Abstract
Background
Alzheimer’s disease (AD) is the most common form of dementia. The incidence of AD rises exponentially with age and its prevalence will increase significantly worldwide in the next few decades. Inflammatory processes have been suspected in the pathogenesis of the disease.
Objectives
To review the efficacy and side effects of aspirin, steroidal and non‐steroidal anti‐inflammatory drugs (NSAIDs) in the treatment of AD, compared to placebo.
Search methods
We searched ALOIS: the Cochrane Dementia and Cognitive Improvement Group’s Specialized Register on 12 April 2011 using the terms: aspirin OR "cyclooxygenase 2 inhibitor" OR aceclofenac OR acemetacin OR betamethasone OR celecoxib OR cortisone OR deflazacort OR dexamethasone OR dexibruprofen OR dexketoprofen OR diclofenac sodium OR diflunisal OR diflusinal OR etodolac OR etoricoxib OR fenbufen OR fenoprofen OR flurbiprofen OR hydrocortisone OR ibuprofen OR indometacin OR indomethacin OR ketoprofen OR lumiracoxib OR mefenamic OR meloxicam OR methylprednisolone OR nabumetone OR naproxen OR nimesulide OR "anti‐inflammatory" OR prednisone OR piroxicam OR sulindac OR tenoxicam OR tiaprofenic acid OR triamcinolone OR NSAIDS OR NSAID. ALOIS contains records of clinical trials identified from monthly searches of a number of major healthcare databases (including MEDLINE, EMBASE, PsycINFO, CINAHL, LILACS), numerous trial registries (including national, international and pharmacuetical registries) and grey literature sources.
Selection criteria
All randomised controlled trials assessing the efficacy of aspirin, steroidal and non‐steroidal anti‐inflammatory drugs in AD.
Data collection and analysis
One author assessed risk of bias of each study and extracted data. A second author verified data selection.
Main results
Our search identified 604 potentially relevant studies. Of these, 14 studies (15 interventions) were RCTs and met our inclusion criteria. The numbers of participants were 352, 138 and 1745 for aspirin, steroid and NSAIDs groups, respectively. One selected study comprised two separate interventions. Interventions assessed in these studies were grouped into four categories: aspirin (three interventions), steroids (one intervention), traditional NSAIDs (six interventions), and selective cyclooxygenase‐2 (COX‐2) inhibitors (five interventions). All studies were evaluated for internal validity using a risk of bias assessment tool. The risk of bias was low for five studies, high for seven studies, and unclear for two studies.There was no significant improvement in cognitive decline for aspirin, steroid, traditional NSAIDs and selective COX‐2 inhibitors. Compared to controls, patients receiving aspirin experienced more bleeding while patients receiving steroid experienced more hyperglycaemia, abnormal lab results and face edema. Patients receiving NSAIDs experienced nausea, vomiting, elevated creatinine, elevated LFT and hypertension. A trend towards higher death rates was observed among patients treated with NSAIDS compared with placebo and this was somewhat higher for selective COX‐2 inhibitors than for traditional NSAIDs.
Authors' conclusions
Based on the studies carried out so far, the efficacy of aspirin, steroid and NSAIDs (traditional NSAIDs and COX‐2 inhibitors) is not proven. Therefore, these drugs cannot be recommended for the treatment of AD.
Keywords: Aged; Aged, 80 and over; Humans; Alzheimer Disease; Alzheimer Disease/drug therapy; Alzheimer Disease/etiology; Anti‐Inflammatory Agents; Anti‐Inflammatory Agents/adverse effects; Anti‐Inflammatory Agents/therapeutic use; Anti‐Inflammatory Agents, Non‐Steroidal; Anti‐Inflammatory Agents, Non‐Steroidal/adverse effects; Anti‐Inflammatory Agents, Non‐Steroidal/therapeutic use; Aspirin; Aspirin/adverse effects; Aspirin/therapeutic use; Cyclooxygenase 2 Inhibitors; Cyclooxygenase 2 Inhibitors/adverse effects; Cyclooxygenase 2 Inhibitors/therapeutic use; Glucocorticoids; Glucocorticoids/adverse effects; Glucocorticoids/therapeutic use; Inflammation; Inflammation/complications; Inflammation/drug therapy; Randomized Controlled Trials as Topic; Treatment Outcome
Plain language summary
Aspirin, steroid and non‐steroidal anti‐inflammatory drugs use for treating Alzheimer's disease
Inflammation may play an important role in the development of Alzheimer’s disease. There is also some evidence from community surveys that people receiving anti‐inflammatory drugs for various medical conditions may be less likely to develop Alzheimer's disease. Fourteen studies met our inclusion criteria for this review and none of the exclusion criteria. Aspirin, steroid and non‐steroidal anti‐inflammatory drugs (NSAIDs) (traditional and the selective cyclooxygenase‐2 (COX‐2) inhibitors) showed no significant benefit in the treatment of Alzheimer's disease. Therefore, the use of these drugs cannot be recommended for the treatment of Alzheimer's disease.
Background
Alzheimer's disease (AD) is the most common form of dementia, and its incidence increases exponentially with age. AD affects 1‐2% of people aged 65‐70 and approximately 20% of those over 80 years (Jorm 2003). It results in a progressive deterioration of intellect, memory and personality. AD is an important health problem that has a significant impact on national economies. In the United States, there are approximately 5.2 million AD patients and the cost of care for an average patient is about 24,500 dollars per year (Wimo 2005). By 2030, an estimated 7.7 million Americans aged 65 and older will have AD (Hebert 2003). The disease is increasing worldwide.
The exact mechanism underlying the pathogenesis of AD is still unclear and remains a topic of intense debate. However, two hypotheses revolving around amyloid and inflammation have been particularly influential in trying to understand the neuropathological processes underlying AD. Beta amyloid (AB) is a proteolytic fragment of 40‐42 residues derived from Amyloid Precursor Protein (APP). In vitro, the longer AB‐42 fragment aggregates much more readily, and is hypothesised to be the main pathological agent in the pathogenesis of AD ( Joachim 1992; Selkoe 1991; Younkin 1995). Altered production, aggregation and deposition of AB may play a critical role in the development of AD (Citron 1992; Haass 1994). Significantly, it is suspected that this deposition of amyloid may directly contribute to the inflammatory environment seen (Ruan 2009; Salminen 2009). The inflammatory hypothesis of AD proposes that specific inflammatory mechanisms, including the cytokine‐driven acute‐phase response, complement activation and microglial activation, contribute to neurodegeneration (Aisen 1994; McGeer 1989). In addition, AD may be associated with loss of the capacity to control inflammation in the brain (Bazan 2009).
In view of the strong association between inflammation and AD, attempts have been made to assess whether anti‐inflammatory pharmaceutical agents may have a role in the management of AD. An important mechanism of attenuating inflammatory processes can be achieved through inhibition of the activity of the enzyme cyclooxygenase (COX) which is critical to the production of prostaglandins (Fiebich 1997). This inhibition can occur through the use of various non‐steroidal anti‐inflammatory drugs (NSAIDs) which might thereby diminish the inflammatory response in degenerative dementias. There is evidence that the enzyme cyclooxygenase‐2 (COX‐2) might be involved in neurodegenerative mechanisms in AD (Ho 1999; Pasinetti 1998). This has given rise to the hypothesis that drugs which inhibit COX‐2 in the brain, including certain steroids, aspirin, traditional non‐steroidal anti‐inflammatory drug (NSAIDs) and selective COX‐2 inhibitors could possibly slow the rate of progression or alleviate the symptoms of AD. Likewise, it has also been suspected that corticosteroids (namely synthetic glucocorticoids) may offer some neuroprotection in AD patients (Aisen 1998) through their well characterised anti‐inflammatory action.
Anti‐inflammatory medications such as non‐selective and selective NSAIDs and corticosteroids have been the subject of epidemiological (Launer 2003) and clinical research in AD. The possibility of benefit from non‐selective COX inhibitors (such as indomethacin, naproxen, ibuprofen and diclofenac) and/or selective COX‐2 inhibitors (such as celecoxib and meloxicam) is supported by several lines of evidence. Less work has been carried out for corticosteroids such as prednisolone, but their potential usefulness is of interest.
Epidemiological studies have found a lower prevalence of dementia in people who have regularly taken NSAIDs, usually for rheumatological disorders (McGeer 1996, Stewart 1997). There has also been a cohort study reporting a lower incidence of AD in users of NSAIDs than in non‐users (In't Veld 2001). There is in vitro evidence that, independently of their inhibition of prostaglandin synthesis, some NSAIDs can directly influence the processing of amyloid precursor protein (APP) which is thought to be implicated in the pathogenesis of AD (Avramovich 2002; Blasko 2001). Likewise, an open‐label pilot study of low‐dose prednisolone showed the suppression of peripheral markers of the acute phase response and complement activation in AD without systemic toxicity (Aisen 1996).
In recent years attempts have been made to determine whether anti‐inflammatory agents may be efficacious in the treatment of AD. The purpose of this review is to establish the effectiveness, or otherwise, of such medication. It is now known that all anti‐inflammatory drugs, including selective COX‐2 inhibitors, can carry significant side effects profile and may occasionally be fatal. Hence, it is now important to ascertain whether there is a place for these drugs in the treatment of AD.
Objectives
To systematically review the evidence examining the efficacy and side effects of aspirin, steroidal and non‐steroidal anti‐inflammatory drugs in the treatment of AD.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials assessing the efficacy of aspirin, steroidal and non‐steroidal anti‐inflammatory drugs in the treatment of AD.
Types of participants
Patients of any age diagnosed with probable AD according to internationally recognised criteria including the 'National Institute of Neurologic and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS‐ADRDA), the Diagnostic and Statistical Manual of Mental Disorders (DSM) and the World Health Organisation classification of mental and behavioural disorders (ICD‐10) (APA 1995; McKhann 1984; WHO 1992) .
Types of interventions
Aspirin, steroidal and non‐steroidal anti‐inflammatory agents (traditional and selective COX‐2 inhibitors) at any dose.
Types of outcome measures
Primary outcomes
Cognition (using objective psychometric rating instruments, e.g. the Alzheimer's disease assessment scale ‐ cognitive sub‐scale or ADAS‐COG) or Mini‐Mental Status Examination (MMSE)
Adverse events
Death
Secondary outcome
Clinical global impression of change
Mood/depression
Behavioural disturbance
Activities of daily living
Quality of life
Caregiver burden
Institutionalization
Search methods for identification of studies
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois) ‐ the Cochrane Dementia and Cognitive Improvement Group’s Specialized Register on 12 April 2011. The search terms used were: aspirin OR "cyclooxygenase 2 inhibitor" OR aceclofenac OR acemetacin OR betamethasone OR celecoxib OR cortisone OR deflazacort OR dexamethasone OR dexibruprofen OR dexketoprofen OR diclofenac sodium OR diflunisal OR diflusinal OR etodolac OR etoricoxib OR fenbufen OR fenoprofen OR flurbiprofen OR hydrocortisone OR ibuprofen OR indometacin OR indomethacin OR ketoprofen OR lumiracoxib OR mefenamic OR meloxicam OR methylprednisolone OR nabumetone OR naproxen OR nimesulide OR "anti‐inflammatory" OR prednisone OR piroxicam OR sulindac OR tenoxicam OR tiaprofenic acid OR triamcinolone OR NSAIDS OR NSAID.
ALOIS is maintained by the Trials Search Co‐ordinator for the Cochrane Dementia Group and contains studies in the areas of dementia prevention, dementia treatment and cognitive enhancement in healthy. The studies are identified from:
Monthly searches of a number of major healthcare databases: Medline, Embase, Cinahl, Psycinfo and Lilacs
Monthly searches of a number of trial registers: ISRCTN; UMIN (Japan's Trial Register); the WHO portal (which covers ClinicalTrials.gov; ISRCTN; the Chinese Clinical Trials Register; the German Clinical Trials Register; the Iranian Registry of Clinical Trials and the Netherlands National Trials Register, plus others)
Quarterly search of The Cochrane Library’s Central Register of Controlled Trials (CENTRAL)
Six‐monthly searches of a number of grey literature sources: ISI Web of Knowledge Conference Proceedings; Index to Theses; Australasian Digital Theses
To view a list of all sources searched for ALOIS see About ALOIS on the ALOIS website.
Details of the search strategies used for the retrieval of reports of trials from the healthcare databases, CENTRAL and conference proceedings can be viewed in the ‘methods used in reviews’ section within the editorial information about the Dementia and Cognitive Improvement Group.
Additional searches were performed in many of the sources listed above to cover the timeframe from the last searches performed for ALOIS to ensure that the search for the review was as up‐to‐date and as comprehensive as possible. The search strategies used can be seen in Appendix 1.
Details of the initial search carried out for this review can be viewed in Appendix 2.
The latest search (April 2011) retrieved a total of 1223 results. After a first‐assess and a de‐duplication of these results the authors were left with 116 references/records to further assess.
Searching other resources
The first authors of important identified RCTs that were potentially suitable for inclusion were contacted to request additional information on related new, unpublished, or in press studies.
Data collection and analysis
Selection of studies
Two authors (DJ, MI) independently examined the titles and abstracts of the trials identified in the search and considered them for inclusion according to the pre‐determined eligibility criteria. Any disparity was resolved by retrieval of the cited articles and further discussion with the third author (NT).
Data extraction and management
A double‐check process was used for risk of bias and outcome data including clinical outcomes and side effects. As mentioned, there were 14 included studies with 15 interventions as one study was comprised two interventions. Initially, the first author (DJ) extracted the data on all 14 included studies (15 interventions) and this was followed by verification by the other two authors (MI and NT). All authors had a data extraction form for this process. Any disagreements were resolved by discussion between DJ and the other author involved (either MI or NT depending on the study in question).
Assessment of risk of bias in included studies
The quality of the methods used in each study was examined by the two reviewers using the domain‐based evaluation as shown in Table 1. In conclusion, risk of bias are summarised for each study as described in Table 2. The Grades of Recommendation, Assessment, Development and Evaluation Working Group (GRADE Working Group) approach was used to define the quality of data presented in this systematic review. The GRADE approach specifies four levels of quality as shown in Table 3.
1. The Cochrane Collaboration’s tool for assessing risk of bias.
| Domain | Description | Review authors’ judgment |
| Sequence generation. | Describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. | Was the allocation sequence adequately generated? |
| Allocation concealment. | Describe the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment. | Was allocation adequately concealed? |
| Blinding of participants, personnel and outcome assessorsAssessments should be made for each main outcome (or class of outcomes). | Describe all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective. | Was knowledge of the allocated intervention adequately prevented during the study? |
| Incomplete outcome dataAssessments should be made for each main outcome (or class of outcomes). | Describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. State whether attrition and exclusions were reported, the numbers in each intervention group (compared with total randomised participants), reasons for attrition/exclusions where reported, and any re‐inclusions in analyses performed by the review authors. | Were incomplete outcome data adequately addressed? |
| Selective outcome reporting. | State how the possibility of selective outcome reporting was examined by the review authors, and what was found. | Are reports of the study free of suggestion of selective outcome reporting? |
| Other sources of bias. | State any important concerns about bias not addressed in the other domains in the tool. If particular questions/entries were pre‐specified in the review’s protocol, responses should be provided for each question/entry. |
Was the study apparently free of other problems that could put it at a high risk of bias? |
2. Risk of bias within a study and across studies.
| Risk of bias | Interpretation | Within a study | Across studies |
| Low risk of bias. | Plausible bias unlikely to seriously alter the results. | Low risk of bias for all key domains. | Most information is from studies at low risk of bias. |
| Unclear risk of bias. | Plausible bias that raises some doubt about the results. | Unclear risk of bias for one or more key domains. | Most information is from studies at low or unclear risk of bias. |
| High risk of bias. | Plausible bias that seriously weakens confidence in the results. | High risk of bias for one or more key domains. | The proportion of information from studies at high risk of bias is sufficient to affect the interpretation of results. |
3. Levels of quality of a body of evidence in the GRADE approach for RCT.
| Underlying methodology | Quality rating | |||
| Randomised trials | High | |||
| Downgraded randomised trials | Moderate | |||
| Double‐downgraded randomised trials | Low | |||
| Triple‐downgraded randomised trials | Very low |
Measures of treatment effect
The data presented in this review included dichotomous and continuous data. In dichotomous data (side effects, institutionalisation and death), each individual outcome comprised two possible categorical responses. Continuous data included the rest of clinical outcomes which were presented in a numerical quantity as a score format.
For dichotomous outcome, we used risk ratio (relative risk) to compare intervention and placebo. Risk ratio describes the probability with which side effects, institutionalisation and death will occur in the intervention group compared to placebo. In addition to risk ratio, 95% confidence interval was also calculated to determine the range of the effect.
For continuous data, we used both the mean difference (MD) and the standardized mean difference (SMD).
Mean difference was used for all outcomes that used the same scale. If an outcome was measured using more than one scale, then we calculated a standardised mean difference (SMD) where
SMD = Difference in mean outcome between group
SD of outcome among participants
Heterogeneity may exist among studies in a meta‐analysis and whenever possible the causes of the heterogeneity need to be explored. One way of exploring this is to carry out a subgroup analysis. However, this approach is problematic when there are very few included studies in each meta‐analysis which is the case in this review Another approach to address the potential consequences of heterogeneity is to use the random‐effects meta‐analysis (Cochrane Handbook). In this review, and in line with current guidelines, I2 was used to quantify inconsistency across studies which then move the focus away from testing whether heterogeneity is present to assessing its impact on the meta‐analysis (Cochrane Handbook). Here, the intention was to use I2 level of >30% (this is a conservative estimate and was preferred to 40%) as an indicator of potential heterogeneity. Hence, for I2<30%, the fixed‐effects meta‐analysis method was determined to be appropriate. For I2>30% the random‐effects meta‐analysis method would be more fitting. This division is not ideal but is in line with current guidelines and does represent an effort to deal with the possible effects of heterogeneity on meta‐analysis results.
Unit of analysis issues
In this review only Aisen 2003a had two active interventions: a traditional NSAIDs and a selective COX‐2 inhibitor group. The approach to overcome a potential unit‐of‐analysis error for such a study which included multiple groups but with clear separation of subgroups was to “split” the shared group (the placebo group) into two subgroups each with a smaller sample size.
Dealing with missing data
Missing data could be a source of bias and affect the validity of the study. For considered studies with potential missing data, the authors were contacted to request more information.
In studies where the SD for continuous outcomes was missing, then SD was calculated from available P value.
For included studies with high risk of bias because of missing data, a sensitivity analyses was conducted to take into account the high bias risk. This was applied to studies with both dichotomous and continuous outcome data.
Subgroup analysis and investigation of heterogeneity
For this review, interventions were grouped according to the established and accepted classification of the different groups of drugs studied (aspirin, steroid and NSAIDs). As aspirin, steroid and NSAID drugs differed significantly in chemical structure and mode of action, overall analyses under "anti‐inflammatory drugs" would not have been useful. Therefore, separate analyses were needed to differentiate between the potential efficacy and adverse events of these three groups of drugs. As far as the NSAID drugs are concerned, a subgroup analysis was justified to differentiate between the traditional NSAIDs and selective COX‐2 inhibitors subgroups. In essence, they were similar enough to be included as one group in the initial analysis and different enough to justify a further subgroup analyses. Hence, analyses undertaken included: aspirin VS Placebo; steroids VS Placebo; NSAIDs VS Placebo, then traditional NSAIDs VS Placebo and selective COX‐2 inhibitors VS Placebo.
Heterogeneity was quantified using the I2 statistic which was interpreted as follows:
0%‐40%: might not be important
30%‐60%: may represent moderate heterogeneity
50%‐90%: may represent substantial heterogeneity
70%‐100%: considerable heterogeneity
Results
Description of studies
Results of the search
604 references were retrieved by the electronic searches, from which 14 relevant RCTs were selected. Kappa statistic for agreement among 2 authors was 0.793 as shown in Appendix 3 which implied excellent agreement.
Included studies
From 15 interventions (14 studies in total), there were three interventions for aspirin, one for steroids, six for traditional NSAIDs and five interventions for selective COX‐2 inhibitors. One study had two interventions including both a traditional NSAIDs and a selective COX‐2 inhibitor (Aisen 2003a). The 14 selected studies enrolled a total of 2445 AD patients. The characteristics of participants in each study are presented in Table 4.
4. Characteristics of participants from each study.
| Study | Mean age (SD) | Female (%) | year of education (SD) | Duration of disease, yr (SD) | ApoE (% >/=1allele) | MMSE (SD) | Hypertension (%) |
| Bentham 2008 (Aspirin, N=156) |
75 | 63 | N/A | N/A | N/A | 19 | 19 |
| Zhou 2004a (Aspirin, N=13) |
71.1 (10.6) | 57.14 | N/A | 2.65 (3.15) | N/A | 14.65 (5.93) | N/A |
| Zhou 2004 (Aspirin, N=8) |
69.5 (9.8) | 37.50 | N/A | N/A | N/A | 14.5 (6.8) | N/A |
| Aisen 2000 (Prednisone, N=69) |
73.4 (7.2) | 49.3 | 14.1 (3) | 3.5 (2.4) | 65.6 | 21.2 (4.4) | N/A |
| Aisen 2002 (Nimesulide, N=21) |
73(2) | 38.09 | 13.1 (11.2) | 2 (0.5) | N/A | 21.1 (1.1) | N/A |
| Aisen 2003 (Naproxen, N=118) |
74.1 (7.8) | 48.3 | 13.8 (3.2) | 4.1 (2.3) | 70.4 | 20.7 (3.6) | N/A |
| Aisen 2003 (Rofecoxib, N=122) |
73.7 (7.2) | 54.9 | 13.8 (3.2) | 4.1 (2.3) | 68.1 | 21.2 (3.8) | N/A |
| de Jong 2008 (Indomethacin, N=26) | 72.7 (6.9) | 53.85 | 2.4 (1.3) | 2.74 (1.75) | 50 | 19.1 (4.1) | N/A |
| Pasqualetti 2009 (Ibuprofen, N=66) | 73.7 (7.3) | 61 | 7.4 (3.7) | 2 (0.5‐5.41) | 20.4 | 19.7 (3.0) | N/A |
| Rogers 1993 (Indoethacin, N=14) |
78 (2) | 35.71 | N/A | N/A | N/A | N/A | N/A |
| Hull 1999 (Piroxicam, N=6) |
range of 55‐75 | N/A | N/A | N/A | N/A | N/A | N/A |
| Scharf 1999 (Diclofenac, N=12) |
71.8 (2.3) | 66.67 | N/A | N/A | N/A | N/A | 18.5 (0.99) |
| Jhee 2004 (Celecoxib, N=15) | 71.17 | 26.67 | N/A | N/A | N/A | N/A | N/A |
| Reines 2004 (Rofecoxib, N=346) |
76 (8) | 54 | N/A | 2.17 (1.83) | N/A | N/A | 21 (4) |
| Soininen 2007 (Celecoxib, N=285) | 73.7 (8.2) | 53 | N/A | 1.37 (1.7) | N/A | 19.8 (4.2) | 31.9 |
Excluded studies
Studies were excluded if they were non‐RCTs, did not include AD patients and did not use one of the drugs under investigation. In total:
529 studies were excluded because they were not RCTs; (Of these 470 studies were excluded at the begining if they were reviews or animal studies, therefore, there were only 59 studies included in the references for the excluded studies)
35 RCTs were excluded because participants were not diagnosed as suffering from AD;
18 RCTs were excluded because the interventions were not aspirin, steroidal or non‐steroidal anti‐inflammatory drugs;
6 RCTs were excluded because they were based on the same data used in other included RCTs;
2 RCTs identified from trial registers were excluded because there was no published data and no response from the authors to a request for information.
Flurbiprofen, a traditional NSAID, was included as a search term. Several studies were identified which proved on closer investigation to have used R‐flurbiprofen. This enantiomer has little activity against cyclooxygenase, the target of traditional NSAIDs, and undergoes minimal chiral conversion in humans to the cyclooxygenase‐inhibiting S‐enantiomer. It cannot, therefore, be classed as an NSAID, but has been investigated as a potential modulator of AB‐42 production in AD (Wilcock 2006a). Trials using R‐flurbiprofen were therefore excluded from this review.
