Abstract
OBJECTIVE:
To analyze the serum metabolic targets of the "Zhibian (BL54) through Shuidao (ST28)" acupuncture technique in cyclophosphamide (CTX)-induced premature ovarian insufficiency (POI) model rats and to elucidate the potential molecular mechanism of acupuncture in improving POI.
METHODS:
We used an intraperitoneal injection of CTX to establish the POI rat model (POI group) and compared serum hormone levels and ovarian histopathological changes to evaluate the effect of the Zhibian (BL54) through Shuidao (ST28) technique (ZS + POI group) on ovarian function. Then, nontargeted metabolomics was performed using rat serum by ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF/MS).
RESULTS:
After acupuncture intervention, the serum hormone levels and ovarian pathological morphology of POI rats were effectively improved. Moreover, UPLC-Q-TOF/MS results showed that the ZS + POI group showed a significant reversal of the levels of 6 differential metabolites. Among them, the levels of four serum metabolic markers, divanillyltetrahydrofuran ferulate, trans-ferulic acid, tryptamine, and neuraminic acid, increased significantly. Further analysis of biological effects showed that all metabolites were involved in the regulation of reproductive hormone levels and antioxidant and antiapoptotic effects.
CONCLUSIONS:
The "Zhibian (BL54) through Shuidao (ST28)" acupuncture method may improve the ovarian function of POI rats by regulating serum metabolite markers to exert antioxidant and antiapoptotic effects, which provides a theoretical basis for the clinical application of acupuncture in the treatment of POI.
Keywords: acupuncture, premature ovarian insufficiency, metabolomics, ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry, Zhibian (BL54) through Shuidao (ST28)
1. INTRODUCTION
Premature ovarian insufficiency (POI) is characterized by menstrual disorders, low estrogen levels, and high gonadotropin levels.1 Mei-row et al 2 found that approximately 42% of female patients treated with alkylating agents developed POI. POI is one of the main long-term complications after radiotherapy and chemotherapy in patients with clinical malignant tumors. High-dose and long-term antitumor treatment can cause ovarian dysfunction and even failure in patients of all ages, resulting in amenorrhea and decreased or lost of reproductive capacity.3,4 As an alkylating agent that is an antitumor drug widely used in chemotherapy,5 cyclophosphamide (CTX) can inhibit cellular immunity and humoral immunity through a series of reactions, damage follicular granulosa cells, affect follicular development and maturation, and lead to ovarian dysfunction or failure.6 Through early diagnosis, intervention, and treatment, the fertility and quality of life of POI patients can be greatly improved.7,8
The acupuncture method of "Zhibian (BL54) through Shuidao (ST28)" was proposed by Professor JI Laixi based on the description of "Jiuzhen" in "Neijing". By using a long needle to make a deep puncture to the pelvic cavity to stimulate the pelvic nerve, one needle and two acupoints dredge Qi and blood, regulate the function of Zang-Fu organs and Chong and Ren, improve the endocrine imbalance of the body, cause the meridian to spread to the disease site, and finally improve the ovarian function and the related symptoms of POI patients. Metabolomics is a systems biology method developed after genomics, transcriptomics, and proteomics.9
Metabolomics is a quantitative analysis of endogenous metabolites (found in blood, urine, stool, and tissue fluid) produced by organisms under stimulation.10 The metabolites of small molecules in different states are analyzed to identify potential candidate biomarkers and reveal their general function in organisms, providing new ideas for the diagnosis and treatment of diseases. The increasing application of metabolomics technology can overcome the limitations of previous research methods for studying the potential therapeutic mechanisms of acupuncture.11 Through metabolomics data processing, a systematic and comprehensive in-depth analysis of metabolites and the construction of related metabolic pathways and pathway networks can clarify biological problems such as changes in biological processes caused by experimental conditions and the pathogenesis of disease development, systematically explain the mechanism of acupuncture and moxibustion and its therapeutic role and promote the scientific development of Traditional Chinese Medicine.12
2. MATERIALS AND METHODS
2.1. Animals
Fifty-five specific pathogen free grade female Sprague-Dawley rats that were 7-8 weeks old and weighed (200 ± 20) g were provided by the Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). The animal production license number SCXK (Beijing) 2016-0011. The rats were routinely raised in the experimental animal room of Shanxi University of Traditional Chinese Medicine [room temperature of (24 ± 3) °C, humidity of 55% ± 5%, 12 h light and 12 h dark alternating light cycle, separate cages]. This experiment was carried out in strict accordance with the Guidelines for the Nursing and Use of Experimental Animals and the Declaration of Helsinki. All experimental programs and procedures were approved by the Animal Research Ethics Committee of Shanxi University of Traditional Chinese Medicine, approval number: 2022DW191.
2.2. Chemicals and reagents
Acupuncture needles (0.18 mm × 25 mm) were purchased from Beijing Zhongyan Taihe Medical Device Company Limited (Beijing, China). CTX (H32020857) was purchased from Jiangsu Hengrui Pharmaceutical Company Limited (Lianyungang, China). Hematoxylin-eosin (HE) dye (DH0006) was purchased from Leagene (Beijing, China). Follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2) enzyme linked immunosorbent assay (ELISA) detection kits (SU-B30447, SU-B3024, SU-B30267) were purchased from WKSUBIO (Shanghai, China). Methanol (67-56-1) and acetonitrile (75-05-8) were purchased from Merck & Co., Inc. (Kenilworth, NJ, USA). L-2-chlorophenylalanine (103616-89-3) was purchased from Aladdin (Shanghai, China). Formic acid (64-18-6) was purchased from TCI (Shanghai, China). UPLC Acquity I-Class PLUS, high-resolution mass spectrometer (UPLC Xevo G2-XS QTOF) and chromatographic column (Acquity UPLC HSS T3 1.8 µm 2.1 mm × 100 mm) were purchased from Waters Associates (Milford, MA, USA).
2.3. Establishment of the POI model and acupuncture treatment
After 1 week of adaptive feeding, 40 rats were randomly selected from 55 rats with normal estrous cycles according to the random number table method. The POI model was established by intraperitoneal injection of CTX solution (2 mg/mL) according to the modeling method proposed by Fu et al.13 CTX (50 mg·kg-1·d-1) was injected intraperitoneally on the first day, and CTX 8 mg·kg-1·d-1 was injected intraperitoneally on the second to fifteenth days. During the modeling period, changes in the estrous cycle in each group were observed, and the disorder of the estrous cycle suggested that the model was successfully established. After modeling, 4 rats without obvious estrous cycle disorder were excluded. Finally, 36 model rats were randomly divided into the POI group (CTX-induced POI model rats), SA + POI group (sham acupuncture intervention POI rats), and ZS + POI group [Zhibian (BL54) through Shuidao (ST28) acupuncture treatment POI rats], with 12 rats in each group. The changes in serum hormone levels were measured in blood from the tail vein of rats. The levels of FSH and LH increased significantly, and the level of E2 decreased significantly, indicating that the model was successfully established.
On the 16th day, the experimental animal acupoints14 combined with the anatomical characteristics of the rats were referenced, and 0.18 mm × 25 mm specification filiform needles were selected for penetration of the Zhibian (BL54) acupoint, which was on the lateral side of the rat's buttocks, at the midpoint of the bone gap between the greater trochanter of the femur and the fourth sacral spine. In the ZS + POI group rats, this acupoint was punctured in the direction of the ipsilateral Shuidao (ST28) acupoint, with a high frequency and small amplitude twisting of the needle, at a depth of approximately 12 mm, and without lifting or thrusting during needle manipulation and continuous twirling for 1 min, once a day, 30 min each time, for a total of 4 weeks. Rats in the SA + POI group were tapped on the skin surface of the Zhibian (BL54) point with a blunt needle once a day for 4 weeks. The rats in the POI group and the SA + POI group were immobile for 30 min, while the ZS + POI group received acupuncture treatment. The experiment lasted for 43 d.
