Abstract
We have isolated a mouse cDNA for a novel dual-specificity phosphatase designated LDP-3 (low-molecular-mass dual-specificity phosphatase 3). The 450 bp open reading frame encodes a protein of 150 amino acids with a predicted molecular mass of 16 kDa. Northern blot and reverse transcription–PCR analyses show that LDP-3 transcripts are expressed in almost all mouse tissues examined. In vitro analyses using several substrates and inhibitors indicate that LDP-3 possesses intrinsic dual-specificity phosphatase activity. When expressed in mammalian cells, LDP-3 protein is localized mainly to the apical submembrane area. Forced expression of LDP-3 does not alter activation of ERK (extracellular-signal-regulated kinase), but rather enhances activation of JNK (c-Jun N-terminal kinase) and p38 and their respective upstream kinases MKK4 (mitogen-activated protein kinase kinase 4) and MKK6 in cells treated with 0.4 M sorbitol. By screening with a variety of stimuli, we found that LDP-3 specifically enhances the osmotic stress-induced activation of JNK and p38.
Keywords: dual-specificity phosphatase, c-Jun N-terminal kinase (JNK), mitogen-activated protein kinase (MAPK), osmotic stress, p38
Abbreviations: ATF, activating transcription factor; DSP, dual-specificity phosphatase; EGF, epidermal growth factor; ERK, extracellular-signal-regulated kinase; GST, glutathione S-transferase; HA, haemagglutinin; JNK, c-Jun N-terminal kinase; JKAP, JNK pathway-associated phosphatase; JSP, JNK stimulatory phosphatase; LDP, low-molecular-mass dual-specificity phosphatase; LMW-DSP, low-molecular-weight DSP; MAPK, mitogen-activated protein kinase; MAPKK, MAPK kinase; MBP, myelin basic protein; MEK, MAPK/ERK kinase; MKK4 (etc.), MAPK kinase 4 (etc.); MKP, MAPK phosphatase; pNPP, p-nitrophenyl phosphate; PTP, protein tyrosine phosphatase; SKRP, stress-activated protein kinase pathway-regulating phosphatase; TNF, tumour necrosis factor; VHR, VH1-related; VHX, VHR-related MKPX
INTRODUCTION
Reversible phosphorylation of tyrosine residues in proteins plays a critical role in regulating cellular responses such as growth, differentiation, metabolism, migration and malignant transformation [1]. Protein tyrosine phosphorylation is controlled through the co-ordinate actions of protein tyrosine kinases and PTPs (protein tyrosine phosphatases). The PTP family can be subdivided into two broad categories: (i) classical phosphotyrosine-specific enzymes, and (ii) DSPs (dual-specificity phosphatases), which dephosphorylate Ser/Thr as well as Tyr residues. The DSPs, which preserve the general catalytic mechanism of classical PTPs but display differences in the architecture of the active site, are thought to be involved in important signalling events, ranging from the control of MAPKs (mitogen-activated protein kinases) in cell proliferation to the regulation of cyclin-dependent kinases in the cell cycle. The DSP family includes: (i) MKPs (MAPK phosphatases), which negatively regulate MAPKs, (ii) members of the CDC25 family, which are positive regulators of G1/S and G2/M cell cycle transitions [2], (iii) members of the CDC14 family, which are indispensable for exit from mitosis [3], and (iv) other small DSPs whose function is currently unknown.
Activation of MAPK cascades plays a key role in transducing various extracellular signals to the nucleus. Three distinct MAPK families comprise ERK (extracellular signal-regulated kinase), JNK (c-Jun N-terminal kinase) and p38. The ERK pathway is activated by growth and differentiation factors, cell adhesion, phorbol esters and some oncogenes. The JNK and p38 pathways are activated by pro-inflammatory cytokines and a variety of environmental stresses, such as heat shock, UV light, irradiation, and osmotic and oxidative stresses. For full activation of these MAPKs, phosphorylation of both threonine and tyrosine residues in TXY (Thr-Xaa-Tyr) motifs found in the activation loop is required by dual-specificity kinases, which are members of the MKK (MAPK kinase) family. MEK1 (MAPK/ERK kinase 1) and MEK2 are specific kinases for ERK, MKK4 and MKK7 are specific for JNK, and MKK3 and MKK6 are kinases for p38 [4].
Negative regulation of MAPKs is achieved by dephosphorylation of the TXY motif by phosphatases, such as MKPs. MKPs are composed primarily of two domains, the DSP catalytic domain and the rhodanese domain, which is responsible for substrate binding and enables MKPs to specifically target individual MAPKs [5]. It has been shown that small DSPs also negatively regulate MAPK activation. For example, VHR (VH1-related) is a small DSP that binds to and dephosphorylates ERK [6,7]. In intact T lymphocytes, JNK as well as ERK are reported to be VHR substrates [8]. We have shown subsequently that VHR dephosphorylates p38 as well as ERK and JNK [9]. LDP-2 (low-molecular-mass dual-specificity phosphatase-2)/SKRP1 (stress-activated protein kinase pathway-regulating phosphatase 1) was identified as a negative regulator of JNK [9,10], and LMW-DSP2 (‘low-molecular-weight DSP2’) was shown to regulate JNK and p38 [11]. We recently characterized the interaction of MKP-7 with MAPKs and demonstrated that there are two MAPK-docking domains in MKP-7 [12]. One is in the rhodanese domain, as reported previously [13], and the other is in the DSP catalytic domain [12]. The latter is characterized by a conserved LxL motif followed by a cluster of hydrophobic residues. Of interest is that the latter MAPK-docking domain is also found in small DSPs [12]. These results strongly suggest that not only MKPs, but also small DSPs, are important regulators of MAPKs.
In the present study, we have isolated and characterized a novel small DSP, named LDP-3. LDP-3 exhibits DSP activity in vitro. However, LDP-3 enhances the activation of JNK and p38 stimulated by several osmotic stresses induced by sorbitol, mannitol, glucose or glycine. We also found that activation of MKK4 and MKK6, which are upstream kinases of JNK and p38 respectively, was enhanced by LDP-3. These data suggested that LDP-3 functions as an enhancer of osmotic stress.
MATERIALS AND METHODS
Isolation and determination of the nucleotide sequences of novel DSP cDNAs
Using nucleotide sequences conserved in DSPs, we screened the expressed-sequence-tag database, dbEST, to identify novel DSPs. A human cDNA clone (accession no. AA159215) and a mouse cDNA clone (accession no. AA048085) were identified encoding the catalytic site motif VXVHCXXGXXRXXTXXXXYL, which is conserved in the DSP family. These clones exhibit fairly high sequence similarity to each other. Both clones were obtained from Research Genetics, Inc. (Huntsville, AL, U.S.A.), and their nucleotide sequences were determined.
Bacterial expression of recombinant proteins
For bacterial expression of LDP-3 as a GST (glutathione S-transferase)-fusion protein, PCR was performed using the primers 5′-ATGAATTCGATGGGCGTGCAGCCCCCCAAC-3′ (primer 1; underlining indicates the EcoRI site) and 5′-ATGTCGACTGGTAGAAGGGTACTAAGG-3′ (primer 2; underlining indicates the SalI site). The PCR product was digested with EcoRI and SalI and subcloned into the corresponding sites of pGEX-6P-3 (Amersham Biosciences, Piscataway, NJ, U.S.A.). Expression and purification of the GST-fusion protein was performed as described previously [14].