Risk of bias in included studies
Risk of bias assessment tool was used to assess the validity of each included study. A range from low (five studies) to high (seven studies) was observed. Two studies had unclear risk of bias (Figure 1 and Figure 2).
1.
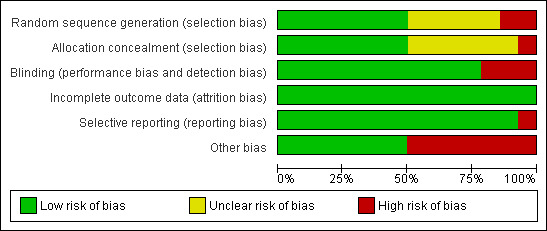
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.
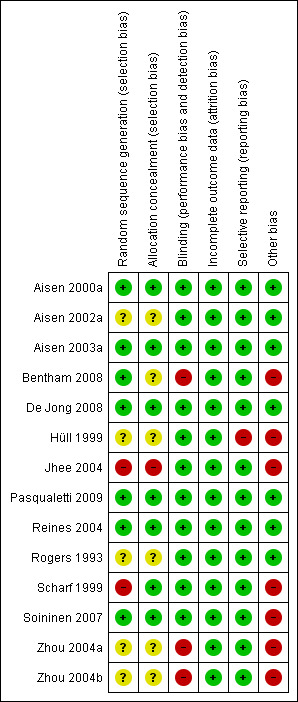
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
There were seven included studies that used proper allocation concealment method, while six studies were unclear and only Jhee 2004 did not apply allocation concealment.
Blinding
Eleven included studies properly blinded participants and outcome assessors. The remaining three studies were open‐label studies (Bentham 2008; Zhou 2004a; Zhou 2004b).
Incomplete outcome data
All studies properly addressed the issue of incomplete outcome data and reasons for missing outcome data were unlikely to be related to true outcome.
Selective reporting
Of all included studies, only Hüll 1999 did not report all stated outcomes. In this particular study the data was presented in an abstract form and no full text article was available. This could have resulted from negative findings or no measurement for these outcomes. This may represent a selective reporting bias.
Effects of interventions
Aspirin
All three selected studies (Bentham 2008; Zhou 2004a; Zhou 2004b) were open‐label and unsuitable for meta‐analysis due to clinical and methodological heterogeneity. Total numbers of participants from both Zhou 2004a and Zhou 2004b were 42, compared to 310 in Bentham 2008. Bentham 2008, Zhou 2004b and Zhou 2004a had different follow up time length which was 3 years, 36 weeks and 6 months respectively. Further, most patients (75%) in Bentham 2008 study took donepezil in the control group, while patients from Zhou 2004a and Zhou 2004b took hyperzine and nigocerline. All three studies also used different doses of aspirin, namely 75 mg, 150 mg and 50 mg per day.
In addition, an issue of lacking and missing data should be taken into account as neither Zhou 2004 nor Zhou 2004a provided data on side effects and detailed data on cognitive improvements. For example, there was no specific p‐value or mean difference shown in the studies.
None of these studies reported significant cognitive improvement in the group treated with aspirin.
However, Bentham 2008 showed significant bleedings from various sites (RR 7.90, 95% CI 2.43 to 25.69; Analysis 1.1), but there was no difference in terms of death (RR 1.01, 95% CI 0.70 to 1.46; Analysis 1.2) and institutionalisation rate (RR 0.93, 95% CI 0.50 to 1.74; Analysis 1.3), compared to aspirin avoidance group.
1.1. Analysis.
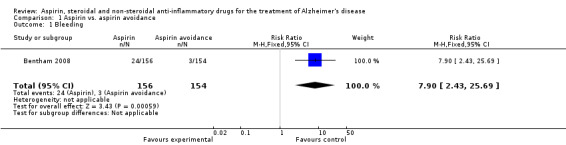
Comparison 1 Aspirin vs. aspirin avoidance, Outcome 1 Bleeding.
1.2. Analysis.
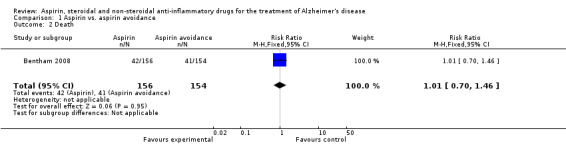
Comparison 1 Aspirin vs. aspirin avoidance, Outcome 2 Death.
1.3. Analysis.
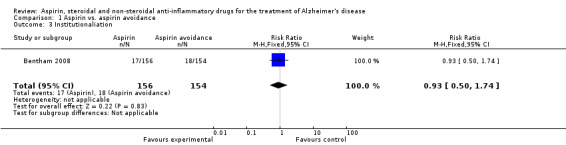
Comparison 1 Aspirin vs. aspirin avoidance, Outcome 3 Institutionaliation.
Steroid
Only one selected study assessed a steroidal anti‐inflammatory agent, prednisone (Aisen 2000a).This study was classified as being at low risk of bias. A total of 138 subjects with probable AD were randomised to either the drug or the placebo groups. This was a double‐blind two‐group parallel design comparing prednisone treatment with placebo. The primary outcome measure for this trial was the 1‐year change in the cognitive subscale of the AD Assessment Scale (ADAS‐cog) score.
No significant effect on cognition or mood at 12 months was observed (Analysis 2.1). Prednisone was associated with hyperglycaemia (RR 5.00, 95% CI 1.14 to 21.99; Analysis 2.3), abnormal lab results (RR 2.55, 95% CI 1.38 to 4.70; Analysis 2.4) and face edema (RR 1.87, 95% CI 1.10 to 3.17; Analysis 2.5).
2.1. Analysis.
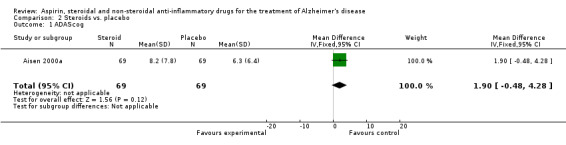
Comparison 2 Steroids vs. placebo, Outcome 1 ADAScog.
2.3. Analysis.
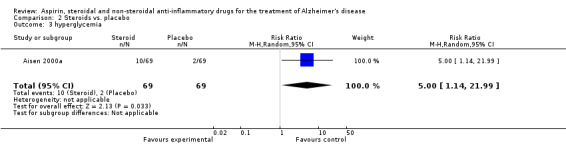
Comparison 2 Steroids vs. placebo, Outcome 3 hyperglycemia.
2.4. Analysis.
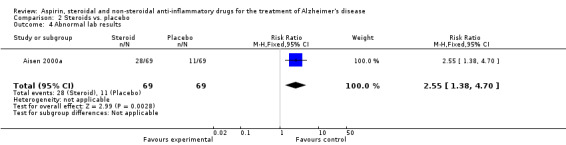
Comparison 2 Steroids vs. placebo, Outcome 4 Abnormal lab results.
2.5. Analysis.
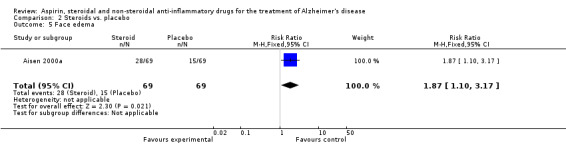
Comparison 2 Steroids vs. placebo, Outcome 5 Face edema.
NSAIDs
Ten studies investigated NSAIDs in AD. There were eleven interventions: six traditional NSAIDs and five selective COX‐2 inhibitors. Analysis was initially undertaken on the NSAIDs group as a whole (all 11 interventions). Subsequently, subgroup analysis was carried out to establish whether any difference existed between traditional NSAIDs and selective COX‐2 inhibitors.
All NSAIDs interventions used ADAS‐cog scores as an outcome measure, but there was not enough data from Jhee 2004 to perform an analysis. In these studies, which included 1745 participants, the meta‐analysis showed no significant difference between the scores of the treatment and placebo groups (MD ‐1.41, 95% CI ‐3.13 to 0.32; Analysis 3.1). Six studies involving 1268 participants also used the MMSE as an outcome measure (De Jong 2008; Pasqualetti 2009; Reines 2004; Rogers 1993; Scharf 1999; Soininen 2007). These studies showed no significant change in rate of MMSE scores decline in the treatment group (MD ‐1.08, 95%CI ‐2.21 to 0.12; Analysis 3.2).
3.1. Analysis.
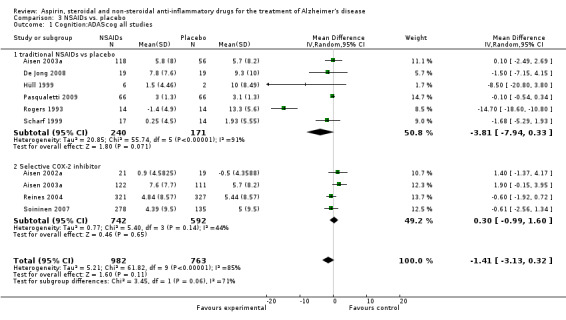
Comparison 3 NSAIDs vs. placebo, Outcome 1 Cognition:ADAScog all studies.
3.2. Analysis.
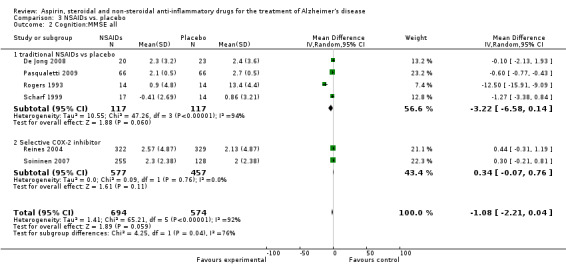
Comparison 3 NSAIDs vs. placebo, Outcome 2 Cognition:MMSE all.
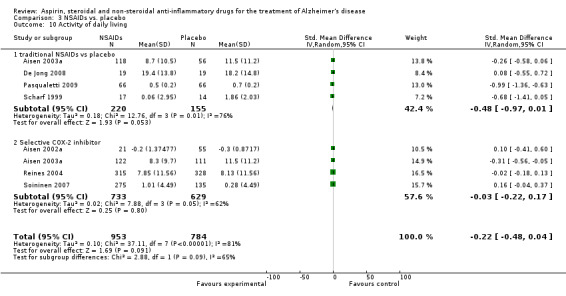
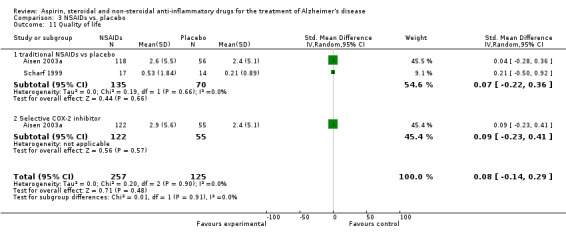
In the subgroup analyses, no significant difference in the rate of cognitive decline overall was observed in the traditional NSAIDs group for ADAScog (MD ‐3.81, 95% CI ‐7.94 to 0.33; Analysis 3.1) and MMSE scores (MD ‐3.22, 95% CI ‐6.58 to 0.14; Analysis 3.2). Similarly, for the selective COX‐2 inhibitors studies, no overall significant change was reported for both ADAScog (MD 0.30, 95% CI ‐0.99 to 1.60; Analysis 3.1) and MMSE scores (MD 0.34, 95% CI ‐0.07 to 0.76; Analysis 3.2). For all NSAIDs, no significant improvement was obtained for Clinician's Interview‐Based Impression of Change (CIBIC+) (MD 0.04; 95% CI ‐0.09 to 0.16: Analysis 3.3). The same also applied for the Clinical Dementia Rating Scale Sum of Boxes (CDR‐SOB), neuropsychiatric inventory (NPI) and other measures where no significant differences between the treatment and the placebo groups were observed (Analysis 3.4 to Analysis 3.12).
3.3. Analysis.
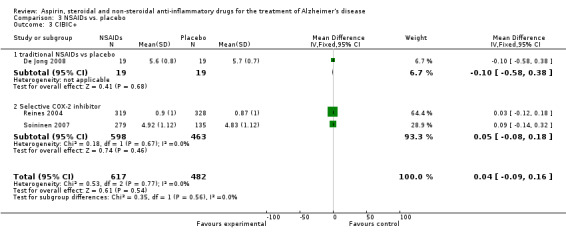
Comparison 3 NSAIDs vs. placebo, Outcome 3 CIBIC+.
3.4. Analysis.
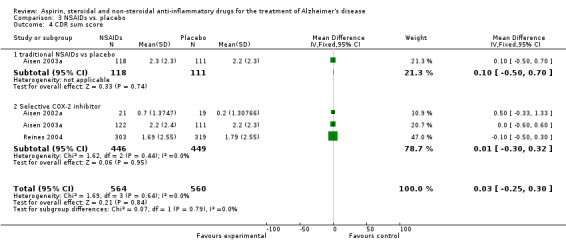
Comparison 3 NSAIDs vs. placebo, Outcome 4 CDR sum score.
3.12. Analysis.
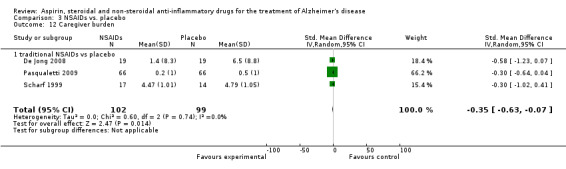
Comparison 3 NSAIDs vs. placebo, Outcome 12 Caregiver burden.
Gastrointestinal side effects were more common in the NSAIDs group (RR 1.94, 95% CI 1.36 to 2.77; Analysis 3.13) compared to placebo based on analysis of data from 1675 participants in 9 studies. Both traditional NSAIDs and selective COX‐2 inhibitors had higher rate of gastrointestinal side effects (RR 1.43, 95% CI 0.68 to 3.00 and RR 2.03, 95% CI 1.37 to 3.03; from Analysis 4.2 and Analysis 5.1, respectively).
3.13. Analysis.
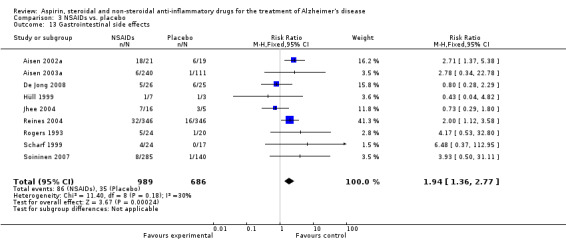
Comparison 3 NSAIDs vs. placebo, Outcome 13 Gastrointestinal side effects.
4.2. Analysis.
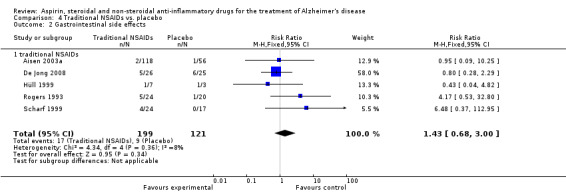
Comparison 4 Traditional NSAIDs vs. placebo, Outcome 2 Gastrointestinal side effects.
5.1. Analysis.
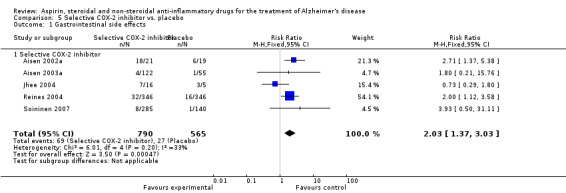
Comparison 5 Selective COX‐2 inhibitor vs. placebo, Outcome 1 Gastrointestinal side effects.
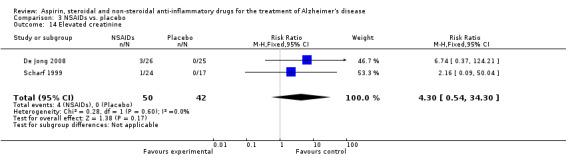
For traditional NSAIDs, common side effects compared to placebo included nausea and vomiting (RR 4.81, 95% CI 0.24 to 95.58; Analysis 4.1), elevated creatinine (RR 4.30, 95% CI 0.54 to 34.30; Analysis 4.3), elevated liver function test (RR 4.04, 95% CI 0.48 to 33.98; Analysis 4.4), and hypertension (RR 3.36, 95% CI 0.84 to 13.34; Analysis 4.5). For selective COX‐2 inhibitors, common side effects compared to placebo included hypertension (RR 8.65, 95% CI 0.51 to 146.03; Analysis 5.2), heart disease (RR 7.52, 95% CI 1.47 to 38.41; Analysis 5.3), and rash (RR 3.47, 95% CI 1.00 to 12.04; Analysis 5.4). The hypertension results came from only 229 participants in Aisen 2003b resulting from the use of rofecoxib. For cardiac side effects, subgroup analysis was performed for rofecoxib and celecoxib separately. There was no direct comparison between the two products. The result showed that celecoxib caused significant heart problems in AD (RR 20.21, 95% CI 1.23 to 331.79; Analysis 5.3), while rofecoxib did not (RR 2.73, 95% CI 0.29 to 25.86; Analysis 5.3).
4.1. Analysis.
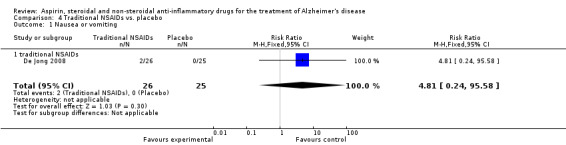
Comparison 4 Traditional NSAIDs vs. placebo, Outcome 1 Nausea or vomiting.
4.3. Analysis.
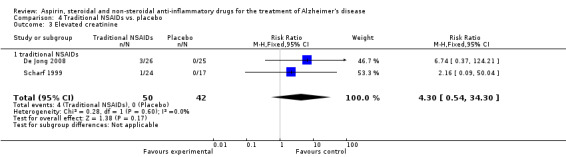
Comparison 4 Traditional NSAIDs vs. placebo, Outcome 3 Elevated creatinine.
4.4. Analysis.
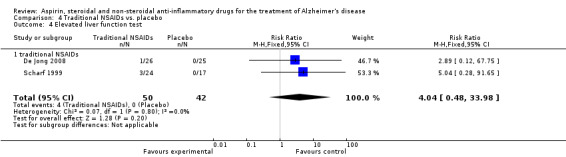
Comparison 4 Traditional NSAIDs vs. placebo, Outcome 4 Elevated liver function test.
4.5. Analysis.
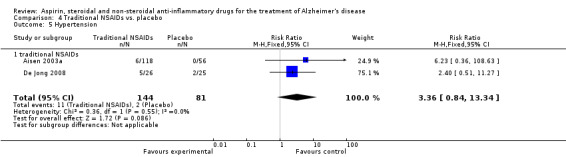
Comparison 4 Traditional NSAIDs vs. placebo, Outcome 5 Hypertension.
5.2. Analysis.
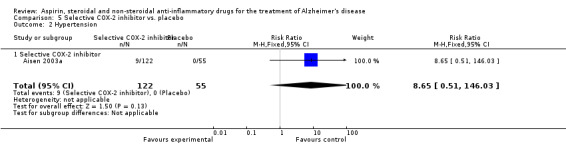
Comparison 5 Selective COX‐2 inhibitor vs. placebo, Outcome 2 Hypertension.
5.3. Analysis.
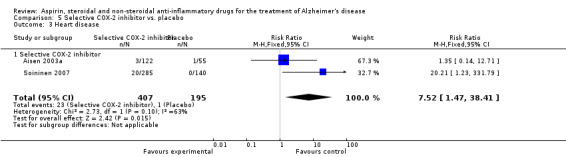
Comparison 5 Selective COX‐2 inhibitor vs. placebo, Outcome 3 Heart disease.
5.4. Analysis.
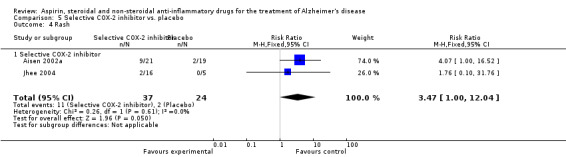
Comparison 5 Selective COX‐2 inhibitor vs. placebo, Outcome 4 Rash.
Death rate was higher in the NSAIDs group although this did not reach statistical significant (RR 1.67, 95% CI 0.85 to 3.31; Analysis 3.3). Death rate for traditional NSAIDs (RR 0.72, 95% CI 0.18 to 2.87; Analysis 4.15) and selective COX‐2 inhibitor groups (RR 1.88, 95% CI 0.87 to 4.07; Analysis 5.14). Data presented in this meta‐analysis was from 1711 participants from 9 included studies.
4.15. Analysis.
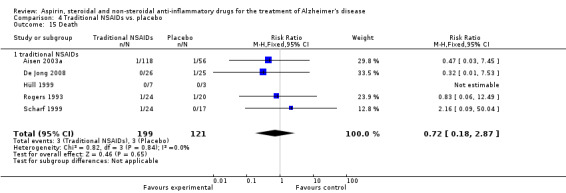
Comparison 4 Traditional NSAIDs vs. placebo, Outcome 15 Death.
5.14. Analysis.
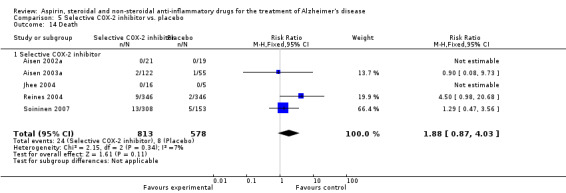
Comparison 5 Selective COX‐2 inhibitor vs. placebo, Outcome 14 Death.
Discussion
Aspirin, steroids and NSAIDs (traditional NSAIDs and selective COX‐2 inhibitors) do not slow down the decline in cognitive function in AD patients and do not improve non‐cognitive and behavioural outcome measures such as depression, behavioral disturbance, activity of daily living, quality of life, clinical global impression of change and caregiver burden.
All anti‐inflammatory agents are known to be associated with a host of side effects. In the selected studies aspirin significantly increased bleeding in AD patients. Steroid medication was associated with hyperglycaemia, abnormal laboratory parameters, and face edema. Traditional NSAIDs were associated with nausea, vomiting, elevated creatinine, elevated liver function tests, and hypertension. Selective COX‐2 inhibitors were associated with hypertension, heart problems, and rash. Celecoxib tended to cause higher rate of heart problems compared to rofecoxib, however the baseline characteristic of celecoxib group had significantly higher rate of hypertension, diabetes and higher numbers of bypass patients. Gastrointestinal side effects were commonly found in both traditional NSAIDs and Selective COX‐2 inhibitors. Significantly, death rate was higher in selective COX‐2 inhibitors, compared to traditional NSAIDs. Meta‐analysis of side effects that included more than 1000 participants included gastrointestinal, cerebrovascular and other side effects as well as death.
There is a wealth of epidemiological data supporting a role for anti‐inflammatory treatment in the protection against the development of cognitive dysfunction. In addition, a role for inflammatory processes in the pathogenesis of AD is now widely recognised. Nevertheless, such data has not been translated into clinical benefit to patients with AD. Anti‐inflammatory drugs do not seem, based on current evidence, to achieve any noticeable improvement in any of the various outcomes assessed, and foremost among them cognitive measures. It cannot be discounted that most, in fact all of the studies assessing the efficacy of anti‐inflammatory agents have been for a relatively short duration not usually exceeding 12 months. Further, patients selected are likely to have well established disease. It is accepted that the disease pathological process begins many years before the start of any symptoms associated with AD. Hence, one cannot comment on any potential efficacy for anti‐inflammatories such as NSAIDs in those with very early stages of asymptomatic and silent disease. In fact epidemiological studies showing a benefit for anti‐inflammatories has tended to assess patients with mainly rheumatological disorders on long term treatment with anti‐inflammatory drugs. This may explain the discrepancy between the widely observed data from epidemiological studies and RCTs.
It is tempting to speculate whether a potential difference may exist between traditional NSAIDs and selective COX‐2 inhibitors. It remains unclear whether this may relate to mechanism of action beyond COX‐2 inhibition. In recent years more interest has been directed at selective COX‐2 inhibitors, in part, due to the earlier perceived supremacy of this class of NSAIDs when it comes to side effects. In recent years, however, the benefit/risk profile of these drugs has been evaluated and earlier enthusiasm about their use has been critically reassessed. It will be of benefit that future work continues to include traditional NSAIDs as well as the newer COX‐2 inhibitors.