On the 44th day, rats were anesthetized by intraperitoneal injection of 20% urethane (5 mL/kg) according to their body weight. The degree of anesthesia was based on the disappearance of the pedal reflex (when the skin between the fingers or toes of the rat is pinched, the animal's legs produce a bent and straight reflex) and forceps tail reflex (clamping the animal's tail with nails or hemostatic forceps will cause the tail to flick and the mouse to occasionally produce sound). After anesthesia, serum and ovarian tissue samples were collected.
2.4. Changes in the estrous cycle
Rat vaginal exfoliated cells were washed and smeared to detect and observe the changes in the estrous cycle of rats. The operator used his hand to control the body torsion of the rats so that the vaginal orifice was fully exposed. Then, 80-100 μL 0.9 % sodium chloride solution was collected with a pipette gun and gently inserted into the vagina of the rats (approximately 1 cm in depth). After repeating 3 times, a small amount of vaginal secretion solution was removed and evenly smeared and marked (approximately 1.5 cm × 2 cm). After HE staining, the sections were observed and photographed under an optical microscope (× 200).
2.5. Analysis of serum hormone levels
To verify the success of the model and clarify the continuous effect of CTX on ovarian function in POI rats and the improvement effect of "Zhibian (BL54) through Shuidao (ST28)" acupuncture on POI rats, changes in serum FSH, LH, and E2 levels were observed after modeling and intervention. The serum samples to be tested were dissolved on ice, and the standard curve was drawn according to the optical density (OD) value of each well detected by ELISA reagent instructions for FSH, LH and E2 to draw the standard curve and calculate the sample concentration.
2.6. Histopathology
The effects of "Zhibian (BL54) through Shuidao (ST28)" acupuncture on the ovaries of POI rats were evaluated by observing and analyzing the general morphology, ovarian coefficient and histopathological changes in the ovaries of rats. Rat ovarian tissue soaked in 10% formalin solution was trimmed and flattened (5 mm × 5 mm). After conventional dehydration, clearing, wax immersion, and embedding, pathological sections with a thickness of approximately 4 μm were made. After conventional dewaxing and hydration, hematoxylin-eosin staining was performed as follows: hematoxylin 5-8 min, rinse 1-2 min, 1% hydrochloric acid ethanol solution 5 s, rinse 1 min, 0.2% ammonia solution 15 s, rinse 1 min, eosin 4-5 min, rinse 1-2 min. Sections were sealed and observed under a microscope after drying (× 100, × 200).
2.7. Ultra performance liquid chromatography-quadrupole-time of flight-mass spectrometry (UPLC-Q-TOF/MS) Nontargeted metabolomics study
2.7.1. UPLC-Q-TOF/MS sample preparation and detection
The serum supernatant was separately added to 1000 μL of extract containing an internal standard (1000∶2) (methanol-acetonitrile volume ratio = 1∶1, internal standard concentration 2 mg/L). After ultrasonic treatment with a 45 Hz grinding instrument, samples were incubated at -20℃for 1 h. The sample was centrifuged at 4 ℃ and 12000 rpm for 15 min, and then the extract was dried in a vacuum concentrator. One hundred and sixty microliters of extract (acetonitrile-water volume ratio: 1∶1) was added to redissolve the samples and vortexed for 30 s. Then, the samples were incubated in an ice water bath ultrasound for 10 min; finally, the samples were centrifuged at 4 °C and 12 000 rpm for 15 min, and 10 μL of each sample was mixed to create QC samples for detection. If the correlation of samples in the same biological replicate group was ≥ 0.7, the expression quantitative index was considered up to standard and repeatable.
2.7.2. UPLC-Q-TOF/MS analysis conditions
Chromatographic conditions: The chromatographic column was an Acquity UPLC HSS T3 1.8 µm (2.1 mm × 100 mm) column; the flow rate was 400 μL/min. In positive ion mode and negative ion mode, mobile phase A was 0.1 % formic acid aqueous solution, and mobile phase B was 0.1 % formic acid acetonitrile. The injection volume was 1 µL. The chromatographic mobile phase conditions were as follows: 0-10 min, 98 % A and 2 % B, gradient settings: 0-0.25 min, 98-98 % A, 2-2 % B; 0.25-10.0 min, 98-2 % A, 2-98 % B; 10.0-13.0 min, 2-2 % A, 98-98 % B; 13.0-13.1 min, 2-98 % A, 98-2 % B; 13.1-15.0 min 98-98 % A, 2-2 % B.
Mass spectrometry conditions: A Waters Xevo G2-XS QToF high-resolution mass spectrometer was used for primary and secondary mass spectrometry data acquisition in MSe mode under the control of acquisition software (MassLynx V 4.2, Waters Associates, Milford, MA, USA). In each data acquisition cycle, dual-channel data acquisition of low collision energy and high collision energy was performed simultaneously. The low collision energy was 2 V, the high collision energy range was 10-40 V, and the scanning frequency was 0.2 s. The following ESI parameters were used: capillary voltage, 2000 V (positive ion mode) or -1500 V (negative ion mode); cone hole voltage, 30 V; ion source temperature, 150 ℃; desolvation gas temperature 500 ℃; reverse blowing gas flow rate, 50 L/h; desolvation gas flow rate, 800 L/h; mass-to-nuclear ratio (m/z) acquisition range 50-1200.
2.7.3. Data processing and multivariate data analysis
The original data collected by MassLynx V 4.2 were processed by Progenesis Qi software for peak extraction, peak alignment, and other data processing operations. Based on the online METLIN database and Biocloud platform self-built database with Progenesis Qi software, metabolite identification was carried out. In addition, theoretical debris identification was executed, and the mass deviation was within 100 ppm. Before the analysis, the data were normalized. To normalize the total peak area, each metabolite in each sample was divided by the total peak area of the sample and then multiplied by the mean of all peak areas. Data analysis was performed using the Biocloud platform (www.biocloud.net) combined with univariate statistical analysis and multivariate statistical analysis, and the differentially expressed metabolites were analyzed and plotted from multiple perspectives according to the data characteristics.
Based on the results of the analysis, the screening criteria were as follows: (a) Fold change (FC) ≥ 1 (when the FC threshold is 1, a screening FC ≥ 1 is upregulated and a screening FC ≤ 1 is downregulated); (b) variable importance in projection (VIP) ≥ 1; (c) P value of t test < 0.01. Metabolites with VIP ≥ 1 and/or P value < 0.05 were considered significantly different.
2.8. Statistical analysis
SPSS 26.0 (IBM Corp., Armonk, NY, USA) was used to analyze the data. The measured data are expressed as the mean ± standard deviation ($\bar{x}±s$). When normality and homogeneity of variance were met, one-way analysis of variance was used for comparisons among groups, and the least significance difference-t test was used for further pairwise comparisons. The Kruskal-Wallis rank sum test was used when normality or homogeneity of variance was not met. Results with P < 0.05 were considered statistically significant. GraphPad Prism 8.0 software (GraphPad Software, San Diego, CA, USA) was used to generate statistical graphs.
3. RESULTS
3.1. Effect of CTX on the basic condition
When the POI model was induced by intraperitoneal injection of CTX, the general behavior and weight changes of the rats were recorded. The body shape (no arch and back curling), fur condition (bright, soft, no hair removal), mental condition (flexibility, activity), fecal condition (dark brown, oval, smooth), and mouth and nose (no abnormal secretions) were normal in the control group rats. After modeling, POI rat body shape (long and thin, arched back, curled up), fur (dry coke, hair removal), mental status (malaise, irritability, less activity), urine (increased), feces (soft, not into shaped), lip color (purple-black), and nose and mouth (increased secretions, occasional nose blood) changed dramatically. Compared with the control group, the body weight of rats in the POI group was significantly decreased (P < 0.05, P < 0.01). After acupuncture intervention, compared with the POI group, the body weight of rats in the SA + POI group was slightly higher (P > 0.05), and the body weight of rats in the ZS + POI group was significantly higher (P < 0.05) (Table 1).