Mammalian expression vectors
The mammalian expression vectors pSRα-HA-ERK2, pSRα-HAJNK1, pMT3-HA-p38α, pFLAG-VHR, pFLAG-MKP-2 and pFLAG-MKP-5 have been described previously [15]. To construct pHA-MKK4KR and pHA-MKK6KA, cDNAs of MKK4(K129R) and MKK6(K82A) respectively were inserted in-frame into pcDNA3HA, which contains a triple HA (haemagglutinin) tag. To construct pFLAG-LDP-3, pFLAG-LDP-3CS, and pFLAG-LDP-3DA, cDNAs of LDP-3, LDP-3(C95S) and LDP-3(D65A) respectively were inserted in-frame into the pFLAG-CMV2 vector (Sigma, Bucks, Switzerland). In vitro mutagenesis was performed using a standard PCR-based method, and all PCR-amplified cDNA fragments were confirmed by sequencing.
Northern blot analysis
Total RNA from various mouse tissues was isolated by acid guanidinium thiocyanate extraction [16]. Poly(A)+ RNA was purified from total RNA using an Oligotex™ dT30 (Super) mRNA purification kit (TaKaRa Bio Inc., Shiga, Japan). A portion of 3 μg of poly(A)+ RNA was fractionated on a formaldehyde/1% (w/v)agarose gel and transferred to nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany). Hybridization with a 32P-labelled fragment of the coding region from mouse LDP-3 cDNA and mouse β-actin cDNA [14] was performed at 42 °C in the presence of 50% (v/v) formamide, 0.65 M NaCl, 5 mM EDTA, 1×Denhardt's solution, 10% (w/v) dextran sulphate, 0.1 M Pipes, pH 6.8, 0.1% SDS and 100 μg/ml denatured salmon sperm DNA. The membranes were washed twice with 2×SSC (1×SSC is 0.15 M NaCl/0.015 M sodium citrate) containing 0.1% SDS at room temperature for 5 min, and then washed twice with 0.1×SSC containing 0.1% SDS at 50 °C for 15 min each. Signals on the filters were then visualized using a bioimaging analyser FLA-3000G (Fuji Photo Film Co. Ltd, Tokyo, Japan).
PCR analysis
cDNA was synthesized utilizing an anchored oligo(dT) primer using SuperScript II (Invitrogen Corp., Carlsbad, CA, U.S.A.). PCR amplification of LDP-3 was performed using the primers 5′-TTGTCCAATAGGAGTCAGAGGG-3′ and 5′-GCAGAGTAGGAAAGGCCAGCC-3′. As a control, PCR amplification of β-actin was performed as described previously [17].
Phosphatase assay
Preparation of the substrate was performed as described previously [14]. A phosphatase assay using pNPP (p-nitrophenyl phosphate) as substrate was performed in 200 μl of assay buffer containing 100 mM sodium acetate, pH 5.0, 1.6 mM dithiothreitol, 10 mM pNPP and enzyme at 30 °C for 10 min. Reactions were terminated by the addition of 500 μl of 0.5 M NaOH, and the absorbance at 410 nm was measured. An assay using phosphorylated MBP (myelin basic protein) was performed in 30 μl of assay buffer containing 50 mM imidazole, pH 7.5, 0.1% 2-mercaptoethanol, GST–LDP-3 and 15 μl of either Ser/Thr(P)-MBP or Tyr(P)-MBP at 30 °C for 10 min. Reactions were terminated and released 32P was measured as previously described [14].
Cell culture and transient transfection
COS-7 cells were maintained in Dulbecco's modified Eagle's medium containing 10% (v/v) fetal bovine serum at 37 °C under 5% CO2. Cells were co-transfected with pFLAG-LDP-3, pFLAG-LDP-3CS, pFLAG-LDP-3DA, pFLAG-VHR, pFLAG-MKP-2 or pFLAG-MKP-5 together with pSRα-HA-ERK2, pSRα-HA-JNK1, pMT3-HA-p38α, pcDNA-HA-MKK4KR or pcDNAHA-MKK6KA using Fugene-6 (Roche Diagnostics, Mannheim, Germany). For ERK activation, at 18 h after transfection cells were maintained without serum for 18 h and then stimulated with 50 ng/ml EGF (epidermal growth factor) for 15 min or 0.4 M sorbitol for 30 min. For activation of JNK and p38, cells were stimulated 36 h after transfection with one of the following: 0.4 M sorbitol for 30 min, 50 μM H2O2 for 30 min, 25 J/m2 UV-C, heat shock at 42 °C for 30 min, 100 ng/ml TNF-α (tumour necrosis factor-α) for 30 min, 0.4 M mannitol for 30 min, 0.4 M glucose for 30 min or 0.4 M glycine for 30 min.
Detection of expressed proteins
Transfected cells were lysed in MAPK lysis buffer, as described previously [9]. Cell lysates were centrifuged at 20000 g for 10 min, and the resulting supernatants were used as cell extracts. Each sample was separated by SDS/PAGE and then transferred to a nitrocellulose membrane. The phosphorylation status of HA–MAPKs and HA–MAPKKs (MAPK kinases) was monitored with anti-ACTIVE JNK (Promega, Madison, WI, U.S.A.), antiphospho-p38 (Cell Signaling Technology, Beverly, MA, U.S.A.) or anti-phospho-ERK (Cell Signaling Technology) antibody, and anti-phospho-MKK4 (Cell Signaling Technology) or antiphospho-MKK3/6 (Cell Signaling Technology) antibody, followed by horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.). The expression levels of HA-tagged kinases and FLAG-tagged phosphatases were monitored with anti-HA monoclonal antibody (12CA5; Roche Diagnostics) and anti-FLAG M2 monoclonal antibody (Sigma) respectively. Signals were detected by enhanced chemiluminescence using the ECL® reagent (Amersham Biosciences).
In vitro kinase assay
To assess the kinase activity of epitope-tagged JNK and p38, in vitro kinase assays were performed as described previously [18].
Cell staining
COS-7 cells were cultured on coverslips treated with VITROGEN 100 (COHESION, Palo Alto, CA, U.S.A.) diluted 30-fold. Cells transfected with FLAG–LDP-3 and cultured for 36 h were fixed in PBS containing 3.7% (v/v) formaldehyde for 10 min, permeabilized with PBS containing 0.25% Triton X-100 for 10 min, and then washed three times with PBS. After blocking with 3% (w/v) BSA in PBS for 2 h at room temperature, cells were incubated with anti-FLAG M2 antibody (Sigma) in PBS containing 3% BSA overnight at 4 °C and washed three times with PBS, followed by incubation with Alexa488-conjugated anti-rabbit IgG secondary antibody (Chemicon) in PBS containing 3% BSA for 30 min at 37 °C. After three washes with PBS, coverslips were mounted with 90% (v/v) glycerol in PBS. For staining of nuclei, transfected cells were treated with 500 ng/ml RNase A for 30 min after permeabilization, followed by staining with 500 ng/ml propidium iodide for 30 min at 37 °C. Fluorescence was visualized under a fluorescence confocal microscope (Olympus). Sequential slices were taken at 0.2 μm depth increments along the optical Z-axis. Three-dimensional images were reconstructed with X–Y stacks of images.