Summary of main results
Aspirin, steroid and NSAIDs (traditional NSAIDs and selective COX‐2 inhibitors) do not slow down cognitive decline in AD patients. They also do not improve behavioural and all other non‐cognitive outcome measures such as depression, behavioral disturbance, activity of daily living, quality of life, clinical global impression of change and caregiver burden.
All anti‐inflammatory agents are known to be associated with a host of side effects. In the selected studies aspirin significantly increased bleeding in AD patients. Steroid medication was associated with hyperglycaemia, abnormal laboratory parameters, and face edema.Traditional NSAIDs were associated with nausea, vomiting, elevated creatinine, elevated liver function tests, and hypertension. Selective COX‐2 inhibitors were associated with hypertension, heart problems and rash. Death rate was higher in selective COX‐2 inhibitors, compared to traditional NSAIDs.
Overall completeness and applicability of evidence
All patients in this review were diagnosed as having probable AD by NINCDS‐ADRDA criteria or DSM 4. MMSE ranged from 10‐26; hence patients in these studies had mild to moderate disease. Most recruitment took place in out‐patient settings, except for two studies that were conducted in in‐patient settings (42 participants) (Zhou 2004b; Zhou 2004a) and one study (Hüll 1999) in both out‐patient and in‐patient settings (10 participants). Minimum participants’ age was 46 years. The studies were conducted in different countries including USA, UK, Netherlands, Australia, Belgium, Finland, France, Germany, Italy and China. Interventions included Aspirin, Steriods and NSAIDs.
Quality of the evidence
The three aspirin studies were rated ‘moderate’ in quality as they were not double‐blind randomised controlled trials. The steroid study had low risk of bias and high quality rating. For NSAIDs, high rating for quality was obtained for four studies (Aisen 2003a; De Jong 2008; Pasqualetti 2009; Reines 2004). Moderate rating for quality was for three studies (Aisen 2002a; Rogers 1993; Soininen 2007) while low rating for quality was achieved by another three studies (Hüll 1999; Jhee 2004; Scharf 1999).
Potential biases in the review process
There were 2 studies by Beck 2000 and Taylor 1999 that met all inclusion criteria, but data could not be retrieved and the authors could not be contacted. Inability to include this unpublished data could have led to publication bias as the articles might not be published due to the nature and direction of the results. However, for this review thorough search of multiple databases was performed and is expected to have identified all registered trials and possible unpublished articles.
Duplicate publication bias
There were 15 studies in this review that were found to be repetitive or overlapped substantially with the included studies. It was crucial to identify all redundant and multiple publication as it can lead to overestimation of intervention effects.
Location bias
It was found that trials published in low or non‐impact factor journals were more likely to report significant results than those published in high‐impact journals and that the quality of the trials was also associated with the journal of publication. In this systematic review, high risk of bias studies such as Zhou 2004b, Hüll 1999 and Scharf 1999 tended to provided more significant positive outcome toward intervention group than those of low risk.
Language bias
Reviews have often been exclusively based on studies published in English. Although the number of systematic reviews that restricted their search to studies reported in English had been decreased from 72% to 16%, it remained a challenge of the review process. In this review there was no language restriction which resulted in including 2 Chinese language studies. Although no total translation for the two Chinese language studies was done, data was extracted using a translation sheet from Cochrane as shown in Appendix 4. The process was done to help reduce language bias in the review.
Outcome reporting bias
Of all included studies only one Hüll 1999 did not report all stated outcomes. A reason for this may have been because the data was presented in an abstract form. For the remainder of the included studies, at least the stated primary outcomes were reported. For Jhee 2002, the ADAScog was not reported and data could not be retrieved although the author could be contacted. For side effects, Soininen 2007 study only reported side effects where more than 10% of participants complained. Hence, less common side effects were not reported.
Selection bias
In Soininen 2007 study the intervention group included higher number of subjects with pre‐existing hypertension, diabetes and heart bypass. Hence, in this sponsored study the results obtained as far as side effects are concerned need to be interpreted with caution.
Agreements and disagreements with other studies or reviews
Results from cross‐sectional, cohort and case‐control studies tended to be positive toward the use of NSAIDs in AD (Imbimbo 2004; In't Veld 2001; Landi 2003; McGeer 1996; Rich 1995). There was also evidence from a systematic review that people who took NSAIDs had a lower risk of developing AD (Etminan 2003). Theoretically, aspirin, steroid and NSAIDs should be able to decrease inflammatory process (Aisen 1994; Aisen 1996; Aisen 1998; Harris 2002; Ho 1999; McGeer 1989; Pasinetti 1998; Thomas 2001) which in turn would help improve AD.
In this systematic review, all three classes of drug were found to be associated with more side effects than placebo. The selective COX‐2 inhibitor group experienced more hypertension and heart problems. Patients treated with NSAIDs, particularly COX‐2 inhibitors had a higher death rate than the placebo group.
In patients with osteoarthritis and rheumatoid arthritis, selective COX‐2 inhibitors have been associated with fewer gastrointestinal side effects than traditional NSAIDs (Deek 2002). Among the AD patients studied, gastrointestinal side effects occurred at a similar rate in both groups for AD.
Authors' conclusions
Implications for practice.
There no evidence to support the use of aspirin, steroidal or NSAIDs (both traditional NSAIDs and selective COX‐2 inhibitors) in AD. None of the assessed drugs can be recommended for the treatment of AD.
Implications for research.
The results of this review show that currently available evidence does not support the use of aspirin, steroid and NSAIDs (traditional NSAIDs and COX‐2 inhibitors) for treating AD. These results are not in line with the majority of epidemiological data supporting a protective role for anti‐inflammatories in the development of cognitive impairment. However, all of the selected studies were of relatively short duration and in symptomatic people with well established disease. As it is now widely accepted that inflammatory processes contribute to the pathogenesis of AD, future clinical trials need to assess a prophylactic role for anti‐inflammatories. Participants should include those with Mild cognitive impairment and those with normal cognition but with evidence of early disease pathology such as amyloid deposits as assessed on Pet imaging and tau/amyloid ratio in the cerebrospinal fluid. Agents used should not be restricted to COX‐2 inhibitors but need to include traditional NSAIDs as well.
History
Protocol first published: Issue 1, 2007 Review first published: Issue 2, 2012
| Date | Event | Description |
|---|---|---|
| 26 August 2008 | Amended | When the full review of "Aspirin and anti‐inflammatory drugs for Alzheimer's Disease" is published, it will replace the previously published reviews "Ibuprofen for Alzheimer's Disease", "Indomethacin for Alzheimer's Disease", and the previously published protocol "Naproxen for Alzheimer's Disease". At that time, these ibuprofen and indomethacin reviews and this naproxen protocol will be withdrawn from the Cochrane Library. |
| 26 August 2008 | Amended | Converted to new review format. |
| 14 November 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors wish to thank the consumer editor, Lynne Ramsay and Ruth Chandler, for their helpful comments. We wish to acknowledge the contribution of Rebecca Quinn as the writer of the protocol of this review. We also wish to thank Dr. Helen Collins. We want to express our thanks for the support of the Cochrane dementia and cognitive improvement group for their help coordinating this project.
We thank Dr. Wanruchada Katchamart, Dr. Paula A. Rochon, Dr. Sunila Kalkar and Wei Wu for their practical advice for the review. We also thank Wei Wu for extracting data and filling the translation sheet for the 2 Chinese studies. We thank Ms. Reem Malouf for statistical advice, Dr. Kittphon Nagaviroj for the PDF file for full text assessment form and Inez Kost and Rita Shaughnessy, the librarians at University of Toronto who helped with the article findings and search term respectively.
Appendices
Appendix 1. Pre‐publication search: April 2011
| Source |
Search strategy | Hits retrieved |
| 1. ALOIS (www.medicine.ox.ac.uk/alois) | Keyword search: aspirin OR "cyclooxygenase 2 inhibitor" OR aceclofenac OR acemetacin OR betamethasone OR celecoxib OR cortisone OR deflazacort OR dexamethasone OR dexibruprofen OR dexketoprofen OR diclofenac sodium OR diflunisal OR diflusinal OR etodolac OR etoricoxib OR fenbufen OR fenoprofen OR flurbiprofen OR hydrocortisone OR ibuprofen OR indometacin OR indomethacin OR ketoprofen OR lumiracoxib OR mefenamic OR meloxicam OR methylprednisolone OR nabumetone OR naproxen OR nimesulide OR "anti‐inflammatory" OR prednisone OR piroxicam OR sulindac OR tenoxicam OR tiaprofenic acid OR triamcinolone OR NSAIDS OR NSAID | 37 |
| 2. MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950‐present (Ovid SP) | 1. Aspirin/ 2. aspirin*.ti,ab. 3. cyclooxygenase 2 inhibitor*.ti,ab. 4. ("anti‐inflammatory agent*" or "antiinflammatory agent" or "antinflammatory agent*").ti,ab. 5. aceclofenac.ti,ab. 6. acemetacin.ti,ab. 7. betamethasone.ti,ab. 8. dexibruprofen.ti,ab. 9. dexketoprofen.ti,ab. 10. "diclofenac sodium".ti,ab. 11. diflunisal.ti,ab. 12. diflusinal.ti,ab. 13. etodolac*.ti,ab. 14. etoricoxib*.ti,ab. 15. (fenbufen* or fenoprofen*).ti,ab. 16. flurbiprofen*.ti,ab. 17. (hydrocortison* or ibuprofen*).ti,ab. 18. (indometacin* or indomethacin*).ti,ab. 19. ketoprofen*.ti,ab. 20. lumiracoxib*.ti,ab. 21. "mefenamic acid".ti,ab. 22. meloxicam*.ti,ab. 23. methylprednisolone.ti,ab. 24. nabumeton*.ti,ab. 25. naproxen.ti,ab. 26. nimesulide.ti,ab. 27. "non‐steroid* anti‐inflammatory agent*".ti,ab. 28. prednisone.ti,ab. 29. piroxicam.ti,ab. 30. sulindac.ti,ab. 31. tenoxicam.ti,ab. 32. "tiaprofenic acid".ti,ab. 33. triamcinolone.ti,ab. 34. Anti‐Inflammatory Agents, Non‐Steroidal/ 35. Anti‐Inflammatory Agents/ 36. NSAID*.ti,ab. 37. or/1‐36 38. Alzheimer Disease/ 39. (alzheimer* or AD or dement*).ti,ab. 40. alzheimer*.ti,ab. 41. (AD or dement*).ti,ab. 42. or/38‐41 43. 37 and 42 44. randomized controlled trial.pt. 45. controlled clinical trial.pt. 46. randomized.ab. 47. placebo.ab. 48. drug therapy.fs. 49. randomly.ab. 50. trial.ab. 51. groups.ab. 52. or/44‐51 53. (animals not (humans and animals)).sh. 54. 52 not 53 55. 43 and 54 56. (2008* or 2009* or 2010* or 2011*).ed. 57. 55 and 56 |
193 |
| 3. EMBASE 1980‐2011 week 15 (Ovid SP) |
1. Aspirin/ 2. aspirin*.ti,ab. 3. cyclooxygenase 2 inhibitor*.ti,ab. 4. ("anti‐inflammatory agent*" or "antiinflammatory agent" or "antinflammatory agent*").ti,ab. 5. aceclofenac.ti,ab. 6. acemetacin.ti,ab. 7. betamethasone.ti,ab. 8. dexibruprofen.ti,ab. 9. dexketoprofen.ti,ab. 10. "diclofenac sodium".ti,ab. 11. diflunisal.ti,ab. 12. diflusinal.ti,ab. 13. etodolac*.ti,ab. 14. etoricoxib*.ti,ab. 15. (fenbufen* or fenoprofen*).ti,ab. 16. flurbiprofen*.ti,ab. 17. (hydrocortison* or ibuprofen*).ti,ab. 18. (indometacin* or indomethacin*).ti,ab. 19. ketoprofen*.ti,ab. 20. lumiracoxib*.ti,ab. 21. "mefenamic acid".ti,ab. 22. meloxicam*.ti,ab. 23. methylprednisolone.ti,ab. 24. nabumeton*.ti,ab. 25. naproxen.ti,ab. 26. nimesulide.ti,ab. 27. "non‐steroid* anti‐inflammatory agent*".ti,ab. 28. prednisone.ti,ab. 29. piroxicam.ti,ab. 30. sulindac.ti,ab. 31. tenoxicam.ti,ab. 32. "tiaprofenic acid".ti,ab. 33. triamcinolone.ti,ab. 34. NSAID*.ti,ab. 35. nonsteroid antiinflammatory agent/ 36. antiinflammatory agent/ 37. or/1‐36 38. ALZHEIMER DISEASE/ 39. (alzheimer* or AD or dement*).ti,ab. 40. or/38‐39 41. 37 and 40 42. randomly.ti,ab. 43. trial.ti,ab. 44. placebo.ab. 45. clinical trial/ 46. "double‐blind*".ti,ab. 47. (2008* or 2009* or 2010* or 2011*).em. 48. or/42‐46 49. 41 and 48 50. 47 and 49 |
460 |
| 4. PSYCINFO 1806‐April week 2 2011 (Ovid SP) |
1. Aspirin/ 2. aspirin*.ti,ab. 3. cyclooxygenase 2 inhibitor*.ti,ab. 4. ("anti‐inflammatory agent*" or "antiinflammatory agent" or "antinflammatory agent*").ti,ab. 5. aceclofenac.ti,ab. 6. acemetacin.ti,ab. 7. betamethasone.ti,ab. 8. dexibruprofen.ti,ab. 9. dexketoprofen.ti,ab. 10. "diclofenac sodium".ti,ab. 11. diflunisal.ti,ab. 12. diflusinal.ti,ab. 13. etodolac*.ti,ab. 14. etoricoxib*.ti,ab. 15. (fenbufen* or fenoprofen*).ti,ab. 16. flurbiprofen*.ti,ab. 17. (hydrocortison* or ibuprofen*).ti,ab. 18. (indometacin* or indomethacin*).ti,ab. 19. ketoprofen*.ti,ab. 20. lumiracoxib*.ti,ab. 21. "mefenamic acid".ti,ab. 22. meloxicam*.ti,ab. 23. methylprednisolone.ti,ab. 24. nabumeton*.ti,ab. 25. naproxen.ti,ab. 26. nimesulide.ti,ab. 27. "non‐steroid* anti‐inflammatory agent*".ti,ab. 28. prednisone.ti,ab. 29. piroxicam.ti,ab. 30. sulindac.ti,ab. 31. tenoxicam.ti,ab. 32. "tiaprofenic acid".ti,ab. 33. triamcinolone.ti,ab. 34. NSAID*.ti,ab. 35. Anti Inflammatory Drugs/ 36. or/1‐35 37. exp Alzheimer's Disease/ 38. (AD or alzheimer* or dement*).ti,ab. 39. or/37‐38 40. 36 and 39 41. (random* or trial or placebo or "double‐blind*" or "single‐blind*").ti,ab. 42. exp Clinical Trials/ 43. 41 or 42 44. 40 and 43 45. (2008* or 2009* or 2010* or 2011*).up. 46. 44 and 45 |
26 |
| 5. CINAHL (EBSCOhost) | S1 (MH "Dementia+") S2 (MH "Delirium") or (MH "Delirium, Dementia, Amnestic, Cognitive Disorders") S3 (MH "Wernicke's Encephalopathy") S4 TX dement* S5 TX alzheimer* S6 TX lewy* N2 bod* S7 TX deliri* S8 TX chronic N2 cerebrovascular S9 TX "organic brain disease" or "organic brain syndrome" S10 or/S1‐S9 S11 MH non‐steroidal anti‐inflammatory agent S12 MH Aspirin S13 TX aspirin S14TX "cyclooxygenase 2 inhibitor*" OR aceclofenac OR acemetacin OR betamethasone OR celecoxib OR cortisone OR deflazacort OR dexamethasone OR dexibruprofen OR dexketoprofen OR diclofenac sodium OR diflunisal OR diflusinal OR etodolac OR etoricoxib OR fenbufen OR fenoprofen OR flurbiprofen OR hydrocortisone OR ibuprofen OR indometacin OR indomethacin OR ketoprofen OR lumiracoxib OR mefenamic OR meloxicam OR methylprednisolone OR nabumetone OR naproxen OR nimesulide OR "anti‐inflammatory" OR prednisone OR piroxicam OR sulindac OR tenoxicam OR tiaprofenic acid OR triamcinolone S15 or/S11‐S14 S16 S10 AND S15 S17 TX random* OR placebo* OR trial OR group OR “double‐blind*” S18 MH Clinical Trial S19 S17 AND S18 S20 S19 AND S16 |
74 |
| 6. ISI Web of Knowledge – all databases [includes: Web of Science (1945‐present); BIOSIS Previews (1926‐present); MEDLINE (1950‐present); Journal Citation Reports] | Topic=(aspirin OR "cyclooxygenase 2 inhibitor*" OR aceclofenac OR acemetacin OR betamethasone OR celecoxib OR cortisone OR deflazacort OR dexamethasone OR dexibruprofen OR dexketoprofen OR diclofenac sodium OR diflunisal OR diflusinal OR etodolac OR etoricoxib OR fenbufen OR fenoprofen OR flurbiprofen OR hydrocortisone OR ibuprofen OR indometacin OR indomethacin OR ketoprofen OR lumiracoxib OR mefenamic OR meloxicam OR methylprednisolone OR nabumetone OR naproxen OR nimesulide OR "anti‐inflammatory" OR prednisone OR piroxicam OR sulindac OR tenoxicam OR tiaprofenic acid OR triamcinolone) AND Topic=(Alzheimer* OR AD) AND Topic=(randomized OR randomised OR placebo OR "double‐blind*" OR randomly OR RCT OR trial OR CCT) AND Year Published=(2008‐2011) |
244 |
| 7. LILACS (BIREME) | aspirin OR anti‐inflammatory [Words] and alzheimer OR AD [Words] | 18 |
| 8. CENTRAL (The Cochrane Library) (Issue 1 of 4, Jan 2011) | #1 aspirin OR aceclofenac OR acemetacin OR betamethasone OR celecoxib OR cortisone OR "cyclooxygenase 2 inhibitor*" OR deflazacort OR dexamethasone OR dexibruprofen OR dexketoprofen OR diclofenac sodium OR diflunisal OR diflusinal OR etodolac OR etoricoxib OR fenbufen OR fenoprofen OR flurbiprofen OR hydrocortisone OR ibuprofen OR indometacin OR indomethacin OR ketoprofen OR lumiracoxib OR mefenamic OR meloxicam OR methylprednisolone OR nabumetone OR naproxen OR nimesulide OR "anti‐inflammatory" OR prednisone OR piroxicam OR sulindac OR tenoxicam OR tiaprofenic OR triamcinolone #2 alzheimer* OR AD #3 (#1 AND #2), from 2008 to 2011 |
88 |
| 9. Clinicaltrials.gov (www.clinicaltrials.gov) | alzheimer OR alzheimers OR AD | aspirin OR anti‐imflammatory OR celecoxib OR cortisone OR ibuprofen | received from 01/01/2008 to 04/20/2011 | 6 |
| 10. ICTRP Search Portal (http://apps.who.int/trialsearch) [includes: Australian New Zealand Clinical Trials Registry; ClinicalTrilas.gov; ISRCTN; Chinese Clinical Trial Registry; Clinical Trials Registry – India; Clinical Research Information Service – Republic of Korea; German Clinical Trials Register; Iranian Registry of Clinical Trials; Japan Primary Registries Network; Pan African Clinical Trial Registry; Sri Lanka Clinical Trials Registry; The Netherlands National Trial Register] | (aspirin OR anti‐inflammatory OR dexamethasone OR dexibruprofen OR dexketoprofen OR diclofenac sodium OR diflunisal OR diflusinal OR etodolac OR etoricoxib OR fenbufen OR fenoprofen OR flurbiprofen OR hydrocortisone OR ibuprofen OR indometacin OR indomethacin OR ketoprofen OR lumiracoxib OR mefenamic OR meloxicam OR methylprednisolone OR nabumetone OR naproxen OR nimesulide OR "anti‐inflammatory" OR prednisone OR piroxicam OR sulindac OR tenoxicam OR tiaprofenic OR triamcinolone OR NSAIDS) AND (rec from: 01/01/2008 to 20/04/2011) |
77 |
| TOTAL before de‐duplication | 1223 | |
| TOTAL after de‐dupe and first‐assess | 116 | |
Appendix 2. Initial search: September 2008
| Source searched | Date of search | Search strategy used |
| PubMed (MEDLINE) | 8 September 2008 | (aspirin OR aceclofenac OR acemetacin OR betamethasone OR celecoxib OR cortisone OR deflazacort OR prednisone OR dexamethasone OR dexibruprofen OR dexketoprofen OR diclofenac sodium OR diflusinal OR etodolac OR etoricoxib OR fenbufen OR fenoprofen OR flurbiprofen OR hydrocortisone OR ibuprofen OR indomethacin OR indometacin OR ketoprofen OR lumiracoxib OR mefenamic acid OR meloxicam OR methylprednisolone OR nabumetone OR naproxen OR nimesulide OR piroxicam OR sulindac OR tenoxicam OR tiaprofenic acid OR triamcinolone OR cyclooxygenase 2 inhibitor* OR anti‐inflammatory agent*) AND (Alzheimer* OR dementia OR ((cognit* or memory* or mental*) and (declin* or impair* or los* or deteriorat*)) AND (randomized OR randomized OR double blind* OR single blind* OR placebo* OR controlled) |
| EMBASE (Ovid SP) | 9 September 2008 | as PubMed |
| CINAHL (Ovid SP) | 9 September 2008 | as PubMed |
| PsycINFO (Ovid SP) | 9 September 2008 | as PubMed |
| LILACS (Bireme) | 9 September 2008 | (aspirin OR aceclofenac OR acemetacin OR betamethasone OR celecoxib OR cortisone OR deflazacort OR prednisone OR dexamethasone OR dexibruprofen OR dexketoprofen OR diclofenac sodium OR diflusinal OR etodolac OR etoricoxib OR fenbufen OR fenoprofen OR flurbiprofen OR hydrocortisone OR ibuprofen OR indomethacin OR indometacin OR ketoprofen OR lumiracoxib OR mefenamic acid OR meloxicam OR methylprednisolone OR nabumetone OR naproxen OR nimesulide OR piroxicam OR sulindac OR tenoxicam OR tiaprofenic acid OR triamcinolone OR cyclooxygenase 2 inhibitor* OR anti‐inflammatory agent*) AND (Alzheimer* OR dementia) |
| CDCIG Specialized Register | 8 September 2008 | (aspirin OR aceclofenac OR acemetacin OR betamethasone OR celecoxib OR cortisone OR deflazacort OR prednisone OR dexamethasone OR dexibruprofen OR dexketoprofen OR diclofenac sodium OR diflusinal OR etodolac OR etoricoxib OR fenbufen OR fenoprofen OR flurbiprofen OR hydrocortisone OR ibuprofen OR indomethacin OR indometacin OR ketoprofen OR lumiracoxib OR mefenamic acid OR meloxicam OR methylprednisolone OR nabumetone OR naproxen OR nimesulide OR piroxicam OR sulindac OR tenoxicam OR tiaprofenic acid OR triamcinolone OR cyclooxygenase 2 inhibitor* OR anti‐inflammatory agent*) |
| CENTRAL | Issue 3/2008 | (aspirin OR aceclofenac OR acemetacin OR betamethasone OR celecoxib OR cortisone OR deflazacort OR prednisone OR dexamethasone OR dexibruprofen OR dexketoprofen OR diclofenac sodium OR diflusinal OR etodolac OR etoricoxib OR fenbufen OR fenoprofen OR flurbiprofen OR hydrocortisone OR ibuprofen OR indomethacin OR indometacin OR ketoprofen OR lumiracoxib OR mefenamic acid OR meloxicam OR methylprednisolone OR nabumetone OR naproxen OR nimesulide OR piroxicam OR sulindac OR tenoxicam OR tiaprofenic acid OR triamcinolone OR cyclooxygenase 2 inhibitor* OR anti‐inflammatory agent*) AND (Alzheimer* OR dementia OR ((cognit* or memory* or mental*) and (declin* or impair* or los* or deteriorat*)) |
| ISI Conference Proceedings | 10 September 2008 | (aspirin OR aceclofenac OR acemetacin OR betamethasone OR celecoxib OR cortisone OR deflazacort OR prednisone OR dexamethasone OR dexibruprofen OR dexketoprofen OR diclofenac sodium OR diflusinal OR etodolac OR etoricoxib OR fenbufen OR fenoprofen OR flurbiprofen OR hydrocortisone OR ibuprofen OR indomethacin OR indometacin OR ketoprofen OR lumiracoxib OR mefenamic acid OR meloxicam OR methylprednisolone OR nabumetone OR naproxen OR nimesulide OR piroxicam OR sulindac OR tenoxicam OR tiaprofenic acid OR triamcinolone OR cyclooxygenase 2 inhibitor* OR anti‐inflammatory agent*) AND (Alzheimer* OR dementia) |
| mRCT including ISRCTN Register, ClinicalTrials.gov |
10 September 2008 | (aspirin OR aceclofenac OR acemetacin OR betamethasone OR celecoxib OR cortisone OR deflazacort OR prednisone OR dexamethasone OR dexibruprofen OR dexketoprofen OR diclofenac sodium OR diflusinal OR etodolac OR etoricoxib OR fenbufen OR fenoprofen OR flurbiprofen OR hydrocortisone OR ibuprofen OR indomethacin OR indometacin OR ketoprofen OR lumiracoxib OR mefenamic acid OR meloxicam OR methylprednisolone OR nabumetone OR naproxen OR nimesulide OR piroxicam OR sulindac OR tenoxicam OR tiaprofenic acid OR triamcinolone OR cyclooxygenase 2 inhibitor* OR anti‐inflammatory agent*) AND (Alzheimer* OR dementia) |
| IFPMA, UMIN Japan trials register, Netherland trials register, Australasian Digital theses, Theses Canada, DATAD | 10 September 2008 | Alzheimer’s disease |
Appendix 3. measurement agreement
Kappa statistic was used to measure agreement between 2 authors who make a decision for inclusion and exclusion.From calculation, kappa is 0.782. It should be noted that this calculation is based on the first decision making of both reviewers.
