3. [PubMed] [Google Scholar]
- Sperzel W D, Glassman H N, Jordan D C, Luther R R. Overall safety of terazosin as an antihypertensive agent. American Journal of Medicine 1986;80(5B):77‐81. [DOI] [PubMed] [Google Scholar]
- Sperzel W D, Luther R R, Glassman H N. Clinical trials with terazosin. General methods. American Journal of Medicine 1986;80(5B):25‐8. [DOI] [PubMed] [Google Scholar]
- Titmarsh S, Monk J P. Terazosin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in essential hypertension. Drugs 1987;33(5):461‐77. [DOI] [PubMed] [Google Scholar]
Cubeddu 1988 {published data only}
- Cubeddu L X, Pool J L, Bloomfield R, Klotman P E, Pickering B I, Wombolt D G, et al. Effect of doxazosin monotherapy on blood pressure and plasma lipids in patients with essential hypertension. American Journal of Hypertension 1988;1(2):158‐67. [DOI] [PubMed] [Google Scholar]
Dauer 1986, Study M81‐059 {published data only}
- Dauer A D. Terazosin: an effective once‐daily monotherapy for the treatment of hypertension. American Journal of Medicine 1986;80(5B):29‐34. [DOI] [PubMed] [Google Scholar]
- Deger G. Comparison of the safety and efficacy of once‐daily terazosin versus twice‐daily prazosin for the treatment of mild to moderate hypertension. American Journal of Medicine 1986;80(5B):62‐7. [DOI] [PubMed] [Google Scholar]
- Deger G. Effect of terazosin on serum lipids. American Journal of Medicine 1986;80(5B):82‐5. [DOI] [PubMed] [Google Scholar]
- Luther R R. Terazosin: a new antihypertensive agent with favorable effects on lipids. International Journal of Clinical Pharmacology, Therapy, & Toxicology 1989;27(7):313‐9. [PubMed] [Google Scholar]
- Luther R R, Glassman H N, Jordan D C, Sperzel W D. Efficacy of terazosin as an antihypertensive agent. American Journal of Medicine 1986;80(5B):73‐6. [DOI] [PubMed] [Google Scholar]
- Luther R R, Glassman H N, Sperzel W D, Steinberg F J, Horton J K, Jordan D C. Terazosin: a new alpha 1‐blocker for the treatment of hypertension: a review of randomized, controlled clinical trials of once‐daily administration as monotherapy. Journal of Hypertension ‐ Supplement 1986;4(5):S494‐7. [PubMed] [Google Scholar]
- Luther R R, Klepper M J, Maurath C J, Glassman H N, Achari R, Laddu A R. Effects of terazosin on serum lipid levels in hypertensive blacks. Journal of Human Hypertension 1990;4(2):154‐6. [PubMed] [Google Scholar]
- Luther R R, Klepper M J, Maurath C J, Glassman H N, Achari R, Laddu A R. Efficacy of terazosin in the treatment of essential hypertension in blacks. Journal of Human Hypertension 1990;4(2):151‐3. [PubMed] [Google Scholar]
- Sperzel W D, Glassman H N, Jordan D C, Luther R R. Overall safety of terazosin as an antihypertensive agent. American Journal of Medicine 1986;80(5B):77‐81. [DOI] [PubMed] [Google Scholar]
- Sperzel W D, Luther R R, Glassman H N. Clinical trials with terazosin. General methods. American Journal of Medicine 1986;80(5B):25‐8. [DOI] [PubMed] [Google Scholar]
- Titmarsh S, Monk J P. Terazosin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in essential hypertension. Drugs 1987;33(5):461‐77. [DOI] [PubMed] [Google Scholar]
Gillenwater 1995 {published data only}
- Gillenwater J Y, Conn R L, Chrysant S G, Roy J, Gaffney M, Ice K, et al. Doxazosin for the treatment of benign prostatic hyperplasia in patients with mild to moderate essential hypertension: a double‐blind, placebo‐controlled, dose‐response multicenter study. Journal of Urology 1995;154(1):110‐5. [PubMed] [Google Scholar]
Harder 1994 {published data only}
- Harder S, Thurmann P. Concentration/effect relationship of bunazosin, a selective alpha 1‐adrenoceptor antagonist in hypertensive patients after single and multiple oral doses. International Journal of Clinical Pharmacology & Therapeutics 1994;32(1):38‐43. [PubMed] [Google Scholar]