RESULTS
Identification and characterization of a novel DSP, LDP-3
We searched the human dbEST database (National Center for Biotechnology Information EST database) with a conserved nucleotide sequence derived from the DSP active-site domain and identified one clone, AA159215, which contains sequences encoding a novel DSP. This clone was sequenced and contained a 450 bp open reading frame with an in-frame termination codon upstream of the initiation ATG. This cDNA encoded a small peptide containing the catalytic-site motif VXVHCXXGXXRXXTXXXXYL, which is conserved in the DSP family. Since previously isolated small DSPs were designated LDP-1 and LDP-2, we named this novel DSP LDP-3. The mouse LDP-3 orthologue was identified after searching the mouse dbEST database. The amino acid sequences of human and mouse LDP-3 are shown in Figure 1(A). Comparison of these sequences shows an amino acid identity of 95%, with seven of 150 amino acids differing. The region between residues 69 and 114 contains the catalytic motif and is completely conserved between mouse and human (Figure 1A). Recently we reported that there are two MAPK-docking domains in MKPs, one in the rhodanese domain and the other in the catalytic domain [12]. The latter has a conserved LxL motif between Pro and Gly, followed by a hydrophobic domain. The sequences of the catalytic domains of small DSPs and those of MKPs were compared (Figure 1B), and the LxL motif was present in LDP-1, LDP-2, VHR and JSP-1 (JNK stimulatory phosphatase-1). By contrast, LDP-3 does not exhibit the corresponding LxL motif, although Pro and Gly residues following the hydrophobic region are present. Such structural differences suggest that LDP-3 has a unique function in the MAPK pathway.
Figure 1. Predicted amino acid sequences of human and mouse LDP-3.
(A) Alignment of amino acid sequences of mouse LDP-3 (mLDP-3) and human LDP-3 (hLDP-3). (B) Alignment of the amino acid sequence of human LDP-3 (LDP-3) with those of other DSPs. Conserved Asp, Cys and Arg residues in the catalytic centre are boxed. Asterisks denote amino acid identity and dots denote amino acid similarity.
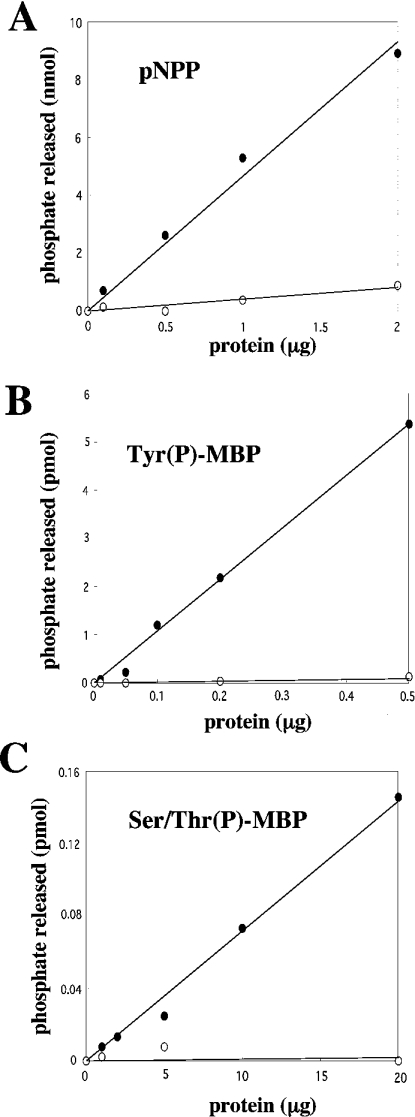
To determine if LDP-3 possesses phosphatase activity, recombinant LDP-3 was produced as a fusion protein with GST in bacteria and purified. GST–LDP-3 was utilized in a phosphatase assay using pNPP as a substrate (Figure 2A). Recombinant LDP-3 dephosphorylated pNPP with a specific activity of 5.3 nmol/μg, depending on the amount of enzyme added. GST alone did not dephosphorylate pNPP (results not shown). Sodium vanadate (1 mM), a specific inhibitor of PTPs, completely inhibited the phosphatase actvity of LDP-3 towards pNPP. Recombinant LDP-3 dephosphorylated both Tyr(P)-MBP and Ser/Thr(P)-MBP, with specific activities of 11 pmol/μg and 0.0073 pmol/μg respectively. Sodium vanadate (1 mM) inhibited LDP-3 activity towards both Tyr(P)-MBP and Ser/Thr(P)-MBP (Figures 2B and 2C), while 1 μM okadaic acid or 40 μM Microcystin LR, an inhibitor of protein phosphatases 2A and 1, did not (results not shown). These data indicate that LDP-3 exhibits the biochemical activity of a DSP.
Figure 2. Phosphatase activity of recombinant LDP-3.
The phosphatase activity of GST-LDP-3 in the presence (○) or absence (•) of 1 mM vanadate was determined. Shown is the dose-dependent dephosphorylation of 10 mM pNPP (A), 15 μM Tyr(P)-MBP (B) and 15 μM Ser/Thr(P)-MBP (C) by increasing amounts GST–LDP-3. Data shown are the means from duplicate experiments.
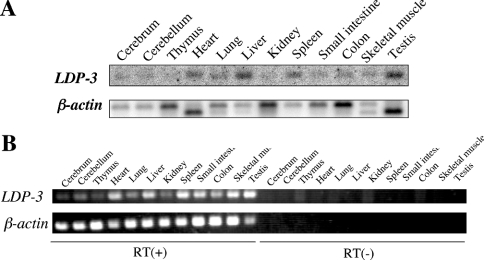
The expression of LDP-3 mRNA in various tissues from adult mouse (5 weeks postnatal) was determined by Northern blot. As shown in Figure 3(A), a 1.6 kb transcript was detected in heart, liver, spleen and testis. Since expression of LDP-3 mRNA in other organs appeared low, its expression was further examined by reverse transcription–PCR. LDP-3 transcripts were amplified not only in heart, liver, spleen and testis, but also in other organs examined (Figure 3B). Therefore we conclude that LDP-3 is expressed in most tissues, but is more abundant in heart, liver, spleen and testis.
Figure 3. Expression of LDP-3 mRNA in mouse tissues.
(A) Poly(A)+ RNAs (3 μg) obtained from several mouse tissues were separated on a 1% (w/v) agarose gel, transferred to a nitrocellulose membrane, and hybridized with a 32P-labelled mouse LDP-3 cDNA. The membrane was reprobed with 32P-labelled β-actin cDNA. (B) Reverse transcription (RT)–PCR amplification was carried out using specific primer sets for LDP-3 and β-actin.
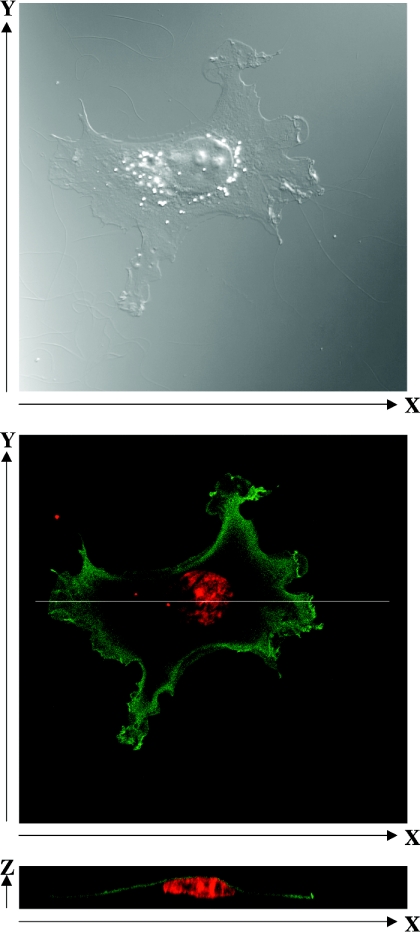
To determine the subcellular localization of LDP-3, COS-7 cells were transiently transfected with pFLAG-LDP-3 and stained using an anti-FLAG antibody. Examination of single cells (Figure 4, top) showed that FLAG–LDP-3 staining was localized to the cytoplasm but not to the nucleus or perinuclear area (Figure 4, middle). X–Z cross-sections indicated that FLAG–LDP-3 was distributed in an apical submembrane area rather than throughout the cytoplasm (Figure 4, bottom).