Table 1.
Changes in body weight of rats (g, $\bar{x}±s$)
| Group | n | Before modeling | After modeling | Post intervention |
|---|---|---|---|---|
| Control | 12 | 245±7 | 266±11 | 316±15 |
| POI | 12 | 244±10 | 253±11a | 286±13b |
| SA+POI | 12 | 244±7 | 255±12a | 288±13 |
| ZS+POI | 12 | 244±9 | 255±14a | 301±17c |
Notes: control: normal feeding, without any treatment; POI: POI model was induced by intraperitoneal injection of CTX (2 mg/mL) for 15 d; SA + POI: sham acupuncture intervention in CTX-induced POI rats for 4 weeks; ZS+POI: Zhibian (BL54) through Shuidao (ST28) acupuncture intervention in CTX-induced POI rats for 4 weeks. The one-way analysis of variance and least significance difference-t test were used. Compared with the control group, aP < 0.05, bP < 0.01; compared with the POI group, cP < 0.05. POI: premature ovarian insufficiency; CTX: cyclophosphamide; SA: sham acupuncture; ZS: Zhibian (BL54) through Shuidao (ST28) acupuncture.
3.2. Changes in the estrous cycle
After sexual maturity, healthy female rats undergo four continuous and regular periodic changes, including proestrus, estrus, metestrus, and diestrus. The estrous cycle is mainly identified by observing the gradually changing vaginal exfoliated cells.15 The morphology and proportion of white blood cells, nucleated epithelial cells, and anucleated keratinocytes in each stage are quite different.16 In proestrus, round or oval nucleated cells are observed (supplementary Figure 1A); in estrus, flake or stacked anuclear keratinocytes are observed (supplementary Figure 1B); in metestrus, nucleated cells, keratinocytes, and white blood cells are observed (supplementary Figure 1C); and in diestrus, white blood cells are observed (supplementary Figure 1D).
After HE staining of rat vaginal exfoliated cells, the chromatin of the nucleus in the smear and the nucleic acid in the cytoplasm were purple-blue, and the components in the cytoplasm and extracellular matrix were different degrees of light red. The red and blue contrast was obvious, and the cell characteristics of each cycle were clear, making them easy to observe and judge. The results showed that the estrous cycle of rats in the control group showed regular changes in proestrus, estrus, metestrus, and diestrus. In the process of intraperitoneal injection of CTX solution, the estrous cycle of the model rats was gradually disordered, and there was no obvious regular cycle. The disorder was most obvious on the 7-15th day of intraperitoneal injection of CTX solution and mainly manifested as estrus or diestrus stagnation, suggesting that the POI model was successfully established (supplementary Figures 1E, 1F). The model was further verified by serum hormone level detection.
3.3. Examinations by ELISA
After modeling, to verify the success of the CTX-induced POI model, we observed the changes in FSH, LH, and E2 levels in the serum of model rats. Compared with the control group, the serum FSH and LH levels of POI model rats were significantly increased (P < 0.01), and the E2 level was significantly decreased (P < 0.01).
After the intervention, to clarify the continuous effect of CTX on the ovarian function of POI rats and the improvement of ovarian function in POI rats by the "Zhibian (BL54) through Shuidao (ST28)" acupuncture, we measured the levels of FSH, LH, and E2 in serum again. Compared with the control group, the serum FSH and LH levels in the POI group were still significantly increased (P < 0.01), and the E2 level was significantly decreased (P < 0.01). The levels of serum FSH, E2, and LH in the SA + POI group were slightly lower or slightly higher than those in the POI group (P > 0.05), while the levels of serum FSH and LH in the ZS + POI group were significantly lower (P < 0.01), and the level of E2 was significantly higher (P < 0.01) (Table 2).
Table 2.
Changes in serum hormone levels ($\bar{x}±s$)
| Group | n | After modeling | Post-intervention | |||||
|---|---|---|---|---|---|---|---|---|
| FSH (IU/L) |
LH (mIU/mL) |
E2 (pmol/L) |
FSH (IU/L) |
LH (mIU/mL) |
E2 (pmol/L) |
|||
| Control | 12 | 3.1±0.9 | 22.7±6.7 | 39.3±9.8 | 4.0±1.1 | 20.9±5.0 | 43.7±6.1 | |
| POI | 12 | 6.0±1.3a | 36.7±5.4a | 26.0±8.7a | 7.4±1.4a | 36.3±4.2a | 28.0±6.0a | |
| SA+POI | 12 | 5.8±0.9a | 38.8±5.1a | 29.6±6.5a | 7.3±0.9 | 35.2±4.4 | 30.6±6.1 | |
| ZS+POI | 12 | 6.3±0.9a | 36.8±6.2a | 27.1±8.6a | 6.1±0.9b | 30.3±4.4b | 38.0±7.0b | |
Notes: control: normal feeding, without any treatment; POI: POI model was induced by intraperitoneal injection of CTX (2 mg/mL) for 15 d; SA + POI: sham acupuncture intervention in CTX-induced POI rats for 4 weeks; ZS + POI: Zhibian (BL54) through Shuidao (ST28) acupuncture intervention in CTX-induced POI rats for 4 weeks. FSH: follicle-stimulating hormone; LH: luteinizing hormone; E2: estradiol. The one-way analysis of variance and least significance difference-t test were used. Compared with the control group, aP < 0.01; compared with the POI group, bP < 0.01. POI: premature ovarian insufficiency; CTX: cyclophosphamide; SA: sham acupuncture; ZS: Zhibian (BL54) through Shuidao (ST28) acupuncture.
3.4. HE staining and ovarian index
By observing and analyzing the changes in ovarian morphology of rats, the effect of the "Zhibian (BL54) through Shuidao (ST28)" acupuncture on the ovaries of POI rats was evaluated. In the control group, the general shape of the ovary was full and large, and the ovary had a ruddy surface color (Figure 1A). HE staining showed many follicles at all levels, including the primary follicle, preantral follicle, early antral follicle, and preovulatory follicle (preantral follicle), and the nucleus was completely visible, with a rich granulosa cell layer arranged in the follicle (Figure 1E). Compared with the control group, the ovaries of the POI group were generally atrophied, the volume was small, the surface color was darker (Figure 1B), the ovarian mass and coefficient were significantly reduced (P < 0.01) (Table 3). The number of growing follicles at all levels was reduced, atresia follicular was increased, the nucleus in the follicles were lysed, and the granulosa cells were disordered and irregularly arranged (Figure 1F).
Figure 1. ovarian tissue morphology.
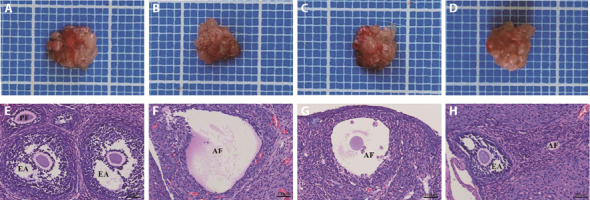
A: gross ovarian tissue specimen in control group; B: gross ovarian tissue specimen in POI group; C: gross ovarian tissue specimen in SA + POI group; D: gross ovarian tissue specimen in ZS + POI group; E: pathomorphological change ovarian tissue in control group; F: pathomorphological change ovarian tissue in POI group; G: pathomorphological change ovarian tissue in SA + POI group; H: pathomorphological change of ovarian tissue in ZS + POI group (HE, × 200). Control: normal feeding, without any treatment. POI: POI model was induced by intraperitoneal injection of CTX (2 mg/mL) for 15 d; SA+POI: sham acupuncture intervention in CTX-induced POI rats for 4 weeks; ZS + POI: Zhibian (BL54) through Shuidao (ST28) acupuncture intervention in CTX-induced POI rats for 4 weeks. PF: primary follicle; EA: early antral follicle; AF: atresia follicle; HE: hematoxylin-eosin staining; POI: premature ovarian insufficiency; CTX: cyclophosphamide; SA: sham acupuncture; ZS: Zhibian (BL54) through Shuidao (ST28) acupuncture.