Meaning of kappa statistic
0.40‐0.59: fair agreement
0.60‐0.74: good agreement
0.75 or more: excellent agreement
From 488 studies, there were 19 studies that we did not agree and required more discussion. Although measuring agreement showed excellent agreement, it should be considered that the discussion is still the main part of the decision making. However, kappa helped us to revisit inclusion criteria again whether it is clear enough for both reviewers in case of poor agreement. Occasionally, we have to revisit and clarify the inclusion and exclusion criteria to assure that all reviewers are on the same page. For example, three reviewers at one point were not sure if we should include all studies of anti‐inflammatory. After the discussion, we understood that we will only focus on aspirin, steroidal and non‐steroidal anti‐inflammatory. This leaded to the change of the review’s title.
| Review Author 1 (DJ) |
Review author 2 (MI) | |||||||
| Include | Exclude | Unsure | ||||||
| Include | 39 (a) | 1 (b) | 1 (c) | |||||
| Exclude | 11 (d) | 546 (e) | 2 (f) | |||||
| Unsure | 0 (g) | 4 (h) | 0 (i) | |||||
| Total | 50 (A2) | 551 (E2) | 3 (U2) | |||||
Po = a+e+i / K Po = 39+546+0 /604 = 0.969
PE = (A1xA2) + (E1+E2) + (U1+U2) / K2 PE = (41x50) + (559x551) + (4x3) /6042 = 0.850
Kappa = Po PE/ 1‐PE Kappa = 0.969‐0.850/ 1‐0.850 = 0.793
Appendix 4. Translation sheet
Cochrane Dementia and Cognitive Improvement Group
Translation sheet.
To be completed by the translator.
Please note: full translations of papers are not necessary. The following questions are designed to assist in the extraction of necessary information for the inclusion of randomised controlled trials.
Date of translation:
Translator:
Language of the paper:
1. Publication details
Authors:
Title (in English):
Original title:
Publication details:
(journal, volume, year, page nos)
If details are not reported, please indicate so.
2. Materials and Methods
Is the trial described as RANDOMISED?
(NB ‐ if the paper is not described as randomised, we do not require any further information, but please give a description of the study, ie, a review article, a case controlled trial, a letter to a journal, a double blind trial in which treatment was not randomised etc).
If YES, the following details are necessary:
Was it a parallel or cross‐over study?
What were the patients described as suffering from?
Diagnostic criteria
Number of patients involved:
Gender ratio:
Age groups (please give Means and SDs) (+/‐ values if reported):
Where were the patients recruited from?
Was the treatment double blinded?
Are the allocation of treatment methods described, if so what were they?
What was the treatment compared with (placebo or standard therapies)?
Where did the study take place (city, multi‐centre, hospital, community)?
How long did the trial last for?
Was there a follow‐up period? If yes, over how long did it take place?
What were the inclusion/exclusion criteria?
What was the dosage involved (if the treatment was pharmacological)?
What were the dosages for the control/placebo group?
3. Results
How were baseline measurements recorded?
What were the outcome measures?
Over what period were values recorded for the outcome measures?
Were there any drop outs reported? If so, how many?
Data:
Were the continuous data reported as Means and SDs/SEMs/Confidence Intervals? Please provide page numbers where results were presented to facilitate double‐checking
What outcomes were presented as binary data?
Were the results reported in tables/graphs?
If, so please indicate on the axes of the copy sent to you, what the headings mean in English, and return with this sheet.
What is the value (e.g. % or otherwise) to describe the increase for each of the outcome measures that are reported? Please give +/‐ values if reported. Please indicate where these values can be found in the original article (this will help to facilitate validation).
Is there any additional information which you consider significant?
Data and analyses
Comparison 1. Aspirin vs. aspirin avoidance.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bleeding | 1 | 310 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.90 [2.43, 25.69] |
| 2 Death | 1 | 310 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.70, 1.46] |
| 3 Institutionaliation | 1 | 310 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.50, 1.74] |
Comparison 2. Steroids vs. placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ADAScog | 1 | 138 | Mean Difference (IV, Fixed, 95% CI) | 1.90 [‐0.48, 4.28] |
| 2 Confusion | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.88, 2.01] |
| 3 hyperglycemia | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [1.14, 21.99] |
| 4 Abnormal lab results | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.55 [1.38, 4.70] |
| 5 Face edema | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.10, 3.17] |
2.2. Analysis.
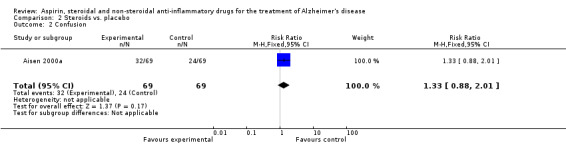
Comparison 2 Steroids vs. placebo, Outcome 2 Confusion.
Comparison 3. NSAIDs vs. placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cognition:ADAScog all studies | 9 | 1745 | Mean Difference (IV, Random, 95% CI) | ‐1.41 [‐3.13, 0.32] |
| 1.1 traditional NSAIDs vs placebo | 6 | 411 | Mean Difference (IV, Random, 95% CI) | ‐3.81 [‐7.94, 0.33] |
| 1.2 Selective COX‐2 inhibitor | 4 | 1334 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.99, 1.60] |
| 2 Cognition:MMSE all | 6 | 1268 | Mean Difference (IV, Random, 95% CI) | ‐1.08 [‐2.21, 0.04] |
| 2.1 traditional NSAIDs vs placebo | 4 | 234 | Mean Difference (IV, Random, 95% CI) | ‐3.22 [‐6.58, 0.14] |
| 2.2 Selective COX‐2 inhibitor | 2 | 1034 | Mean Difference (IV, Random, 95% CI) | 0.34 [‐0.07, 0.76] |
| 3 CIBIC+ | 3 | 1099 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.09, 0.16] |
| 3.1 traditional NSAIDs vs placebo | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.58, 0.38] |
| 3.2 Selective COX‐2 inhibitor | 2 | 1061 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.08, 0.18] |
| 4 CDR sum score | 3 | 1124 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.25, 0.30] |
| 4.1 traditional NSAIDs vs placebo | 1 | 229 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.50, 0.70] |
| 4.2 Selective COX‐2 inhibitor | 3 | 895 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.30, 0.32] |
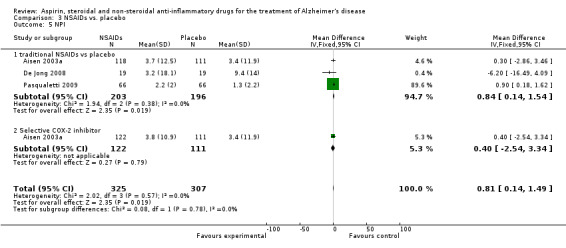
| 5 NPI | 3 | 632 | Mean Difference (IV, Fixed, 95% CI) | 0.81 [0.14, 1.49] |
| 5.1 traditional NSAIDs vs placebo | 3 | 399 | Mean Difference (IV, Fixed, 95% CI) | 0.84 [0.14, 1.54] |
| 5.2 Selective COX‐2 inhibitor | 1 | 233 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐2.54, 3.34] |
| 6 Mood/depression | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.96, 0.29] |
| 6.1 selective COX‐2 inhibitor vs placebo | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.96, 0.29] |
| 7 Clinical global impression: GDS | 1 | 31 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐1.15, 0.29] |
| 7.1 traditional NSAIDs vs placebo | 1 | 31 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐1.15, 0.29] |
| 8 Clinical global impression: CGIC and NOSGER 6 months | 2 | 441 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.49, 0.35] |
| 8.1 traditional NSAIDs vs placebo | 1 | 31 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐1.16, 0.27] |
| 8.2 Selective COX‐2 inhibitor | 1 | 410 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.16, 0.26] |
| 9 Behavioral disturbance | 3 | 479 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.29, 0.46] |
| 9.1 traditional NSAIDs vs placebo | 2 | 69 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.89, 0.82] |
| 9.2 Selective COX‐2 inhibitor | 1 | 410 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.04, 0.37] |
| 10 Activity of daily living | 7 | 1737 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.48, 0.04] |
| 10.1 traditional NSAIDs vs placebo | 4 | 375 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.97, 0.01] |
| 10.2 Selective COX‐2 inhibitor | 4 | 1362 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.22, 0.17] |
| 11 Quality of life | 2 | 382 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.14, 0.29] |
| 11.1 traditional NSAIDs vs placebo | 2 | 205 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.22, 0.36] |
| 11.2 Selective COX‐2 inhibitor | 1 | 177 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.23, 0.41] |
| 12 Caregiver burden | 3 | 201 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.63, ‐0.07] |
| 12.1 traditional NSAIDs vs placebo | 3 | 201 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.63, ‐0.07] |
| 13 Gastrointestinal side effects | 9 | 1675 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.36, 2.77] |
| 14 Elevated creatinine | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.30 [0.54, 34.30] |
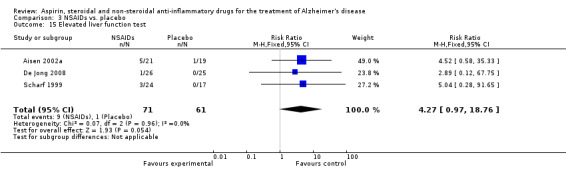
| 15 Elevated liver function test | 3 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.27 [0.97, 18.76] |
| 16 Headache | 4 | 577 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.34, 3.44] |
| 17 Psychiatric side effects | 4 | 586 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.67, 1.86] |
| 18 Bleeding | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.45 [0.18, 66.35] |
| 19 Heart disease | 2 | 776 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.58 [1.48, 38.90] |
| 20 Cerebrovascular side effects | 4 | 1555 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.54, 2.02] |
| 21 Hypertension | 2 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.41 [1.36, 21.60] |
| 22 Hyperglycemia | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [0.19, 19.90] |
| 23 Rash | 2 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.47 [1.00, 12.04] |
| 24 Respiratory side effects | 1 | 461 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.73 [0.61, 12.17] |
| 25 Dry mouth | 1 | 351 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.24 [0.40, 26.00] |
| 26 Fatigue | 1 | 351 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.31 [1.06, 5.04] |
| 27 Dizziness | 3 | 423 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.40 [1.10, 5.25] |
| 28 Abnormal labs other than Cr. and LFT | 2 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.21, 8.69] |
| 29 Withdrawal due to side effects | 3 | 1083 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.89, 1.65] |
| 30 Abdominal pain or dyspepsia | 8 | 994 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.97, 3.22] |
| 31 Constipation or diarrhea | 3 | 527 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.84, 4.88] |
| 32 Nausea or vomiting | 3 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.39, 7.38] |
| 33 Death | 9 | 1711 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.85, 3.31] |
3.5. Analysis.
Comparison 3 NSAIDs vs. placebo, Outcome 5 NPI.
3.6. Analysis.
Comparison 3 NSAIDs vs. placebo, Outcome 6 Mood/depression.
3.7. Analysis.
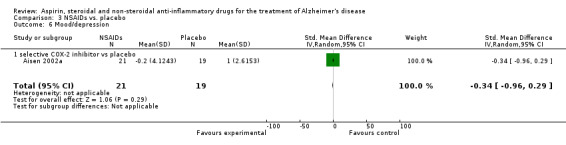
Comparison 3 NSAIDs vs. placebo, Outcome 7 Clinical global impression: GDS.
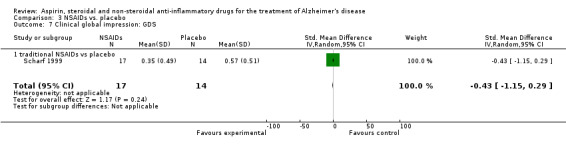
3.8. Analysis.
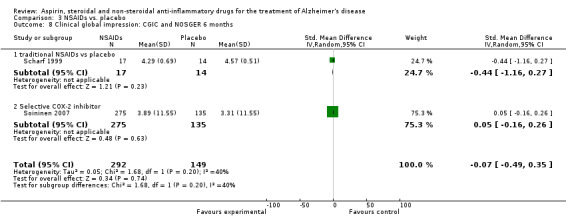
Comparison 3 NSAIDs vs. placebo, Outcome 8 Clinical global impression: CGIC and NOSGER 6 months.
3.9. Analysis.
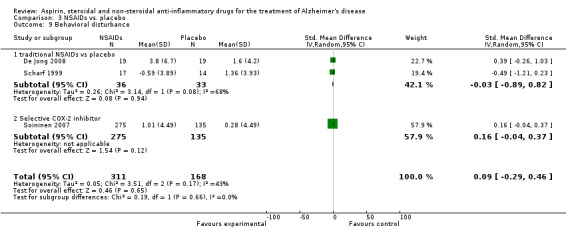
Comparison 3 NSAIDs vs. placebo, Outcome 9 Behavioral disturbance.
3.10. Analysis.
Comparison 3 NSAIDs vs. placebo, Outcome 10 Activity of daily living.
3.11. Analysis.
Comparison 3 NSAIDs vs. placebo, Outcome 11 Quality of life.
3.14. Analysis.
Comparison 3 NSAIDs vs. placebo, Outcome 14 Elevated creatinine.
3.15. Analysis.
Comparison 3 NSAIDs vs. placebo, Outcome 15 Elevated liver function test.
3.16. Analysis.
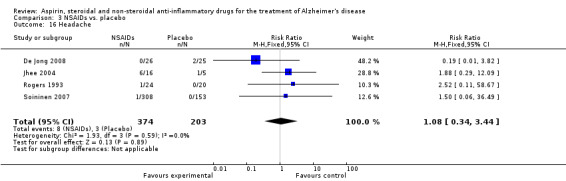
Comparison 3 NSAIDs vs. placebo, Outcome 16 Headache.
3.17. Analysis.
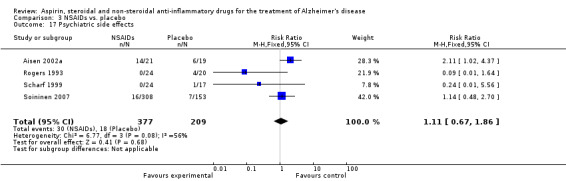
Comparison 3 NSAIDs vs. placebo, Outcome 17 Psychiatric side effects.
3.18. Analysis.
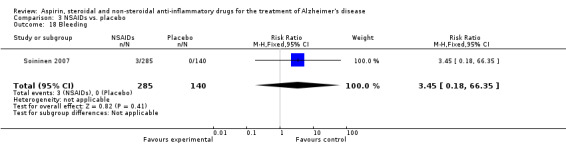
Comparison 3 NSAIDs vs. placebo, Outcome 18 Bleeding.
3.19. Analysis.
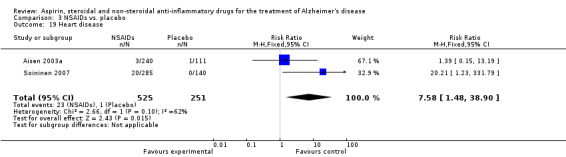
Comparison 3 NSAIDs vs. placebo, Outcome 19 Heart disease.
3.20. Analysis.
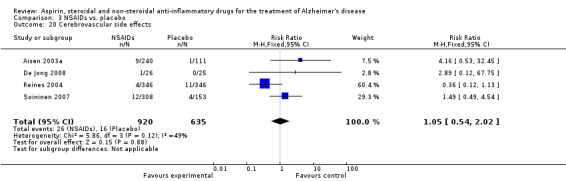
Comparison 3 NSAIDs vs. placebo, Outcome 20 Cerebrovascular side effects.
3.21. Analysis.
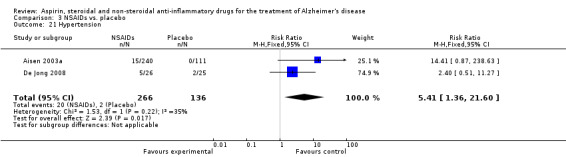
Comparison 3 NSAIDs vs. placebo, Outcome 21 Hypertension.
3.22. Analysis.
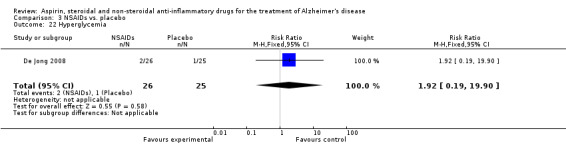
Comparison 3 NSAIDs vs. placebo, Outcome 22 Hyperglycemia.
3.23. Analysis.
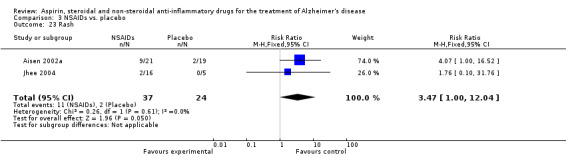
Comparison 3 NSAIDs vs. placebo, Outcome 23 Rash.
3.24. Analysis.
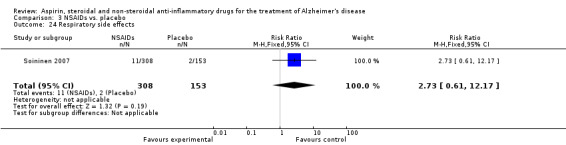
Comparison 3 NSAIDs vs. placebo, Outcome 24 Respiratory side effects.
3.25. Analysis.
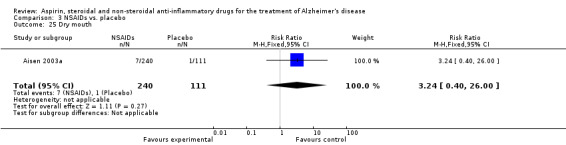
Comparison 3 NSAIDs vs. placebo, Outcome 25 Dry mouth.
3.26. Analysis.
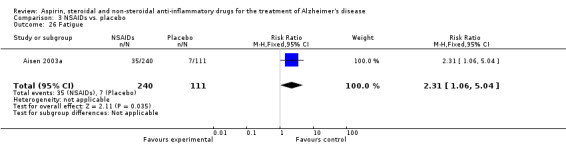
Comparison 3 NSAIDs vs. placebo, Outcome 26 Fatigue.
3.27. Analysis.
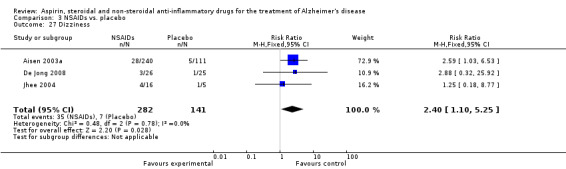
Comparison 3 NSAIDs vs. placebo, Outcome 27 Dizziness.
3.28. Analysis.
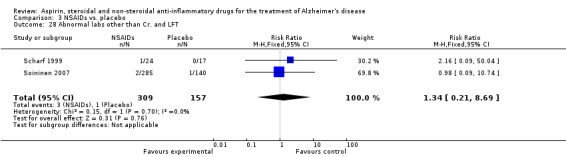
Comparison 3 NSAIDs vs. placebo, Outcome 28 Abnormal labs other than Cr. and LFT.
3.29. Analysis.
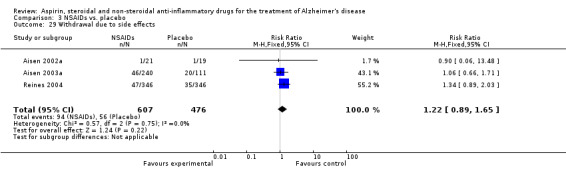
Comparison 3 NSAIDs vs. placebo, Outcome 29 Withdrawal due to side effects.
3.30. Analysis.
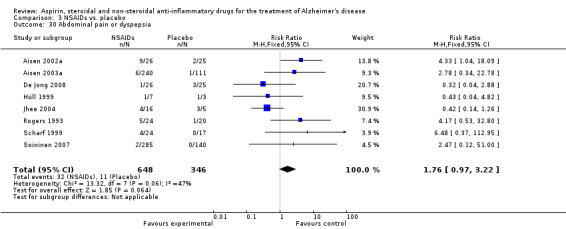
Comparison 3 NSAIDs vs. placebo, Outcome 30 Abdominal pain or dyspepsia.
3.31. Analysis.
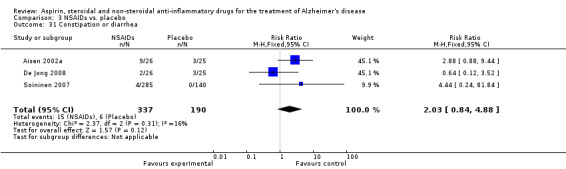
Comparison 3 NSAIDs vs. placebo, Outcome 31 Constipation or diarrhea.
3.32. Analysis.
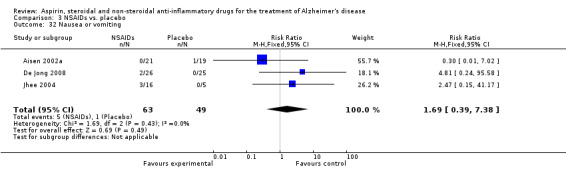
Comparison 3 NSAIDs vs. placebo, Outcome 32 Nausea or vomiting.
3.33. Analysis.
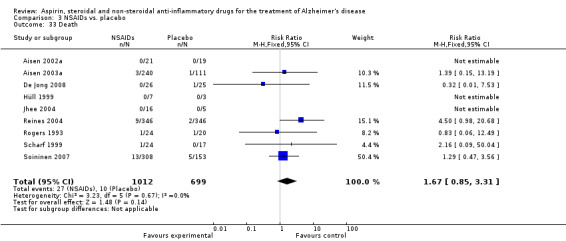
Comparison 3 NSAIDs vs. placebo, Outcome 33 Death.