Figure 4. Subcellular localization of FLAG–LDP-3.
COS-7 cells were transfected with 1.5 μg of pFLAG-LDP-3 and cultured for 36 h. Cells were stained with anti-FLAG M2 antibody, followed by Alexa488-conjugated anti-mouse IgG and propidium iodide. The top panel shows a differential interference contrast image. The middle panel shows confocal microscopy of FLAG–LDP-3 stained with anti-FLAG M2 antibody, followed by Alexa488-conjugated anti-mouse IgG and propidium iodide. The bottom panel shows an X–Z cross-section.
LDP-3 enhances the activation of JNK and p38 in COS-7 cells
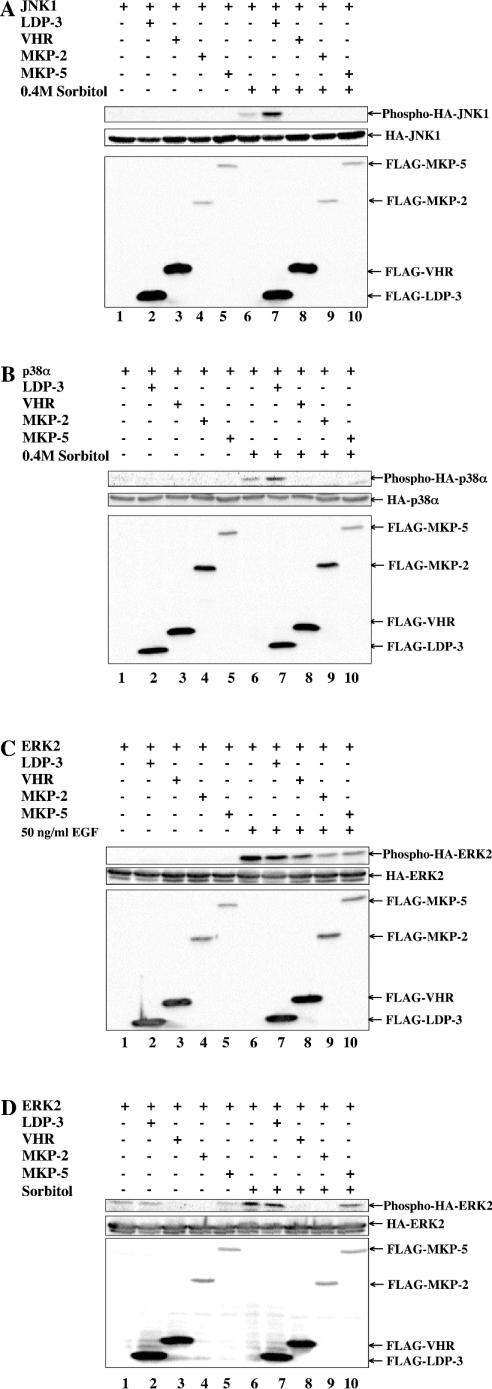
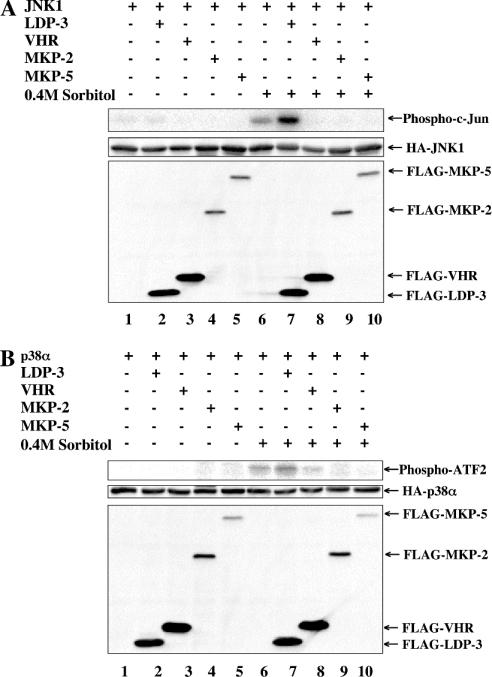
We next asked whether LDP-3 has phosphatase activity towards MAPKs. To address this question, we determined the effects of FLAG–LDP-3 on the activation of HA–JNK1 by sorbitol (Figure 5A), on HA–p38α activation by sorbitol (Figure 5B), on HA–ERK2 activation by EGF (Figure 5C) and on HA–ERK2 activation by sorbitol (Figure 5D). Co-transfection of pFLAG-VHR, pFLAG-MKP-2 and pFLAG-MKP-5 decreased the phosphorylation levels of HA–MAPKs (Figures 5A–5D, lanes 8–10), as has been reported [19,20]. By contrast, under the same conditions co-transfection of pFLAG-LDP-3 enhanced the levels of phosphorylation of HA–JNK1 and HA–p38α induced by sorbitol (Figures 5A and 5B, lane 7), but did not affect the phosphorylation level of HA–ERK2 following activation by EGF or sorbitol (Figures 5C and 5D, lane 7). Overexpression of FLAG–LDP-3 alone did not induce the phosphorylation of JNK and p38 (Figures 5A and 5B, lane 2). These observations suggest that LDP-3 selectively up-regulates levels of phosphorylation of JNK and p38 induced by sorbitol.
Figure 5. Phosphorylation of JNK and p38 upon sorbitol treatment is enhanced by LDP-3.
COS-7 cells were co-transfected with 0.5 μg of pSRα-HA-JNK1 (A), pMT3-HA-p38α (B) or pSRα-HA-ERK2 (C and D) together with 1.5 μg of pFLAG-CMV, pFLAG-LDP-3, pFLAG-VHR, pFLAG-MKP-2 or pFLAG-MKP-5. After 36 h, transfected cells were stimulated with 0.4 M sorbitol for 30 min (A, B and D) or 50 ng/ml EGF for 15 min (C). Phosphorylated HA–JNK1, HA–p38α and HA–ERK2 were detected by anti-ACTIVE JNK, anti-phospho-p38α and anti-phospho-ERK antibodies respectively. The expression levels of HA–MAPKs (HA–JNK1, HA–p38α and HA–ERK2) and FLAG-tagged phosphatases (FLAG–LDP-3, FLAG–VHR, FLAG–MKP-2 and FLAG–MKP-5) were assessed by immunoblot using anti-HA (12CA5) and anti-FLAG M2 antibodies respectively.
We next examined the effects of LDP-3 on JNK and p38 activities using an in vitro kinase assay. Enzyme activities of JNK and p38 towards c-Jun and ATF2 (activating transcription factor 2) were not detected in the absence of sorbitol, but were apparent following sorbitol treatment (Figures 6A and 6B, lane 6). Whereas VHR, MKP-2 and MKP-5 suppressed the activities of JNK and p38, LDP-3 up-regulated the activities of both (Figures 6A and 6B, lane 8). These results strongly suggest that LDP-3 selectively enhances the phosphorylation and activation of JNK and p38 following activation by sorbitol.