Table 3.
Changes in ovarian weight and coefficient ($\bar{x}±s$)
| Group | n | Left ovary | Right ovary | |||
|---|---|---|---|---|---|---|
| Weight (mg) | Coefficient (mg/g) | Weight (mg) | Coefficient (mg/g) | |||
| Control | 12 | 73.333±6.513 | 0.233±0.023 | 71.667±12.673 | 0.227±0.037 | |
| POI | 12 | 44.167±12.401a | 0.153±0.039a | 45.000±7.977a | 0.157±0.026a | |
| SA+POI | 12 | 45.833±9.962 | 0.159±0.033 | 43.333±10.731 | 0.150±0.032 | |
| ZS+POI | 12 | 56.667±6.513b | 0.188±0.020b | 58.333±11.146b | 0.194±0.037b | |
Notes: control: normal feeding, without any treatment; POI: POI model was induced by intraperitoneal injection of CTX (2 mg/mL) for 15 d.; SA + POI: sham acupuncture intervention in CTX-induced POI rats for 4 weeks; ZS + POI: Zhibian (BL54) through Shuidao (ST28) acupuncture intervention in CTX-induced POI rats for 4 weeks. POI: premature ovarian insufficiency; CTX: cyclophosphamide; SA: sham acupuncture; ZS: Zhibian (BL54) through Shuidao (ST28) acupuncture. The one-way analysis of variance and least significance difference-t test were used. Compared with the control group, aP < 0.01; compared with the POI group, bP < 0.01.
Compared with the POI group, the ovarian general morphology (Figure 1C), weight, coefficient (Table 3) and pathological morphology (Figure 1G) in the SA + POI group were not significantly improved (P > 0.05). However, the general morphology of the ovaries in the ZS + POI group was larger than that in the POI group (Figure 1D), and the ovarian weight and coefficient were significantly increased (Table 3). Under the microscope, it was observed that the follicles at all levels had different degrees of growth, and the number of atresia follicles was reduced (Figure 1H), indicating that "Zhibian (BL54) through Shuidao (ST28)" acupuncture had a significant improvement on the ovary.
3.5. Quality control (QC) in UPLC/Q-TOF MS analysis
The internal standard (2-chlorophenylalanine) concen-tration introduced by the external source and the internal standard concentration of the QC sample were the same. Positive ion mode: mass-to-charge ratio (m/z): 200.0488, retention time (R.T.): 2.424, relative standard deviation: 0.16%. Negative ion mode: mass-to-charge ratio (m/z): 198.0315, retention time (R.T.): 2.417, relative standard deviation: 1.23%. The smaller response difference of the internal standard (RSD ≤ 20%) indicates a more stable system and higher quality data.
3.6. Metabolic profiling of total ion flow chromatograms of serum
UPLC-Q-TOF/MS was used to analyze the total ion current chromatograms of endogenous metabolites in the serum of rats in the control group, POI group, SA + POI group, and ZS + POI group in positive and negative ion modes. The results showed that the total ion current chromatograms of each group were similar, but there were some differences in the peak type and peak area of each group, indicating that some metabolites changed among the rat groups. The retention times of chro-matographic peaks were mainly concentrated in 0-15 min, and differences among the groups were found by further analysis (supplementary Figure 2A-2H).
3.7. Multivariate statistical analysis
3.7.1. Principal component analysis (PCA) score plot of serum metabolites
The PCA score plot showed that compared with the control group, the profiles of serum metabolites in the POI group had a clear separated trend, indicating that the metabolic profiles of the ZS + POI group were clearly different from those in the POI group, and the degree of variation between groups was significant, indicating that the "Zhibian (BL54) through Shuidao (ST28)" acupuncture had a regulatory effect on the metabolites in the serum of POI rats (supplementary Figures 3A-3F). However, PCA is a multidimensional data statistical analysis method for unsupervised pattern recognition. It is limited to the principal component analysis of samples grouped by differences to understand the overall differences between samples and the variation between samples in the group. It is not sensitive to variables with small sample correlations and cannot distinguish between groups of samples well. To highlight the differences between groups to the greatest extent, we further used orthogonal partial least squares-dis-crimination analysis (OPLS-DA).
3.7.2. OPLS-DA score plot and model replacement test plot of serum metabolites
OPLS is a latent variable regression method based on the covariance between predictors and responses. It can effectively be used to analyze data sets with multiple collinear predictors. The dimension of the data is reduced, the regression model is established, and the regression results are discriminated. To check the reliability of the OPLS-DA model, the samples were tested by a permutation test, and the R2γ and Q2γ were calculated. The permutation test was repeated 200 times, and the results of multiple modeling were graphed using a scatter plot for analysis. The prediction parameters of the evaluation model are R2γ and Q2γ, which represent the interpretation and prediction ability of the model, respectively. The data of the OPLS-DA model was verified by a 200× permutation test as follows: POI group compared with control group: serum positive ion mode: R2γ (cum) = 0.993, Q2γ (cum) = 0.805; negative ion mode: R2γ (cum) = 0.993, Q2γ (cum) = 0.765.
The prediction parameters showed that in the positive and negative ion modes, the R2γ and Q2γ values of the control group vs the POI group and the POI group vs the ZS + POI group were close to 1, indicating that the model was stable and reliable. A Q2γ value of > 0.5 indicates that the model is effective, that is, the interpretability and predictive ability of the model are suitable, and no overfitting occurs. The permutation test executed by the OPLS-DA model showed that the R2γ and Q2γ values of the control group vs the POI group and the POI group vs the ZS + POI group were generally smaller than those of the original model in positive and negative ion modes; that is, all points on the left side of the figure (permutation test) were lower than the points at the x-axis of 1 (the original model), and the slope of the fitted regression line was positive, indicating that the model was meaningful, so this model was used to screen differentially expressed metabolites (Figures 2A-2D).
Figure 2. OPLS-DA score plots and model replacement test plots of rat serum samples.
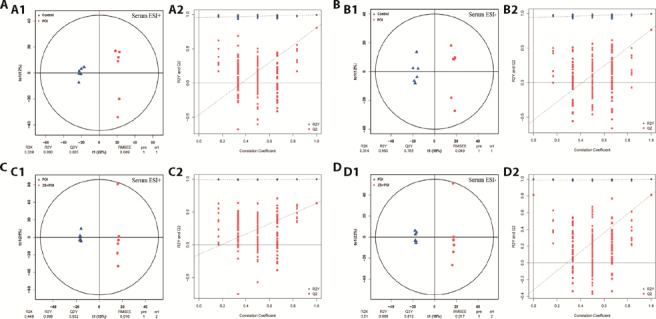
A: OPLS-DA score plot and model replacement test plot of the control group vs the POI group in positive ion mode; B: OPLS-DA score plot and model replacement test plot of the control group vs the POI group in negative ion mode; C: OPLS-DA score plot and model replacement test plot of the POI group vs the ZS + POI group in positive ion mode; D: OPLS-DA score plot and model replacement test plot of the POI group vs the ZS+POI group in negative ion mode; A1, B1, C1, D1: OPLS-DA score plot; A2, B2, C2, D2: model replacement test plot. In the OPLS-DA score plots, the x-axis (t1) represents the prediction component (intergroup difference component), the y-axis (t2) represents the orthogonal component (intragroup difference component), and the percentage of the horizontal y-axis represents the proportion of this component in the total variance. In the model replacement test plots, the x-axis represents the correlation between the permutation group and the original model group, the y-axis represents the value of R2Y or Q2Y (where R2γ and Q2γ with 1 in the x-axis are the values of the original model), the blue point and the red point represent the R2γ and Q2γ of the replaced model, respectively, and the imaginary line is the fitted regression line. Control: normal feeding, without any treatment; POI: POI model was induced by intraperitoneal injection of CTX (2 mg/mL) for 15 d; ZS+POI: Zhibian (BL54) through Shuidao (ST28) acupuncture intervention in CTX-induced POI rats for 4 weeks. ESI: electron spray ionization; RMSEE: root mean square error, pre: number of predicted components, ort: number of orthogonal components; OPLS-DA: orthogonal partial least squares-discrimination analysis; POI: premature ovarian insufficiency; CTX: cyclophosphamide; ZS: Zhibian (BL54) through Shuidao (ST28) acupuncture.