Comparison 4. Traditional NSAIDs vs. placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nausea or vomiting | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.81 [0.24, 95.58] |
| 1.1 traditional NSAIDs | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.81 [0.24, 95.58] |
| 2 Gastrointestinal side effects | 5 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.68, 3.00] |
| 2.1 traditional NSAIDs | 5 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.68, 3.00] |
| 3 Elevated creatinine | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.30 [0.54, 34.30] |
| 3.1 traditional NSAIDs | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.30 [0.54, 34.30] |
| 4 Elevated liver function test | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.04 [0.48, 33.98] |
| 4.1 traditional NSAIDs | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.04 [0.48, 33.98] |
| 5 Hypertension | 2 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.36 [0.84, 13.34] |
| 5.1 traditional NSAIDs | 2 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.36 [0.84, 13.34] |
| 6 Headache | 2 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.10, 3.62] |
| 6.1 traditional NSAIDs | 2 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.10, 3.62] |
| 7 Psychiatric side effects | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.02, 1.07] |
| 7.1 traditional NSAIDs | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.02, 1.07] |
| 8 Heart disease | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 3.86] |
| 8.1 traditional NSAIDs | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 3.86] |
| 9 Cerebrovascular side effects | 2 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.51 [0.43, 14.63] |
| 9.1 traditional NSAIDs | 2 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.51 [0.43, 14.63] |
| 10 Hyperglycemia | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [0.19, 19.90] |
| 10.1 traditional NSAIDs | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [0.19, 19.90] |
| 11 Dry mouth | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [0.35, 23.09] |
| 11.1 traditional NSAIDs | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [0.35, 23.09] |
| 12 Fatigue | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.51, 2.62] |
| 12.1 traditional NSAIDs | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.51, 2.62] |
| 13 Dizziness | 2 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.64, 3.69] |
| 13.1 traditional NSAIDs | 2 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.64, 3.69] |
| 14 Abnormal labs other than Cr. and LFT | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.16 [0.09, 50.04] |
| 14.1 traditional NSAIDs | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.16 [0.09, 50.04] |
| 15 Death | 5 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.18, 2.87] |
| 15.1 traditional NSAIDs | 5 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.18, 2.87] |
| 16 Withdrawal due to side effects | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.30, 0.84] |
| 16.1 traditional NSAIDs | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.30, 0.84] |
| 17 Abdominal pain or dyspepsia | 5 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.61, 3.68] |
| 17.1 traditional NSAIDs | 5 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.61, 3.68] |
| 18 Constipation or diarrhea | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.12, 3.52] |
| 18.1 traditional NSAIDs | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.12, 3.52] |
4.6. Analysis.
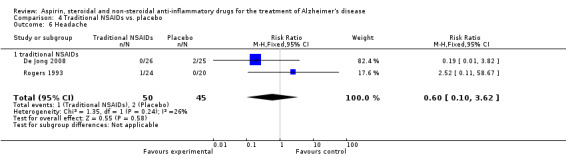
Comparison 4 Traditional NSAIDs vs. placebo, Outcome 6 Headache.
4.7. Analysis.
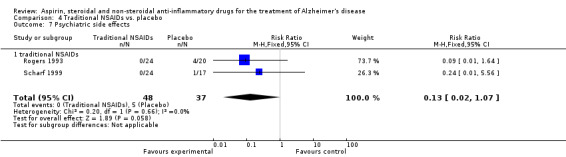
Comparison 4 Traditional NSAIDs vs. placebo, Outcome 7 Psychiatric side effects.
4.8. Analysis.
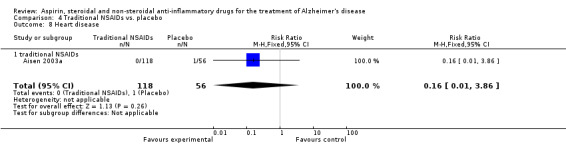
Comparison 4 Traditional NSAIDs vs. placebo, Outcome 8 Heart disease.
4.9. Analysis.
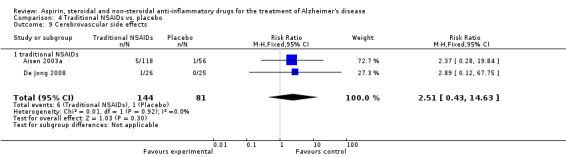
Comparison 4 Traditional NSAIDs vs. placebo, Outcome 9 Cerebrovascular side effects.
4.10. Analysis.
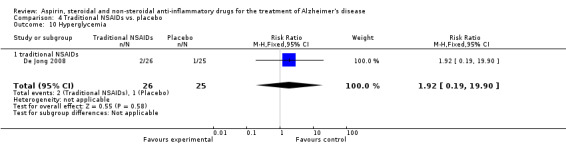
Comparison 4 Traditional NSAIDs vs. placebo, Outcome 10 Hyperglycemia.
4.11. Analysis.
Comparison 4 Traditional NSAIDs vs. placebo, Outcome 11 Dry mouth.
4.12. Analysis.
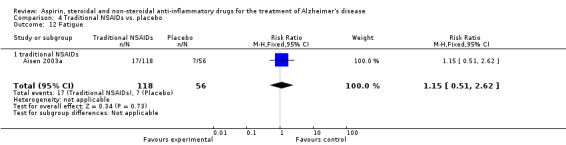
Comparison 4 Traditional NSAIDs vs. placebo, Outcome 12 Fatigue.
4.13. Analysis.
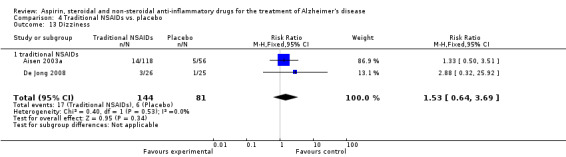
Comparison 4 Traditional NSAIDs vs. placebo, Outcome 13 Dizziness.
4.14. Analysis.
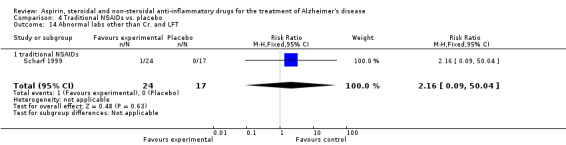
Comparison 4 Traditional NSAIDs vs. placebo, Outcome 14 Abnormal labs other than Cr. and LFT.
4.16. Analysis.
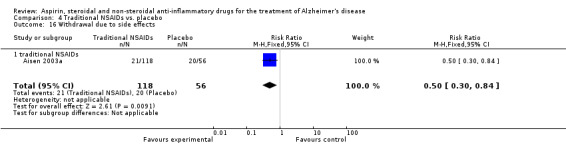
Comparison 4 Traditional NSAIDs vs. placebo, Outcome 16 Withdrawal due to side effects.
4.17. Analysis.
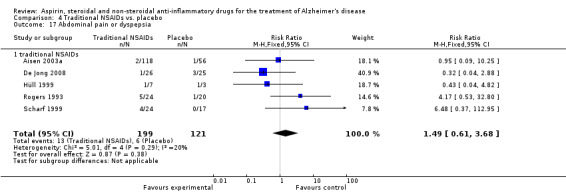
Comparison 4 Traditional NSAIDs vs. placebo, Outcome 17 Abdominal pain or dyspepsia.
4.18. Analysis.
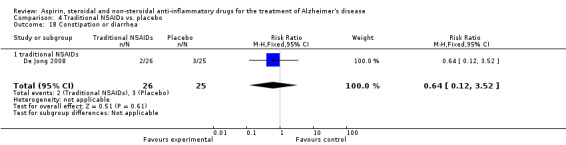
Comparison 4 Traditional NSAIDs vs. placebo, Outcome 18 Constipation or diarrhea.
Comparison 5. Selective COX‐2 inhibitor vs. placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gastrointestinal side effects | 5 | 1355 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [1.37, 3.03] |
| 1.1 Selective COX‐2 inhibitor | 5 | 1355 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [1.37, 3.03] |
| 2 Hypertension | 1 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.65 [0.51, 146.03] |
| 2.1 Selective COX‐2 inhibitor | 1 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.65 [0.51, 146.03] |
| 3 Heart disease | 2 | 602 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.52 [1.47, 38.41] |
| 3.1 Selective COX‐2 inhibitor | 2 | 602 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.52 [1.47, 38.41] |
| 4 Rash | 2 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.47 [1.00, 12.04] |
| 4.1 Selective COX‐2 inhibitor | 2 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.47 [1.00, 12.04] |
| 5 Headache | 2 | 482 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.35, 8.81] |
| 5.1 Selective COX‐2 inhibitor | 2 | 482 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.35, 8.81] |
| 6 Psychiatric side effects | 2 | 501 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.87, 2.69] |
| 6.1 Selective COX‐2 inhibitor | 2 | 501 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.87, 2.69] |
| 7 Bleeding | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.45 [0.18, 66.35] |
| 7.1 Selective COX‐2 inhibitor | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.45 [0.18, 66.35] |
| 8 Cerebrovascular side effects | 3 | 1330 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.40, 1.64] |
| 8.1 Selective COX‐2 inhibitor | 3 | 1330 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.40, 1.64] |
| 9 Respiratory side effects | 1 | 461 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.73 [0.61, 12.17] |
| 9.1 Selective COX‐2 inhibitor | 1 | 461 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.73 [0.61, 12.17] |
| 10 Dry mouth | 1 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.06, 33.01] |
| 10.1 Selective COX‐2 inhibitor | 1 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.06, 33.01] |
| 11 Fatigue | 1 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.51, 2.61] |
| 11.1 Selective COX‐2 inhibitor | 1 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.51, 2.61] |
| 12 Dizziness | 2 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.53, 3.01] |
| 12.1 Selective COX‐2 inhibitor | 2 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.53, 3.01] |
| 13 Abnormal labs other than Cr. and LFT | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.09, 10.74] |
| 13.1 Selective COX‐2 inhibitor | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.09, 10.74] |
| 14 Death | 5 | 1391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.87, 4.03] |
| 14.1 Selective COX‐2 inhibitor | 5 | 1391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.87, 4.03] |
| 15 Withdrawal due to side effects | 4 | 1370 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.71, 1.31] |
| 15.1 Selective COX‐2 inhibitor | 4 | 1370 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.71, 1.31] |
| 16 Abdominal pain or dyspepsia | 4 | 663 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.79, 3.55] |
| 16.1 Selective COX‐2 inhibitor | 4 | 663 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.79, 3.55] |
| 17 Constipation or diarrhea | 2 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [1.04, 9.66] |
| 17.1 Selective COX‐2 inhibitor | 2 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [1.04, 9.66] |
| 18 Nausea or vomiting | 2 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.16, 6.29] |
| 18.1 Selective COX‐2 inhibitor | 2 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.16, 6.29] |
| 19 Abnormal liver function test | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.52 [0.58, 35.33] |
5.5. Analysis.
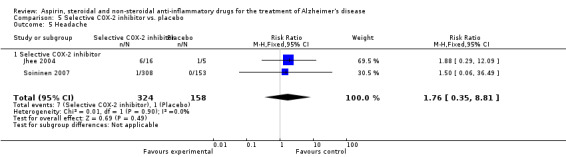
Comparison 5 Selective COX‐2 inhibitor vs. placebo, Outcome 5 Headache.
5.6. Analysis.
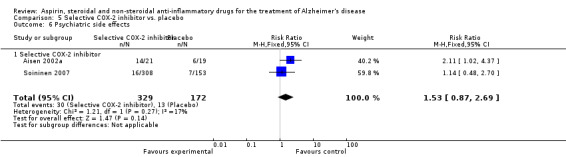
Comparison 5 Selective COX‐2 inhibitor vs. placebo, Outcome 6 Psychiatric side effects.
5.7. Analysis.
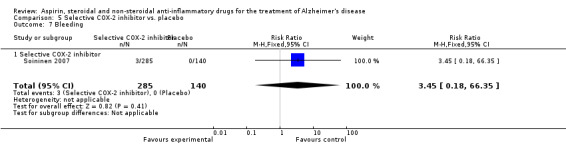
Comparison 5 Selective COX‐2 inhibitor vs. placebo, Outcome 7 Bleeding.
5.8. Analysis.
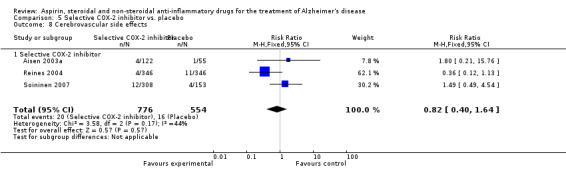
Comparison 5 Selective COX‐2 inhibitor vs. placebo, Outcome 8 Cerebrovascular side effects.
5.9. Analysis.
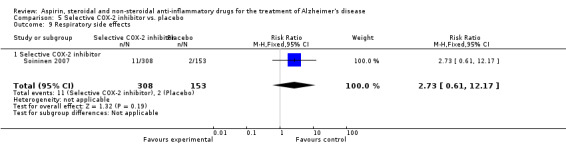
Comparison 5 Selective COX‐2 inhibitor vs. placebo, Outcome 9 Respiratory side effects.
5.10. Analysis.
Comparison 5 Selective COX‐2 inhibitor vs. placebo, Outcome 10 Dry mouth.
5.11. Analysis.
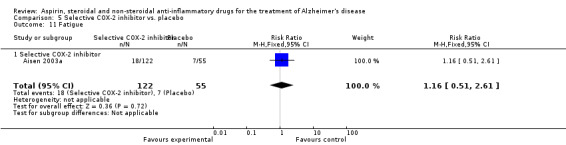
Comparison 5 Selective COX‐2 inhibitor vs. placebo, Outcome 11 Fatigue.
5.12. Analysis.
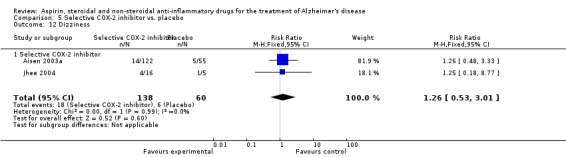
Comparison 5 Selective COX‐2 inhibitor vs. placebo, Outcome 12 Dizziness.
5.13. Analysis.
Comparison 5 Selective COX‐2 inhibitor vs. placebo, Outcome 13 Abnormal labs other than Cr. and LFT.
5.15. Analysis.
Comparison 5 Selective COX‐2 inhibitor vs. placebo, Outcome 15 Withdrawal due to side effects.
5.16. Analysis.
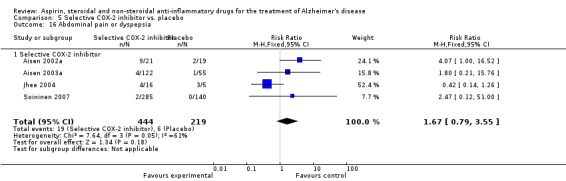
Comparison 5 Selective COX‐2 inhibitor vs. placebo, Outcome 16 Abdominal pain or dyspepsia.
5.17. Analysis.
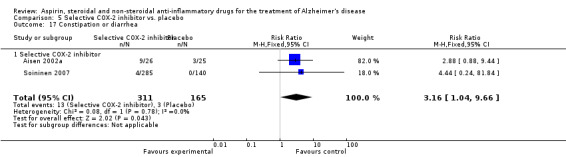
Comparison 5 Selective COX‐2 inhibitor vs. placebo, Outcome 17 Constipation or diarrhea.
5.18. Analysis.
Comparison 5 Selective COX‐2 inhibitor vs. placebo, Outcome 18 Nausea or vomiting.
5.19. Analysis.
Comparison 5 Selective COX‐2 inhibitor vs. placebo, Outcome 19 Abnormal liver function test.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aisen 2000a.
| Methods | Double blind randomised controlled trial, 12 months | |
| Participants | 138 participants (69 intervention, 69 control) who had MMSE 13‐26, aged>50 years, were out‐patient in USA | |
| Interventions | Intervention: Prednisone 20 mg oral x 4 weeks then 10 mg x1year, follow by gradual taper over an additional 16 weeks of observation Control: placebo |
|
| Outcomes | Cognition (ADAScog 0‐70 lower score = improvement) at 12 months (CDR‐sob lower score = improvement) at 12 months Mood and depression (BPRS 7‐126 lower score = improvement at 12 months (Ham‐D 0‐54 lower score = improvement) at 12 months (BDRS lower score = improvement) at 12 months |
|
| Notes | Supported by a grant from the national institutes of health. Prednisone and matching placebo were provided by Pharmacia and Upjohn, Inc | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote “Codes were randomised at the packaging centre (supposed it is computer), the randomisation scheme was approved by the Alzheimer’s disease Cooperative study (ADCS) statistic core. Randomization was stratified by site and utilized a block size of eight” Comment: Codes were generated at the packaging centre, probably using computer. |
| Allocation concealment (selection bias) | Low risk |
Quote 1 “Scratch‐off code breakers were used so that instances of unbinding would be documented. All code breakers were collected at the end of the trial” Quote 2 “Randomization codes were broken in two instances. In each case, the code was broken by investigators at local sites to determine whether stress‐dose glucocorticoid treatment was necessary for subjects who required surgical procedures” Comment: It mentioned about using the code as a number. Probably done |
| Blinding (performance bias and detection bias) All outcomes | Low risk |
Quote 1“Matching placebo tablets were assembled into identical containers with coded labels to blind participants” Quote 2 “Adequacy of masking was assesses by questionnaires completed by subjects, caregivers, psychometrists, and site investigators” Comment: Blinding of participants ensured and unlikely that the blinding could have been broken |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The reasons for early discontinuation of study medication were caregiver issues and perceived lack of efficacy. No subjects discontinued medication because of serious adverse events attributed to the treatment 1 year: 19/69 missing from intervention group, 11/69 missing from placebo Comment: Reasons for missing outcome data likely to be related to true outcome |
| Selective reporting (reporting bias) | Low risk | Report all outcome state in methodology Comment: The study protocol is not available, but all of the study’s pre‐specified outcomes have been reported in the pre‐specified way in methodology |
| Other bias | Low risk |
Comment: The study appears to be free of other sources of bias |
Aisen 2002a.
| Methods | Double blind randomised controlled trial, 12 weeks | |
| Participants | 40 participants (21 intervention, 19 placebo) who has probable AD with stable medical condition, were out‐patient in USA, age‐not limited | |
| Interventions | Intervention: Nimesulide 100 mg oral twice Control: Placebo |
|
| Outcomes | Cognition (ADAScog 0‐70 lower score = improvement) (CDR‐sob lower score = improvement) (MMSE 0‐30 higher score = improvement) Mood and depression (BPRS 7‐126 lower score = improvement) (Ham‐D 0‐54 lower score = improvement) Activity of daily living (Blessed ADL score higher score = improvement) |
|
| Notes | Sources of funding not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: The randomisation schedule was generated in the research pharmacy Comment: Insufficient information about the sequence generation process to permit judgement of yes or no |
| Allocation concealment (selection bias) | Unclear risk |
Comment Insufficient information to permit judgement of Yes or No |
| Blinding (performance bias and detection bias) All outcomes | Low risk |
Quote 1 : All investigators and study personnel remained blind to group assignment of participants until completion of data collection Quote 2 : Participants were treated twice daily with Nimesulide or identical placebo tablets Comment: done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Reasons for missing outcome data unlikely to be related to true outcome |
| Selective reporting (reporting bias) | Low risk |
Comment: The study protocol is not available, but all of the study’s pre‐specified outcomes have been reported in the pre‐specified way in methodology |
| Other bias | Low risk |
Comment: The study appears to be free of other sources of bias |
Aisen 2003a.
| Methods | Double blind randomised controlled trial, 12 months | |
| Participants | 351 participants (240 intervention, 111 control) who had MMSE 13‐26, aged > 50 years, were out‐patient in USA | |
| Interventions | Intervention 1: Naproxen sodium 220 mg oral twice (118 participants) Intervention 2: Rofecoxib 25 mg oral once (122 participants) Control: Placebo |
|
| Outcomes | Cognition (ADAScog 0‐70 lower score = improvement) at 6, 12 months (CDR‐sob lower score = improvement) at 6, 12 months (NPI 0‐144 lower score = improvement) at 6, 12 months Activity of daily living (ADCS‐ADL 0‐78 higher score = improvement) at 6, 12 months Qulity of life (QoL‐AD higher score = improvement) at 6, 12 months |
|
| Notes | source of funding: a grant from the national institute on aging and the general clinical research centre program of the national centre for research resources | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: The randomisation process used a permuted block design with block size of 3 stratified by site. The randomisation sequence was generated by the ADCS data centre Comment: done |
| Allocation concealment (selection bias) | Low risk |
Quote 1: Scratch‐off code‐breakers were used so that instances of unbinding would be documented. All code breakers were collected at the end of the trial. Comment: Probably done |
| Blinding (performance bias and detection bias) All outcomes | Low risk |
Quote 1: Rofecoxib tablets were over‐encapsulated to allow preparation of an identical placebo capsule. Naproxen sodium tablets and identical placebo tables were supplied by?.. Quote 2: Adequacy of masking was assessed by questionnaires completed by participants, caregivers, psychometrists, and site investigators. The results of questionnaires at 12 months indicated that the percentage of participants and informants who believed they were taking active study medication and active study medication also did not differ significantly across the treatment group, indicating that blinding was adequately maintained. Quote3: the randomisation code was broken in 1 instance, based on clinical need for the management of an acute medical problem Comment: acceptable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quote 1: Reason for loss follow up are caregiver issue and adverse event Comment: Reasons for missing outcome data unlikely to be related to true outcome Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across group |
| Selective reporting (reporting bias) | Low risk | All primary outcomes were reported, but not all secondary outcome such as institutionalisation Comment: The study protocol is not available, but all of the study’s pre‐specified outcomes have been reported in the pre‐specified way in methodology |
| Other bias | Low risk |
Comment: The study appears to be free of other sources of bias |
Bentham 2008.
| Methods | Open‐label randomised controlled trial, 3 years | |
| Participants | 310 participants (156 intervention, 154 control) who had a DSM‐4 diagnosis of probable dementia of AD without a coexisting diagnosis of vascular dementia, aged 46‐90, were out‐patient in the UK. 75% took Donepezil. | |
| Interventions | Intervention: aspirin 75 mg oral once Control: avoid aspirin |
|
| Outcomes | Cognition (MMSE 0‐30 higher score = improvement) at 12 wk, 1,2,3 years (NPI 0‐144 lower score = improvement) at 12 wk, 1,2,3 years Activity of daily living (BALDS 0‐60 higher score = improvement ) at 12 wk, 1,2,3 years Caregiver burden (GHQ for care give 0‐30 lower score = improvement) at 12 wk, 1,2,3 years |
|
| Notes | funding from research and development directorate of the west midlands region or national health service executive | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: Eligible patients were randomly assigned to take open‐label aspirin or to take no aspirin. Treatment allocation was obtained by telephone from the central trial office and used minimised randomisation generated by a computer program to balance allocations by age, severity of dementia, the presence of absence of vascular dementia, parkinsonian and psychotic symptoms. Comment: done |
| Allocation concealment (selection bias) | Unclear risk | No statement Comment: Probably not done |
| Blinding (performance bias and detection bias) All outcomes | High risk |
Quote 1: No blinding and the outcome or outcome measurement is likely to be influenced by lack of blinding. This is an open‐label study Quote 2: The potential for biased assessment by patients or raters was judged insufficient to justify the cost of packaging aspirin and placebo for this long‐term study. Comment: Not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quote 1 Comment: Reasons for missing outcome data unlikely to be related to true outcome Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk |
Comment: The study protocol is not available, but all of the study’s pre‐specified outcomes have been reported in the pre‐specified way in methodology |
| Other bias | High risk |
Comment: Had a potential source of bias related to the specific study design used |
De Jong 2008.