Mersey 1984 {published data only}
- Dauer A D. Terazosin: an effective once‐daily monotherapy for the treatment of hypertension. American Journal of Medicine 1986;80(5B):29‐34. [DOI] [PubMed] [Google Scholar]
- Deger G. Comparison of the safety and efficacy of once‐daily terazosin versus twice‐daily prazosin for the treatment of mild to moderate hypertension. American Journal of Medicine 1986;80(5B):62‐7. [DOI] [PubMed] [Google Scholar]
- Deger G. Effect of terazosin on serum lipids. American Journal of Medicine 1986;80(5B):82‐5. [DOI] [PubMed] [Google Scholar]
- Luther R R. Terazosin: a new antihypertensive agent with favorable effects on lipids. International Journal of Clinical Pharmacology, Therapy, & Toxicology 1989;27(7):313‐9. [PubMed] [Google Scholar]
- Luther R R, Glassman H N, Jordan D C, Sperzel W D. Efficacy of terazosin as an antihypertensive agent. American Journal of Medicine 1986;80(5B):73‐6. [DOI] [PubMed] [Google Scholar]
- Luther R R, Glassman H N, Sperzel W D, Steinberg F J, Horton J K, Jordan D C. Terazosin: a new alpha 1‐blocker for the treatment of hypertension: a review of randomized, controlled clinical trials of once‐daily administration as monotherapy. Journal of Hypertension ‐ Supplement 1986;4(5):S494‐7. [PubMed] [Google Scholar]
- Luther R R, Klepper M J, Maurath C J, Glassman H N, Achari R, Laddu A R. Effects of terazosin on serum lipid levels in hypertensive blacks. Journal of Human Hypertension 1990;4(2):154‐6. [PubMed] [Google Scholar]
- Luther R R, Klepper M J, Maurath C J, Glassman H N, Achari R, Laddu A R. Efficacy of terazosin in the treatment of essential hypertension in blacks. Journal of Human Hypertension 1990;4(2):151‐3. [PubMed] [Google Scholar]
- Mersey J H, Elkayam U. Effect of terazosin on blood pressure and lipids in hypertension. Clinical Research 1984;32(3):668A. [Google Scholar]
- Sperzel W D, Glassman H N, Jordan D C, Luther R R. Overall safety of terazosin as an antihypertensive agent. American Journal of Medicine 1986;80(5B):77‐81. [DOI] [PubMed] [Google Scholar]
- Sperzel W D, Luther R R, Glassman H N. Clinical trials with terazosin. General methods. American Journal of Medicine 1986;80(5B):25‐8. [DOI] [PubMed] [Google Scholar]
- Titmarsh S, Monk J P. Terazosin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in essential hypertension. Drugs 1987;33(5):461‐77. [DOI] [PubMed] [Google Scholar]
Os 1999 {published data only}
- Os I, Stokke H P. Doxazosin GITS compared with doxazosin standard and placebo in patients with mild hypertension. Blood Pressure 1999;8(3):184‐91. [DOI] [PubMed] [Google Scholar]
- Os I, Stokke H P. Effects of doxazosin in the gastrointestinal therapeutic system formulation versus doxazosin standard and placebo in mild‐to‐moderate hypertension. Doxazosin Investigators' Study Group. Journal of Cardiovascular Pharmacology 1999;33(5):791‐7. [DOI] [PubMed] [Google Scholar]
Schnaper 1975, Study 2 {published data only}
- Schnaper H W, Oberman A. Double‐blind studies of the clinical effectiveness of prazosin. Postgraduate Medicine 1975;Spec No:81‐7. [PubMed] [Google Scholar]
Singleton 1989 {published data only}
- Singleton W, Dix R K, Monsen L, Moisey D, Levenstein M, Bottiglieri D F, et al. Efficacy and safety of Minipress XL, a new once‐a‐day formulation of prazosin. American Journal of Medicine 1989;87(2A):45S‐52S. [DOI] [PubMed] [Google Scholar]
Weber 1991 {published data only}
- Weber M A, Cheung D G, Laddu A R, Luther R R. Antihypertensive dose‐response relationships: studies with the selective alpha 1‐blocking agent terazosin. American Heart Journal 1991;122(3 Pt 2):905‐10. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Achari 2000 {published data only}