Figure 6. Activation of JNK and p38 upon sorbitol treatment is enhanced by LDP-3.
COS-7 cells were co-transfected with 0.5 μg of pSRα-HA-JNK1 (A) or pMT3-HA-p38α (B) together with 1.5 μg of pFLAG-CMV, pFLAG-LDP-3, pFLAG-VHR, pFLAG-MKP-2 or pFLAG-MKP-5. After 36 h, transfected cells were stimulated with 0.4 M sorbitol for 30 min. The kinase activity of HA–JNK1 and HA–p38α was determined by an in vitro kinase assay using GST–c-Jun (A) and GST–ATF2 (B) respectively as substrates. The expression levels of HA–MAPKs (HA–ERK2, HA–JNK1 and HA–p38α) and FLAG-tagged phosphatases (FLAG–LDP-3, FLAG–VHR, FLAG–MKP-2 and FLAG–MKP-5) were assessed by immunoblot using anti-HA (12CA5) and anti-FLAG M2 antibodies respectively.
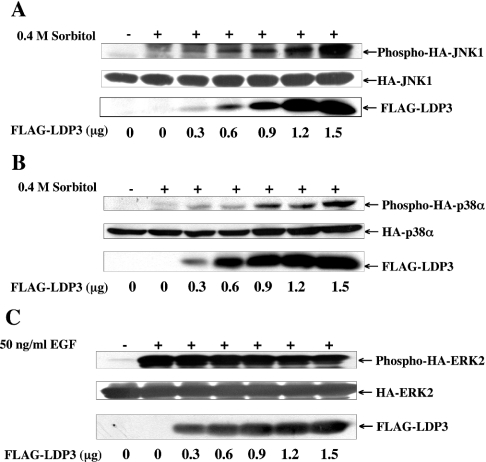
The dose dependency of FLAG–LDP-3 was also examined (Figure 7). Co-transfection of 0.3 μg of pFLAG-LDP-3 expression plasmid enhanced the phosphorylation levels of HA–JNK1 and HA–p38α, and further enhancement was observed in a dose-dependent manner up to 1.5 μg of plasmid (Figures 7A and 7B). In contrast, FLAG–LDP-3 at the amounts indicated did not affect the phosphorylation level of HA–ERK2 (Figure 7C).
Figure 7. Dose-dependent effects of LDP-3 on JNK and p38 activation.
COS-7 cells were co-transfected with 0.5 μg of pSRα-HA-JNK1 (A), pMT3-HA-p38α (B) or pSRα-HA-ERK2 (C) together with 0.3, 0.6, 0.9, 1.2 or 1.5 μg of pFLAG-LDP-3. The total amount of DNA in each transfection was adjusted to 1.5 μg with pFLAG-CMV. At 36 h after transfection, cells were stimulated with 0.4 M sorbitol for 30 min (A and B) or 50 ng/ml EGF for 15 min (C). Phosphorylated HA–JNK1, HA–p38α and HA–ERK2 were detected by anti-ACTIVE JNK, anti-phospho-p38α and anti-phospho-ERK antibodies respectively. The expression levels of HA–MAPKs (HA–ERK2, HA–JNK1 and HA–p38α) and FLAG-tagged phosphatases (FLAG–LDP-3, FLAG–VHR, FLAG–MKP-2 and FLAG–MKP-5) were assessed by immunoblot using anti-HA (12CA5) and anti-FLAG M2 antibodies respectively.
Inactive LDP-3 enhances the activation of JNK and p38 in COS-7 cells
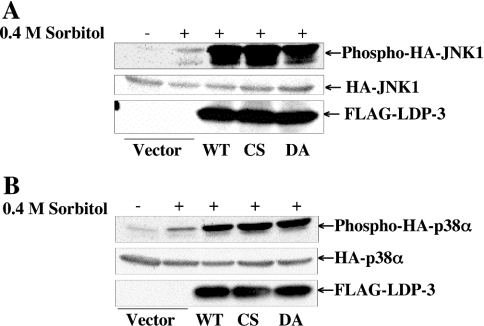
We then tested the effects of two inactive LDP-3 mutants, C95S and D65A, on the phosphorylation state of JNK and p38 in cells following stimulation by 0.4 M sorbitol (Figure 8). The phosphatase activities of the C95S and D65A mutants of LDP-3 expressed in COS-7 cells were determined using pNPP. Neither mutant exhibited phosphatase activity in this assay (results not shown). The expression levels of FLAG–LDP-3 wild type and FLAG–LDP-3 C95S and D65A mutant proteins were similar. Both mutant proteins enhanced phosphorylation of HA–JNK1 and HA–p38α to the same extent as did the wild-type protein.
Figure 8. Activation of JNK and p38 by inactive mutants of LDP-3.
COS-7 cells were co-transfected with 0.5 μg of pSRα-HA-JNK1 (A) or pMT3-HA-p38α (B) together with 1.5 μg of pFLAG-CMV, pFLAG-LDP-3, pFLAG-LDP-3CS or pFLAG-LDP-3DA. After 36 h, cells were stimulated with 0.4 M sorbitol for 30 min. Phosphorylated HA–JNK1 and HA–p38α were detected by anti-ACTIVE JNK and anti-phospho-p38 antibodies respectively. The expression levels of HA–JNK1 and HA–p38α were determined by immunoblot with anti-HA (12CA5) antibody. The expression levels of wild-type FLAG–LDP-3 (WT) and the mutants FLAG–LDP-3CS (CS) and FLAG–LDP-3DA (DA) were determined by immunoblot with anti-FLAG antibody.
LDP-3 enhances the activation of MKK4 and MKK6 in COS-7 cells
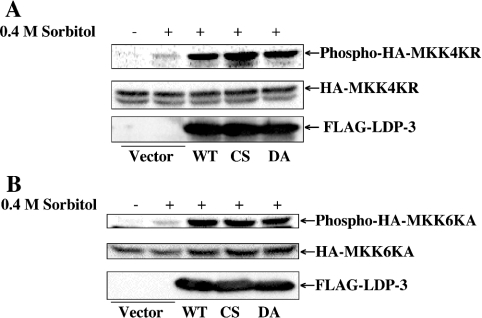
To determine whether hyperphosphorylation of JNK and p38 in the presence of LDP-3 is due to the activation of upstream kinases, we analysed the effects of LDP-3 on the phosphorylation levels of MAPKKs (Figure 9). For this purpose, HA–MKK4KR and HA–MKK6KA, both of which have a mutation at the ATP binding site, were used. Forced expression of LDP-3 wild type and the C95S and D65A mutants enhanced phosphorylation levels of HA–MKK4KR and HA–MKK6KA. Enhancement of MAPKK activation by LDP-3 is similar to that seen with MAPKs, suggesting that enhanced phosphorylation of JNK and p38 by LDP-3 results from the activity of their respective upstream kinases.
Figure 9. Effects of LDP-3 on MKK4 and MKK6 activation.