3.8. Screening and pathway analysis of serum differential metabolites in POI rats treated with "Zhibian (BL54) through Shuidao (ST28)" acupuncture
Based on the results of the OPLS-DA model, VIP ≥ 1 was selected for the preliminary screening of metabolites, and then differential metabolites were screened by combining FC = 1 and univariate analysis P value < 0.01. The volcano plot of differential metabolites showed that compared with the POI group, 223 differential metabolites (ESI+ mode, 65; ESI- mode, 158) were detected in the serum of rats in the ZS+POI group, of which 164 differential metabolites were upregulated and 59 differential metabolites were downregulated (Figures 3A, 3B). KEGG pathway enrichment analysis (P value < 0.05) showed that these above serum differential metabolites were mainly involved in bile secretion, pyrimidine metabolism, steroid hormone biosynthesis, benzoate degradation, and other metabolic pathways (Figures 3C, 3D).
Figure 3. Screening of metabolic differentials and KEGG pathway enrichment maps in serum of POI model intervened by acupuncture intervention.
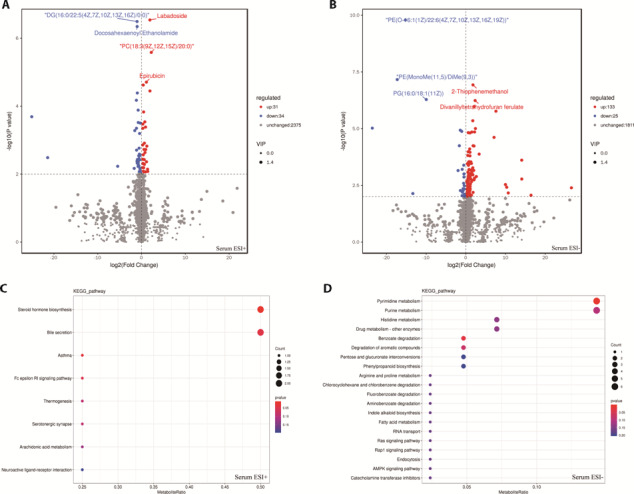
A: volcano map in positive ion mode, B: volcano map in negative ion mode, C: KEGG pathway enrichment map in positive ion mode, D: KEGG pathway enrichment map in negative ion mode. In the volcano map, the x-axis represents the difference multiple, the y-axis represents the P-value, and the scatter size represents the VIP value. Blue dots indicate down-regulation, red dots indicate up-regulation, and gray dots indicate no significant difference. In the KEGG pathway enrichment map, the x-axis is the ratio of the number of differential metabolites to the total number of metabolites, and the y-axis is the name of the pathway. The larger the point, the more the number of metabolites enriched, and the redder the color of the point, the more significant the enrichment pathway. KEGG: Kyoto Encyclopedia of Genes and Genomes; POI: premature ovarian insufficiency.
3.9. The "Zhibian (BL54) through Shuidao (ST28)" acupuncture method significantly reversed the potential metabolic markers in serum
As shown in the Venn diagram (supplementary Figure 4 and Table 4), by intersecting the serum differential metabolites between the POI group vs the ZS + POI group and the control group vs the POI group, it was found that the "Zhibian (BL54) through Shuidao (ST28)" acu-puncture method regulates 39 serum biomarkers (ESI+ mode, 25; ESI- mode, 14). The levels of 6 biomarkers, including neuraminic acid, divanilly-ltetrahydrofuran ferulate, eupatilin, trans-ferulic acid, tryptamine, and rolitetracycline, were significantly callbacked.
Table 4.
Effect of "Zhibian (BL54) through Shuidao (ST28)" acupuncture on potential biomarkers in the serum of rats with POI
| Name | Formula | Mass-to-charge ratio (m/z) |
RT (min) |
Control vs POI | POI vs ZS+POI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FC |
P
value |
VIP | Trend | FC | P value | VIP | Trend | |||||
| Divanillyltetrahydrofuran ferulate | C30H32O8 | 1099.4293 | 11.4854 | 0.2409 | 0.0001 | 2.1464 | ↓a | 4.4102 | 0.0000 | 2.2110 | ↑c | |
| Rolitetracycline | C27H33N3O8 | 1099.4546 | 13.5905 | 0.1087 | 0.0011 | 2.0491 | ↓b | 6.5169 | 0.0000 | 2.1883 | ↑c | |
| Trans-ferulic acid | C10H10O4 | 239.0564 | 2.9167 | 0.6022 | 0.0006 | 1.9304 | ↓a | 1.5964 | 0.0027 | 1.8209 | ↑b | |
| Tryptamine | C10H12N2 | 159.0912 | 2.4098 | 0.7189 | 0.0023 | 1.8795 | ↓b | 4.2828 | 0.0000 | 2.2682 | ↑c | |
| Neuraminic acid | C9H17NO8 | 288.0653 | 2.0602 | 0.4761 | 0.0038 | 1.8148 | ↓b | 1.6599 | 0.0095 | 1.6740 | ↑b | |
| Eupatilin | C18H16O7 | 343.0840 | 4.2009 | 0.5563 | 0.0030 | 1.7867 | ↓b | 2.0420 | 0.0015 | 1.8760 | ↑b | |
Notes: control: normal feeding, without any treatment; POI: POI model was induced by intraperitoneal injection of CTX (2 mg/mL) for 15 d; ZS + POI: Zhibian (BL54) through Shuidao (ST28) acupuncture intervention in CTX-induced POI rats for 4 weeks. POI: premature ovarian insufficiency; CTX: cyclophosphamide; ZS: Zhibian (BL54) through Shuidao (ST28) acupuncture; FC: fold change; VIP: variable importance in projection; RT: retention time. The difference in metabolites between the control and experimental groups was more than 1 time, and the difference was considered significant. FC ≥ 1 (when the FC threshold is 1, a screening FC ≥ 1 is upregulated and a screening FC ≤ 1 is downregulated). The VIP value represents the influence intensity of the difference between the corresponding metabolites in the classification and discrimination of each group of samples in the model. It is generally believed that the metabolites with VIP ≥ 1 are significantly different. P value is the P value of t test, metabolites with P value < 0.05 are generally considered to be significantly different, in this study, P value < 0.01 of t test was used to screen differential metabolites, aP < 0.001, bP < 0.01, cP < 0.0001.
According to biological significance, 4 metabolic markers neuraminic acid, divanillyltetrahydrofuran ferulate, trans-ferulic acid, tryptamine, were finally determined as the metabolic targets of the "Zhibian (BL54) through Shuidao (ST28)" acupuncture intervention in POI model rats. If the area under roc (AUC) is between 0.5 (the two classes are statistically the same) and 1.0 (there is a threshold to achieve perfect separation between classes), the closer the value is to 1, the more obvious the separation of substances in the control group and the experimental group (that is, control group vs The POI group and the POI group vs the ZS+POI group were always close to 1.0, indicating that the separation of metabolites in different comparison control group vs The POI group and the POI group vs the ZS + POI group were always close to 1.0, indicating that the separation of metabolites in different comparison potential biomarkers).The AUCs of 4 metabolites in the groups was very obvious (Figure 4). Therefore, these metabolites may can be determined as potential biomarkers for the intervention of the "Zhibian (BL54) through Shuidao (ST28)" acupuncture on serum metabolic differences in POI rats.