- Achari R, Hosmane B, Bonacci E, O'Dea R. The relationship between terazosin dose and blood pressure response in hypertensive patients. Journal of Clinical Pharmacology 2000;40:1166‐72. [PubMed] [Google Scholar]
Ames 1989 {published data only}
- Ames R P, Chrysant S G, Gonzalez F, Schnaper H W, Spann S, Velasquez M T. Effectiveness of doxazosin in systemic hypertension. American Journal of Cardiology 1989;64(3):203‐8. [DOI] [PubMed] [Google Scholar]
Ames 1989a {published data only}
- Ames R P, Kiyasu J Y. Alpha‐1 adrenoceptor blockade with doxazosin in hypertension: effects on blood pressure and lipoproteins. Journal of Clinical Pharmacology 1989;29(2):123‐7. [DOI] [PubMed] [Google Scholar]
Aronow 1977 {published data only}
- Aronow W S, Tobis J, Hughes D, Siegel J, Easthope J. Comparison of trimazosin and methyldopa in hypertension. Clinical Pharmacology & Therapeutics 1977;22(4):425‐9. [DOI] [PubMed] [Google Scholar]
Aronow 1978 {published data only}
- Aronow W S, Oberman A, Pool P E, Schnaper H W, Seagren S C, Tobis J, Hughes D, Siegel J, Easthope J. Effect of trimazosin, methyldopa, and placebo on hypertension. Current Therapeutic Research 1978;23(4):448‐54. [Google Scholar]
Baez 1986 {published data only}
- Baez M A, Garg D C, Jallad N S, Weidler D J. Antihypertensive effect of doxazosin in hypertensive patients: comparison with atenolol. British Journal of Clinical Pharmacology 1986;21 Suppl 1:63S‐7S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chrysant 1981 {published data only}
- Chrysant S G, Miller R F, Brown J L, Danisa K. Long‐term hemodynamic and metabolic effects of trimazosin in essential hypertension. Clinical Pharmacology & Therapeutics 1981;30(5):600‐4. [DOI] [PubMed] [Google Scholar]
Cohen 1987 {published data only}
- Cohen A. Placebo‐controlled study of once‐daily administration of terazosin to patients with mild‐to‐moderate hypertension. Current Therapeutic Research, Clinical & Experimental 1987;41(1):105‐13. [Google Scholar]
Courtney 2003 {published data only}
Gould 1981 {published data only}
- Gould B A, Mann S, Davies A B, Altman D G, Raftery E B. Can placebo therapy influence arterial blood pressure?. Clinical Science 1981;61 Suppl 7:487s‐90s. [DOI] [PubMed] [Google Scholar]
Materson 1993 {published data only}
- Materson BJ, Reda DJ, Cushman WC, Massie BM, Freis ED, Kochar MS, Hamburger RJ, Fye C, Lakshman R, Gottdiener J. Single‐drug therapy for hypertension in men: A comparison of six antihypertensive agents with placebo. New England Journal of Medicine 1993;328(13):914‐21. [DOI] [PubMed] [Google Scholar]
Mroczek 1974 {published data only}
- Mroczek W J, Fotiu S, Davidov M E, Finnerty F A Jr. Prazosin in hypertension: a double‐blind evaluation with methyldopa and placebo. Current Therapeutic Research, Clinical & Experimental 1974;16(8):769‐77. [PubMed] [Google Scholar]
Nash 1987 {published data only}
- Nash D T, Schonfeld G, Reeves R L, Black H, Weidler D J. A double‐blind parallel trial to assess the efficacy of doxazosin, atenolol and placebo in patients with mild to moderate systemic hypertension. American Journal of Cardiology 1987;59(14):87G‐90G. [DOI] [PubMed] [Google Scholar]
Romero 1992 {published data only}
- Romero E, Angeli M, Velasco M, Azar E, Bueno O, Lema G, et al. Double‐blind placebo‐controlled trial of terazosin effect on blood pressure and urinary output of dopamine in hypertensive patients. Journal of Clinical Pharmacology 1992;32(9):816‐21. [DOI] [PubMed] [Google Scholar]
Shionoiri 1997 {published data only}
- Shionoiri H, Ashino K, Yamanaka K, Shindo K, Hiroto S, Arita T. Effect of doxazosin therapy on glucose tolerance and lipid metabolism in hypertensive patients with impaired glucose tolerance. Clinical Therapeutics 1997;19(3):527‐36. [DOI] [PubMed] [Google Scholar]