COS-7 cells were co-transfected with 0.5 μg of pcDNA-HA-MKK4KR (A) or pcDNA-HA-MKK6KA (B) together with 1.5 μg of pFLAG-CMV, pFLAG-LDP-3, pFLAG-LDP-3CS or pFLAG-LDP-3DA. After 36 h, cells were stimulated with 0.4 M sorbitol for 30 min. Phosphorylated HA–MKK4KR and HA–MKK6KA were determined by immunoblot with phospho-SEK1/MKK4 and phospho-MKK3/MKK6 antibodies respectively (SEK is stress-activated protein kinase/ERK kinase). The expression levels of HA–MKK4KR and HA–MKK6KA were determined by immunoblot with anti-HA antibody. The expression levels of FLAG-tagged wild-type LDP-3 (WT) and its CS and DA mutants were assessed by immunoblot using anti-FLAG antibody.
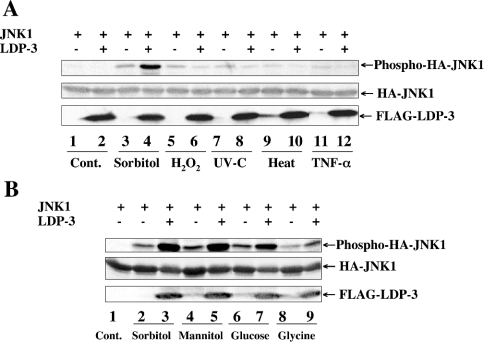
Specific enhancement by LDP-3 of osmotic stress-induced JNK and p38 activation
We next asked whether LDP-3 enhances JNK activation when cells are treated with other stimuli, including H2O2, UV-C, heat shock and TNF-α. As shown in Figure 10(A), these external stimuli induced phosphorylation of HA–JNK1 to similar levels. Under these conditions, LDP-3 showed an enhancing effect on JNK1 activation only when cells were treated with sorbitol (Figure 10A, lane 4). Similar results were observed for p38α activation (results not shown). We then analysed the effects of other osmotic stresses, such as mannitol, glucose and glycine (Figure 10B). FLAG–LDP-3 enhanced the activation of JNK1 induced by mannitol, glucose or glycine as well as sorbitol. These results suggest that enhancement of JNK1 and p38α activation by LDP-3 is specific to osmotic stress.
Figure 10. Enhancing effect of LDP-3 on JNK is selective for osmotic stress.
(A) COS-7 cells were co-transfected with 0.5 μg of pSRα-HA-JNK1 together with 1.5 μg of pFLAG-CMV (lanes 1, 3, 5, 7, 9 and 11) or pFLAG-LDP-3 (lanes 2, 4, 6, 8, 10 and 12). After 36 h of culture, cells were not treated (Cont.; lanes 1 and 2), or treated with 0.4 M sorbitol for 30 min (lanes 3 and 4), 50 μM H2O2 for 30 min (lanes 5 and 6), 25 J/m2 UV-C (lanes 7 and 8), heat shock at 42 °C for 30 min (lanes 9 and 10) or 100 ng/ml TNF-α for 30 min (lanes 11 and 12). (B) COS-7 cells were co-transfected with 0.5 μg of pSRα-HA-JNK1 together with 1.5 μg of pFLAG-CMV (lanes 1, 2, 4, 6 and 8) or pFLAG-LDP-3 (lanes 3, 5, 7 and 9). After culture for 36 h, cells were not treated (lane 1), or treated with 0.4 M sorbitol for 30 min (lanes 2 and 3), 0.4 M mannitol for 30 min (lanes 4 and 5), 0.4 M glucose for 30 min (lanes 6 and 7) or 0.4 M glycine for 30 min (lanes 8 and 9). Phosphorylated HA–JNK1 was detected by anti-ACTIVE JNK antibody. The expression levels of HA–JNK1 and FLAG–LDP-3 were assessed by immunoblot using anti-HA and anti-FLAG antibodies respectively.
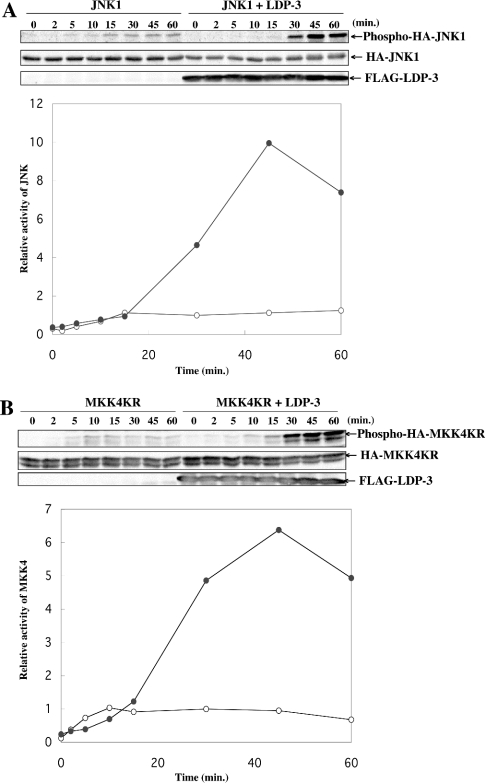
Time course of the enhancement of JNK and MKK4 activation by LDP-3
We examined the time courses of JNK1 and MKK4 activation induced by 0.4 M sorbitol with or without LDP-3 (Figure 11). In the absence of LDP-3, JNK1 activation reached a maximum level within 15 min. By contrast, increased phosphorylation of JNK1 in the presence of LDP-3 was observed after 15 min, and the value continued to increase, reaching an approx. 10-fold increase at 45 min (Figure 11A). Similarly, phosphorylation of MKK4 in the absence of LDP-3 reached a maximum level at 10 min, whereas phosphorylation of MKK4 in the presence of LDP-3 continued to increase, reaching an approx. 6.5-fold increase at 45 min (Figure 11B).
Figure 11. Time courses of JNK and MKK4 activation in the presence or absence of LDP-3 upon sorbitol stimulation.
COS-7 cells were co-transfected with 0.5 μg of pSRα-HA-JNK1 (A) or pcDNA-HA-MKK4KR (B) together with 1.5 μg of pFLAG-CMV or pFLAG-LDP-3. After 36 h of culture, cells were treated with 0.4 M sorbitol and incubated for the indicated times. Phosphorylated HA–JNK1 and HA–MKK4KR were detected by anti-ACTIVE JNK and phospho-SEK1/MKK4 antibodies respectively (SEK is stress-activated protein kinase/ERK kinase). The levels of expression of HA–JNK1 and HA–MKK4KR were determined by immunoblot with anti-HA antibody. Expression levels of FLAG–LDP-3 were assessed by immunoblot using anti-FLAG antibody. Signals were detected with a Fuji LAS 1000 image analyser and quantified. The graph shows the relative intensity of phosphorylated JNK and MKK4 in the absence (○) or presence (•) of LDP-3, at various time points. Intensities relative to that seen in cells stimulated with 0.4 M sorbitol for 30 min without forced expression of FLAG–LDP-3 are presented. Data are representative of three independent experiments.
DISCUSSION
The present study demonstrates that LDP-3 is a novel member of the small DSP family, which also contains VHR [6], LDP-1/TMDP (testis- and skeletal-muscle-specific DSP). [14], LDP-2/SKRP1/LMW-DSP3 [9,10,21], DUSP-18 [22], and JSP-1/VHX (VHR-related MKPX)/LMW-DSP2 and its splice variant, JKAP (JNK pathway-associated phosphatase) [11,23–25]. In vitro characterization of the enzyme activity of bacterially expressed LDP-3 revealed its preferential dephosphorylation of tyrosine residues in proteins, as reported previously for LDP-2, DUSP-18 and JSP-1 [9,21,23].