Figure 4. ROC curve and box plot of acupuncture on potential biomarkers in serum of rats with POI.
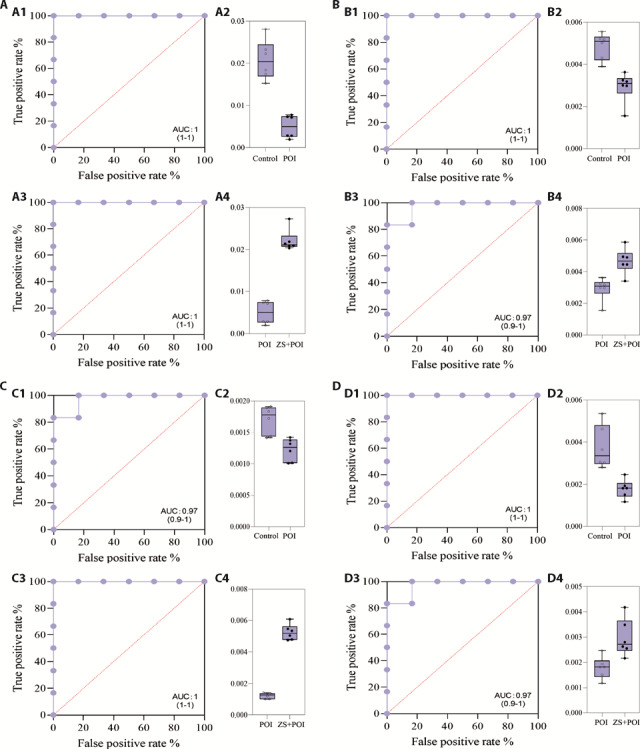
A: divanillyltetrahydrofuran ferulate; B: trans-ferulic acid; C: tryptamine; D: neuraminic acid. Each plot represents the ROC curve and metabolic box plot of the same metabolite in the Control vs POI group (above) and ZS + POI vs POI group (below). A1, B1, C1, D1: ROC curve plot in the Control vs POI group; A2, B2, C2, D2: box plot in the Control vs POI group; A3, B3, C3, D3: ROC curve plot in the ZS+POI vs POI group; A4, B4, C4, D4: box plot in the ZS + POI vs POI group. In the ROC curve plot, the area surrounded by the blue dot solid line and the straight line (x = 1, y = 0) is AUC, the closer the AUC value is to 1, the higher the accuracy of the prediction. In the box plot, the content distribution of metabolites in each group was shown. Control: normal feeding, without any treatment; POI: POI model was induced by intraperitoneal injection of CTX (2 mg/mL) for 15 d; SA + POI: sham acupuncture intervention in CTX-induced POI rats for 4 weeks; ZS + POI: Zhibian (BL54) through Shuidao (ST28) acupuncture intervention in CTX-induced POI rats for 4 weeks. ROC: receiver operating characteristic curve; POI: premature ovarian insufficiency; ZS: Zhibian (BL54) through Shuidao (ST28) acupuncture; AUC: area under roc; CTX: cyclophosphamide.
4. DISCUSSION
POI is mostly classified into the categories of "blood dryness", "amenorrhea", "premature rupture of menstruation", "infertility", and other diseases in ancient Chinese medicine books. Traditional Chinese Medicine believes that POI is a multivisceral dysfunction caused by various factors, such as congenital deficiency, deficiency of Qi and blood, invasion of pathogenic Qi, and emotional disorders. It is based on kidney deficiency, and its main pathological factors are Qi stagnation, phlegm coagulation, and blood stasis2 . Based on the description of "Nine Needles" in Huang Di Nei Jing,17 including "Pricking Deep Pathogen Far Bi", "If the disease is in the middle, take Long Needle" and "Pricking Sacral with Long Needle", Professor Ji Laixi proposed the "Zhibian (BL54) through Shuidao (ST28)" acupuncture method. This acupuncture method is a unique Chinese medicine technique that uses long needles for deep acupuncture. Through the stimulation of "one needle and two points", the meridians and collaterals are transmitted, and Qi is brought to the disease, enhancing the body's immune function and regulating endocrine disorders. It breaks through the limitations of the traditional one-needle-one-point treatment of diseases and has been widely recognized in the clinical treatment of POI. Initially, it was mainly used for the clinical treatment of chronic nonbacterial prostatitis, benign prostatic hyperplasia, impotence, and other male diseases.18 In recent years, it has been gradually extended to clinical application and basic experimental research in urinary system diseases and gynecological reproductive system diseases.
In this study, UPLC-Q-TOF/MS metabolomics technology was used for the first time to screen the serum metabolic marker targets of "Zhibian (BL54) through Shuidao (ST28)" acupuncture intervention in a CTX-induced POI model. By observing the regulation of this acupuncture method on the internal environment of the POI model and from the perspective of the apoptosis mechanism and metabolic disorder, the mechanism of acupuncture on the POI model was discussed, which provided a certain experimental basis for subsequent related animal experiments. Metabolites are the final downstream products of metabolic reactions and more accurately reflect the current metabolic state of organisms than genomics, transcriptomics, or proteomics. Early studies have confirmed the reliability of metabolic changes in evaluating oocyte quality and studying ovarian diseases using metabolic techniques. In this study, we used serum UPLC-Q-TOF/MS nontargeted metabolomics technology combined with multivariate statistical analysis to fully reflect the changes in endogenous metabolites and identified metabolites that changed significantly after CTX-induced POI and acupuncture intervention. Finally, four metabolites, divanillyltetrahydrofuran ferulate, trans-ferulic acid, tryptamine, and neuraminic acid were identified as potential biomarkers for the effect of the "Zhibian (BL54) through Shuidao (ST28)" acupuncture on the recovery of damaged ovarian function in CTX-induced POI rats, providing additional insights for the treatment of POI diseases by this acupuncture technique.