Smyth 1988 {published data only}
- Smyth P, Pringle S, Jackson G, Lorimer A R. 24‐hour control of blood pressure by once daily doxazosin: a multicentre double‐blind comparison with placebo. European Journal of Clinical Pharmacology 1988;34(6):613‐8. [DOI] [PubMed] [Google Scholar]
Svetkey 1988 {published data only}
- Svetkey L P, Brobyn R, Deedwania P, Graham R M, Morganroth J, Klotman P E. Double‐blind comparison of doxazosin, nadolol, and placebo in patients with mild‐to‐moderate hypertension. Current Therapeutic Research 1988;43(5):969‐78. [Google Scholar]
Torvik 1986 {published data only}
- Torvik D, Madsbu H P. Multicentre 12‐week double‐blind comparison of doxazosin, prazosin and placebo in patients with mild to moderate essential hypertension. British Journal of Clinical Pharmacology 1986;21 Suppl 1:69S‐75S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Venter 1991 {published data only}
- Venter CP, Venter HL, Muntingh GL. The effect of enalapril and prazosin on mild to moderate hypertension in black Africans. Results of a clinical trial [Die Effekte Van Enalapril En Prasosien Op Geringe Tot Matige Hipertensie in Swart Suid‐Afrikaners. Resultate Van 'N Kliniese Proef]. South African Medical Journal 1991;80:324‐6. [PubMed] [Google Scholar]
Yasunari 2006 {published data only}
- Yasunari K, Matsui T, Maeda K, Nakamura M, Watanabe T, Kiriike N. Anxiety‐induced plasma norepinephrine augmentation increases reactive oxygen species formation by monocytes in essential hypertension. American Journal of Hypertension 2006;19(6):573‐8. [DOI] [PubMed] [Google Scholar]
Additional references
ALLHAT 2000
- Davis B R, Furberg C D, Wright J T, Cutler J A, Alderman M, Black H, Cushman W, et al. Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: The antihypertensive and lipid‐lowering treatment to prevent heart attack trial (ALLHAT). Journal of the American Medical Association 2000;283(15):1967‐75. [PubMed] [Google Scholar]
Bucher 1997
- Bucher H C, Guyatt G H, Griffith L E, Walter D. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. Journal of Clinical Epidemiology 1997;50(6):683‐91. [DOI] [PubMed] [Google Scholar]
Cochrane Handbook 2008
- Higgins J P T, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration 2008. Available from www.cochrane‐handbook.org.
Dauer 1986
- Dauer A D. Terazosin: an effective once‐daily monotherapy for the treatment of hypertension. American Journal of Medicine 1986;80(5B):29‐34. [DOI] [PubMed] [Google Scholar]
Heran 2008a
- Heran BS, Wong MMY, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin converting enzyme (ACE) inhibitors for primary hypertension. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003823.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heran 2008b
- Heran BS, Wong MMY, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin receptor blockers for primary hypertension. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003822.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Musini 2008
- Musini VM, Fortin PM, Bassett K, Wright JM. Blood pressure lowering efficacy of renin inhibitors for primary hypertension. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD007066.pub2] [DOI] [PubMed] [Google Scholar]
Song 2003
- Song F, Altman D G, Glenny A M, Deeks J J. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta‐analyses. British Medical Journal 2003;326:472‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wright 2009
- Wright J M, Musini V M. First‐line drugs for hypertension. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD001841.pub2] [DOI] [PubMed] [Google Scholar]