Some small DSPs have been shown to be involved in negative regulation of the MAPK pathway [7–10]. However, the function of one of these, JSP-1/VHX/LMW-DSP2/JKAP, in the MAPK pathway remains controversial. VHX has been shown to possess a higher dephosphorylation activity than VHR [24], and LMW-DSP2 can dephosphorylate JNK and p38 in vitro and in vivo [11]. By contrast, overexpression of JKAP or JSP-1 results in activation of JNK in the absence of stimuli, but phosphatase-dead mutants do not show such effects [23,25]. A deficiency of JKAP abolishes JNK activation [25]. These results indicate that JKAP/JSP-1 acts as a positive regulator of the JNK pathway and that its phosphatase activity is required for this effect. On the other hand, overexpression of SKRP1 activates the JNK pathway, but its enzyme activity is not required for such an effect [26]. Coimmunoprecipitation experiments suggest that SKRP1 positively activates the JNK pathway, possibly as a scaffold protein, by interacting with JNK2, MKK7 and ASK1 (apoptosis signal-regulating kinase 1). In the present study, we showed that LDP-3 is a positive regulator of the JNK and p38 kinase pathways. Since JNK and p38 respond to a variety of cell stresses, we undertook a detailed analysis of the specificity of LDP-3 function with regard to JNK and p38 activation. Overexpression of LDP-3 in the absence of stimuli did not induce activation of JNK or p38, showing that overexpression of LDP-3 in itself does not constitute a stress that induces JNK and p38 activation (Figures 5A and 5B). However, overexpression of LDP-3 significantly enhanced the phosphorylation of JNK and p38 induced by 0.4 M sorbitol, mannitol, glucose or glycine (Figures 5A, 5B and 10B). Such an enhancing effect of LDP-3 on the JNK and p38 pathways was not observed when cells were treated with oxidative stress, heat shock, UV irradiation or TNF-α (Figure 10B). Therefore we conclude that LDP-3 is a specific enhancer of osmotic stress-induced JNK and p38 activation.
Two potential mechanisms for how LDP-3 enhances activation of the JNK pathway induced by osmotic stress are possible. One is that LDP-3 functions as a scaffold protein, enabling efficient MAPK activation driven by osmotic stress; the other is that LDP-3 functions as an inhibitor of negative regulators of this pathway. Observations derived from the time course experiment (Figure 11) provide support for the latter mechanism. Since the time course of MKK4 activation in the presence of LDP-3 is similar to that of JNK1, target(s) of LDP-3 could be either MAPKKs or targets upstream of MAPKKs. In the absence of LDP-3, levels of activation of MKK4 were maximal at 10 min and constant until 60 min, suggesting the activity at 10 min of a negative regulator that blocks the hyperactivation of MKK4. Interestingly, LDP-3 did not enhance activation of MKK4 until 10 min, suggesting that LDP-3 does not amplify physiological positive regulation. Enhancement was apparent only after 10 min, suggesting that LDP-3 inhibits negative regulation upstream of MKK4 by dephosphorylating unidentified protein(s). Figures 8 and 9 show that the C95S and D65A mutants of LDP-3 enhanced the activation of JNK and p38 pathways in the same manner as the wild-type protein. It has been reported that both C95S and D65A mutants can function as substrate-trapping mutants [27,28], and that misexpression of these mutants can promote the same phenotypes as the wild-type protein in certain pathways [29,30]. Therefore it is likely that the C95S and D65A mutants of LDP-3 also inhibit the negative regulation of MAPKs induced by osmotic stress by associating with a putative substrate(s). Alternatively, it is possible that LDP-3 functions as a competitor of a negative regulator(s). If this were the case, the phosphatase activity of LDP-3 would not necessarily be essential.
Although the substrates of LDP-3 are not known, FLAG–LDP-3 was shown here to be cytoplasmic and localized predominantly in the submembrane fraction. Recently it was reported that Rac, MEKK3 (MEK kinase 3) and OSM (osmosensing scaffold for MEKK3) localize to actin ruffles, and that osmotic stress is transmitted to the MAPK cascade via Rac GTPases [31]. It is important, therefore, to determine whether LDP-3 interacts with this signalling complex.
Acknowledgments
We thank Dr J.M. Kryiakis for pMT-HA-p38α, and Dr M. Karin for pSRα-HA-JNK1 and pSRα-HA-ERK2. We are grateful to Dr H. Saito for advice and suggestions. We also thank Mrs E. Yoshida for secretarial assistance. This work was supported by Grant-in-Aids for Scientific Research (B) and (C) provided by the Japanese Society for the Promotion of Science, and a grant-in-aid for Scientific Research on Priority Areas (C) provided by the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and in part by a grant from the Akiyama Foundation.
References
- 1.Tonks N. K., Neel B. G. Combinatorial control of the specificity of protein tyrosine phosphatases. Curr. Opin. Cell Biol. 2001;13:182–195. doi: 10.1016/s0955-0674(00)00196-4. [DOI] [PubMed] [Google Scholar]
- 2.Iliakis G., Wang Y., Guan J., Wang H. DNA damage checkpoint control in cells exposed to ionizing radiation. Oncogene. 2003;22:5834–5847. doi: 10.1038/sj.onc.1206682. [DOI] [PubMed] [Google Scholar]
- 3.Geymonat M., Jensen S., Johnston L. H. Mitotic exit: the Cdc14 double cross. Curr. Biol. 2002;12:R482–R484. doi: 10.1016/s0960-9822(02)00963-6. [DOI] [PubMed] [Google Scholar]
- 4.Pearson G., Robinson F., Beers Gibson T., Xu B. E., Karandikar M., Berman K., Cobb M. H. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 5.Muda M., Theodosiou A., Gillieron C., Smith A., Chabert C., Camps M., Boschert U., Rodrigues N., Davies K., Ashworth A., Arkinstall S. The mitogen-activated protein kinase phosphatase-3 N-terminal noncatalytic region is responsible for tight substrate binding and enzymatic specificity. J. Biol. Chem. 1998;273:9323–9329. doi: 10.1074/jbc.273.15.9323. [DOI] [PubMed] [Google Scholar]
- 6.Ishibashi T., Bottaro D. P., Chan A., Miki T., Aaronson S. A. Expression cloning of a human dual-specificity phosphatase. Proc. Natl. Acad. Sci. U.S.A. 1992;89:12170–12174. doi: 10.1073/pnas.89.24.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Todd J. L., Tanner K. G., Denu J. M. Extracellular regulated kinases (ERK) 1 and ERK2 are authentic substrates for the dual-specificity protein-tyrosine phosphatase VHR. A novel role in down-regulating the ERK pathway. J. Biol. Chem. 1999;274:13271–13280. doi: 10.1074/jbc.274.19.13271. [DOI] [PubMed] [Google Scholar]