| Methods | Double blind randomised controlled trial, 12 months | |
| Participants | 51 participants (26 intervention, 25 control) who had a diagnosis of probable AD by NINCDS/ADRDA with MMSE 10‐26, were out‐patient in Netherlands, aged‐not limited |
|
| Interventions | Intervention: Indomethacin 50 mg oral twice Control: Placebo |
|
| Outcomes | Cognition (ADAScog 0‐70 lower score = improvement) at 6,12 months (MMSE higher score = improvement) at 6,12 months (CIBIC+ 1‐7 lower score = improvement) at 6,12 months (NPI 0‐144 lower score = improvement) at 6,12 months Activity of daily living (IDDD lower score = improvement) at 6,12 months Caregiver burden (NPI‐D lower score = improvement) at 6,12 months |
|
| Notes | Funding from grants from Zon‐MW Innovational Research, Hersenstichting Nederland | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: the statistician provided computer‐generated lists of random numbers allocating patients in a 1:1 ratio to receive indomethacin or placebo. For each centre, a separate randomisation list was provided Comment: done |
| Allocation concealment (selection bias) | Low risk |
Quote 1: Randomization codes were held by the pharmacy of the Radboud University Nijmegen Medical Centerthat labelled and dispensed all trial medication. Allocation was concealed from all investigators and patients. Comment: done |
| Blinding (performance bias and detection bias) All outcomes | Low risk |
Quote 1: The indomethacin and placebo tablets were of identical appearance. Neither the patients nor the investigators knew which treatment they received or dispensed. The blinding process remained complete until all data was entered in the trial database and the accuracy of the data and the database was confirmed. Afterward, the database was forwarded to the statistician for analysis Comment: done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quote 1: Comment: Reasons for missing outcome data unlikely to be related to true outcome Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across group |
| Selective reporting (reporting bias) | Low risk |
Comment: The study protocol is not available, but all of the study’s pre‐specified outcomes have been reported in the pre‐specified way in methodology |
| Other bias | Low risk |
Comment: The study appears to be free of other sources of bias |
Hüll 1999.
| Methods | Double blind randomised controlled trial, 6 months | |
| Participants | 10 Participants (7 intervention, 3 control) who had AD diagnosis, unclear if they were out‐patient or in‐patient, in Germany, aged 55‐75 | |
| Interventions | Intervention: Piroxicam‐B‐cyclodextrin 10 mg oral once Control: Placebo |
|
| Outcomes | Cognition (ADAScog 0‐70 lower score = improvement) at 6 months | |
| Notes | Sources of funding not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
No information Comment: probably not done |
| Allocation concealment (selection bias) | Unclear risk |
No information Comment: Probably not done |
| Blinding (performance bias and detection bias) All outcomes | Low risk |
Quote 1: all patients received either 10 mg piroxicam B Cyclodextrin or placebo Comment: Probably done, but there is only information for participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Comment: Reasons for missing outcome data unlikely to be related to true outcome |
| Selective reporting (reporting bias) | High risk | Only report ADAS‐cog Comment: not all of the study’s pre‐specified primary outcomes have been reported. The study report fails to include results for a key outcome that would be expected to have been reported for such a study |
| Other bias | High risk |
Comment: had a potential source of bias related to the specific study design used Selection bias due to baseline imbalance |
Jhee 2004.
| Methods | Double blind randomised controlled trial, 4 weeks | |
| Participants | 21 participants (16 intervention, 5 control) who had a diagnosis of probable AD. Subjects had to meet the NINCDS‐ADRDA and DSM4 criteria for probable AD, had modified Hachinski Ischemia Scale score of less than or equal to 4, MMSE score between 10‐24 inclusive and had been diagnosed with probable AD for at least a six month period. They were out‐patient, aged>/=60 y in USA | |
| Interventions | Interventions: Celecoxib dose 50. 200 and 400 mg oral twice Control: Placebo |
|
| Outcomes | Cognition (ADAScog 0‐70 lower score = improvement) at 4 weeks (MMSE higher score = improvement) at 4 weeks |
|
| Notes | Sources of funding not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk |
Quote: Subjects with AD were randomly allocated a treatment arm in the order in which they were enrolled in the study Comment: Sequence generated by some rule based on the number they enrolled in the study |
| Allocation concealment (selection bias) | High risk |
No information Comment: Probably not done |
| Blinding (performance bias and detection bias) All outcomes | Low risk |
Quote 1: All study medication had the same appearance and was provided as celecoxib 50 mg and 200 mg capsules and matching placebo. Comment: Probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Comment: Reasons for missing outcome data unlikely to be related to true outcome |
| Selective reporting (reporting bias) | Low risk |
Comment: The study protocol is not available, but all of the study’s pre‐specified outcomes have been reported in the pre‐specified way in methodology |
| Other bias | High risk |
Comment: The study appears to have some degrees of selective bias as patients' age in each group were difference. |
Pasqualetti 2009.
| Methods | Double blind randomised control trial, 52 weeks | |
| Participants | 132 participants (66 intervention, 66 control) who had a diagnosis of probable AD by ADRdA‐NINCDS criteria with MMSE 16‐25 and Clinical Dementia Rating =0.5‐1, in Italy, aged 65 years or older and be cared for by a reliable caregiver, Out‐patient setting | |
| Interventions | Intervention: Ibuprofen 400 mg twice a day plus esomeprazole 20 mg Control: Placebo |
|
| Outcomes | Cognition (MMSE 0‐30 higher score = improvement) at 52 weeks (ADAScog 0‐70 lower score = improvement) at 52 weeks (CDR‐sob lower score = improvement) at 52 weeks (CIBIC+ 1‐7 lower score = improvement) at 52 weeks (NPI 0‐144 lower score = improvement) at 52 weeks Mood (GDS 0‐15 lower score = improvement) at 52 weeks Basic and instrumental activities of Daily LIving Scales (lower score = improvement) at 52 weeks Caregiver burden (NPI‐stress CG subscale lower score = improvement) at 52 weeks (STAI‐Y1, STAI‐Y2) at 52 weeks (Beck Depression Inventory) at 52 weeks (CBI) at 52 weeks |
|
| Notes | The study was supported by a grant from the Italian Health Department. Active drug tablets and relative placebos were supplied by Angelini SpA for Ibuprofen and by Astra‐Zeneca Pharmaceuticals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: Randomization was performed with a permuted block design with a block size of 10, allocation rate 1:1, stratified by recruitment site. The randomisation sequence was provided by the Statistical Analysis Center, according to a pseudo‐random computerized generator. Comment: Sequence generated by some rule based on the number they enrolled in the study |
| Allocation concealment (selection bias) | Low risk |
Quote: Randomization was performed with a permuted block design with a block size of 10, allocation rate 1:1, stratified by recruitment site. Comment: Done |
| Blinding (performance bias and detection bias) All outcomes | Low risk |
Quote 1: Tablets of the active drug and the placebo were undistinguishable, as were the gastroprotective agent and the relative placebo. Code‐breaking was possible only at the level of the statistical Analysis Center an upon motivated request, resulting in simultaneous discontinuation of the patient from the study. Comment: Done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Comment: Reasons for missing outcome data unlikely to be related to true outcome |
| Selective reporting (reporting bias) | Low risk |
Comment: The study protocol is not available, but all of the study’s pre‐specified outcomes have been reported in the pre‐specified way in methodology |
| Other bias | Low risk | Comment: The study appears to be free of other sources of bias |
Reines 2004.
| Methods | Double blind randomised controlled trial, 12 months | |
| Participants | 692 participants (346 intervention, 346 control) who aged at least 50 years, met standard research criteria for possible or probable AD and had mild or moderate dementia as measured by MMSE score from 14‐26 inclusive, unclear if they were out‐patient or in‐patient, in USA | |
| Interventions | Intervention: Rofecoxib 25 mg oral once Control: Placebo |
|
| Outcomes | Cognition (ADAScog 0‐70 lower score = improvement) at 6, 12 months (CDR‐sob lower score = improvement) at 6, 12 months (MMSE 0‐30 higher score = improvement) at 6, 12 months (CIBIC+ 1‐7 lower score = improvement) at 6, 12 months Activity of daily living (ADCS‐ADL 0‐78 higher score = improvement) at 6, 12 months |
|
| Notes | Funding from Merck Research Laboratories | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: Randomization of patients at each study site was determined by a computer‐generated allocation schedule and was stratified according to MMSE score (14‐20 and > 20) to try to ensure equal distributions of dementia severity in each treatment group Comment: done |
| Allocation concealment (selection bias) | Low risk |
Quote: A statistician at Merck Research Laboratories generated the allocation schedule according to in‐house blinding conditions. The rofecoxib and placebo tables were visually identical. Comment: done |
| Blinding (performance bias and detection bias) All outcomes | Low risk |
Quote 1: A The rofecoxib and placebo tables were visually identical. Quote 2: Any adverse events occurring during the study were recorded and rated by the investigator, while still blinded. Any serious vascular events including cardiac, peripheral vascular and cerebrovascular events were reviewed by independent blinded adjudication committees Comment: done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Comment: Reasons for missing outcome data unlikely to be related to true outcome Reason for missing outcome data unlikely to be related to true outcome |
| Selective reporting (reporting bias) | Low risk |
Comment: The study protocol is not available, but all of the study’s pre‐specified outcomes have been reported in the pre‐specified way in methodology |
| Other bias | Low risk |
Comment: The study appears to be free of other sources of bias |
Rogers 1993.
| Methods | Double blind randomised control trial, 6 months | |
| Participants | 28 participants (14 intervention, 14 control) who had a diagnosis of probable AD and MMSE of 16 or more, unclear if they are out‐patient or in‐patient, in USA, aged, not limited | |
| Interventions | Intervention: Indomethacin oral once 100 mg/day for weight < 100 pounds, 125 mg/day for weight 101‐150 pounds, 150 mg/day for weight > 150 pounds Control: Placebo |
|
| Outcomes | Cognition (MMSE 0‐30 higher score = improvement) at 6 months (ADAScog 0‐70 lower score = improvement) at 6 months (BNT 0‐60 higher score = improvement) at 6 months (TK higher score = improvement) at 6 months |
|
| Notes | Sources of funding not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were then randomly assigned to one of two treatment conditions in a double‐blind protocol, no information about sequence generation Comment: probably not done |
| Allocation concealment (selection bias) | Unclear risk | No information Comment: Probably not done |
| Blinding (performance bias and detection bias) All outcomes | Low risk |
Quote 1: Patients were then randomly assigned to one of two treatment conditions in a double‐blind protocol Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Comment: Reasons for missing outcome data unlikely to be related to true outcome |
| Selective reporting (reporting bias) | Low risk |
Comment: The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Other bias | Low risk |
Comment: The study appears to be free of other sources of bias |
Scharf 1999.
| Methods | Double blind randomised control trial, 25 weeks | |
| Participants | 27 participants (12 intervention, 15 placebo) who had a diagnosis of AD according to DSM‐4 criteria that was of mild to moderate severity, defined by a MMSE score of 11‐25, aged >/= 50 years, were out‐patient in Australia | |
| Interventions | Intervention: Unknown dose of diclofenac and misoprostol oral Control: Placebo |
|
| Outcomes | Cognition (ADAScog 0‐70 lower score = improvement) at 12, 25 weeks (MMSE 0‐30 higher score = improvement) at 12, 25 weeks Clinical global impression of change at 12, 25 weeks (GDS lower score = improvement) at 12, 25 weeks (CGIC lower score = improvement) at 12, 25 weeks Behavioral disturbance (ADAS‐noncog lower score = improvement) at 12, 25 weeks (ADAS‐total lower score = improvement) at 12, 25 weeks Activity of daily living (IADL lower score = improvement) at 12, 25 weeks Quality of life (PSMS lower score = improvement) at 12, 25 weeks Caregiver burden (CGIC lower score = improvement) at 12, 25 weeks |
|
| Notes | source of funding from postgraduate medical research scholarship | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk |
Quote: Randomization was performed by the manufacturer of the medication before delivery to the study centre. For patients 1 to 20, 50% received diclofenac/misoprostol (D/M) and 50% placebo; for patients 21‐41, two thirds received D/M and one third placebo. Comment: Sequence generated by some rule based on hospital or clinic record number |
| Allocation concealment (selection bias) | Low risk |
Quote: The identity code was concealed in sequentially numbered opaque envelopes. Quote 2: The medication and envelopes were stored in a pharmacy separate from the study site. The medication was supervised and dispensed by independent pharmacist trained in the conduct of randomised double‐blind clinical trials. Quote 3: Medication codes were broken after completion of the trial or if a patient was withdrawn prematurely due to an adverse event. Comment: Done |
| Blinding (performance bias and detection bias) All outcomes | Low risk |
Quote 1: Patients were randomly assigned in a double‐blind fashion to receive either D/M as a single table twice a day or identical placebo twice daily for 25 weeks. Comment: Blinding of participants ensured and unlikely that the blinding could have been broken |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Side effects are more significant in D/M group. Comment: Reasons for missing outcome data unlikely to be related to true outcome |
| Selective reporting (reporting bias) | Low risk | Report all outcome state in methodology Comment: The study protocol is not available, but all of the study’s pre‐specified outcomes have been reported in the pre‐specified way in methodology |
| Other bias | High risk |
Comment: Selection bias: baseline are different between intervention and placebo |
Soininen 2007.
| Methods | Double blind randomised control trial, 12 months | |
| Participants | 461 participants (308 intervention, 153 control) who aged >/= 50 years with early to moderate AD by MMSE and global deterioration scale (GDS) scores of 3‐5, meeting NINCDS or DSM 4 criteria for probable AD with symptoms present for at least one year, were out‐patient in USA, Australia, Belgium, Finland, France, Germany, Netherlands and the UK. | |
| Interventions | Intervention: Celecoxib 200 mg oral twice Control: Placebo |
|
| Outcomes | Cognition (ADAScog 0‐70 lower score = improvement) at 6, 12 months (CIBIC+ 1‐7 lower score = improvement) at 6, 12 months Clinical global impression of change (NOSGER 30‐150 lower score = improvement ) at 6, 12 months Behavioral disturbance (Behave‐AD 0‐75 lower score = improvement) at 6, 12 months |
|
| Notes | Funding from Pfizer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: Patients were assigned to receive the study medication in the order of enrolment according to a computer‐generated randomisation schedule prepared by the sponsor prior to the start of the study. Comment: done |
| Allocation concealment (selection bias) | Low risk |
Quote: Patients and study personnel were blinded to the allocation of treatments throughout, and the randomisation code was only to be broken for a specific patient in an emergency situation when the investigator’s opinion required this action. Comment: Probably done |
| Blinding (performance bias and detection bias) All outcomes | Low risk |
Quote 1: Eligible patients were randomised to receive the 52‐week dosing regimen of either celecoxib 200 mg bid or matching placebo in the ration of 2:1 Quote 2: Patients and study personnel were blinded to the allocation of treatments throughout. Comment: Blinding of participants and unlikely to introduce bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Comment: Reasons for missing outcome data unlikely to be related to true outcome |
| Selective reporting (reporting bias) | Low risk | All primary outcomes were reported, but not all secondary outcomes were reported Comment: The study protocol is not available, but all of the study’s pre‐specified outcomes have been reported in the pre‐specified way in methodology |
| Other bias | High risk | Celecoxib group has more hypertension, diabetes and by pass patients, trans cerebral ischemias, coronary artery disease and aspirin use, compared to placebo Comment: Selection bias |
Zhou 2004a.
| Methods | Open‐label randomised controlled trial, 6 months | |
| Participants | 16 participants (8 intervention, 8 control) who had a diagnosis of AD, were in‐patient. in China, aged‐not limited | |
| Interventions | Intervention: Hyperzine A 0.15 mg oral twice, Nicegoline 20 mg oral twice and enteric‐coated aspirin oral 50 mg once Control: Hyperzine A 0.15 mg oral twice, Nicegoline 20 mg oral twice |
|
| Outcomes | Cognition (MMSE 0‐30 higher score = improvement) at 3,6 months Activity of daily living (Blessed ADL score higher score = improvement) at 3,6 months |
|
| Notes | Sources of funding not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
No information Comment: probably not done |
| Allocation concealment (selection bias) | Unclear risk |
No information Comment: Probably not done |
| Blinding (performance bias and detection bias) All outcomes | High risk |
Quote 1: This is an open‐label trial Comment: Only outcome assessors were blinded, incomplete blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Comment: Reasons for missing outcome data unlikely to be related to true outcome |
| Selective reporting (reporting bias) | Low risk |
Comment: The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Other bias | High risk |
Comment: Performance bias due to the nature of open‐label trial |
Zhou 2004b.
| Methods | Open‐label randomised controlled trial, 36 weeks | |
| Participants | 26 participants (13 intervention, 13 control) who had a diagnosis of AD, were in‐patient in China, aged‐not limited | |
| Interventions | Intervention: Hyperzine A 0.15 mg oral twice and aspirin oral 150 mg once Control: Hyperzine A 0.15 mg oral twice |
|
| Outcomes | Cognition (MMSE 0‐30 higher score = improvement) at 36 weeks Activity of daily living (Blessed ADL score higher score = improvement) at 36 weeks |
|
| Notes | Sources of funding not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
No information Comment: probably not done |
| Allocation concealment (selection bias) | Unclear risk |
No information Comment: Probably not done |
| Blinding (performance bias and detection bias) All outcomes | High risk |
Quote 1: This is an open‐label trial Comment: Only outcome assessors were blinded, incomplete blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Comment: Reasons for missing outcome data unlikely to be related to true outcome |
| Selective reporting (reporting bias) | Low risk |
Comment: The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Other bias | High risk |
Comment: Performance bias due to the nature of open‐label trial |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ADAPT 2008 | not RCT |
| Aisen 1996a | no comparison group |
| Aisen 2000b | Repeat study to Aisen2000a |
| Aisen 2000c | not RCT |
| Aisen 2002b | not RCT |
| Aisen 2003b | not RCT |
| Aisen 2008a | not RCT |
| Aisen 2008b | not RCT |
| Aizen 2005 | not RCT |
| Allain 2007 | not RCT |
| Anthony 2000a | not RCT |
| Anthony 2000b | not RCT |
| Asthana 1999 | RCT, AD, but intervention is physostigmine which is not aspirin, steroids or NSAIDS |
| Baum 2008 | RCT, AD, but intervention is curcurmin which is not aspirin, steroids or NSAIDS |
| Beck 2000 | This study met all inclusion criteria, but information of the study from the authors could not be obtained. |
| Belanoff 2002a | RCT, AD, but intervention is mifepristone which is not aspirin, steroids or NSAIDS |
| Belanoff 2002b | not RCT |
| Bernardi 2000 | not RCT |
| Bertozzi 1996 | not RCT |
| Black 2006 | RCT, AD, but intervention is R‐flurbiprofen which is not aspirin, steroids or NSAIDS |
| Breitner 1995 | not RCT |
| Breitner 2000 | RCT, but for AD prevention |
| Breitner 2009 | RCT, but in MCI and normal elderly |
| Browne 1999 | not RCT |
| Bruce‐Jones 1994 | RCT, but for healthy elderly |
| Brunner 2006 | not RCT |
| Budge 2000 | RCT, but for non‐specified dementia, not AD |
| Bullock 2004 | not RCT |
| Bussiere 2005 | RCT, but for non‐specified dementia |
| Canigueral 2008 | RCT, but not in AD |
| Chang 2005 | RCT, AD, but intervention is Ginko biloba which is not aspirin, steroids or NSAIDS |
| Clarke 2003a | RCT, but for non‐specified dementia, not AD |
| Clostre 1999 | not RCT |
| Diener 2007 | RCT, but for ischemic stroke, not AD |
| Diener 2008 | RCT, but ofr ischemic stroke, not AD |
| Doraiswamy 1996 | not RCT |
| Fisk 2000 | Repeat study to Bentham2008 |
| Forssell 1989 | RCT, AD, but intervention is choline chloride and lecithin which is not aspirin, steroids or NSAIDS |
| Fotuhi 2008 | not RCT |
| Geldmacher 2004 | RCT, AD, but intervention is pioglitazone which is not aspirin, steroids or NSAIDS |
| Gomez‐Isla 2008 | RCT, but in MCI, not AD |
| Green 2009 | RCT, AD, but intervention is Tarenflurbil |
| Group 2007 | RCT, but in elderly with family history of AD, not AD |
| Hollander 1987 | RCT, AD, but intervention is RS 86 (Cholinomimetic drug) which is not aspirin, steroids or NSAIDS |
| Jackson 2005 | RCT, AD, but intervention is propranolol which is not aspirin, steroid, or NSAIDS |
| Jacobs 2009 | not RCT |
| Jacoby 2002 | RCT, but for non‐specified dementia |
| Jeong 2004 | not RCT |
| Jiang 2008 | not RCT |
| Jin 2008 | not RCT |
| Kimball 2008 | RCT, but in atopic dermatitis |
| Landi 2003 | not RCT |
| Laughlin 2006 | RCT, AD, but intervention is R‐flurbiprofen which is not aspirin, steroid, or NSAIDS |
| Leblhuber 2004 | not RCT |
| Leuchtenberger 2006 | not RCT |
| Lucca 1994 | not RCT |
| Martin 2006 | RCT, but in patients with family history of AD |
| Martin 2008 | RCT, but in patients with family history of AD |
| McIntyre 2006 | not RCT |
| Meinert 2009 | not RCT |
| Meinerta 2008 | RCT, but in TIA or Stroke patients |
| Meyer 1989 | not RCT |
| Meyer 2002 | RCT, but in multi‐infarct dementia |
| Nawata 2002 | RCT, but in vascular dementia |
| Nourhashemi 1998 | not RCT |
| O'Shea 2007 | RCT, but in premature infact |
| Pasinetti 2002 | not RCT |
| Pfizer 2004 | Repeat study to Soininen2007 |
| Pfizer 2005 | Repeat study to Soininen2007 |
| Pomara 1985 | RCT, AD, but intervention is naltrexone which is not aspirin, steroid or NSAIDs |
| Pomara 2006 | RCT, AD, but intervention is mifepristone which is not aspirin, steroid or NSAIDs |
| Reid 2005 | RCT, but in cocaine dependent subjects |
| Relkin 2003 | RCT, AD, but intervention is donepezil which is not aspirin, steroid or NSAIDs |
| Sainati 2000 | Repeat study to Soininen2007 |
| Simon 1998 | RCT, but in osteoarthritis and rheumatoid arthritis patients |
| Small 1999 | RCT, but in MCI |
| Small 2008 | RCT, but in non demented volunteer with mild age‐related memory decline |
| Solomon 2008 | not RCT |
| Souza‐Talarico 2008 | not RCT |
| Steiger 1991 | RCT, but in healthy men |
| Stevenson 2004 | Repeat study to Jhee2004 |
| Stewart 1997 | not RCT |
| Stoppe 1996 | not RCT |
| Szekely 2008 | not RCT |
| Szekely 2010 | not RCT |
| Taylor 1999 | This study met all inclusion criteria, but information of the study from the authors could not be obtained. |
| Thal 2005 | RCT, but in MCI |
| Tsartsalis 2011 | not RCT |
| Tuppo 2005 | not RCT |
| Turini 2002 | not RCT |
| Usui 2006 | not RCT |
| Uttner 2005 | RCT, but in multiple sclerosis |
| Van Niekerk 2001 | RCT, but in men aged 62‐76 |
| van Reekum 1999 | not RCT |
| Visser 2005 | not RCT |
| Vlad 2008 | not RCT |
| Walker 2005 | not RCT |
| Walther 2011 | RCT, but in 2 patients with unspecified dementia |
| Watson 2004 | RCT, but in both AD and MCI and intervention is rosiglitasone which is not aspirin, steroid or NSAIDs |
| Whitehouse 1998 | not RCT |
| Widimsky 2008 | RCT, but in stable angina group A |
| Wilcock 1999 | RCT, but in non‐specified AD |
| Wilcock 2004 | RCT, AD, but intervention is R‐flurbiprofen which is not aspirin, steroid and NSAIDs |
| Wilcock 2006 | not RCT |
| Wilcock 2008 | RCT, AD, but intervention is tarenflurbil which is not aspirin, steroid and NSAIDs |
| Williams 2004 | not RCT |
| Windisch 2000 | not RCT |
| Wisloff 1996 | RCT, but in multiple myeloma |
| Wolkowitz 2003 | RCT, AD, but intervention is a steroid that does not have anti‐inflammatory effect |
| Wollheim 2000 | not RCT |
| Woodward 2002 | not RCT |
| Xia 2007 | RCT, but in premature infant |
| Yaqub 1999 | not RCT |
| Yip 2005 | not RCT |
| Young 1999 | RCT, but in normal male volunteers |
| Zandi 2005 | not RCT |
| Zaragoza 2005 | not RCT |
| Zerovnik 2010 | not RCT |
| Zhai 2010 | RCT, but in vascular cognitive impairment |
| Ziebell 2010 | not RCT |
Differences between protocol and review
1) Background about steroidal anti‐inflammatory drugs was added in the review.