Ferulic acid (FA) is a widely existing phenolic hydroxy-cinnamic acid that has both cis and trans isomers. and the trans isomers is the main isomer in natural compounds.19 FA plays an antioxidant role by scavenging reactive oxygen species and regulating the nuclear factor erythroid2-related factor 2/heme oxygenase-1 axis and other signaling pathways. It can also play an anti-apoptotic or autophagy role by regulating the level of oxidative stress and participate in the regulation of a series of apoptosis or autophagy-related factors mediated by c-Jun N-terminal kinase (JNK) and phosphoinositide 3-kinase.20,⇓-22 Finally, FA can also improve vasodilation and protect the vascular endothelium through extracellular regulated protein kinases1/2 and nitrous oxide/endothelin 1 signaling.23 Divanilylltetrahy-drofuran ferulate is an FA ester derivative. FA reacts with alcohol compounds to form FA ester derivatives, which maintain the antioxidation and antiapoptotic characteristics of FA.24,⇓-26
Tryptamine, also known as TrpN, is a common precursor molecule for many hormones and neurotransmitters, mainly serotonin and melatonin.27 Serotonin, also known as 5-hydroxytryptamine, is an important inhibitory neurotransmitter28 that is widely distributed in the hypothalamus of mammals and is also the main source of estrogen29,30 in the reproductive system of female mammals. It is involved in the regulation of physiological functions, including the secretion and synthesis of reproductive hormones (gonadotropin-releasing hormone and LH),27 the growth and development of follicular cells in ovarian tissue,29 and the production of steroids in follicular fluid.30 Melatonin (MT) is an indoleamine hormone present in mammals. It is produced by the pineal gland and secreted in tissues such as the ovary and placenta.31 Studies have also shown that the ovary can directly synthesize MT.32 MT mediates the reproductive function of the hypothalamic-pituitary-ovarian axis at the gonadal level by regulating gonadotropins33 and has strong antioxidant and anti-apoptotic effects. It can reduce oxidative stress in ovarian follicles by scavenging reactive oxygen species (ROS),34,35 activating antioxidant enzymes such as glutathione pero-xidase, superoxide dismutase, and catalase; and reducing apoptosis by mediating various physiological pathways, thereby protecting oocytes and granulosa cells.36,37
Neuraminic acid, the most common sialic acid, is a key acidic monosaccharide in the early development of mammals. It is present in glycoprotein hormones such as LH and FSH and is involved in regulating the estrous cycle.38 Most sialic acids share the core neuraminic acid Neu structure and are N-acylated with N-acetyl or N-glycoacyl at the C-5 position. Among them, N-acetylneuraminic acid is the main metabolic precursor of animal sialic acid,39 which is widely found in mammalian tissues and regulates innate immune function, protects red blood cells, reduces free radical production, and executes antioxidation.40,41
Serum metabolomics results showed that the levels of divanillyltetrahydrofuran ferulate, trans-ferulic acid, tryptamine, and neuraminic acid were significantly increased, indicating that the "Zhibian (BL54) through Shuidao (ST28)" acupuncture method could cause differential expression of serum metabolites. Its mechanism is mainly manifested in two aspects: on the one hand, serum metabolic markers are involved in regulating levels of reproductive hormone such as FSH, LH, and E2. The changes in levels of serum sex hormones FSH, LH and E2 are important indicators for the clinical diagnosis and evaluation of ovarian quality in POI.42 Estrogen and progesterone secreted by the ovary and gonadotropin (FSH and LH) secreted by the hypothalamus and pituitary are mutual feedback regulatiors that jointly promote the growth, development, and maturation of follicles.43,44 In this experiment, the changes in four serum metabolic markers were negatively correlated with the changes in FSH and LH levels detected by ELISA and positively correlated with the changes in E2 levels, indicating that acupuncture may improve the serum hormone levels of POI rats by regulating the above metabolic markers. On the other hand, serum metabolic markers are involved in improving vasodilation and protecting the vascular endothelium while exerting antioxidant and anti-apoptotic effects. The main pathogenesis of POI is that the apoptosis of ovarian granulosa cells leads to follicular atresia, which leads to the gradual failure of ovarian function.45 Apoptosis based on oxidative stress levels can destroy the important structure of oocytes and granulosa cells in the ovary, and accelerate oocyte aging, and follicular atresia affects ovarian reserve function, which is consistent with the previous experimental results of the research group.46,47 The "Zhibian (BL54) through Shuidao (ST28)" acupuncture method can not only increase vascular permeability by upregulating the content of vascular endothelial growth factors in serum48 but also promote the angiogenesis and the blood circulation supply of the ovary and surrounding tissues.49 It may also inhibit the expression of the JNK-mediated apoptosis pathway-related factors under oxidative stress by downregulating ROS levels in ovarian tissue,50 slowing down the process of follicular atresia, and improving ovarian function in POI model rats. The main pathogenesis of POI is follicular atresia caused by apoptosis of ovarian granulosa cells, which leads to the gradual failure of ovarian function, indicating that acupuncture may participate in the blood circulation and apoptosis factor expression of ovarian tissue by regulating metabolic markers.
In summary, we analyzed the endogenous metabolic changes in rat serum by UPLC-Q-TOF/MS-based metabolomics technology and comprehensively analyzed the biological mechanism of four serum metabolic markers divanillyltetrahydrofuran ferulate, trans-ferulic acid, tryptamine, and neuraminic acid. These four marker may be the target of "Zhibian (BL54) through Shuidao (ST28)" acupuncture in improving the ovarian reserve function of POI rats. Although this study provides new insights, the significance of these individual metabolites in the pathophysiology of POI remains to be further studied.
5. SUPPORTING INFORMATION
Supporting data to this article can be found online at http://journaltcm.cn.
Footnotes
Supported by the Research Grant from the Natural Science Research Program of Shanxi Province: Based on Ultra-Performance Liquid Chromatography-Mass Spectrometry, the effect of "Zhibian (BL54) through Shuidao (ST28)" Needle Method on Ovarian Metabolism and Apoptosis Pathways in rats with Premature Ovarian Insufficiency was Discussed (No. 2022030211216)
References
- 1. Wesevich V, Kellen AN, Pal L. Recent advances in understanding primary ovarian insufficiency. F1000Res 2020; 9: F1000 Faculty Rev-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Meirow D, Biederman H, Anderson RA, Wallace WH. Toxicity of chemotherapy and radiation on female reproduction. Clin Obstet Gynecol 2010; 53: 727-39. [DOI] [PubMed] [Google Scholar]
- 3. Oktem O, Oktay K. Quantitative assessment of the impact of chemotherapy on ovarian follicle reserve and stromal function. Cancer 2007; 110: 2222-9. [DOI] [PubMed] [Google Scholar]
- 4. Spears N, Lopes F, Stefansdottir A, et al. Ovarian damage from chemotherapy and current approaches to its protection. Hum Reprod Update 2019; 25: 673-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xu H, Bao X, Kong H, Yang J, Li Y, Sun Z. Melatonin protects against cyclophosphamide-induced premature ovarian failure in rats. Hum Exp Toxicol 2022; 41: 9603271221127430. [DOI] [PubMed] [Google Scholar]
- 6. Emadi A, Jones RJ, Brodsky RA. Cyclophosphamide and cancer: golden anniversary. Nat Rev Clin Oncol 2009; 6: 638-47. [DOI] [PubMed] [Google Scholar]
- 7. Chen SL, Zhou XY. Clinical research advances on premature ovarian insufficiency. Shandong Da Xue Xue Bao (Yi Xue Ban) 2018; 56: 1-7. [Google Scholar]
- 8. Torrealday S, Kodaman P, Pal L. Premature ovarian insufficiency-an update on recent advances in understanding and management. F1000Res 2017; 6: 2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nicholson JK, Lindon JC. Systems biology: metabonomics. Nature 2008; 455: 1054-6. [DOI] [PubMed] [Google Scholar]