- 8.Alonso A., Saxena M., Williams S., Mustelin T. Inhibitory role for dual specificity phosphatase VHR in T cell antigen receptor and CD28-induced Erk and Jnk activation. J. Biol. Chem. 2001;276:4766–4771. doi: 10.1074/jbc.M006497200. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura K., Tanoue K., Satoh T., Takekawa M., Watanabe M., Shima H., Kikuchi K. A novel low-molecular-mass dual-specificity phosphatase, LDP-2, with a naturally occurring substitution that affects substrate specificity. J. Biochem. (Tokyo) 2002;132:463–470. doi: 10.1093/oxfordjournals.jbchem.a003244. [DOI] [PubMed] [Google Scholar]
- 10.Zama T., Aoki R., Kamimoto T., Inoue K., Ikeda Y., Hagiwara M. A novel dual specificity phosphatase SKRP1 interacts with the MAPK kinase MKK7 and inactivates the JNK MAPK pathway. Implication for the precise regulation of the particular MAPK pathway. J. Biol. Chem. 2002;277:23909–23918. doi: 10.1074/jbc.M200837200. [DOI] [PubMed] [Google Scholar]
- 11.Aoyama K., Nagata M., Oshima K., Matsuda T., Aoki N. Molecular cloning and characterization of a novel dual specificity phosphatase, LMW-DSP2, that lacks the cdc25 homology domain. J. Biol. Chem. 2001;276:27575–27583. doi: 10.1074/jbc.M100408200. [DOI] [PubMed] [Google Scholar]
- 12.Masuda K., Shima H., Katagiri C., Kikuchi K. Activation of ERK induces phosphorylation of MAPK phosphatase-7, a JNK specific phosphatase, at Ser-446. J. Biol. Chem. 2003;278:32448–32456. doi: 10.1074/jbc.M213254200. [DOI] [PubMed] [Google Scholar]
- 13.Alonso A., Rojas A., Godzik A., Mustelin T. The dual-specific protein tyrosine phosphatase family. In: Ariño J., Alexander D. R., editors. Protein Phosphatases. Berlin, Heiderberg and New York: Springer Verlag; 2004. pp. 333–358. [Google Scholar]
- 14.Nakamura K., Shima H., Watanabe M., Haneji T., Kikuchi K. Molecular cloning and characterization of a novel dual-specificity protein phosphatase possibly involved in spermatogenesis. Biochem. J. 1999;344:819–825. [PMC free article] [PubMed] [Google Scholar]
- 15.Masuda K., Shima H., Watanabe M., Kikuchi K. MKP-7, a novel mitogen-activated protein kinase phosphatase, functions as a shuttle protein. J. Biol. Chem. 2001;276:39002–39011. doi: 10.1074/jbc.M104600200. [DOI] [PubMed] [Google Scholar]
- 16.Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 17.Shnyra A., Brewington R., Alipio A., Amura C., Morrison D. C. Reprogramming of lipopolysaccharide-primed macrophages is controlled by a counterbalanced production of IL-10 and IL-12. J. Immunol. 1998;160:3729–3736. [PubMed] [Google Scholar]
- 18.Takekawa M., Posas F., Saito H. A human homolog of the yeast Ssk2/Ssk22 MAP kinase kinase kinases, MTK1, mediates stress-induced activation of the p38 and JNK pathways. EMBO J. 1997;16:4973–4982. doi: 10.1093/emboj/16.16.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu Y., Solski P. A., Khosravi-Far R., Der C. J., Kelly K. The mitogen-activated protein kinase phosphatases PAC1, MKP-1, and MKP-2 have unique substrate specificities and reduced activity in vivo toward the ERK2 sevenmaker mutation. J. Biol. Chem. 1996;271:6497–6501. doi: 10.1074/jbc.271.11.6497. [DOI] [PubMed] [Google Scholar]
- 20.Tanoue T., Moriguchi T., Nishida E. Molecular cloning and characterization of a novel dual specificity phosphatase, MKP-5. J. Biol. Chem. 1999;274:19949–19956. doi: 10.1074/jbc.274.28.19949. [DOI] [PubMed] [Google Scholar]
- 21.Cheng H., Gao Q., Jiang M., Ma Y., Ni X., Guo L., Jin W., Cao G., Ji C., Ying K., et al. Molecular cloning and characterization of a novel human protein phosphatase, LMW-DSP3. Int. J. Biochem. Cell Biol. 2003;35:226–234. doi: 10.1016/s1357-2725(02)00127-9. [DOI] [PubMed] [Google Scholar]
- 22.Wu Q., Gu S., Dai J., Wang L., Li Y., Zeng L., Xu J., Ye X., Zhao W., Ji C., et al. Molecular cloning and characterization of a novel dual-specificity phosphatase18 gene from human fetal brain. Biochim. Biophys. Acta. 2003;1625:296–304. doi: 10.1016/s0167-4781(02)00629-2. [DOI] [PubMed] [Google Scholar]
- 23.Shen Y., Luche R., Wei B., Gordon M. L., Diltz C. D., Tonks N. K. Activation of the Jnk signaling pathway by a dual-specificity phosphatase, JSP-1. Proc. Natl. Acad. Sci. U.S.A. 2001;98:13613–13618. doi: 10.1073/pnas.231499098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alonso A., Merlo J. J., Na S., Kholod N., Jaroszewski L., Kharitonenkov A., Williams S., Godzik A., Posada J. D., Mustelin T. Inhibition of T cell antigen receptor signaling by VHR-related MKPX (VHX), a new dual specificity phosphatase related to VH1 related (VHR) J. Biol. Chem. 2002;277:5524–5528. doi: 10.1074/jbc.M107653200. [DOI] [PubMed] [Google Scholar]
- 25.Chen A. J., Zhou G., Juan T., Colicos S. M., Cannon J. P., Cabriera-Hansen M., Meyer C. F., Jurecic R., Copeland N. G., Gilbert D. J., et al. The dual specificity JKAP specifically activates the c-Jun N-terminal kinase pathway. J. Biol. Chem. 2002;277:36592–36601. doi: 10.1074/jbc.M200453200. [DOI] [PubMed] [Google Scholar]
- 26.Zama T., Aoki R., Kamimoto T., Inoue K., Ikeda Y., Hagiwara M. Scaffold role of a mitogen-activated protein kinase phosphatase, SKRP1, for the JNK signaling pathway. J. Biol. Chem. 2002;277:23919–23926. doi: 10.1074/jbc.M200838200. [DOI] [PubMed] [Google Scholar]
- 27.Bliska J. B., Clemens J. C., Dixon J. E., Falkow S. The Yersinia tyrosine phosphatase: specificity of a bacterial virulence determinant for phosphoproteins in the J774A.1 macrophage. J. Exp. Med. 1992;176:1625–1630. doi: 10.1084/jem.176.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garton A. J., Flint A. J., Tonks N. K. Identification of p130(cas) as a substrate for the cytosolic protein tyrosine phosphatase PTP-PEST. Mol. Cell. Biol. 1996;16:6408–6418. doi: 10.1128/mcb.16.11.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LaMontagne K. R., Jr, Flint A. J., Franza B. R., Jr, Pandergast A. M., Tonks N. K. Protein tyrosine phosphatase 1B antagonizes signalling by oncoprotein tyrosine kinase p210 bcr-abl in vivo. Mol. Cell. Biol. 1998;18:2965–2975. doi: 10.1128/mcb.18.5.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myers M. P., Andersen J. N., Cheng A., Tremblay M. L., Horvath C. M., Parisien J. P., Salmeen A., Barford D., Tonks N. K. TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J. Biol. Chem. 2001;276:47771–47774. doi: 10.1074/jbc.C100583200. [DOI] [PubMed] [Google Scholar]
- 31.Uhlik M. T., Abell A. N., Johnson N. L., Sun W., Cuevas B. D., Lobel-Rice K. E., Horne E. A., Dell'Acqua M. L., Johnson G. L. Rac-MEKK3-MKK3 scaffolding for p38 MAPK activation during hyperosmotic shock. Nat. Cell Biol. 2003;5:1104–1110. doi: 10.1038/ncb1071. [DOI] [PubMed] [Google Scholar]