2) Inclusion criteria was adjusted to include any types of RCT and not only double‐blinded
3) Two reviewers (DJ, MI) instead of 3 reviewers independently examined the titles and abstracts of the trials identified in the search and considered them for inclusion according to the pre‐determined eligibility criteria. Any disparity was resolved by retrieval of the cited articles and further discussion with the third reviewer (NT).
4) Review topic was changed from aspirin and anti‐inflammatory agents for Alzheimer’s disease to aspirin, steroidal and non‐steroidal anti‐inflammatory agents for Alzheimer’s disease.
5) Data was not extracted independently by two authors. The first author extracted the data and this was followed by verification by a second author.
Contributions of authors
DJ: Wrote, coordinated, searched and selected trials for inclusion in this review. Also extracted, interpreted and entered data on to RevMan.
MI and NT: contributed to trial searches, obtained copies of trial reports and assessed trials for inclusion/exclusion. They also were involved in data extraction and interpretation, and providing general advice on the review
JMcC: contributed to design of analysis, data interpretation and drafting of review
Contact editors: Leon Flicker and Gordon Wilcock Consumer editor: Lynne Ramsay
Sources of support
Internal sources
New Source of support, Not specified.
External sources
No sources of support supplied
Declarations of interest
All authors declare no conflict of interest in this research project.
New
References
References to studies included in this review
Aisen 2000a {published data only}
- Aisen PS, Davis KL, Berg JD, Schafer K, Campbell K, Thomas RG, et al. A randomized controlled trial of prednisone in Alzheimer's disease. Alzheimer's Disease Cooperative Study. Neurology 2000;54(3):588‐93. [DOI] [PubMed] [Google Scholar]
Aisen 2002a {published data only}
- Aisen PS, Schmeidler J, Pasinetti GM. Randomized pilot study of nimesulide treatment in Alzheimer's disease. Neurology 2002;58(7):1050‐4. [DOI] [PubMed] [Google Scholar]
Aisen 2003a {published data only}
- Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, et al. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA 2003;289(21):2819‐26. [DOI] [PubMed] [Google Scholar]
Bentham 2008 {published data only}
- Bentham P, Gray R, Sellwood E, Hills R, Crome P, Raftery J. Aspirin in Alzheimer's disease (AD2000): a randomised open‐label trial. Lancet Neurology 2008;7(1):41‐9. [DOI] [PubMed] [Google Scholar]
De Jong 2008 {published data only}
- Jong D, Jansen R, Hoefnagels W, Jellesma‐Eggenkamp M, Verbeek M, Borm G, et al. No effect of one‐year treatment with indomethacin on Alzheimer's disease progression: a randomized controlled trial. PLoS ONE 2008;3:e1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hüll 1999 {published data only}
- Hüll M, Bauer J. Treatment with the non‐steroidal anti‐inflammatory drug piroxicam‐B‐cyclodextrin in Alzheimer's disease. Ninth Congress of the International Psychogeriatric Association, August 15‐20, 1999, Vancouver Convention & Exhibition Centre Vancouver, British Columbia, Canada. 1999:107.
Jhee 2004 {published data only}
- Jhee SS, Frackiewicz E, Tolbert D, Sainati S, Karim A, Zolnouni PP, et al. A Pharmacokinetic, Pharmacodynamic, and Safety Study of Celecoxib in Subjects with Probable Alzheimer’s Disease. Clinical Research and Regulatory Affairs 2004;21(1):49‐66. [Google Scholar]
Pasqualetti 2009 {published data only}
- Pasqualetti P, Bonomini C, Dal Forno G, Paulon L, Sinforiani E, Marra C, et al. A randomized controlled study on effects of ibuprofen on cognitive progression of Alzheimer's disease. Aging Clinical & Experimental Research 2009;21(2):102‐10. [DOI] [PubMed] [Google Scholar]
Reines 2004 {published data only}
- Reines SA, Block GA, Morris JC, Liu G, Nessly ML, Lines CR, et al. Rofecoxib ‐ no effect on Alzheimer's disease in a 1‐year, randomized, blinded, controlled study. Neurology 2004;62:66‐71. [DOI] [PubMed] [Google Scholar]
Rogers 1993 {published data only}
- Rogers J, Kirby LC, Hempelman SR, Berry DL, McGeer PL, Kaszniak AW, et al. Clinical trial of indomethacin in Alzheimers disease. Neurology 1993;43(8):1609‐11. [DOI] [PubMed] [Google Scholar]
Scharf 1999 {published data only}
- Scharf S, Mander A, Ugoni A, Vajda F, Christophidis N. A double‐blind, placebo‐controlled trial of diclofenac/misoprostol in Alzheimer's disease. Neurology 1999;53:197‐201. [DOI] [PubMed] [Google Scholar]
Soininen 2007 {published data only}
- Soininen H, West C, Robbins J, Niculescu L. Long‐term efficacy and safety of celecoxib in Alzheimer's disease. Dementia and Geriatric Cognitive Disorders 2007;23(1):8‐21. [DOI] [PubMed] [Google Scholar]
Zhou 2004a {published data only}
- Zhou BR, Xu ZQ, Kuang YF, Deng YH, Liu ZF. Effectiveness of polydrug therapy for senile dementia. Chinese Journal of Clinical Rehabilitation 2004;8:1214‐5. [Google Scholar]
Zhou 2004b {published data only}
- Zhou BR, Xu ZQ, Kuang YF, Deng YH, Qian CY. Curative effect of aspirin for postponing the development of Alzheimer's disease. Chinese Journal of Clinical Rehabilitation 2004;8:3020‐1. [Google Scholar]
References to studies excluded from this review
ADAPT 2008 {published data only}
- Cognitive function over time in the Alzheimer's Disease Anti‐Inflammatory Prevention trial (ADAPT). Results of a randomized, controlled trial of naproxen and celecoxib. Archives of Neurology 2008;65(7):896‐905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aisen 1996a {published data only}
- Aisen PS, Marin D, Altstiel L, Goodwin C, Baruch B, Jacobson R, et al. A pilot study of prednisone in Alzheimer's disease. Dementia 1996;7:201‐6. [DOI] [PubMed] [Google Scholar]
Aisen 2000b {published data only}
- Aisen PS, Davis KL, Berg JD, Schafer K, Campbell K, Thomas RG, et al. A randomized controlled trial of prednisone in Alzheimer's disease. Neurology 2000;54:588‐93. [DOI] [PubMed] [Google Scholar]
Aisen 2000c {published data only}
- Aisen PS. Anti‐inflammatory therapy for Alzheimer's disease: implications of the prednisone trial. Acta Neurologica Scandinavica Supplement 2000;102:85‐9. [DOI] [PubMed] [Google Scholar]
Aisen 2002b {published data only}
- Aisen PS. Evaluation of selective COX‐2 inhibitors for the treatment of Alzheimer's disease. Journal of Pain and Symptom Management 2002;23(4 Suppl):S35‐40. [DOI] [PubMed] [Google Scholar]
Aisen 2003b {published data only}
- Aisen PS, Berg JD, Craft S, Peskind ER, Sano M, Teri L, et al. Steroid‐induced elevation of glucose in Alzheimer's disease: relationship to gender, apolipoprotein E genotype and cognition. Psychoneuroendocrinology 2003;28:113‐20. [DOI] [PubMed] [Google Scholar]
Aisen 2008a {published data only}
- Aisen PS, Thal LJ, Ferris SH, Assaid C, Nessly ML, Giuliani MJ, et al. Rofecoxib in patients with mild cognitive impairment: further analyses of data from a randomized, double‐blind, trial. Current Alzheimer Research 2008;5:73‐82. [DOI] [PubMed] [Google Scholar]
Aisen 2008b {published data only}
- Aisen PS, Thal LJ, Ferris SH, Assaid C, Nessly ML, Giuliani MJ, et al. Rofecoxib in patients with mild cognitive impairment: further analyses of data from a randomized, double‐blind, trial. Current Alzheimer Research 2008;5(1):73‐82. [DOI] [PubMed] [Google Scholar]
Aizen 2005 {published data only}
- Aizen E, Kagan G, Assy B, Iobel R, Bershadsky Y, Gilhar A. Effect of non‐steroidal anti‐inflammatory drugs on natural killer cell activity in patients with dementia. The Israel Medical Association Journal 2005;7(2):78‐81. [PubMed] [Google Scholar]
Allain 2007 {published data only}
- Allain H, Bentue‐Ferrer D, Akwa Y. Treatment of the mild cognitive impairment (MCI). Human Psychopharmacology 2007;22:189‐97. [DOI] [PubMed] [Google Scholar]
Anthony 2000a {published data only}
- Anthony JC, Breitner JC, Zandi PP, Meyer MR, Jurasova I, Norton MC, et al. Reduced prevalence of AD in users of NSAIDs and H2 receptor antagonists: the Cache County study. Neurology 2000;54(11):2066‐71. [DOI] [PubMed] [Google Scholar]
Anthony 2000b {published data only}
- Anthony JC, Breitner JCS, Zandi PP, Meyer MR, Jurasova I, Norton MC, et al. Alzheimer disease: current therapy and future therapeutic strategies. Alzheimer Disease and Associated Disorders 2000;14(Suppl 1):S11‐7. [DOI] [PubMed] [Google Scholar]
Asthana 1999 {published data only}
- Asthana S, Raffaele KC, Greig NH, Schapiro MB, Blackman MR, Soncrant TT. Neuroendocrine responses to intravenous infusion of physostigmine in patients with Alzheimer disease. Alzheimer Disease and Associated Disorders 1999;13:102‐8. [DOI] [PubMed] [Google Scholar]
Baum 2008 {published data only}
- Baum L, Lam CW, Cheung SK, Kwok T, Lui V, Tsoh J, et al. Six‐month randomized, placebo‐controlled, double‐blind, pilot clinical trial of curcumin in patients with Alzheimer disease. Journal of Clinical Psychopharmacology 2008;28(1):110‐3. [DOI] [PubMed] [Google Scholar]
Beck 2000 {published data only}
- Beck K. The safety and efficacy of an Investigational Drug (VIOXX) in Delaying the progression of Alzheimer's Disease. ClinicalTrials.gov 2000. [MEDLINE: ]
Belanoff 2002a {published data only}
- Belanoff JK, Jurik J, Schatzberg LD, DeBattista C, Schatzberg AF. Slowing the progression of cognitive decline in Alzheimer's disease using mifepristone. Journal of Molecular Neuroscience 2002;19:201‐6. [DOI] [PubMed] [Google Scholar]
Belanoff 2002b {published data only}
- Belanoff JK, Jurik J, Schatzberg LD, DeBattista C, Schatzberg AF. Reduced incidence of AD with NSAID but not H2 receptor antagonists: The Cache County study. Neurology 2002;59(6):880‐886. [DOI] [PubMed] [Google Scholar]
Bernardi 2000 {published data only}
- Bernardi F, Lanzone A, Cento RM, Spada RS, Pezzani I, Genazzani AD, et al. Allopregnanolone and dehydroepiandrosterone response to corticotropin‐releasing factor in patients suffering from Alzheimer's disease and vascular dementia. European Journal of Endocrinology 2000;142:466‐71. [DOI] [PubMed] [Google Scholar]
Bertozzi 1996 {published data only}
- Bertozzi B, Barbisoni P, Franzoni S, Frisoni GB, Rozzini R, Trabucchi M. Association of chronic non‐steroidal anti‐inflammatory drugs use and cognitive decline in non‐demented elderly patients admitted to a Geriatric Evaluation and Rehabilitation Unit. Archives of Gerontology and Geriatrics 1996;23(1):71‐9. [DOI] [PubMed] [Google Scholar]
Black 2006 {published data only}
- Black S, Wilcock GK, Haworth J, Hendrix S, Zavitz K, Christensen D, et al. Efficacy and safety of MPC‐7869 (R‐flurbiprofen), a selective ab42‐lowering agent, in mild Alzheimer's disease (AD): Results of a 12‐month Phase 2 trial and 1‐year follow‐on study. Neurology 2006;66(5):A347. [Google Scholar]
Breitner 1995 {published data only}
- Breitner JCS, Welsh KA, Helms MJ, Gaskell PC, Gau BA, Roses AD, et al. Delayed onset of Alzheimers disease with non‐steroidal anti‐inflammatory and histamine (H2) blocking drugs. Neurobiology of Aging 1995;16(4):523‐30. [DOI] [PubMed] [Google Scholar]
Breitner 2000 {published data only}
- Breitner J. Randomized controlled trial of two non‐steroidal anti‐inflammatory drugs for prevention of Alzheimer's disease. 39th Annual Meeting of the American College of Neuropsychopharmacology. 2000.
Breitner 2009 {published data only}
- Breitner JCS, Haneuse SJPA, Walker R, Dublin S, Crane PK, Gray SL, et al. Risk of dementia and AD with prior exposure to NSAIDs in an elderly community‐based cohort. Neurology 2009; Vol. 72, issue 22:1899‐905. [DOI] [PMC free article] [PubMed]
Browne 1999 {published data only}
- Browne SM, Halligan PW, Wade DT, Taggart DP. Cognitive performance after cardiac operation: implications of regression toward the mean. Journal of Thoracic and Cardiovascular Surgery 1999;117(3):481‐5. [DOI] [PubMed] [Google Scholar]
Bruce‐Jones 1994 {published data only}
- Bruce‐Jones PN, Crome P, Kalra L. Indomethacin and cognitive function in healthy elderly volunteers. British Journal of Clinical Pharmacology 1994;38:45‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brunner 2006 {published data only}
- Brunner R, Schaefer D, Hess K, Parzer P, Resch F, Schwab S. Effect of high‐dose cortisol on memory functions. Annals of the New York Academy of Sciences 2006;1071:434‐7. [DOI] [PubMed] [Google Scholar]
Budge 2000 {published data only}
- Budge M. A pilot study for the VITAL trial (Vitamins and Aspirin for the Treatment of Dementia). National Research Register 2000.
Bullock 2004 {published data only}
- Bullock R. DHEA treatment of Alzheimer's disease: a randomized, double‐blind, placebo‐controlled trial. Neurology 2004;62(6):1030. [DOI] [PubMed] [Google Scholar]
Bussiere 2005 {published data only}
- Bussiere M, Wiebe S. Effect of acetaminophen on behavior, well‐being, and psychotropic medication use in nursing home residents with moderate‐to‐severe dementia. Journal of the American Geriatrics Society 2005;53(11):1921‐9. [DOI] [PubMed] [Google Scholar]
Canigueral 2008 {published data only}
- Canigueral S, Tschopp R, Ambrosetti L, Vignutelli A, Scaglione F, Petrini O. Cardiovascular risk of celecoxib in 6 randomized placebo‐controlled trials: The cross trial safety analysis. Circulation 2008;117(16):2104‐2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2005 {published data only}
- Chang J, Wei WS, Li YJ. A randomized, double‐blind, placebo‐controlled trial of two doses of Ginkgo biloba extract in dementia of the Alzheimer's type. Current Alzheimer Research 2005;2(5):541‐51. [DOI] [PubMed] [Google Scholar]
Clarke 2003a {published data only}
- Clarke R, Harrison G, Richards S. Effect of vitamins and aspirin on markers of platelet activation, oxidative stress and homocysteine in people at high risk of dementia. Journal of Internal Medicine 2003;254(1):67‐75. [DOI] [PubMed] [Google Scholar]
Clostre 1999 {published data only}
- Clostre F. Ginkgo biloba extract (EGb 761) a state of art at the dawn of the year 2000. Annales Pharmaceutiques Francaises 1999;57(Suppl 1):1S8‐88. [PubMed] [Google Scholar]
Diener 2007 {published data only}
- Diener HC, Sacco R, Yusuf S. Rationale, design and baseline data of a randomized, double‐blind, controlled trial comparing two antithrombotic regimens (a fixed‐dose combination of extended‐release dipyridamole plus ASA with clopidogrel) and telmisartan versus placebo in patients with strokes: The prevention regimen for effectively avoiding second strokes trial (PRoFESS). Cerebrovascular Diseases 2007;23(5‐6):368‐80. [DOI] [PubMed] [Google Scholar]
Diener 2008 {published data only}
- Diener HC, Sacco RL, Yusuf S, Cotton D, Ounpuu S, Lawton WA, et al. Effects of aspirin plus extended‐release dipyridamole versus clopidogrel and telmisartan on disability and cognitive function after recurrent stroke in patients with ischaemic stroke in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial: a double‐blind, active and placebo‐controlled study. Lancet Neurology 2008;7(10):875‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Doraiswamy 1996 {published data only}
- Doraiswamy PM, Krishen A, Stallone F, Martin WL, Potts NL, Metz A, et al. NSAIDs and cognition in Alzheimer's disease. Neurology 1996;46(4):1194. [DOI] [PubMed] [Google Scholar]
Fisk 2000 {published data only}
- Fisk J. AD2000, a reliable assessment of the efficacy and safety of donepezil and aspirin in Alzheimer's Disease. National Research Register 2000.
Forssell 1989 {published data only}
- Forssell LG, Sjokvist B, Winblad B. Early stages of late onset Alzheimer's disease III Double blind treatment with choline chloride and lecithin with and without L‐dopa and L‐tryptophan, alternatively placebo. Acta Neurologica Scandinavica 1989;79:43‐66. [Google Scholar]
Fotuhi 2008 {published data only}
- Fotuhi M, Zandi PP, Hayden KM, Khachaturian AS, Szekely CA, Wengreen H, et al. Better cognitive performance in elderly taking antioxidant vitamins E and C supplements in combination with nonsteroidal anti‐inflammatory drugs: the Cache County Study. Alzheimer's and Dementia 2008;4(3):223‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Geldmacher 2004 {published data only}
- Geldmacher DS, Landreth GE. Pioglitazone In Alzheimer's disease: rationale and clinical trial design. NeuroBiology of Aging 2004;25:211. [Google Scholar]
Gomez‐Isla 2008 {published data only}
- Gomez‐Isla T, Blesa R, Boada M, Clarimon J, Ser T, Domenech G, et al. A randomized, double‐blind, placebo controlled‐trial of triflusal in mild cognitive impairment: the TRIMCI study. Alzheimer Disease & Associated Disorders 2008;22(1):21‐9. [DOI] [PubMed] [Google Scholar]
Green 2009 {published data only}
- Green RC, Schneider LS, Amato DA, Beelen AP, Wilcock G, Swabb EA, et al. Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial. JAMA 2009;302(23):2557‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Group 2007 {published data only}
- Group AR, Lyketsos CG, Breitner JC, Green RC, Martin BK, Meinert C, et al. Naproxen and celecoxib do not prevent AD in early results from a randomized controlled trial. Neurology 2007;68(21):1800‐8. [DOI] [PubMed] [Google Scholar]
Hollander 1987 {published data only}
- Hollander E, Davidson M, Mohs RC, Horvath TB, Davis BM, Zemishlany Z, et al. RS 86 in the treatment of Alzheimer's disease: cognitive and biological effects. Biological Psychiatry 1987;22:1067‐78. [DOI] [PubMed] [Google Scholar]
Jackson 2005 {published data only}
- Jackson S, Ham RJ, Wilkinson D. Propranolol for disruptive behaviors in nursing home residents with probable or possible Alzheimer disease: A placebo‐controlled study. Alzheimer Disease and Associated Disorders 2005;19(1):23‐8. [DOI] [PubMed] [Google Scholar]
Jacobs 2009 {published data only}
- Jacobs LG, Billett HH, Freeman K, Dinglas C, Jumaquio L. Anticoagulation for stroke prevention in elderly patients with atrial fibrillation, including those with falls and/or early‐stage dementia: a single‐center, retrospective, observational study. American Journal of Geriatric Pharmacotherapy 2009;7(3):159‐66. [DOI] [PubMed] [Google Scholar]
Jacoby 2002 {published data only}
- Jacoby RJ. A pilot study for the VITAL trial (Vitamins and Aspirin for the treatment of Dementia). National Research Register 2002.
Jeong 2004 {published data only}
- Jeong YH, Woo RS, Park CH, Suh YH. Therapeutic potentials of mefenamic acid for the treatment of Alzheimer's disease. Neurobiology of Aging 2004;25:S589. [Google Scholar]
Jiang 2008 {published data only}
- Jiang Q, Heneka M, Landreth G. The role of peroxisome proliferator‐activated receptor‐ (PPAR) in Alzheimer's disease: therapeutic implications. CNS Drugs 2008;22(1):1‐14. [DOI] [PubMed] [Google Scholar]
Jin 2008 {published data only}
- Jin D‐Q, Sung J‐Y, Hwang YK, Kwon, Kyoung J, Han S‐H, et al. Dexibuprofen (s(+)‐isomer ibuprofen) reduces microglial activation and impairments of spatial working memory induced by chronic lipopolysaccharide infusion. Pharmacology, Biochemistry and Behavior 2008;89(3):404‐11. [DOI] [PubMed] [Google Scholar]
Kimball 2008 {published data only}
- Kimball AB, Gold MH, Zib B, Davis MW. Clobetasol propionate emulsion formulation foam 0.05%: review of phase II open‐label and phase III randomized controlled trials in steroid‐responsive dermatoses in adults and adolescents. Journal of the American Academy of Dermatology 2008;59(3):448‐54. [DOI] [PubMed] [Google Scholar]
Landi 2003 {published data only}
- Landi F, Cesari M, Onder G, Russo A, Torre S, Bernabei R. Non‐steroidal anti‐inflammatory drug (NSAID) use and Alzheimer disease in community‐dwelling elderly patients. Am J Geriatr Psychiatry 2003;11(2):179‐85. [PubMed] [Google Scholar]
Laughlin 2006 {published data only}
- Laughlin M, Black SE, Wilcock GK, Haworth J, Hendrix S, Zavitz KH, et al. Efficacy and safety of MPC‐7869 (R‐flurbiprofen), a Selective AB42‐Lowering Agent, in Alzheimer's disease (AD): results of a 12‐month phase 2 trial and 1‐year follow‐on study. Journal of the American Geriatrics Society 2006;54(4):S7. [Google Scholar]
Leblhuber 2004 {published data only}
Leuchtenberger 2006 {published data only}
- Leuchtenberger S, Beher D, Weggen S. Selective modulation of A beta 42 production in Alzheimer's disease: non‐steroidal anti‐inflammatory drugs and beyond. Current Pharmaceutical Design 2006;12(33):4337‐55. [DOI] [PubMed] [Google Scholar]
Lucca 1994 {published data only}
- Lucca U, Tettamanti M, Forloni G, Spagnoli A. Nonsteroidal antiinflammatory drug use in Alzheimer's disease. Biological Psychiatry 1994;36(12):854‐6. [DOI] [PubMed] [Google Scholar]
Martin 2006 {published data only}
- Martin BK, Breitner JCS, Evans D, Lyketsos CG, Meinert CL. Cardiovascular and cerebrovascular events in the randomized, controlled Alzheimer's disease Anti‐inflammatory Prevention Trial (ADAPT). PLoS Clinical Trials 2006;1(7):e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin 2008 {published data only}
- Martin BK, Szekely C, Brandt J, Piantadosi S, Breitner JC, Craft S, et al. Cognitive function over time in the Alzheimer's Disease Anti‐inflammatory Prevention Trial (ADAPT): results of a randomized, controlled trial of naproxen and celecoxib. Archives of Neurology 2008;65(7):896‐905. [DOI] [PMC free article] [PubMed] [Google Scholar]
McIntyre 2006 {published data only}
- McIntyre JA, Castaner J, Fernandez‐Forner D. MPC‐7869 ‐ prostate cancer therapy ‐ treatment of Alzheimer's dementia. Drugs of the Future 2006;31(1):22‐7. [Google Scholar]
Meinert 2009 {published data only}
- Meinert CL, Mccaffrey LD, Breitner JCS, Adapt RG. Alzheimer's disease anti‐inflammatory prevention trial: design, methods, and baseline results. Alzheimers & Dementia 2009;5(2):93‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meinerta 2008 {published data only}
- Meinert CL, Breitner JCS. Triflusal versus aspirin for the prevention of stroke. Progress in Neurotherapeutics and Neuropsychopharmacology 2008;3(1):13‐33. [Google Scholar]
Meyer 1989 {published data only}
- Meyer JS, Rogers RL, McClintic K, Mortel KF, Lotfi J. Randomized clinical trial of daily aspirin therapy in multi‐infarct dementia. A pilot study. Journal of the American Geriatrics Society 1989;37(6):549‐55. [DOI] [PubMed] [Google Scholar]
Meyer 2002 {published data only}
- Meyer JS, Chowdhury MH, Quach M. Randomized clinical trial of daily aspirin therapy in multi‐infarct dementia. 2nd International Congress on Vascular dementia, January, Salzburg, Austria, 2002. 2002.