- 10. Nicholson JK, Lindon JC, Holmes E. 'Metabonomics': understanding the metabolic responses of living systems to patho-physiological stimuli via multivariate statistical analysis of biolo-gical NMR spectroscopic data. Xenobiotica 1999; 29: 1181-9. [DOI] [PubMed] [Google Scholar]
- 11. Zhang Y, Zhang A, Yan G, et al. High-throughput metabolomic approach revealed the acupuncture exerting intervention effects by perturbed signatures and pathways. Mol Biosyst 2014; 10: 65-73. [DOI] [PubMed] [Google Scholar]
- 12. Zhang HL, Si YM. Significance and practice exploration of Traditional Chinese Medicine syndrome research based on metabolomics. Zhong Hua Zhong Yi Yao Xue Kan 2021; 39: 21-4. [Google Scholar]
- 13. Fu XF, He YL. Establishment of ratmodel of chemotherapy-induced premature ovarian failure. Guangdong Yi Xue 2008: 1952-4. [Google Scholar]
- 14. China Association for Acupuncture and Moxibustion. Name and location of commonly used acupoints in experimental animals part 2: rats. Zhen Ci Yan Jiu 2021; 46: 351-2. [Google Scholar]
- 15. Burke AW, Broadhurst PL. Behavioural correlates of the oestrous cycle in the rat. Nature 1966; 209: 223-4. [DOI] [PubMed] [Google Scholar]
- 16. Scandroglio R. Sugli aspetti dell'epitelio della bagina del ratto nel corso del ciclo estrale. Ricerche istologiche ed istochimiche [On aspects of the epithelium of the rat vagina during the estrus cycle. Histological and histochemical studies]. Atti Accad Fisiocrit Siena Med Fis 1967; 16: 629-47. [PubMed] [Google Scholar]
- 17. Tian DH, Liu GS. Ling Shu Jing. Beijing: People's Medical Publishing House, 2021: 1- 4+22. [Google Scholar]
- 18. Hao CY, Zhang TS, Jin XF, Ji LX. Clinical application and expansion of 'Zhibian (BL54) through Shuidao (ST28)' acupuncture needling system. Zhong Guo Zhong Yi Ji Chu Yi Xue Za Zhi 2015; 21: 1433-4. [Google Scholar]
- 19. Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 1996; 20: 933-56. [DOI] [PubMed] [Google Scholar]
- 20. Gupta A, Singh AK, Loka M, Pandey AK, Bishayee A. Ferulic acid-mediated modulation of apoptotic signaling pathways in cancer. Adv Protein Chem Struct Biol 2021; 125: 215-57. [DOI] [PubMed] [Google Scholar]
- 21. Haddad JJ. Redox and oxidant-mediated regulation of apoptosis signaling pathways: immuno-pharmaco-redox conception of oxidative siege versus cell death commitment. Int Immunopharmacol 2004; 4: 475-93. [DOI] [PubMed] [Google Scholar]
- 22. Roy S, Metya SK, Rahaman N, Sannigrahi S, Ahmed F. Ferulic scid in the treatment of post-diabetes testicular damage: relevance to the down regulation of apoptosis correlates with antioxidant status via modulation of TGF-β1, IL-1β and Akt signalling. Cell Biochem Funct 2014; 32: 115-24. [DOI] [PubMed] [Google Scholar]
- 23. Li D, Rui YX, Guo SD, Luan F, Liu R, Zeng N. Ferulic acid: a review of its pharmacology, pharmacokinetics and derivatives. Life Sci 2021; 284: 119921. [DOI] [PubMed] [Google Scholar]
- 24. Cione E, Tucci P, Senatore V, et al. Synthesized esters of ferulic acid induce release of cytochrome c from rat testes mitochondria. J Bioenerg Biomembr 2008; 40: 19-26. [DOI] [PubMed] [Google Scholar]
- 25. Lin QL, Wen QB, Ou SY, Wu LY, Lai FR. Scavenging of in vitro hydroxyl free radical using feruloyl-arabinose. Hua Nan Li Gong Da Xue Xue Bao (Zi Ran Ke Xue Ban) 2010; 38: 110-4. [Google Scholar]
- 26. Zhang X, Gao ZP. Research progress in ferulic acid. Zhong Guo Xian Dai Zhong Yao 2020; 22: 138-47. [Google Scholar]
- 27. Ayala ME. Brain serotonin, psychoactive drugs, and effects on reproduction. Cent Nerv Syst Agents Med Chem 2009; 9: 258-76. [DOI] [PubMed] [Google Scholar]
- 28. Li SQ, Yang B, Xu CT. The research status for neurotransmitter 5-hydroxytryptamine. Lin Chuang Yi Xue Gong Cheng 2010; 17: 145-7. [Google Scholar]
- 29. Nikishin DA, Alyoshina NM, Shmukler YB. Synthesis and membrane transport of serotonin in the developing ovarian follicle of mouse. Dokl Biochem Biophys 2018; 478: 4-7. [DOI] [PubMed] [Google Scholar]
- 30. Tanaka E, Baba N, Toshida K, Suzuki K. Serotonin stimulates steroidogenesis in rat preovulatory follicles: involvement of 5-HT2 receptor. Life Sci 1993; 53: 563-70. [DOI] [PubMed] [Google Scholar]
- 31. Cruz MH, Leal CL, da Cruz JF, Tan DX, Reiter RJ. Role of melatonin on production and preservation of gametes and embryos: a brief review. Anim Reprod Sci 2014; 145: 150-60. [DOI] [PubMed] [Google Scholar]
- 32. Ezzati M, Velaei K, Kheirjou R. Melatonin and its mechanism of action in the female reproductive system and related malignancies. Mol Cell Biochem 2021; 476: 3177-90. [DOI] [PubMed] [Google Scholar]
- 33. Tamura H, Takasaki A, Taketani T, et al. The role of melatonin as an antioxidant in the follicle. J Ovarian Res 2012; 5: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Keshavarzi S, Salehi M, Farifteh-Nobijari F, et al. Melatonin modifies histone acetylation during in vitro maturation of mouse oocytes. Cell J 2018; 20: 244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kilic U, Kilic E, Reiter RJ, Bassetti CL, Hermann DM. Signal transduction pathways involved in melatonin-induced neuroprotection after focal cerebral ischemia in mice. J Pineal Res 2005; 38: 67-71. [DOI] [PubMed] [Google Scholar]
- 36. Barberino RS, Lins TLBG, Monte APO, et al. Melatonin attenuates cyclophosphamide-induced primordial follicle loss by interaction with MT1 receptor and modulation of PTEN/Akt/FOXO3a proteins in the mouse ovary. Reprod Sci 2022; 29: 2505-14. [DOI] [PubMed] [Google Scholar]
- 37. Tang J, Chen R, Wang L, et al. Melatonin attenuates thrombin-induced inflammation in BV2 cells and then protects HT22 cells from apoptosis. Inflammation 2020; 43: 1959-70. [DOI] [PubMed] [Google Scholar]
- 38. Varki A. Sialic acids in human health and disease. Trends Mol Med 2008; 14: 351-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lewis AL, Chen X, Schnaar RL, Varki A. Sialic acids and other nonulosonic acids. In: Varki A, Cummings RD, Esko JD, et al., eds. Essentials of glycobiology. 4th ed. New York: Cold Spring Harbor Laboratory Press, 2022: 185- 204. [Google Scholar]
- 40. Bhide GP, Colley KJ. Sialylation of N-glycans: mechanism, cellular compartmentalization and function. Histochem Cell Biol 2017; 147: 149-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhou X, Yang G, Guan F. Biological functions and analytical strategies of sialic acids in tumor. Cells 2020; 9: 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang X, Chen ZJ. A decade of discovery: the stunning progress of premature ovarian insufficiency research in China. Biol Reprod 2022; 107: 27-39. [DOI] [PubMed] [Google Scholar]
- 43. Shoham Z, Schachter M. Estrogen biosynthesis-regulation, action, remote effects, and value of monitoring in ovarian stimulation cycles. Fertil Steril 1996; 65: 687-701. [DOI] [PubMed] [Google Scholar]
- 44. Liu DE. Gynecological sex hormone regulation mechanism. Zhong Guo Shi Yong Fu Ke Yu Chan Ke Za Zhi 2000: 18-20. [Google Scholar]
- 45. Monniaux D, Huet C, Pisselet C, Mandon-Pépin B, Monget P. Mechanism, regulation, and manipulations of follicular atresia. Contracept Fertil Sex 1998; 26: 528-35. [PubMed] [Google Scholar]
- 46. Waetzig V, Herdegen T. Context-specific inhibition of JNKs: overcoming the dilemma of protection and damage. Trends Pharmacol Sci 2005; 26: 455-61. [DOI] [PubMed] [Google Scholar]
- 47. Bode AM, Dong ZG. The functional contrariety of JNK. Mol Carcinog 2007; 46: 591-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jin XF, Yan J, Zhao JY, Yin LY, Ma MN, Wang HY. Effect of penetrative needling of "Zhibian" (BL54) through "Shuidao" (ST28) on expression of TRAIL and its receptors in rats with premature ovrian insufficiency. Zhen Ci Yan Jiu 2023; 48: 259-66+80. [DOI] [PubMed] [Google Scholar]
- 49. Malamitsi-puchner A, Sarandakou A, Tziotis J, Stavreus-Evers A, Tzonou A, Landgren BM. Circulating angiogenic factors during periovulation and the luteal phase of normal menstrual cycles. Fertil Steril 2004; 81: 1322-7. [DOI] [PubMed] [Google Scholar]
- 50. Yan J, Zhao JY, Yin LY, Yan XQ, Jin XF. Effect of acupuncture at "Zhibian" (BL54) through "Shuidao" (ST28) on the expression of apoptosis-related factors in rats with premature ovarian insufficieney based on oxidative stress. Zhong Guo Zhen Jiu 2023; 43: 454-60. [DOI] [PubMed] [Google Scholar]