Nawata 2002 {published data only}
- Nawata H, Yanase T, Goto K, Okabe T, Ashida K. An open‐label pilot study comparing rivastigmine and low‐dose aspirin for the treatment of symptoms specific to patients with subcortical vascular dementia. Current Therapeutic Research ‐ Clinical and Experimental 2002;63(7):443‐458. [Google Scholar]
Nourhashemi 1998 {published data only}
- Nourhashemi F, Ousset PJ, Reyes G, Micas M, Adoue D, Vellas BJ, et al. Non steroidal anti‐inflammatory drugs in Alzheimer disease. Presse Medicale 1998;27:25‐8. [PubMed] [Google Scholar]
O'Shea 2007 {published data only}
- O'Shea TM, Washburn LK, Nixon PA, Goldstein DJ. Followup of a randomized, placebo‐controlled trial of dexamethasone to decrease the duration of ventilator dependency in very low birth weight infants: Neurodevelopmental outcomes at 4 to 11 years of age. Pediatrics 2007;120(3):594‐602. [DOI] [PubMed] [Google Scholar]
Pasinetti 2002 {published data only}
- Pasinetti GM. Cyclooxygenase as a target for the antiamyloidogenic activities of nonsteroidal anti‐inflammatory drugs in Alzheimer's disease. Neurosignals 2002;11(5):293‐7. [DOI] [PubMed] [Google Scholar]
Pfizer 2004 {published data only}
- Pfizer [Sponsor]. A double‐blind, randomized, placebo‐controlled, comparative study of celecoxib (SC‐58635) for the inhibition of progression of Alzheimer's disease. http://www.clinicalstudyresults.org/documents/company‐study_76_0.pdf 2004.
Pfizer 2005 {published data only}
- Pfizer [Sponsor]. A placebo‐controlled evaluation of the long‐term efficacy and safety of celecoxib (SC‐58635) In Alzheimer’s disease. http://www.clinicalstudyresults.org/documents/company‐study_76_1.pdf 2005.
Pomara 1985 {published data only}
- Pomara N, Roberts R, Rhiew HB, Stanley M, Gershon S. Multiple, single‐dose naltrexone administrations fail to effect overall cognitive functioning and plasma cortisol in individuals with probable Alzheimer's disease. Neurobiology of Aging 1985;6:233‐6. [DOI] [PubMed] [Google Scholar]
Pomara 2006 {published data only}
- Pomara N, Hernando RT, Pena CB, Sidtis JJ, Cooper TB, Ferris S. The effect of mifepristone (RU 486) on plasma cortisol in Alzheimer's disease. Neurochemical Research 2006;31(5):585‐8. [DOI] [PubMed] [Google Scholar]
Reid 2005 {published data only}
- Reid MS, Angrist B, Baker S, Woo C, Schwartz M, Montgomery A, et al. A placebo‐controlled screening trial of celecoxib for the treatment of cocaine dependence. Addiction 2005;100:32‐42. [DOI] [PubMed] [Google Scholar]
Relkin 2003 {published data only}
- Relkin NR, Reichman WE, Orazem J, McRae T. A large, community‐based, open‐label trial of donepezil in the treatment of Alzheimer's disease. Dementia and Geriatric Cognitive Disorders 2003;16(1):15‐24. [DOI] [PubMed] [Google Scholar]
Sainati 2000 {published data only}
- Sainati SM, Ingram DM, Talwalker S, Geis GS. Results of a double‐blind, randomized, placebo‐controlled study of celecoxib in the treatment of progression of Alzheimer's disease. Proceedings of the 6th International Stockholm/Springfield Symposium on Advances in Alzheimer Therapy; 2000 Apr 5‐8, Stockholm, Sweden. 2000:180.
Simon 1998 {published data only}
- Simon LS, Lanza FL, Lipsky PE, Hubbard RC, Talwalker S, Schwartz BD, et al. Preliminary study of the safety and efficacy of SC‐58635, a novel cyclooxygenase 2 inhibitor ‐ Efficacy and safety in two placebo‐controlled trials in osteoarthritis and rheumatoid arthritis, and studies of gastrointestinal and platelet effects. Arthritis and Rheumatism 1998;41(9):1591‐602. [DOI] [PubMed] [Google Scholar]
Small 1999 {published data only}
- Small GW. Anti‐inflammatory treatment for age‐associated memory impairment: a double‐blind placebo‐controlled trial. Alzheimer's Disease Education and Referral Center (ADEAR) 1999.
Small 2008 {published data only}
- Small GW, Siddarth P, Silverman DHS, Ercoli LM, Miller KJ, Lavretsky H, et al. Cognitive and cerebral metabolic effects of celecoxib versus placebo in people with age‐related memory loss: randomized controlled study. The American Journal of Geriatric Psychiatry 2008;16(12):999‐1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Solomon 2008 {published data only}
- Solomon SD, Wittes J, Finn PV, Fowler R, Viner J, Bertagnolli MM, et al. Cardiovascular risk of celecoxib in 6 randomized placebo‐controlled trials: the cross trial safety analysis. Circulation 2008;117(16):2104‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Souza‐Talarico 2008 {published data only}
- Souza‐Talarico JN, Caramelli P, Nitrini R, Chaves EC. Effect of cortisol levels on working memory performance in elderly subjects with Alzheimer's disease. Arquivos de Neuro‐Psiquiatria 2008;66(3B):619‐24. [DOI] [PubMed] [Google Scholar]
Steiger 1991 {published data only}
- Steiger A, Guldner J, Knisatschek H, Rothe B, et al. Effects of an ACTH/MSH(4‐9) analog (HOE 427) on the sleep EEG and nocturnal hormonal secretion in humans. Peptides 1991;12(5):1007‐1010. [DOI] [PubMed] [Google Scholar]
Stevenson 2004 {published data only}
- Stevenson DD. A pharmacokinetic, pharmacodynamic, and safety study of celecoxib in subjects with probable Alzheimer's disease. Clinical Research and Regulatory Affairs 2004;21(1):49‐66. [Google Scholar]
Stewart 1997 {published data only}
- Stewart WF, Kawas C, Corrada M, Metter EJ. Risk of Alzheimer's disease and duration of NSAID use. Neurology 1997;48:626‐32. [DOI] [PubMed] [Google Scholar]
Stoppe 1996 {published data only}
- Stoppe G, Sandholzer H, Staedt J, Winter S, Kiefer J, Ruther E. Prescribing practice with cognition enhancers in outpatient care: Are there differences regarding type of dementia? Results of a representation survey in lower Saxony, Germany. Pharmacopsychiatry 1996;29(4):150‐155. [DOI] [PubMed] [Google Scholar]
Szekely 2008 {published data only}
- Szekely CA, Green RC, Breitner JCS, Ostbye T, Beiser AS, Corrada MM, et al. No advantage of A beta(42)‐lowering NSAIDs for prevention of Alzheimer dementia in six pooled cohort studies. Neurology 2008;70(24):2291‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Szekely 2010 {published data only}
- Szekely CA, Zandi PP. Non‐steroidal anti‐inflammatory drugs and Alzheimer's disease: the epidemiological evidence. CNS and Neurological Disorders ‐ Drug Targets 2010;9(2):132‐9. [DOI] [PubMed] [Google Scholar]
Taylor 1999 {published data only}
- Taylor D. 52 week double‐blind, randomised, placebo‐controlled comparative study of celecoxib (SC‐58635) for the inhibition of progression of Alzheimers disease. National Research Register 1999.
Thal 2005 {published data only}
- Thal LJ, Ferris SH, Kirby L, Block GA, Lines CR, Yuen E, et al. A randomized, double‐blind, study of rofecoxib in patients with mild cognitive impairment. Neuropsychopharmacology 2005;30(6):1204‐15. [DOI] [PubMed] [Google Scholar]
Tsartsalis 2011 {published data only}
- Tsartsalis S, Panagopoulos PK, Mironidou‐Tzouveleki M. Non‐cholinergic pharmacotherapy approaches to Alzheimer's disease: the use of non‐steroidal anti‐inflammatory drugs. CNS & Neurological Disorders‐Drug Targets 2011;10(1):133‐9. [DOI] [PubMed] [Google Scholar]
Tuppo 2005 {published data only}
- Tuppo EE, Arias HR. The role of inflammation in Alzheimer's disease. International Journal of Biochemistry & Cell Biology 2005;37(2):289‐305. [DOI] [PubMed] [Google Scholar]
Turini 2002 {published data only}
- Turini ME, Dubois RN. Cyclooxygenase‐2: a therapeutic target. Annual Review of Medicine 2002;53:35‐57. [DOI] [PubMed] [Google Scholar]
Usui 2006 {published data only}
- Usui T. Pharmaceutical prospects of phytoestrogens. Endocrine Journal 2006;53(1):7‐20. [DOI] [PubMed] [Google Scholar]
Uttner 2005 {published data only}
- Uttner I, Muller S, Zinser C, Maier M, Sussmuth S, Claus A, et al. Reversible impaired memory induced by pulsed methylprednisolone in patients with MS. Neurology 2005;64(11):1971‐1973. [DOI] [PubMed] [Google Scholar]
Van Niekerk 2001 {published data only}
- Niekerk JK, Huppert FA, Herbert J. Salivary cortisol and DHEA: Association with measures of cognition and well‐being in normal older men, and effects of three months of DHEA supplementation. Psychoneuroendocrinology 2001;26:591‐612. [DOI] [PubMed] [Google Scholar]
van Reekum 1999 {published data only}
- Reekum R, Simard M, Cohen T. The prediction and prevention of Alzheimer's disease ‐ towards a research agenda. Journal of Psychiatry & Neuroscience 1999;24(5):413‐30. [PMC free article] [PubMed] [Google Scholar]
Visser 2005 {published data only}
- Visser PJ, Scheltens P, Verhey FR. Do MCI criteria in drug trials accurately identify subjects with predementia Alzheimer's disease?. Journal of Neurology, Neurosurgery & Psychiatry 2005;76(10):1348‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vlad 2008 {published data only}
- Vlad SC, Miller DR, Kowall NW, Felson DT. Protective effects of NSAIDs on the development of Alzheimer disease. Neurology 2008;70(19):1672‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 2005 {published data only}
- Walker PC, Alrawi A, Mitchell JF, Regal RE, Khanderia U. Medication use as a risk factor for falls among hospitalized elderly patients. American Journal of Health‐System Pharmacy 2005;62(23):2495‐9. [DOI] [PubMed] [Google Scholar]
Walther 2011 {published data only}
- Walther S, Schupbach B, Seifritz E, Homan P, Strik W. Randomized, controlled crossover trial of dronabinol, 2.5 mg, for agitation in 2 patients with dementia. Journal of Clinical Psychopharmacology 2011;31(2):256‐8. [DOI] [PubMed] [Google Scholar]
Watson 2004 {published data only}
- Watson GS, Reger MA, Cholerton BA, Asthana S, Baker LD, Rhoads KW, et al. Rosiglitazone preserves cognitive functions in patients with early Alzheimer's disease. Neurobiology of Aging 2004;25:83. [Google Scholar]
Whitehouse 1998 {published data only}
- Whitehouse PJ, Kittner B, Roessner M, Rossor M, Sano M, Thal L, et al. Clinical trial designs for demonstrating disease course altering effects in dementia. Alzheimer Disease and Associated Disorders 1998;12:281‐94. [DOI] [PubMed] [Google Scholar]
Widimsky 2008 {published data only}
- Widimsky P, Motovska Z, Simek S, Kala P, Pudil R, Holm F, et al. Clopidogrel pre‐treatment in stable angina: for all patients > 6 H before elective coronary angiography or only for angiographically selected patients a few minutes before PCI? A randomized multicentre trial Prague‐8. European Heart Journal 2008;29(12):1495‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilcock 1999 {published data only}
- Wilcock GK. A pilot study for a large simple randomised trial to assess the effects of aspirin, anti‐oxidant vitamins (C & E) and folic acid and Vitamin B12 in patients with dementia. National Research Register 1999.
Wilcock 2004 {published data only}
- Wilcock GK. MPC‐7869 effect on cognitive function in Alzheimer's. National Research Register 2004.
Wilcock 2006 {published data only}
- Wilcock GK. Disease‐modifying treatments for Alzheimer's disease: a perspective based on experience with r‐flurbiprofen. Alzheimer's and Dementia 2006;2:150‐2. [DOI] [PubMed] [Google Scholar]
Wilcock 2008 {published data only}
- Wilcock GK, Black SE, Hendrix SB, Zavitz KH, Swabb EA, Laughlin MA. Efficacy and safety of tarenflurbil in mild to moderate Alzheimer's disease: a randomised phase II trial. Lancet Neurology 2008;7(6):483‐93. [DOI] [PubMed] [Google Scholar]
Williams 2004 {published data only}
- Williams B. Protection against stroke and dementia: an update on the latest clinical trial evidence. Current Hypertension Reports 2004;6(4):307‐13. [DOI] [PubMed] [Google Scholar]
Windisch 2000 {published data only}
- Windisch M. Approach towards an integrative drug treatment of Alzheimer's disease. Journal of Neural Transmission, Supplement 2000;59:301‐13. [DOI] [PubMed] [Google Scholar]
Wisloff 1996 {published data only}
- Wisloff F, Hjorth M, Kaasa S, Westin J. Effect of interferon on the health‐related quality of life of multiple myeloma patients: results of a Nordic randomized trial comparing melphalan‐prednisone to melphalan‐prednisone + alpha‐interferon. The Nordic Myeloma Study Group. British Journal of Haematology 1996;94(2):324‐32. [DOI] [PubMed] [Google Scholar]
Wolkowitz 2003 {published data only}
- Wolkowitz OM, Kramer JH, Reus VI, Costa MM, Yaffe K, Walton P, et al. DHEA treatment of Alzheimer's disease: a randomized, double‐blind, placebo‐controlled study. Neurology 2003;60:1071‐6. [DOI] [PubMed] [Google Scholar]
Wollheim 2000 {published data only}
- Wollheim FA. Selective Cox‐2 inhibition in man ‐ therapeutic breakthrough or cosmetic advance?. Rheumatology 2000;39(9):935‐8. [DOI] [PubMed] [Google Scholar]
Woodward 2002 {published data only}
- Woodward MC. The potential of anti‐inflammatory drugs for the treatment of Alzheimer's disease. Lancet Neurology 2002;1(5):279‐284. [DOI] [PubMed] [Google Scholar]
Xia 2007 {published data only}
- Xia W, Han E. Follow‐up of a randomized, placebo‐controlled trial of dexamethasone to decrease the duration of ventilator dependency in very low birth weight infants: neurodevelopmental outcomes at 4 to 11 years of age. Pediatrics 2007;120(3):594‐602. [DOI] [PubMed] [Google Scholar]
Yaqub 1999 {published data only}
- Yaqub BA. New horizons in management of Alzheimer's disease. Saudi Medical Journal 1999;20(9):671‐7. [PubMed] [Google Scholar]
Yip 2005 {published data only}
- Yip AG, Green RC, Huyck M, Cupples LA, Farrer LA. Nonsteroidal anti‐inflammatory drug use and Alzheimer's disease risk: the MIRAGE Study. BMC Geriatriatrics 2005;5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 1999 {published data only}
Zandi 2005 {published data only}
- Zandi P. Pharmacological strategies for prevention of Alzheimer's disease: the epidemiologic evidence. Research and Practice in Alzheimer's Disease 2005;10:200‐4. [Google Scholar]
Zaragoza 2005 {published data only}
- Zaragoza JL. Attitude before COX‐2 selective inhibitors. Revista De Investigacion Clinica 2005;57(1):6‐12. [PubMed] [Google Scholar]
Zerovnik 2010 {published data only}
Zhai 2010 {published data only}
- Zhai Q‐J, Yue X‐Y, Hong Z, Xu G‐L, Liu X‐F. Efficacy observation of batroxobin for treatment of vascular cognitive impairment. Chinese Journal of Cerebrovascular Diseases 2010;7(2):73‐6. [Google Scholar]
Ziebell 2010 {published data only}
- Ziebell JM, Morganti‐Kossmann MC. Involvement of pro‐ and anti‐inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics 2010;7(1):22‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Aisen 1994
- Aisen. Inflammatory mechanisms in Alzheimer's disease: Implications for therapy. American Journal of Psychiatry 1994;151:1105‐13. [DOI] [PubMed] [Google Scholar]
Aisen 1996
- Aisen PS, Marin D, Altstiel L. A pilot study of prednisone in Alzheimer's disease. Dementia 1996;7:201‐206. [DOI] [PubMed] [Google Scholar]
Aisen 1998
- Aisen PS, Pasinetti GM. Glucocorticoids in Alzheimer's disease: the story so far. Drugs Aging 1998;12:1‐6. [DOI] [PubMed] [Google Scholar]
APA 1995
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. IV. Washington, DC: American Psychiatric Association, 1995. [Google Scholar]
Avramovich 2002
- Avramovich Y, Amit T, Youdim MB. Non‐steroidal anti‐inflammatory drugs stimulate secretion of non‐amyloidogenic precursor protein. Journal of Biological Chemistry 2002;277(35):31466‐73. [DOI] [PubMed] [Google Scholar]
Bazan 2009
- Bazan NG. Neuroprotectin D1‐mediated anti‐inflammatory and survival signaling in stroke, retinal degenerations, and Alzheimer's disease. Journal of Lipid Research 2009;50:S400‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blasko 2001
- Blasko I, et al. Ibuprofen decreases cytokine‐induced amyloid beta production in neuronal cells. Neurobiology of Disease Dec 2001;8(6):1094‐101. [DOI] [PubMed] [Google Scholar]
Citron 1992
- Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, Seubert P, Vigo‐Pelfrey C, Lieberburg I, Selkoe DJ. Mutation of the beta‐amyloid precursor protein in familial Alzheimer's disease increases beta‐protein production. Nature 1992;360(6405):672‐4. [DOI] [PubMed] [Google Scholar]
Deek 2002
- Deeks JJ, Smith LA, Bradley MD. Efficacy, tolerability, and upper gastrointestinal safety of celecoxib for treatment of osteoarthritis and rheumatoid arthritis: systematic review of randomised controlled trials. BMJ 2002;325:619. [DOI] [PMC free article] [PubMed] [Google Scholar]
Etminan 2003
- Etminan M, Gill S, Samii A. Effect of non‐steroidal anti‐inflammatory drugs on risk of Alzheimer's disease: systematic review and meta‐analysis of observational studies . BMJ 2003;327:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fiebich 1997
- Fiebich BL, et al. Prostaglandin E2 Induces Interleukin‐6 Synthesis in Human Astrocytoma cells. Journal of Neurochemistry 1997;68(2):704‐9. [DOI] [PubMed] [Google Scholar]
Haass 1994
- Haass C, Hung AY, Selkoe DJ, Teplow DB. Mutations associated with a locus for familial Alzheimer's disease result in alternative processing of amyloid beta‐protein precursor. Journal of Biological Chemistry 1994;269(26):17741‐8. [PubMed] [Google Scholar]
Harris 2002
- Harris JR. In vitro fibrillogenesis of the amyloid B1‐42 peptide: cholesterol potentiation and aspirin inhibition. Micron 2002;33:609‐26. [DOI] [PubMed] [Google Scholar]
Hebert 2003
- Hebert LE, Scherr PA, Bienias JL, et al. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Archives of Neurology 2003;60(8):1119‐22. [DOI] [PubMed] [Google Scholar]
Ho 1999
- Ho L, Pieroni C, Winger D, Purohit DP, Aisen PS, Pasinetti GM. Regional distribution of cyclooxygenase‐2 in the hippocampal formation in Alzheimer's disease. Journal of Neuroscience Research 1999;57:295‐303. [DOI] [PubMed] [Google Scholar]
In't Veld 2001
- In't Veld. Non‐steroidal anti‐inflammatory drugs and the risk of Alzheimer's disease. The New England Journal of Medicine 2001;345:1515‐21. [DOI] [PubMed] [Google Scholar]
Joachim 1992
- Joachim CL, Selkoe DJ. The seminal role of beta‐amyloid in the pathogenesis of Alzheimer disease. Alzheimer Disease & Associated Disorders 1992;6(1):7‐34. [DOI] [PubMed] [Google Scholar]
Jorm 2003
- Jorm. The incidence of dementia: a meta‐analysis. Neurology 2003;51:728‐733. [DOI] [PubMed] [Google Scholar]
McGeer 1989
- Mcgeer PL, Akiyama H, Itagaki S, McGeer EG. Immune system response in Alzheimer's disease. Canadian Journal Neurological Sciences 1989;16:516‐527. [DOI] [PubMed] [Google Scholar]
McGeer 1996
- McGeer. Arthritis and anti‐inflammatory agents as possible protective factors for Alzheimer's disease: a review of 17 epidemiologic studies. Neurology 1996;47(2):425‐432. [DOI] [PubMed] [Google Scholar]
McKhann 1984
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services task force on Alzheimer's disease. Neurology 1984;34:939‐44. [DOI] [PubMed] [Google Scholar]
Pasinetti 1998
- Pasinetti GM, Aisen PS. Cyclooxygenase‐2 expression is increased in frontal cortex of Alzheimer's disease brain. Neuroscience 1998;87:319‐424. [DOI] [PubMed] [Google Scholar]
Rich 1995
- Rich. Nonsteroidal anti‐inflammatory drugs in Alzheimer's disease. Neurology 1995;45:51‐55. [DOI] [PubMed] [Google Scholar]
Ruan 2009
- Ruan L, Kang Z, Pei G, Le Y. Amyloid deposition and inflammation in APPswe/PS1dE9 mouse model of Alzheimer's disease. Current Alzheimer Research 2009;6(6):531‐40. [DOI] [PubMed] [Google Scholar]
Salminen 2009
- Salminen A, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T. Inflammation in Alzheimer's disease: amyloid‐beta oligomers trigger innate immunity defence via pattern recognition receptors. Progress in Neurobiology 2009;87(3):181‐94. [DOI] [PubMed] [Google Scholar]
Selkoe 1991
- Selkoe DJ. Alzheimer's disease. In the beginning. Nature 1991;354(6353):432‐3. [DOI] [PubMed] [Google Scholar]
Thomas 2001
- Thomas T, Nadackal TG, Thomas K. Aspirin and non‐steroidal anti‐inflammatory drug inhibit amyloid‐B aggregation. Neuroreport 2001;12:3263‐67. [DOI] [PubMed] [Google Scholar]
WHO 1992
- World Health Organisation. The ICD‐10 Classification of Mental and Behavioural disorders: clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organisation, Division of Mental Health, 1992. [Google Scholar]
Wilcock 2006a
- Wilcock G, et al. Efficacy and safety of MPC‐7869 (R‐flurbiprofen), a selective Ab42‐lowering agent, in Alzheimers disease (AD): Results of a 12‐month phase 2 trial and 1‐year follow‐on study.. Alzheimer and Dementia 2006;2(3):S81‐S82. [Google Scholar]
Wimo 2005
- Wimo A, Winblad B, Jonsson L. An estimate of the total worldwide societal costs of dementia in 2005. Alzheimer's & dementia 2007;3:81‐91. [DOI] [PubMed] [Google Scholar]
Younkin 1995
- Younkin SG. Evidence that A beta 42 is the real culprit in Alzheimer's disease. Annals of Neurology 1995;37(3):287‐8. [DOI] [PubMed] [Google Scholar]


